Introduction
Diffuse large B cell lymphoma (DLBCL) represents the
most common subtype of non-Hodgkin lymphoma (1) and accounts for ~40% of newly
diagnosed cases of non-Hodgkin lymphoma annually in China (2). DLBCL is an aggressive form of
lymphoma, with a highly variable clinical manifestation, histology,
outcome and prognosis (1). Despite
the fact that patients with DLBCL who receive comprehensive
treatment, including radiotherapy, chemotherapy and combined
therapy with molecular targeted therapy, have a high chance of
achieving partial or complete remission, some patients are
resistant to first line therapy or relapse, leading to reduced
survival rates, psychological and physical pain (1). Furthermore, the medical cost of DLBCL
is high (1). Therefore,
identifying new strategies for the treatment of DLBCL is
needed.
B lymphocyte receptor (BCR) signaling is important
for B cell lymphoma proliferation (3). BCR signaling can activate the PI3K
signaling pathway, leading to the production of
phosphatidylinositol 3,4,5-triphosphate, which initiates a large
number of signaling cascades, including those involving
serine/threonine kinase AKT (4).
This can lead to uncontrolled growth by inhibiting the function of
the cell cycle inhibitors p21 and p27 (5–7). AKT
can indirectly activate mammalian target of rapamycin (mTOR), a
serine/threonine kinase downstream of the PI3K/AKT pathway
(8). Activated mTOR can increase
the phosphorylation levels of the translational repressor
eukaryotic translation initiation factor 4E-binding protein 1
(4EBP1) and ribosomal protein S6 kinase β-1 (p70S6K), which
increases mRNA translation, protein synthesis and cell
proliferation (9,10). Therefore, mTOR is able to influence
cell cycle progression and the homeostasis of metabolism, and
enhance cell growth. The PI3K/AKT-mTOR axis also plays an important
role in the negative regulation of autophagy (11). Previous studies have reported the
aberrant activation of the PI3K/AKT/mTOR pathway, and its role in
controlling tumor cell proliferation and survival in DLBCL
(4,8). Moreover, patients with DLBCL with
active PI3K/AKT/mTOR signaling experience a more rapid
deterioration, with poor treatment response and shortened survival
times (12). The suppression of
active PI3K/AKT/mTOR signaling has been studied, and several
inhibitors have been discovered; mTOR inhibitors have been shown to
be effective in preclinical and clinical settings for the treatment
of autoimmune diseases, cancer and infectious diseases, such as HIV
(13–24). Furthermore, dual inhibitors of PI3K
and mTOR, including GSK2126458A and NVP-BEZ235, are being developed
for cancer that show promising chemotherapeutic profiles in
different settings (25–27). However, the mTOR inhibitor
rapamycin (RAPA) and other analogues, such as everolimus, exhibit
various side effects, including stomatitis, rashes,
myelosuppression and metabolic complications, such as
hyperglycemia, hypertriglyceridemia, hypercholesterolemia and
fatigue (28,29). In the case of serious side effects,
dose adjustment, interruption or permanent discontinuation are
required to reduce the toxicity of these medications (28). The combination of RAPA or other
analogues with additional medications to reduce toxicity may be an
effective way to treat cancer.
1,25-Dihydroxyvitamin D3
[1,25(OH)2D3] has an important role in bone
homeostasis and calcium metabolism (30,31).
Furthermore, 1,25(OH)2D3 has been shown to
have antiproliferative and proapoptotic effects, and promote
differentiation in cancer cells, including pancreatic, breast and
prostate cancer (32–38). 1,25(OH)2D3
inhibits the mTOR signaling pathway by stimulating the expression
of DNA damage-inducible transcript 4 (DDIT4), a potent suppressor
of mTOR activity (39). This may a
novel strategy for the treatment of cancer.
1,25(OH)2D3 induces autophagy in SH-SY5Y
cells to attenuate rotenone-induced neurotoxicity (40) and inhibits the proliferation of
keratinocytes by suppressing the mTOR signaling pathway (41). However, whether
1,25(OH)2D3 inhibits the progression of DLBCL
by suppressing the mTOR signaling pathway is unclear.
The concentration of
1,25(OH)2D3 available in tissues depends on
the balance between its synthesis and degradation, carried out by
25-hydroxyvitamin D-1 α hydroxylase and 25-hydroxyvitamin
D-24-hydroxylase (CYP24A1), respectively. A previous study found
that the basal expression of CYP24A1 was higher in several tumor
types, including colon, breast, lung, ovarian and cervical tumors
(42). The high expression of
CYP24A1 was found to be associated with the increased expression of
replication licensing factors and tumor progression (43–47).
However, little is known concerning the relationship between the
clinical features of patients with DLBCL and the expression of
CYP24A1, or how the antitumor effect of
1,25(OH)2D3 in DLBCL is influenced by the
expression of CYP24A1.
The present study aimed to investigate the
relationship between the clinical features of patients with DLBCL
and the expression of CYP24A1. In addition, the roles of the
PI3K/AKT/mTOR signaling pathway and autophagy in the anticancer
effects of 1,25(OH)2D3 in DLBCL cells were
investigated.
Materials and methods
Reagents
1,25(OH)2D3 and RAPA were
purchased from Sigma-Aldrich; Merck KGaA. 3-Methyladenine was
purchased from Selleck Chemicals. The FITC Annexin V Apoptosis
Detection kit, propidium iodide (PI) and ribonuclease A (RNase A)
were purchased from BD Biosciences. The Cell Counting Kit-8 (CCK-8)
was purchased from 7Sea Biotech. The ECL Western kit was purchased
from Advansta, Inc. The following primary antibodies were purchased
from Cell Signaling Technology, Inc.: mTOR (cat. no. 2972),
phosphorylated (p)-mTOR (Ser2448; cat. no. 2971), AKT (cat. no.
4691), p-AKT (Ser473; cat. no. 4060), p-4EBP1 (Thr37/46; cat. no.
2855), 4E-BP1 (cat. no. 9452), p-p70S6K (Thr389; cat. no. 9234),
p70S6K (cat. no. 2708), ubiquitin binding protein P62 (P62; cat.
no. 8025), LC3I/II (cat. no. 4108). The goat monoclonal antibody
against CYP24A1 was purchased from Abcam (cat. no. ab189322). A
rabbit monoclonal antibody against the vitamin D receptor (VDR) was
purchased from Santa Cruz Biotechnology (cat. no. sc-13133), Inc.
α-Tubulin was purchased from ProteinTech Group (cat. no.
11224-1-AP), Inc. Horseradish peroxidase (HRP)-conjugated
goat-anti-mouse IgG (cat. no. A-11001) and HRP-conjugated
goat-anti-rabbit IgG (cat. no. A-11034) were obtained from Thermo
Fisher Scientific, Inc. 1,25(OH)2D3 was
dissolved and stored in ethanol and RAPA was dissolved and stored
in DMSO at −20°C. A biotinylated goat-anti-mouse antibody (cat. no.
PV-9003) was purchased from Origene Technologies, Inc. A
fluorescein-conjugated goat anti-rabbit secondary antibody (cat.
no. Andy Fluor™ 594) was purchased from GeneCopoeia, Inc. Fetal
calf serum was purchased from Biological Industries.
Clinical specimens
In total, 57 formalin-fixed (overnight at 4°C),
paraffin-embedded (FFPE) DLBCL lymph node tissues from diagnostic
biopsies (age, 31–70 years; male:female, 30:27) and 21 FFPE
lymphnoditis lymph node tissues from diagnostic biopsies (age,
35–66 years; male:female, 12:9) were collected. All the samples
were obtained between January 2010 to June 2013 from Xiangya
Hospital. The Institutional Ethical Review Board of Xiangya
Hospital approved the present study and written informed consent
was obtained from all patients for the use of their biopsy samples.
No patient had received any antitumor treatments before the biopsy
sample was collected. The Ann Arbor staging system was used to
assess the stage of the patient and the International Prognostic
Index (IPI) was used to assess the risk of the patient (48). The serum lactate dehydrogenase
(LDH) level was measured by clinical laboratory staff using the
Hitachi 7170 biochemical analyzer (Hitachi, Ltd.).
Cell culture
The human Pfeiffer DLBCL cell line was purchased
from the American Type Culture Collection (cat. no.
ATCC® CRL-2632™) and cultured in RPMI-1640 (HyClone; GE
Healthcare Life Sciences) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 100 U/ml penicillin and streptomycin
(HyClone; GE Healthcare Life Sciences) at 37°C in a humidified 5%
CO2 incubator. Cells were passaged every 2–3 days to
maintain a density between 1–2×106 cells/ml.
Immunohistochemistry
Sections (thickness, 4 mm) obtained from the 57 FFPE
DLBCL specimens were deparaffinized, rehydrated and the endogenous
peroxidase activity was blocked using 3% H2O2
in methanol for 15 min at room temperature. The sections were
microwaved for 4 min with trisodium citrate dihydrate solution
(0.125%, pH 6.0) and then soaked with PBS three times for 5 min for
citrate-mediated high-temperature antigen retrieval. Non-specific
binding was blocked by pre-incubating with 5% BSA for 30 min at
room temperature. Sections were incubated with anti-CYP24A1 (1:300)
at 4°C overnight and then incubated with a biotinylated secondary
antibody (1:5,000) for 30 min at room temperature. Sections were
incubated with a streptavidin-HRP complex (Origene Technologies,
Inc.) at room temperature for 5 min and 3,3′-diaminobenzidine
(Origene Technologies, Inc.) at room temperature for 5 min,
sections were then counterstained with hematoxylin at room
temperature for 20. Positive cells were counted in 10 randomly
selected fields with a ×40 objective (Olympus CX23; Olympus
Corporation). All sections were scored by two pathologists. The
staining index was calculated as the product of staining intensity
(Score: 0, no staining; 1, weak/light yellow; 2,
moderate/yellow-brown; 3, strong/brown). The number of stained
cells was scored as 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) or
4 (76–100%). The sum of the intensity and number scores was used as
the final staining score (0–7). Low expression was defined as a
final staining score of ≤3. High expression was defined as a final
staining score >3.
Cell viability assay
Pfeiffer cells in exponential phase growth were
plated at a density of 1×104 cells/well in 96-well
plates. The wells were divided into three groups (five
wells/group): Control group (0.1% ethanol); 10 nM
1,25(OH)2D3 group; 100 nM
1,25(OH)2D3 group. The concentrations of
1,25(OH)2D3 used were similar to previous
studies (49,50). Cytotoxicity was determined using
the CCK-8 assay, according to the manufacturer's instructions.
After 48 h of treatment, cells were incubated with 10 µl CCK-8
reagent for a further 3 h. The optical density of the samples was
determined using a microplate reader at 450 nm. Each experiment was
performed in triplicate.
Cell cycle analysis
Pfeiffer cells were plated into 6-well plates at a
density of 3×105 cells/well and divided into six groups:
Control group (0.1% ethanol); 10 nM
1,25(OH)2D3; 100 nM
1,25(OH)2D3; 10 nM RAPA; combination group
(10 nM or 100 nM 1,25(OH)2D3 + 10 nM RAPA).
After incubation for 48 h, the cells were washed twice with PBS and
fixed with ice-cold 70% ethanol at −20°C overnight. Subsequently,
the cells were centrifuged at 111.8 × g for 5 min at 4°C, washed
once with PBS and the DNA was labeled with 0.5 ml cold PI solution
(0.1% Triton X-100, 0.1 mM EDTA, 50 µg/ml RNase A, 50 µg/ml PI in
PBS) on ice for 30 min in the dark. The cell cycle distribution was
determined using a FACSCalibur flow cytometer (BD Biosciences)
using ModFit LT software version 3.0 (Verity Software House).
LC3II immunofluorescence staining
Cells were fixed for 15 min with 4% paraformaldehyde
at 4°C and permeabilization with 0.25% Triton X-100 in PBS for 5
min at room temperature. After blocking with fetal calf serum for 1
h at room temperature, the slides were incubated with an LC3I/II
primary antibody (1:200) overnight at 4°C. The slides were then
incubated with a fluorescein-conjugated goat anti-rabbit secondary
antibodies (1:1,000) for 1 h at 37°C. The nuclei of cells were
stained using DAPI (1 µg/ml) at room temperature. The fluorescent
staining was imaged using a confocal microscope (IX71; Olympus
Corporation). In total, 10 random fields were selected for analyzed
(magnification, ×400 and ×600).
Transmission electron microscopy
To morphologically observe the induction of
autophagy in 1,25(OH)2D3 treated Pfeiffer
cells, ultrastructural analysis was performed. After treatment with
100 nM 1,25(OH)2D3 for 48 h, cells were
washed twice with PBS and fixed with ice-cold glutaraldehyde (3% in
0.1 M cacodylate buffer, pH 7.4) for 30 min. Cells were post-fixed
in 1% osmium tetroxide at room temperature for 2 h and embedded in
Epon812 (SERVA Electrophoresis GmbH) before being cut (60 nm) and
stained with 2% uranyl acetate/2.5% lead citrate and incubated for
15 min at room temperature. The formation of autophagosomes was
assessed using the Tecnai electron microscope (FEI; Thermo Fisher
Scientific, Inc.) at a magnification of ×1,700 and ×5,000.
Western blot analysis
Proteins were extracted using a nuclear and
cytoplasmic protein extraction kit (Beyotime Institute of
Biotechnology). Protein concentrations were determined using the
bicinchoninic acid method. Proteins (30 µg) were separated using 6%
SDS-PAGE under reducing conditions and then transferred onto PVDF
membranes. The membranes were blocked in 5 mg/ml BSA for 2 h at
room temperature. The following primary antibodies were used: VDR
(1:1,000), CYP24A1 (1:1,000), AKT (1:1,000), p-AKT (1:1,000), mTOR
(1:1,000), p-mTOR (1:1,000), LC3II (1:1,000) and P62 (1:1,000),
P70S6K (1:1,000), p-p70S6K (1:1,000), 4EBP1 (1:1,000) and p-4EBP1
(1:1,000). α-Tubulin (1:5,000) was used as a loading control. The
primary antibodies were incubated with the membranes overnight at
4°C. HRP-conjugated secondary antibodies (1:5,000) were incubated
with the membranes at room temperature for 1–2 h. Protein bands
were visualized using ECL and the Chemi Doc™ MP Imaging System
(Bio-Rad Laboratories, Inc.). Band densitometry was assessed using
ImageJ software 1.8.0 (National Institutes of Health).
Statistical analysis
All statistical analysis was performed using SPSS
22.0 software (IBM Corp.). Data are present as the mean ± SD. Data
were analyzed using the χ2 test, Student's t-test, or
one- or two-way ANOVA and the Tukey's test when applicable. Data
were representative of three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of CYP24A1 is different in
patients with DLBCL and lymphnoditis
To examine whether the expression of CYP24A1 protein
in DLBCL and lymphnoditis tissues, immunohistochemistry was
performed on the 57 paraffin-embedded DLBCL lymph node tissue
sections and 21 lymphnoditis lymph node tissue sections to evaluate
the expression of CYP24A1 (Fig.
1A-D). High levels of CYP24A1 expression were detected in 9.5%
of patients with lymphnoditis (n=21), whereas high levels of
CYP24A1 expression were detected in 35.1% of patients with DLBCL
(n=57). Low CYP24A1 expression was detected in 90.5% of patients
with lymphnoditis, while low CYP24A1 expression was detected in
74.9% of patients with DLBCL. The expression levels of CYP24A1
between patients with lymphnoditis and DLBCL was significantly
different (P<0.001). The patients with DLBCL were stratified
into different groups, using factors such as age and sex, to
investigate associations with the expression of CYP24A1 (Table I). There was no significant
different in the expression of CYP24A1 between the Ann Arbor stage
I/II patients and the Ann Arbor stage III/IV patients (P=0.28),
however, the expression of CYP24A1 was significantly higher in
patients with a higher IPI (P<0.05). Serum LDH levels were also
significantly associated with CYP24A1 expression. The patient whose
clinical treatment response was stable or showed complete remission
had a lower level of CYP24A1 (P<0.05). The expression of CYP24A1
was significantly higher in patients over the age of 60
(P<0.05).
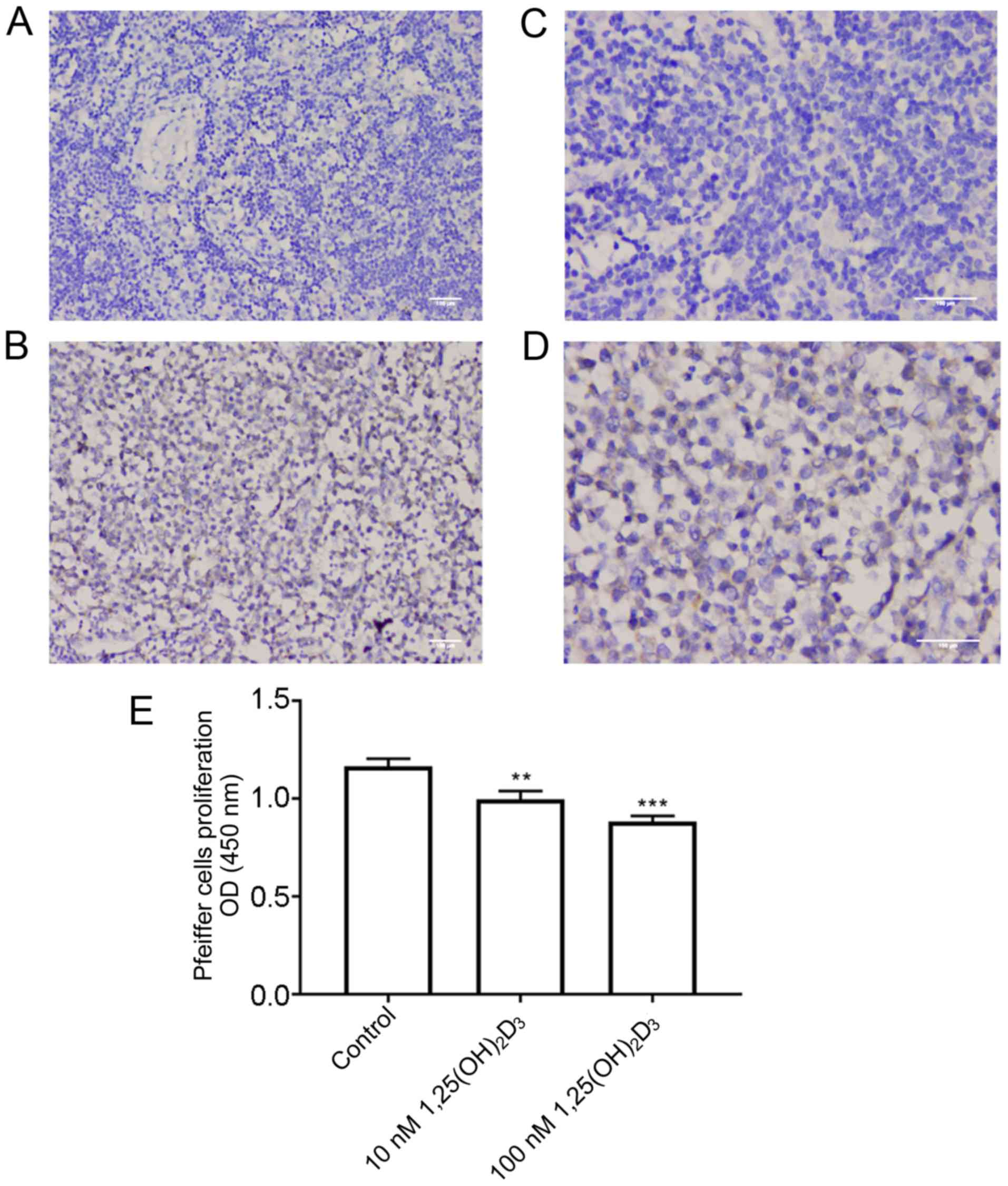 | Figure 1.CYP24A1 is differently expressed in
patients with DLBCL and lymphnoditis, and
1,25(OH)2D3 inhibits the proliferation of
Pfeiffer cells. Sections of paraffin-embedded lymph node tissues
from the diagnostic biopsies of 20 patients with lymphnoditis and
57 patients with DLBCL were processed for immunohistochemical
staining with an antibody against CYP24A1. Representative images of
CYP24A1 immunohistochemical staining in lymphnoditis samples at (A)
×200 and (C) ×400 magnification. Representative images of CYP24A1
immunohistochemical staining in DLBCL lymph node tissue at (B) ×200
and (D) ×400 magnification. Scale bar, 100 µm. (E) Pfeiffer cells
were cultured with 1,25(OH)2D3 (10–100 nM)
for 48 h. After treatment, cell viability was determined using a
Cell Counting Kit-8. Pfeiffer cell proliferation was significantly
inhibited by 10 and 100 nM 1,25(OH)2D3. The
rate of inhibition was calculated to be 16.8±3.4% for cells treated
with 10 nM 1,25(OH)2D3 and 28.2±2.9% for
cells treated with 100 nM 1,25(OH)2D3 for 48
h. Each experiment was performed in triplicate. **P<0.01,
***P<0.001 vs. control. CYP24A1, 25-hydroxyvitamin
D-24-hydroxylase; 1,25(OH)2D3,
1,25-dihydroxyvitamin D3; OD, optical density; DLBCL,
diffuse large B cell lymphoma. |
 | Table I.Clinical features and CYP24A1
expression of patients with diffuse large B cell lymphoma. |
Table I.
Clinical features and CYP24A1
expression of patients with diffuse large B cell lymphoma.
|
| CYP24A1
expression |
|
|---|
|
|
|
|
|---|
| Parameter | Low | High | P-value |
|---|
| Age, years |
|
<60 | 32 | 11 | 0.008a |
|
≥60 | 5 | 9 |
|
| Sex |
|
Male | 18 | 12 | 0.37 |
|
Female | 19 | 8 |
|
| Ann Arbor
stage |
|
I/II | 10 | 3 | 0.28 |
|
III/IV | 27 | 17 |
|
| LDH |
|
Normal | 32 | 11 | 0.015a |
|
Elevated | 5 | 9 |
|
| IPI |
|
0–2 | 18 | 8 | 0.008a |
|
3–5 | 19 | 20 |
|
| Clinical treatment
response |
|
CR/PR | 26 | 8 | 0.028a |
|
SD/PD | 11 | 12 |
|
1,25(OH)2D3
inhibits the proliferation of Pfeiffer cells
To investigate the growth inhibitory properties of
1,25(OH)2D3, CCK-8 assays were performed,
which is based on the ability of viable cells to reduce CCK-8 to
formazan crystals. As shown in Fig.
1E, Pfeiffer cell proliferation was significantly inhibited by
10 nM (P<0.01) and 100 nM 1,25(OH)2D3
(P<0.001). The rate of inhibition was calculated as 16.8±3.4%
for cells treated with 10 nM 1,25(OH)2D3 and
28.2±2.9% for cells treated with 100 nM
1,25(OH)2D3 for 48 h.
1,25(OH)2D3
induces cell cycle arrest in Pfeiffer cells
To determine whether
1,25(OH)2D3 induces cell cycle arrest in
Pfeiffer cells, the effect of 1,25(OH)2D3 on
the cell cycle distribution was analyzed using PI staining.
Pfeiffer cells were treated with different concentrations of
1,25(OH)2D3 for 48 h and then subjected to
cell cycle analysis. As shown in Fig.
2A and B, 22.70±0.40% of cells in the control group were in the
G1 phase, while the cells treated with 100 nM
1,25(OH)2D3 showed a significant increase in
the proportion of cells in the G1 phase (26.11±0.64%; P<0.05).
In addition, whether 1,25(OH)2D3 increased
the G1 phase arrest induced by RAPA was investigated. It was found
the addition of RAPA increased the number of cells in the G1 phase
compared with 1,25(OH)2D3 treatment alone
(Table II; Fig. 2A and B).
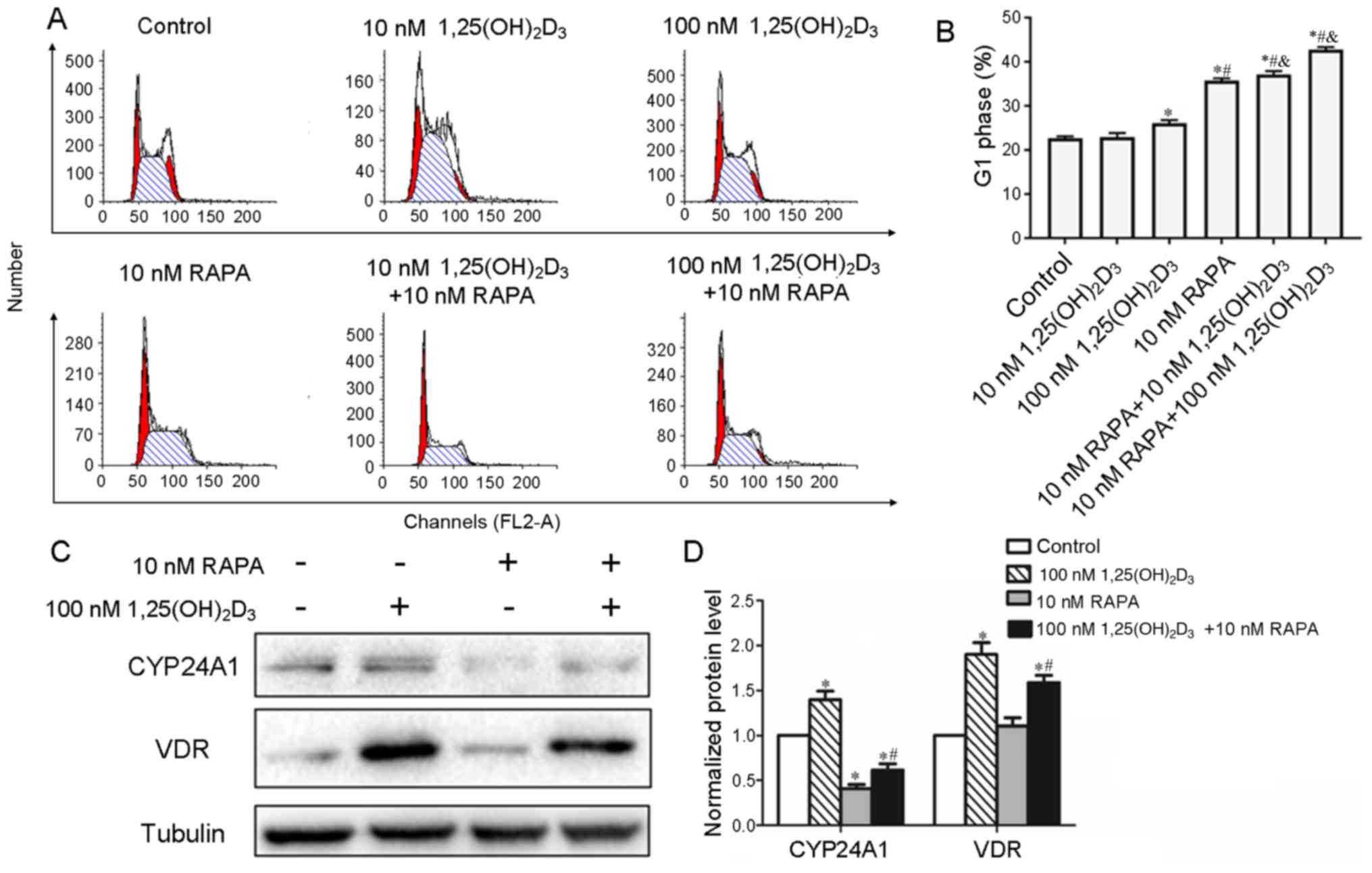 | Figure 2.Cell cycle distribution of Pfeiffer
cells after treatment with 1,25(OH)2D3 and
RAPA, and the expression of CYP24A1 and VDR in Pfeiffer cells. (A)
Pfeiffer cells were cultured with 10 or 100 nM
1,25(OH)2D3 and RAPA (10 nM). After 48 h, the
cell cycle distribution was analyzed. The figure shows
representative examples of three independent experiments. (B)
Quantification of the G1 population of Pfeiffer cells. (C) After
treatment with 1,25(OH)2D3 and/or RAPA for 48
h, cells were harvested and the protein levels of CYP24A1 and VDR
were analyzed via western blotting. A representative of three
independent experiments is shown. (D) Quantification of western
blotting results. *P<0.05 vs. control; #P<0.05 vs.
100 nM 1,25(OH)2D3; &P<0.05
vs. 10 nM RAPA. VDR, vitamin D receptor; CYP24A1, 25-hydroxyvitamin
D-24-hydroxylase; 1,25(OH)2D3,
1,25-dihydroxyvitamin D3; RAPA, rapamycin. |
 | Table II.Cell cycle distribution of Pfeiffer
cells treated with 1,25(OH)2D3 and RAPA. |
Table II.
Cell cycle distribution of Pfeiffer
cells treated with 1,25(OH)2D3 and RAPA.
| Group | G1 phase (%) |
|---|
| Control | 22.70±0.40 |
| 10 nM
1,25(OH)2D3 | 22.92±0.93 |
| 100 nM
1,25(OH)2D3 |
26.11±0.64a |
| 10 nM RAPA |
35.74±0.55a,b |
| 10 nM RAPA + 10 nM
1,25(OH)2D3 |
37.13±0.75a,b |
| 10 nM RAPA + 100 nM
1,25(OH)2D3 |
42.75±0.61a,c |
1,25(OH)2D3 induced the
expression of CYP24A1 mediated by VDR, which is the receptor of
1,25(OH)2D3, and CYP24A1 causes the
degradation of 1,25(OH)2D3. This is an
important regulatory mechanism to control the level of
1,25(OH)2D3 (30,31).
In order to investigate whether RAPA increases the cell cycle
arrest induced by 1,25(OH)2D3 by reducing the
degradation of 1,25(OH)2D3, the levels of
CYP24A1 and VDR were determined in Pfeiffer cells after exposure to
either 1,25(OH)2D3 or RAPA using western blot
analysis. It was found that RAPA suppressed the expression of
CYP24A1 and VDR induced by 1,25(OH)2D3
(Fig. 2C and D). This suggested
that RAPA increased the cell cycle arrest in Pfeiffer cells induced
by 1,25(OH)2D3 by suppressing the expression
of CYP24A1 and VDR.
1,25(OH)2D3
induces autophagy in Pfeiffer cells
In order to investigate whether
1,25(OH)2D3 regulates autophagy in Pfeiffer
cells, the induction of autophagy was analyzed. The induction of
autophagy in Pfeiffer cells after treatment with or without
1,25(OH)2D3 (10 nM, 100 nM) for 48 h was
analyzed. The extent of autophagosome formation is associated with
the modification of LC3I to LC3II and the degradation of P62. A
significant increase in LC3II/LC3I and a reduction in P62 was
induced by 100 nM 1,25(OH)2D3, as assessed
using western blot analysis (Fig. 3C
and D). Furthermore, the aggregation and localization of LC3
plays an important role in the maturation and transport of the
autophagosome; therefore, immunofluorescence experiments were
performed to observe changes in LC3 aggregation. A notable increase
in green LC3 puncta was observed in the 100 nM
1,25(OH)2D3 treatment group compared with the
control group (Fig. 3B). In
addition, it was found that 100 nM
1,25(OH)2D3 enhanced the formation of
autophagosomes, as observed using transmission electron microscopy
(Fig. 3A). These findings
indicated that 1,25(OH)2D3 activates
autophagy in Pfeiffer cells.
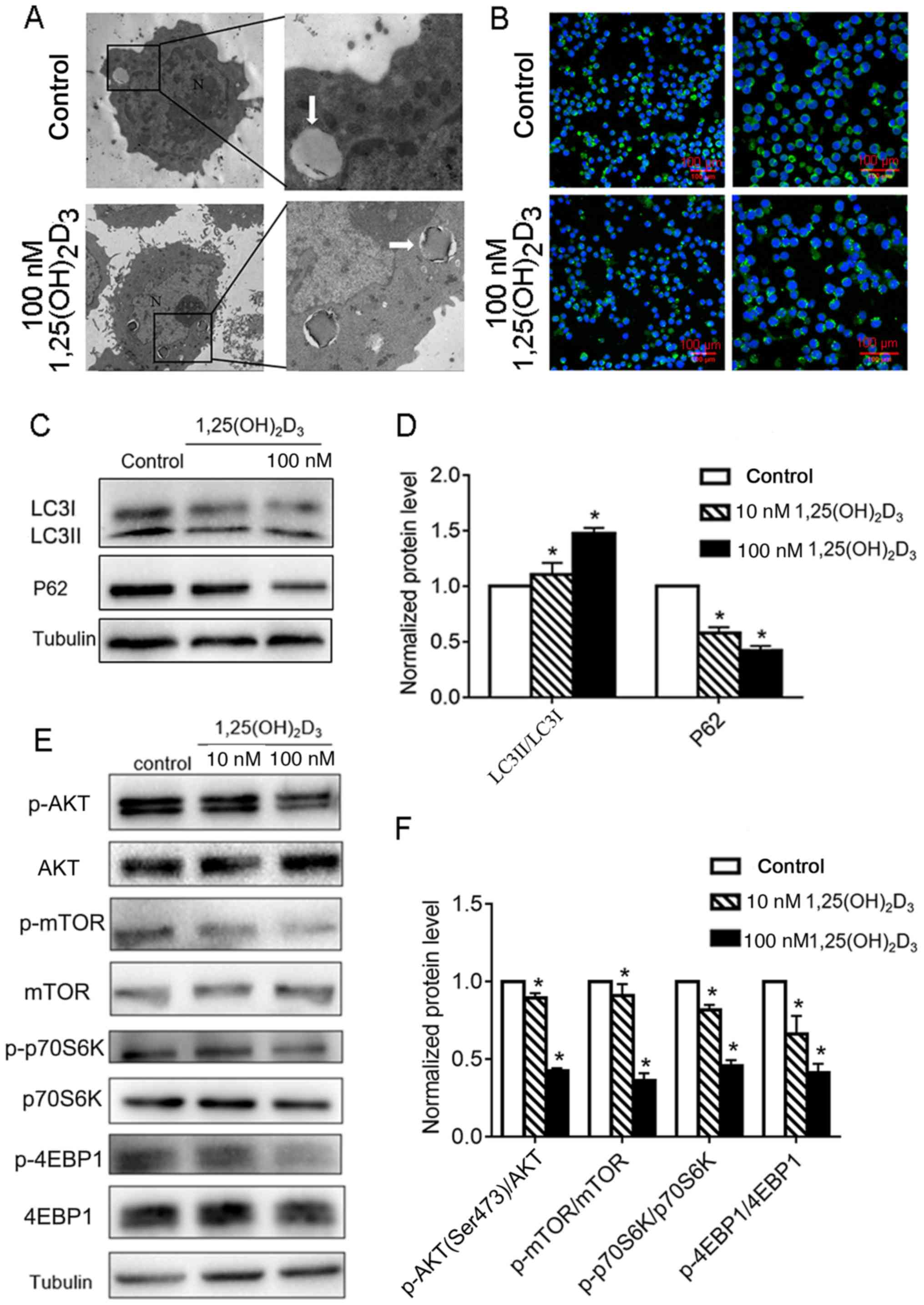 | Figure 3.Analysis of autophagy and the
AKT/mTOR signaling pathway in Pfeiffer cells after treatment with
1,25(OH)2D3. (A) Representative transmission
electron microscope images [magnification, ×1,700 (left) and ×5,000
(right)] of the ultrastructure of Pfeiffer cells after treatment
with or without 100 nM 1,25(OH)2D3 for 48 h.
(B) Changes in the level and localization of LC3 examined by
confocal microscopy after treatment with or without 100 nM
1,25(OH)2D3 for 48 h. Scale bar =100 µm. (C)
Western blot analysis of the expression of the autophagy related
proteins LC3 and P62 after treatment with 100 nM 1,25-D3
for 48 h and (D) the quantification. (E) Pfeiffer cells were
cultured without or with 1,25(OH)2D3 for 1 h.
The phosphorylation levels of AKT/mTOR signaling proteins were
evaluated using western blotting. A representative example of three
independent experiments is shown. (F) Quantification of western
blotting. *P<0.05 vs. control. LC3, protein light chain 3;
1,25(OH)2D3, 1,25-dihydroxyvitamin
D3; mTOR, target of rapamycin; p-, phosphorylated; 4EBP,
eukaryotic translation imitation factor 4E-binding protein 1;
p70SK6, ribosomal protein S6 kinase β-1; P62, ubiquitin binding
protein P62. |
1,25(OH)2D3
inhibits the AKT/mTOR signaling pathway
Having established that
1,25(OH)2D3 inhibited proliferation and
induced autophagy in Pfeiffer cells, the molecular mechanisms
underlying these biological effects were investigated. Activation
of the AKT/mTOR signaling pathway increases the proliferation of
Pfeiffer cells and is one of the major targets in the process of
autophagy (51). Western blot
analysis showed that p-AKT and p-mTOR were downregulated after
1,25(OH)2D3 exposure. The activation of
downstream targets of mTOR, including p70S6K and 4EBP1, were also
decreased following 1,25(OH)2D3 treatment
(Fig. 3E and F).
Discussion
In the present study, it was found that the levels
of CYP24A1 in DLBCL lymph node tissues were higher than in
lymphnoditis tissues. There was no significant difference in the
expression of CYP24A1 between Ann Arbor stage I/II patients and Ann
Arbor stage III/IV patients (P=0.28), however, the expression of
CYP24A1 was significantly higher in patients with a higher IPI.
Moreover, CYP24A1 expression was negatively associated with
clinical treatment response. Patients over the age of 60 had a
higher level of CYP24A1 expression. Furthermore, it was found
1,25(OH)2D3 inhibited proliferation and
increased the G1 phase population of Pfeiffer cells. RAPA led to a
further increase in the G1 population and decreased the expression
of CYP24A1 and VDR induced by 1,25(OH)2D3.
1,25(OH)2D3 induced the formation of
autophagosomes and increased the levels of the autophagy related
protein LC3II/LC3I and reduced the expression of P62. In addition,
1,25(OH)2D3 decreased the phosphorylation of
AKT, mTOR, and its downstream targets 4EBP and p70S6K in Pfeiffer
cells.
To the best of our knowledge, the present study was
the first to show higher levels of the vitamin D-degrading enzyme
CYP24A1 in DLBCL tissues compared with lymphnoditis tissues, which
was used as a normal control for the lymphoma tissues. CYP24A1
degrades 1,25(OH)2D and 25(OH)D, which is a substrate
required for the synthesis of 1,25(OH)2D. Therefore,
CYP24A1 is an important factor in determining the local
concentration of 1,25(OH)2D and regulates the
antiproliferative effect of 1,25(OH)2D (52). The higher expression of CYP24A1 may
be one explanation for the significantly lower serum concentration
of 25(OH)D in patients with DLBCL (53). In addition, previous studies have
described the moderate impact of 1,25(OH)2D on the
proliferation of certain DLBCL cell lines, including DOHH2 and K442
(50,49). The present study found higher
levels of CYP24A1 expression in DLBCL lymph node tissues,
suggesting that the actual concentration of vitamin D available and
the concentration of 1,25(OH)2D in these tissues may be
low, and that this may attenuate the antitumor effects of
1,25(OH)2D in vivo. The increased expression of
CYP24A1 was also found in some solid tumors, including colon, lung,
prostate, thyroid, breast and ovarian cancer cells (43–47).
However, little is known regarding the mechanism underlying the
increased expression of CYP24A1 in these cancer cells.
Understanding the mechanism that leads to the upregulation of
CYP24A1 in tumors may provide strategies for targeting CYP24A1 in
the future. A combination of CYP24A1 inhibitors, such as liarozole,
genistein [a soy isoflavone that directly inhibits CYP24A1 activity
and increases the sensitivity of cells to 1,25(OH)2D] or
ritonavir (which inhibits the expression of CYP24A1), may increase
the level and calcemic activity of 1,25(OH)2D,
increasing the risk of hypercalcemic side effects (54,55).
Structural non-calcemic action analogs of calcitriol that resist
CYP24A1 may be more biologically active and more useful in cancer
therapy and for the treatment of DLBCL, which expresses a high
level of CYP24A1.
In the present study, it was found that
1,25(OH)2D3 inhibits the proliferation of
DLBCL Pfeiffer cell line. In a previous study into the
antiproliferative effect of 1,25(OH)2D3 in
other DLBCL cell lines, 1,25(OH)2D3 was found
to have an antiproliferative effect on the Pfeiffer cell line
(50).
CYP24A1, which degrades
1,25(OH)2D3 and 25(OH)D, is important for
lowering the levels of 1,25(OH)2D3 and
25(OH)D. Vitamin D deficiency is a risk factor for autoimmune
diseases, such as rheumatoid arthritis and system lupus
erythematosus (SLE) (56,57). A recent study reported that vitamin
D deficiency and the upregulation of CYP24A1 have a combined role
in the transition to SLE (58).
Patients with autoimmune diseases, in particular those mediated by
B lymphocytes, have a higher risk of developing DLBCL, with a more
aggressive nature, possibly due to the misregulated production of
several cytokines, including interleukin (IL)-6, IL-10 and tumor
necrosis factor-α, that contribute to the pathogenesis of DLBCL
(59–62). 1,25(OH)2D3
also plays an important role in the regulation of immune function
by inhibiting the production of cytokines (63,64).
In future research, whether the increased production of cytokines
contribute to the pathogenesis of DLBCL, and whether this is
associated with the upregulation of CYP24A1, should be
investigated. Furthermore, whether inhibiting the production of
cytokines using 1,25(OH)2D3 is beneficial for
the treatment of some cases of DLBCL should be investigated in
in vivo studies.
Autophagic cell death is a survival mechanism to
deal with metabolic stress and is a caspase-independent mechanism
of cell death (65). Nevertheless,
previous studies have indicated that autophagy and apoptosis may be
interconnected process (66,67).
The present study found that 1,25(OH)2D3
induced autophagy in Pfeiffer cells. These results suggested that
1,25(OH)2D3 induced cell killing in a
caspase-independent autophagy-mediated manner in Pfeiffer
cells.
Activation of the PI3K/AKT/mTOR signaling pathway
suppresses autophagy (68,69). The data from the present study
showed that 1,25(OH)2D3 decreased the
phosphorylation levels of AKT and mTOR in Pfeiffer cells,
consistent with a previous study that found that
1,25(OH)2D3 is involved in mTOR signaling
(70). These results suggested
that 1,25(OH)2D3 induces autophagy in
Pfeiffer cells by inhibiting the PI3K/AKT/mTOR signaling pathway.
In addition, the PI3K/AKT/mTOR signaling pathway also increases
mRNA translation, protein synthesis and cellular proliferation
(9,10). The aberrant activation of mTOR is
frequently associated with poorer prognosis and has been well
described in non-Hodgkin lymphoma (4,71–73).
Suppressing mTOR in Pfeiffer cells may extend the therapeutic
applications of 1,25(OH)2D to the treatment of DLBCL. A
previous study reported that 1,25(OH)2D3
inhibits the mTOR signaling pathway by stimulating the expression
of DDIT4, a potent suppressor of mTOR activity (39). The present study showed that
1,25(OH)2D3 decreased the phosphorylation of
downstream factors in the PI3K/AKT/mTOR signaling pathway in
Pfeiffer cells, including 4EBP and p70S6K. p70S6K is an important
regulator of protein synthesis; blocking the activation of p70S6K,
for example using Saquinavir-NO, interrupts protein synthesis,
leading to decreased cancer cell proliferation (74–77).
The inhibition of Pfeiffer cell proliferation by
1,25(OH)2D3 may be due to the decreased
phosphorylation of p70S6K. In future studies, the anticancer
effects of other p70S6K inhibitors should be investigated in DLBCL,
and their potential synergism with
1,25(OH)2D3 should be tested.
A previous study reported that the mTOR signaling
inhibitor RAPA and its analogs increase the antitumor effects of
1,25(OH)2D in breast cancer cells and acute myelogenous
leukemia (78,79). This effect of RAPA and its analogs
may be due to the increased expression, or nuclear translocation,
of VDR (79). Consistent with
these previous studies, the data from the present study showed that
1,25(OH)2D3 increased the G1 phase population
of Pfeiffer cells and that this was potentiated by RAPA. However,
it was found that RAPA blocked the increase in VDR and CYP24A1
expression induced by 1,25(OH)2D3.
1,25(OH)2D3 induced the expression of
CYP24A1, mediated by VDR, which is the receptor of 1,25(OH)2D3, and
CYP24A1 causes the degradation of
1,25(OH)2D3. This is an important
autoregulatory mechanism of 1,25(OH)2D3. RAPA
may potentiate the effect of 1,25(OH)2D3 by
reducing its degradation.
In conclusion, the results of the present study
suggested that the expression of CYP24A1 may be a novel prognostic
indicator for DLBCL. 1,25(OH)2D3 inhibited
the proliferation, and induced the autophagy, of Pfeiffer cells. In
addition, 1,25(OH)2D3 increased the G1 phase
population of Pfeiffer cells. These effects may be mediated by
inhibiting the PI3K/AKT/mTOR signaling pathway. RAPA may potentiate
the cell cycle arrest caused by 1,25(OH)2D3
by inhibiting the expression of CYP24A1 and VDR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81570200) and was
partially supported by the fund from Guangzhou Institute of
Pediatrics/Guangzhou Women and Children's Medical Center (grant no.
YIP-2018-005). The funders had no role in the study concept, study
design, data analysis, interpretation or reporting of the results.
The authors had full control of the data and information submitted
for publication.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WW and JH contributed to the design of the study
and were involved in performing the experiments, data analysis and
the preparation of the manuscript. YT contributed to the data
analysis. MZ contributed to data analysis and manuscript
preparation. All authors contributed to the data interpretation and
approved the final version of the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The Institutional Ethical Review Board of the
Xiangya Hospital approved the present study, and written informed
consent was obtained from all patients for the use of their biopsy
samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
1,25(OH)2D3
|
1,25-dihydroxyvitamin
D3
|
|
4EBP
|
eukaryotic translation imitation
factor 4E-binding protein 1
|
|
CYP24A1
|
25-hydroxyvitamin
D-24-hydroxylase
|
|
DLBCL
|
diffuse large B cell lymphoma
|
|
IPI
|
International Prognostic Index
|
|
LC3
|
Protein light chain 3
|
|
mTOR
|
mammalian target of rapamycin
|
|
FFPE
|
Paraffin-embedded
|
|
RAPA
|
rapamycin
|
|
VDR
|
vitamin D receptor
|
References
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lymphoma. Pathology. 50:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li JM, Wang L, Shen Y, Xia ZG, Chen Y,
Chen QS, Chen Y, Zeng XY, You JH, Qian Y and Shen ZX: Rituximab in
combination with CHOP chemotherapy for the treatment of diffuse
large B cell lymphoma in Chinese patients. Ann Hematol. 86:639–645.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He F, Wang L, Hu XB, Yin DD, Zhang P, Li
GH, Wang YC, Huang SY, Liang YM and Han H: Notch and BCR signaling
synergistically promote the proliferation of Raji B-lymphoma cells.
Leuk Res. 33:798–802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uddin S, Hussain AR, Siraj AK, Manogaran
PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A,
El-Solh H, et al: Role of phosphatidylinositol 3′-kinase/AKT
pathway in diffuse large B-cell lymphoma survival. Blood.
108:4178–4186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang J, Zubovitz J, Petrocelli T,
Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C,
Beniston R, et al: PKB/Akt phosphorylates p27, impairs nuclear
import of p27 and opposes p27-mediated G1 arrest. Nat Med.
8:1153–1160. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Héron-Milhavet L, Franckhauser C, Rana V,
Berthenet C, Fisher D, Hemmings BA, Fernandez A and Lamb NJ: Only
Akt1 is required for proliferation, while Akt2 promotes cell cycle
exit through p21 binding. Mol Cell Biol. 26:8267–8280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brazil DP, Yang ZZ and Hemmings BA:
Advances in protein kinase B signalling: AKTion on multiple fronts.
Trends Biochem Sci. 29:233–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai SL, Tee AR, Short JD, Bergeron JM, Kim
J, Shen J, Guo R, Johnson CL, Kiguchi K and Walker CL: Activity of
TSC2 is inhibited by AKT-mediated phosphorylation and membrane
partitioning. J Cell Biol. 173:279–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discov Today. 16:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu ZZ, Xia ZG, Wang AH, Wang WF, Liu ZY,
Chen LY and Li JM: Activation of the PI3K/AKT/mTOR pathway in
diffuse large B cell lymphoma: Clinical significance and inhibitory
effect of rituximab. Ann Hematol. 92:1351–1358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ott G, Ziepert M, Klapper W, Horn H,
Szczepanowski M, Bernd HW, Thorns C, Feller AC, Lenze D, Hummel M,
et al: Immunoblastic morphology but not the immunohistochemical
GCB/nonGCB classifier predicts outcome in diffuse large B-cell
lymphoma in the RICOVER-60 trial of the DSHNHL. Blood.
116:4916–4925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahadevan D, Chiorean EG, Harris WB, Von
Hoff DD, Stejskal-Barnett A, Qi W, Anthony SP, Younger AE, Rensvold
DM, Cordova F, et al: Phase I pharmacokinetic and pharmacodynamic
study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in
patients with advanced solid tumours and B-cell malignancies. Eur J
Cancer. 48:3319–3327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bendell JC, Rodon J, Burris HA, de Jonge
M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, et
al: Phase I, dose-escalation study of BKM120, an oral pan-Class I
PI3K inhibitor, in patients with advanced solid tumors. J Clin
Oncol. 30:282–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Zhang Y, Zhang L, Ren X,
Huber-Keener KJ, Liu X, Zhou L, Liao J, Keihack H, Yan L, et al:
MK-2206, a novel allosteric inhibitor of Akt, synergizes with
gefitinib against malignant glioma via modulating both autophagy
and apoptosis. Mol Cancer Ther. 11:154–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Donia M, Mangano K, Amoroso A, Mazzarino
MC, Imbesi R, Castrogiovanni P, Coco M, Meroni P and Nicoletti F:
Treatment with rapamycin ameliorates clinical and histological
signs of protracted relapsing experimental allergic
encephalomyelitis in Dark Agouti rats and induces expansion of
peripheral CD4+CD25+Foxp3+
regulatory T cells. J Autoimmun. 33:135–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bagherpour B, Salehi M, Jafari R, Bagheri
A, Kiani-Esfahani A, Edalati M, Kardi MT and Shaygannejad V:
Promising effect of rapamycin on multiple sclerosis. Mult Scler
Relat Disord. 26:40–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen QD, Merrill PT, Clark WL, Banker
AS, Fardeau C, Franco P, LeHoang P, Ohno S, Rathinam SR, Thurau S,
et al: Intravitreal sirolimus for noninfectious uveitis: A Phase
III sirolimus study assessing double-masKed uveitis TReAtment
(SAKURA). Ophthalmology. 123:2413–2423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steelman LS, Martelli AM, Cocco L, Libra
M, Nicoletti F, Abrams SL and McCubrey JA: The therapeutic
potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol.
82:1189–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.PubMed/NCBI
|
|
23
|
Donia M, McCubrey JA, Bendtzen K and
Nicoletti F: Potential use of rapamycin in HIV infection. Br J Clin
Pharmacol. 70:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicoletti F, Lapenta C, Donati S, Spada M,
Ranazzi A, Cacopardo B, Mangano K, Belardelli F, Perno C and Aquaro
S: Inhibition of human immunodeficiency virus (HIV-1) infection in
human peripheral blood leucocytes-SCID reconstituted mice by
rapamycin. Clin Exp Immunol. 155:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caporali S, Alvino E, Lacal PM, Levati L,
Giurato G, Memoli D, Caprini E, Antonini Cappellini GC and D'Atri
S: Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating
effect of dabrafenib on the invasive behavior of melanoma cells
with acquired resistance to the BRAF inhibitor. Int J Oncol.
49:1164–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Yu X, Ma J, Tong Y and Yao J:
Effects of NVP-BEZ235 on the proliferation, migration, apoptosis
and autophagy in HT-29 human colorectal adenocarcinoma cells. Int J
Oncol. 49:285–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang Z, Shi G, Jin J, Guo H, Guo X, Luo
F, Song Y and Jia X: Dual PI3K/mTOR inhibitor NVP-BEZ235-induced
apoptosis of hepatocellular carcinoma cell lines is enhanced by
inhibitors of autophagy. Int J Mol Med. 31:1449–1456. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paplomata E, Zelnak A and O'Regan R:
Everolimus: Side effect profile and management of toxicities in
breast cancer. Breast Cancer Res Treat. 140:453–462. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lew S and Chamberlain RS: Risk of
metabolic complications in patients with solid tumors treated with
mTOR inhibitors: Meta-analysis. Anticancer Res. 36:1711–1718.
2016.PubMed/NCBI
|
|
30
|
Marcinkowska E, Wallace GR and Brown G:
The use of 1α,25-dihydroxyvitamin D3 as an anticancer
agent. Int J Mol Sci. 17:E7292016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holick MF: Vitamin D and bone health. J
Nutr. 126 (Suppl 4):1159S–1164S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abu El Maaty MA and Wölfl S: Vitamin D as
a novel regulator of tumor metabolism: Insights on potential
mechanisms and implications for anti-cancer therapy. Int J Mol Sci.
18:E21842017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barreto SG and Neale RE: Vitamin D and
pancreatic cancer. Cancer Lett. 368:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castronovo C, Castronovo V, Nikkels A and
Peulen O: Vitamin D anti-cancer activities: Observations, doubts
and certainties. Rev Med Liege. 70:495–500. 2015.(In French).
PubMed/NCBI
|
|
35
|
Duffy MJ, Murray A, Synnott NC, O'Donovan
N and Crown J: Vitamin D analogues: Potential use in cancer
treatment. Crit Rev Oncol Hematol. 112:190–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li MX, Li LF, Zhang L, Xiao ZG, Shen J, Hu
W, Zeng Q and Cho CH: Vitamin D and cancer stem cells in the
gastrointestinal tract. Curr Med Chem. 24:918–927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Harbeck N, Jeschke U and
Doisneau-Sixou S: Influence of vitamin D signaling on hormone
receptor status and HER2 expression in breast cancer. J Cancer Res
Clin Oncol. 143:1107–1122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn J, Park S, Zuniga B, Bera A, Song CS
and Chatterjee B: Vitamin D in prostate cancer. Vitam Horm.
100:321–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lisse TS, Liu T, Irmler M, Beckers J, Chen
H, Adams JS and Hewison M: Gene targeting by the vitamin D response
element binding protein reveals a role for vitamin D in osteoblast
mTOR signaling. FASEB J. 25:937–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jang W, Kim HJ, Li H, Jo KD, Lee MK, Song
SH and Yang HO: 1,25-Dyhydroxyvitamin D3 attenuates
rotenone-induced neurotoxicity in SH-SY5Y cells through induction
of autophagy. Biochem Biophys Res Commun. 451:142–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Datta Mitra A, Raychaudhuri SP, Abria CJ,
Mitra A, Wright R, Ray R and Kundu-Raychaudhuri S:
1α,25-Dihydroxyvitamin-D3-3-bromoacetate regulates AKT/mTOR
signaling cascades: A therapeutic agent for psoriasis. J Invest
Dermatol. 133:1556–1564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gröschel C, Tennakoon S and Kállay E:
Cytochrome P450 Vitamin D hydroxylases in inflammation and cancer.
Adv Pharmacol. 74:413–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tannour-Louet M, Lewis SK, Louet JF,
Stewart J, Addai JB, Sahin A, Vangapandu HV, Lewis AL, Dittmar K,
Pautler RG, et al: Increased expression of CYP24A1 correlates with
advanced stages of prostate cancer and can cause resistance to
vitamin D3-based therapies. FASEB J. 28:364–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun H, Wang C, Hao M, Sun R, Wang Y, Liu
T, Cong X and Liu Y: CYP24A1 is a potential biomarker for the
progression and prognosis of human colorectal cancer. Hum Pathol.
50:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sakaki T, Yasuda K, Kittaka A, Yamamoto K
and Chen TC: CYP24A1 as a potential target for cancer therapy.
Anticancer Agents Med Chem. 14:97–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Osanai M and Lee GH: CYP24A1-induced
vitamin D insufficiency promotes breast cancer growth. Oncol Rep.
36:2755–2762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu N and Zhang H: CYP24A1 depletion
facilitates the antitumor effect of vitamin D3 on thyroid cancer
cells. Exp Ther Med. 16:2821–2830. 2018.PubMed/NCBI
|
|
48
|
Ng AK, Weiss L and LaCasce AS: Chapter 74
- Hodgkin's LymphomaClinical Radiation Oncology. 3rd. Gunderson LL
and Tepper JE: W.B. Saunders; Philadelphia: pp. 1527–1543. 2012,
View Article : Google Scholar
|
|
49
|
Kozielewicz P, Grafton G, Kutner A, Curnow
SJ, Gordon J and Barnes NM: Novel vitamin D analogues; cytotoxic
and anti-proliferative activity against a diffuse large B-cell
lymphoma cell line and B-cells from healthy donors. J Steroid
Biochem Mol Biol. 164:98–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hickish T, Cunningham D, Colston K, Millar
BC, Sandle J, Mackay AG, Soukop M and Sloane J: The effect of
1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of
vitamin D receptor in lymphoma. Br J Cancer. 68:668–672. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chung I, Karpf AR, Muindi JR, Conroy JM,
Nowak NJ, Johnson CS and Trump DL: Epigenetic silencing of CYP24 in
tumor-derived endothelial cells contributes to selective growth
inhibition by calcitriol. J Biol Chem. 282:8704–8714. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Drake MT, Maurer MJ, Link BK, Habermann
TM, Ansell SM, Micallef IN, Kelly JL, Macon WR, Nowakowski GS,
Inwards DJ, et al: Vitamin D insufficiency and prognosis in
non-Hodgkin's lymphoma. J Clin Oncol. 28:4191–4198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ikezoe T, Bandobashi K, Yang Y, Takeuchi
S, Sekiguchi N, Sakai S, Koeffler HP and Taguchi H: HIV-1 protease
inhibitor ritonavir potentiates the effect of 1,25-dihydroxyvitamin
D3 to induce growth arrest and differentiation of human myeloid
leukemia cells via down-regulation of CYP24. Leuk Res.
30:1005–1011. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ly LH, Zhao XY, Holloway L and Feldman D:
Liarozole acts synergistically with 1alpha,25-dihydroxyvitamin D3
to inhibit growth of DU 145 human prostate cancer cells by blocking
24-hydroxylase activity. Endocrinology. 140:2071–2076. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Adorini L and Penna G: Control of
autoimmune diseases by the vitamin D endocrine system. Nat Clin
Pract Rheumatol. 4:404–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Agmon-Levin N, Theodor E, Segal RM and
Shoenfeld Y: Vitamin D in systemic and organ-specific autoimmune
diseases. Clin Rev Allergy Immunol. 45:256–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Young KA, Munroe ME, Guthridge JM, Kamen
DL, Niewold TB, Gilkeson GS, Weisman MH, Ishimori ML, Kelly J,
Gaffney PM, et al: Combined role of vitamin D status and CYP24A1 in
the transition to systemic lupus erythematosus. Ann Rheum Dis.
76:153–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kleinstern G, Averbuch M, Abu Seir R,
Perlman R, Ben Yehuda D and Paltiel O: Presence of autoimmune
disease affects not only risk but also survival in patients with
B-cell non-Hodgkin lymphoma. Hematol Oncol. 36:457–462. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Barcellini W, Rizzardi GP, Borghi MO,
Nicoletti F, Fain C, Del Papa N and Meroni PL: In vitro type-1 and
type-2 cytokine production in systemic lupus erythematosus: Lack of
relationship with clinical disease activity. Lupus. 5:139–145.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Narazaki M and Kishimoto T: The two-faced
cytokine IL-6 in host defense and diseases. Int J Mol Sci.
19:E35282018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dlouhy I, Filella X, Rovira J, Magnano L,
Rivas-Delgado A, Baumann T, Martínez-Trillos A, Balagué O, Martínez
A, González-Farre B, et al: High serum levels of soluble
interleukin-2 receptor (sIL2-R), interleukin-6 (IL-6) and tumor
necrosis factor alpha (TNF) are associated with adverse clinical
features and predict poor outcome in diffuse large B-cell lymphoma.
Leuk Res. 59:20–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ysmail-Dahlouk L, Nouari W and Aribi M:
1,25-dihydroxyvitamin D3 down-modulates the production
of proinflammatory cytokines and nitric oxide and enhances the
phosphorylation of monocyte-expressed STAT6 at the recent-onset
type 1 diabetes. Immunol Lett. 179:122–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang M, Xu J, Yu J, Yang B, Gan H, Li S
and Li X: Anti-inflammatory effects of 1,25-dihydroxyvitamin D3 in
monocytes cultured in serum from patients with type 2 diabetes
mellitus and diabetic nephropathy with uremia via Toll-like
receptor 4 and nuclear factor-κB p65. Mol Med Rep. 12:8215–8222.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lockshin RA and Zakeri Z:
Caspase-independent cell deaths. Curr Opin Cell Biol. 14:727–733.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Takagi H, Matsui Y, Hirotani S, Sakoda H,
Asano T and Sadoshima J: AMPK mediates autophagy during myocardial
ischemia in vivo. Autophagy. 3:405–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Maiuri MC, Tasdemir E, Criollo A, Morselli
E, Vicencio JM, Carnuccio R and Kroemer G: Control of autophagy by
oncogenes and tumor suppressor genes. Cell Death Differ. 16:87–93.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lisse TS and Hewison M: Vitamin D: A new
player in the world of mTOR signaling. Cell Cycle. 10:1888–1889.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Peponi E, Drakos E, Reyes G, Leventaki V,
Rassidakis GZ and Medeiros LJ: Activation of mammalian target of
rapamycin signaling promotes cell cycle progression and protects
cells from apoptosis in mantle cell lymphoma. Am J Pathol.
169:2171–2180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rudelius M, Pittaluga S, Nishizuka S, Pham
TH, Fend F, Jaffe ES, Quintanilla-Martinez L and Raffeld M:
Constitutive activation of Akt contributes to the pathogenesis and
survival of mantle cell lymphoma. Blood. 108:1668–1676. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hasselblom S, Hansson U, Olsson M, Torén
L, Bergström A, Nilsson-Ehle H and Andersson PO: High
immunohistochemical expression of p-AKT predicts inferior survival
in patients with diffuse large B-cell lymphoma treated with
immunochemotherapy. Br J Haematol. 149:560–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Maksimovic-Ivanic D, Fagone P, McCubrey J,
Bendtzen K, Mijatovic S and Nicoletti F: HIV-protease inhibitors
for the treatment of cancer: Repositioning HIV protease inhibitors
while developing more potent NO-hybridized derivatives? Int J
Cancer. 140:1713–1726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rothweiler F, Michaelis M, Brauer P, Otte
J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, et
al: Anticancer effects of the nitric oxide-modified saquinavir
derivative saquinavir-NO against multidrug-resistant cancer cells.
Neoplasia. 12:1023–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Maksimovic-Ivanic D, Mojic M, Bulatovic M,
Radojkovic M, Kuzmanovic M, Ristic S, Stosic-Grujicic S, Miljkovic
D, Cavalli E, Libra M, et al: The NO-modified HIV protease
inhibitor as a valuable drug for hematological malignancies: Role
of p70S6K. Leuk Res. 39:1088–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pearce LR, Alton GR, Richter DT, Kath JC,
Lingardo L, Chapman J, Hwang C and Alessi DR: Characterization of
PF-4708671, a novel and highly specific inhibitor of p70 ribosomal
S6 kinase (S6K1). Biochem J. 431:245–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Guo LS, Li HX, Li CY, Zhang SY, Chen J,
Wang QL, Gao JM, Liang JQ, Gao MT and Wu YJ: Synergistic antitumor
activity of vitamin D3 combined with metformin in human breast
carcinoma MDA-MB-231 cells involves m-TOR related signaling
pathways. Pharmazie. 70:117–122. 2015.PubMed/NCBI
|
|
79
|
Yang J, Ikezoe T, Nishioka C, Ni L,
Koeffler HP and Yokoyama A: Inhibition of mTORC1 by RAD001
(everolimus) potentiates the effects of 1,25-dihydroxyvitamin D(3)
to induce growth arrest and differentiation of AML cells in vitro
and in vivo. Exp Hematol. 38:666–676. 2010. View Article : Google Scholar : PubMed/NCBI
|

















