Introduction
Prostate cancer is the most common reported
malignancy of men in developed countries (1). A total of 307,500 men worldwide
succumb to prostate cancer annually (1). The pathogenesis of prostate cancer
involves multiple factors, including age, race, familial heredity
and androgen levels (2). Androgen
deprivation therapy is the most common clinical approach to treat
prostate cancer. However, in the majority of patients
castration-resistant prostate cancer (CRPC) develops, for which
current anticancer treatments have yet to report positive clinical
outcomes (3,4). Therefore, examining possible novel
therapies is of great importance for the clinical treatment of
prostate cancer.
In the recent years, the role of long noncoding RNAs
(lncRNAs) in cancer pathogenesis and progression has gained
increasing attention. LncRNAs are a class of endogenous noncoding
RNAs with >200 nucleotides (5).
LncRNA metastasis-associated lung adenocarcinoma transcript 1
(MALAT1), also known as nuclear-enriched abundant transcript 2, was
first indicated to be highly expressed in non-small cell lung
cancer with a potential metastatic promoting role (6). Subsequently, MALAT1 was reported to
be a nuclear-enriched transcript and widely conserved among 33
mammalian species (7). MALAT1 has
been demonstrated to promote proliferation, migration, invasion,
cell cycle transition and tumorigenesis in multiple types of
malignant tumors, including ovarian cancer, cervical cancer and
gastric cancer (8–11). MALAT1 is also indicated to be
overexpressed in prostate cancer and silencing of MALAT1 inhibits
proliferation, migration and invasion, and induces cell cycle
arrest in prostate cancer cells (12).
MALAT1 has been reported to serve a binding role to
microRNAs (miRs/miRNAs) by acting as a miRNA sponge or a
competitive endogenous RNA (ceRNA). For instance, MALAT1 sponges
miR-22 and upregulates the miR-22 targets matrix metallopeptidase
14 and Snail, and consequently enhances malignant melanoma growth
and metastasis (13). In breast
cancer cells, MALAT1 sponges miR-1-3p in order to promote
malignancies (14,15). In addition, miR-1-3p is
downregulated in prostate cancer specimens and its expression is
negatively associated with prognosis. The overexpression of
miR-1-3p inhibits proliferation and invasion in prostate cancer
cells (16). Therefore, the aim of
the present study is to examine whether the function of MALAT1 in
prostate cancer is associated with miR-1-3p. This study
investigated the association between MALAT1 and miR-1-3p in
prostate cancer, and examined the underlying mechanism of
MALAT1.
Materials and methods
Plasmid construction
To construct the lncRNA MALAT1 knockdown plasmid,
two primers containing a sequence targeting the
5′-GGAAGATAGAAACAAGATA-3′ in the MALAT1 transcript were
synthesized. The two primers were annealed and inserted into the
pRNAH1.1 vector (Genscript, Nanjing, Jiangsu, China) at
BamHI and HindIII sites. The sequence containing a
negative control (NC) segment was inserted into the pRNAH1.1 vector
and served as the control. The sequence information of the primers
is presented in Table I.
 | Table I.The information of knockdown,
amplification PCR and qPCR primers used in this study. |
Table I.
The information of knockdown,
amplification PCR and qPCR primers used in this study.
| Name | Sequence
(5′-3′) |
|---|
| shMALAT1 S |
GATCCCCGGAAGATAGAAACAAGATATTCAAGAGATATCTTGTTTCTATCTTCCTTTTT |
| shMALAT1 AS |
AGCTAAAAAGGAAGATAGAAACAAGATATCTCTTGAATATCTTGTTTCTATCTTCCGGG |
| shRNA NC S |
GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT |
| shRNA NC AS |
AGCTAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG |
| CORO1C CDS F |
CAAGGATCCATGAGGCGAGTGGTACGACAG |
| CORO1C CDS R |
CGCCTCGAGTCAGGCTGCTATCTTTGCCAT |
| MALAT1
amplification PCR F |
CACGGATCCGATAAAGGCTGAGTGTTGAG |
| MALAT1
amplification PCR F |
CAACTCGAGCTTCGCATACGTGTGTCTGC |
| MALAT1 qPCR F |
GACTTCAGGTCTGTCTGTTCT |
| MALAT1 qPCR R |
CAACAATCACTACTCCAAGC |
| GAPDH qPCR F |
GAAGGTCGGAGTCAACGGAT |
| GAPDH qPCR R |
CCTGGAAGATGGTGATGGGAT |
| miR-1-3p RT |
GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACATACAT |
| miR-1-3p qPCR
F |
GCGGGCTGGAATGTAAAG |
| U6 RT | GTTGGCTCTG
GTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAAAATATGG |
| U6 qPCR F |
GCTTCGGCAGCACATATACT |
| qPCR Reverse |
GTGCAGGGTCCGAGGTATTC |
To establish the overexpression plasmid of coronin
1C (CORO1C), the coding sequence (CDS) was amplified with human
cDNA as the template, in the presence of CORO1C CDS primers.
Following sequence analysis, the amplification segment was inserted
into pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with BamHI and XhoI sites. The
MALAT1 sequence targeted by miR-1-3p was also cloned into the
pcDNA3.1 vector with BamHI and XhoI sites, in the
presence of amplification PCR primers. The information regarding
the CORO1C CDS and MALAT1 amplification PCR primers is presented in
Table I.
Cell culture and transfection
Prostatic epithelial cell line RWPE2 was purchased
from Shanghai Zhongqiaoxinzhou Biotech (Shanghai, China) and
cultured with prostatic epithelial cell medium (Shanghai
Zhongqiaoxinzhou Biotech) at 37°C in 5% CO2. Prostate
cancer cell lines DU145 and PC-3 were purchased from Procell Life
Science & Technology Co., Ltd., (Wuhan, China), and cultured
with minimum essential medium (MEM) or Ham's F-12K medium (Procell
Life Science & Technology Co., Ltd.) supplemented with 10%
fetal bovine serum (FBS; Biological Industries, Kibbutz Beit
Haemek, Israel). Prostate cancer cell lines LNCaP and 22RV1 were
purchased from Shanghai Zhongqiaoxinzhou Biotech and cultured with
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% FBS. The cells were seeded in 6-well
plates and transfected with short hairpin RNA (sh)MALAT1 plasmid
(~6.8 kb) with Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific Inc.). Following 24 h, the cells were
treated with 100 µg/ml G418 for 2 weeks and returned to normal
medium. Subsequent to culturing for ~2 weeks, the monoclonal cell
masses were selected. The MALAT1-knocked down cells were
obtained.
After culturing for 24 h, the LNCaP and 22RV1 cells
were transfected with miR-1-3p mimics or inhibitor in serum-free
medium, in the presence of Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). A total of 4 h later, the
transfection medium was replaced with normal medium and the cells
were cultured for other experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using a TRIpure RNA
extraction kit (BioTek China, Beijing, China) and the concentration
was measured with an ultraviolet spectrophotometer (Thermo Fisher
Scientific Inc.). The RNA was reverse transcribed into cDNA with
M-MLV reverse transcriptase (BioTek China) and 2×Power Taq PCR
Master Mix buffer (BioTeke), in the presence of oligo(dT) and
random primers, or specific miRNA RT primers (Sangon Biotech Co.,
Ltd., Shanghai, China): Denaturation at 70°C for 2 min, annealing
at 25°C for 10 min, and extension at 42°C for 50 min. Subsequently,
the cDNA was used for RT-qPCR with SYBR Green (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) to detect the
expression levels of MALAT1 and miR-1-3p, with GAPDH or U6 as the
respective internal control. The procedure was as follows: 94°C for
5 min 10 sec, 60°C for 20 sec, 72°C for 30 sec, and 40 cycles of
72°C for 2 min 30 sec, 40°C for 1 min 30 sec, melting from 60–94°C
each 1°C for 1 sec, and finally incubation at 25°C for several
minutes. The data were calculated using the 2−ΔΔCq
method (17). The information on
primers is presented in Table
I.
Western blot analysis
The protein was extracted with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and the concentration was determined
with a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Following protein denaturation by boiling, the
proteins were separated with SDS-PAGE (5, 8, 10 and 14% separation
gel for different sized proteins) and transferred onto a
polyvinylidene difluoride (PVDF) membrane (EMD Millipore,
Billerica, MA, USA). Following blocking with 5% skimmed milk at
room temperature for 1 h, the PVDF membrane was incubated with one
of the following antibodies at 4°C overnight: Rabbit anti-CORO1C
(1:500; cat. no. 14749-1-AP; Wuhan Sanying Biotechnology, Wuhan,
China), mouse anti-epithelial (E)-cadherin (1:1,000; cat. no.
14472), rabbit anti-neural (N)-cadherin (1:1,000; cat. no. 4061),
rabbit anti-vimentin (1:500; cat. no. 5741; all Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-Slug (1:500; cat.
no. ab27568; Abcam, Cambridge, UK), rabbit anti-Snail (1:1,000;
cat. no. 3879; Cell Signaling Technology, Inc.). Following rinsing
with TBST (TBS buffer with 0.15% Tween-20), the PVDF membrane was
incubated with the corresponding secondary antibody labeled with
IgG at 37°C for 45 min. The PVDF membrane was reacted with enhanced
chemiluminescent reagent (Beyotime Institute of Biotechnology) for
5 min, followed by signal exposure. The immunoblotting bands were
analyzed with Gel-Pro-Analyzer 4 software (Media Cybernetics,
Rockville, MD, USA). GAPDH served as the internal control. The
GAPDH level was detected via incubation with rabbit anti-GAPDH
(1:500; cat. no. bs-2188R; Bioss, Beijing, China) and corresponding
secondary antibody as described above.
Bioinformatic analysis
StarBase v2.0 (http://starbase.sysu.edu.cn/) was used to predict the
candidate miRNAs of MALAT1. TargetScan 7.2 (http://www.targetscan.org/vert_72/) was subsequently
used to predict the targeted genes of miR-1-3p.
Dual-luciferase reporter assay
To demonstrate the binding of MALAT1 to miR-1-3p,
the DNA segment containing the sequence miR-1-3p, was cloned into
the dual-luciferase reporter, pmirGLO (Promega Cooperation,
Madison, WI, USA). The 293T cells (Procell Life Science &
Technology Co., Ltd.) were transfected with the dual-luciferase
reporter containing the MALAT1 segment or its mutant sequence and
miR-1-3p mimics, in presence of Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific Inc.). Following 48
h, the cells were treated with dual-luciferase reporter assay
system (Promega Cooperation, Madison, WI, USA) and the firefly and
the Renilla luciferase activities were detected.
Similarly, the DNA segment containing CORO1C 3′UTR
sequence targeted by miR-1-3p, was cloned into dual-luciferase
reporter constructs. The firefly and Renilla luciferase
activities were detected.
Wound healing assay
The LNCaP and 22RV1 cells were cultured until they
reached 80% confluence, and treated with 1 µg/ml mitomycin C
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h. The wound
was made with a 200 µl pipette tip and the cells were subsequently
cultured. At 0 and 24 h, the wound size was viewed under an
inverted light microscope (magnification ×100; Olympus Corporation,
Tokyo, Japan).
Transwell assay
The Transwell assay was performed and membrane
precoated with Matrigel to detect the invasive ability of LNCaP and
22RV1 cells. The Transwell chamber with an 8.0 µm pore
polycarbonate membrane (Corning Inc., Corning, NY, USA) was
pre-coated with Matrigel (Becton, Dickinson and Company; BD
Biosciences, Franklin Lakers, NJ, USA) at 37°C. A total of 200 µl
cell suspension containing 2×104 cells was added into
the upper chamber and 800 µl medium containing 30% FBS was added
into the lower chamber. After 24 h culture at 37°C, the cells on
the reverse surface of the polycarbonate membrane were fixed with
4% paraformaldehyde at room temperature for 20 min, stained with
0.5% cresol violet at room temperature for 5 min and images were
captured under an inverted light microscope (×200
magnification).
Statistical analysis
The data in the present study were presented as the
mean ± standard deviation replicated in triplicate. Results were
analyzed with one-way or two-way analysis of variance with
Bonferroni's post hoc multiple comparisons. GraphPad Prism
5.01 (GraphPad Software, Inc., La Jolla, CA, USA) was used for
analysis of data in this study. P<0.05 was considered to
indicate a statistically significant difference.
Results
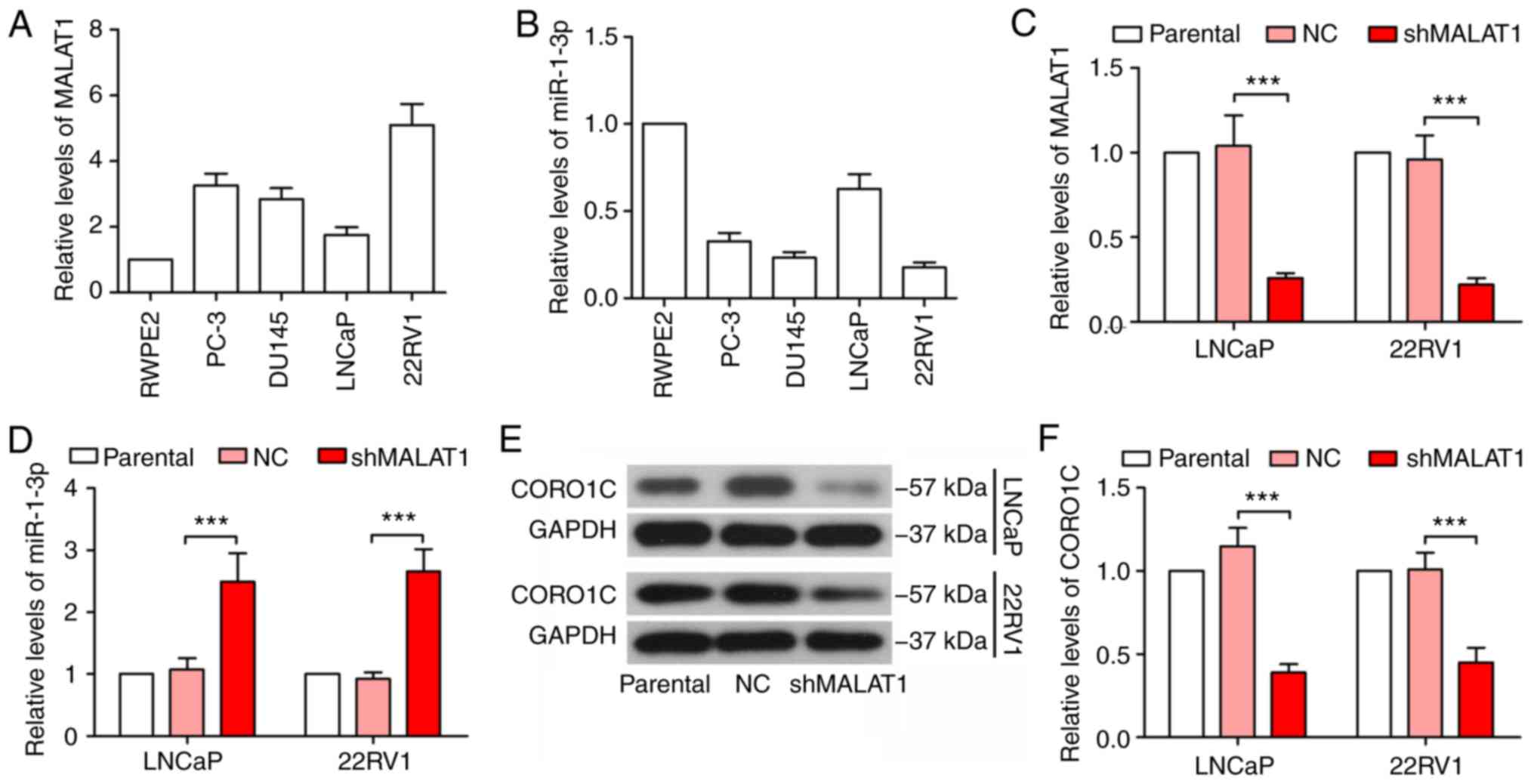
MALAT1 is increased in prostate cancer
cells and inhibits miR-1-3p expression
The expression levels of MALAT1 and miR-1-3p were
detected in the normal prostatic epithelial cell line, RWPE2 and
prostate cancer cell lines, PC-3, DU145, LNCaP and 22RV1. As
indicated in Fig. 1A and B, the
expression of MALAT1 was variably upregulated in prostate cancer
cells, compared with RWPE2 cells, while miR-1-3p was downregulated
in prostate cancer cells. LNCaP and 22RV1 cells were selected to
investigate the role of MALAT1, since the expression levels of
MALAT1 and miR-1-3p differed between LNCaP and 22RV1 cells. In
addition, LNCaP cells are androgen-dependent, whereas 22RV1 cells
are androgen-independent (18).
MALAT1 was significantly silenced in LNCaP and 22RV1 cells, and its
effectiveness was verified by RT-qPCR (P<0.001; Fig. 1C). The expression level of miR-1-3p
was significantly increased following MALAT1 knockdown (P<0.001;
Fig. 1D). At the same time, CORO1C
was decreased in MALAT1-silenced cells (Fig. 1E and F), suggesting that MALAT1
served a regulatory role in miR-1-3p and CORO1C expression
levels.
MALAT1 knockdown inhibits migration,
invasion and epithelial-mesenchymal transition (EMT) in prostate
cancer cells
Phenotypic experiments were performed to detect the
effect of MALAT1 on prostate cancer cell malignancy. As indicated
in Fig. 2, the knockdown of MALAT1
significantly decreased the migratory ability of prostate cancer
cells (P<0.01; Fig. 2A-C). In
addition, the MALAT1-silenced LNCaP and 22RV1 cells indicated a
significant decrease in invasive ability (P<0.01; Fig. 2D and E). Taking into consideration
the important role of EMT on cell migration and invasion in cancer
cells, a number of EMT-associated proteins were detected. The
results on the expression levels of E-cadherin indicated a
>3-fold increase and the expression levels of N-cadherin,
vimentin, Slug and Snail were decreased by ≥50% in MALAT1-silenced
prostate cancer cells compared with NC (Fig. 2F and H).
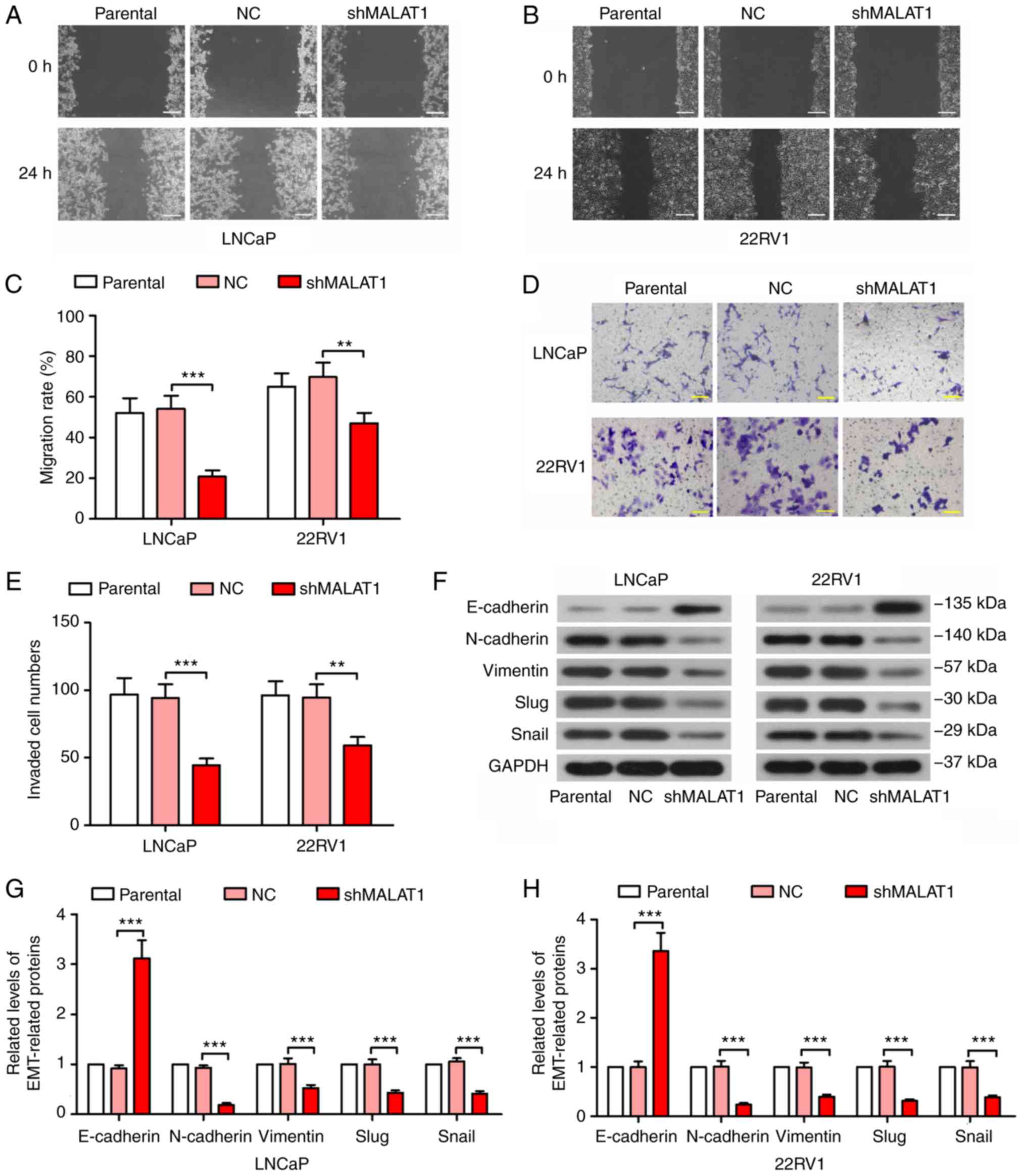 | Figure 2.MALAT1 knockdown inhibited migration,
invasion and EMT in prostate cancer cells. A wound healing assay
was performed to measure the migratory ability of (A) LNCaP and (B)
22RV1 cells following MALAT1 knockdown (Scale bar, 200 µm). (C) The
migration rate in A and B was quantified. A Transwell assay with
Matrigel was performed to detect the invasive ability of
MALAT1-silenced (D) LNCaP and (E) 22RV1 cells (Scale bar, 100 µm).
(F) The expression levels of several EMT-associated proteins,
E-cadherin, N-cadherin, vimentin, Slug and Snail in (G) LNCaP and
(H) 22RV1 cells. **P<0.01 and ***P<0.001. MALAT1,
metastasis-associated lung adenocarcinoma transcript 1; EMT,
epithelial-mesenchymal transition; NC, negative control; n, neural;
E, epithelial; sh, short hairpin. |
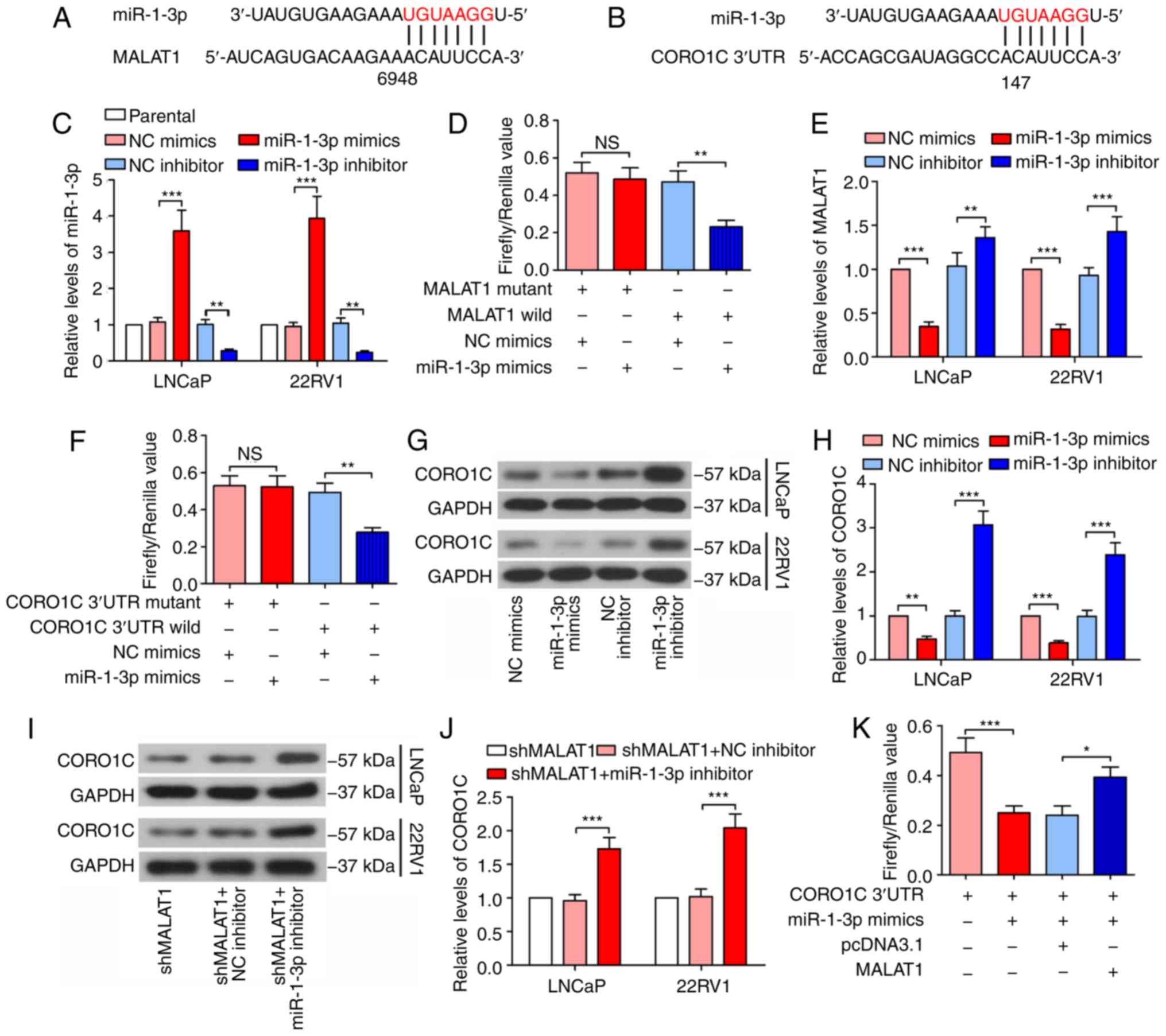
MALAT1is bound to miR-1-3p and
regulates CORO1C expression
Bioinformatics analysis was conducted using starBase
v2.0 (http://starbase.sysu.edu.cn/) to
predict the candidate miRNAs of MALAT1 and miR-1-3p was identified
(Fig. 3A). TargetScan 7.2
(http://www.targetscan.org/vert_72/)
was subsequently used and it was indicated that miR-1-3p seed
sequence directly binds to 3′UTR of CORO1C mRNA (Fig. 3B). Following confirmation of the
efficacy of the miR-1-3p mimic and inhibitor (Fig. 3C), a dual-luciferase reporter assay
results demonstrated that the firefly/Renilla value of the
wild type MALAT1 was decreased by 51% by the miR-1-3p mimic, and
the firefly/Renilla value of CORO1C 3′UTR was decreased by
43% by the miR-1-3p mimic in 293T cells (Fig. 3D and F), demonstrating the binding
of miR-1-3p to MALAT1 and 3′UTR of the CORO1C mRNA. As indicated in
Fig. 3E, the expression levels of
MALAT1 were decreased following transfection of with the miR-1-3p
mimic, while they decreased following miR-1-3p inhibition,
suggesting that MALAT1 may be a degradable sponge. The CORO1C
expression level decreased following miR-1-3p overexpression and
increased following miR-1-3p inhibition (Fig. 3G and H), in addition, the MALAT1
silencing-induced decrease of the CORO1C expression level was
rescued by the inhibition of miR-1-3p (Fig. 3I and J). In addition, the
dual-luciferase reporter assay demonstrated that MALAT1 attenuated
the binding of miR-1-3p to the CORO1C 3′UTR (the luciferase
activity of CORO1C 3′UTR + miR-1-3p mimics + MALAT1 group was
compared with that of CORO1C 3′UTR + miR-1-3p mimics + pcDNA3.1
group; Fig. 3K), suggesting that
MALAT1 additionally competes with CORO1C for the binding sites of
miR-1-3p.
The MALAT1 knockdown-induced
malignancy changes are recovered by miR-1-3p inhibition
To confirm the underlying mechanism of action of
MALAT1, phenotypic experiments were performed in MALAT1-silenced
cells following miR-1-3p inhibition. The wound healing assay
results revealed that the suppression of the migratory ability of
MALAT1 knockdown-induced cells was significantly recovered by
miR-1-3p in prostate cancer cells (P<0.01; Fig. 4A-C). Similar results were observed
in the Transwell assay (Fig. 4D and
E). Additionally, the expression level of E-cadherin was
decreased by ≥70% and the expression levels of N-cadherin,
vimentin, Slug and Snail indicated a 2-fold increase following
transfection with the miR-1-3p inhibitor (Fig. 4F and H), suggesting enhanced
EMT.
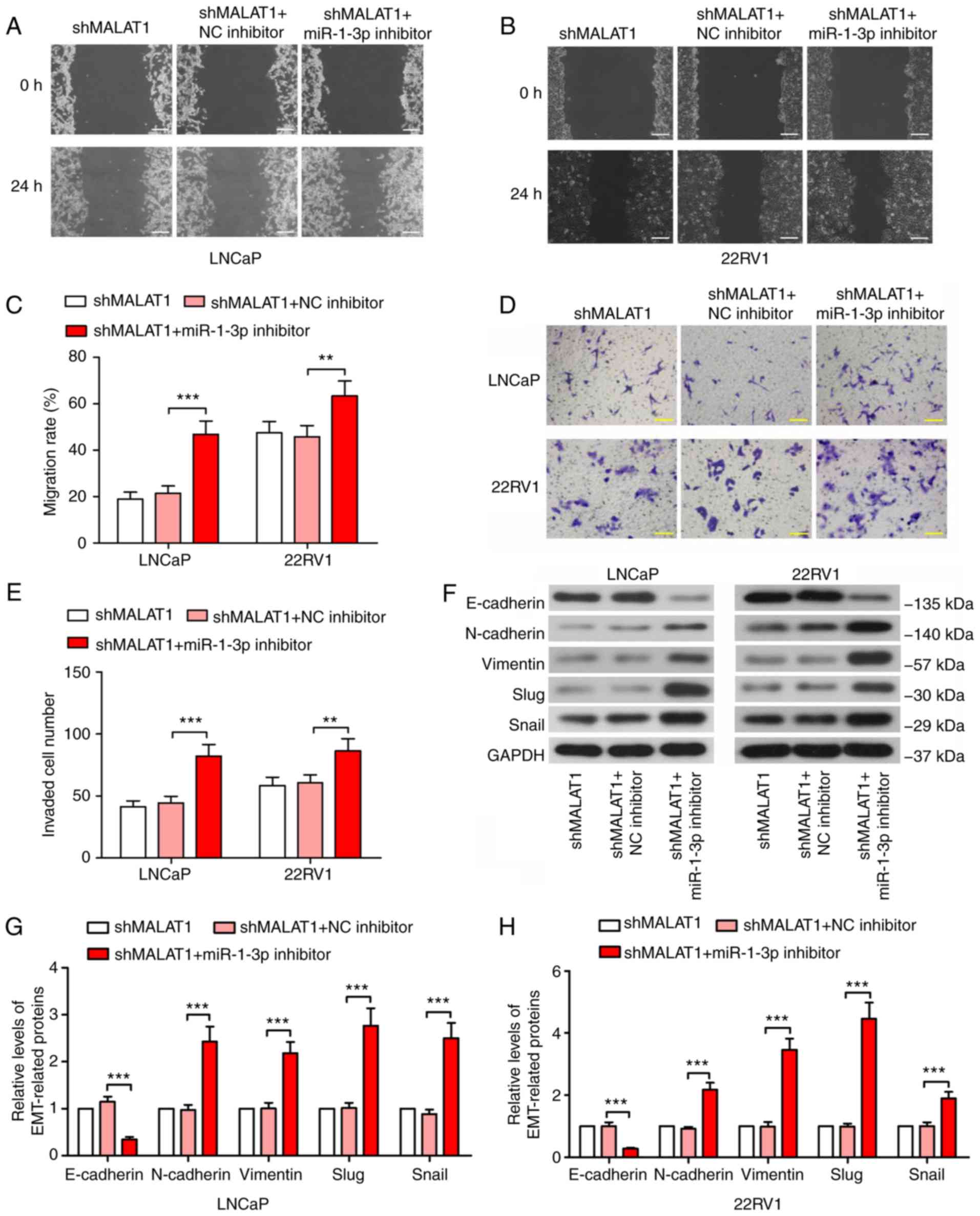 | Figure 4.MALAT1-knockdown-induced malignancy
changes are recovered by inhibition of miR-1-3p. A wound healing
assay was performed to measure the migratory ability of (A) LNCaP
and (B) 22RV1 cells following inhibition of MALAT1 and miR-1-3p
(Scale bar, 200 µm). (C) The migration rate in LNCaP and 22RV1
cells was quantified. (D) A Transwell assay with Matrigel and (E)
quantitative analysis to detect the invasive ability of prostate
cancer cells following silencing of MALAT1 and miR-1-3p (Scale bar,
100 µm). (F) Western blotting of the expression levels of
EMT-associated proteins, E-cadherin, N-cadherin, vimentin, Slug and
Snail in (G) LNCaP and (H) 22RV1. **P<0.01 and ***P<0.001.
MALAT1, metastasis-associated lung adenocarcinoma transcript 1;
EMT, epithelial-mesenchymal transition; N, neural; e, epithelial;
sh, short hairpin; NC, negative control; miR, microRNA. |
The phenotypic alterations induced by
silencing MALAT1 are abolished by forced expression of CORO1C
In order to confirm the competitive association
between MALAT1 and CORO1C, CORO1C was overexpressed in
MALAT1-silenced cells. CORO1C expression was significantly
increased following transfection with a CORO1C overexpression
plasmid (Fig. 5A and B), which
proved the effectiveness of the CORO1C ectopic expression plasmid.
The wound healing assay revealed that the shMALAT1-induced decline
in migratory ability was rescued by forced expression of CORO1C
(Fig. 5C-E). The Transwell assay
demonstrated similar results for the invasion of prostate cancer
cells (Fig. 5F and G). As
presented in Fig. 5H-J, E-cadherin
expression level was significantly decreased (P<0.01) and
N-cadherin, vimentin, Slug and Snail was significantly increased
following CORO1C overexpression, compared with the MALAT1-silenced
cells (P<0.001), suggesting that shMALAT1-induced EMT inhibition
was ceased by forced expression of CORO1C.
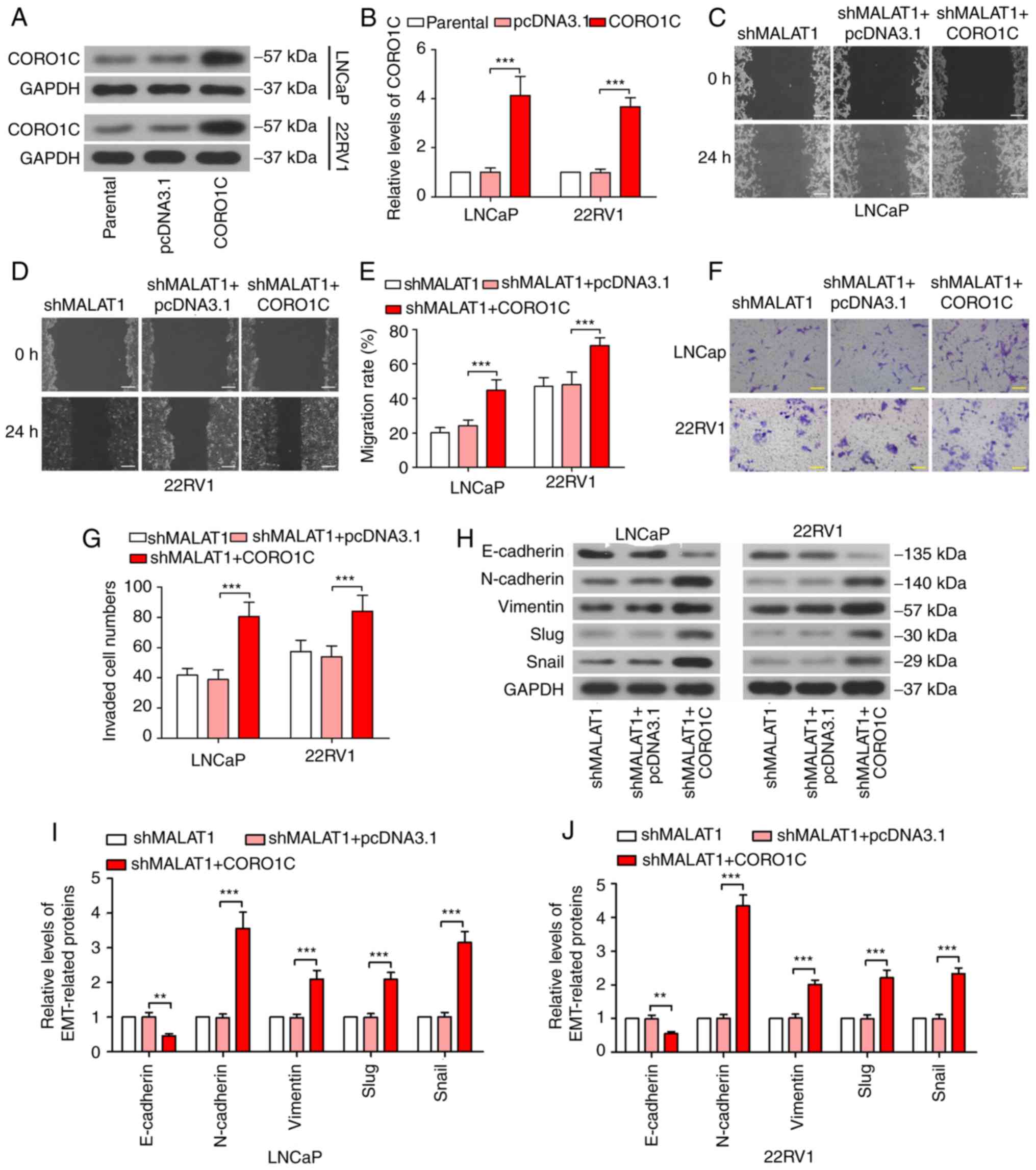 | Figure 5.Phenotypic alterations induced by
silencing of MALAT1 is abolished by forced expression of CORO1C.
(A) Western blotting and (B) quantitative analysis of the CORO1C
expression levels were detected following CORO1C overexpression.
Wound healing assay was performed to measure the migratory rate in
(C) LNCaP and (D) 22RV1 cells following MALAT1 knockdown or/and
CORO1C overexpression (Scale bar, 200 µm) and (E) quantitative
analysis. (F) Transwell assay and (G) quantitative analysis to
investigate the invasive ability of LNCaP and 22RV1 cells (Scale
bar, 100 µm). (H) Western blotting of the expression levels of
EMT-associated proteins, E-cadherin, N-cadherin, vimentin, Slug and
Snail in (I) LNCaP and (J) 22RV1. ***P<0.001. MALAT1,
metastasis-associated lung adenocarcinoma transcript 1; EMT,
epithelial-mesenchymal transition; CORO1C, coronin 1C; N, neural;
e, epithelial; sh, short hairpin; NC, negative control; miR,
microRNA. |
Discussion
MALAT1 is located at the nuclear speckles that are
enriched with pre-mRNA splicing factors (7,19).
MALAT1 has been demonstrated to interact with a set of
serine/arginine-rich (SR) family splicing regulators (SRSF1),
including SRSF1/2/3 (20,21). The depletion of MALAT1 alters the
phosphorylation status of SR proteins and causes mislocalization of
splicing factors in nuclear speckles. The repression of MALAT1 in
HeLa cells alters the pattern of alternative splicing of particular
pre-mRNAs (20). These results
suggest that MALAT1 indirectly controls alternative splicing by
changing the distribution of splicing regulators in the nuclear
speckles. However, the Malat1 knockout mouse does not
exhibit similar phenotypes. In the embryo fibroblasts isolated from
the Malat1 knockout mouse, the formation/structure of
nuclear speckles and the phosphorylation status/localization of SR
proteins are presented correctly (22). The discrepancy between MALAT1
function in vitro and in vivo could be explained as
follows: MALAT1 only serves certain roles under specific conditions
(23).
MALAT1 also can post-transcriptionally control gene
expression through sponging miRNAs as a ceRNA (24). Through this mechanism, MALAT1
sequesters miRNAs through miRNA responsive elements located in its
sequence, therefore, relieving the inhibitory effects of tumor
suppressor miRNAs on oncogenic targets and leading to phenotypic
alterations, including proliferation and invasion (7). In the present study, it was
demonstrated that MALAT1 served as a miRNA sponge, bound to the
seed sequence of miR-1-3p and attenuated the binding of miR-1-3p to
CORO1C 3′UTR. Additionally, MALAT1 expression levels were affected
by the miR-1-3p mimic or inhibitor, suggesting that MALAT1 may be a
degradable sponge. Nevertheless, the effect of MALAT1 on miR-1-3p
and CORO1C in vivo has not been investigated. The authors
plan to next establish a Malat1 knockout rodent model and
detect the expression levels of miR-1-3p and CORO1C, to confirm
whether the effect of MALAT1 in vivo and in vitro is
consistent.
CORO1C is an actin-binding protein that serves a key
role in cell motility (25).
Directional cell migration is essential for multiple physiological
processes. For instance, the migration of leukocytes into damaged
or infected tissues is crucial for an effective immune response and
the migration of keratinocytes and dermal fibroblasts is a key
aspect of wound healing (26).
Additionally, migration is of significance for metastasis of
malignant tumors and multiple reports have demonstrated the
function of CORO1C in cancer (27–29).
For instance, CORO1C is highly expressed in diffuse gliomas and
CORO1C knockdown inhibits proliferation, motility and invasion in
glioblastoma cells (29). The role
of CORO1C in prostate cancer, to the best of our knowledge, has yet
to be reported. The inhibition of miR-1-3p led to increased
expression of CORO1C, accompanied by an increased migration,
invasion and EMT rate in prostate cancer, which suggested the
metastatic promoting role of CORO1C. The tumor suppressing role of
miR-1-3p has been reported in multiple tumors (30,31),
including prostate cancer (16).
In the present study, it was demonstrated that silencing MALAT1
inhibited the expression of CORO1C by relieving the binding to
miR-1-3p. The shMALAT1-induced phenotypic changes may be reversed
by the miR-1-3p inhibitor or the CORO1C overexpression plasmid.
Recently, another team reported similar results (32). They indicated that MALAT1 served as
a sponge to bind to miR-1-3p and sequestered miR-1-3p away from its
target KRAS, and the knockdown of MALAT1 accelerated KRAS-induced
apoptosis in prostate cancer (32).
Prostate cancer is an androgen-dependent tumor,
although it always develops to CRPC. In the present study, LNCaP
and 22RV1 cells were selected to perform experiments. LNCaP cells
were androgen-dependent, while 22RV1 cells were
androgen-independent (18).
However, the results of LNCaP and 22RV1 cells were similar,
suggesting that the effect of MALAT1 on phenotypes of prostate
cancer cells and expression of miR-1-3p and CORO1C, may be
independent of androgens. The present study also demonstrated that
the expression of MALAT1 was increased in several prostate cancer
cell lines, including LNCaP and 22RV1, compared with normal
prostatic epithelial cells, and the expression of miR-1-3p was
decreased. It is well known that androgen and androgen receptor
(AR) signaling are essential for the progression of prostate
cancer, so the results suggested that the functions of MALAT1 may
be associated with androgen and AR signaling. Therefore, further
experiments with androgens are essential to deeply expound the
mechanism of MALAT1 in prostate cancer.
In conclusion, the present study demonstrated that
the silencing of MALAT1 inhibited migration, invasion and EMT by
serving as a miRNA sponge and impairing the binding of miR-1-3p to
CORO1C in prostate cancer cells. The present study identified the
role of CORO1C in prostate cancer cells and demonstrated that
MALAT1 regulated the CORO1C expression by competing with CORO1C for
the binding sites of miR-1-3p. These findings may provide novel
insight for clinical therapies for prostate cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and XD designed the research and conducted the
experiments. ZL, LL, KG and SX performed experiments, and analyzed
the data. XD drafted the manuscript. All authors read the
manuscript and approved the submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Attard G, Parker C, Eeles RA, Schröder F,
Tomlins SA, Tannock I, Drake CG and de Bono JS: Prostate cancer.
Lancet. 387:70–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan X, Cai C, Chen S, Chen S, Yu Z and
Balk SP: Androgen receptor functions in castration-resistant
prostate cancer and mechanisms of resistance to new agents
targeting the androgen axis. Oncogene. 33:2815–2825. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke NW: Landmarks in non-hormonal
pharmacological therapies for castration-resistant prostate cancer.
BJU Int. 110 (Suppl 1):S14–S22. 2012. View Article : Google Scholar
|
|
5
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amodio N, Raimondi L, Juli G, Stamato MA,
Caracciolo D, Tagliaferri P and Tassone P: MALAT1: A druggable long
non-coding RNA for targeted anti-cancer approaches. J Hematol
Oncol. 11:632018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu L, Wang X and Guo Y: Long non-coding
RNA MALAT1 is upregulated and involved in cell proliferation,
migration and apoptosis in ovarian cancer. Exp Ther Med.
13:3055–3060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Bai HS, Deng Y and Fan L: High
MALAT1 expression predicts a poor prognosis of cervical cancer and
promotes cancer cell growth and invasion. Eur Rev Med Pharmacol
Sci. 19:3187–3193. 2015.PubMed/NCBI
|
|
10
|
Sun R, Qin C, Jiang B, Fang S, Pan X, Peng
L, Liu Z, Li W, Li Y and Li G: Down-regulation of MALAT1 inhibits
cervical cancer cell invasion and metastasis by inhibition of
epithelial-mesenchymal transition. Mol Biosyst. 12:952–962. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N,
Bin J, Liao Y and Liao W: Long non-coding RNA MALAT1 promotes
gastric cancer tumorigenicity and metastasis by regulating
vasculogenic mimicry and angiogenesis. Cancer Lett. 395:31–44.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F,
Wei M, Shen J, Hou J, Gao X, et al: Long noncoding RNA MALAT-1 is a
new potential therapeutic target for castration resistant prostate
cancer. J Urol. 190:2278–2287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luan W, Li L, Shi Y, Bu X, Xia Y, Wang J,
Djangmah HS, Liu X, You Y and Xu B: Long non-coding RNA MALAT1 acts
as a competing endogenous RNA to promote malignant melanoma growth
and metastasis by sponging miR-22. Oncotarget. 7:63901–63912. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou J, Wang B, Zheng T, Li X, Zheng L, Hu
J, Zhang Y, Xing Y and Xi T: MALAT1 induced migration and invasion
of human breast cancer cells by competitively binding miR-1 with
cdc42. Biochem Biophys Res Commun. 472:262–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin C, Yan B, Lu Q, Lin Y and Ma L:
Reciprocal regulation of Hsa-miR-1 and long noncoding RNA MALAT1
promotes triple-negative breast cancer development. Tumour Biol.
37:7383–7394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hudson RS, Yi M, Esposito D, Watkins SK,
Hurwitz AA, Yfantis HG, Lee DH, Borin JF, Naslund MJ, Alexander RB,
et al: MicroRNA-1 is a candidate tumor suppressor and prognostic
marker in human prostate cancer. Nucleic Acids Res. 40:3689–3703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Bokhoven A, Varella-Garcia M, Korch C,
Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ and Lucia
MS: Molecular characterization of human prostate carcinoma cell
lines. Prostate. 57:205–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshimoto R, Mayeda A, Yoshida M and
Nakagawa S: MALAT1 long non-coding RNA in cancer. Biochim Biophys
Acta. 1859:192–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tripathi V, Ellis JD, Shen Z, Song DY, Pan
Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al: The
nuclear-retained noncoding RNA MALAT1 regulates alternative
splicing by modulating SR splicing factor phosphorylation. Mol
Cell. 39:925–938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schor IE, Llères D, Risso GJ, Pawellek A,
Ule J, Lamond AI and Kornblihtt AR: Perturbation of chromatin
structure globally affects localization and recruitment of splicing
factors. PLoS One. 7:e480842012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Arun G, Mao YS, Lazar Z, Hung G,
Bhattacharjee G, Xiao X, Booth CJ, Wu J, Zhang C and Spector DL:
The lncRNA Malat1 is dispensable for mouse development but its
transcription plays a cis-regulatory role in the adult. Cell Rep.
2:111–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Hamblin MH and Yin KJ: The long
noncoding RNA Malat1: Its physiological and pathophysiological
functions. RNA Biol. 14:1705–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Humphries CL, Balcer HI, D'Agostino JL,
Winsor B, Drubin DG, Barnes G, Andrews BJ and Goode BL: Direct
regulation of Arp2/3 complex activity and function by the actin
binding protein coronin. J Cell Biol. 159:993–1004. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vicente-Manzanares M, Webb DJ and Horwitz
AR: Cell migration at a glance. J Cell Sci. 118:4917–4919. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mataki H, Enokida H, Chiyomaru T, Mizuno
K, Matsushita R, Goto Y, Nishikawa R, Higashimoto I, Samukawa T,
Nakagawa M, et al: Downregulation of the microRNA-1/133a cluster
enhances cancer cell migration and invasion in lung-squamous cell
carcinoma via regulation of Coronin 1C. J Hum Genet. 60:53–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim JP, Shyamasundar S, Gunaratne J,
Scully OJ, Matsumoto K and Bay BH: YBX1 gene silencing inhibits
migratory and invasive potential via CORO1C in breast cancer in
vitro. BMC Cancer. 17:2012017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thal D, Xavier CP, Rosentreter A, Linder
S, Friedrichs B, Waha A, Pietsch T, Stumpf M, Noegel A and Clemen
C: Expression of coronin-3 (coronin-1C) in diffuse gliomas is
related to malignancy. J Pathol. 214:415–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang A, Yang M, Shen F, Wang J, Wei J,
Wang W, Lu W and Wang C and Wang C: MiR-1-3p suppresses the
proliferation, invasion and migration of bladder cancer cells by
up-regulating SFRP1 expression. Cell Physiol Biochem. 41:1179–1188.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Wang J, Chen Z, Wang K and Shi L:
MicroRNA-1-3p inhibits the proliferation and migration of oral
squamous cell carcinoma cells by targeting DKK1. Biochem Cell Biol.
96:355–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang J, Xu W, Du X and Hou J: MALAT1
silencing suppresses prostate cancer progression by upregulating
miR-1 and downregulating KRAS. Onco Targets Ther. 11:3461–3473.
2018. View Article : Google Scholar : PubMed/NCBI
|