Introduction
Opioids are useful in medicine for alleviating acute
and chronic pains. However, they are limited by their associated
side effects, particularly allodynia and hyperalgesia. The
underlying cellular and molecular mechanisms of opioid tolerance
and chronic neuropathic pain remain to be understood. Evidence
suggests that opioid tolerance and chronic neuropathic pain share a
general cellular mechanism (1)
that increases sensitivity to pain via spinal cord microglia
activation (2). Therefore, it is
necessary to understand the detailed mechanisms of opioid tolerance
and chronic neuropathic pain in order to develop new pain
therapies.
Previous studies reported that glia-modulating
agents, such as ionized calcium binding adaptor molecule 1, cluster
of differentiation 11b (CD11b) (3)
and pro-inflammatory cytokines (4)
can decrease morphine tolerance by inhibiting rat spinal microglia
activity. In impaired central nervous systems, injury induces a
gradual transformation of resting microglia to the active state.
Activating microglia not only changes their morphology, but also
enhances their adhesion and chemotaxis mobility (5). Those processes depend on the velocity
of cytoskeleton assembly and disassembly. It was demonstrated that
the histone deacetylase 6 (HDAC6)/heat shock protein 90 (HSP90)
signaling pathway was involved in the microglia migration process
(6). It has been suggested that
activated microglia play a role in the development of morphine
tolerance and/or hyperalgesia. Our previous study and other
previous studies demonstrated that co-administration of an
anti-inflammatory drug with morphine is a strategy for enhancing
the antinociception of morphine, which also attenuates tolerance
(7,8) and neuropathic pain (9) by suppressing pro-inflammatory
cytokine expression and microglia activation.
Hericium erinaceus (H. erinaceus) is an
edible mushroom with notable medicinal properties. It has been
consumed as food and used in traditional Chinese herbal medicine in
China without toxic side effects (10). Evidence shows that H.
erinaceus has several beneficial biological effects, such as
neuroprotection (11),
anti-oxidation (12), immune
modulation and anticancer activity (13). H. erinaceus suppresses
synthesis of inflammatory mediators by inhibiting oxidative stress
(14) and the inducible nitric
oxide synthase/p38 MAPK pathway (15), which suggested that H.
erinaceus may have analgesic properties. Additionally, in
Alzheimer's APPswe/PS1dE9 transgenic mice, H. erinaceus
significantly diminished the number of amyloid-β plaque-activated
microglia (16). The ethanol
extracts of H. erinaceus (EHE) mycelium, especially
Erinacine A, have demonstrated epinephrine-stronger nerve growth
factor-inducing activities in vitro and in vivo
(17). This result suggested that
EHE has a potential effect on pain management in patients who
develop morphine tolerance. This is the first study, to the best of
our knowledge, to evaluate the effect of EHE on morphine-induced
BV2 microglial cell activation and migration. It was observed that
EHE suppresses BV2 microglial cell activation and migration by
regulating the HDAC6 and HSP90 deacetylation signaling pathway.
Materials and methods
Hericium erinaceus
H. erinaceus, which was purchased from The
Bioresources Collection and Research Center (BCRC no. 35669) in The
Food Industry Research and Development Institute, was maintained on
potato dextrose agar at 26°C for 15 days (18). After incubation, a mycelial agar
block (1 cm3) was removed, transferred to a 2-L
Erlenmeyer flask containing 1.3 L synthetic medium (composed of
0.25% yeast extract, 4.5% glucose, 0.5% soybean powder, 0.25%
peptone and 0.05% MgSO4, adjusted to pH 4.5) and
incubated for 5 days at 26°C on a rotary shaker (1.12 × g; Firstek;
cat. no. S203). The fermentation process was then scaled up from a
2-L shake flask to 500-L and 20-ton fermenters for 5 and 12 days,
respectively. At the end of the fermentation process, the mycelia
were then harvested, lyophilized, ground into a powder and stored
in a desiccator at room temperature (18). The dry powder was extracted four
times with 90% ethanol under reflux. The H. erinaceus powder
was then extracted four times with 95% ethanol at 40 KHz and 26°C
with 1 h of sonication each time. All ethanol solutions were
concentrated under a vacuum in order to obtain brown extracts.
Cell culture and reagents
The mouse BV2 microglial cell line was provided by
Professor Chih-Shung Wong (Fu-Jen Catholic University). The cell
line was verified in our laboratory; it was positive for MAC1 and
CD11b expression, but negative for the expression of the astrocyte
marker glial fibrillary acidic protein and oligodendrocyte marker
galactocerebrosidase. BV2 mouse microglia cells were grown in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) and supplemented with 100
µg/ml penicillin-streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in a 5% CO2 incubator. The medium was changed every 3
days.
Drug delivery for culture cells
Cultured cells were washed 3 times with serum-free
DMEM (blank medium) and starved for 4 h. Then, the cells were
incubated with different concentrations (ranging between 1 ng and 1
µg) of EHE for 30 min, followed by a 2-h incubation with morphine
at different concentrations (10–100 µM) for 2 h. (Sigma-Aldrich;
Merck KGaA). Subsequently, the cells were gently harvested in PBS/1
mM EDTA and used for a migration assay and western blot
analysis.
Chemotaxis assay
The cells were incubated for 30 min in serum-free
DMEM with or without 100 ng/ml EHE, and then incubated for 2 h with
1 µM morphine. After incubation, cell survival was further
determined by trypan blue staining (0.4% trypan blue and 3 min at
room temperature) followed by cell counting. The treated cells
(1×103) in serum-free DMEM were seeded in the top wells
of a 48-well microchemotaxis chamber (Neuro Probe, Inc.), and DMEM
containing 10% FBS was added to the bottom wells of the chamber.
The cells in both chambers were incubated for 1 h at 37°C in 5%
CO2. The number of BV2 microglial cells that migrated to
the underside of the filter (25×80 mm polycarbonate membrane, 3.0
µm pore size; Osmonics; cat. no. K80SH58050) was counted. The
membranes were rinsed with PBS, and the migrated cells were then
fixed with 100% methanol at room temperature for 10 min and stained
with crystal violet for 20 min at room temperature (Sigma-Aldrich;
Merck KGaA). In total, 6 random fields were counted for each
condition using an Olympus BX 50 fluorescence microscope (Olympus
Corporation; magnification, ×20).
Image assay
Cell images were captured under a light microscope
at ×10 and ×40 magnification and at least three wells from each
experimental group were selected. (Olympus Corporation).
Cell lysis and western blotting
The cells were lysed in 200 µl 1X Laemmli sample
buffer (Bio-Rad Laboratories, Inc.) comprised of 2-mercaptoethanol.
The protein concentration of the samples was measured using the
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific Inc.;
cat. no. 23225). In total, 15–25 µg protein was boiled at 95°C for
5 min, separated by SDS-PAGE on 10% gels, and transferred to
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked with 5% non-fat milk in buffer (50 mM
Tris-HCl, 154 mM NaCl and 0.05% Tween 20) at room temperature for 1
h. Then, the membranes were incubated at 4°C overnight with various
primary antibodies; goat polyclonal antibodies against mouse HDAC6
(Santa Cruz Biotechnology, Inc.; cat. no. sc-5258; 1:200 in
blocking buffer) and rabbit polyclonal antibodies against HSP90,
CD11b (Abcam; cat. no. ab13495 and ab128797; 1:200 in blocking
buffer) and acetylated-HSP90 (LifeSpan BioSciences, Inc.; cat. no.
LS-C380587; 1:100 in blocking buffer). The membranes were then
incubated for 1 h at room temperature with the corresponding
horseradish peroxidase-conjugated donkey anti-goat or goat
anti-rabbit immunoglobulin G antibodies, as appropriate (all from
Abcam; cat. nos. ab205723 and ab205718; 1:2,000 in 5% non-fat milk
in TBS with 0.05% Tween-20). Membrane-bound secondary antibodies
were detected using Chemiluminescence Plus reagent (PerkinElmer,
Inc.) and visualized using a chemiluminescence imaging system
(Syngene Europe). Finally, the blots were incubated for 18 min at
56°C in stripping buffer (62.6 mM Tris-HCl, pH 6.7, 2% SDS, 100 mM
mercaptoethanol) and re-probed with mouse monoclonal anti-β-actin
antibody (Sigma-Aldrich; Merck KGaA; cat. no. A1978; 1:10,000 in
blocking buffer) at room temperature for 1 h and horseradish
peroxidase-conjugated goat anti-mouse IgG antibody (Abcam; cat. no.
ab205719; 1:3,000 in 5% non-fat milk in TBS with 0.05% Tween-20) as
the loading control. The relative densities of the protein bands
were analyzed using a computer-assisted imaging analysis system
(GeneTools Match software, 4.3.7, Syngene).
Statistical analysis
All experiments were conducted at least three times,
and data are presented as the mean ± SEM. For immunoreactivity
data, the intensity of each test band was expressed as the optical
density relative to that of the average optical density for the
corresponding control band. For statistical analysis,
immunoreactivity was analyzed using one-way ANOVA, followed by
multiple comparisons with the Student-Newman-Keuls post hoc test.
The statistical analysis was performed using SigmaStat 3.0 software
(Systat Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
EHE mycelium inhibits morphine-induced
microglia chemotaxis
BV2 microglial Morphine stimulated cell migration in
a concentration-dependent manner up to 1 µM, and then reduced cell
migration at higher doses (Fig.
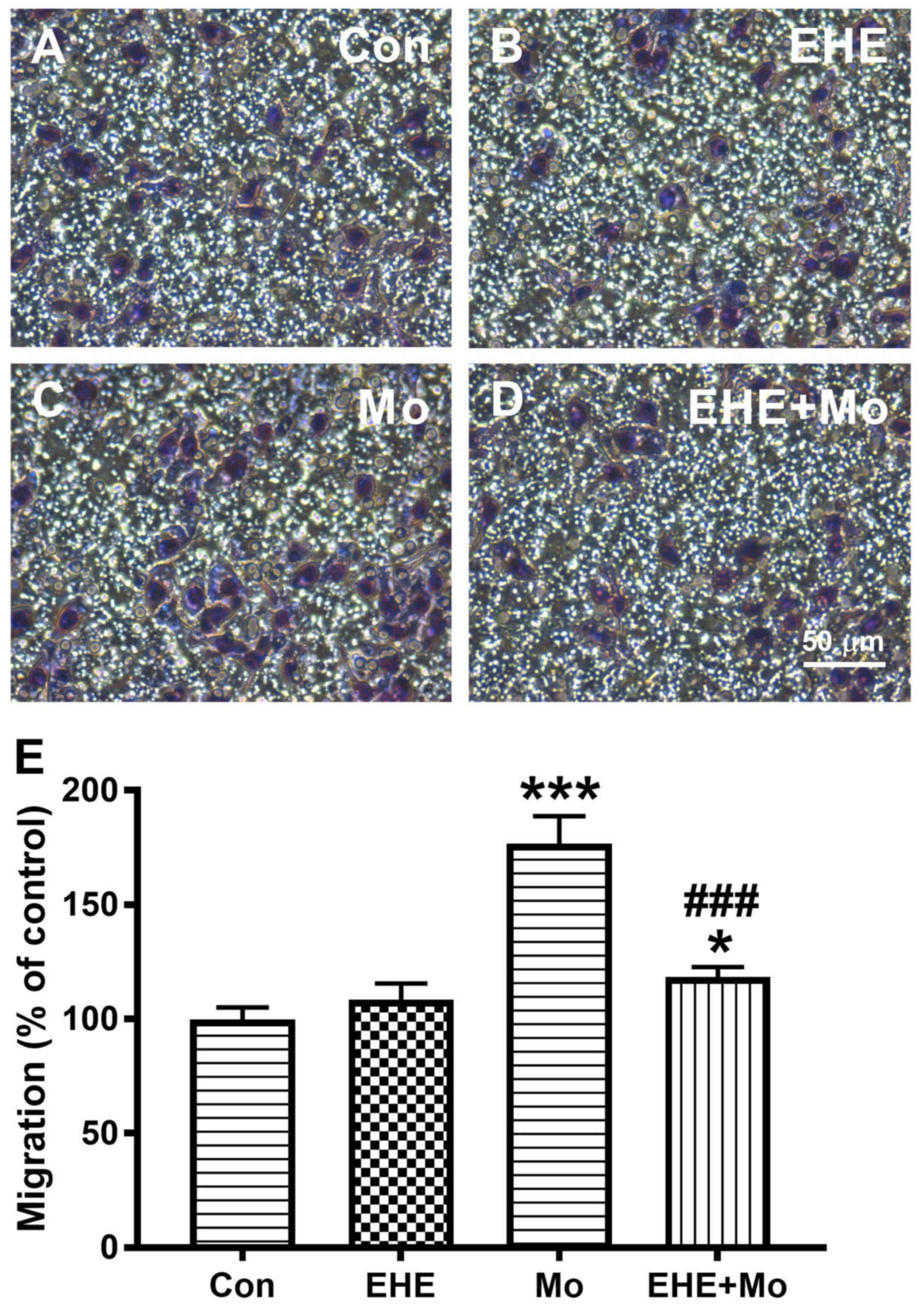
1A). Pretreatment with 100 ng/ml EHE significantly inhibited
morphine-induced microglia migration from 181.7±7.63 to 103.0±6.08%
(P=0.0002; Figs. 1B and 2). All subsequent experiments were
performed using 1 µM morphine and 100 ng/ml EHE.
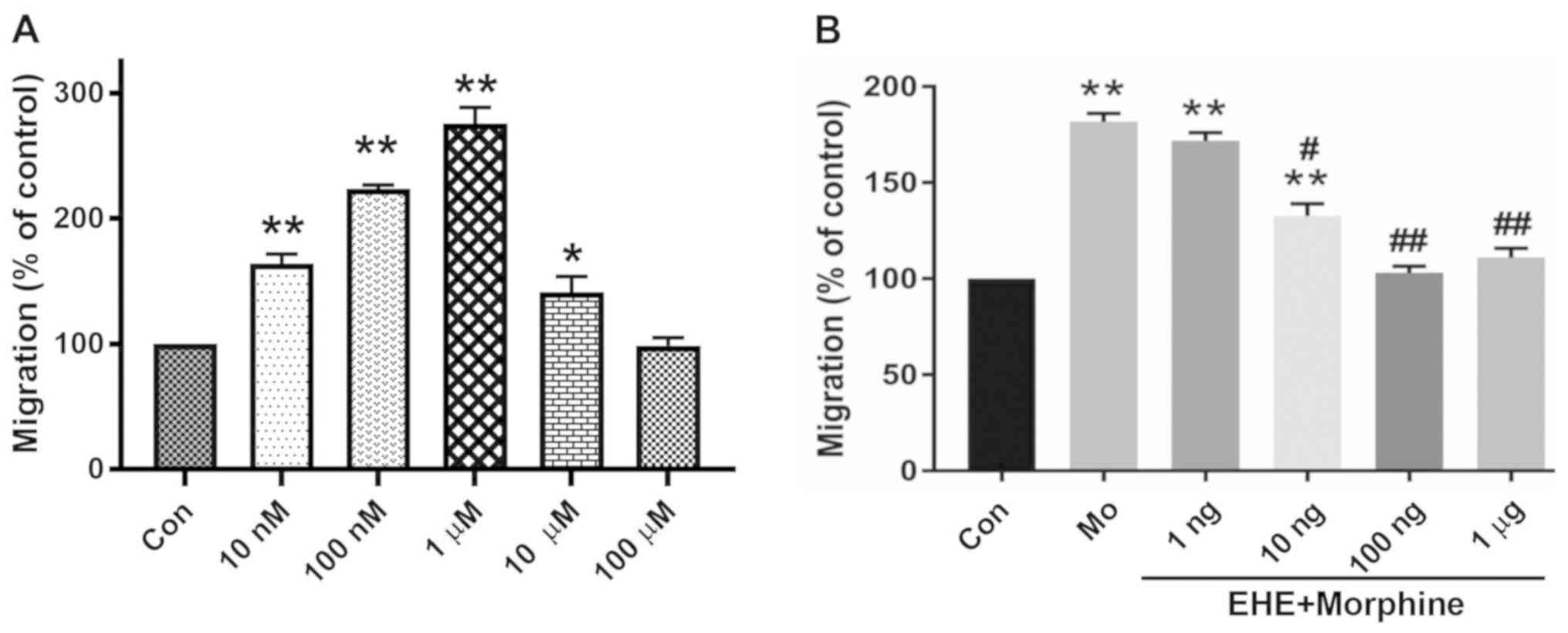 | Figure 1.Effect of EHE on morphine-induced BV2
cell activation. (A) Concentration-response effect of morphine on
BV2 cell migration. The cells were incubated for 2 h with
serum-free medium alone (Con) or with medium containing 10 and 100
nM, and 1, 10 or 100 µM Mo. *P<0.05, **P<0.001 vs. Con group.
(B) Concentration-response effect of EHE on morphine-induced BV2
cell migration. The cells were incubated for 2 h with blank medium
alone (Con), incubated with 1 µM Mo, or pretreated for 30 min with
a particular concentration of EHE and then incubated with 1 mM Mo
(EHE+Mo). A cell migration assay was performed, and the migrated
cells were counted. Data are presented as the mean ± SEM. n=18.
**P<0.001 vs. Con group; #P<0.05,
##P<0.001 vs. Mo group. EHE, ethanol extracts of
H. erinaceus; Mo, morphine; Con, control. |
Morphine-induced microglia activation
is inhibited by EHE
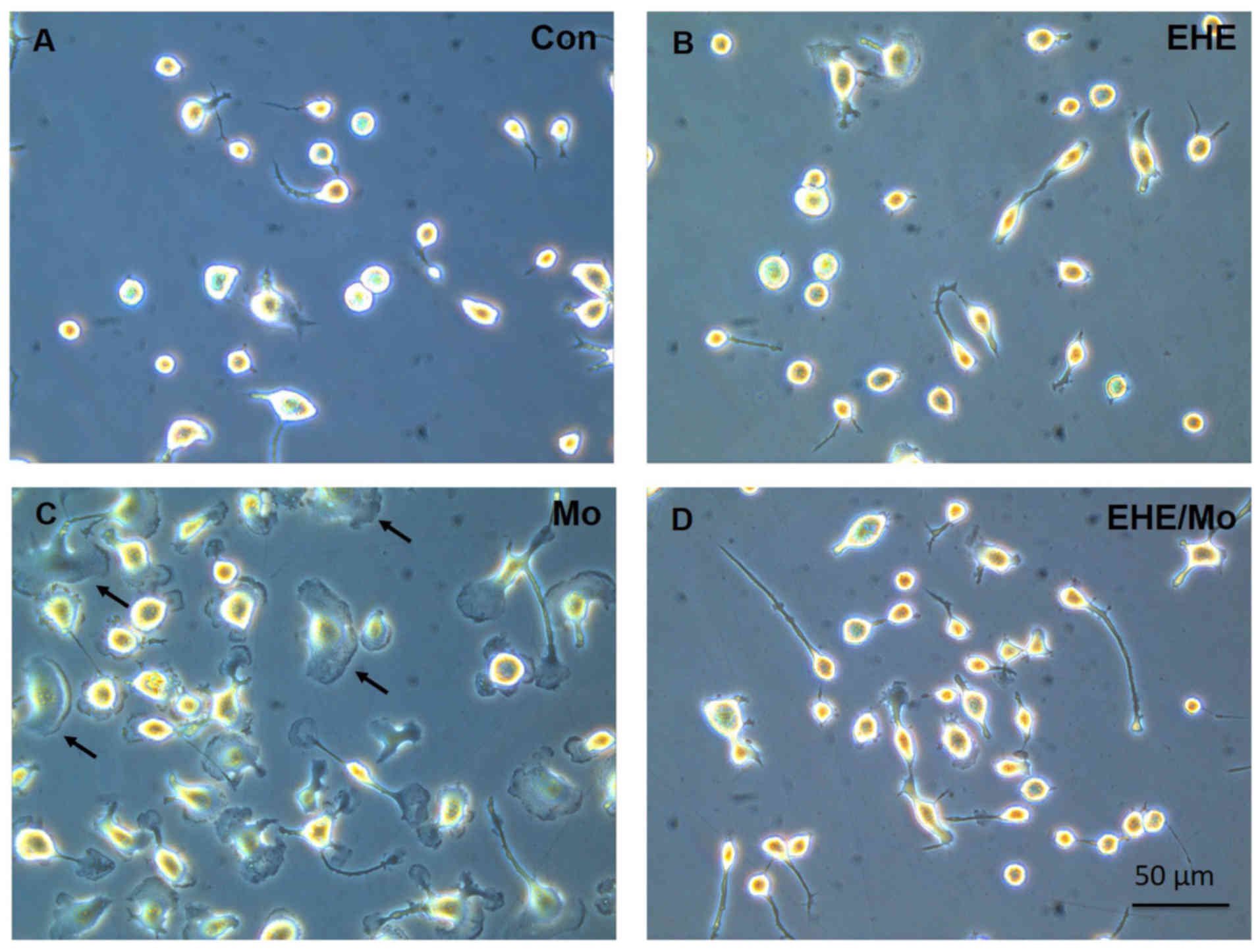
In the cell morphology study, BV2 cells exhibited
shapes that were bipolar rod-like or globular after seeding
overnight. At 24 h after seeding, the cells were starved for 4 h
prior to treatment. They were pre-incubated with blank medium or
with 100 ng/ml EHE for 30 min; then they were incubated for 2 h
after morphine was added. As shown in Fig. 3, the cell morphology was altered
from ramified cells (Fig. 3A and
B) to an amoeboid shape with a larger cell body and retracted
processes (Fig. 3C). Moreover,
morphine treatment not only induced BV2 cell activation with
membrane ruffling (black arrows), it activated the microglia as
well. In addition, EHE pretreatment markedly inhibited microglia
activation and cell membrane ruffling (Fig. 3D).
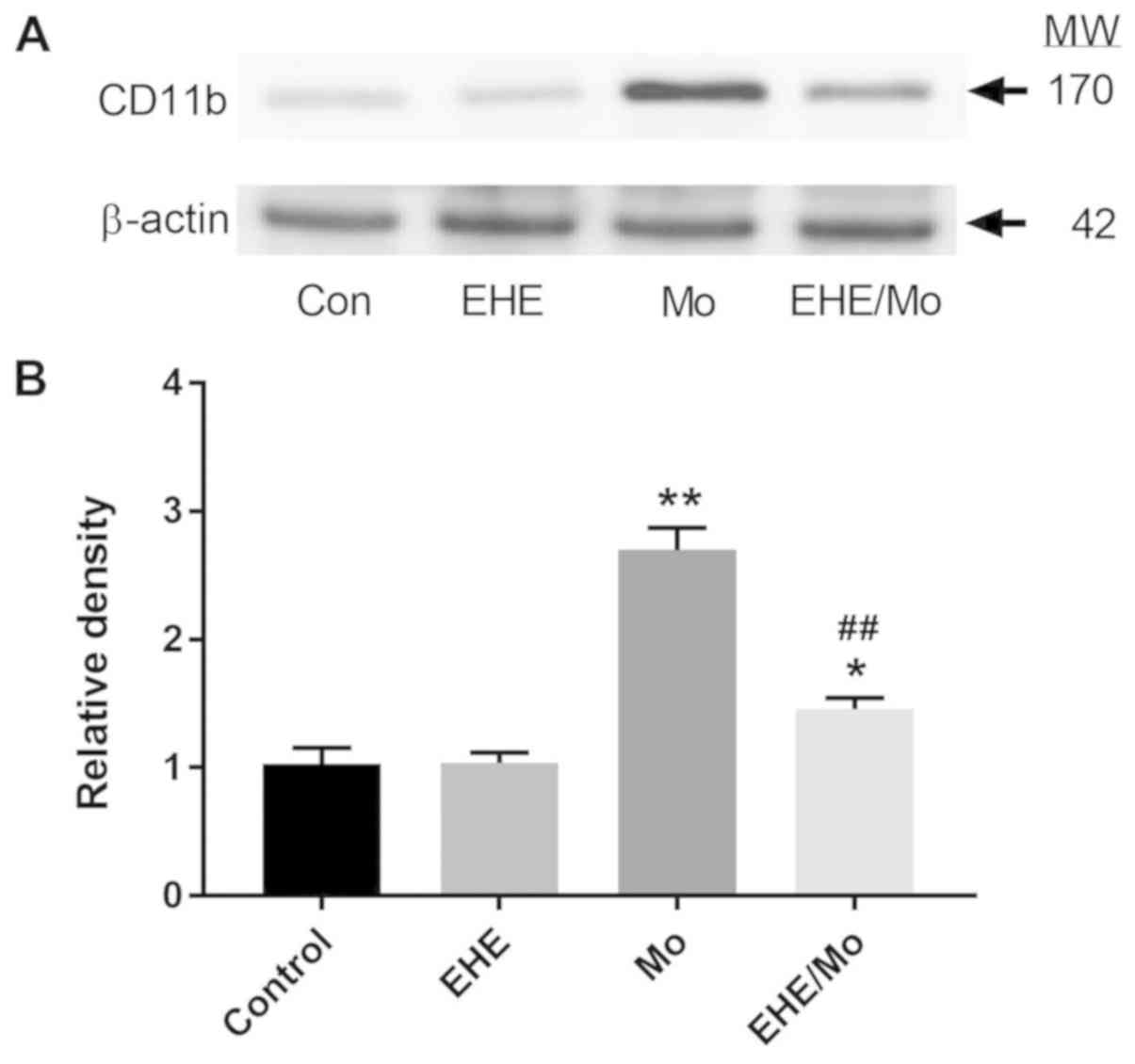
As shown in Fig. 4,
the immunoblot analysis of BV2 protein extracts showed that CD11b
expression was significantly increased (2.70±0.23; P<0.001) in
the morphine-treated cells, compared with the control group. This
effect was significantly decreased by EHE pre-treatment (1.46±0.12;
P<0.05 compared with the control group; P<0.001 compared with
the morphine-treated group).
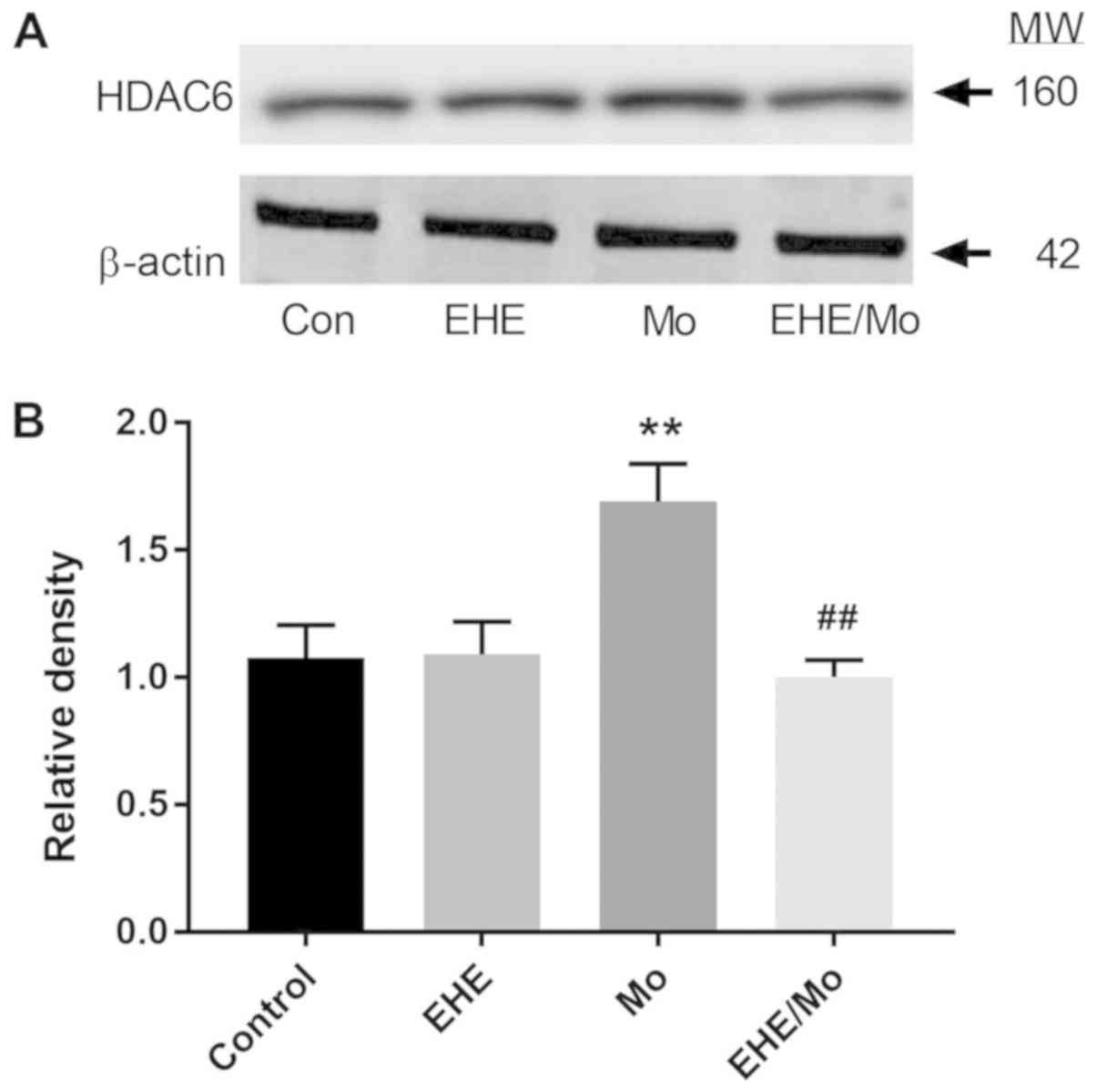
Morphine induces HDAC6 expression in
BV2 cells
HDAC6 has been documented to affect cell migration
through regulation of HSP90 (19).
As shown in Fig. 5, morphine
treatment increased HDAC6 expression compared with the control
group from 1 to 1.6±0.13 (P=0.0003) and it was inhibited by EHE
pretreatment (1.02±0.05; P<0.001 compared with the morphine
group). The present results demonstrated that morphine affects
HDAC6-mediated processes.
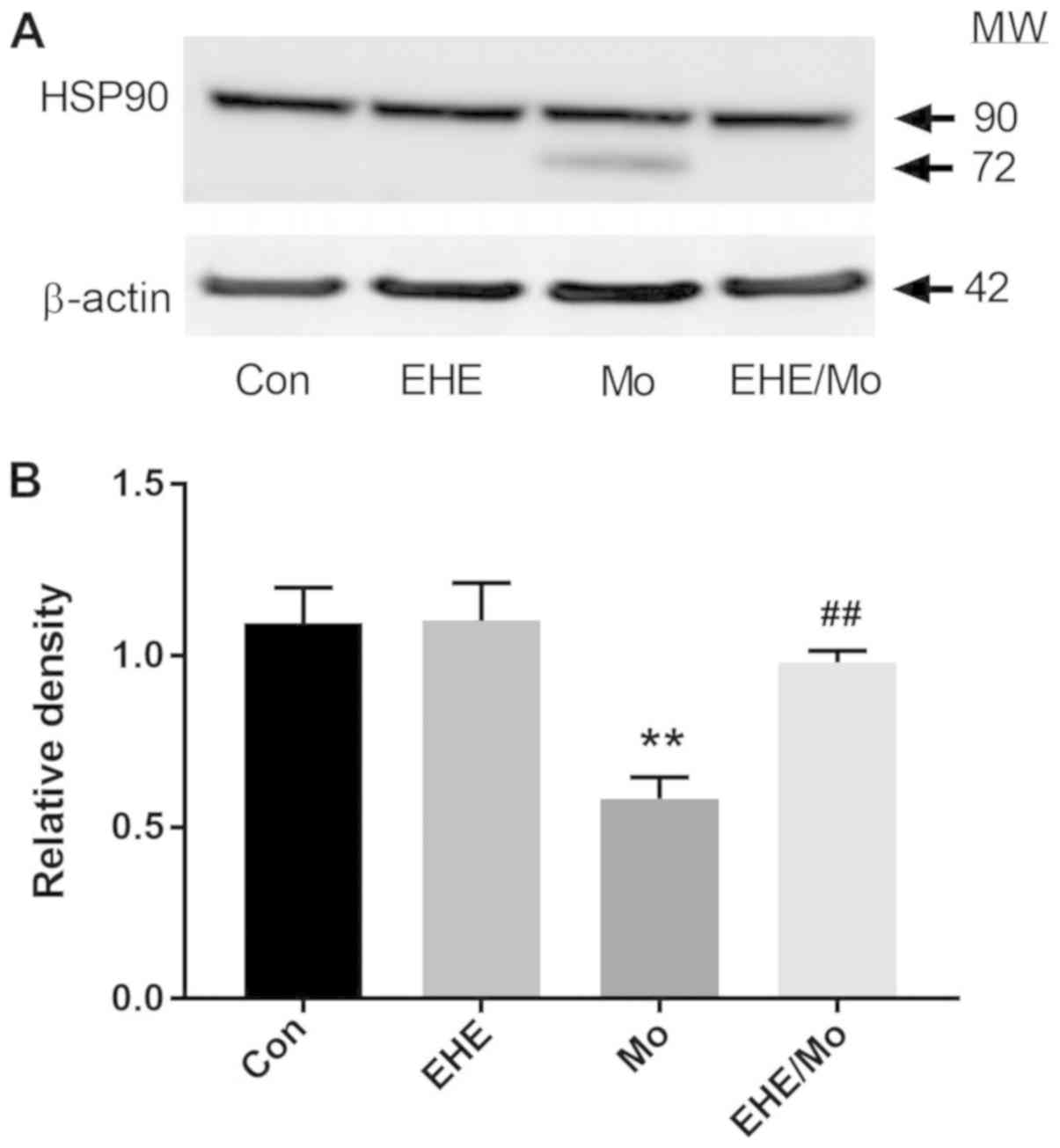
EHE reverses morphine-stimulated HSP90
cleavage and deacetylation
Morphine treatment not only increased HDAC6
expression, it additionally decreased HSP90 expression (0.68±0.012;
P=0.00001) compared with the control group and further led to HSP90
fragmentation (Fig. 6). Compared
with the morphine group, EHE pretreatment significantly inhibited
the morphine-induced cleavage of HSP90 and lower HSP90 expression
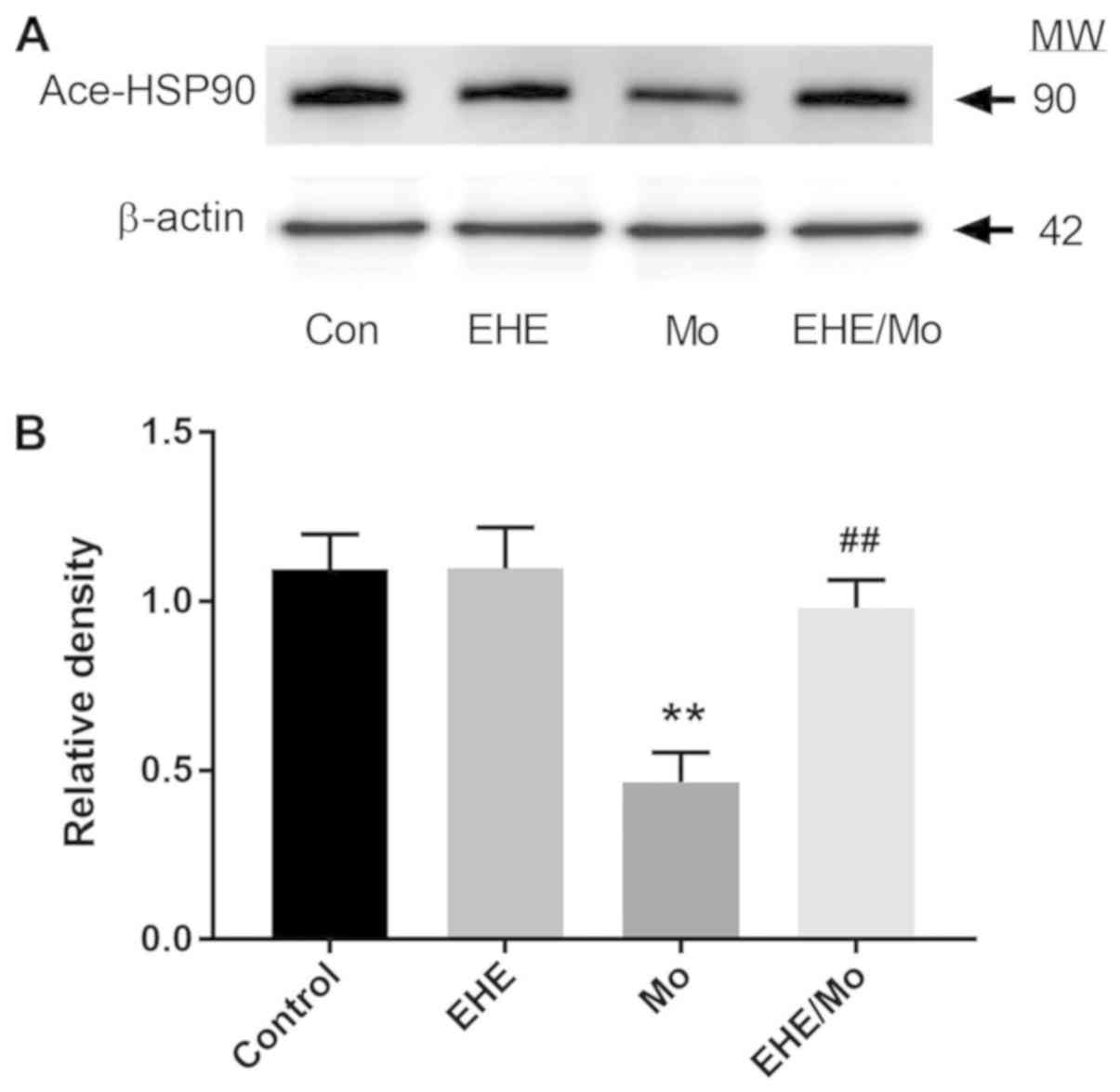
(0.98±0.06; P<0.05). As Fig. 7
shows, the morphine treatment stimulated deacetylation of HSP90
(0.42±0.06; P=0.0006, compared with the untreated control), which
was significantly inhibited by EHE pretreatment (0.95±0.076;
P<0.005, compared with the morphine control).
Discussion
Microglia are now recognized for playing key roles
in the formation and maintenance of morphine tolerance, and are
associated with hyperalgesia (20). Activated microglia are a source of
production of pro-inflammatory cytokines, such as tumor necrosis
factor-α, interleukin (IL)1-β and Il-6 (7), which attenuate antinociception
induced by morphine, and thus contribute to the development of
morphine tolerance and associated pain sensitization. Therefore,
suppressing microglia activation has been considered an effective
therapeutic approach in mitigating morphine tolerance and
neuropathic pain. In the present study, H. erinaceus
mycelium demonstrated potent inhibitory effects on
morphine-stimulated BV2 cells in multiple aspects; it markedly
attenuated morphine-induced membrane ruffling and migration of BV2
microglial cells. The present data suggested that EHE has potential
as an analgesic adjuvant in pain management.
Reactive microgliosis is associated with changes of
cell morphology, enhanced adhesion and migration processes
(6). A previous study demonstrated
that microtubule stabilization plays an active role in axon
formation in primary hippocampal neurons of rats (21). These findings suggested that the
stabilization of α-tubulin and microtubules plays a crucial role in
cellular processes, as well as morphogenesis, compartmentalization
and organelle movement. It has been reported that chronic morphine
treatment results in a significant decrease in both mRNA and
protein levels of α-tubulin, and the microtubule-associated protein
τ in rat striatum (22). Long-term
morphine administration markedly decreases neurofilament light
polypeptide expression, a major component of neuronal cytoskeleton
neurofilaments, and results in neuronal damage (23). In our previous study, morphine
treatment decreased acetylated α-tubulin levels in EOC13.31
microglia cells, and these effects were correlated with an increase
in activation and migration of microglia (6). In addition, it was observed that
α-tubulin stabilization directly correlated with cell function.
According to these results, it was hypothesized that chronic
morphine administration directly affects microglia cell
cytoskeleton stabilization and consequent activation.
Cell chemotaxis plays an essential role in immune
surveillance, tissue localization and tumor metastasis (19). Most migrating cells are polarized
and show a leading edge or frontal flat lamella, which is the site
of protrusive activity. A previous study used microglia cells to
examine the effect of morphine on microglia chemotaxis and to
explore the role of immunocompetent cells in the actions of opioids
(24). The results of that
previous study showed that morphine stimulated microglia migration
by modulating P2X purinoceptor 4 receptor signaling. Our previous
study demonstrated that morphine changes the morphology of EOC13.31
microglia, and causes membrane ruffling and chemotaxis (6). Membrane ruffling exists at the lead
edge of a migrating cell as a driving force of chemotaxis (25). Similar results were found in the
present study. The results demonstrated that morphine increased BV2
microglial cell migration and membrane ruffling. Pretreatment with
EHE inhibited BV2 cell migration and activation. This suggested
that EHE may regulate cytoskeleton dynamics.
HDAC6 is a member of the class IIb HDAC family.
HDAC6 removes acetyl groups from substrates other than histones; it
is a unique isoenzyme with specific physiological roles. HDAC6
deacetylates various non-histone substrates, such as α-tubulin and
HSP90, and is involved in protein trafficking and degradation, and
cell shaping and migration (19,26).
A previous study indicated that decreased tubulin and HSP90
acetylation was correlated with cell migration, cell growth and
survival (26). Similarly, our
recent study showed that HDAC6 overexpression in morphine-treated
microglial cells was accompanied by inhibited acetylation of
α-tubulin and enhanced microglia activity (6). In the present study, it was also
demonstrated that chronic morphine treatment of BV2 microglial
cells induced upregulation of HDAC6, promoted HSP90 deacetylation
and enhanced microglia reactivity. It was hypothesized that HDAC6
deacetylase serves an important in microtubule configuration and
cell migration. A previous study found that HDAC6 is localized in
the protrusive structures of polarized cells as the leading edge
and membrane ruffles (27). In
agreement, the present study observed that EHE suppressed
morphine-induced HDAC6 expression. According to these results, it
was hypothesized that the inhibitory effect of EHE on
morphine-stimulated microglia activation and migration is by
inhibiting the HDAC6/HSP90 deacetylation signaling transduction
pathway.
HSP90 is an ATP-dependent molecular chaperone, an
essential cytosolic protein under normal conditions, and it
markedly increases during stress (28). HSP90 works together with a group of
co-chaperones. Co-chaperones modulate HSP90 activity, which
facilitates client protein maturation. Post-translational
modifications of HSP90 contribute to protein folding and serve
various cellular processes, such as signal transduction and protein
degradation (29). Acetylation is
a reversible modification regulated through the opposing action of
acetyltransferases and deacetylases (30). In general, HSP90 serves to regulate
both the inactivation and activation of client proteins. Therefore,
acetylation weakens HSP90 interaction with client proteins, which
results in the inactivation of the client proteins (31). In the present study, it was
determined that that morphine induced microglial activation and
migration, upregulated HDAC6 expression, and induced HSP90
fragmentation and deacetylation; this may explain the action of
HSP90 related to cytoskeleton stabilization. A previous study
showed that HSP90 protected tubulin against thermal denaturation
and keeps it in a state compatible with microtubule polymerization
(32) in nephrotoxin-induced
injuries of renal cell models. This result suggests that HSP90 may
contribute to the regulation of microtubule cytoskeleton
polymerization and depolymerization. HSP90 is a protein associated
with a variety of stress responses and defense mechanisms of cells
(33). Based on the results of the
present study, morphine treatment downregulated HSP90 expression
and induced HSP90 fragmentation in BV2 microglia cells, which were
accompanied with cell activation and migration. Therefore, it was
suggested that morphine treatment may cause a stress response in
BV2 cells. Pretreatment with EHE significantly inhibited this
stress response. In conclusion, the present data revealed a novel
mechanism in which morphine mediates BV2 migration via the
HDAC6/HSP90 deacetylation signaling pathway.
The limitation of the present study is that there is
no in vitro method available yet that includes all hallmarks
of homeostatic microglia. A large collection of microglia cell
lines has been generated over the years, including BV2, N9,
EOC-13.31 and IMG (34,35). Most microglia cell lines obtained
from neonatal or embryonic central nervous system sources are
unlikely to reflect the phenotype of adult or elderly microglia.
Despite these limitations, the BV2 cells have been used for many
years to study neuroinflammation and neurodegenerative disorders
(34,35). The neuroscience field is
increasingly acknowledging that modulation of neuroinflammation is
a promising strategy to beneficially alter the disease course of
neuroinflammation and neurodegenerative disease. Although there are
advantages and limitations of existing models across different
species, microglia remain key components of the neuroinflammatory
response, leading to an intensive research interest in this
particular cell type.
In conclusion, in the present study, it was
demonstrated that EHE inhibits morphine-induced BV2 activations by
regulating the HDAC6/HSP90 deacetylation signaling transduction
pathway. The present findings suggested that EHE, as an analgesic
adjuvant, may be a potential therapeutic agent for pain management
in morphine-treated patients.
Acknowledgements
The authors are grateful for the use of the
laboratories provided to us by the Proteomics Laboratory, Cathay
Medical Research Institute, Cathay General Hospital, Xizhi, New
Taipei City, Taiwan.
Funding
The present study was supported by grants provided
by The Grape King Bio Ltd.-Da Yeh University Collaboration Project
(grant nos. DON-0411 and DON-0604) and by Da Yeh University (grant
no. ORD-105032).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHY and RYT participated in the design of the
present study and performed the statistical analysis. LWS, CML, CMC
and CCC conducted the study and analyses, and collected important
background information. TPY, JNL, SLT and WPC analyzed and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mao J, Price DD and Mayer DJ: Mechanisms
of hyperalgesia and morphine tolerance: A current view of their
possible interactions. Pain. 62:259–274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang ZJ, Jiang BC and Gao YJ: Chemokines
in neuron-glial cell interaction and pathogenesis of neuropathic
pain. Cell Mol Life Sci. 74:3275–3291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mika J, Zychowska M, Popiolek-Barczyk K,
Rojewska E and Przewlocka B: Importance of glial activation in
neuropathic pain. Eur J Pharmacol. 716:106–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao
CM, Chen PX and Feng JQ: A novel role of minocycline: Attenuating
morphine antinociceptive tolerance by inhibition of p38 MAPK in the
activated spinal microglia. Brain Behav Immun. 22:114–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horvath RJ, Nutile-McMenemy N, Alkaitis MS
and Deleo JA: Differential migration, LPS-induced cytokine,
chemokine, and NO expression in immortalized BV-2 and HAPI cell
lines and primary microglial cultures. J Neurochem. 107:557–569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai RY, Cheng YC and Wong CS:
(+)-Naloxone inhibits morphine-induced chemotaxis via prevention of
heat shock protein 90 cleavage in microglia. J Formos Med Assoc.
114:446–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsai RY, Chou KY, Shen CH, Chien CC, Tsai
WY, Huang YN, Tao PL, Lin YS and Wong CS: Resveratrol regulates
N-methyl-D-aspartate receptor expression and suppresses
neuroinflammation in morphine-tolerant rats. Anesth Analg.
115:944–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeh YC, Lin TF, Chang HC, Chan WS, Wang
YP, Lin CJ and Sun WZ: Combination of low-dose nalbuphine and
morphine in patient-controlled analgesia decreases incidence of
opioid-related side effects. J Formos Med Assoc. 108:548–553. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cherng CH, Lee KC, Chien CC, Chou KY,
Cheng YC, Hsin ST, Lee SO, Shen CH, Tsai RY and Wong CS: Baicalin
ameliorates neuropathic pain by suppressing HDAC1 expression in the
spinal cord of spinal nerve ligation rats. J Formos Med Assoc.
113:513–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ulziijargal E and Mau JL: Nutrient
compositions of culinary-medicinal mushroom fruiting bodies and
mycelia. Int J Med Mushrooms. 13:343–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuo HC, Lu CC, Shen CH, Tung SY, Hsieh MC,
Lee KC, Lee LY, Chen CC, Teng CC, Huang WS, et al: Hericium
erinaceus mycelium and its isolated erinacine A protection from
MPTP-induced neurotoxicity through the ER stress, triggering an
apoptosis cascade. J Transl Med. 14:782016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malinowska E, Krzyczkowski W, Łapienis G
and Herold F: Improved simultaneous production of mycelial biomass
and polysaccharides by submerged culture of Hericium
erinaceum: Optimization using a central composite rotatable
design (CCRD). J Ind Microbiol Biotechnol. 36:1513–1527. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Yu K, Li F, Xu K, Li J, He S, Cao S
and Tan G: Anticancer potential of Hericium erinaceus
extracts against human gastrointestinal cancers. J Ethnopharmacol.
153:521–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin M, Geng Y, Lu Z, Xu H, Shi JS, Xu X
and Xu ZH: Anti-inflammatory effects of ethanol extract of lion's
mane medicinal mushroom, Hericium erinaceus
(Agaricomycetes), in mice with ulcerative colitis. Int J Med
Mushrooms. 18:227–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KF, Chen JH, Teng CC, Shen CH, Hsieh
MC, Lu CC, Lee KC, Lee LY, Chen WP, Chen CC, et al: Protective
effects of Hericium erinaceus mycelium and its isolated
erinacine A against ischemia-injury-induced neuronal cell death via
the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int J Mol Sci.
15:15073–15089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai-Teng T, Chin-Chu C, Li-Ya L, Wan-Ping
C, Chung-Kuang L, Chien-Chang S, Chi-Ying HF, Chien-Chih C and
Shiao YJ: Erinacine A-enriched Hericium erinaceus mycelium
ameliorates Alzheimer's disease-related pathologies in
APPswe/PS1dE9 transgenic mice. J Biomed Sci. 23:492016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori K, Inatomi S, Ouchi K, Azumi Y and
Tuchida T: Improving effects of the mushroom Yamabushitake
(Hericium erinaceus) on mild cognitive impairment: A
double-blind placebo-controlled clinical trial. Phytother Res.
23:367–372. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li IC, Chen YL, Lee LY, Chen WP, Tsai YT,
Chen CC and Chen CS: Evaluation of the toxicological safety of
erinacine A-enriched Hericium erinaceus in a 28-day oral
feeding study in Sprague-Dawley rats. Food Chem Toxicol. 70:61–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valenzuela-Fernández A, Cabrero JR,
Serrador JM and Sánchez-Madrid F: HDAC6: A key regulator of
cytoskeleton, cell migration and cell-cell interactions. Trends
Cell Biol. 18:291–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bekhit MH: Opioid-induced hyperalgesia and
tolerance. Am J Ther. 17:498–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Witte H, Neukirchen D and Bradke F:
Microtubule stabilization specifies initial neuronal polarization.
J Cell Biol. 180:619–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shoji F, Shirabe K, Yano T and Maehara Y:
Surgical resection of solitary cardiophrenic lymph node metastasis
by video-assisted thoracic surgery after complete resection of
hepatocellular carcinoma. Interact Cardiovasc Thorac Surg.
10:446–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia-Sevilla JA, Ventayol P, Busquets X,
La Harpe R, Walzer C and Guimón J: Marked decrease of
immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of
opiate addicts. Neuroreport. 8:1561–1565. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX,
Liu WS, Qin HD, Feng QS, Chen LZ, Yao SY and Zeng YX: Traditional
Cantonese diet and nasopharyngeal carcinoma risk: A large-scale
case-control study in Guangdong, China. BMC Cancer. 10:4462010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deming PB, Campbell SL, Baldor LC and Howe
AK: Protein kinase A regulates 3-phosphatidylinositide dynamics
during platelet-derived growth factor-induced membrane ruffling and
chemotaxis. J Biol Chem. 283:35199–35211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caron C, Boyault C and Khochbin S:
Regulatory cross-talk between lysine acetylation and
ubiquitination: Role in the control of protein stability.
Bioessays. 27:408–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cabrero JR, Serrador JM, Barreiro O,
Mittelbrunn M, Naranjo-Suárez S, Martín-Cófreces N,
Vicente-Manzanares M, Mazitschek R, Bradner JE, Avila J, et al:
Lymphocyte chemotaxis is regulated by histone deacetylase 6,
independently of its deacetylase activity. Mol Biol Cell.
17:3435–3445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taipale M, Jarosz DF and Lindquist S:
HSP90 at the hub of protein homeostasis: Emerging mechanistic
insights. Nat Rev Mol Cell Biol. 11:515–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J and Buchner J: Structure, function
and regulation of the hsp90 machinery. Biomed J. 36:106–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aoyagi S and Archer TK: Modulating
molecular chaperone Hsp90 functions through reversible acetylation.
Trends Cell Biol. 15:565–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scroggins BT, Robzyk K, Wang D, Marcu MG,
Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, et al:
An acetylation site in the middle domain of Hsp90 regulates
chaperone function. Mol Cell. 25:151–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weis F, Moullintraffort L, Heichette C,
Chretien D and Garnier C: The 90-kDa heat shock protein Hsp90
protects tubulin against thermal denaturation. J Biol Chem.
285:9525–9534. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohtani H, Wakui H, Komatsuda A, Satoh K,
Miura AB, Itoh H and Tashima Y: Induction and intracellular
localization of 90-kilodalton heat-shock protein in rat kidneys
with acute gentamicin nephropathy. Lab Invest. 72:161–165.
1995.PubMed/NCBI
|
|
34
|
Gao F, Chen D, Hu Q and Wang G: Rotenone
directly induces BV2 cell activation via the p38 MAPK pathway. PLoS
One. 8:e720462013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Griciuc A, Serrano-Pozo A, Parrado AR,
Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT and
Tanzi RE: Alzheimer's disease risk gene CD33 inhibits microglial
uptake of amyloid beta. Neuron. 78:631–643. 2013. View Article : Google Scholar : PubMed/NCBI
|