Introduction
A balance between bone formation and resorption
maintains the integrity of skeleton, and such a balance is realized
by osteoclasts and osteoblasts (1,2).
Osteoblast differentiation stimulates bone formation and repair
(3). Therefore, osteogenic
differentiation has a connection with bone disease (bone fracture
and osteoporosis) and bone implantation (4–7). The
differentiation of bone marrow mesenchymal stem cells (BMSCs)
involves a number of signaling pathways, including the
hypoxia-inducible factor 1α pathway, mechano-growth factor
signaling, the leukemia inhibitory factor/STAT/suppressor of
cytokine signaling 3 pathway and NF-κB signaling (8–10).
MicroRNAs (miRNAs), small non-protein coding RNAs
(~22 nucleotides), serve important roles in the regulation of gene
expression by binding to the 3′-untranslated region (3′-UTR) of
target messenger RNAs (mRNAs) (11,12).
Located at cancer-related gene regions 9q21.1-q22.3 (13), a previous study reported that
miR-204 regulated cancer cell proliferation and invasion by
targeting cyclin D2 and matrix metalloproteinase-9 (14). In addition, prior evidence
suggested that miR-204 overexpression inhibited osteogenic
differentiation and promoted adipogenic differentiation (14,15);
however, the main mechanisms underlying the effects of miR-204 on
BMSCs are yet to be determined. The present study investigated the
role of the Runt-related transcription factor 2/alkaline
phosphatase/bone morphogenic protein 2 (Runx2/ALP/BMP2) signaling
pathway in the effects of miR-204 on BMSCs.
It was previously demonstrated that increased Runx2
levels were observed in mesenchymal stem cells derived from
multiple myeloma patients compared with normal MSCs, and that
upregulation of Runx2 resulted in bone defects (16). ALP is a glycoprotein involved in
mineral formation in bone tissue; ALP activity has a positive
effect on the mineralization process for cellular cementum
formation (17). Increased serum
alkaline phosphatase (ALP) activity has been reported in rheumatoid
arthritis (RA) (18). BMP2 belongs
to the BMP family, which possess diverse biological functions
during osteogenesis and osteogenic differentiation, including the
maintenance of normal bone and bone regeneration (19–22).
Furthermore, the BMP family regulates osteogenic differentiation
(23,24).
The aim of the present study was to investigate
whether miR-204 affected osteoblast differentiation and the
potential underlying mechanisms. The effects of miR-204 on the
osteogenic differentiation of MSCs were determined by evaluating
Runx2, BMP2 and ALP expression. Additionally, the regulatory target
of miR-204 was explored.
Materials and methods
Cell identification and cell
culture
BMSCs were isolated from rat bone marrow. Male
Sprague-Dawley (SD) rats aged 4 weeks were purchased from Shangdong
Laboratory Animal Center (Jinan, China). The rat bone marrow
tissues were extracted after the rats were sacrificed. The femur
and tibia were washed with phosphate buffered saline (PBS; Gibco;
Thermo Fisher Scientific, Inc.) and cut into 5 mm pieces,
high-glucose DMEM (basic medium; Thermo Fisher Scientific, Inc.)
was used to wash the bone fragments, and the solution was
centrifuged at 675 × g for 5 min at room temperature prior to
resuspension of cells in PBS. The cells were separated using
lymphocyte separation medium (Beijing Solarbio Science &
Technology Co., Ltd.) and the BMSCs were acquired. The animal study
was approved by the Institutional Animal Care and Use Committee of
Yantai Yuhuangding Hospital. BMSCs were seeded at
3.5×105 cells/dish in 75-mm culture dishes (Corning
Inc.) in basic medium with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin/100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a 5%
CO2 atmosphere at 37°C. BMSCs were subcultured when the
cells reached 90% confluency. The cells were centrifuged at 750 × g
for 5 min at room temperature after digestion using 0.25%
trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.) at 37°C for 5
min in an incubator (Thermo Fisher Scientific, Inc.). Purified
BMSCs (2×107 cells/ml) were seeded in 6-well plates and
cluster of differentiation (CD) 90-allophycocyanin (APC; 1:20; cat.
no. 17-5900-42; Invitrogen; Thermo Fisher Scientific, Inc.),
CD45-phycoerythrin/cyanine7 (PE/CY7; 0.1 mg/ml; cat. no. 1660-17,
SouthernBiotech) and CD11b/c-FITC (30 µg/ml, cat. no. 130-105-273;
Miltenyi Biotec, Inc.) antibodies were used to stain the samples in
a final volume of 100 µl at 4°C for 20 min. Then, BMSCs in solution
were identified via flow cytometry and analyzed with CellQuest pro
software version 5.1 (BD Biosciences), by detecting the absorbance
at 647 nm for CD90-APC, 532 nm for CD45-PE/CY7 and 488 nm for
CD11b/c-FITC.
Cell transfection
BMSCs were seeded in 75-mm culture dishes at
3.5×105 cells/dish and incubated for 24 h. miR-204
agomir (5′-UUCCCUUUGUCAUCCUAUGCC-3′), miR-204 antagomir
(5′-AGGCAUGGAUGACAAAGGGAA-3′) and negative control (NC,
5′-CAGUACUUUUGUGUAGUACAA-3′) sequences were purchased from
Guangzhou RiboBio Co., Ltd. pLVX-AcGFP-C1-BMP2 was constructed by
cloning amplified BMP2 into pLVX-AcGFP-C1 (Sangon Biotech Co.,
Ltd.). pLVX-AcGFP-C1 was used as the negative control.
Transfections were performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Transfection
complexes were added to the medium at a final concentration of 50
nM. The transfection solution was added to BMSCs for 3, 5 and 7
days at 37°C in a 5% CO2 in an incubator.
Western blotting
BMSCs were transfected for 3 days. Then, the total
proteins were extracted using RIPA buffer (Invitrogen; Thermo
Fisher Scientific, Inc.) at 4°C. Protein concentration were
determined using the BCA method. Proteins (20 µg/lane) were
electrophoresed on 10% SDS-PAGE gels and transferred to
polyvinylidene fluoride membranes (Thermo Fisher Scientific, Inc.).
After blocking with 5% non-fat milk at room temperature for 2 h,
membranes were incubated overnight at 4°C with antibodies against
Runx2 (1:1,000; cat. no. 12556; Cell Signaling Technology, Inc.),
ALP (1:1,000; cat. no. ab83259; Abcam), BMP2 (1:1,000; cat. no.
ab14933; Abcam) and β-actin (1:1,000; cat. no. 4970; Cell Signaling
Technology, Inc.). After the membranes were washed three times in
TBS-0.5% Tween-20 (TBST), a horseradish peroxidase-conjugated
secondary antibody (1:10,000; cat. no. 10285-1-AP; ProteintTech
Group, Inc.) was used to incubate the membranes for 2–3 h at room
temperature. The membranes were then washed 2–3 times in TBST
solution. The blots were visualized using ECL (Thermo Fisher
Scientific, Inc.). An ECL system (Amersham; GE Healthcare) was used
to visualize the bands. Quantity one software version 4.6.2
(Bio-Rad Laboratories, Inc.) was used for densitometry
analysis.
Bioinformatics analysis
TargetScan (http://www.targetscan.org/vert_72/) was used to
predict the targets of miR-204.
Dual-luciferase assay
The BMP2-3′-UTR and mutant (mut) BMP2-3′-UTR were
inserted into psi-CHECK-2 plasmids (Promega Corporation), and then
BMSCs were incubated with BMP2-3′-UTR or BMP2-3′-UTR mut plasmids
at 37°C with 5% CO2 in an incubator for 72 h. The total
protein of BMSCs transfected with luciferase plasmids was extracted
using RIPA buffer (Invitrogen; Thermo Fisher Scientific, Inc.) on
ice. Prior to analysis using a luciferase reporter assay kit
(BioVision, Inc.), cells (2×105 cells/well) were plated
in 24-well plates and were co-transfected with miR plasmids at a
final concentration of 50 nM using Lipofectamine 2000®.
The luciferase activity of BMSCs was measured 3 days after
transfection using a luciferase reporter assay kit (BioVision,
Inc.). Firefly luciferase activity was normalized with
renilla luciferase activity (Promega Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was collected as follows: BMSCs were
treated with TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) via centrifugation at 3,000 × g for 10 min at
room temperature. RT was performed to synthesize cDNA using 2 µl
FQ-RT Primer Mix, 2 µl 10X Fast RT Buffer, 1 µl RT Enzyme Mix and
RNA-Free ddH2O (to 10 µl; all Tiangen Biotech Co.,
Ltd.); RT was conducted at 42°C for 15 min and 95°C for 3 min. qPCR
was conducted using cDNA, forward and reverse primers, and 2X PCR
Taq Master Mix (MedChemExpress LLC) under the following conditions:
40 cycles of 94°C for 5 min, 94°C for 30 sec, 60°C for 40 sec and
72°C for 50 sec.
The following primers were used for qPCR: U6,
forward 5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-ACGCTTCACGAATTTGCGT-3′;
BMP2, forward 5′-ACCAGCATTAGCATCACG-3′, reverse
5′-AGGTCCTTGGGTTGTTTT-3′; Runx2, forward 5′-CTCGCTTCGGCAGCACA-3′,
reverse 5′-AACGCTTCACGAATTTGCGT-3′; ALP, forward
5′-CTGATCAGTGTGCCCCTGCAG-3′, reverse 5′-GGAGCTTGGAACGAATGTTCTG-3′;
and miR-204, forward 5′-CTGATCAGTGTGCCCCTGCA-3′ and reverse
5′-GGAGCTTGGAACGAATGTTCTG-3′. U6 was used as an internal reference
for qPCR, and the 2−∆∆Cq method was applied to calculate
relative expression levels (25).
Alizarin red stain assays
BMSCs were seeded into 24-well plates at 1,000
cells/well for 24 h. NC, miR-204 agomir, BMP2 and miR-204 agomir +
BMP2 were transfected as aforementioned when the BMSCs reached 30%
confluency. After 12 h, basic medium was replaced with culture
medium containing vitamin C (50 µg/ml; Invitrogen; Thermo Fisher
Scientific, Inc.) and β-phosphoglycerol (10 mmol/l; Invitrogen;
Thermo Fisher Scientific, Inc.), and BMSCs were cultured with the
aforementioned transfection reagents for a further 15 days. BMSCs
were stained with 0.1% alizarin red at 37°C for 30 min following
fixation with 10% glutaraldehyde for 10 min. Images were acquired
using an inverted light microscope (magnification, ×100; Olympus
Corporation). For each sample 5 fields were analyzed. Images were
analyzed using ImageJ software version 2.0.0 (National Institutes
of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6.0 (GraphPad Software, Inc.). Data were presented as
the mean ± standard deviation and all experiments were repeated at
least three times. Groups were analyzed by ANOVA followed by a
Tukey-Kramer post hoc multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of Runx2, ALP and miR-204
in BMSCs isolated from rats
The phenotype of BMSCs was measured using flow
cytometry using CD90-APC, CD45-PE/CY7 and CD11b/c-FITC kits. It was
revealed that the phenotype of the BMSCs was CD90+,
CD45− and CD11b− (Fig. 1Aa-c). The expression levels of
Runx2 and ALP in BMSCs increased in a time-dependent manner,
whereas those of miR-204 decreased (Fig. 1B-D).
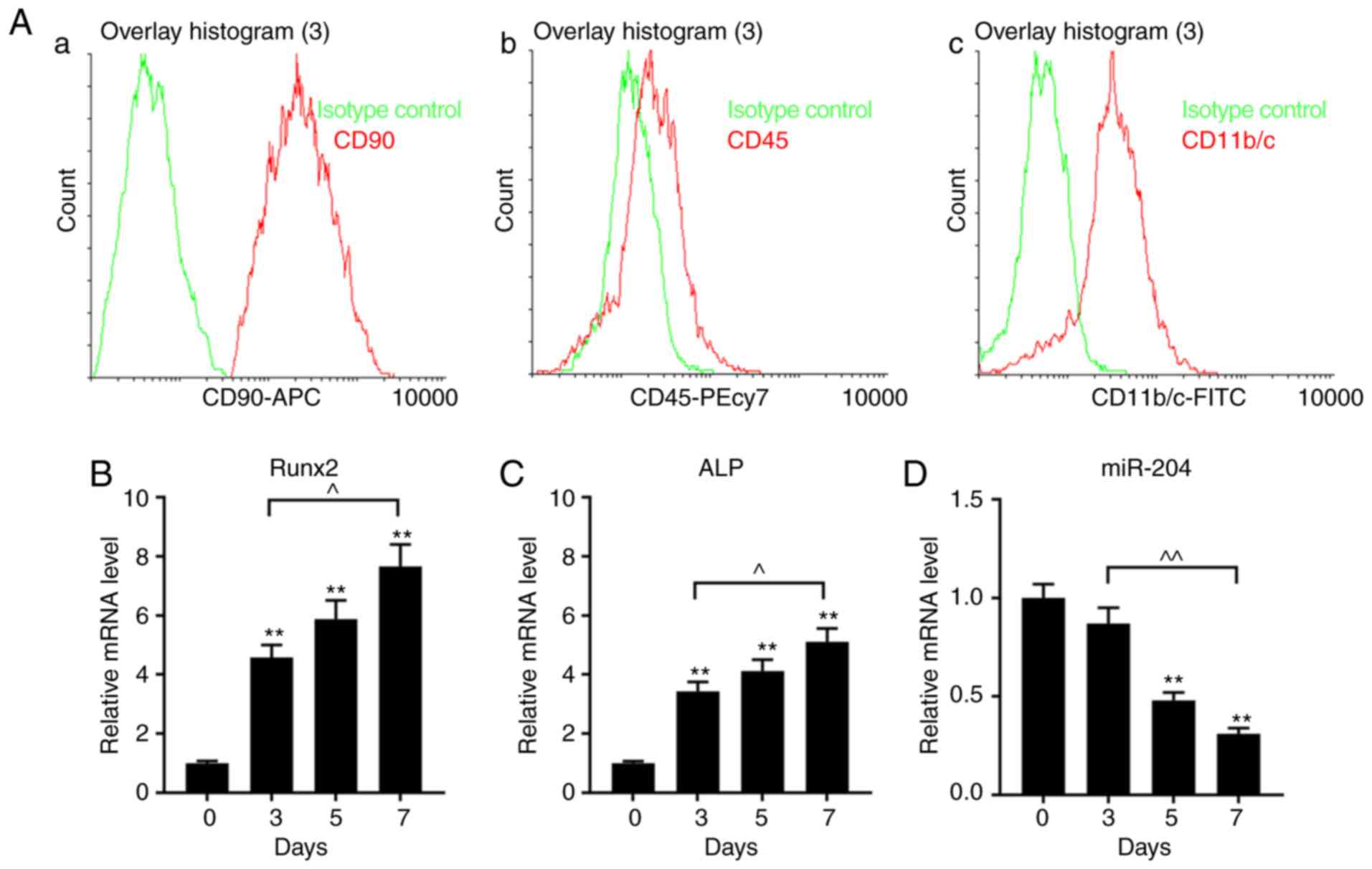 | Figure 1.Properties of cultured BMSCs isolated
from rat bone marrow. (A) CD expression phenotypes of BMSCs were
measured using flow cytometry with (Aa) CD90-APC, (Ab) CD45-PE/CY7
and (Ac) CD11b/c-FITC. The green lines represent the
isotype/negative control. (B-D) Expression of Runx2, ALP and
miR-204 in BMSCs cultured for 0, 3, 5 and 7 days, as determined via
reverse transcription-quantitative PCR analysis. Data are presented
as the mean ± standard deviation and analyzed by ANOVA followed by
Tukey-Kramer multiple comparison post hoc tests. **P<0.01 vs.
day 0; ^P<0.05, ^^P<0.01. BMSC, bone
marrow mesenchymal stem cell; miR-204, microRNA-204; CD, cluster of
differentiation; ALP, alkaline phosphatase; Runx2, Runt-related
transcription factor 2; APC, allophycocyanin; PE, phycoerythrin;
Cy7, cyanine7. |
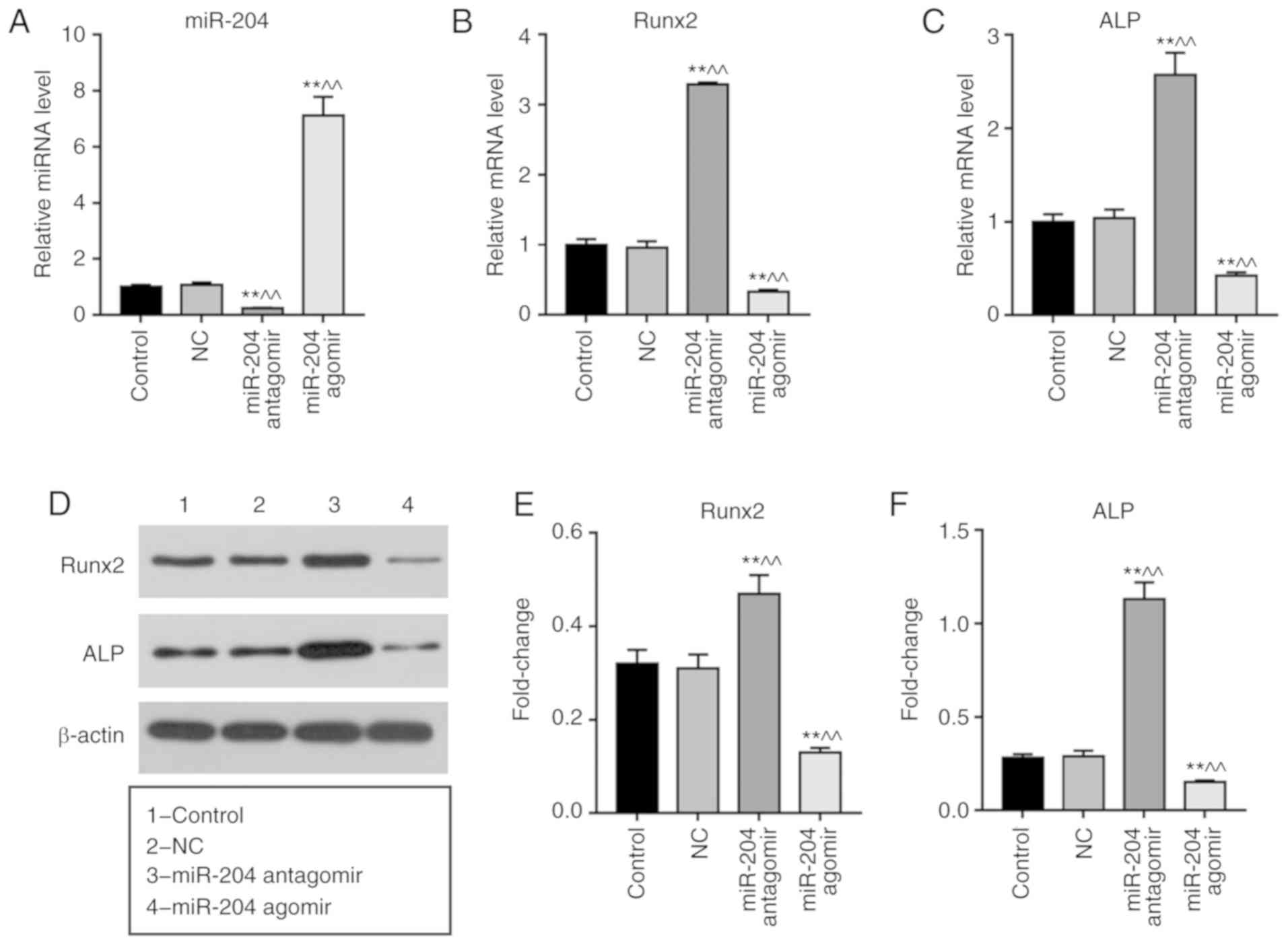
Effects of miR-204 agomir on the
expression levels of Runx2 and ALP in BMSCs
Transfection with miR-204 antagomir significantly
inhibited the expression of miR-204 in BMSCs, whereas miR-204
agomir induced opposing effects (Fig.
2A). Cells transfected with miR-204 agomir exhibited
significantly downregulated expression of Runx2 and ALP at the mRNA
and protein levels; conversely, miR-204 antagomir promoted the
expression of Runx2 and ALP (Fig.
2B-F).
Effects of miR-204 on BMP2 in
BMSCs
A luciferase assay was performed to investigate the
potential association between miR-204 and BMP2. Co-transfection
with miR-204 + BMP2-3′-UTR resulted in significantly decreased
relative luciferase activity compared with control + BMP2-3′-UTR or
miR-204 + BMP2-3′-UTR mut co-transfections (Fig. 3D and E). Additionally, transfection
with miR-204 agomir revealed that upregulation of miR-204
significantly decreased the expression of BMP2 at the mRNA and
protein levels compared with the NC, whereas miR-204 antagomir
induced opposing effects (Fig.
3F-H).
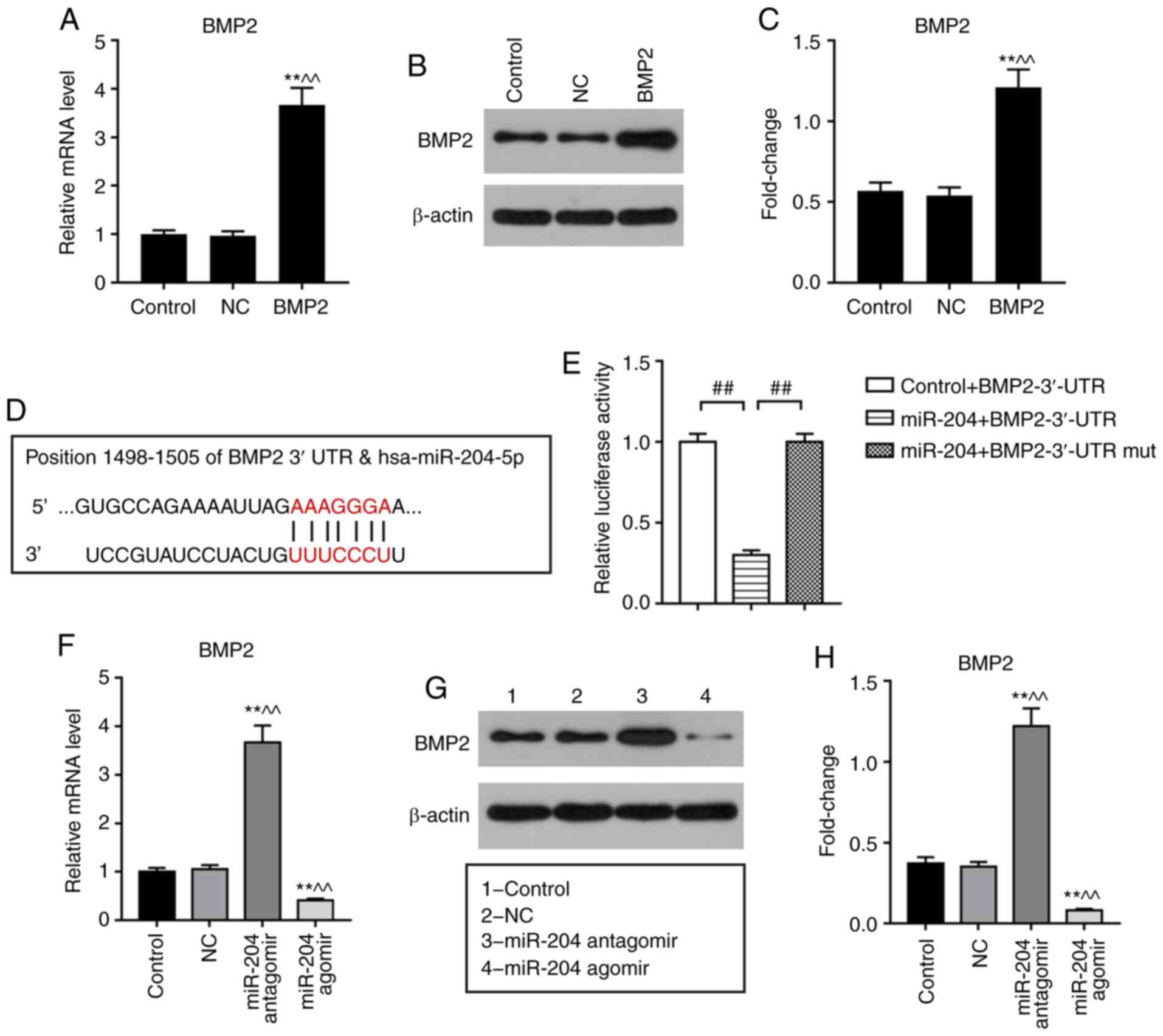 | Figure 3.Effects of miR-204 agomir and BMP2 on
the expression levels of Runx2, ALP and BMP2 in BMSCs. BMSCs were
treated with NC, miR-204 agomir, miR-204 antagomir, BMP2 vector or
miR-204 agomir + BMP2 vector for 3 days. β-actin and U6 were used
as internal references. (A-C) BMP2 expression in BMSCs transfected
with BMP2 vector as determined via RT-qPCR and western blot
analyses. (D) Putative target genes and binding sites of miR-204
were predicted using TargetScan. (E) Interactions between miR-204
and the BMP2 3′-UTR were evaluated using dual-luciferase assays.
(F) mRNA and (G and H) protein expression of BMP2 in BMSCs
transfected with miR-204 agomir or antagomir. (I-K) mRNA levels of
BMP2, Runx2 and ALP in BMSCs transfected with miR-204 agomir and/or
BMP2 vector, as determined via RT-qPCR analysis. (L) Protein levels
of BMP2, Runx2 and ALP in BMSCs transfected with miR-204 agomir
and/or BMP2 vector, as determined via western blotting. Data are
presented as the mean ± standard deviation and analyzed by ANOVA
followed by Tukey-Kramer multiple comparison post hoc tests.
*P<0.05, **P<0.01 vs. control; ^P<0.05,
^^P<0.01 vs. NC; #P<0.05,
##P<0.01. BMSC, bone marrow mesenchymal stem cell;
miRNA/miR, microRNA; BMP2, bone morphogenic protein 2; ALP,
alkaline phosphatase; Runx2, Runt-related transcription factor 2;
NC, negative control; 3′-UTR, 3′-untranslated region; mut,
mutant. |
Effects of miR-204 agomir and BMP2 on
the expression of Runx2, ALP and BMP2 in BMSCs
To investigate the role of BMP2 in the effects of
miR-204 on BMSCs, a BMP2 overexpression vector was used.
Transfection with the BMP2 vector upregulated the expression of
BMP2 at the mRNA and protein levels (Fig. 3A-C). Following transfection of
BMSCs with miR-204 agomir and/or BMP2 for 3 days, the expression
levels of Runx2, ALP and BMP2 were determined via RT-qPCR and
western blot analyses. It was observed that BMSCs transfected with
miR-204 agomir exhibited significantly downregulated expression of
BMP2, Runx2 and ALP compared with the NC (Fig. 3I-L). BMP2 overexpression
significantly upregulated the expression of Runx2 and ALP in BMSCs
compared with the NC; additionally, BMP2 overexpression
significantly increased the expression levels of BMP2, Runx2 and
ALP in miR-204 agomir-treated BMSCs compared with miR-204 agomir
treatment alone (Fig. 3I-L).
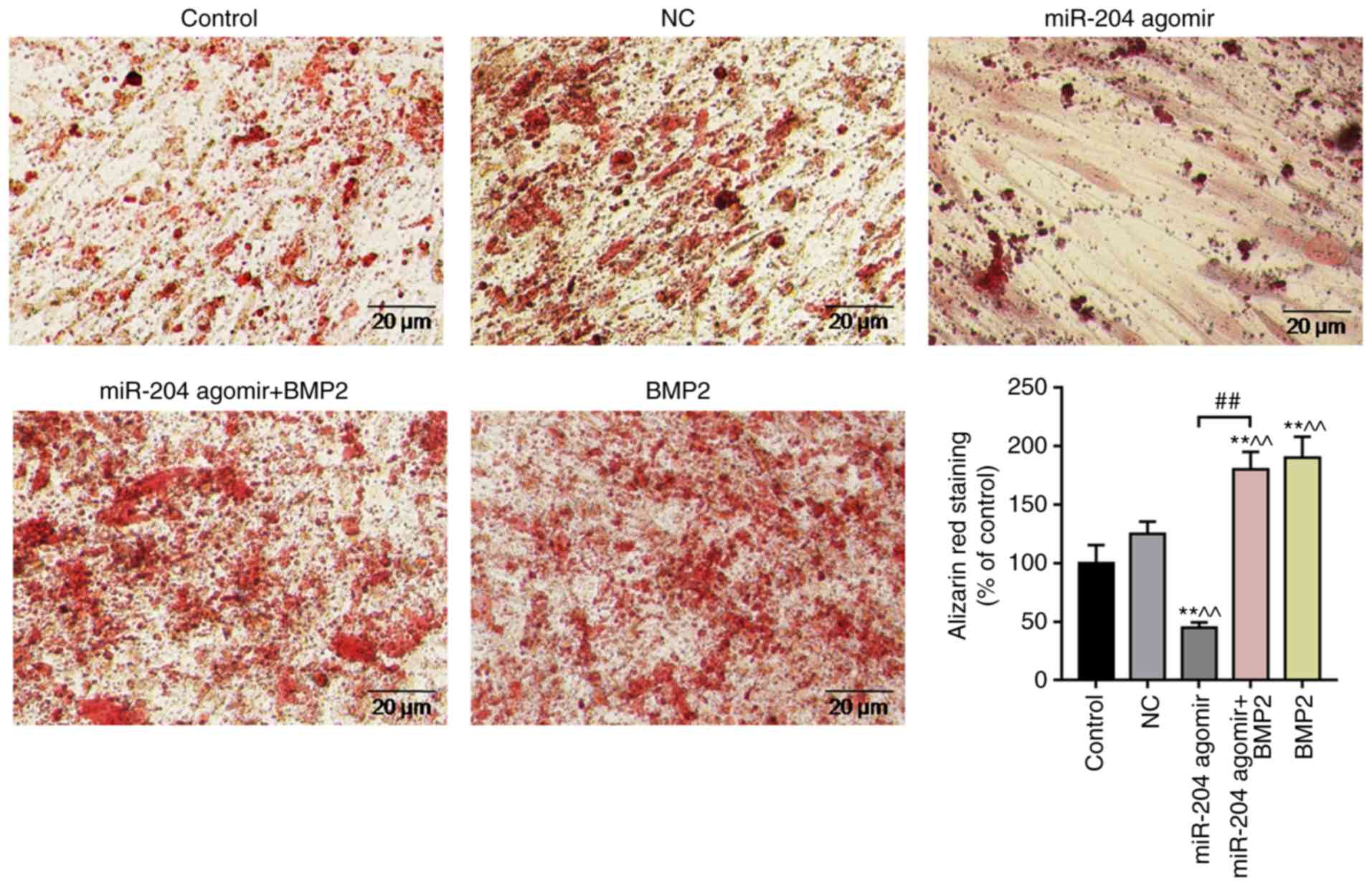
Effects of miR-204 agomir and BMP2 on
calcification in BMSCs
BMSCs were treated with NC, miR-204 agomir, miR-204
agomir + BMP2 or BMP2 for 15 days. BMSCs were stained with alizarin
red. It was observed that the intensity of alizarin red staining
was significantly increased in the BMP2-overexpressing group
compared with in the other groups, and that the intensity in the
miR-204 agomir group was significantly reduced compared with the NC
(Fig. 4). The findings indicated
that BMP2 promoted the calcification of BMSCs, whereas miR-204
agomir inhibited BMSCs calcification.
Discussion
Previous studies have reported that miR-204
inhibited thyroid carcinoma cell proliferation and esophageal
cancer cell invasion (26,27). Additionally, downregulation of
miR-204 enhanced osteogenesis in rat BMSCs (28). In the present study, it was
observed that miR-204 agomir inhibited Runx2, ALP and BMP2
expression, and inhibited MSC calcification, the underlying
mechanisms of these inhibitory effects were investigated.
The BMSCs isolated from rat bone marrow in the
present study exhibited a
CD90+/CD45−/CD11b− phenotype
(Fig. 1A). Runx2 regulates a
series of cell cycle genes in endothelial cells, including
cyclin-dependent kinase (CDK)4, CDK1 and cyclin B1 (29); additionally, reducing Runx2 levels
decreased breast tumor cell viability and inhibited cell migration
(30). The loss of Runx2 in
chondrocytes impaired osteoprotegerin-receptor signaling and
chondroclast development (30,31).
Increased bone turnover resulted in elevated serum ALP in females,
and ALP levels are routinely used in the assessment of Paget's
disease of bone (32,33). High serum ALP levels were reported
in patients with RA (18). The
present findings revealed that the expression levels of Runx2 and
ALP increased with longer durations of BMSC culturing, suggesting
that differentiation occurred in BMSCs when the cells were cultured
for a long period of time. Additionally, miR-204 expression
decreased in a time-dependent manner. Transfection with miR-204
agomir downregulated the expression of Runx2 and ALP, suggesting
that increased miR-204 levels inhibited the osteogenic
differentiation of MSCs.
BMP2 has been reported to promote bone regeneration,
and abnormal BMP2 levels result in bone diseases, as BMP2 promotes
chondrogenesis, myogenesis, osteogenesis and bone mineral density
(34,35). Additionally, BMP2-deficient embryos
exhibited defects in cardiac development, which manifested as the
abnormal development of the heart in the exocoelomic cavity
(19). Reduced BMP2 in both
embryonic and maternal tissues affected neural tube closure and
body wall closure to varying degrees (22). In humans, there was a reduction in
BMP2 expression in certain parts of unfractured bones compared with
fractures in the process of healing, whereas BMP2 was expressed
strongly in areas of healing; BMP2 levels gradually decreased as
healing progressed (36–38). The results of the present study
indicated that miR-204 agomir inhibited the expression of BMP2 in
BMSCs. It was proposed that miR-204 agomir inhibited Runx2 and ALP
expression by regulating BMP2. To investigate this hypothesis, the
expression levels of Runx2 and ALP were determined in BMSCs
transfected with a BMP2 overexpression vector. It was demonstrated
that overexpression of BMP2 increased the levels of Runx2 and ALP
in BMSCs; however, miR-204 agomir downregulated the expression of
Runx2 and ALP in BMP2-overexpressing BMSCs.
Skeletal mineralization requires connections between
cellular activity and the extracellular environment; skeletal
formation promotes the mineralization of the matrix (39). Numerous studies reported that the
alizarin red stain assay can be used to evaluate the osteogenic
capacity of BMSCs as determined by the extent of mineralization
(40–43). The present findings suggested that
BMP2 promoted the osteogenesis of BMSCs, and that miR-204 agomir
reduced the osteogenic capacity of BMSCs by inhibiting BMP2;
however, the present study did not include the use an animal model
to further investigate whether miR-204 overexpression negatively
affected osteogenic differentiation. Additionally, bioinformatics
analysis was not conducted to identify other putative target genes
of miR-204.
In conclusion, the results of the present study
suggested that miR-204 upregulation inhibited the BMP2/Runx2/ALP
signaling pathway by regulating BMP2. Additionally, the study
provided preliminary evidence that miR-204 inhibited the
differentiation of osteogenesis in BMSCs by targeting BMP2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets used and/or anlayzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
XJ and ZZ made substantial contributions to the
conception and design of the present study. TP, GW, QX and GL were
involved in data acquisition, analysis and interpretation. XJ and
ZZ drafted the manuscript and critically revised it for important
intellectual content. All authors gave final approval of the
version to be published. All authors agreed to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The animal study was approved by the Institutional
Animal Care and Use Committee of Yantai Yuhuangding Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim BS, Kang HJ, Park JY and Lee J:
Fucoidan promotes osteoblast differentiation via JNK- and
ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem
cells. Exp Mol Med. 47:e1282015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon JS, Kim SH, Oh SH, Jeong YW, Kang JH,
Park JC, Son HJ, Bae S, Park BI, Kim MS, et al: Relaxin augments
BMP-2-induced osteoblast differentiation and bone formation. J Bone
Miner Res. 29:1586–1596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li KL, Chen J, Li ZH, Zhao L and He YN:
p53 negatively regulates the osteogenic differentiation of vascular
smooth muscle cells in mice with chronic kidney disease. Cardiovasc
J Afr. 23:e1–e9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian K, Xu H, Dai T and Shi K: Effects of
Tanshinone IIA on osteogenic differentiation of mouse bone marrow
mesenchymal stem cells. Naunyn Schmiedebergs Arch Pharmacol.
388:1201–1209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Papathanasopoulos A, Kouroupis D, Henshaw
K, McGonagle D, Jones EA and Giannoudis PV: Effects of
antithrombotic drugs fondaparinux and tinzaparin on in vitro
proliferation and osteogenic and chondrogenic differentiation of
bone-derived mesenchymal stem cells. J Orthop Res. 29:1327–1335.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng S, Gao D, Gao C, Wei P, Niu M and
Shuai C: MicroRNAs regulate signaling pathways in osteogenic
differentiation of mesenchymal stem cells (Review). Mol Med Rep.
14:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang C, Sun J, Dai Y, Cao P, Zhang L,
Peng S, Zhou Y, Li G, Tang J and Xiang J: HIF-1A and C/EBPs
transcriptionally regulate adipogenic differentiation of bone
marrow-derived MSCs in hypoxia. Stem Cell Res Ther. 6:212015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui H, Yi Q, Feng J, Yang L and Tang L:
Mechano growth factor E peptide regulates migration and
differentiation of bone marrow mesenchymal stem cells. J Mol
Endocrinol. 52:111–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsushita K, Itoh S, Ikeda S, Yamamoto Y,
Yamauchi Y and Hayashi M: LIF/STAT3/SOCS3 signaling pathway in
murine bone marrow stromal cells suppresses osteoblast
differentiation. J Cell Biochem. 115:1262–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li T, Pan H and Li R: The dual regulatory
role of miR-204 in cancer. Tumour Biol. 37:11667–11677. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doench JG and Sharp PA: Specificity of
microRNA target selection in translational repression. Genes Dev.
18:504–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu X, Shen H, Yin X, Long L, Chen X, Feng
F, Liu Y, Zhao P, Xu Y, Li M, et al: IL-6R/STAT3/miR-204 feedback
loop contributes to cisplatin resistance of epithelial ovarian
cancer cells. Oncotarget. 8:39154–39166. 2017.PubMed/NCBI
|
|
14
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: MiR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting CyclinD2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Wang C, Song Y and Fang B: Arsenic
trioxide and microRNA-204 display contrary effects on regulating
adipogenic and osteogenic differentiation of mesenchymal stem cells
in aplastic anemia. Acta Biochim Biophys Sin (Shanghai).
46:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mansurabadi R, Abroun S, Hajifathali A,
Asri A, Atashi A and Haghighi M: Expression of hsa-MIR-204, RUNX2,
PPARγ and BCL2 in bone marrow derived mesenchymal stem cells from
multiple myeloma patients and normal individuals. Cell J. 19 (Suppl
1):S27–S36. 2017.
|
|
17
|
Groeneveld MC, Everts V and Beertsen W:
Alkaline phosphatase activity in the periodontal ligament and
gingiva of the rat molar: Its relation to cementum formation. J
Dent Res. 74:1374–1381. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nanke Y, Kotake S, Akama H and Kamatani N:
Alkaline phosphatase in rheumatoid arthritis patients: Possible
contribution of bone-type ALP to the raised activities of ALP in
rheumatoid arthritis patients. Clin Rheumatol. 21:198–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H and Bradley A: Mice deficient for
BMP2 are nonviable and have defects in amnion/chorion and cardiac
development. Development. 122:2977–2986. 1996.PubMed/NCBI
|
|
20
|
Ducy P and Karsenty G: The family of bone
morphogenetic proteins. Kidney Int. 57:2207–2214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosen V: BMP2 signaling in bone
development and repair. Cytokine Growth Factor Rev. 20:475–480.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh AP, Castranio T, Scott G, Guo D,
Harris MA, Ray M, Harris SE and Mishina Y: Influences of reduced
expression of maternal bone morphogenetic protein 2 on mouse
embryonic development. Sex Dev. 2:134–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamplot JD, Qin J, Nan G, Wang J, Liu X,
Yin L, Tomal J, Li R, Shui W, Zhang H, et al: BMP9 signaling in
stem cell differentiation and osteogenesis. Am J Stem Cells.
2:1–21. 2013.PubMed/NCBI
|
|
24
|
Peng Y, Kang Q, Cheng H, Li X, Sun MH,
Jiang W, Luu HH, Park JY, Haydon RC and He TC: Transcriptional
characterization of bone morphogenetic proteins (BMPs)-mediated
osteogenic signaling. J Cell Biochem. 90:1149–1165. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Wang J, Li X, Ma J, Shi C, Zhu H,
Xi Q, Zhang J, Zhao X and Gu M: MiR-204-5p suppresses cell
proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma.
Biochem Biophys Res Commun. 457:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Yu X and Bai Q: miR-204 inhibits
invasion and epithelial-mesenchymal transition by targeting FOXM1
in esophageal cancer. Int J Clin Exp Pathol. 8:12775–12783.
2015.PubMed/NCBI
|
|
28
|
Shang G, Wang Y, Xu Y, Zhang S, Sun X,
Guan H, Zhao X, Wang Y, Li Y and Zhao G: Long non-coding RNA
TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of
rat bone marrow mesenchymal stem cell by targeting miR-204-5p and
miR-125a-3p. J Cell Physiol. 233:6041–6051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pierce AD, Anglin IE, Vitolo MI, Mochin
MT, Underwood KF, Goldblum SE, Kommineni S and Passaniti A:
Glucose-activated RUNX2 phosphorylation promotes endothelial cell
proliferation and an angiogenic phenotype. J Cell Biochem.
113:282–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taipaleenmaki H, Browne G, Akech J, Zustin
J, van Wijnen AJ, Stein JL, Hesse E, Stein GS and Lian JB:
Targeting of Runx2 by miR-135 and miR-203 impairs progression of
breast cancer and metastatic bone disease. Cancer Res.
75:1433–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Ghori-Javed FY, Rashid H, Adhami
MD, Serra R, Gutierrez SE and Javed A: Runx2 regulates endochondral
ossification through control of chondrocyte proliferation and
differentiation. J Bone Miner Res. 29:2653–2665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mukaiyama K, Kamimura M, Uchiyama S,
Ikegami S, Nakamura Y and Kato H: Elevation of serum alkaline
phosphatase (ALP) level in postmenopausal women is caused by high
bone turnover. Aging Clin Exp Res. 27:413–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Magnusson P, Davie MW and Sharp CA:
Circulating and tissue-derived isoforms of bone alkaline
phosphatase in Paget's disease of bone. Scand J Clin Lab Invest.
70:128–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rogers MB, Shah TA and Shaikh NN: Turning
bone morphogenetic protein 2 (BMP2) on and off in mesenchymal
cells. J Cell Biochem. 116:2127–2138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng M, Liu P, Xiao H, Zhang Y, Wang Y,
Zhao J and Xu J: Improving the osteogenic efficacy of BMP2 with
mechano growth factor by regulating the signaling events in BMP
pathway. Cell Tissue Res. 361:723–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwong FN, Hoyland JA, Evans CH and
Freemont AJ: Regional and cellular localisation of BMPs and their
inhibitors' expression in human fractures. Int Orthop. 33:281–288.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Myers TJ, Longobardi L, Willcockson H,
Temple JD, Tagliafierro L, Ye P, Li T, Esposito A, Moats-Staats BM
and Spagnoli A: BMP2 regulation of CXCL12 cellular, temporal, and
spatial expression is essential during fracture repair. J Bone
Miner Res. 30:2014–2027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwong FN, Hoyland JA, Freemont AJ and
Evans CH: Altered relative expression of BMPs and BMP inhibitors in
cartilaginous areas of human fractures progressing towards
nonunion. J Orthop Res. 27:752–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bensimon-Brito A, Cardeira J, Dionisio G,
Huysseune A, Cancela ML and Witten PE: Revisiting in vivo staining
with alizarin red S-a valuable approach to analyse zebrafish
skeletal mineralization during development and regeneration. BMC
Dev Biol. 16:22016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ovchinnikov D: Alcian blue/alizarin red
staining of cartilage and bone in mouse. Cold Spring Harb Protoc.
2009:pdb.prot5170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagy A, Gertsenstein M, Vintersten K and
Behringer R: Alizarin red staining of post-natal bone in mouse.
Cold Spring Harb Protoc. 2009:pdb.prot5171. 2009. View Article : Google Scholar
|
|
42
|
Reynaud L and Jocteur-Monrozier A:
Skeletal examination by alizarin staining. Methods Mol Biol.
947:201–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bruneel B and Witten PE: Power and
challenges of using zebrafish as a model for skeletal tissue
imaging. Connect Tissue Res. 56:161–173. 2015. View Article : Google Scholar : PubMed/NCBI
|