Introduction
Allergic rhinitis (AR) is a non-infectious nasal
mucosal disease caused by the exposure of susceptible individuals
to exogenous allergens and the release of IgE-mediated histamine
and other mediators, and it is accompanied by immune cell
activation and cytokine secretion (1). As a common otolaryngology disease,
the incidence of AR has increased greatly in the past decades
(2). AR has become a global public
health problem and caused huge economic burden to individuals,
families and society. At present, the treatment of AR mainly
includes avoidance of contact with susceptible allergens,
anti-inflammation, antihistamine and other symptomatic treatments,
which are far from satisfactory. Allergen-specific immunotherapy
(AIT) is the only WHO approved aetiological treatment for allergic
diseases that can effectively change the natural process of AR
(3). At present, the clinically
used desensitization agents are mainly from allergen crude
extracts, which contain complex components, resulting in poor
stability, difficulty in standardization, and serious side effects
(4). Therefore, it is particularly
important to develop new, safe and efficient methods for the
prevention and treatment of AR.
The dust mite is an important allergen of AR, and
studies have revealed that approximately 70–80% of patients with
upper respiratory tract allergy have positive reactions to dust
mites, among which Der f2 and Der p2 have the highest serum
positive rate (5,6). A20, also known as tumour necrosis
factor-induced protein 3 (TNFAIP3), is a ubiquitin-modified enzyme
protein encoded by the TNFAIP3 gene in the cytoplasm that regulates
a variety of immune cell functions and is involved in maintaining
immune homeostasis (7). Mutations
in the A20 gene are associated with inflammation, allergic disease
and tumourigenesis (8). Mice with
A20 gene deletion have severe inflammation and tissue damage, and
A20 overexpression improves the tolerance of intestinal mucosa to
LPS and enhances the intestinal mucosal barrier function. A20 plays
an important role in the pathogenesis of allergic diseases, and its
absence can lead to the occurrence of allergic asthma (8).
Studies have revealed that a DNA vaccine displays
not only immunogenicity and safety but also greater flexibility
than previous protein vaccines for its convenient modification and
construction (9). Compared with
the AIT and recombinant allergen vaccines, a DNA vaccine induces
Th1 cell reaction, reduces allergen-specific IgE, and increases the
concentration of IgG2a by endogenous expression of allergens
(10), thus becoming a promising
strategy for AR-specific immune therapy. Further studies have
indicated that the combination of an allergen gene with a
regulatory molecule through genetic engineering technology improves
the therapeutic effect of a DNA vaccine. The fusion of allergen
ovalbumin with heat shock protein (Hsp65) has been revealed to
attenuate an established Th2 upper respiratory tract allergic
inflammation in mice (10), and a
fusion DNA vaccine constructed by malaria antigen and chemokine
MIP3 CCL20 has been revealed to effectively prevent the infection
of malaria parasites (11). In the
present study, a DNA vaccine co-expressing Der p2 and A20 was
constructed and encapsulated into PLGA nanoparticles, and its
specific immunotherapeutic effect on allergic inflammation in the
Der p2-induced AR mouse model and potential mechanism were
evaluated.
Materials and methods
Reagents
Biotin-labelled sheep anti-mouse IgE antibody (cat.
no. 1110-08) was purchased from Southern Biotech. HRP-labelled
sheep anti-mouse IgG1 antibody (cat. no. A10551) and HRP-labelled
sheep anti-mouse IgG2a antibody (cat. no. A10685) was purchased
from Life Technologies; Thermo Fisher Scientific, Inc. HRP-labelled
streptavidin (cat. no. A0303) was obtained from Beyotime Institute
of Biotechnology. Mouse IL-4, IL-10, IL-13, TNF-α, IFN-γ and TGF-β
ELISA Kits were purchased from R&D Systems. Cholera toxin and
poly (lactic-co-glycolic acid) were purchased from SigmaAldrich;
Merck KGaA. APC rat anti-mouse CD4 antibody (cat. no. 553051), FITC
rat anti-mouse CD25 antibody (cat. no. 558689) and PE rat
anti-mouse Foxp3 (cat. no. 560408) were obtained from BD
Pharmingen; BD Biosciences. Anti-A20 antibody (product code
ab74037) was purchased from Abcam, and anti-p65 antibody (product
no. 3033S) and anti-β-actin antibody (4967S) were obtained from
Cell Signaling Technology. The 293T cell line (CBP60439) was
obtained from Cobioer.
Construction of DNA vaccine
For construction of the DNA vaccine co-expressing
Der p2 and A20 (pVAX1-Der p2-A20), the coding sequence of Der p2
(FM177223.1) and TNFAIP3 (GenBank: KJ892292.1) were synthesized by
GeneCreate Biotech and inserted into the pVAX1 vector. The mCherry
gene sequence, which is a fluorescent protein derived from the
tetrameric Discosoma sp., was amplified by polymerase chain
reaction (PCR) from the pmCherry-N1 vector (presented by Jinan
University, China) and subcloned into pVAX1-Der p2-A20 to establish
the pVAX1-mCherry-Der p2-A20 expression vector. The recombinant
pVAX1-Der p2-A20 and pVAX1-mCherry-Der p2-A20 expression vectors
were encapsulated into poly(L-lactide-co-glycolide) (PLGA)
(Sigma-Aldrich; Merck KGaA) to form nanoparticles via the emulsion
method before intranasal administration (Fig. 1). In addition, pET-32a-Der p2 was
also constructed for recombinant Der p2 expression and purification
(>95%). The coding sequence of the Der p2 from Genebank was
synthesized and inserted into pET-32a vector (Thermo Fisher
Scientific, Inc.). Then the recombinant vector was transfected into
BL21 Escherichia coli for Der p2 expression, purification
and identification (data not shown). For evaluation of the
transfection effect of constructed DNA vaccine in vitro, the
293T cell line was cultured and transfected in DMEM (product no.
10-013-CVRC; Corning Incorporated) supplemented with 10% fetal
bovine serum (Sigma-Aldrich; Merck KGaA) and
penicillin-streptomycin (Corning Incorporated) in a 125 ml
polycarbonate flask (Corning Incorporated) under the following
conditions: 37°C, 5% CO2 and 80% humidity.
Mice
In total, 30 female BALB/c mice (6–8 weeks old) were
purchased from Guangzhou Experimental Animal Centre and were housed
under pathogen-free conditions. The experimental procedures were
conducted in accordance with the approval and guidelines of the
Institutional Animal Care and Use Committee of the ENT Institute of
Shenzhen (no. 201702013). Animal suffering and the number of
animals used in this study were minimized. Mice were acclimated for
7 days before experiments and were monitored daily for signs of
distress and advanced nasal inflammation during experiments.
Induction of nasal allergic
inflammation in mice
The allergic rhinitis (AR) murine model was
performed according to other and our previous procedures (12–14).
Mice were sensitized with recombinant Der p2 (10 µg) and cholera
toxin (CT, 1 µg/mouse) diluted in sterile normal saline (0.1
ml/mouse) three times by intraperitoneal injection (ip) on days 0,
7 and 14. The sensitized mice in different groups (n=6 per group)
were intranasally treated as follows (20 µl/mouse) every 3 days for
a total of 5 treatments from day 21: Sterile normal saline, AR
group; pVAX1-Der p2-A20 nanoparticles (100 µg), PpDA group; blank
PLGA treatment (100 µg), PLGA group; and naked pVAX1-Der p2-A20
(100 µg; no encapsulation), pDA group. Mice were intranasally
challenged three times daily with recombinant Der p2 (10 µg; 20 µl
of 0.5 mg/ml Der p2) after the final treatment. Control groups
(Con) were treated with normal saline by ip and nasal challenge.
The frequencies of nose scratching and sneezing in each group were
recorded within 30 min after the last challenge. Twenty-four hours
after the last challenge, mice were sacrificed by cervical
dislocation under 4% isoflurane (Youcheng Biotech) anesthesia.
Samples were collected from each mouse for further evaluation
(Fig. 2).
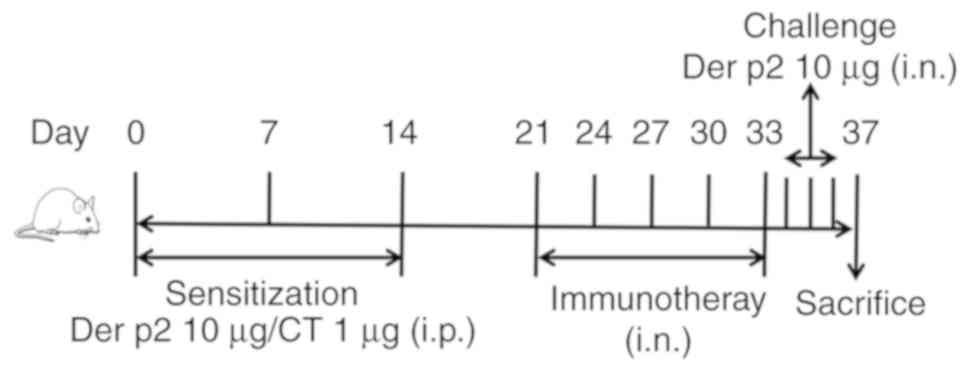 | Figure 2.Schematic outline of allergic
rhinitis induction and treatment. BALB/c mice were sensitized with
recombinant Der p2 and CT three times by ip on days 0, 7 and 14.
The sensitized mice in different groups (n=6 per group) were then
intranasally treated every 3 days for a total of 5 treatments from
day 21 as follows: Sterile normal saline in the AR group, pVAX1-Der
p2-A20 nanoparticles in the PpDA group (100 µg, pVAX1-Der p2-A20),
blank PLGA treatment in the PLGA group (100 µg, PLGA) and naked
pVAX1-Der p2-A20 treatment in the pDA group (100 µg, pVAX1-Der
p2-A20 without being encapsulated into PLGA). Mice were
intranasally challenged three times daily with recombinant Der p2
(10 µg) after the final treatment. The Con group was treated with
normal saline by ip and nasal challenge. CT, cholera toxin; ip,
intraperitoneal injection; Con, control; AR, allergic rhinitis;
PLGA, poly(L-lactide-co-glycolide). |
Symptom scores
The frequencies of nose scratching and sneezing in
each group were counted within 30 min after the last intranasal
challenge by recombinant Der p2 to evaluate the symptom scores
according to a reported procedure (15).
Histology
The nasal mucosa was collected and fixed in 4%
paraformaldehyde overnight and processed for paraffin embedding.
Sections (4 µm thick) were prepared and stained with haematoxylin
and eosin (H&E). Images were obtained using a microscope (Nikon
Corporation; magnification of ×200) for detection of inflammatory
cell infiltration.
Enzyme-linked immunosorbent assay
(ELISA)
Mouse sera from each group were collected after the
last challenge. Serum concentrations of Der p2-specific antibodies
including IgE (cat. no. 1110-08), IgG1(cat. no. A10551) from
Southern Biotech, IgG2a (cat. no. A10685; Life Technologies; Thermo
Fisher Scientific, Inc.), and cytokines including IL-4 (cat. no.
M4000B), IL-10 (cat. no. M1000B), IL-13 (cat. no. M1300CB), IFN-γ
(cat. no. MIF00) and TGF-β1 (cat. no. MB100B) were detected using
ELISA kits (R&D Systems) following the manufacturers'
instructions.
Flow cytometry
Splenic mononuclear cells were collected from
culture, fixed with 1% formaldehyde and permeabilization buffer
(0.1% Triton X-100) for 30 min at 4°C, washed three times with 1%
bovine serum albumin (BSA)/PBS and blocked for 30 min at 4°C with
1% BSA. Cells were incubated with APC-conjugated rat anti-mouse
CD4, FITC-conjugated rat anti-mouse CD25 and PE-conjugated rat
anti-mouse Foxp3 antibodies or isotype IgG for 1 h at room
temperature. After washing with PBS, cells were analysed with a
flow cytometer (FACSCanto II; BD Biosciences) and FlowJo V10
software (FlowJo, LLC).
Statistical analysis
SPSS 13.0 software (SPSS, Inc.) was used for
statistical analysis. All values are presented as the mean ±
standard deviation (SD) of a minimum of three independent
experiments. Data were analysed using one-way analysis of variance
followed by Tukey's post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Der p2 and A20 DNA vaccine
construction and in vitro transfection identification
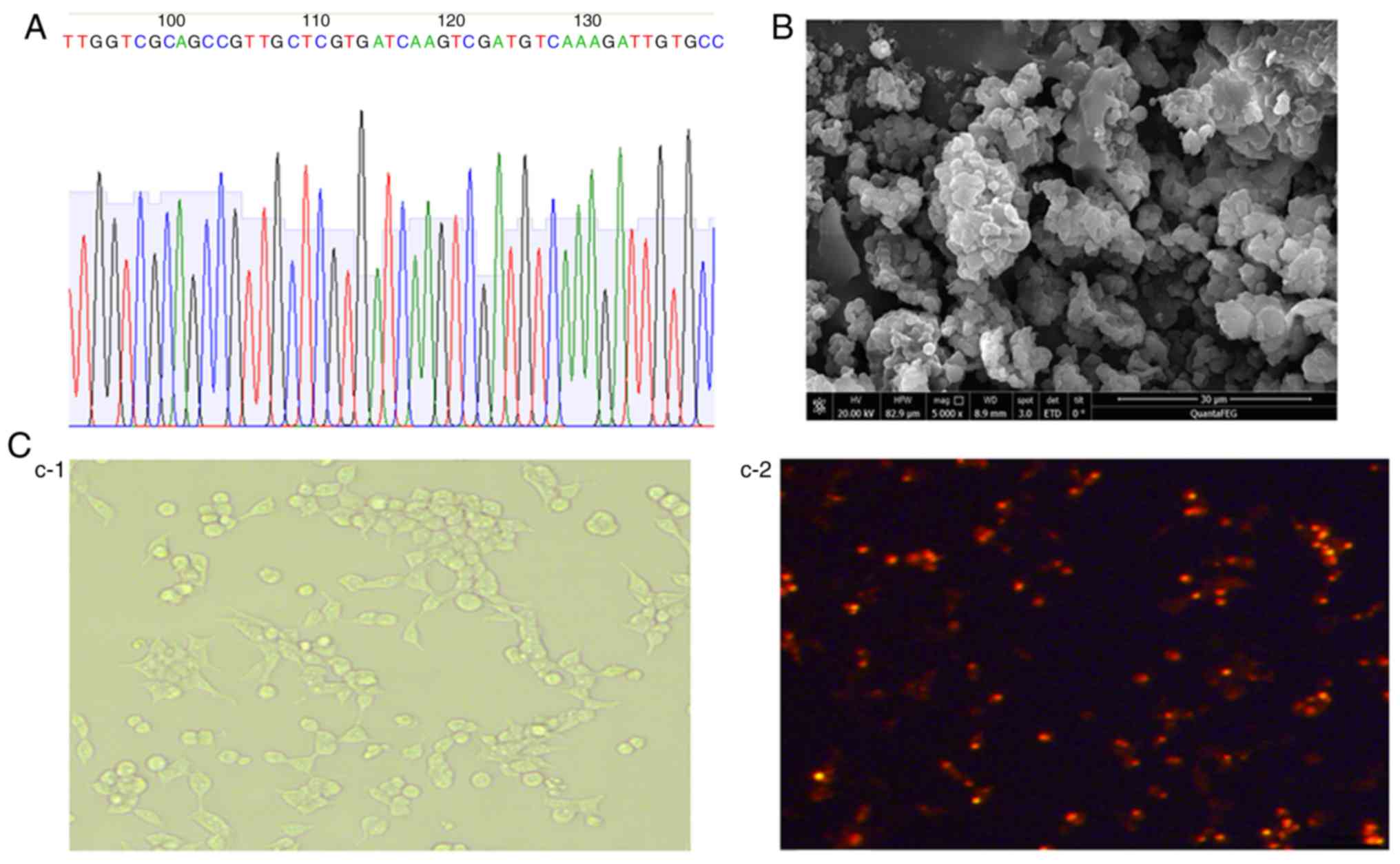
The coding sequences of Der p2 [437 base pairs (bp)]
and A20 (2,373 bp) were cloned into pVAX1 (3,000 bp). The
constructed recombinant pVAX1-Der p2-A20 vector was confirmed by
plasmid sequencing (Fig. 1A). The
pVAX1-Der p2-A20 vector was then encapsulated into poly
L-lactide-co-glycolide (PLGA) after construction to form a
nanoparticle (Fig. 1B). To
evaluate the transfection effect of the DNA vaccine, a
pVAX1-mCherry-Der p2-A20 vector was also constructed by subcloning
the mCherry-coding gene into pVAX1-Der p2-A20. The transfection
effect of pVAX1-mCherry-Der p2-A20 in vitro and the
expression of Der p2-A20-mCherry fusion protein in the 293T cell
line were evaluated. Obvious red fluorescence was observed in the
293T cells after transfection with pVAX1-mCherry-Der p2-A20 for 48
h (Fig. 1C). This result indicated
that pVAX1-Der p2-A20 was successfully constructed and efficiently
transfected into 293T cells for fusion protein expression.
Der p2-A20 DNA vaccine inhibits Der
p2-induced nasal allergic inflammation
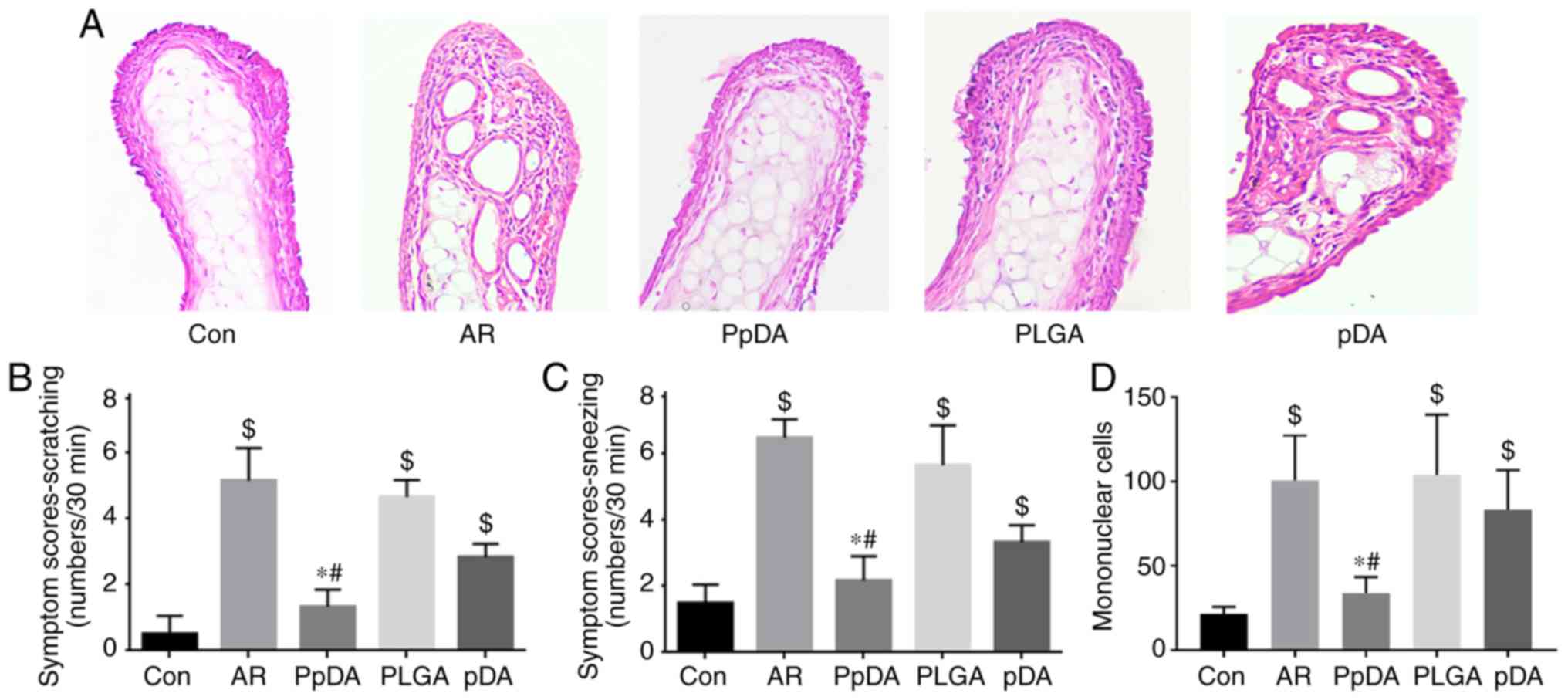
A mouse model with AR was established, and the mice
were treated with the Der p2-A20 DNA vaccine (Fig. 2). The results revealed that mice in
the AR group had more denuded skin around the nose, increased
scratching and sneezing frequencies (Fig. 3B and C), mononuclear cell
infiltration in the nasal mucosa (Fig.
3A and D), and increased serum Der p2 specific IgE, IL-4 and
IL-13 levels (Fig. 4) compared
with mice in the control group. Der p2-A20 DNA vaccine
administration in the PpDA group significantly reduced the
scratching and sneezing events compared with administration in the
AR and PLGA treatment groups (Fig. 3B
and C). Histopathologic analysis of the AR group revealed
evident nasal mucosal inflammation and increased mononuclear cells,
and the Der p2-A20 DNA vaccine significantly inhibited the allergic
inflammation compared with analysis of the AR and PLGA groups
(Fig. 3A and D). Der p2-A20 DNA
vaccine treatment in the PpDA group decreased scratching events,
decreased sneezing events and inhibited nasal inflammation more
efficiently than treatment with the naked plasmid DNA in the pDA
group. These results indicated that the mouse model with AR was
established successfully, and the Der p2-A20 DNA vaccine inhibits
Der p2-induced nasal allergic inflammation.
Der p2-A20 DNA vaccine modulates the
serum levels of cytokines and antibodies involved in nasal allergic
inflammation
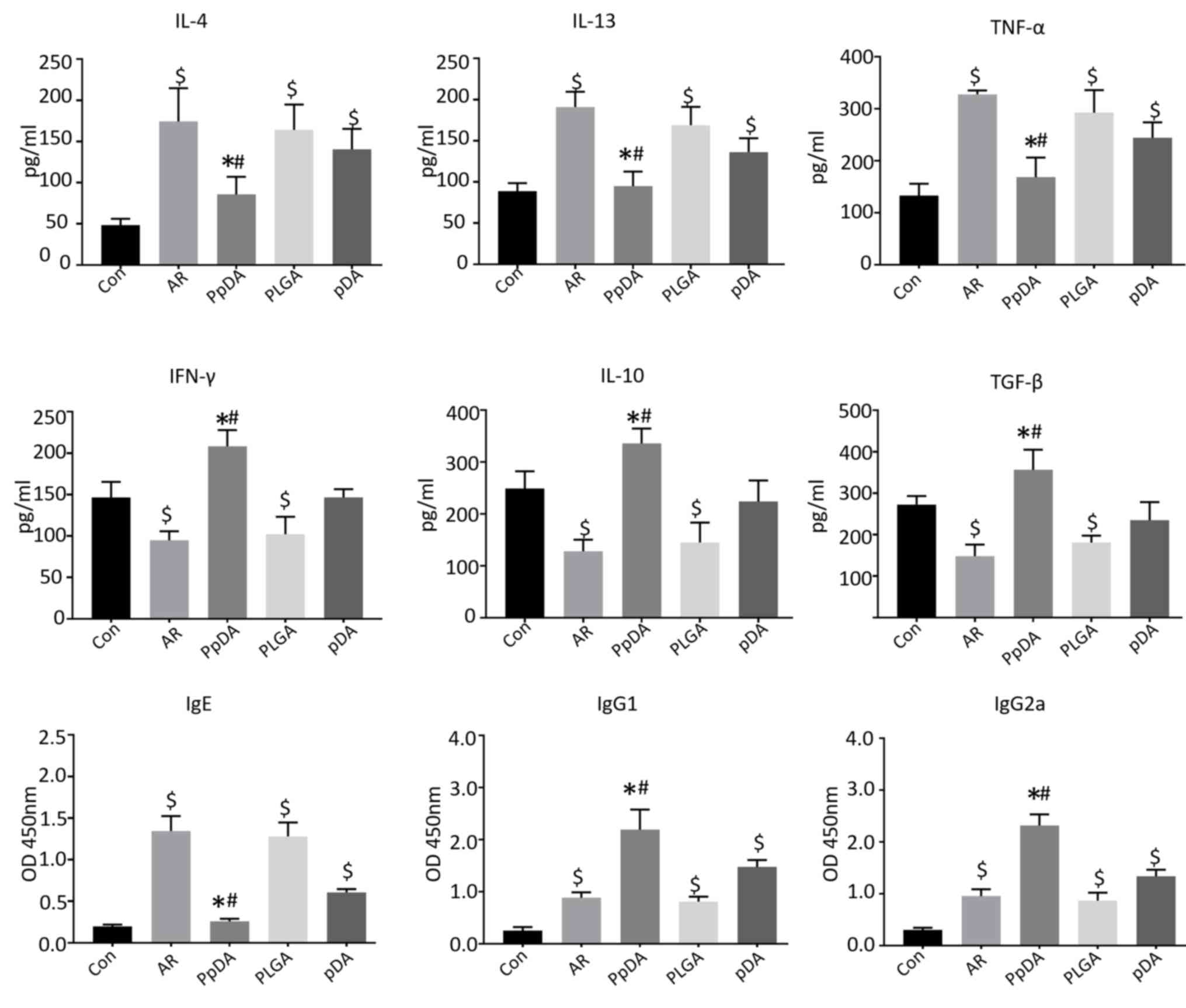
The serum levels of IL-4, IL-13, and TNF-α were
increased while the levels of IL-10, IFN-γ and TGF-β were decreased
in the AR group compared with those in the control group after the
Der p2 challenge (Fig. 4).
Intranasal treatment with the Der p2-A20 DNA vaccine suppressed
IL-4, IL-13 and TNF-α secretion but improved the levels of IL-10,
IFN-γ and TGF-β expression in the serum compared with the AR group
(Fig. 4). Moreover, serum Der
p2-specific IgE (sIgE), sIgG1 and sIgG2a antibodies were also
evaluated. sIgE was significantlyy increased, whereas sIgG1 and
sIgG2a levels were increased slightly in the AR group compared with
those in the control group. The DNA vaccine enhanced sIgG1 and
sIgG2a expression but decreased sIgE levels compared with the AR or
pDA group (Fig. 4). These results
indicated that the Der p2-A20 DNA vaccine may regulate Th1/Th2
cytokine expression and increase IL-10, TGF-β, sIgG1 and sIgG2a
neutralizing antibody levels.
Der p2-A20 DNA vaccine increases
splenic Treg population
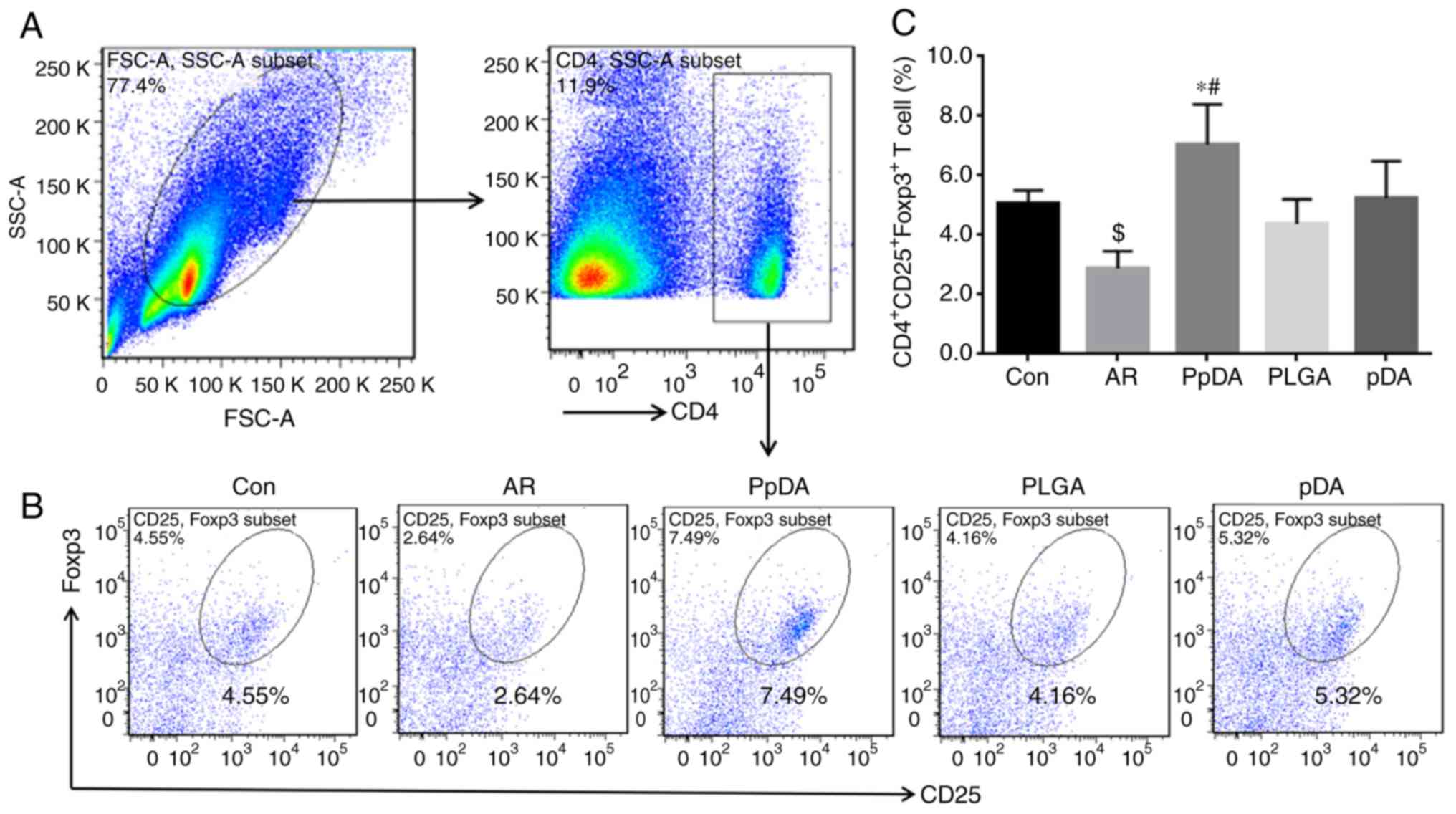
To elucidate whether the inhibitory effects of the
Der p2-A20 DNA vaccine on nasal inflammation in mice with allergic
rhinitis were related to the induction of Tregs, the Treg
population in the splenic CD4+T cells was further
analysed by flow cytometry (Fig.
5A). The results revealed that the frequency of
CD4+CD25+Foxp3+T cells in splenic
mononuclear cells was decreased in the AR group (2.64%) compared
with that in the control group (4.55%), while this frequency was
increased in the PpDA group (7.49%) after DNA vaccine treatment
(Fig. 5B and C). These results
indicated that the Der p2-A20 DNA vaccine promotes Treg
proliferation in mice with allergic rhinitis.
Discussion
As a common and frequently occurring disease in
clinical practice, AR is a type of IgE-mediated non-infectious
inflammation characterized by Th2 cell polarization (16). Currently, the symptomatic treatment
based on histamine and other anti-inflammatory drugs cannot achieve
a satisfactory therapeutic effect. The present study revealed that
intranasal administration of the Der p2-A20 DNA vaccine markedly
ameliorated Der p2-induced nasal mucosal proinflammatory cell
infiltration. Serum Der p2-specific IgE, IL-4 and IL-13 expression
levels were decreased, while serum Der p2-specific IgG1, IgG2a,
IFN-γ, IL-10 and TGF-β expression levels as well as the splenic
CD4+CD25+Foxp3+Treg population
were significantly increased after Der p2-A20 DNA vaccine
treatment. These results indicated that the Der p2-A20 DNA vaccine
alleviates nasal allergic inflammation in mice with allergic
rhinitis and promotes splenic Treg population. Therefore, DNA
vaccine is a promising new direction for prevention and treatment
of various pathogens. Compared with traditional protein-based
vaccines, DNA vaccines are more stable, inexpensive, and can be
conveniently produced. Biomaterial-based delivery systems that
encapsulate plasmid DNA represent a promising strategy that can
increase DNA vaccine internalization, transfection efficiency and
mucosal uptake (10). Genetic
immunization with a non-viral vector DNA vaccine has been employed
in a broad range of therapeutic applications, including infectious
diseases, cancers and other diseases (17,18).
Treatment strategies using plasmid DNA vaccine-encoding allergens
in allergic diseases have been revealed to induce Th1 cell
response, IFN-γ production and IgG2a production as well as to
reduce the desensitization-induced IgE production and anaphylactic
reactions compared with crude extracts used in allergen-specific
immunotherapy (19,20). CryJ-LAMP DNA vaccines co-expressing
CryJ1 or CryJ2 from Japanese red cedar combined with
lysosomal-associated membrane protein-1 (LAMP-1) were revealed to
induce robust Th1-type immune responses as well as IgG2a and IgG1
antibody secretion in an allergic murine model (21). DNA-encoding Der p2 prevented the
development of house dust mite-induced respiratory allergies by
enhancing the Th1 cell immune response, reducing allergen-specific
IgE, reducing IL-5, inducing IgG2a and inducing IFN-γ (22). As a negative regulator of NF-κB,
A20 not only inhibited the activity of NF-κB but also further
blocked the release of various inflammatory factors induced by
NF-κB (23). Loss of A20 in lung
epithelium abolished the protective effect of farm dust and
endotoxin to allergy and asthma (8), and deficiency of A20 promoted antigen
transportation across airway epithelial cells (24). Consistent with findings of other
studies, the present results revealed that intranasal
administration of the DNA vaccine co-expressing an antigen and a
regulatory molecular A20 ameliorated Der p2-induced nasal mucosal
allergic inflammation. NF-κB is a key transcription factor for Th2
cell differentiation, and its continuous activation is involved in
the occurrence and development of airway allergic diseases
(25). Furthermore, in our
experiments, DNA vaccine treatment suppressed serum Der p2-specific
IgE secretion and Th2-type cytokine (IL-4 and IL-13) expression.
Neutralizing IgG subclass antibodies can inhibit IgE-mediated
inflammatory responses by competing with IgE (26). IgG1 constitutes approximately 65%
of total IgG, and IgG2 constitutes approximately 22% of total IgG.
However, the contribution of allergen-specific IgG to the
development of allergic inflammation is controversial (27). Data from murine experiments
revealed that Th1 cells promoted IgG1 and IgG2a production by
secreting IFN-γ and that Th2 cells promoted IgG4 production
(26). Allergen immunotherapy
increased protective IgG1 and IgA antibodies (27,28).
The results of the present study revealed that IgG1 and IgG2a
production induced by DNA vaccine intranasal immunization may play
a protective role in nasal allergic inflammation. Eosinophilia and
mononuclear cell infiltration in the nasal mucosa are important in
characterizing allergic rhinitis. The limitation of the present
study is that we did not analyze eosinophilia since the amount of
available nasal mucosa was not sufficient.
Regulatory T cells (Tregs) are negative regulatory
cells of the immune response and are involved in the regulation of
a variety of immune responses, including allergy, autoimmunity and
graft-versus-host response (29).
A previous study revealed that peripheral blood
CD4+CD25highFoxp3+Tregs were
decreased in patients with mild asthma compared with those in
non-asthmatic controls (30).
Animal experiments have revealed that removing Tregs from mice
before sensitization aggravates airway inflammation and airway
hyperresponsiveness (AHR) (31).
Tregs inhibit effector T cells and regulate allergic diseases by
secreting IL-10 and TGF-β, leading to reduced production of Th2
cytokines (IL-4, IL-5 and IL-13) (32). An approach to enhance the
anti-allergic efficacy of therapeutic DNA vaccines was performed by
fusion of a modulating cytokine such as GM-CSF, IFN-γ, IL-1β or an
immunosuppressive molecule to the cDNA of a certain allergen
(10), thus driving the immune
response toward a Th1 direction. Intranasal immunization of DNA
vaccine co-expressing Der p1 and ubiquitin elicited a Th1 type
response, lower level of specific IgE and increased IgG in an
allergic rhinitis mouse model (14). In the present study, fusion of
NF-κB signaling negative regulator (A20) with Der p2 not only
induced a Th1 response, but also significantly increased serum
levels of IL-10 and TGF-β. In addition, the
CD4+CD25+Foxp3+Treg population in
the spleen was also significantly increased after Der p2-A20 DNA
vaccine treatment, indicating that the DNA vaccine may inhibit the
inflammatory response of AR by promoting Tregs. In the present
experiment, the splenic Treg population was evaluated in each group
according to published studies, because we could not collect enough
cells from the nasal mucosa for flow cytometric analysis.
In conclusion, allergic rhinitis is a common disease
that requires more convenient, safe and effective antigen-specific
immunotherapies. A DNA vaccine co-expressing Der p2 and A20 was
successfully constructed. Intranasal administration of this DNA
vaccine greatly ameliorated Der p2-induced nasal mucosal
proinflammatory cell infiltration by inhibiting specific IgE, IL-4
and IL-13 secretion and by increasing IgG1, IgG2a, IFN-γ, IL-10 and
TGF-β expression as well as the
CD4+CD25+Foxp3+Treg
population.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grants from
the Natural Science Foundation of China (grant nos. 81700888,
81773978, 81870706) and the Innovation of Science and Technology
Commission of Shenzhen Municipality (grant nos.
JCYJ20160429091935720, JCYJ20170302165727389, JCYJ20170412103841386
and ZDSYS201506050935272).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH, LM, TH, XZ, JL, BC and GY performed experiments
and analyzed the data. HZ collected the data and revised the
manuscript. ZL organized the study, supervised the experiments,
designed the study and wrote the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experiments of the present study were approved
by the local Ethics Committee and comply with the current Chinese
laws and are in accordance with the Declaration of Shenzhen. The
experimental procedures were conducted in accordance with the
approval and guidance of the Institutional Animal Care Use
Committee of the ENT Institute of Shenzhen.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Small P, Keith PK and Kim H: Allergic
rhinitis. Allergy Asthma Clin Immunol. 14:512018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y and Zhang L: Increasing prevalence
of allergic rhinitis in china. Allergy Asthma Immunol Res.
11:156–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffmann HJ, Valovirta E, Pfaar O,
Moingeon P, Schmid JM, Skaarup SH, Cardell LO, Simonsen K, Larché
M, Durham SR and Sørensen P: Novel approaches and perspectives in
allergen immunotherapy. Allergy. 72:1022–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jutel M, Kosowska A and Smolinska S:
Allergen immunotherapy: Past, present, and future. Allergy Asthma
Immunol Res. 8:191–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui Y, Wang Q and Jia H: Consideration of
methods for identifying mite allergens. Clin Transl Allergy.
8:142018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller JD: The role of dust mites in
allergy. Clin Rev Allergy Immunol. Jun 23–2018.(Epub ahead of
print). PubMed/NCBI
|
|
7
|
Reihill JA, Malcomson B, Bertelsen A,
Cheung S, Czerwiec A, Barsden R, Elborn JS, Dürkop H, Hirsch B,
Ennis M, et al: Induction of the inflammatory regulator A20 by
gibberellic acid in airway epithelial cells. Br J Pharmacol.
173:778–789. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schuijs MJ, Willart MA, Vergote K, Gras D,
Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, et
al: Farm dust and endotoxin protect against allergy through A20
induction in lung epithelial cells. Science. 349:1106–1110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tulic MK: Allergen-free immunotherapy
using DNA vaccines in treatment of established allergic disease.
Clin Exp Allergy. 42:3–4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiss R, Hammerl P, Hartl A, Hochreiter R,
Leitner WW, Scheiblhofer S and Thalhamer J: Design of protective
and therapeutic DNA vaccines for the treatment of allergic
diseases. Curr Drug Targets Inflamm Allergy. 4:585–597. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fonseca DM, Wowk PF, Paula MO, Campos LW,
Gembre AF, Turato WM, Ramos SG, Dias-Baruffi M, Barboza R, Gomes E,
et al: Recombinant DNA immunotherapy ameliorate established airway
allergy in a IL-10 dependent pathway. Clin Exp Allergy. 42:131–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu T, Fan X, Ma L, Liu J, Chang Y, Yang P,
Qiu S, Chen T, Yang L and Liu Z: TIM4-TIM1 interaction modulates
Th2 pattern inflammation through enhancing SIRT1 expression. Int J
Mol Med. 40:1504–1510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wikstrom ME, Batanero E, Smith M, Thomas
JA, von Garnier C, Holt PG and Stumbles PA: Influence of mucosal
adjuvants on antigen pass age and CD4+ T cell activation during the
primary response to airborne allergen. J Immunol. 177:913–924.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ou J, Shi W, Xu Y and Tao Z: Intranasal
immunization with DNA vaccine coexpressing Der p 1 and ubiquitin in
an allergic rhinitis mouse model. Ann Allergy Asthma Immunol.
113:658–665.e651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Tao ZZ, Zhou XH, Wu TT and Ye LF:
Immunosuppressive effect of sinomenine in an allergic rhinitis
mouse model. Exp Ther Med. 13:2405–2410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rothenberg ME, Saito H and Peebles RS Jr:
Advances in mechanisms of allergic disease in 2016. J Allergy Clin
Immunol. 140:1622–1631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gholami E, Oliveira F, Taheri T, Seyed N,
Gharibzadeh S, Gholami N, Mizbani A, Zali F, Habibzadeh S, Bakhadj
DO, et al: DNA plasmid coding for Phlebotomus sergenti salivary
protein PsSP9, a member of the SP15 family of proteins, protects
against Leishmania tropica. PLoS Negl Trop Dis. 13:e00070672019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asbach B, Kibler KV, Kostler J, Perdiguero
B, Yates NL, Stanfield-Oakley S, Tomaras GD, Kao SF, Foulds KE,
Roederer M, et al: Priming with a potent HIV-1 DNA vaccine frames
the quality of immune responses prior to a poxvirus and protein
boost. J Virol. 93:e015292019.PubMed/NCBI
|
|
19
|
Dantzer JA and Wood RA: Next-generation
approaches for the treatment of food allergy. Curr Allergy Asthma
Rep. 19:52019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scheiblhofer S, Thalhamer J and Weiss R:
DNA and mRNA vaccination against allergies. Pediatr Allergy
Immunol. 29:679–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su Y, Connolly M, Marketon A and Heiland
T: CryJ-LAMP DNA vaccines for Japanese red cedar allergy induce
robust Th1-Type immune responses in murine model. J Immunol Res.
2016:48578692016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pulsawat P, Pitakpolrat P, Prompetchara E,
Kaewamatawong T, Techakriengkrai N, Sirivichayakul S,
Buranapraditkun S, Hannaman D, Ruxrungtham K and Jacquet A:
Optimization of a Der p 2-based prophylactic DNA vaccine against
house dust mite allergy. Immunol Lett. 151:23–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Das T, Chen Z, Hendriks RW and Kool M:
A20/tumor necrosis factor α-induced protein 3 in immune cells
controls development of autoinflammation and autoimmunity: Lessons
from mouse models. Front Immunol. 9:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li TL, Zhang SY, Du YC and Yang PC:
Deficiency of ubiquitin A20 promotes antigen transport across
airway epithelial cells via a transcellular pathway. Anal Biochem.
433:86–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kelly C, Shields MD, Elborn JS and Schock
BC: A20 regulation of nuclear factor-κB: Perspectives for
inflammatory lung disease. Am J Respir Cell Mol Biol. 44:743–748.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scott-Taylor TH, Axinia SC, Amin S and
Pettengell R: Immunoglobulin G; structure and functional
implications of different subclass modifications in initiation and
resolution of allergy. Immun Inflamm Dis. 6:13–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams JW, Tjota MY and Sperling AI: The
contribution of allergen-specific IgG to the development of
th2-mediated airway inflammation. J Allergy (Cairo).
2012:2360752012.PubMed/NCBI
|
|
28
|
Wisniewski J, Agrawal R and Woodfolk JA:
Mechanisms of tolerance induction in allergic disease: Integrating
current and emerging concepts. Clin Exp Allergy. 43:164–176. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maloy KJ and Powrie F: Regulatory T cells
in the control of immune pathology. Nat Immunol. 2:816–822. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baatjes AJ, Smith SG, Watson R, Howie K,
Murphy D, Larché M, Denburg JA, Inman MD and O'Byrne PM: T
regulatory cell phenotypes in peripheral blood and bronchoalveolar
lavage from non-asthmatic and asthmatic subjects. Clin Exp Allergy.
45:1654–1662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewkowich IP, Herman NS, Schleifer KW,
Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid
Y and Wills-Karp M: CD4+CD25+ T cells protect
against experimentally induced asthma and alter pulmonary dendritic
cell phenotype and function. J Exp Med. 202:1549–1561. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaisri U, Tungtrongchitr A, Indrawattana
N, Meechan P, Phurttikul W, Tasaniyananda N, Saelim N, Chaicumpa W
and Sookrung N: Immunotherapeutic efficacy of liposome-encapsulated
refined allergen vaccines against Dermatophagoides
pteronyssinus allergy. PLoS One. 12:e01886272017. View Article : Google Scholar : PubMed/NCBI
|