The aryl hydrocarbon receptor (AhR) is
ligand-dependent and mediates nuclear receptors that react with
heterologous substances of phases I and II (Fig. 1). The theory of polycyclic aromatic
compound (PAC) metabolic reactions was postulated in the late 1950s
(1). The carcinogenic dye
3-methylcholanthrene induces the synthesis of a specific enzyme
that detoxifies 3-methylcholanthrene by promoting the synthesis of
the liver microsomal enzyme P450, which is an aryl hydrocarbon
hydroxylase (AHH) (1). Other
carcinogens, including insecticides and phenobarbital, have a
similar effect. This increase in synthesis meets the criteria of an
adaptive response, as the upregulated enzyme oxidizes the PAH
inducer when it is re-exposed within a short timeframe (2–4).
Genetic studies have revealed that AHH is regulated by multiple
alleles. These alleles were originally called Ah and used to
describe the reaction of aromatic hydrocarbons (5).
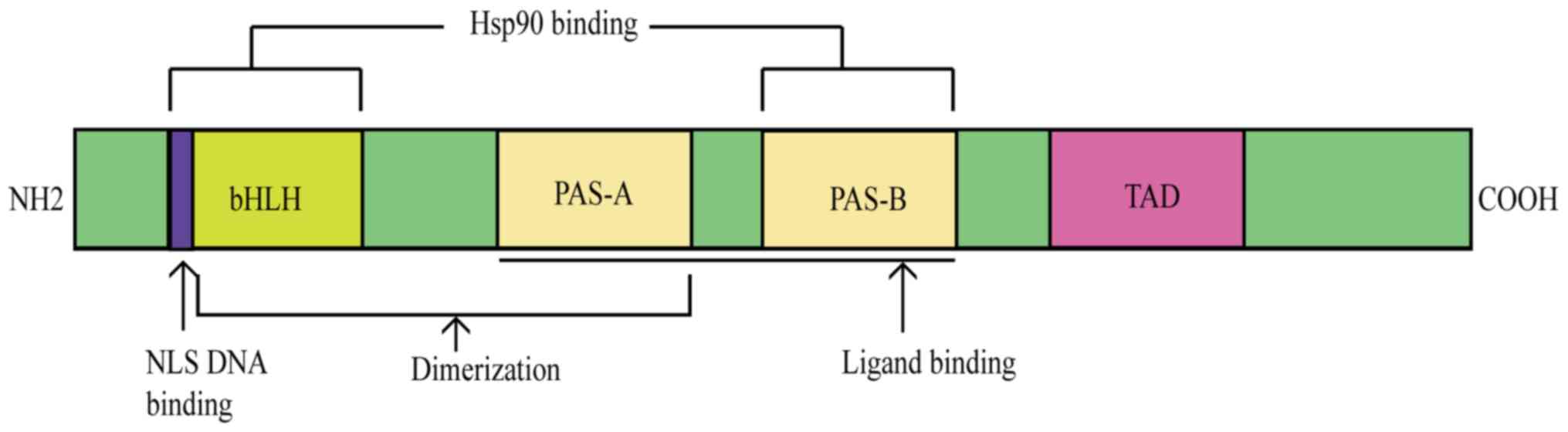
The AhR belongs to the basic helix-loop-helix (bHLH)
family and has a Per-Arnt-Sim (PAS) domain that binds to a variety
of endogenous and exogenous chemicals. It binds specific auxiliary
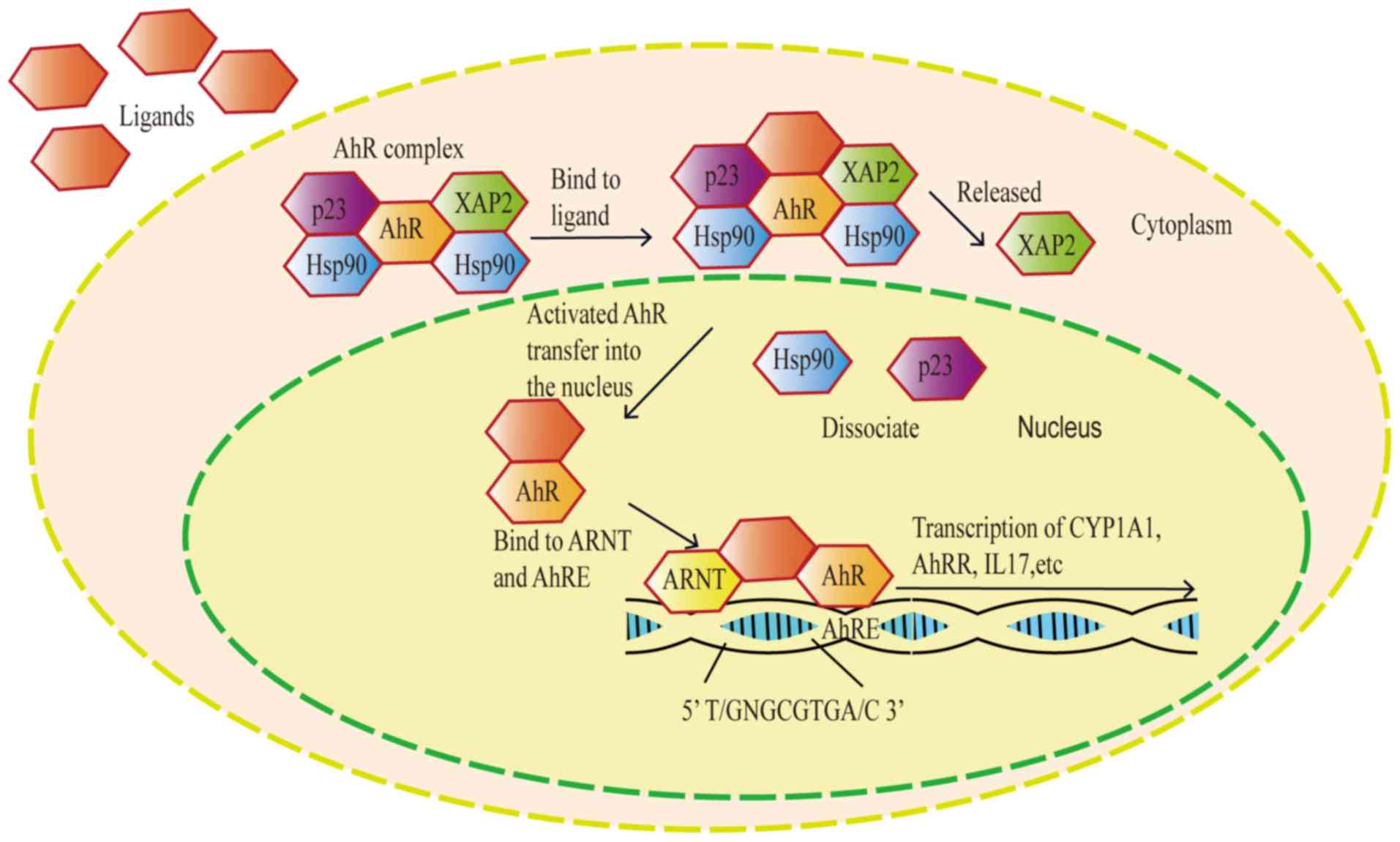
proteins, including heat shock protein 90 and hepatitis B virus
X-associated protein, in the cytoplasm of resting state cells
(6,7). When the AhR is transferred to the
nucleus, it combines with the aryl hydrocarbon receptor nuclear
translocator and triggers the transcription of several downstream
genes, including cytochrome P450 family 1 subfamily A member 1
(CYP1A1) and cytochrome P450 family 1 subfamily B member 1
(CYP1B1), resulting in a variety of physiological and toxicological
effects (8) (Fig. 2). Aryl hydrocarbon receptors are
polymorphic. Known alleles include AhRb-1–3 and AhRd (9). The receptors have different
affinities, however; all four proteins are alkaline and contain a
bHLH as well as PAS and transactivation domains (10).
The sensitivity of AhR to
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is species-specific, and
its persistence in different organs in vivo varies according
to the expression pattern of AhR in the specific organ (11). The AhR is most highly expressed in
the human placenta, followed by the lungs, heart, pancreas and
liver. Its lowest expression levels are in the kidney, brain and
skeletal muscle (9). The AhR is
transcribed from highly conserved sequences and plays a regulatory
role in system development and physiological processes of different
organs. Therefore, its expression is of great importance. The AhR
is activated by the high-affinity exogenous ligands HAH and PAH as
well as low-affinity endogenous ligands such as arachidonic acid,
pyrene, and tryptophan and flavonoid derivatives (12–14).
The endogenous ligands activate the AhR to participate in the
regulation of cardiac functions, vascular development and blood
pressure (15–19). In addition, the AhR signaling
pathway senses changes in the circadian rhythm, oxygen tension and
redox potential to regulate neural development and vacularization
(20). For example, exposure to
polycyclic aromatic hydrocarbons in cigarette smoke results in
oxidative stress and the production of oxidized low-density
lipoprotein (ox-LDL). ox-LDL accumulation in macrophages and smooth
muscle-derived pro-inflammatory foam cells is a hallmark of
atherosclerosis (21).
The aim of the present review was to discuss the
nature of the AhR, mediation of exogenous drugs, and potential
targets for modification of cardiovascular genes. The role of the
AhR receptor in the cardiovascular system, particularly the
mechanism of action of AhR in atherosclerosis, is discussed in the
present review. The role of the AhR in the development of novel
therapeutic agents for the treatment of cardiovascular diseases is
also presented.
Atherosclerosis is an inflammatory immune disease;
it's inflammatory etiology was first proposed by Ross (28). Nuclear factor κ-B (NF-κB) is a key
signal transduction factor and plays a central role in inflammatory
cytokine-mediated inflammatory responses. When cells are stimulated
by various internal factors including SRC-1 and p300, the NF-κB
signaling pathway is activated, and nuclear factors combine with
the corresponding genes, thereby regulating the expression of
target genes that magnify the inflammatory response, such as
chemokines, inflammatory cytokines (including TNF-α, IL-1 and IL-6)
and adhesion molecules [including intercellular cell adhesion
molecule-1 and vascular cell adhesion molecule-1 (VCAM-1)]
(29,38). In the cytoplasm, AhR competitively
binds to the RELA proto-oncogene, NF-κB subunit (RELA) in NF-κB in
a ‘tethered’ manner, preventing the AhR from combining with a
required synergistic activator (39). By binding to the promoter of
AhR-NF-κB1, RELA regulates the promoter sequence, affecting the
expression of the AhR (40). In
the coronary endothelium, whether the AhR signaling pathway exerts
adverse effects on physiological functions through the NF-κB
signaling pathway remains unreported (41).
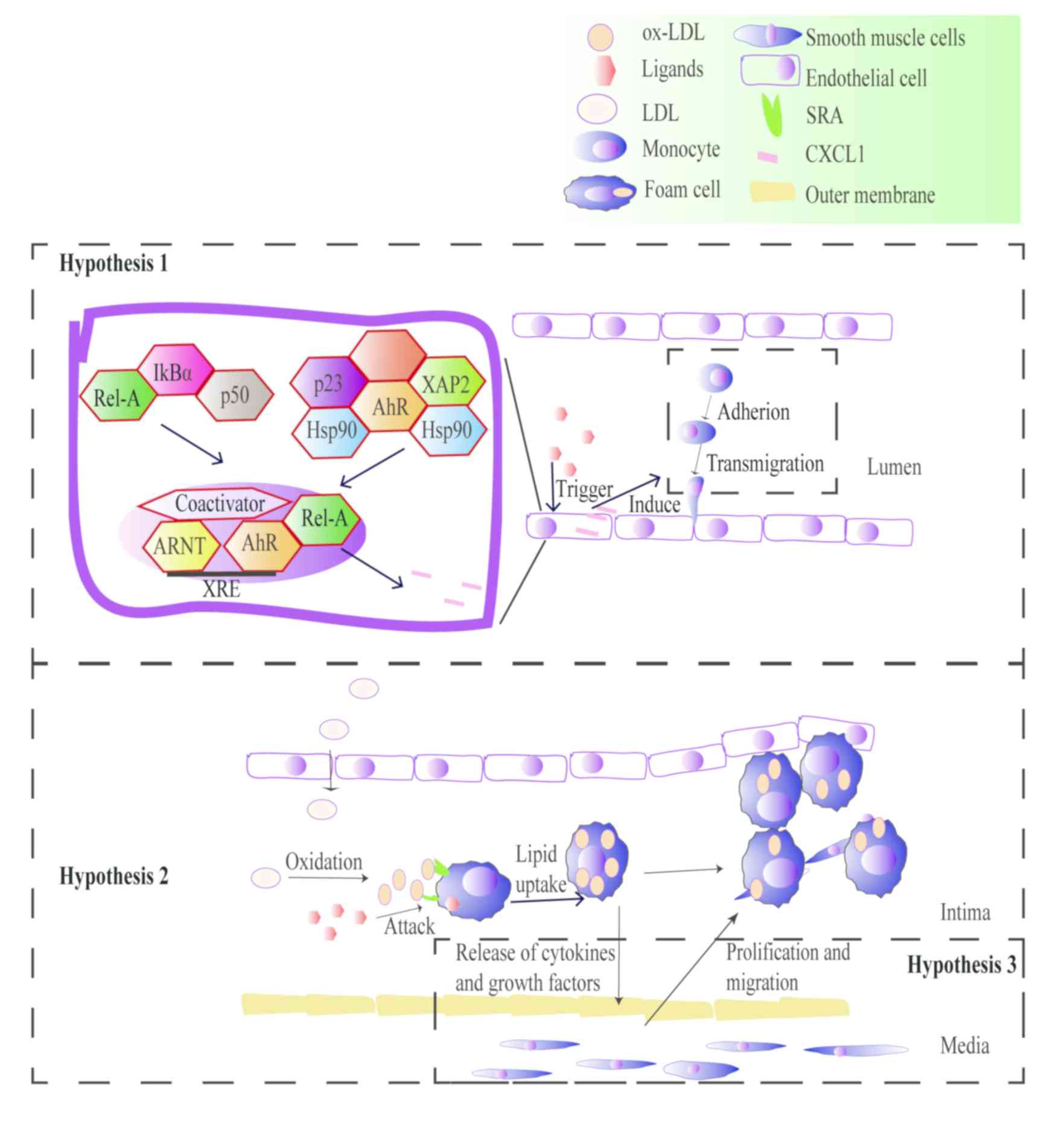
There are three hypotheses underlying the AhR
signaling pathways that mediate inflammation and promote
atherosclerosis. The first hypothesis involves the signaling of
downstream inflammatory factors such as VCAM-1 via the AhR/NF-κB
signaling pathway, which leads to monocyte chemotaxis (42). Macrophages and monocytes are
targeted by polycyclic aromatic hydrocarbons involved in the
physiological and pathological processes of atherosclerosis
(43). The second hypothesis
postulates that the AhR promotes macrophages absorption of ox-LDL
to form foam cells by mediating endogenous and exogenous ligands
such as ox-LDL, lipopolysaccharides and TCDD. In vitro
studies have revealed that cholesterol accumulation in foam cells
caused by particulate matter-induced inflammation is an early sign
of cardiovascular disease (30,44).
However, the inhibitory effect of AhR inhibitors on foam cells and
inflammation have not been investigated. It is believed that these
mechanisms will be elucidated by extensive research of the AhR. The
third hypothesis involves the increased proliferation of vascular
smooth muscle cells (VSMCs), which is a critical factor in the
occurrence of vascular complications. Yisireyili et al
(45) exposed VSMCs to indoxyl
sulfate, an agonist of AhR. Indoxyl sulfate induces VSMC
proliferation via the activation of the AhR, the NF-κB signaling
pathway and reactive oxygen species (ROS) production (Fig. 3).
AhR mediates exogenous chemicals, such as TCDD and
dioxin-like planar polychlorinated biphenyls (PCBs), and endogenous
substances, including indoxyl sulfate and arachidonic acid, by
activating NADPH oxidase to produce ROS that directly damages
vascular endothelial cells. This may result in a cellular oxidative
stress/antioxidant imbalance that leads to cell damage and reduces
the integrity of the vascular endothelium (46,47).
Previous studies have revealed that ROS mediate the transcription
of specific genes, such as NF-κB, which mediate the transcription
of inflammatory inducible nitric oxide synthase (44,48).
Lipid deposition can be induced by several AhR
ligands. TCDD is a classical AhR ligand. A previous study revealed
that α-endosulfan and 2,3,7,8-TCDD jointly downregulate the
expression of glucose- and lipid-associated genes in the liver,
such as nuclear receptor subfamily 1 group H member 4 and nuclear
receptor binding factor 2 (53).
Lipoxin A4 (LXA4), an endogenous ligand of AhR, is induced by
homocysteine in patients with hyperhomocysteinemia. LXA4 promotes
the binding of the AhR to the promoter of CD36 in hepatocytes and
promotes CD36 expression, which increases the uptake of fatty acids
and lipid accumulation by hepatocytes (54). Previous studies revealed that AhR
activation affects the systemic metabolic functions of mice,
including suppressed tricarboxylic acid cycle, disrupted lipid
metabolism, amino acids metabolism, glycogenolysis,
gluconeogenesis, thereby increased hepatic lipogenesis, and
promotedinflammatory signaling pathways (23,55,56).
AhR knockout mice are widely used to study the role
of the AhR in physiological functions. Activation of AhR protects
against fatty liver induced by insulin resistance by activating
fibroblast growth factor 21 (FGF21) to regulate lipid and energy
metabolism in such mice (57). AhR
knockout mice have increased levels of energy metabolism compared
with normal mice, which protects against insulin resistance,
hepatic steatosis, obesity and inflammation caused by a high-fat
diet (HFD) (23). By contrast, the
AhR protects against hepatic steatosis induced by a HFD and
subsequent lipotoxicity. The AhR protects against fatty liver
induced by insulin resistance by activating FGF21. The endocrine
signaling pathway of AhR and FGF21 suggests that AhR is a crucial
environmental modifier that combines signals from chemical exposure
to regulate lipid and energy metabolism (57). In vivo experiments have
revealed that locked nucleic acid 29, an inhibitor of microRNA
(miR)-29, inhibits lipid deposition in the liver, and whole-genome
analysis demonstrated increased AhR and sirtuin1 expression
(58). AhR is a direct target gene
of miR-29. Therefore, it may be an alternative therapeutic target
for treating metabolic disorders such as dyslipidemia (58). PCB 153, mediated by AhR, can be
considered as a ‘secondary strike’ mechanism for
obesity/non-alcoholic fatty liver disease in the context of a HFD
(59).
Studies investigating the association of the AhR
signaling pathway and its downstream genes, glutathione
S-transferase μ1 (GSTM1) and glutathione S-transferase θ1 (GSTT1),
with the risk and complications of atherosclerosis-associated
diseases have yielded inconclusive results (60,61).
Recent studies revealed that AhR may be associated with
atherosclerosis-associated diseases, including coronary artery
disease (CAD), ischemic stroke and type 2 diabetes mellitus (T2DM)
(62–64) (Table
I). The current review presents clinical research to reveal
their association.
CAD may result in mortality and is associated with
atherosclerosis and thrombosis (65). There is no clinically relevant
research on AhR gene polymorphisms and atherosclerosis, to the best
of our knowledge, and few studies on AhR gene polymorphism and CAD
(66–68). Receiver operating characteristic
analysis of 939 patients with confirmed CAD and 868 normal subjects
indicated that the AhR is a potential marker for objective
measurement and evaluation of CAD in addition to other cardiac
markers, such as creatine kinase-MB (69). Genotype frequencies of AhR
rs2066853 reveal significant differences between CAD and control
subjects, and hyperlipidemia and smoking significantly increased
the risk of CAD associated with AhR polymorphism (69). Furthermore, the four subtypes of
CAD with varying severity show significant differences in the
distribution of AhR variants (70). Previous studies have investigated
the association of CAD and the downstream mediators of the AhR
signaling pathway, CYP1A1, GSTT1 and GSTM1, that mediate the
metabolism of allogenic toxic substances (Table I). Previous studies in China have
demonstrated significant associations of CYP1A1, GSTM1, GSTT1 and
peroxisome proliferator activated receptor γ with CAD, particularly
in smokers (70–72).
A cross-sectional study in Croatia included 252
adult subjects with suspected exposure to PAHs; it was revealed
that CYP1A1, GSTM1 and GSTT1 gene polymorphisms had no association
with the risk of CAD (73).
Previous studies in India have demonstrated an
association between GSTM1/GSTT1/glutathione S-transferase π1
(GSTP1) polymorphism, coronary heart disease and blood lipid
parameters (74–76). These findings suggest that blood
lipid parameters in patients with coronary angiography are
significantly associated with GSTM1/T1/P1 genotype distribution and
GSTT1 deletion polymorphisms. However, a case-control study in the
Republic of Korea revealed that GSTM1/T1 had no effect on the
degree of lumen stenosis in CAD (77). Smokers carrying a GSTM1/T1 mutation
have a higher risk of CAD (77).
Regarding the association between genetic
polymorphisms of GSTM1 and smoking-related CAD, smokers with the
GSTM1 null genotype have a greater risk of CAD compared with
non-smokers with the GSTM1-positive genotype [odds ratio (OR),
2.07; 95% confidence interval (CI), 1.06–4.07]. The association
between genetic polymorphisms of GSTT1 and smoking related-CAD
smoking shares the same tendency as that for GSTM1 (OR, 2.00; 95%
CI, 1.05–3.84). The association between GSTM1 and GSTT1 null
genotypes in smoking-related CAD was also augmented when genetic
polymorphisms of GSTM1 and GSTT1 were considered simultaneously
(OR, 2.76; 95% CI, 1.17–6.52) (77). On the basis of several
epidemiological studies, AhR downstream genes are significantly
associated with CAD, particularly in smokers with the GSTT1/M1
knockout gene (78–80).
AhR mRNA and the mRNA level of its allele are higher
in the peripheral blood of patients with CAD compared with controls
(69). Smoking increases the risk
of CAD in patients with AhR gene polymorphisms. This may be due to
the aromatic hydrocarbons present in smoke which cause lipid
metabolism through the AhR signaling pathway (81). Furthermore, nicotine exposure
induces VCAM-1, matrix metalloprotein (MMP)-2 and MMP-9 production
in VSMCs and macrophages (82) and
promotes vascular oxidative stress, leading to vascular damage
(83).
A case-control study in Turkey, which included 226
patients with ischemic stroke and 113 controls, showed significant
differences between 6235C allele and the risk of ischemic stroke,
particularly in smokers and patients with hypertension (87).
To investigate the association between stroke and
polymorphism of the CYP1A1 gene, Sultana et al (88) selected 215 patients with ischemic
stroke and 162 age-matched controls. The results indicated that
ischemic stroke had a significant association with the CYP1A1
genotype ‘CC’ (P=0.01; OR, 5.14; 95% CI, 1.14–23.14) in south
Indian population, whereas Zhang et al (89) showed that CYP1A1 decreased the risk
of disease in the eastern Han of China, and this contradiction
showed CYP1A1 gene to display distinct alleles distribution among
populations. In conclusion, CYP1A1 was shown to be significantly
associated with ischemic stroke in certain clinical studies;
however, further investigation is required to verify this
association.
T2DM, a metabolic disease, is associated with
oxidative stress and chronic inflammation in adipose tissue
(90). Previous epidemiological
studies have revealed associations between oxidative
stress-associated enzymes, such as GSTT1 and GSTM1, and diabetes
(Table I). GSTT1 and GSTM1 are AhR
downstream genes.
A case-control study in India revealed that GSTM1
and GSTT1 are associated with gene polymorphism-associated fat mass
and obesity. GSTM1-positive and GSTM1 null genotypes had
significant associations with T2DM, but there was no significant
association with FTO α-ketoglutarate-dependent dioxygenase
polymorphism (91). Other
epidemiological studies revealed that GSTM1 and GSTT1 did not have
significant effects on T2DM (92,93).
Hori et al (94) did not
reveal a significant association between T2DM and GSTT1/M1 gene
alleles, but the incidence rate of T2DM in GSTT1 and GSTM1 null
genotypes was 1.5-fold higher than that in GSTM1 and GSTT1 positive
genotypes. The aforementioned clinical studies demonstrated that
GSTT1/M1 and diabetes are not highly associated and that further
investigation is required to determine their associations.
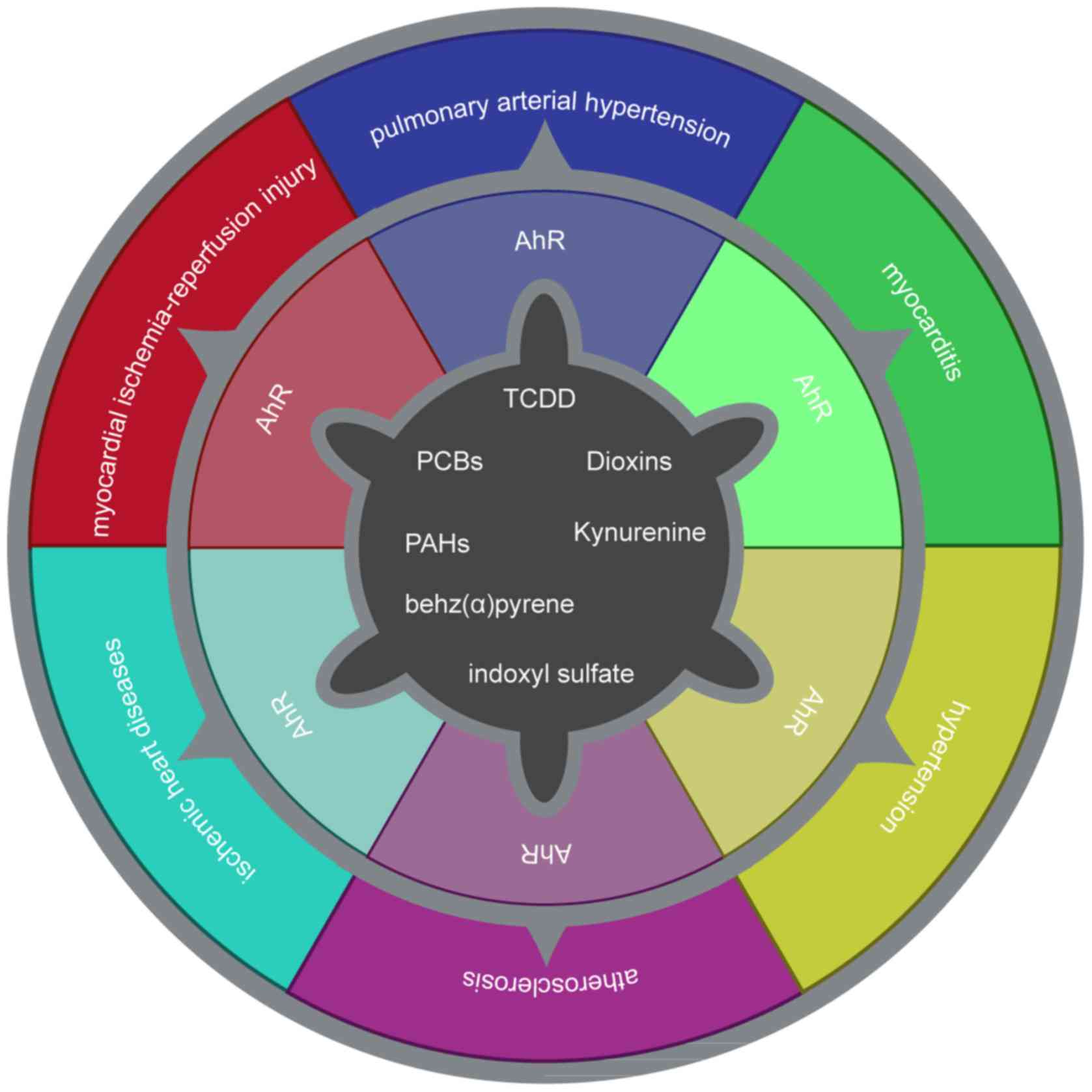
The current review presented the association between
the AhR and inflammation, oxidative stress, lipid infiltration and
atherosclerosis. The AhR is closely associated with cardiovascular
disease in terms of cardiac function, vascular development and
blood pressure regulation (Fig.
4). In certain atherosclerosis-associated diseases, the AhR may
serve a role as an oxidative stress signal transmitter. The AhR may
be a potential target for the clinical treatment of cardiovascular
disease. However, some important questions remain unanswered. The
regulation of the AhR at the gene level has not been elucidated in
humans. There are currently no drugs targeting the AhR in the
clinic. The AhR is associated with other signaling pathways,
including the Wnt and E2 factor signaling pathways, and further
basic experiments are required to elucidate the roles of the AhR.
The identification of novel endogenous ligands and the application
of AhR knockout mice may clarify the role, regulation and
intervention of the AhR in the treatment of atherosclerosis.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81700269 and
81741129), the Collaborative Innovation and Platform Environment
Construction Projects of Guangdong Province (grant no.
2015A050502049) and the Characteristic Innovation Project of
Department of Education of Guangdong Province (grant no.
2016KTSCX048).
Not applicable.
WL and JZ conceived and designed the review. KZ
retrieved the relevant literature and wrote the manuscript. TY
provided a figure. QM, ZZ and YH reviewed and edited the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Conney AH, Miller EC and Miller JA: The
metabolism of methylated aminoazo dyes v. evidence for induction of
enzyme synthesis in the rat by 3-methylcholanthre. Cancer Res.
16:450–459. 1956.PubMed/NCBI
|
|
2
|
Hu B, Huang H, Wei Q, Ren M, Mburu DK,
Tian X and Su J: Transcription factors CncC/Maf and AhR/ARNT
coordinately regulate the expression of multiple GSTs conferring
resistance to chlorpyrifos and cypermethrin in spodoptera exigua.
Pest Manag Sci. 75:2009–2019. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins LG and Hayes JD: Mechanisms of
induction of cytosolic and microsomal glutathione transferase (GST)
genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev.
43:92–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Anna JS, Leggieri LR, Arias Darraz L,
Cárcamo JG, Venturino A and Luquet CM: Effects of sequential
exposure to water accommodated fraction of crude oil and
chlorpyrifos on molecular and biochemical biomarkers in rainbow
trout. Comp Biochem Physiol C Toxicol Pharmacol. 212:47–55. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davisson MT: Rules and guidelines for
genetic nomenclature in mice: Excerpted version. Transgenic Res.
6:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baricza E, Tamási V, Marton N, Buzás EI
and Nagy G: The emerging role of aryl hydrocarbon receptor in the
activation and differentiation of Th17 cells. Cell Mol Life Sci.
73:95–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt JV and Bradfield CA: Ah receptor
signaling pathways. Annu Rev Cell Dev Biol. 12:55–89. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Go RE, Hwang KA and Choi KC: Cytochrome
P450 1 family and cancers. J Steroid Biochem Mol Biol. 147:24–30.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang N: The role of endogenous aryl
hydrocarbon receptor signaling in cardiovascular physiology. J
Cardiovasc Dis Res. 2:91–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Curran CP, Altenhofen E, Ashworth A, Brown
A, Kamau-Cheggeh C, Curran M, Evans A, Floyd R, Fowler J, Garber H,
et al: AhrdCyp1a2(−/−) mice show increased
susceptibility to PCB-induced developmental neurotoxicity.
Neurotoxicology. 33:1436–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bock KW: From TCDD-mediated toxicity to
searches of physiologic AHR functions. Biochem Pharmacol.
155:419–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian Y, Rabson AB and Gallo MA: Ah
receptor and NF-κB interactions: Mechanisms and physiological
implications. Chem Biol Interact. 141:97–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stejskalova L, Dvorak Z and Pavek P:
Endogenous and exogenous ligands of aryl hydrocarbon receptor:
Current state of art. Curr Drug Metab. 12:198–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamas B, Natividad JM and Sokol H: Aryl
hydrocarbon receptor and intestinal immunity. Mucosal Immunol.
11:1024–1038. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wakamatsu T, Yamamoto S, Ito T, Sato Y,
Matsuo K, Takahashi Y, Kaneko Y, Goto S, Kazama JJ, Gejyo F and
Narita I: Indoxyl sulfate promotes macrophage IL-1β production by
activating aryl hydrocarbon receptor/NF-κ/MAPK cascades, but the
NLRP3 inflammasome was not activated. Toxins (Basel). 10:E1242018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishiumi S, Yamamoto N, Kodoi R, Fukuda I,
Yoshida K and Ashida H: Antagonistic and agonistic effects of
indigoids on the transformation of an aryl hydrocarbon receptor.
Arch Biochem Biophys. 470:187–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peter Guengerich F, Martin MV, McCormick
WA, Nguyen LP, Glover E and Bradfield CA: Aryl hydrocarbon receptor
response to indigoids in vitro and in vivo. Arch Biochem Biophys.
423:309–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Li Y, Sun R, Zhou S, Li M, Feng M
and Xie Y: Dual character of flavonoids in attenuating and
aggravating ischemia-reperfusion-induced myocardial injury. Exp
Ther Med. 14:1307–1314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hubbard TD, Murray IA and Perdew GH:
Indole and tryptophan metabolism: Endogenous and dietary routes to
Ah Receptor activation. Drug Metab Dispos. 43:1522–1535. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kewley RJ, Whitelaw ML and Chapman-Smith
A: The mammalian basic helix-loop-helix/PAS family of
transcriptional regulators. Int J Biochem Cell Biol. 36:189–204.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bock KW: Human AHR functions in vascular
tissue: Pro- and anti-inflammatory responses of AHR agonists in
atherosclerosis. Biochem Pharmacol. 159:116–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt JV, Su GH, Reddy JK, Simon MC and
Bradfield CA: Characterization of a murine ahR null allele:
Involvement of the Ah receptor in hepatic growth and development.
Proc Natl Acad Sci USA. 93:6731–6736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu CX, Wang C, Zhang ZM, Jaeger CD, Krager
SL, Bottum KM, Liu J, Liao DF and Tischkau SA: Aryl hydrocarbon
receptor deficiency protects mice from diet-induced adiposity and
metabolic disorders through increased energy expenditure. Int J
Obes (Lond). 39:1300–1309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gutiérrez-Vázquez C and Quintana FJ:
Regulation of the immune response by the aryl hydrocarbon receptor.
Immunity. 48:19–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brinchmann BC, Skuland T Rambøl MH, Szoke
K, Brinchmann JE, Gutleb AC, Moschini E, Kubátová A, Kukowski K, Le
Ferrec E, et al: Lipophilic components of diesel exhaust particles
induce Pro-inflammatory responses in human endothelial cells
through AhR dependent pathway(s). Part Fibre Toxicol. 15:212018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Liu Q, Guo S, Zhang Q, Tang J, Liu
G, Kong D, Li J, Yan S, Wang R, et al:
2,3,7,8-Tetrachlorodibenzo-p-dioxin promotes endothelial cell
apoptosis through activation of EP3/p38MAPK/Bcl-2 pathway. J Cell
Mol Med. 21:3540–3551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan ZQ and Hansson GK: Innate immunity,
macrophage activation, and atherosclerosis. Immunol Rev.
219:187–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vogel CF, Khan EM, Leung PS, Gershwin ME,
Chang WL, Wu D, Haarmann-Stemmann T, Hoffmann A and Denison MS:
Cross-talk between aryl hydrocarbon receptor and the inflammatory
response: A role for nuclear factor-κB. J Biol Chem. 289:1866–1875.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogel CF, Sciullo E, Wong P, Kuzmicky P,
Kado N and Matsumura F: Induction of proinflammatory cytokines and
C-reactive protein in human macrophage cell line U937 exposed to
air pollution particulates. Environ Health Perspect. 113:1536–1541.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ledda A, González M, Gulfo J, Díaz
Ludovico I, Ramella N, Toledo J, Garda H, Grasa M and Esteve M:
Decreased OxLDL uptake and cholesterol efflux in THP1 cells
elicited by cortisol and by cortisone through 11β-hydroxysteroid
dehydrogenase type 1. Atherosclerosis. 250:84–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Y, Du KL, Guo PY, Zhao RM, Wang B,
Zhao XL and Zhang CQ: IL-16 regulates macrophage polarization as a
target gene of mir-145-3p. Mol Immunol. 107:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sieve I, Ricke-Hoch M, Kasten M, Battmer
K, Stapel B, Falk CS, Leisegang MS, Haverich A Scherr M and
Hilfiker-Kleiner D: A positive feedback loop between IL-1β, LPS and
NEU1 may promote atherosclerosis by enhancing a pro-inflammatory
state in monocytes and macrophages. Vascul Pharmacol. 103:16–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdolmaleki F, Gheibi Hayat SM, Bianconi
V, Johnston TP and Sahebkar A: Atherosclerosis and immunity: A
perspective. Trends Cardiovasc Med. 28:S1050–1738. 2018.(Epub ahead
of print).
|
|
35
|
Ohtsuka M, Miyashita Y and Shirai K:
Lipids deposited in human atheromatous lesions induce apoptosisof
human vascular smooth muscle cells. J Atheroscler Thromb.
13:256–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baumer Y, McCurdy S, Weatherby TM, Mehta
NN, Halbherr S, Halbherr P, Yamazaki N and Boisvert WA:
Hyperlipidemia-induced cholesterol crystal production by
endothelial cells promotes atherogenesis. Nat Commun. 8:11292017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barnes MJ and Farndale RW: Collagens and
atherosclerosis. Exp Gerontol. 34:513–525. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chitra P, Saiprasad G, Manikandan R and
Sudhandiran G: Berberine inhibits smad and non-smad signaling
cascades and enhances autophagy against pulmonary fibrosis. J Mol
Med (Berl). 93:1015–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ke S, Rabson AB, Germino JF, Gallo MA and
Tian Y: Mechanism of suppression of cytochrome P-4501A1 expression
by tumor necrosis Factor-α and lipopolysaccharide. J Biol Chem.
276:39638–39644. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aung HH, Lame MW, Gohil K, He G, Denison
MS, Rutledge JC and Wilson DW: Comparative gene responses to
collected ambient particles in vitro: Endothelial responses.
Physiol Genomics. 43:917–929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vogel CF and Matsumura F: A new cross-talk
between the aryl hydrocarbon receptor and RelB, a member of the
NF-ĸB family. Biochem Pharmacol. 77:734–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vogel CF, Sciullo E, Li W, Wong P,
Lazennec G and Matsumura F: RelB, a new partner of aryl hydrocarbon
receptor-mediated transcription. Mol Endocrinol. 21:2941–2955.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang C, Petriello MC, Zhu B and Hennig B:
PCB 126 induces monocyte/macrophage polarization and inflammation
through ahR and NF-κB pathways. Toxicol Appl Pharmacol. 367:71–81.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hennig B, Meerarani P, Slim R, Toborek M,
Daugherty A, Silverstone AE and Robertson LW: Proinflammatory
properties of coplanar PCBs: In vitro and in vivo evidence. Toxicol
Appl Pharmacol. 181:174–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yisireyili M, Saito S, Abudureyimu S,
Adelibieke Y, Ng HY, Nishijima F, Takeshita K, Murohara T and Niwa
T: Indoxyl sulfate-induced activation of (Pro)renin receptor
promotes cell proliferation and tissue factor expression in
vascular smooth muscle cells. PLoS One. 9:e1092682014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hennig B, Hammock BD, Slim R, Toborek M,
Saraswathi V and Robertson LW: PCB-induced oxidative stress in
endothelial cells: Modulation by nutritients. Int J Hyg Environ
Health. 205:95–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Watanabe I, Tatebe J, Namba S, Koizumi M,
Yamazaki J and Morita T: Activation of aryl hydrocarbon receptor
mediates indoxyl sulfate-induced monocyte chemoattractant Protein-1
expression in human umbilical vein endothelial Cells. Circ J.
77:224–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Puga A, Barnes SJ, Chang C, Zhu H, Nephew
KP, Khan SA and Shertzer HG: Activation of transcription factors
activator protein-1 and nuclear factor-κB by
2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Pharmacol.
59:997–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med. 95:1153–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan DC, Watts F, Ooi EM, Ji J, Johnson AG
and Barrett PH: Atorvastatin and fenofibrate have comparable
effects on VLDL-Apolipoprotein C-III kinetics in men with the
metabolic syndrome. Arterioscler Thromb Vasc Biol. 28:1831–1837.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mesnage R, Biserni M, Balu S, Frainay C,
Poupin N, Jourdan F, Wozniak E, Xenakis T, Mein CA and Antoniou MN:
Integrated transcriptomics and metabolomics reveal signatures of
lipid metabolism dysregulation in hepaRG liver cells exposed to PCB
126. Arch Toxicol. 92:2533–2547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Podechard N, Le Ferrec E, Rebillard A,
Fardel O and Lecureur V: NPC1 repression contributes to lipid
accumulation in human macrophages exposed to environmental aryl
hydrocarbons. Cardiovasc Res. 82:361–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ambolet-Camoit A, Ottolenghi C, Leblanc A,
Kim MJ, Letourneur F, Jacques S, Cagnard N, Guguen-Guillouzo C,
Barouki R and Aggerbeck M: Two persistent organic pollutants which
act through different xenosensors (alpha-endosulfan and 2,3,7,8
tetrachlorodibenzo-p-dioxin) interact in a mixture and downregulate
multiple genes involved in human hepatocyte lipid and glucose.
Biochimie. 116:79–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yao L, Wang C, Zhang X, Peng L, Liu W,
Zhang X, Liu Y, He J, Jiang C, Ai D and Zhu Y: Hyperhomocysteinemia
activates the aryl hydrocarbon receptor/CD36 pathway to promote
hepatic steatosis in mice. Hepatology. 64:92–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Van Breda SGJ, Claessen SMH, van Herwijnen
M, Theunissen DHJ, Jennen DGJ, de Kok TMCM and Kleinjans JCS:
Integrative omics data analyses of repeated dose toxicity of
valproic acid in vitro reveal new mechanisms of steatosis
induction. Toxicology. 393:160–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang L, Hatzakis E, Nichols RG, Hao R,
Correll J, Smith PB, Chiaro CR, Perdew GH and Patterson AD:
Metabolomics reveals that aryl hydrocarbon receptor activation by
environmental chemicals induces systemic metabolic dysfunction in
mice. Environ Sci Technol. 49:8067–8077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu P, Yan J, Liu K, Garbacz WG, Wang P, Xu
M, Ma X and Xie W: Activation of aryl hydrocarbon receptor
dissociates fatty liver from insulin resistance by inducing FGF21.
Hepatology. 61:1908–1919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kurtz CL, Fannin EE, Toth CL, Pearson DS,
Vickers KC and Sethupathy P: Inhibition of miR-29 has a significant
lipid-lowering benefit through suppression of lipogenic programs in
liver. Sci Rep. 5:129112015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wahlang B, Falkner KC, Gregory B, Ansert
D, Young D, Conklin DJ, Bhatnagar A, McClain CJ and Cave M:
Polychlorinated biphenyl 153 is a diet-dependent obesogen that
worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J
Nutr Biochem. 24:1587–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Han SG, Han SS, Toborek M and Hennig B:
EGCG protects endothelial cells against PCB 126-induced
inflammation through inhibition of AhR and induction of
Nrf2-regulated genes. Toxicol Appl Pharmacol. 261:181–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Z, Yang H, Ramesh A, Roberts LJ, Zhou
L, Lin X, Zhao Y and Guo Z: Overexpression of Cu/Zn-superoxide
dismutase and/or catalase accelerates benzo(a)pyrene detoxification
by upregulation of the aryl hydrocarbon receptor in mouse
endothelial cells. Free Radic Biol Med. 47:1221–1229. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim JB, Pjanic M, Nguyen T, Miller CL,
Iyer D, Liu B, Wang T, Sazonova O, Carcamo-Orive I, Matic LP, et
al: TCF21 and the environmental sensor aryl-hydrocarbon receptor
cooperate to activate a pro-inflammatory gene expression program in
coronary artery smooth muscle cells. PLoS Genet. 13:e10067502017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li X, Liu N, Wang Y, Liu J, Shi H, Qu Z,
Du T, Guo B and Gu B: Brain and muscle aryl hydrocarbon receptor
nuclear translocator-like protein-1 cooperates with glycogen
synthase kinase-3β to regulate osteogenesis of bone-marrow
mesenchymal stem cells in type 2 diabetes. Mol Cell Endocrinol.
440:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wnuk A and Kajta M: Steroid and xenobiotic
receptor signalling in apoptosis and autophagy of the nervous
system. Int J Mol Sci. 18:E23942017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: American heart association council on
epidemiology and prevention statistics committee and stroke
statistics subcommittee: Heart disease and stroke statistics-2018
update: A report from the american heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zou JG, Ma YT, Xie X, Yang YN, Pan S, Adi
D, Liu F and Chen BD: The association between CYP1A1 genetic
polymorphisms and coronary artery disease in the uygur and han of
China. Lipids Health Dis. 13:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Taspinar M, Aydos S, Sakiragaoglu O, Duzen
IV, Yalcinkaya A, Oztuna D, Bardakci H, Tutar E and Sunguroglu A:
Impact of genetic variations of the CYP1A1, GSTT1, and GSTM1 genes
on the risk of coronary artery disease. DNA Cell Biol. 31:211–218.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Manfredi S, Federici C, Picano E, Botto N,
Rizza A and Andreassi MG: GSTM1, GSTT1 and CYP1A1 detoxification
gene polymorphisms and susceptibility to smoking-related coronary
artery disease: A case-only study. Mutat Res. 621:106–112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huang S, Shui X, He Y, Xue Y, Li J, Li G,
Lei W and Chen C: AhR expression and polymorphisms are associated
with risk of coronary arterial disease in Chinese population. Sci
Rep. 5:80222015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Peng DD, Xie W and Yu ZX: Impact of
interaction between CYP1A1 genetic polymorphisms and smoking on
coronary artery disease in the han of China. Clin Exp Hypertens.
39:339–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang X, Lv S, Guo C, Shi C, Chi Y, Zhao
L, Wang G and Wang Z: Gene-gene interaction between PPARG and
CYP1A1 gene on coronary artery disease in the Chinese han
population. Oncotarget. 8:34398–34404. 2017.PubMed/NCBI
|
|
72
|
Zhang ZX and Zhang Y: Glutathione
S-transferase M1 (GSTM1) null genotype and coronary artery disease
risk: A meta-analysis. Int J Clin Exp Med. 7:3378–3384.
2014.PubMed/NCBI
|
|
73
|
Pašalić D and Marinković N: Genetic
polymorphisms of the CYP1A1, GSTM1, and GSTT1 enzymes and their
influence on cardiovascular risk and lipid profile in people who
live near a natural gas plant. Arh Hig Rada Toksikol. 68:46–52.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mir R, Bhat MA, Javaid J, Shah N, Kumar P,
Sharma E, Jhu C, Basak S, Amle D, Ray PC, et al: Glutathione
S-transferase M1 and T1 (rs4025935 and rs71748309) null genotypes
are associated with increased susceptibility to coronary artery
disease in Indian Populations. Acta Cardiol. 71:678–684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bhat MA and Gandhi G: Association of GSTT1
and GSTM1 gene polymorphisms with coronary artery disease in north
Indian punjabi population: A case-control study. Postgrad Med J.
92:701–706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ramprasath T, Senthil Murugan P,
Prabakaran AD, Gomathi P, Rathinavel A and Selvam GS: Potential
risk modifications of GSTT1, GSTM1 and GSTP1
(glutathione-S-transferases) variants and their association to CAD
in patients with type-2 diabetes. Biochem Biophys Res Commun.
407:49–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim SJ, Kim MG, Kim KS, Song JS, Yim SV
and Chung JH: Impact of glutathione s-transferase M1 and T1 gene
polymorphisms on the smoking-related coronary artery disease. J
Korean Med Sci. 23:365–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Manfredi S, Calvi D, del Fiandra M, Botto
N, Biagini A and Andreassi MG: Glutathione S-transferase T1- and
M1-null genotypes and coronary artery disease risk in patients with
type 2 diabetes mellitus. Pharmacogenomics. 10:29–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang LS, Tang JJ, Tang NP, Wang MW, Yan
JJ, Wang QM, Yang ZJ and Wang B: Association of GSTM1 and GSTT1
gene polymorphisms with coronary artery disease in relation to
tobacco smoking. Clin Chem Lab Med. 46:1720–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Masetti S, Botto N, Manfredi S, Colombo
MG, Rizza A, Vassalle C, Clerico A, Biagini A and Andreassi MG:
Interactive effect of the glutathione S-transferase genes and
cigarette smoking on occurrence and severity of coronary artery
risk. J Mol Med (Berl). 81:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kalpana C, Rajasekharan KN and Menon VP:
Modulatory effects of curcumin and curcumin analog on circulatory
lipid profiles during nicotine-induced toxicity in wistar rats. J
Med Food. 8:246–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li ZZ, Guo ZZ, Zhang Z, Cao QA, Zhu YJ,
Yao HL, Wu LL and Dai QY: Nicotine-induced upregulation of VCAM-1,
MMP-2, and MMP-9 through the α7-nAChR-JNK pathway in RAW264.7 and
MOVAS cells. Mol Cell Biochem. 399:49–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Abdel Fattah S, Rizk AAE, Motawie AG, Abd
El-Galil TI and El Sebaie M: Effects of nicotine on rat adrenal
gland: Crosstalk between oxidative and inflammatory markers, and
amelioration by melatonin. Biotech Histochem. 19:1–10. 2018.
|
|
84
|
O'Donnel MJ, Chin SL, Rangarajan S, Xavier
D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapary S, et
al: Global and regional effects of potentially modifiable risk
factors associated with acute stroke in 32 countries (INTERSTROKE):
A case control study. Lancet. 388:761–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhu H, Li Z, Lv J and Zhao R: Effects of
cerebral small vessel disease on the outcome of patients with
ischemic stroke caused by large artery atherosclerosis. Neurol Res.
40:381–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Moon KS, Lee HJ, Hong SH, Kim HM and Um
JY: CYP1A1 and GSTM1/T1 genetic variation in predicting risk for
cerebral infarction. J Mol Neurosci. 32:155–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Demirdöğen BC, Adali AÇ, Bek S, Demirkaya
Ş and Adali O: Cytochrome P4501A1 genotypes and smoking- and
hypertension-related ischemic stroke risk. Hum Exp Toxicol.
32:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sultana S, Kolla VK, Peddireddy V,
Jeedigunta Y, Penagaluru PK, Joshi S, Penagaluru UR and Penagaluru
PR: Association of cyp1a1 gene polymorphism with ischemic stroke in
south Indian population. Transl Stroke Res. 2:26–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang M, Wu JM, Zhang QS, Yan DW, Ren LJ
and Li WP: The association of CYP1A1 genetic polymorphisms and
additional gene-gene interaction with ischemic stroke in the
eastern han of China. Neurol Sci. 37:1679–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sindhu S, Akhter N, Kochumon S, Thomas R,
Wilson A, Shenouda S, Tuomilehto J and Ahmad R: Increased
expression of the innate immune receptor TLR10 in obesity and
Type-2 diabetes: Association with ROS-mediated oxidative stress.
Cell Physiol Biochem. 45:575–590. 2018. View Article : Google Scholar
|
|
91
|
Raza ST, Abbas S, Ahmad A, Ahmed F, Zaidi
Zh and Mahdi F: Association of glutathione-s-transferase (GSTM1 and
GSTT1) and FTO gene polymorphisms with type 2 diabetes mellitus
cases in northern India. Balkan J Med Genet. 17:47–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Etemad A, Vasudevan R, Aziz AF, Yusof AK,
Khazaei S, Fawzi N, Jamalpour S, Arkani M, Mohammad NA and Ismail
P: Analysis of selected glutathione S-transferase gene
polymorphisms in malaysian type 2 diabetes mellitus patients with
and without cardiovascular disease. Genet Mol Res. 15:2016.
View Article : Google Scholar :
|
|
93
|
Porojan MD, Bala C, Ilies R, Catana A,
Popp RA and Dumitrascu DL: Combined glutathione S transferase M1/T1
null genotypes is associated with type 2 diabetes mellitus. Clujul
Med. 88:159–163. 2015.PubMed/NCBI
|
|
94
|
Hori M, Oniki K, Ueda K, Goto S, Mihara S,
Marubayashi T and Nakagawa K: Combined glutathione S-transferase T1
and M1 positive genotypes afford protection against type 2 diabetes
in japanese. Pharmacogenomics. 8:1307–1314. 2007. View Article : Google Scholar : PubMed/NCBI
|