Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancer in humans, and in China alone accounts for
53% of all liver cancer-related deaths worldwide (1,2),
representing the second most common cause of cancer-related
mortalities (3). Potential
therapies for HCC include local ablative therapies, liver
transplantation and resection. However, these therapies are
effective only for treating HCC at early stages (4). For advanced stages, systemic therapy
with the tyrosine kinase inhibitor sorafenib is the best available
option, as indicated by current data (5). Treatment with sorafenib presents
various side effects, such as diarrhea, hypertension and skin
toxicity (6). Therefore,
understanding the molecular mechanisms involved in development of
HCC is essential to uncover novel drugs for targeted therapies.
Berberine (BBR), a natural isoquinoline alkaloid (Fig. 1), is found in the roots, rhizome
and stem bark of a number of important medicinal plants (7). In the clinical setting, BBR has been
used for decades to treat patients who have diarrhea (8), acute radiation intestinal syndrome
(9) and type 2 diabetes mellitus
(10), with doses of 0.1–0.3 g
three times a day, 20 mg/kg once a day and 0.9 g once a day with
negligible adverse reactions. Previous studies also have shown that
BBR exhibits antitumor properties (11) against ovarian cancer (12), breast cancer (13), nasopharyngeal carcinoma (14), thyroid carcinoma (15) and prostate cancer (16), with the IC50 of BBR of
2.79 mg/l, 26.5 µM, 11.7 µg/ml, 125.6 and 220.36 µM, respectively.
BBR has been proposed to exhibit several antitumor activities that
target multiple signaling pathways, for example the PI3K/AKT
pathway, that are crucial for tumor progression (17). Despite several investigations, the
precise cellular and molecular targets of BBR remain unknown
(18). In addition, the molecular
mechanisms of action of BBR in HCC have yet to be fully elucidated.
Therefore, the present study investigated the potential anti-HCC
effect of BBR and examined its molecular mechanism of action using
computer-aided approaches.
Computer-aided approaches have been widely used in
drug research to improve the efficiency of drug discovery.
Systematic pharmacology is an emerging field that combines multiple
drug target prediction, oral bioavailability prediction and network
analysis to understand the active compounds and therapeutic targets
of Traditional Chinese medicine (TCM) (19). As a receptor-based
computer-assisted drug design approach, molecular docking mainly
predicts the interactions between two or several molecules based on
both geometry and energy match (20). As for other tumors, many emerging
treatments of HCC need further evaluation (5). Efforts are underway to explore the
molecular mechanisms for the treatment of HCC and for the
development of novel therapeutic approaches with lower toxicity.
The present study provided in vitro experimental evidence to
validate the mechanisms of action of BBR in treating HCC at the
molecular level, as were predicted by systems pharmacology and
molecular docking results.
Materials and methods
Evaluation of pharmacokinetic
properties by TCM systems pharmacology (TCMSP)
TCMSP (http://lsp.nwu.edu.cn/tcmsp.php; version 2.3) is an
open-source systems pharmacology platform for TCM that provides
information on the interactions among drugs, targets and diseases.
This database includes herbal products, chemicals, targets,
drug-target networks and drug-target-disease networks; as well as
pharmacokinetic properties of natural ingredients including
drug-likeness (DL), oral bioavailability (OB), fractional negative
accessible surface area (FASA-), blood-brain barrier (BBB), Caco-2
permeability (Caco-2) and Lipinski's rule of five [molecular weight
(MW), total polar surface area (TPSA), octanol-water partition
coefficient (ALogP), H-bond donor (Hdon) and H-bond acceptor
(Hacc)] (21). In the present
study, the molecular name ‘berberine’ was searched in TCMSP and the
drug-like properties of BBR were analyzed at the molecular
level.
Target fishing by TCMSP and
PharmMapper
The putative targets of BBR were collected from two
databases, TCMSP and PharmMapper (http://www.lilab-ecust.cn/pharmmapper/; updated: April
10, 2019) (22). TCMSP is a useful
analysis platform and knowledge repository. Drug-target mappings
were collected from two sources in TCMSP: Experimentally validated
targets were obtained from the database for Herb Ingredients'
Target (http://lifecenter.sgst.cn/hit/; downloaded on July 28,
2018) and the Systems Drug Targeting (https://targetingsystems.net/; downloaded on July 30,
2018) model construction was used to predict potential targets that
were not validated. Support Vector Machine score (SVM) ≥0.7 and
Random Forest score (RF) ≥0.8 were set as the threshold to identify
candidate targets (23).
PharmMapper, a free web server, can identify potential drug targets
using the pharmacophore mapping approach. A mol2 file for BBR was
obtained from TCMSP database and uploaded in PharmMapper.
HCC-significant target selection
HCC-significant targets were obtained from two
databases including OncoDB.HCC (http://oncodb.hcc.ibms.sinica.edu.tw/index.htm;
downloaded on August 13, 2018) and Liverome (http://liverome.kobic.re.kr/; downloaded on August 14,
2018). OncoDB.HCC is a comprehensive tumor genomic database that
displays abnormal cancer related target genes and loci seen in HCC
(24); while Liverome is a
database of genes associated with HCC. In these databases, gene
signatures are mostly from proteomics studies and published
microarray data manually assembled and annotated (24).
Protein-protein interaction (PPI)
data
The protein-protein interaction (PPI) data were
obtained from Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (http://string-db.org/; version 10) (25), with the species limited to ‘Homo
sapiens’.
Gene Ontology (GO) and pathway
enrichment analysis
GO and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway enrichment analysis were performed using Database
for Annotation, Visualization and Integrated Discovery (DAVID;
http://david.abcc.ncifcrf.gov/; version
6.8) (26,27).
Network construction and analysis
Construction of BBR-BBR targets network and PPI
networks were visualized using Cytoscape (http://cytoscape.org/; version 3.6.0) (28).
Molecular docking algorithm
Before the docking process, the three-dimensional
(3D) crystal structures of AKT were obtained in the PDB format from
RCSB Protein Data Bank (http://www.pdb.org/; PDB-ID:3MVH; Released: June 2,
2010) and prepared with Autodock 4.2 (http://autodock.scripps.edu/;version 1.2) for flexible
docking studies. The 3D structure of BBR (Pubchem CID: 2335) was
obtained from NCBI-PubChem (https://pubchem.ncbi.nlm.nih.gov/), energy
optimization was performed with ChemOffice (http://www.cambridgesoft.com; version 17.0) and saved
in the PDB format. Gasteiger charges were added, rotatable bonds
were set by the AutoDock tools and all torsions were allowed to
rotate. Polar hydrogen atoms were added to the protein using
AutoDock tools. The grid map was centered at the active site pocket
of the protein by Autogrid (version 1.4.5) (29). The root-mean-square deviation value
was less than 2.0 Å. Briefly, to perform docking in AutoDock 4.2,
the grid dimensions were established using the grid center, number
of points (X: 60, Y: 60, Z: 60) and spacing (0.375 Å). Molecular
docking was performed and analyzed using AutoDock 4.2. A Lamarckian
genetic algorithm method (Runs 100) was implemented in the program
suite to identify appropriate binding modes and conformation of the
ligand molecules (30). A maximum
of 25 million energy evaluations were applied for the experiment.
The results were clustered using a tolerance of 2.0 Å. Analysis of
BBR and target protein docking results using Pymol (https://pymol.org/2/; version 2.3) and ligplot
(https://www.ebi.ac.uk/thornton-srv/software/LigPlus/;
version 1.4).
Cell lines, culture conditions and
reagents
MHCC97-H and HepG2 cell lines were obtained from the
Cell Bank of the Chinese Academy of SciencHes (Shanghai, China).
The cells were cultured in DMEM (Thermo Fisher Scientific, Inc.)
containing 10% heat inactivated FBS (Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin and
maintained as monolayer in a humidified atmosphere of 95% air and
5% CO2 at 37°C. Culture medium was replaced every 2
days. BBR was purchased from Chengdu Desite Chemical Co. Ltd. and a
stock solution of 10 mM was prepared in DMSO (Sigma-Aldrich; Merck
KGaA). The solution was serially diluted in DMEM immediately before
the experiments. Pancreatin, penicillin and streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc.). All the
reagents were of analytical grade.
Western blotting
Following treatment with different concentrations of
BBR for 24 h, MHCC97-H and HepG2 cells were harvested and washed
with ice-cold PBS. Total cellular protein was extracted by lysing
cells in buffer containing 50% glycerol, 10% SDS, 0.5 M Tris/HCl
(pH 6.8), 1:100 proteases and phosphatases inhibitor cocktail.
Protein concentrations were determined using the BCA method
(Beyotime Institute of Biotechnology). Then, 50 µg protein was
separated by 10% SDS PAGE and transferred onto PVDF membranes
(Bio-Rad Laboratories, Inc.). Membranes were blocked with 5% BSA
(Gibco; Thermo Fisher Scientific, Inc.) in TBST (0.1% TWEEN) for 2
h at room temperature. The membranes were incubated overnight at
4°C with appropriate primary antibodies including anti-PI3K p85-α
(1:1,000; cat. no. ab182651; Abcam), anti-AKT (1:1,000; cat. no.
9272; Cell Signaling Technology, Inc.), anti-phosphorylated (p-)
AKT (1:1,000; Cell Signaling Technology, Inc.) and β-actin
(1:6,000; ab8227; Abcam). After being incubated with HRP-conjugated
goat anti-rabbit (1:1,000; ab181662; Abcam) or anti-mouse IgG
(1:1,000; ab205719; Abcam) for 1 h at room temperature, immune
complexes were detected using enhanced chemiluminescence (ECL kit;
EMD Millipore). Protein expression levels were normalized to
β-actin and quantified using Image Lab software version 6.0.1 (Bio
Rad Laboratories, Inc).
Cell viability assay
Cells were collected by trypsinization (GE
Healthcare Life Sciences) after replacing the medium twice. The
cells were cultured in 96-well plates at a density of
5×104 cells per well. After 24 h of incubation, cells
were treated with various concentrations of BBR (50, 100 and 200
µM) and cultured for 24 h. The cells in the logarithmic growth
phase were used for detecting cell viability using the MTT assay.
At the end of the incubation, 10 µl of MTT solution (Sigma-Aldrich;
Merck KGaA) was added to each well and incubated for another 2.5 h.
DMSO (100 µl) was used to dissolve formazan crystals and absorbance
was detected at wavelength of 570 nm with a microplate reader.
Colony formation assay
MHCC97-H and HepG2 cells in the logarithmic phase
were dispersed into single cells by trypsin digestion. Cell
suspensions containing 1,000 cells were seeded in six-well plates
with complete medium and the plates were incubated for two weeks.
The medium was removed and the plates were washed twice with PBS,
after which the cells were fixed with paraformaldehyde for 15 min
and stained with crystal violet solution for 15 min at room
temperature. After washing, the cells were air-dried and the number
of colonies was calculated for each group.
Flow cytometry for cell apoptosis
analysis
BBR-induced apoptosis in MHCC97-H and HepG2 cells
was quantitatively determined by flow cytometry using the Annexin
V-FITC apoptosis detection kit (Vazyme) and a flow cytometer
(FACSCalibur; BD Biosciences). Briefly, after treatment with BBR
for 24 h, cells were collected by using 0.25% trypsin and washed
twice with cold PBS for 5 min, washed with PBS and incubated with
Alexa 488 and propidium iodide (PI) for cellular staining at room
temperature for 10 min in the dark. The stained cells were analyzed
by Cell Quest acquisition software (version 3.3; BD
Biosciences).
Wound healing assay
Wound healing assay was performed to evaluate the
migration ability of cells. Cells were seeded 5×105
cells per well in 6-well plates and allowed to adhere for 24 h.
Confluent monolayer cells were scratched using a 20 µl pipette tip
and then washed three times with 1X PBS to clear cell debris and
suspension cells. Medium containing 1% FBS and BBR (50–200 µM) was
added and the cells were allowed to close the wound for 48 h.
Images were acquired under an fluorescence microscope (Model DMi8,
Leica Microsystems GmbH) at 0, 24 and 48 h at the same position of
the wound (magnification, ×10). The migration distance was
calculated by the change in wound size during the 24 and 48 h
period using Adobe Photoshop CS6 software (Adobe Systems, Inc.).
The experiment was performed in triplicate.
Transwell assay
A 24-well Transwell plate (8-mm pore size; Corning,
Inc.) was used to measure the migratory and invasive ability of
each cell line. The inserts in the Transwell were coated with a
thin layer of 0.25 mg/ml Matrigel Basement Membrane Matrix (BD
Biosciences) at 37°C for 30 min. MHCC97-H and HepG2 cells were
treated with BBR for 24 h, trypsinized and seeded into the upper
Transwell chamber (2.5×104 cells) in 100 µl of
serum-free medium. Complete medium (600 µl) containing 10% FBS was
added to the lower chamber. Triplicate wells were used for each
group. The cells were allowed to migrate through the filters for 48
h at 37°C in a humidified incubator with 5% CO2. Cells
attached to the lower surface of the membrane were fixed in 4%
paraformaldehyde for 30 min and stained with 0.1% crystal violet at
room temperature. The cells on the upper surface of the filters
were removed by wiping with a cotton swab. The number of stained
cells on the lower surface of the filters was counted at ×20
magnification under a fluorescence microscope (Model DMi8; Leica
Microsystems GmbH). A total of five fields of view were counted for
each Transwell filter.
Statistical analysis
Data are presented as the mean ± SEM. All
statistical analyses were performed with SPSS 19.0 statistical
software (IBM Corp.). The data are expressed as the mean ± SEM.
Statistical analysis was performed by one-way ANOVA followed by
Tukey's multiple comparison test. Western blot analysis was
repeated at least three times. P<0.05 was considered to indicate
a statistically significant difference.
Results
Pharmacokinetic properties and
putative targets of BBR
Pharmacokinetic properties play a vital role in drug
discovery. Pharmacokinetic properties for BBR including Caco-2
permeability, DL, FASA-, OB, BBB and Lipinski's rule of five (MW,
TPSA, AlogP, Hdon, Hacc) were obtained from TCMSP (Table I).
 | Table I.Pharmacokinetic properties of
berberine. |
Table I.
Pharmacokinetic properties of
berberine.
| Name | OB (%) | DL | BBB | Caco-2 | MW | TPSA | AlogP | Hdon | Hacc | FASA- |
|---|
| Berberine | 36.86 | 0.78 | 0.57 | 1.24 | 336.39 | 40.80 | 3.45 | 0 | 4 | 0.19 |
A total of 230 putative targets were predicted for
BBR by TCMSP and PharmMapper, and duplicated potential targets were
removed. Detailed information on candidate targets are provided in
Table SI.
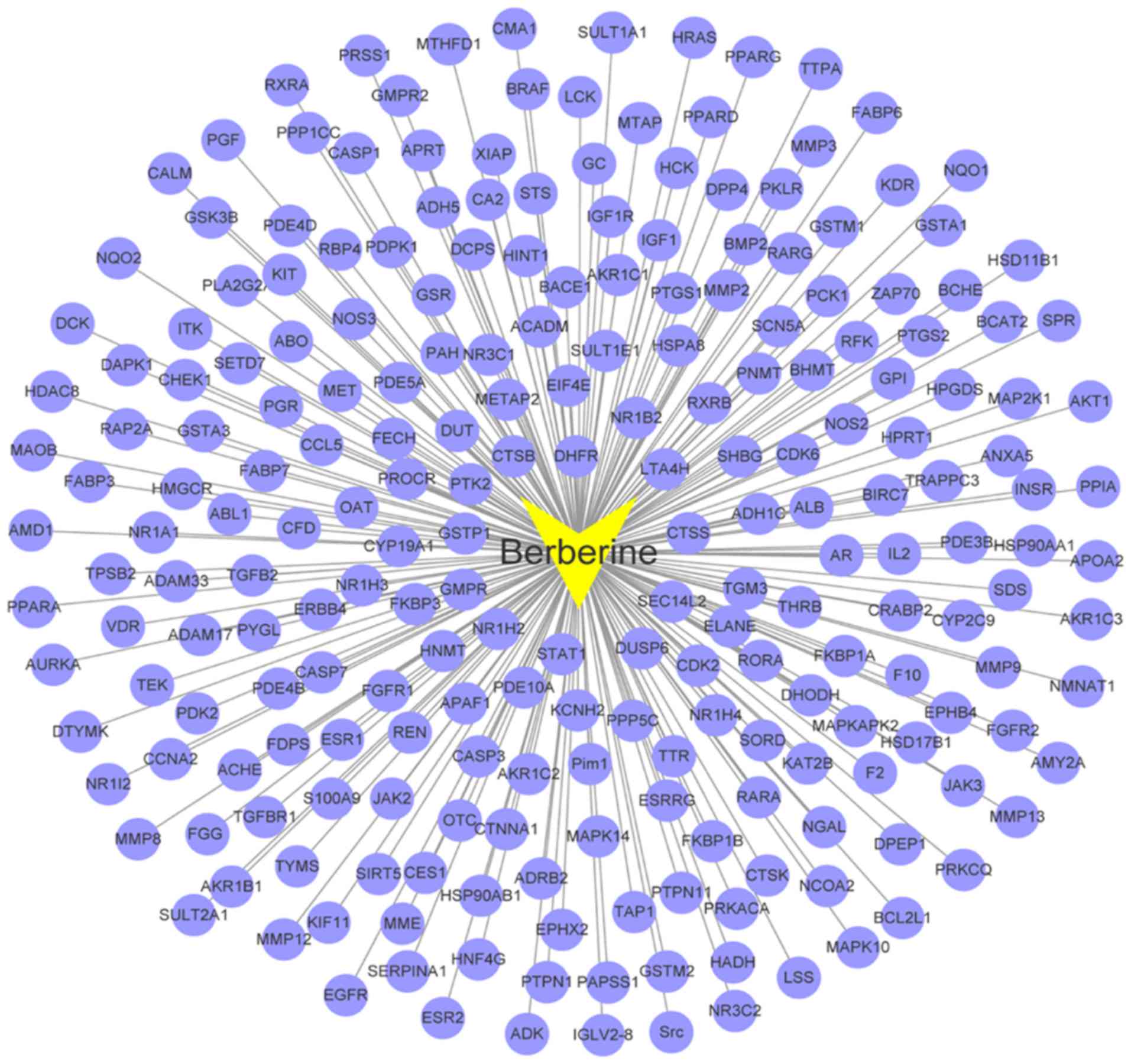
BBR-BBR targets network analysis
As shown in Fig. 2,
a graph of BBR-BBR targets interaction was constructed based on 231
nodes (one compound and 230 candidate targets) and 230 edges.
BBR-BBR targets network analysis displayed that BBR targeted
proteins including AKT1, PTGS2, PDK2, MMP2, MMP8, CDK2, CDK6,
CASP3, CASP1, ALB and GSK3B. Multiple targets predicted as hits for
BBR in this network may be critical to the treatment of HCC.
Previous research has shown MMP2 may act as an oncogene in HCC
(31), and high expression of CDK2
may be more aggressive in HCC (32).
Identification of targets related to
HCC
The present study identified 611 and 6,927
HCC-significant targets from OncoDB. HCC and Liverome,
respectively. As displayed in Table
SII, 167 candidate targets were obtained, and duplicated
candidate targets were deleted.
In order to validate the molecular mechanisms of BBR
acting on HCC, a PPI network analysis and a GO and pathway
enrichment analysis on the common targets of BBR and
HCC-significant targets were conducted.
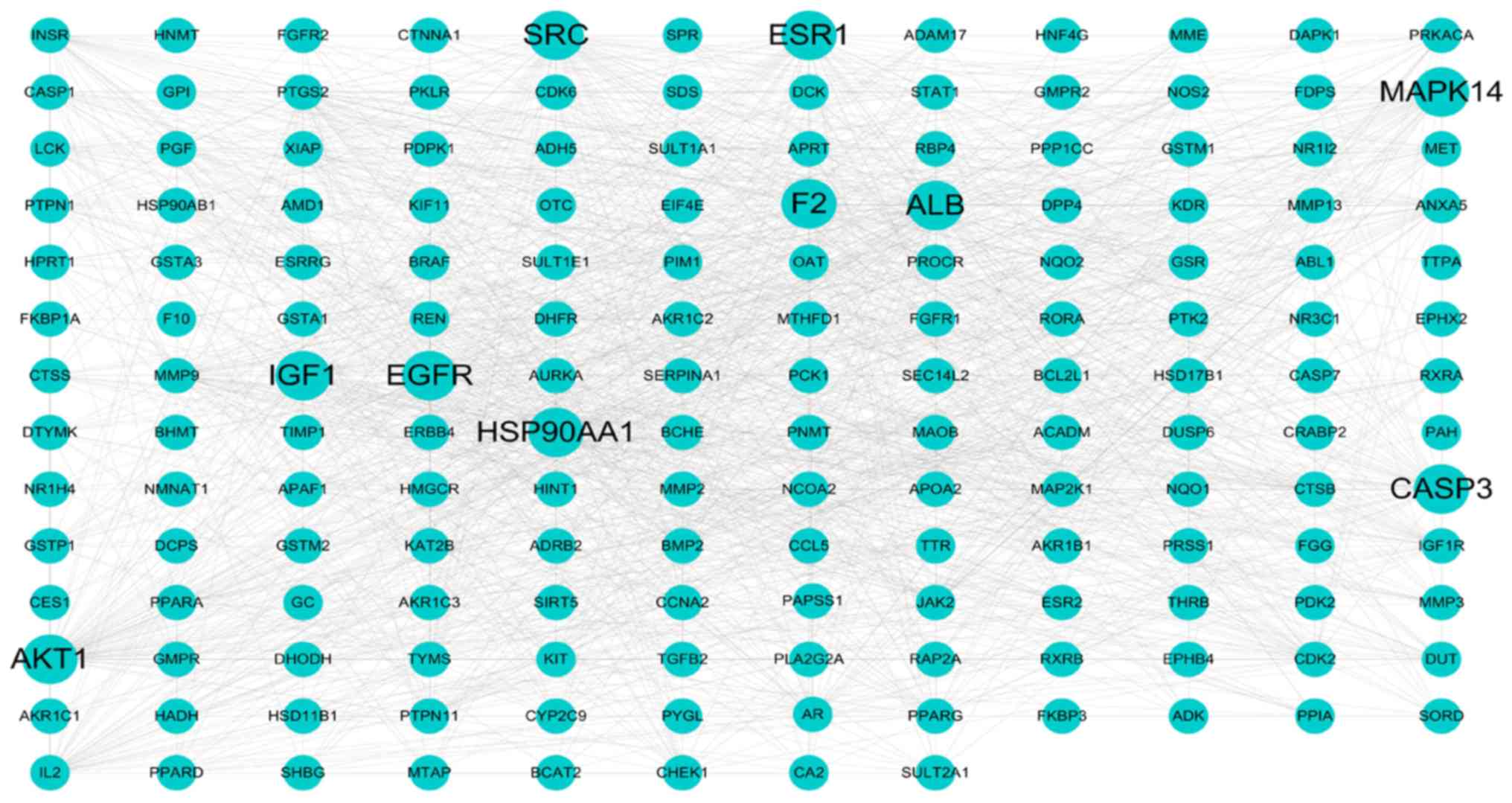
PPI network of HCC-significant
targets
The protein-protein interaction network of the
common targets was constructed using the STRING database. Only one
target did not show any protein-protein interactions, while two
targets could not be found in the above database. A total of 164
nodes and 1,292 edges (Fig. 3) are
shown in this network. In total, 12 genes, including ALB, AKT1,
SRC, IGF1, EGFR, HSP90AA1, ESR1, CASP3, F2, MAPK14, MMP9 and
PTGS2, with degree ≥40, were selected as key genes. A large
number of edges were obtained for each node (101 for ALB, 74
for AKT1, 62 for SRC, 57 for IGF1, 57 for
EGFR, 56 for HSP90AA1, 53 for ESR1, 47 for
CASP3, 44 for F2, 44 for MAPK14, 43 for
MMP9 and 41 for PTGS2). The results of the PPI
network analysis revealed that these key genes may play important
roles in the treatment of HCC.
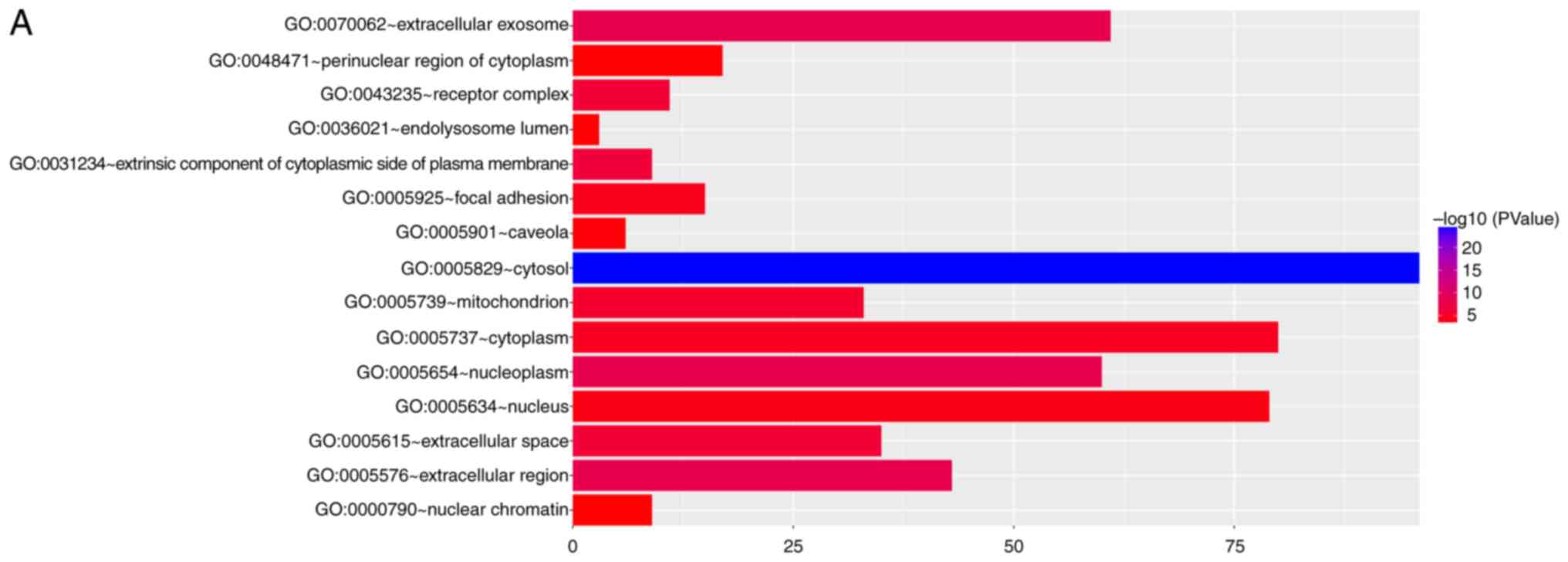
GO and pathway enrichment analysis for
target genes
To identify the biological function of the candidate
targets, functional enrichment analysis was performed using DAVID
database. The detailed results of GO terms and pathways are
presented in Table SIII. GO
analysis suggested that the cellular components were related to
cytosol, extracellular exosome, mitochondrion, nucleoplasm,
extracellular region, extracellular space, cytoplasm, mitochondrial
matrix, focal adhesion and receptor complex (Fig. 4A). The biological processes in
which the targets were involved were related to protein
autophosphorylation, steroid hormone mediated signaling pathway,
transcription initiation from RNA polymerase II promoter, response
to drug, peptidyl-tyrosine phosphorylation, positive regulation of
phosphatidylinositol 3-kinase signaling, positive regulation of
ERK1 and ERK2 cascade, purine-containing compound salvage and
intracellular receptor signaling pathway (Fig. 4B). The molecular function terms
were associated with steroid hormone receptor activity, protein
tyrosine kinase activity, drug binding, protein homodimerization
activity, transmembrane receptor protein tyrosine kinase activity,
ATP binding, enzyme binding, identical protein binding and steroid
binding (Fig. 4C). These results
suggested that these biological characteristics played a critical
role in the treatment of HCC by BBR. Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathways indicated that pathways in cancer,
PI3K-Akt signaling pathway, proteoglycans in cancer, prostate
cancer, metabolism of xenobiotics by cytochrome P450, chemical
carcinogenesis, small cell lung cancer, progesterone-mediated
oocyte maturation, Ras signaling pathway and thyroid hormone
signaling pathway, were involved in the therapy of HCC with BBR
(Fig. 4D). Taken together, the
above results showed that PI3K/AKT signaling pathway may
potentially serve a key role in the BBR treatment process of
HCC.
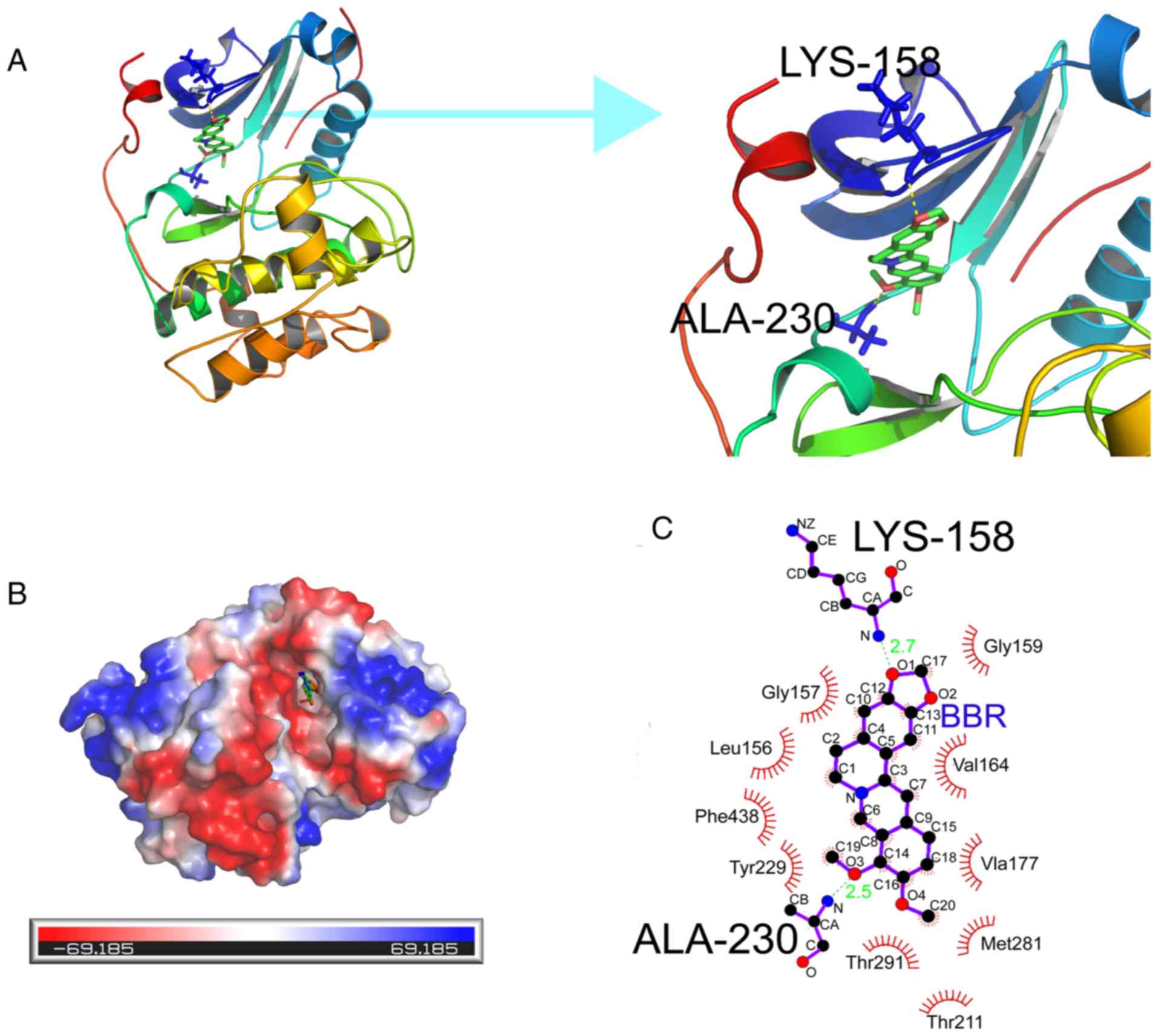
Docking of BBR to AKT
After applying the molecular docking approach
according to the visual ligand-protein docking results, an in
silico analysis was used to determine the binding site of BBR
on AKT. The binding energy of BBR and AKT (PDB-ID:3MVH) was −8.83;
BBR could be docked into the active site of AKT. Secondly, BBR was
found to compete with ATP for the ATP-binding site of AKT, to which
it bound tightly by forming hydrogen bonds with the backbone amino
and carbonyl groups of the corresponding residues (Lys-158,
Ala-230) and also by establishing hydrophobic contacts (Gly-159,
Gly-157, Leu-156, Val-164, Ala-177, Met-281, Tlu-211, Tlu-291,
Tyr-229, Phe-438) with side chains of surrounding residues. The
inhibitory activities were confirmed with molecular docking
analysis when considering H-bond interaction, electrostatic
potential energy, hydrophobic interaction of ligand molecules in
the active pocket of AKT (Fig. 5).
These results demonstrated that the binding of BBR to the active
site of AKT could potentially suppress its activation, which could
contribute to the inhibitory effects of BBR on proliferation and
migration of HCC.
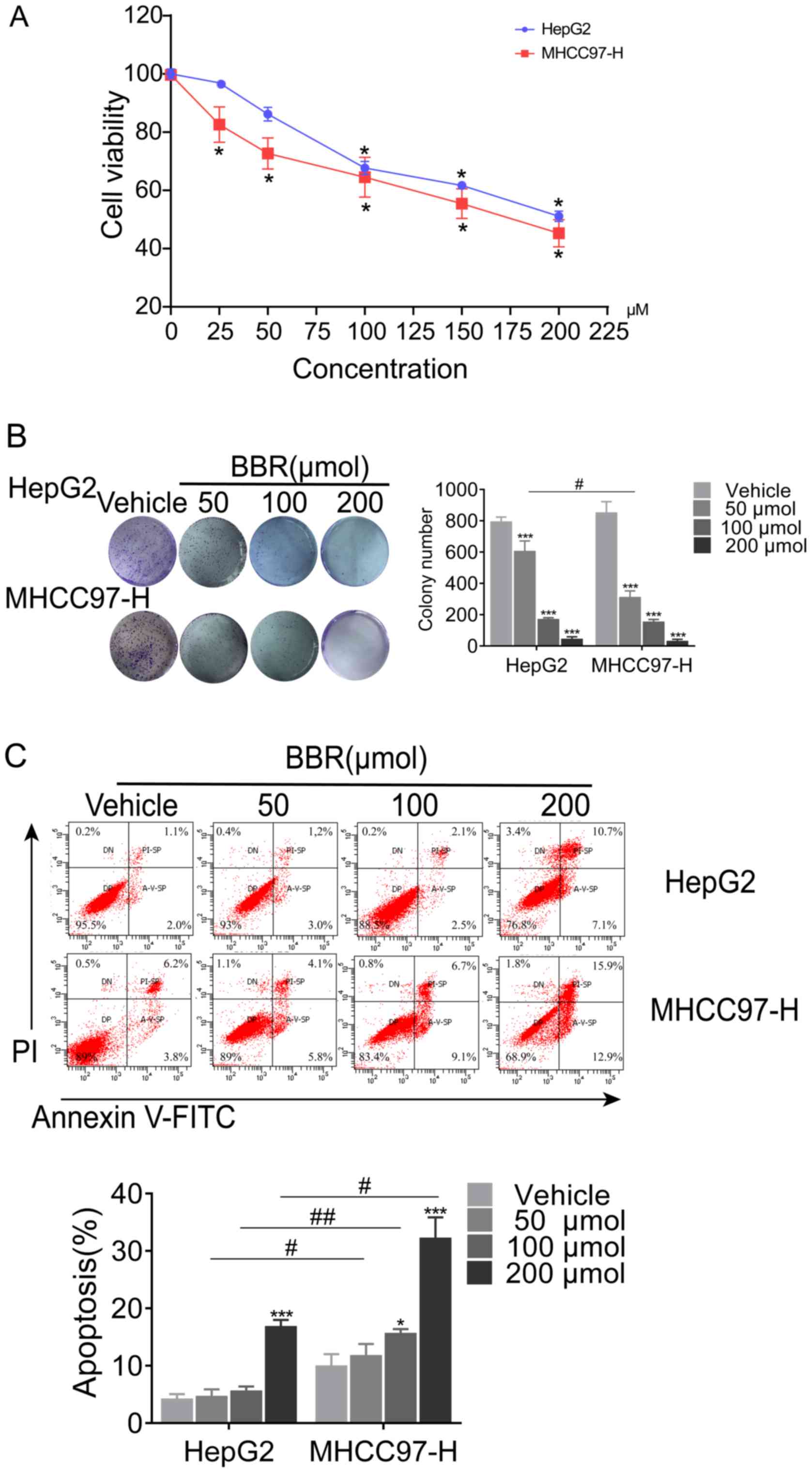
BBR inhibits cell growth and induces
cell apoptosis of MHCC97-H and HepG2 cells
Viability and proliferation of MHCC97-H and HepG2
cells were assessed by MTT and colony formation assays,
respectively. BBR treatment significantly inhibited the growth of
MHCC97-H and HepG2 cells (Fig. 6A)
when treated BBR for 24 h. With the increase of concentration, the
inhibition rate of cells increased significantly. Consistently, BBR
decreased colony numbers of MHCC97-H and HepG2 cells at all
indicated doses (Fig. 6B). These
data suggested that the inhibitory effects of BBR could contribute
to the suppression of HCC cell viability and proliferation. To
further investigate the effect of BBR treatment on cell apoptosis,
flowcytometry was performed using an Annexin V-FITC/PI kit. In the
present study, both early (A-V-SP) and late (PI-SP) apoptotic cells
were counted, as shown in the lower right and the upper right
quadrant of the scatter plots, respectively. As demonstrated in
Fig. 6C, after a 24-h treatment of
HepG2 cells with BBR, the percentages of total apoptotic cells
were: 4.2% (0 µM, vehicle treated control), 4.7% (50 µM), 5.6% (100
µM) and 16.9% (200 µM). For MHCC97-H cells, the percentages of
total apoptotic cells were: 10% (0 µM, vehicle treated control),
11.8% (50 µM), 16% (100 µM) and 33% (200 µM).
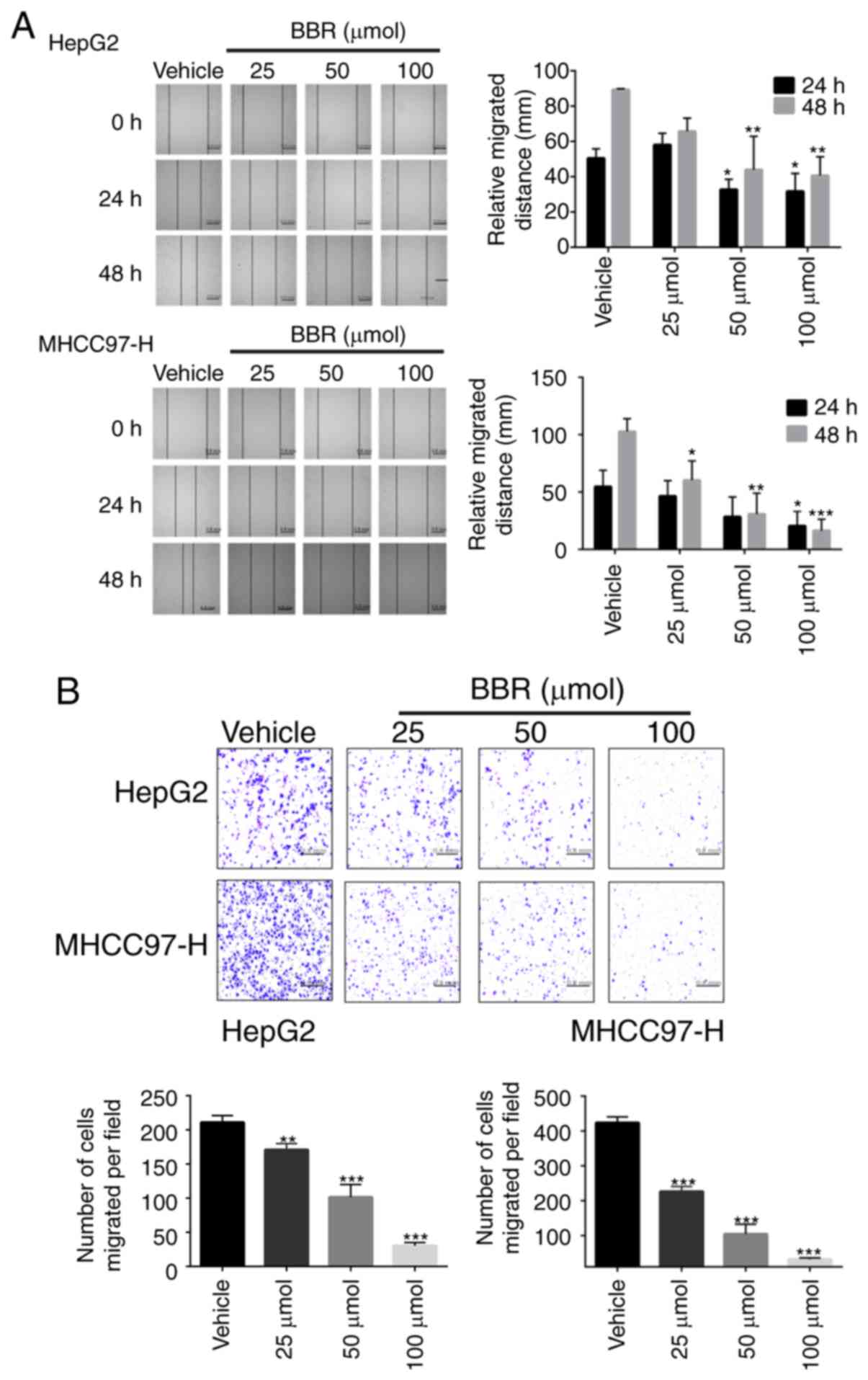
BBR suppresses cell migration and
invasion of MHCC97-H and HepG2 cells
As PI3K/AKT pathway plays a critical role in cancer
cell migration, the effects of BBR on the invasion and migration
ability of HCC cells were investigated. To this end, wound healing
and Transwell chamber migration assays were performed in
vitro. The relative migrated distance in BBR treated group was
significantly smaller than that in the non-treated group, at
indicated time points (P<0.05; n=3; Fig. 7A). Matrigel matrix invasion assay
further indicated BBR treatment suppressed HCC cell migration
(Fig. 7B).
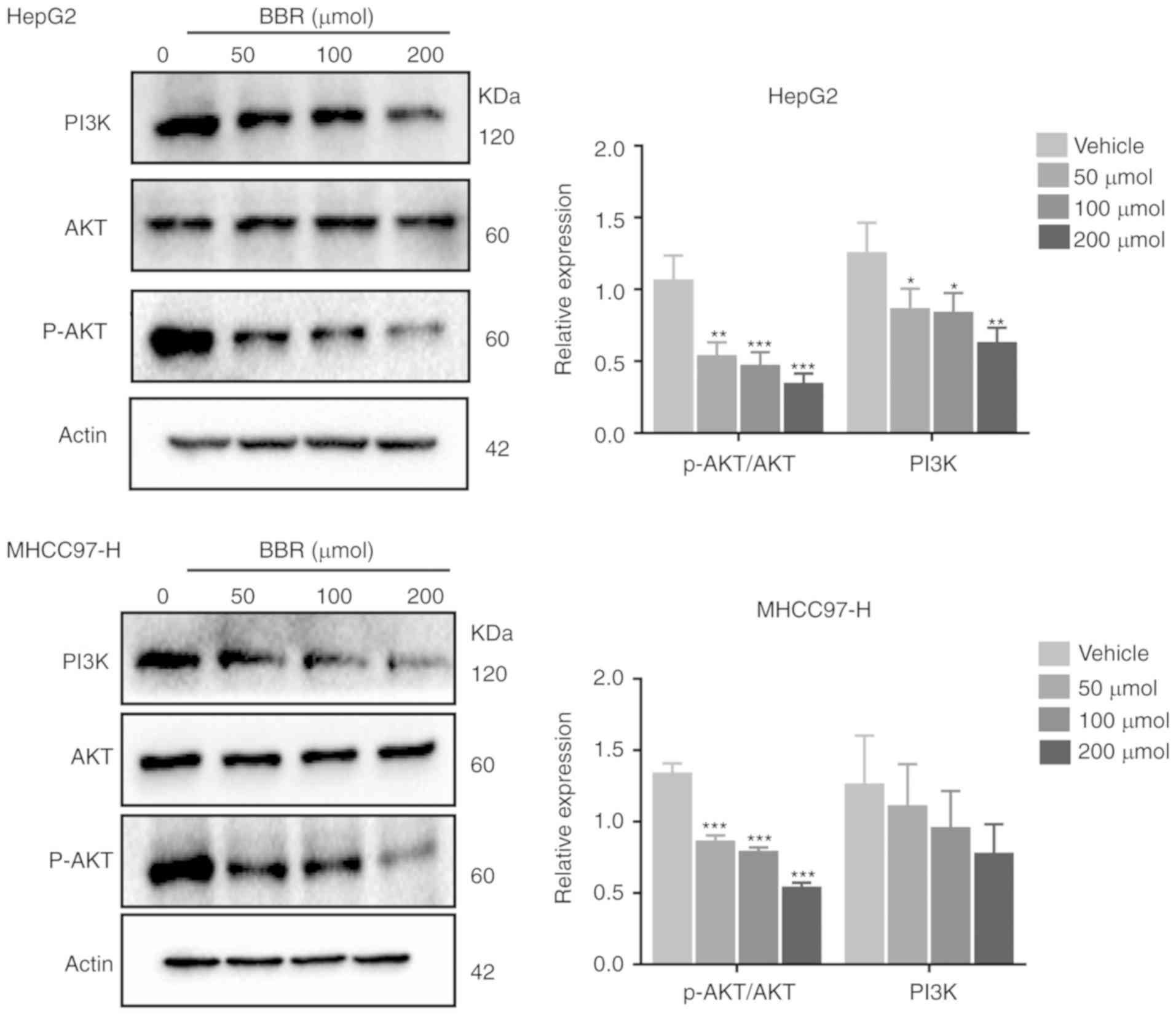
BBR inhibits PI3K/AKT signaling
pathway in HCC cells
To confirm the effect of BBR on PI3K/AKT signaling
pathway, changes in PI3K/AKT effector proteins in HCC cells were
analyzed by western blotting. As shown in Fig. 8, HCC cells possessed a high basal
level of PI3K and p-AKT, indicating that HCC cells potentially rely
on PI3K/AKT for proliferation and migration. However, BBR treatment
significantly inhibited the levels of PI3K and p-AKT without
affecting the total AKT protein. Further, AKT phosphorylation was
suppressed in a BBR concentration-dependent manner (Fig. 8). This implied a potential
relationship between the inhibitory effects of BBR on cancer cell
growth and migration and the suppression of the PI3K/AKT
pathway.
Discussion
Over the past few decades, there has been a
significant decline in the rate of novel phytochemicals that have
been translated into effective drugs. Currently, the most important
problem for novel drug development is a lack of therapeutic
efficacy in clinical trials accounting for 33% of failures
(33). Thus, to maximize drug
efficacy in pharmaceutical development, network pharmacology has
been recently introduced to analyze the biological network of drug
candidates, in order to design poly-target phytochemicals. Previous
studies have shown that BBR potentially represses tumor progression
by inhibiting cell proliferation (34), inducing cell cycle arrest and cell
death, in various cancer cells (35,36).
In the present study, PPI network analysis revealed that AKT1,
ALB, SRC, IGF1, EGFR, HSP90AA1, ESR1, CASP3, F2, MAPK14, MMP9
and PTGS2 could be the key genes that are essential for the
survival of HCC. Pathway analysis elucidated that PI3K/AKT
signaling pathway in cancer may be closely related to the progress
of HCC. The present study hypothesized that BBR potentially
suppresses HCC progression by regulating AKT, based on the network
pharmacology study results.
AKT is a serine-threonine kinase and a vital
component of the PI3K/AKT/mammalian target of rapamycin signaling
pathway. It is over-expressed in most types of cancer (37). Activated AKT phosphorylates
downstream signaling molecules such as GSK3β, PRAS40, BAD and
p70S6K, that promote survival, proliferation, growth and metastasis
of cancer cells (38). Due to its
central role in these pathways, inhibition of AKT is an attractive
intervention strategy for the treatment of cancer (39). In the present study, the docking
approach indicated that the binding potential between BBR and AKT
(RAC-α serine/threonine-protein kinase) could lead to suppression
of AKT activity. The inhibitory effect of BBR on PI3K/AKT pathway
in HCC was validated and it was found that BBR could downregulate
the expressions of p-AKT and PI3K in MHCC97-H and HepG2 cells,
which could then inhibit the growth, cell migration and invasion of
MHCC97-H and HepG2 cells in a dose-dependent manner. In addition,
AKT pathway inhibition by BBR also contributed to cell apoptosis in
MHCC97-H and HepG2 cells. Based the current study, 100 µM is the
ideal concentration of BBR in potential treatment. However, there
are some limitations in the present study. For example, a lack of
caspase inhibition experiment, which may also be relevant for the
anti-HCC mechanism. In the future, further systematic and in-depth
studies investigating the mechanism of anti-HCC will be conducted.
In conclusion, the use of BBR as a sensitizer of cancer cells to
AKT inhibitors should be further explored by in vivo
experiments and in preclinical studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81673627, 81673717 and
81774199), Guangzhou Science Technology and Innovation Commission
Research Projects (grant nos. 201805010005 and 201803010047).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS, YL and XW contributed to experimental study,
data analysis and drafted the manuscript. MMA, HP, WL and YL
participated in the experiments. MH and QW designed and supervised
all aspects of the study. All authors critically reviewed and
revised the manuscript and approved the final manuscript as
submitted.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 (Suppl 1):S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Estimates of the worldwide mortality from 25 cancers in 1990. Int J
Cancer. 83:18–29. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pinter M and Peck-Radosavljevic M: Review
article: Systemic treatment of hepatocellular carcinoma. Aliment
Pharm Ther. 48:598–609. 2018. View Article : Google Scholar
|
|
6
|
Abdel-Rahman O and Lamarca A: Development
of sorafenib-related side effects in patients diagnosed with
advanced hepatocellular carcinoma treated with sorafenib: A
systematic-review and meta-analysis of the impact on survival.
Expert Rev Gastroenterol Hepatol. 11:75–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imenshahidi M and Hosseinzadeh H: Berberis
vulgaris and berberine: An update review. Phytother Res.
30:1745–1764. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Wang X, Sha S, Liang S, Zhao L,
Liu L, Chai N, Wang H and Wu K: Berberine increases the expression
of NHE3 and AQP4 in sennosideA-induced diarrhoea model.
Fitoterapia. 83:1014–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Yu H, Zhang C, Cheng Y, Hu L, Meng
X and Zhao Y: Protective effects of berberine on radiation-induced
lung injury via intercellular adhesion molecular-1 and transforming
growth factor-beta-1 in patients with lung cancer. Eur J Cancer.
44:2425–2432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rezaeiamiri E, Bahramsoltani R and Rahimi
R: Plant-derived natural agents as dietary supplements for the
regulation of glycosylated hemoglobin: A review of clinical trials.
Clin Nutr. Feb 10–2019.(Epub ahead of print). doi:
10.1016/j.clnu.2019.02.006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tillhon M, Guamán Ortiz LM, Lombardi P and
Scovassi AI: Berberine: New perspectives for old remedies. Biochem
Pharmacol. 84:1260–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin P, Zhang C and Li N: Berberine
exhibits antitumor effects in human ovarian cancer cells.
Anticancer Agents Med Chem. 15:511–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pierpaoli E, Arcamone AG, Buzzetti F,
Lombardi P, Salvatore C and Provinciali M: Antitumor effect of
novel berberine derivatives in breast cancer cells. Biofactors.
39:672–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CH, Wu DF, Ding H, Zhao Y, Zhou KY and
Xu DE: Berberine hydrochloride impact on physiological processes
and modulation of twist levels in nasopharyngeal carcinoma CNE-1
cells. Asian Pac J Cancer Prev. 15:1851–1857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Wang X, Sharvan R, Gao J and Qu S:
Berberine could inhibit thyroid carcinoma cells by inducing
mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing
migration via PI3K-AKT and MAPK signaling pathways. Biomed
Pharmacother. 95:1225–1231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Zhang C, Yang X, Yang B, Wang J,
Kang Y, Wang Z, Li D, Huang G, Ma Z, et al: Berberine inhibits the
expression of hypoxia induction factor-1alpha and increases the
radiosensitivity of prostate cancer. Diagn Pathol. 9:982014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kou Y, Li L, Li H, Tan Y, Li B, Wang K and
Du B: Berberine suppressed epithelial mesenchymal transition
through cross-talk regulation of PI3K/AKT and RARα/RARβ in melanoma
cells. Biochem Biophys Res Commun. 479:290–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo P, Cai C, Wu X, Fan X, Huang W, Zhou
J, Wu Q, Huang Y, Zhao W, Zhang F, et al: An insight into the
molecular mechanism of berberine towards multiple cancer types
through systems pharmacology. Front Pharmacol. 10:8572019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao DC and Xiao PG: Network pharmacology:
A Rosetta stone for traditional Chinese medicine. Drug Develop Res.
75:299–312. 2014. View Article : Google Scholar
|
|
20
|
Zoete V, Grosdidier A and Michielin O:
Docking, virtual high throughput screening and in silico
fragment-based drug design. J Cell Mol Med. 13:238–248. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminform. 6:132014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Ouyang S, Yu B, Liu Y, Huang K,
Gong J, Zheng S, Li Z, Li H and Jiang H: PharmMapper server: A web
server for potential drug target identification using pharmacophore
mapping approach. Nucleic Acids Res. 38:W609–W614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H, Chen J, Xu X, Li Y, Zhao H, Fang Y,
Li X, Zhou W, Wang W and Wang Y: A systematic prediction of
multiple drug-target interactions from chemical, genomic and
pharmacological data. PLoS One. 7:e376082012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su WH, Chao CC, Yeh SH, Chen DS, Chen PJ
and Jou YS: OncoDB.HCC: An integrated oncogenomic database of
hepatocellular carcinoma revealed aberrant cancer target genes and
loci. Nucleic Acids Res. 35:D727–D731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang DW, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chuang CH, Cheng TC, Leu YL, Chuang KH,
Tzou SC and Chen CS: Discovery of Akt kinase inhibitors through
Structure-Based virtual screening and their evaluation as potential
anticancer agents. Int J Mol Sci. 16:3202–3212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Renata DP, Christian VQ, Ruiz DD, Gargano
F and de Souza ON: A selective method for optimizing ensemble
docking-based experiments on an InhA Fully-Flexible receptor model.
BMC Bioinformatics. 19:2352018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Li X, Huang J and Liu Y: Matrix
metalloproteinase 2 knockdown suppresses the proliferation of HepG2
and Huh7 cells and enhances the cisplatin effect. Open Med (Wars).
14:384–391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu C, Zhang W, Zhang X, Zhou D, Qu L, Liu
J, Xiao M, Ni R, Jiang F, Ni W and Lu C: Coupling function of
cyclin-dependent kinase 2 and Septin2 in the promotion of
hepatocellular carcinoma. Cancer Sci. 110:540–549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong M, Li S, Wang N, Tan HY, Cheung F and
Feng Y: A biomedical investigation of the hepatoprotective effect
of radix salviae miltiorrhizae and network Pharmacology-Based
prediction of the active compounds and molecular targets. Int J Mol
Sci. 18:E6202017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong M, Li S, Tan H, Cheung F, Wang N,
Huang J and Feng Y: A Network-Based pharmacology study of the
Herb-induced liver injury potential of traditional hepatoprotective
Chinese herbal medicines. Molecules. 22:E6322017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Wang N, Li H, Liu M, Cao F, Yu X,
Zhang J, Tan Y, Xiang L and Feng Y: Up-Regulation of PAI-1 and
Down-regulation of uPA are involved in suppression of invasiveness
and motility of hepatocellular carcinoma cells by a natural
compound berberine. Int J Mol Sci. 17:5772016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang N, Feng Y, Zhu M, Tsang CM, Man K,
Tong Y and Tsao SW: Berberine induces autophagic cell death and
mitochondrial apoptosis in liver cancer cells: The cellular
mechanism. J Cell Biochem. 111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|