Introduction
Primary hepatocellular carcinoma (HCC) is a
malignancy with high mortality rate, and it is common in developing
countries (1). HCC is a leading
cause of tumor-associated mortality in China, partly due to the
high occurrence rate of chronic hepatitis B virus infection
(2). Transcatheter arterial
embolization (TAE) has been widely used for the treatment of
inoperable hepatocellular carcinoma, and it has been shown to
decrease the tumor volume with promising effects (3); however, tumor recurrence and
metastasis are common after TAE (4,5), and
these processes may be caused by circulating tumor cells (CTCs).
CTCs have the ability to invade distant tissues and to survive in
various microenvironmental contexts (6). The expression of epidermal growth
factor receptor (EGFR), a transmembrane tyrosine kinase receptor
(7), has been identified to have
an important role in CTCs (6).
EGFR is dysregulated in many types of cancer, including HCC
(8). Although EGFR signaling was
identified to be critical for HCC growth and metastasis, and a
previous study identified that treatment with the EGFR inhibitor
cetuximab could reduce HCC growth following TAE, the effects of
other EGFR inhibitors, such as afatinib, and their possible
regulatory mechanisms in HCC cells following TAE remain unknown
(9,10). Afatinib, an EGFR-tyrosine kinase
inhibitor (TKI), was previously identified to repress cancer cell
growth by mitigating the activation of EGFR in tumor cells
(11). A previous study reported
that epithelial-mesenchymal transition (EMT) has a critical role in
the shedding of CTCs from primary tumors into blood vessels, and
EMT is an essential process in embryonic development and cancer
progression (12). EMT is
characterized by a loss of cell polarity, cell-cell adhesion, and
increased migratory and invasive abilities, which facilitate the
metastasis of cancer cells (13,14).
E-cadherin and Vimentin are involved in the EMT process (15). In addition, metastasis-associated
gene 1 (MTA1) (16,17) and T lymphoma invasion and
metastasis inducing factor 1 (TIAM1) are responsible for cell
migration and adhesion in tumorigenesis (18–20).
Therefore, afatinib may be involved in preventing EMT in HCC
following TAE.
Vascular endothelial growth factor (VEGF) is a
potent inducer of angiogenesis and was identified to be associated
with tumor angiogenesis (21).
Matrix metalloproteinase (MMPs) play critical roles in proteolytic
degradation and in altering cell adhesion, cell migration and EMT
during cancer-associated angiogenesis (22–24).
ERK is a member of the mitogen-activated protein kinase (MAPK)
signaling cascade (25), and was
identified to be involved in multiple biological processes
regulated by phosphorylation cascades, including gene expression,
cell survival and cell migration (26–28).
Therefore, the present study aimed to investigate the effects of
afatinib on HCC after TAE and the potential mechanisms underlying
its function. The present results suggested that afatinib was able
to effectively suppress the overactivation of EGFR. Moreover, the
expression levels of genes involved in proliferation, migration,
invasion and EMT were decreased following treatment with afatinib
through EGFR inhibition in HCC cells, and these effects may be
associated with the ERK-VEGF/MMP signaling pathway. The present
results may provide new insights into the mechanisms underlying the
prevention of HCC recurrence after TAE.
Materials and methods
Tissue specimens
In total, 50 patients with HCC underwent TAE
intervention therapy in Tiantai County People's Hospital between
June 2014 and April 2016. The inclusion criteria were the
following: i) All patients with HCC were diagnosed by histological
analysis; ii) all patients underwent curative surgery with no
presurgical treatment resulting in tissue necrosis; iii) the
patients did not receive radiotherapy or chemotherapy before
surgical intervention; and iv) no patient had concurrent presence
of another liver carcinoma. Signed written informed consent was
obtained from 42 patients for the use of their clinical tissues.
Eight patients did not provide written informed consent and were
thus excluded from the present study. The adjacent normal tissues
were collected at a distance of 5 cm from the tumor margin. The
median age was 50 years (range, 30–89 years). The tumor length was
between 0.5 and 8.5 cm (median, 5 cm). The protocols were performed
according to the Declaration of Helsinki. Paired tissues were
divided into two groups. Sections were stored in 4% formaldehyde at
−80°C for routine pathological diagnosis, whereas the other part
was frozen by immersion into liquid nitrogen and stored at −80°C.
Reverse transcription-quantitative PCR (RT-qPCR) and western blot
analysis were performed to examine the tissues. The association
between the expression level of EGFR and clinicopathological
features is presented in Table I.
The present study was approved by The Ethics Committee of Tiantai
County People's Hospital.
 | Table I.Association between EGFR expression
and various clinicopathological features. |
Table I.
Association between EGFR expression
and various clinicopathological features.
|
|
| Expression of
EGFR |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | n | Positive, n
(%) | Negative, n
(%) | P-value |
|---|
| Sex |
|
|
| 0.798 |
|
Male | 32 | 11 (34.4) | 21 (65.6) |
|
|
Female | 10 | 3 (30.0) | 7 (70.0) |
|
| Age, years |
|
|
| 0.172 |
|
≤50 | 15 | 7 (46.7) | 8 (53.3) |
|
|
>50 | 27 | 7 (25.9) | 20 (74.1) |
|
| Histology
differentiation |
|
|
| 0.127 |
|
High | 8 | 5 (62.5) | 3 (37.5) |
|
|
Moderate | 19 | 7 (36.8) | 12 (63.2) |
|
|
Low | 15 | 2 (20.0) | 13 (80.0) |
|
| Liver
cirrhosis |
|
|
| 0.35 |
|
Yes | 36 | 11 (30.6) | 25 (69.4) |
|
| No | 6 | 3 (50.0) | 3 (50.0) |
|
| α-fetoprotein |
|
|
| 0.469 |
|
≤400 | 30 | 11 (36.7) | 19 (63.3) |
|
|
>400 | 12 | 3 (25.0) | 9 (75.0) |
|
| Tumor diameter,
cm |
|
|
| 0.116 |
| ≤5 | 26 | 11 (42.3) | 15 (57.7) |
|
|
>5 | 16 | 3 (18.8) | 13 (81.3) |
|
| TNM stage |
|
|
| 0.075 |
|
I/II | 25 | 11 (44.0) | 14 (56.0) |
|
|
III/IV | 17 | 3 (17.6) | 14 (82.4) |
|
| Intravascular tumor
thrombus |
|
|
| 0.028a |
|
Yes | 19 | 3 (15.8) | 16 (84.2) |
|
| No | 23 | 11 (47.8) | 12 (52.2) |
|
| Portal vein tumor
thrombus |
|
|
| 0.736 |
|
Yes | 5 | 2 (40.0) | 3 (60.0) |
|
| No | 37 | 12 (32.4) | 25 (67.6) |
|
| Involving the liver
capsule |
|
|
| 0.005a |
|
Yes | 32 | 7 (21.9) | 25 (78.1) |
|
| No | 10 | 7 (70.0) | 3 (30.0) |
|
Cell culture and treatment
Huh-7 cells were purchased from Thermo Fisher
Scientific, Inc. The cells were maintained at 37°C in an incubator
with 5% CO2 and cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), streptomycin and penicillin (CoWin Biosciences
Co., Ltd.). Cells were plated in 6-well plates at a density of
1×105 cells/well and were starved overnight before
treatment. On the following day, the cells were treated with 25
ng/ml recombinant human EGF (PeproTech, Inc.) for 6 h to mimic the
overactivation of EGFR in HCC after TAE.
Cell transfection
Subsequently, the cells were transfected with small
interfering RNAs (siRNAs). EGFR siRNA (si-EGFR) and scrambled siRNA
(si-CTR) were purchased from Shanghai GenePharma Co., Ltd. In
total, 0.25 µg siRNA was transfected into Huh-7 cells, using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) as
transfection reagent. 24 h after transfection, cells were used for
following detection. Subsequently, 10 nmol afatinib (Selleck
Chemicals) was added to 100 µl cell culture medium for 18 h at
37°C. Treatment with DMSO (0.1%) was used as the control.
Assessment of cell viability
After 18 h of treatment with afatinib, the cells
were plated at a density of 1×103 cells/well in 96-well
plates. Then, cell viability was detected using a Cell Cycle Kit-8
(CCK-8) according to the manufacturer's protocol (Beyotime
Institute of Biotechnology). The CCK-8 solution was added and
incubated at 37°C. After 4 h, the medium was removed and a
microplate reader (Bio-Rad Laboratories, Inc.) was used for
determining absorbance values at 450 nm.
Detection of migratory and invasive
abilities of Huh-7 cells
Matrigel-coated Transwell inserts (Corning, Inc.)
were used to measure the migratory and invasive abilities of cancer
cells, as previously described (29). For the migration assay, the inserts
were not coated with Matrigel. Cells were plated at a concentration
of 2×105 cells/ml in the upper chamber and incubated for
24 h. Crystal violet (0.1%) was used for staining at 37°C for 20
min. The migrated or invaded cells were counted for 200 fold using
a light microscope (Nikon Corporation; magnification, ×200), and
the averages were calculated.
RT-qPCR
The relative gene expression data were analyzed by
RT-qPCR. Total RNA was extracted using RNeasy kit (Qiagen GmbH).
Subsequently, 1 µg RNA was reversely transcribed to cDNA using
High-capacity cDNA Reverse Transcription Kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The qPCR was performed using the ChamQ SYBR qPCR master
mix (Vazyme) on a Bio-Rad CFX96 system. The thermocycling
conditions were the following: Initial denaturation at 95°C for 15
sec, followed by 40 cycles of 95°C for 25 sec, 55°C for 25 sec and
72°C for 30 sec. RT-qPCR data were quantified using the
2−ΔΔCq method (30).
GAPDH was used as internal control gene. The sequences of the
primers used in the present study are listed in Table II.
 | Table II.Reverse transcription-quantitative
PCR primers. |
Table II.
Reverse transcription-quantitative
PCR primers.
| Gene symbol | Primer sequences
(5′-3′) |
|---|
| EGFR | F:
GCGCTACCTTGTCATTCAGG |
|
| R:
TATCAATGCAAGCCACGGTG |
| E-cadherin | F:
TCACATCCTACACTGCCCAG |
|
| R:
AGTGTCCCTGTTCCAGTAGC |
| MMP9 | F:
GCGTCTTCCCCTTCACTTTC |
|
| R:
ATAGGGTACATGAGCGCCTC |
| VEGF | F:
TTGCTGTGCTTTGGGGATTC |
|
| R:
CTGTCATGGGCTGCTTCTTC |
| Vimentin | F:
GAGAGGAAGCCGAAAACACC |
|
| R:
TTCCTGAATCTGAGCCTGCA |
| MTA1 | F:
ACAGACAAGCAGATCGACCA |
|
| R:
GGCCTTGGAGATGTCGTAGA |
| TIAM1 | F:
ACTGTCTCTCTGAAGGTGCC |
|
| R:
GGTGAGTAGCTGGAGTTGGT |
| GAPDH | F:
CACAGTCCATGCCATCACTG |
|
| R:
ATCTCGCTCCTGGAAGATGG |
Western blotting
Total protein was extracted using RIPA buffer
(Boster Biological Technology) and 1 mmol/l PMSF and separated by
SDS-PAGE on 12% gels. Next, the proteins were transferred to PVDF
membranes (EMD Millipore). Subsequently, 2% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) was added to block nonspecific
binding. The PVDF membranes were incubated overnight at 4°C with
primary antibodies (all from Cell Signaling Technology, Inc.)
against phosphorylated (p-)EGFR (cat. no. 4407, 1:1,000), EGFR
(cat. no. 3197; 1,000), MMP9 (cat. no. 3852; 1,000), p-ERK (cat.
no. 9101; 1,000), ERK (cat. no. 9102; 1,000), VEGF (cat. no. 2463;
1,000), E-cadherin (cat. no. 3195; 1,000), Vimentin (cat. no. 5741;
1,000), TIAM1 (cat. no. 63647; 1,000), MTA1 (cat. no. 5646; 1,000)
and GAPDH (cat. no. 2118; 1:2,000). After being washed with PBS,
the membranes were incubated with a horseradish peroxidase-labeled
secondary antibody (cat. no. 7074; 1:5,000; Cell Signaling
Technology, Inc.). The bands were visualized using an ECL kit
(Pierce; Thermo Fisher Scientific, Inc.). Digital images of
immunoreactive bands were analyzed using the Bio-Rad ChemiDoc XRS+
System with Image Lab Software (version 1708265; Bio-Rad
Laboratories, Inc.).
Statistical analysis
SPSS 22.0 (IBM Corp.) and GraphPad Prism 6 software
(GraphPad Software, Inc.) were used for statistical analysis. Data
are presented as the mean ± SD. The χ2 test was used to
analyze the association between the expression level of EGFR and
various clinical features or between the expression levels of EGFR
and E-cadherin. One-way ANOVA followed by Dunnett's post-hoc test
was used to compare multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression level of EGFR in HCC after
TAE
The mRNA and protein expression level of EGFR in HCC
tissues was investigated using RT-qPCR and western blot analysis,
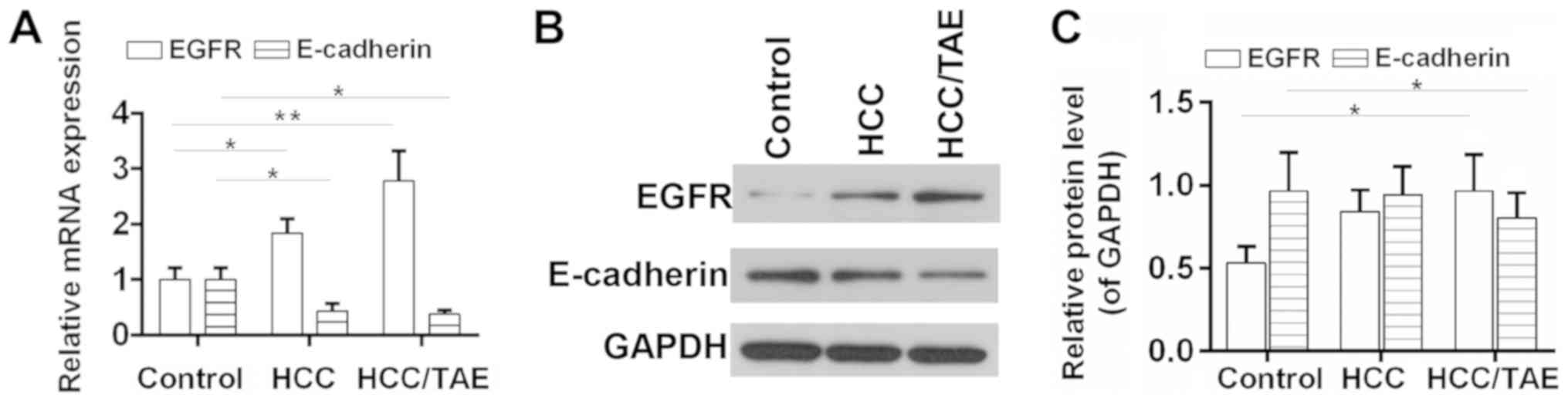
respectively. The results suggested that EGFR was upregulated in
HCC tissues and increased following TAE compared with the control
group (Fig. 1). The association
between the expression level of EGFR and various
clinicopathological features was also examined (Table I). The expression level of EGFR was
significantly associated with intravascular tumors and the
retraction/lesion of the liver capsule, but not with gender, age
and tumor diameter. In addition, the expression level of E-cadherin
was significantly reduced in HCC tissues, in particular after TAE
(Fig. 1). Although the expression
levels of EGFR and E-cadherin were not significantly associated
with each other, 18 patients (66.7%) exhibited positive expression
of EGFR and negative expression of E-cadherin (Table III). Sample exhibiting higher
levels than the control group were considered positive, whereas
samples with lower levels were considered negative.
 | Table III.Association between the expression
levels of EGFR and E-cadherin. |
Table III.
Association between the expression
levels of EGFR and E-cadherin.
|
| E-cadherin
expression |
|
|
|---|
|
|
|
|
|
|---|
| EGFR
expression | - (%) | + (%) | n | P-value |
|---|
| − | 7 (46.7) | 8 (53.3) | 15 | 0.206 |
| + | 18 (66.7) | 9 (33.3) | 27 |
|
Afatinib modulates the expression
levels of EMT- and metastasis-associated genes in HCC cells
EMT is involved in the migration of tumor cells,
thus increasing the tumor metastatic ability (31). RT-qPCR and western blot analysis
showed that knockdown of EGFR expression by siRNA decreased the
protein expression level ratio of p-EGFR/EGFR. Moreover, afatinib
could effectively suppress the phosphorylation of EGFR; whereas the
knockdown of EGFR expression by siRNA decreased the ratio of
p-EGFR/EGFR. Moreover, afatinib could effectively decrease the
ratio of p-EGFR/EGFR (Fig. 2A-C).
Additionally, the mRNA and protein expression levels of EMT- and
metastasis-associated genes were examined by RT-qPCR and western
blot, respectively. The expression levels of E-cadherin and TIAM1,
which are negative regulators of EMT (32,33),
were increased following EGFR knockdown or afatinib-mediated
inhibition of EGFR. However, the expression levels of Vimentin and
MTA1, which are positive regulators of EMT (34,35),
were decreased in EGFR knockdown group or the afatinib group
(Fig. 2D-F).
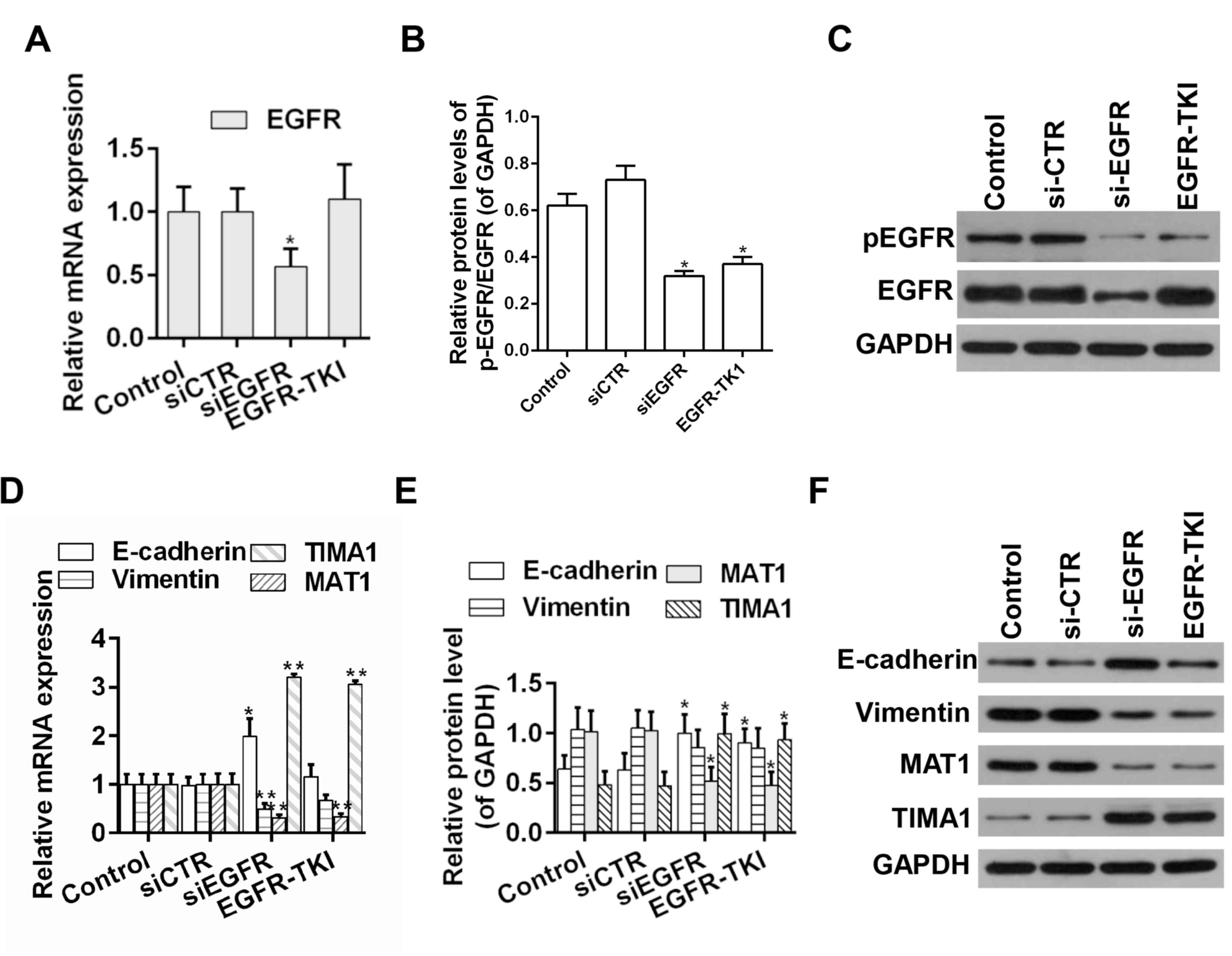 | Figure 2.Detection of the expression levels of
EGFR, E-cadherin, Vimentin, MTA1 and TIAM1 in HCC tumor samples.
(A) mRNA and (B) protein expression level of EGFR. (C) Western blot
analysis of EGFR and p-EGFR. (D) mRNA and (E) protein expression
levels of E-cadherin, Vimentin, MTA1 and TIAM1. (F) Western blot
analysis of E-cadherin, Vimentin, MTA1 and TIAM1. n=3. *P<0.05,
**P<0.01 vs. corresponding control. EGFR, epidermal growth
factor receptor; MTA1, metastasis associated 1; TIAM1, T cell
lymphoma invasion and metastasis 1; si-CTR, scrambled siRNA;
si-EGFR, siRNA targeting EGFR; siRNA, small interference RNA; p-,
phosphorylated; EGFR-TKI, afatinib treatment; TKI, tyrosine-kinase
inhibitor. |
Afatinib inhibits viability, migration
and invasion of HCC cells
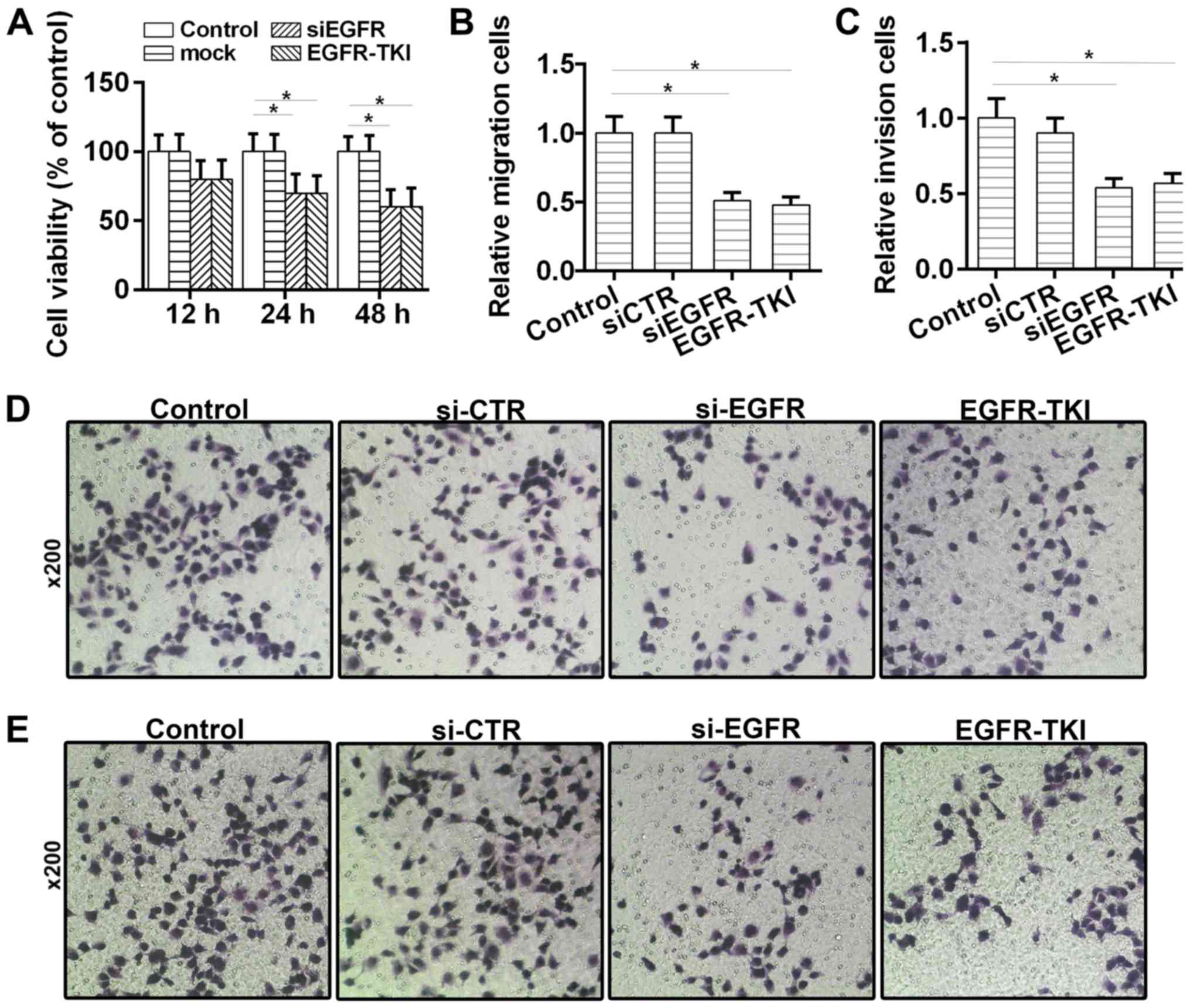
The effects of afatinib on HCC tumorigenesis were
investigated in vitro. The CCK-8 results suggested that cell
viability was reduced (Fig. 3A) in
the EGFR-TKI group. Moreover, the Transwell assays suggested that
the migratory and invasive abilities of HCC cells were
significantly decreased following treatment with afatinib (Fig. 3B-E). Collectively, the present
results suggested that afatinib could inhibit the viability,
migration and invasion of HCC cells through EGFR inhibition.
Activity of the ERK-VEGF/MMP9
signaling pathway is decreased by afatinib through EGFR
inhibition
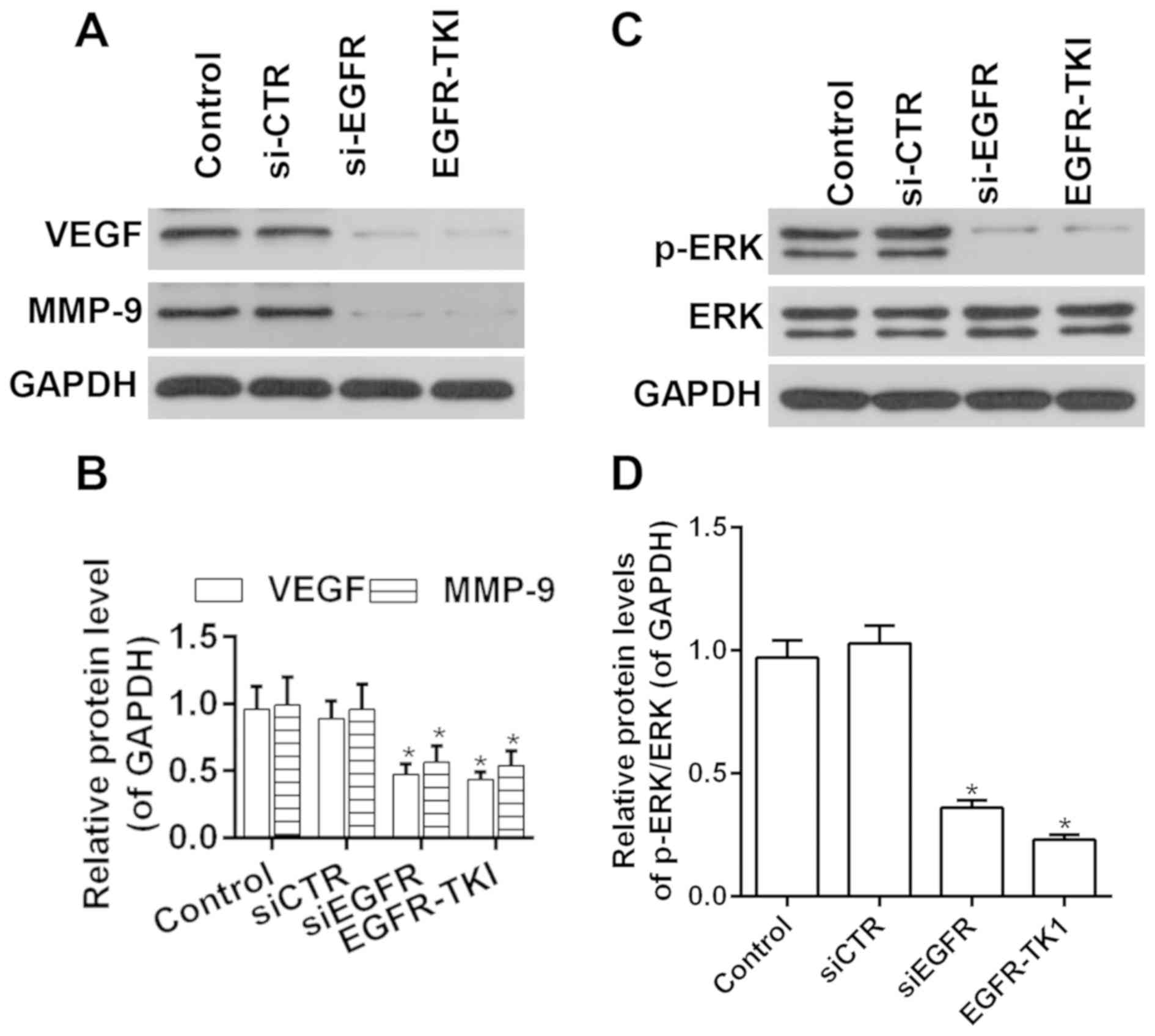
To investigate the molecular mechanisms underlying
afatinib function, the activity of ERK and the expression levels of
VEGF and MMP9 were investigated following treatment with afatinib.
RT-qPCR and western blot analysis results suggested that the
expression levels of VEGF and MMP9 were decreased by afatinib
through EGFR inhibition (Fig. 4A and
B). Moreover, the phosphorylation level of ERK was
significantly decreased after treatment with afatinib through EGFR
inhibition, and the ratio of p-ERK/ERK was significantly decreased
after treatment with afatinib through EGFR inhibition (Fig. 4C and D). The present results
suggested that afatinib decreased the activity of the ERK-VEGF/MMP9
signaling pathway in vitro.
Discussion
HCC is a type of malignant cancer with high
incidence rates worldwide (1). TAE
is an effective palliative treatment for inoperable HCC.
Nevertheless, a high incidence of HCC recurrence and metastasis was
reported after TAE (5). Multiple
intracellular signals are altered in HCC cells following TAE,
leading to an increased number of CTCs, thus promoting metastasis
and tumor recurrence (4,5). As one of the most widely investigated
receptor tyrosine kinase families, EGFRs serve important roles in
signal transduction and oncogenesis (36). Previous studies reported that EGFR
signaling is critical for HCC growth, metastasis and invasion
(37,38). Additionally, dysregulation of EGFR
is common in certain types of tumors (39). Accumulating evidence suggested that
EGFR is overactivated or mutated in HCC; Ikeda et al
(40) performed next-generation
sequencing to investigate circulating tumor DNA, and found that 14%
of patients with HCC presents an increased number of copies of
EGFR. EGFR was activated in HCC cells (41). In addition, Panvichian et al
(42) demonstrated that EGFR
overexpression and mutations in the EGFR gene are present in HCC.
EMT contributes to the growth and metastasis of cancer cells in the
liver (31). VEGF has an important
role in the proliferation and differentiation of endothelial cells
(21). In addition, MMPs serve key
roles in the degradation of basement membrane collagen and
extracellular matrix (43).
Interestingly, afatinib, a TKI able to suppress the activity of
EGFR, can interact with the ATP-binding site of EGFR, blocking its
enzymatic activity (44). In a
previous study, EGFRWT and EGFRL858R/T790M
kinase inhibition assays identified that afatinib has significant
inhibitory activities, and its IC50 values, tested in
A549, HepG2, MCF-7 and PC-3 cell lines, are in the nanomolar range
(45). Moreover, afatinib was
reported to have inhibitory effects on non-small-cell lung cancer
(NSCLC) and has been approved for the therapy of metastatic NSCLC
by the Food and Drug Administration (45,46).
Therefore, the present study aimed to investigate the effects of
afatinib on HCC and its underlying molecular mechanism.
Fang et al (47), reported that possible mechanisms
underlying the metastatic potential of HCC cells following TAE are
the acquisition of EMT features and the increased number of CTCs.
The present study not only examined the expression levels of genes
involved in the mechanism underlying HCC metastasis, but also
suggested a possible EGFR-targeted therapeutic strategy.
E-cadherin is downregulated during cell migration
and metastasis of tumor cells, which are associated with EMT
(48–51). In the present study, the
association between the expression levels of EGFR and E-cadherin
was analyzed, and the present results suggested that the expression
level of EGFR was not significantly associated with the expression
level of E-cadherin; however, 18 patients (66.7%) were
EGFR-positive and E-cadherin-negative. A higher number of samples
are required to confirm the association between the expression
levels of these two genes. The present results suggested that EGFR
could be used as a target for the prevention of EMT in HCC after
TAE. In the present study, the effects of afatinib on the activity
of EGFR signaling were investigated. Additionally, the expression
levels of E-cadherin and Vimentin, two genes associated with EMT,
increased and decreased following afatinib-mediated EGFR
inhibition, respectively. Moreover, the expression levels of the
metastasis-associated genes MTA1 and TIAM1 were decreased and
increased in the EGFR-TKI group, respectively. Wang et al
(52) suggested that silencing
EGFR or inhibiting the phosphorylation of molecules downstream of
the EGFR pathway could inhibit EMT and metastasis in HCC. Deng
et al (53) reported that
reducing the activity of HER2, a member of the EGFR family, can
decrease the promotion of EMT by increasing the expression level of
MTA1 in HCC both in vitro and in vivo, suggesting
that the EGFR-MTA-EMT1 pathway may be context-specific. In
addition, the acquisition of EMT features was observed in lung
cancer cell lines, and is associated with an increased resistance
to afatinib (54). Nevertheless,
EMT-associated resistance to afatinib in the treatment of HCC
requires further validation in clinical studies. A previous study
reported that the effects of combined treatment with bufalin and
afatinib is associated with the inhibition of EMT in lung cancer
cells (55). Therefore, combined
treatments and conversion therapies may be used in the treatment of
HCC. In addition, CCK-8 and Transwell assays were performed to
investigate the effects of afatinib on tumorigenesis. The present
results suggested that afatinib decreased the proliferation,
migration and invasion of HCC cells through EGFR inhibition. The
inhibitory effect of afatinib on the migration and metastasis of
HCC cells was in accordance with a previous study describing the
effects of afatinib on NSCLC (46). In the present study, the molecular
mechanism of afatinib was investigated in HCC cells.
The MAPK/ERK pathway is involved in numerous
biological events, including cancer progression (26,28,56).
In the present study, the protein expression level of p-ERK was
identified to be reduced by afatinib in vitro. The present
results suggested that afatinib may exert its inhibitory effect on
tumorigenesis by inactivating the ERK signaling pathway. The
present findings are in line with a previous study that showed
induction of apoptosis in Eca109 cells following knockdown of ERK2
(8). Nevertheless, a previous
study reported that the effect of the ERK signaling pathway depends
on the cellular context and the crosstalk with other signaling
pathways (46). In addition, VEGF
and MMPs are responsible for tumor angiogenesis, growth and
metastasis (57–59). Zhang et al (60) investigated the effects of metformin
in combination with curcumin on the growth and progression of HCC,
and the downregulation of the expression levels of MMP2/9, VEGF and
VEGF receptor-2 were identified to be associated with the
suppression of EGFR signaling. This previous study is in line with
the present study, which suggested that the expression levels of
VEGF and MMP9 were decreased by afatinib through EGFR inhibition in
HCC cells. Previous studies demonstrated that the VEGF signaling
pathway promotes the activation of MMPs in endothelial cells
(57,58,61).
However, the present study did not investigate whether VEGF
regulated MMP9, which were previously shown to be associated
(62). Additionally, previous
studies showed that the MAPK/ERK pathway is downstream of the VEGF
signaling pathway (63,64); in contrast, a previous study showed
that the upregulation of VEGF is dependent on ERK activation
(65). Therefore, ERK may regulate
VEGF signaling in a positive feedback mechanism. Therefore,
examining the hierarchy between ERK and VEGF could improve the
understanding of the molecular mechanism investigated in the
present study. However, the present findings may increase the
understanding of HCC, facilitating the development of novel
treatments. The present study presented certain limitations,
including the lack of clinical studies. In addition, to examine the
effects of agonists of the ERK signaling pathway may validate the
molecular mechanism identified in the present study.
Collectively, the present study identified that
afatinib could not only effectively suppress the proliferation,
migration and invasion of HCC cells, but also regulated the
expression levels of EMT- and metastasis-associated genes through
EGFR inhibition. Furthermore, the inhibitory effects of afatinib on
tumorigenesis were identified to be associated with the
ERK-VEGF/MMP9 signaling pathway. The present findings may
facilitate the development of a novel therapeutic strategy for the
recurrence of HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC conceived and designed the present study. XC and
XD performed the experiments. YW analyzed, collected and
interpreted the data.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Tiantai County People's Hospital. Written informed
consent was obtained from 42 patients for the use of their clinical
tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burkhart RA, Ronnekleiv-Kelly SM and
Pawlik TM: Personalized therapy in hepatocellular carcinoma:
Molecular markers of prognosis and therapeutic response. Surg
Oncol. 26:138–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagao E, Hirakawa M, Soeda H, Tsuruta S,
Sakai H and Honda H: Transcatheter arterial embolization for chest
wall metastasis of hepatocellular carcinoma. World J Radiol.
5:45–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang R, Zhao N, Li S, Fang JH, Chen MX,
Yang J, Jia WH, Yuan Y and Zhuang SM: MicroRNA-195 suppresses
angiogenesis and metastasis of hepatocellular carcinoma by
inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology.
58:642–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Germano D and Daniele B: TIE2-expressing
monocytes as a diagnostic marker for hepatocellular carcinoma
correlates with angiogenesis. Hepatobiliary Surg Nutr. 3:166–167.
2014.PubMed/NCBI
|
|
6
|
Serrano MJ, Alvarez-Cubero MJ, De Miguel
Pérez D, Rodríguez-Martínez A, Gonzalez-Herrera L, Robles-Fernandez
I, Hernandez JE, Puche JLG and Lorente JA: Significance of EGFR
expression in circulating tumor cells. Adv Exp Med Biol.
994:285–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komposch K and Sibilia M: EGFR signaling
in liver diseases. Int J Mol Sci. 17:E302015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong L, Liao D, Zhang M, Zeng C, Li X,
Zhang R, Ma H and Kang T: YTHDF2 suppresses cell proliferation and
growth via destabilizing the EGFR mRNA in hepatocellular carcinoma.
Cancer Lett. 442:252–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu L, Liu R, Zhang W, Qian S and Wang J:
Application of EGFR inhibitor reduces circulating tumor cells
during transcatheter arterial embolization. Clin Transl Oncol.
20:639–646. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keating GM: Afatinib: A review in advanced
non-small cell lung cancer. Target Oncol. 11:825–835. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li JY, Huang WX, Zhou X, Chen J and Li Z:
Numb inhibits epithelial-mesenchymal transition via
RBP-Jκ-dependent Notch1/PTEN/FAK signaling pathway in tongue
cancer. BMC Cancer. 19:3912019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng H and Li H: The encouraging role of
long noncoding RNA small nuclear RNA host gene 16 in
epithelial-mesenchymal transition of bladder cancer via directly
acting on miR-17-5p/metalloproteinases 3 axis. Mol Carcinog.
2019.(Epub ahead of print). View
Article : Google Scholar
|
|
15
|
Felipe Lima J, Nofech-Mozes S, Bayani J
and Bartlett JM: EMT in breast carcinoma-a review. J Clin Med.
5:E652016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toh Y, Pencil SD and Nicolson GL: A novel
candidate metastasis-associated gene, mta1, differentially
expressed in highly metastatic mammary adenocarcinoma cell lines.
cDNA cloning, expression, and protein analyses. J Biol Chem.
269:22958–22963. 1994.PubMed/NCBI
|
|
17
|
Toh Y, Pencil SD and Nicolson GL: Analysis
of the complete sequence of the novel metastasis-associated
candidate gene, mta1, differentially expressed in mammary
adenocarcinoma and breast cancer cell lines. Gene. 159:97–104.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minard ME, Kim LS, Price JE and Gallick
GE: The role of the guanine nucleotide exchange factor Tiam1 in
cellular migration, invasion, adhesion and tumor progression.
Breast Cancer Res Treat. 84:21–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen B, Ding Y, Liu F, Ruan J, Guan J,
Huang J, Ye X, Wang S, Zhang G, Zhang X, et al: Tiam1,
overexpressed in most malignancies, is a novel tumor biomarker. Mol
Med Rep. 5:48–53. 2012.PubMed/NCBI
|
|
20
|
Yu LN, Zhang QL, Li X, Hua X, Cui YM,
Zhang NJ, Liao WT and Ding YQ: Tiam1 transgenic mice display
increased tumor invasive and metastatic potential of colorectal
cancer after 1,2-dimethylhydrazine treatment. PLoS One.
8:e730772013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gomes E and Rockwell P: p38 MAPK as a
negative regulator of VEGF/VEGFR2 signaling pathway in serum
deprived human SK-N-SH neuroblastoma cells. Neurosci Lett.
431:95–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Liu T, Nishioka M, Aguirre RL, Win
SS and Okada N: Activation of ERK1/2 and cyclin D1 expression in
oral tongue squamous cell carcinomas: Relationship between
clinicopathological appearances and cell proliferation. Oral Oncol.
42:625–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q and Yang Z: Expression of
phospho-ERK1/2 and PI3-K in benign and malignant gallbladder
lesions and its clinical and pathological correlations. J Exp Clin
Cancer Res. 28:652009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Park H, Yang Y, Kim TS, Bang SI
and Cho D: Enhancement of cell migration by corticotropin-releasing
hormone through ERK1/2 pathway in murine melanoma cell line,
B16F10. Exp Dermatol. 16:22–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henry C, Llamosas E, Knipprath-Meszaros A,
Schoetzau A, Obermann E, Fuenfschilling M, Caduff R, Fink D, Hacker
N, Ward R, et al: Targeting the ROR1 and ROR2 receptors in
epithelial ovarian cancer inhibits cell migration and invasion.
Oncotarget. 6:40310–40326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jayachandran A, Dhungel B and Steel JC:
Epithelial-to- mesenchymal plasticity of cancer stem cells:
Therapeutic targets in hepatocellular carcinoma. J Hematol Oncol.
9:742016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Wu Q, Zhang Y, Zhang HN, Wang YB
and Wang W: TGF-β1-induced epithelial-mesenchymal transition in
lung cancer cells involves upregulation of miR-9 and downregulation
of its target, E-cadherin. Cell Mol Biol Lett. 22:222017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu G, Zhang Y, Wang Q, Che S, Yang Y,
Chen L and Lin Z: The prognostic value of Tiam1 correlates with its
roles in epithelial-mesenchymal transition progression and
angiogenesis in lung adenocarcinoma. Cancer Manag Res.
11:1741–1752. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015.PubMed/NCBI
|
|
35
|
Lin X, Zheng L, Song H, Xiao J, Pan B,
Chen H, Jin X and Yu H: Effects of microRNA-183 on
epithelial-mesenchymal transition, proliferation, migration,
invasion and apoptosis in human pancreatic cancer SW1900 cells by
targeting MTA1. Exp Mol Pathol. 102:522–532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roskoski R Jr: Small molecule inhibitors
targeting the EGFR/ErbB family of protein-tyrosine kinases in human
cancers. Pharmacol Res. 139:395–411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding D, Huang H, Jiang W, Yu W, Zhu H, Liu
J, Saiyin H, Wu J, Huang H, Jiang S and Yu L: Reticulocalbin-2
enhances hepatocellular carcinoma proliferation via modulating the
EGFR-ERK pathway. Oncogene. 36:6691–6700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye QH, Zhu WW, Zhang JB, Qin Y, Lu M, Lin
GL, Guo L, Zhang B, Lin ZH, Roessler S, et al: GOLM1 modulates
EGFR/RTK cell-surface recycling to drive hepatocellular carcinoma
metastasis. Cancer Cell. 30:444–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: A model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ikeda S, Tsigelny IF, Skjevik ÅA, Kono Y,
Mendler M, Kuo A, Sicklick JK, Heestand G, Banks KC, Talasaz A, et
al: Next-generation sequencing of circulating tumor DNA reveals
frequent alterations in advanced hepatocellular carcinoma.
Oncologist. 23:586–593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li R, Yanjiao G, Wubin H, Yue W, Jianhua
H, Huachuan Z, Rongjian S and Zhidong L: Secreted GRP78 activates
EGFR-SRC-STAT3 signaling and confers the resistance to sorafeinib
in HCC cells. Oncotargt. 8:19354–19364. 2017.
|
|
42
|
Panvichian R, Tantiwetrueangdet A,
Sornmayura P and Leelaudomlipi S: Missense mutations in exons 18–24
of EGFR in hepatocellular carcinoma tissues. Biomed Res Int.
2015:1718452015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen BH and Giudice LC: Dysfunctional
uterine bleeding. West J Med. 169:280–284. 1998.PubMed/NCBI
|
|
44
|
Solca F, Dahl G, Zoephel A, Bader G,
Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E and Adolf
GR: Target binding properties and cellular activity of afatinib
(BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp
Ther. 343:342–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tu Y, Wang C, Yang Z, Zhao B, Lai L, Yang
Q, Zheng P and Zhu W: Discovery of novel quinazoline derivatives
bearing semicarbazone moiety as potent EGFR kinase inhibitors.
Comput Struct Biotechnol J. 16:462–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Roof AK and Gutierrez-Hartmann A: Consider
the context: Ras/ERK and PI3K/AKT/mTOR signaling outcomes are
pituitary cell type-specific. Mol Cell Endocrinol. 463:87–96. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fang ZT, Wang GZ, Zhang W, Qu XD, Liu R,
Qian S, Zhu L, Zhou B and Wang JH: Transcatheter arterial
embolization promotes liver tumor metastasis by increasing the
population of circulating tumor cells. OncoTargets Ther.
6:1563–1572. 2013.
|
|
48
|
Ishiyama N, Lee SH, Liu S, Li GY, Smith
MJ, Reichardt LF and Ikura M: Dynamic and static interactions
between p120 catenin and E-cadherin regulate the stability of
cell-cell adhesion. Cell. 141:117–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang WG, Grimshaw D, Martin TA, Davies G,
Parr C, Watkins G, Lane J, Abounader R, Laterra J and Mansel RE:
Reduction of stromal fibroblast-induced mammary tumor growth, by
retroviral ribozyme transgenes to hepatocyte growth factor/scatter
factor and its receptor, c-MET. Clin Cancer Res. 9:4274–4281.
2003.PubMed/NCBI
|
|
50
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Puisieux A: Role of epithelial-mesenchymal
transition in tumor progression. Bull Acad Natl Med. 193:2017–2032;
discussion 2032–2034. 2009.(In French). PubMed/NCBI
|
|
52
|
Wang ZC, Gao Q, Shi JY, Guo WJ, Yang LX,
Liu XY, Liu LZ, Ma LJ, Duan M, Zhao YJ, et al: Protein tyrosine
phosphatase receptor S acts as a metastatic suppressor in
hepatocellular carcinoma by control of epithermal growth factor
receptor-induced epithelial-mesenchymal transition. Hepatology.
62:1201–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Deng L, Tang J, Yang H, Cheng C, Lu S,
Jiang R and Sun B: MTA1 modulated by miR-30e contributes to
epithelial-to-mesenchymal transition in hepatocellular carcinoma
through an ErbB2-dependent pathway. Oncogene. 36:3976–3985. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hashida S, Yamamoto H, Shien K, Miyoshi Y,
Ohtsuka T, Suzawa K, Watanabe M, Maki Y, Soh J, Asano H, et al:
Acquisition of cancer stem cell-like properties in non-small cell
lung cancer with acquired resistance to afatinib. Cancer Sci.
106:1377–1384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kang X, Lu P, Cui Y, Wang Y, Zhang Q, Gong
Y and Xu Z: Bufalin reverses hepatocyte growth factor-induced
resistance to afatinib in H1975 lung cancer cells. Zhonghua Zhong
Liu Za Zhi. 37:490–496. 2015.(In Chinese). PubMed/NCBI
|
|
56
|
Song L, Li W, Zhang H, Liao W, Dai T, Yu
C, Ding X, Zhang L and Li J: Over-expression of AEG-1 significantly
associates with tumour aggressiveness and poor prognosis in human
non-small cell lung cancer. J Pathol. 219:317–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ng EW, Shima DT, Calias P, Cunningham ET
Jr, Guyer DR and Adamis AP: Pegaptanib, a targeted anti-VEGF
aptamer for ocular vascular disease. Nat Rev Drug Discov.
5:123–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mukhopadhyay D, Nagy JA, Manseau EJ and
Dvorak HF: Vascular permeability factor/vascular endothelial growth
factor-mediated signaling in mouse mesentery vascular endothelium.
Cancer Res. 58:1278–1284. 1998.PubMed/NCBI
|
|
60
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Brown DM and Regillo CD: Anti-VEGF agents
in the treatment of neovascular age-related macular degeneration:
Applying clinical trial results to the treatment of everyday
patients. Am J Ophthalmol. 144:627–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang M, Huang B, Li G and Zeng S:
Apatinib affect VEGF-mediated cell proliferation, migration,
invasion via blocking VEGFR2/RAF/MEK/ERK and PI3K/AKT pathways in
cholangiocarcinoma cell. BMC Gastroenterol. 18:1692018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fearnley GW, Smith GA, Abdul-Zani I,
Yuldasheva N, Mughal NA, Homer-Vanniasinkam S, Kearney MT, Zachary
IC, Tomlinson DC, Harrison MA, et al: VEGF-A isoforms program
differential VEGFR2 signal transduction, trafficking and
proteolysis. Biol Open. 5:571–583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Almalki SG and Agrawal DK: ERK signaling
is required for VEGF-A/VEGFR2-induced differentiation of porcine
adipose-derived mesenchymal stem cells into endothelial cells. Stem
Cell Res Ther. 8:1132017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mu X, Zhao T, Xu C, Shi W, Geng B, Shen J,
Zhang C, Pan J, Yang J, Hu S, et al: Oncometabolite succinate
promotes angiogenesis by upregulating VEGF expression through
GPR91-mediated STAT3 and ERK activation. Oncotarget. 8:13174–13185.
2017. View Article : Google Scholar : PubMed/NCBI
|