Introduction
Aging is an important risk factor for the
development of cardiovascular disease (1), and this association may be caused by
a continuum of cardiac structural and functional alterations that
occur with age (2). These cardiac
changes are relevant in age-associated diseases (3). Since aging increases the risk of
cardiac disease and reduces organ function, studies that aim to
develop interventions that combat cardiac aging and elucidate the
disease mechanisms have significant preclinical and clinical
implications. Swimming exercise has recently been shown to improve
structural abnormalities and upregulate the antioxidant defense
capacity of senescent female rat hearts (4). Oxidative stress, due to excessive
reactive oxygen species (ROS) production and impaired antioxidant
defense mechanisms, induces a range of pathologies that are
believed to be important contributors to the cardiovascular aging
process (2,5). Recently, Belaya et al
(6) reported that long-term wheel
running can protect against age-related cellular stress. The
endoplasmic reticulum (ER) is a specialized organelle where the
folding and post-translational maturation of almost all membrane
proteins, and most secreted proteins, occur (7). Although exercise significantly
improves cardiorespiratory fitness, little is known about the
impact of physical activity on myocardial function. Many of the
pathological changes associated with aging have been attributed to
oxidative stresses (8). It has
been proposed that endurance exercise training is associated with
altered ER function (9). The
unfolded protein response (UPR) is a crucial process in maintaining
ER homeostasis or inducing cell death in chronically damaged cells;
the UPR causes ER stress. ER stress is initiated by the activation
of at least three types of stress sensors: i) Inositol-requiring
enzyme-1; ii) activating transcription factor 6; and iii) PKR-like
ER kinase (PERK) (7).
Additionally, a previous report demonstrated that levels of the ER
chaperones glucose-regulated protein 78 (GRP78) are decreased,
whereas levels of the pro-apoptotic mediator C/EBP homologous
protein (CHOP) are increased in aged brains (10,11).
These previous findings suggested that the ability to maintain ER
homeostasis may be disrupted during aging; however, the functional
significance of these processes in aged hearts remains unclear.
Both oxidative stress and ER stress are involved in physiological
and pathophysiological processes associated with aging. Therefore,
strategies designed to reduce the aberrant activation of oxidative
stress and ER stress in the aged heart are of great interest.
cGMP is a ubiquitous second messenger involved in
many cardiovascular processes and is produced by guanylate cyclases
(12). The biological activity of
cGMP is regulated by cGMP-specific phosphodiesterase type 5 (PDE5)
through hydrolytic degradation (13). Previous studies have indicated that
protein kinase G (PKG) activation by cGMP has a role in
cGMP-induced myocardial functions (13–15).
It has also been reported that PKG activation decreases with aging
(15). However, the actions of
cGMP-PKG signaling in the aged heart are not fully understood.
Therefore, the present study was designed with two aims: i) To
determine whether exercise training improves myocardial function
via the cGMP-PKG pathway; and ii) to examine whether the endogenous
cGMP-PKG system attenuated aged-induced myocardial ER stress.
Materials and methods
Animals and treatment
A total of 64 male C57Bl/6J mice were obtained from
the animal center of the Fourth Military Medical University. All
animal experimental procedures and protocols were approved by the
Ethics Committee of The Fourth Military Medical University. The
animals were studied at 4 (young) and 20 (aged) months of age
(ranging approximately 25–40 g). They were housed under a 12-h
light/dark cycle in temperature (22±2°C) and humidity
(55±10%)-controlled rooms with free access to food and water. The
mice were assigned to three groups: i) Young (n=16); ii) aged
(n=24); and iii) aged + exercise (n=24). The animals in the
exercise group performed swimming exercise, free of any loading, 5
days/week for 8 weeks in water maintained at 32–35°C. The mice swam
for 15 min on the first day, with the swimming duration increased
gradually over a 1 week period to 60 min continuously every day on
one protocol. All exercise sessions were performed between 8:00 and
11:00 a.m., as previously described (10,14).
The aged mice were intraperitoneally injected with sildenafil (3
mg/kg/day for 3 weeks) or tunicamycin (TM; 2 mg/kg/day for 2 days)
(13,16). Sildenafil and TM were purchased
from Sigma-Aldrich; Merck KGaA. The compounds were dissolved in
0.9% saline for injection. All mice were anaesthetized by inhaling
oxygen with 5% isoflurane at the rate of 1 l/min after 24 h of the
last drug administration. The mice were confirmed to be deeply
anesthetized after they were immobile for 1 min. To euthanatize the
mice, a 25% volume of CO2 gas was allowed to constantly
flow of 0.2 l/min into the chamber until the lack of respiration
and heart beat were detected. The heart tissue was isolated
post-mortem.
Echocardiography
Echocardiographic examinations were performed using
an ACUSON Sequoia 512 ultrasound machine (Siemens AG) using 2.5%
isoflurane as an inhalant anesthetic. The measurements were
performed using M-mode tracings from the papillary muscles and the
apical four-chamber view.
Pathological examination
The excised heart samples were fixed with 4%
paraformaldehyde at 4°C for 24 h and incubated in 80, 90 and 100%
ethanol. Each incubation was performed for 30 min. Subsequently,
samples were incubated in 100% ethanol for 15 min at room
temperature, and were subsequently embedded in paraffin wax. The
samples were longitudinally sectioned to a thickness of 5 µm. The
sections were stained with hematoxylin and eosin (Sigma-Aldrich:
Merck KGaA) and assessed using an optical microscope (Olympus
Corporation; magnification, 40–400×). Sections were incubated with
haematoxylin (0.2%) for 5 min at room temperature and with eosin
(1%) for 2 min temperature.
Measurements of the level of
superoxide dismutase (SOD) activity and malondialdehyde (MDA)
content
The hearts were homogenized with saline and the
activity of the antioxidant enzyme SOD and the concentration of MDA
were determined using commercially available kits (cat. nos. A001
and A003 respectively; Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's instructions.
Detection of ROS
A reactive oxygen species (ROS) assay kit (cat. no.
E004, Nanjing Jiancheng Bioengineering Institute) was used to
determine the level of ROS generation. The myocardial tissue was
trypsinized with trypsin (0.4%) at 37°C for 3 min, and then washed
with PBS and stained with 10 µM 2′,7′-dichlorodihydrofluorescein
diacetate for 30 min at 37°C. After centrifugation at 1,000 × g for
5 min at room temperature, the tissues were washed three times with
PBS and the fluorescence intensity was analyzed using a
fluorescence microplate reader (Molecular Devices, LLC) at an
excitation wavelength of 480 nm and an emission wavelength of 530
nm.
cGMP measurement
The cGMP content in the myocardium was measured
using the cGMP complete ELISA kit (cat. no. ADI900164, Assay
Designs, Inc.), according to the manufacturer's instructions and as
previously described (14). The
results were reported as pmol cGMP/mg tissue.
Western blot analysis
Western blot analysis was performed as described in
our previous study (17). Briefly,
frozen animal heart tissues were homogenized in ice-cold RIPA lysis
buffer [500 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Nonidet P-40,
0.02% sodium azide, 0.1% SDS, 100 µg/ml PMSF, 1 µg/ml aprotinin and
0.5% sodium deoxycholate]. The cell extracts were centrifuged for
30 min at 15,000 × g at 4°C and the supernatants were collected.
Protein lysates (35 µg/lane) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes. Membranes were blocked with 5%
non-fat milk for 1 h at room temperature, and then incubated with
the following primary antibodies at 4°C overnight: GRP78 (cat. no.
3177, Cell Signaling Technology, Inc.), CHOP (cat. no. 5554, Cell
Signaling Technology, Inc.), PKG (cat. no. 3248, Cell Signaling
Technology, Inc.), PDE5 (cat. no. SAB2500767, Sigma-Aldrich; Merck
KGaA) and β-actin (cat. no. 3700, Cell Signaling Technology, Inc.).
Membranes were then incubated with a peroxidase-conjugated
secondary antibody (goat-anti-rabbit; 1:5,000; cat. no. A0208;
goat-anti-mouse; 1:5,000; cat. no. A0216; Beyotime Institute of
Biotechnology) for 2–3 h at room temperature before visualization
using an Immobilon western ECL hydrogen peroxidase substrate (EMD
Millipore; cat. no. WBKLS0500) and analyzed using the Discovery
Series Quantity One software (version, 4.52; Bio-Rad Laboratories,
Inc.).
Statistical analysis
The results are presented as the mean ± SEM.
Statistical analyses were performed using Student's t-test for
comparing two groups or two-way ANOVA for comparing ≥3 groups
followed by Bonferroni's post hoc test. All statistical analyses
were performed using GraphPad Prism software version 5.0 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference. All the experiments were
repeated for three times.
Results
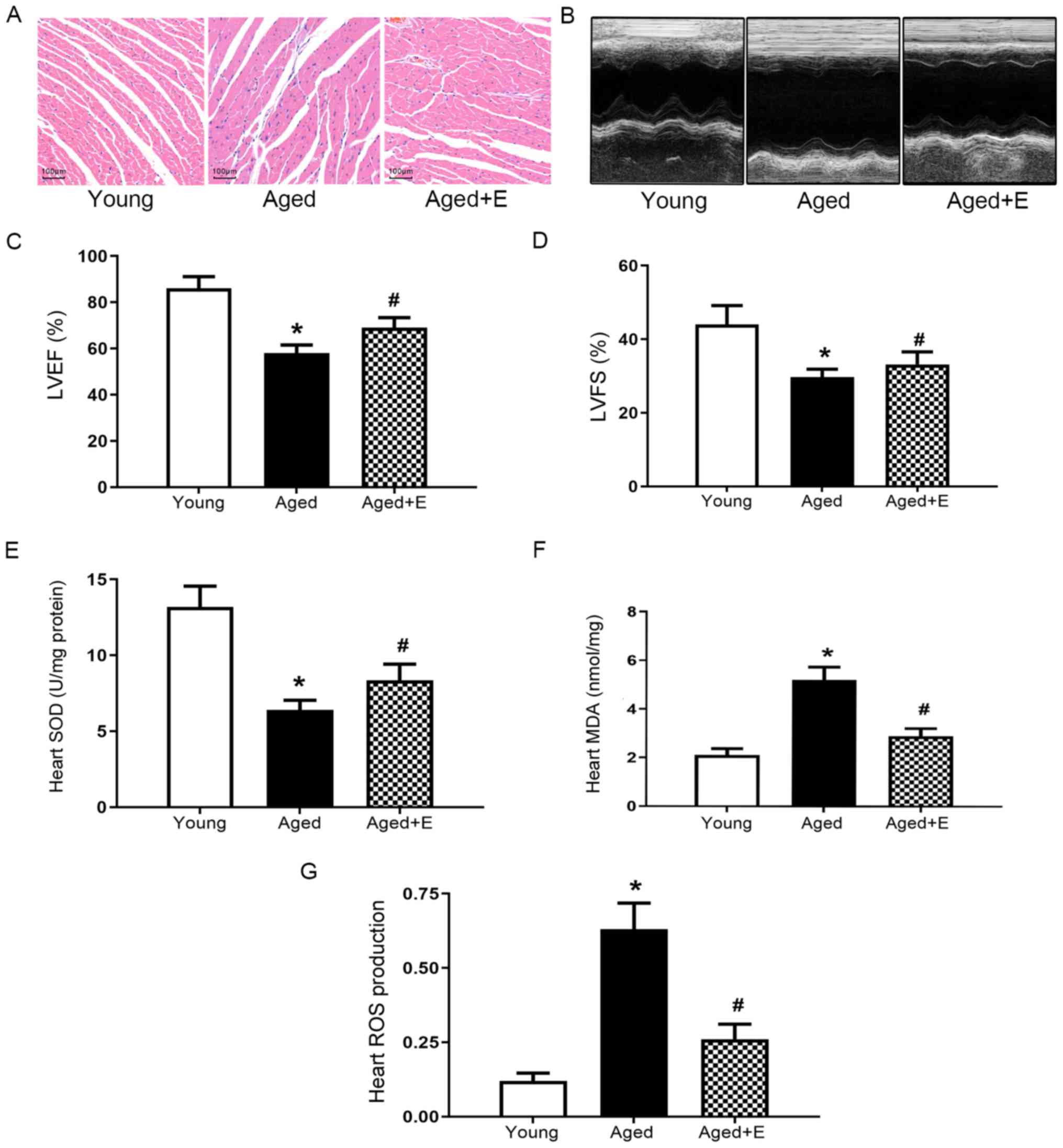
Swimming exercise improves cardiac
structure and function in aged mice
Swimming exercise attenuated the irregularly
arranged cardiomyocytes that are caused by aging (Fig. 1A). A decrease in cardiac function
is a hallmark of aging. The left ventricular ejection fraction
(LVEF) and left ventricular fraction shortening (LVFS) were
measured using echocardiography to determine the cardiac function
of mice in the young, aged and aged + exercise groups (Fig. 1B). A significantly lower LVEF and
LVFS were observed in aged mice compared with young mice (Fig. 1C and D). Swimming exercise
significantly improved the heart function of aged mice (Fig. 1B-D). Additionally, the levels of
several oxidative stress indicators, including SOD, MDA and ROS,
were measured. Lower SOD activity was detected in hearts from the
aged group compared with the young group, and swimming exercise
significantly increased SOD activity (Fig. 1E). In addition, swimming exercise
ameliorated age-induced alterations in the levels of MDA (Fig. 1F) and ROS (Fig. 1G). Based on these results, swimming
exercise was found to exert beneficial effects on the heart that
were found to be associated with an attenuation of oxidative stress
in aged mice.
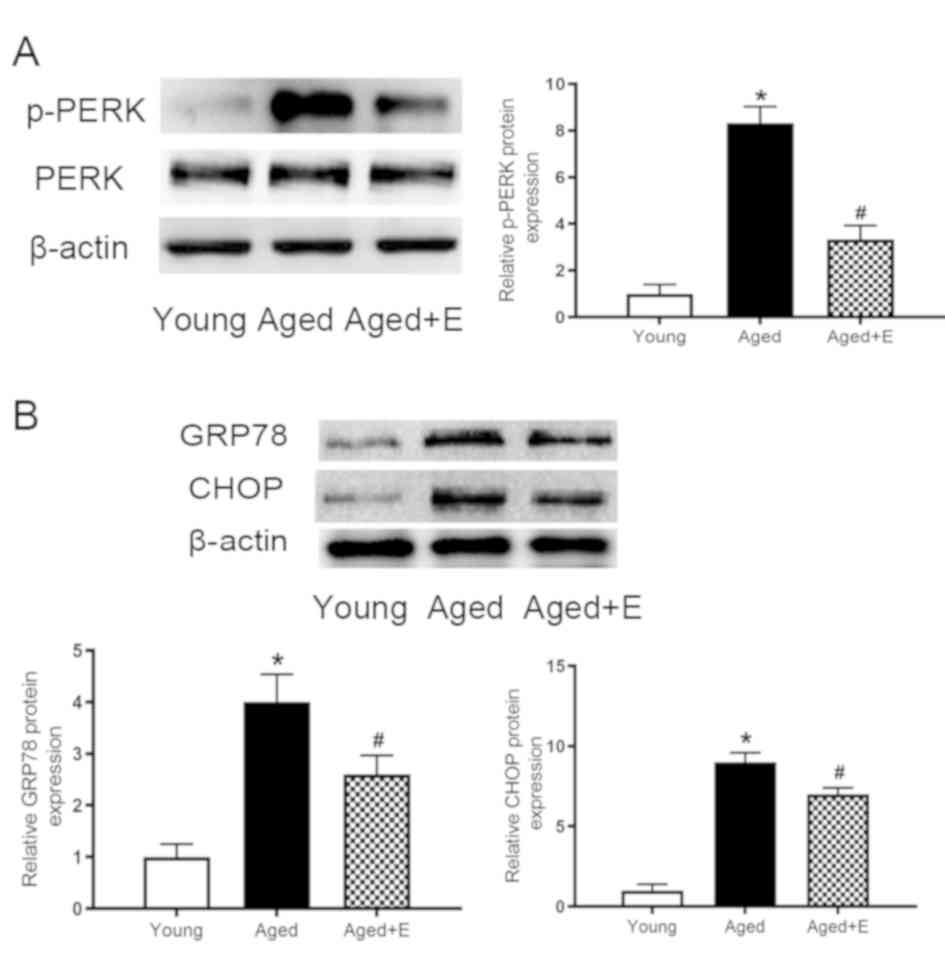
Swimming exercise inhibits ER stress
in the hearts of aged mice
ER stress and oxidative stress are closely related
processes (18). Therefore,
experiments were conducted to examine the levels of ER
stress-related proteins. ER stress was induced in the aged hearts,
as indicated by the increased level of p-PERK (Fig. 2A). Furthermore, the levels of GRP78
and CHOP were also increased (Fig.
2B). The increased levels of p-PERK, GRP78 and CHOP were all
suppressed by swimming exercise. Therefore, swimming exercise was
found to inhibit ER stress in aged mice.
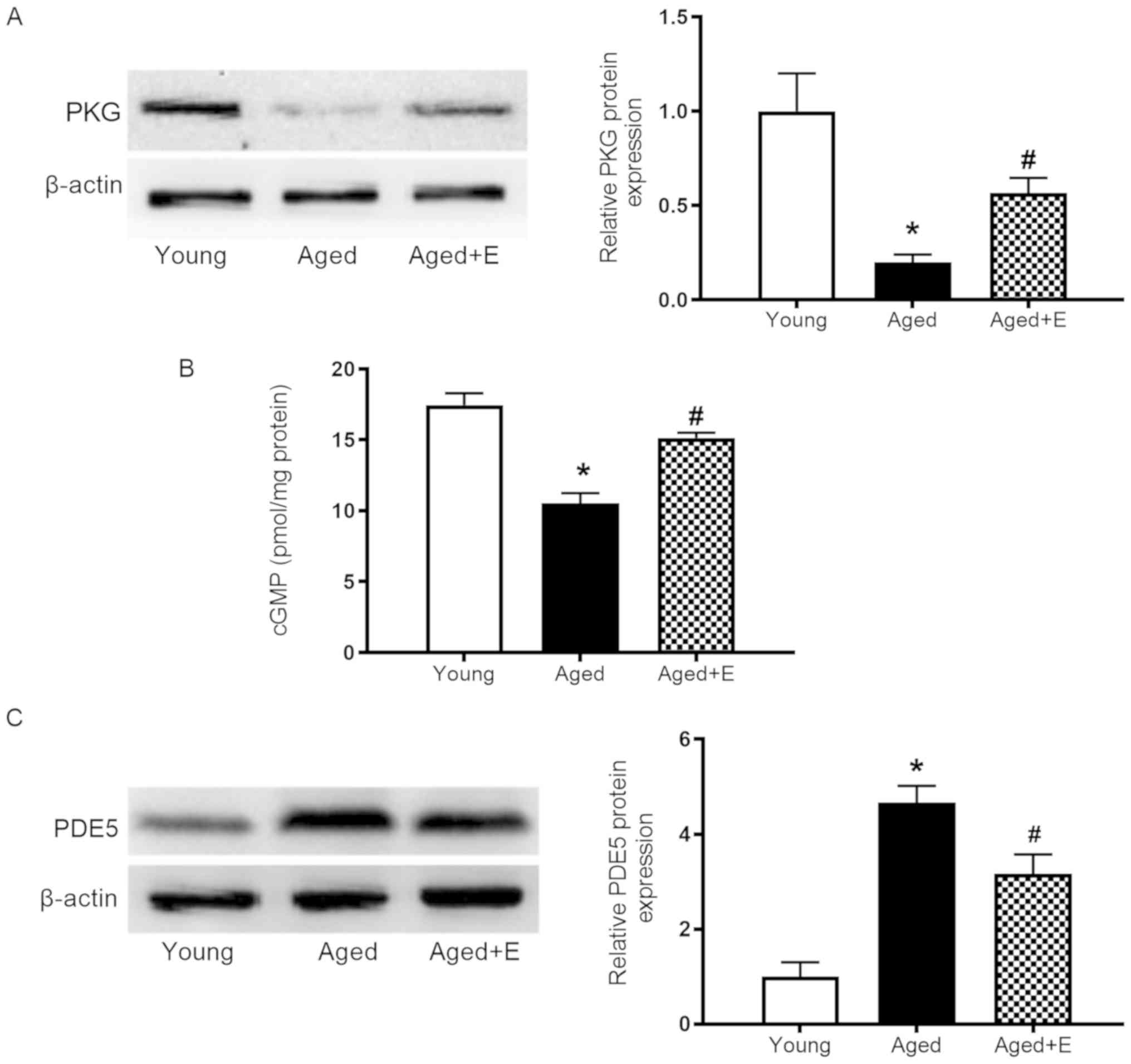
Swimming exercise reverses the
downregulation of cGMP-PKG signaling in the hearts of aged
mice
The effect of swimming exercise on myocardial
cGMP-PKG signaling was evaluated to investigate the
cardioprotective mechanism of swimming exercise in aged hearts.
Significantly lower PKG expression was observed in aged hearts
compared with young hearts, and this effect was significantly
reversed by swimming exercise (Fig.
3A). cGMP levels in the myocardium were higher in aged mice
subjected to swimming exercise than in the aged group (Fig. 3B). Furthermore, higher levels of
PDE5 were detected in aged hearts compared with young hearts, and
swimming exercise reduced the increase of PDE5 levels in aged
hearts (Fig. 3C). Based on these
results, swimming exercise was found to reverse the downregulation
of cGMP-PKG signaling in aged hearts by increasing PKG and cGMP
levels and reducing PDE5 levels.
Effects of the cGMP signaling pathway
on cardiac function, ROS production and ER stress
To determine whether cGMP-PKG signaling regulated
the anti-ER stress effect of swimming exercise in aged hearts, the
aged group were treated with sildenafil, a specific PDE5 inhibitor,
and the aged + exercise group were treated with TM, an ER stress
inducer. The heart function in all groups was observed by
echocardiography (Fig. 4A). The
LVEF and LVFS values were slightly increased in the hearts of aged
mice treated with sildenafil or swimming exercise compared with
aged hearts (Fig. 4B and C).
Additionally, sildenafil and swimming exercise significantly
reduced the levels of ROS in the aged hearts (Fig. 4D). Exposure to the ER stress
inducer TM, which increased the expression levels of GRP78 and CHOP
proteins to mimic ER stress-induced injuries, abolished the
beneficial effects of swimming exercise on cardiac function in aged
mice and increased ROS production compared with swimming exercise
in aged mice (Fig. 4A-D).
Sildenafil decreased the levels of the ER stress-related proteins
GRP78 and CHOP (Fig. 4E),
increased levels of PKG and reduced the level of PDE5 (Fig. 4F), while reducing ROS production
(Fig. 4A-D). The present data
suggested that the activation of cGMP-PKG signaling can mimic the
protective effect of exercise in aged mice. TM did not affect PKG
and PDE5 expression (Fig. 4F).
However, TM increased ROS production and reduced cardiac function
in aged mice after exercise (Fig.
4A-D). Collectively, the present data suggested that activation
of ER stress abolished the protective effect of exercise in aged
mice. In summary, swimming exercise exerted its cardioprotective
effect by ameliorating ER stress through the cGMP-PKG signaling
pathway.
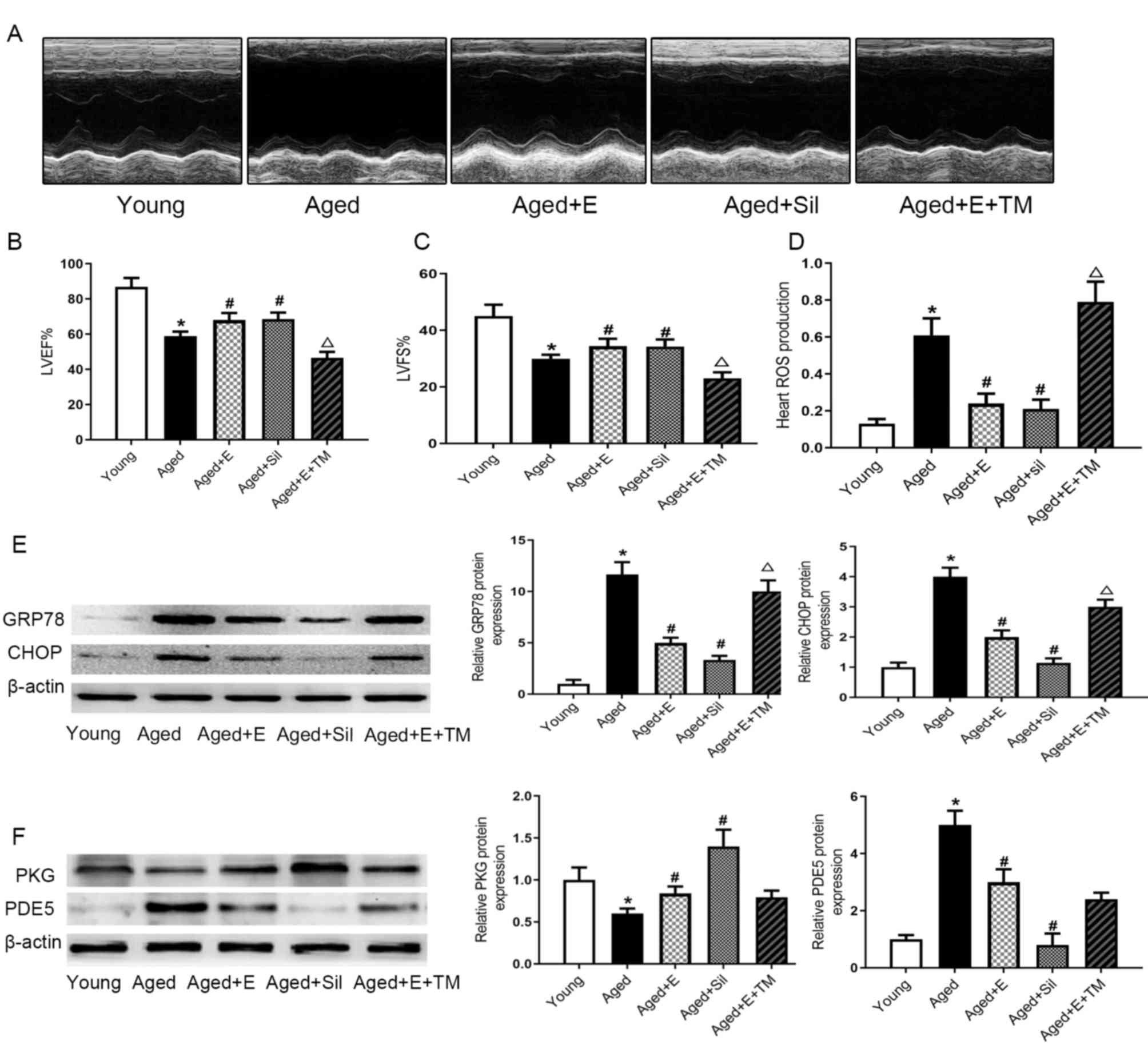 | Figure 4.Activation of cGMP inhibits ROS
production and endoplasmic reticulum stress. (A) Echocardiography
of hearts from mice in each group. (B) LVEF and (C) LVFS of hearts
from mice in each group. (D) ROS production. (E) Levels of GRP78
and CHOP. (F) Levels of PKG and PDE5. Proteins were normalized to
β-actin. *P<0.05 vs. young mice; #P<0.05 vs. aged
mice; ΔP<0.05 vs. aged mice + E. Data are presented
as the mean ± SEM of three independent experiments. LVEF, left
ventricle ejection fraction; LVFS, left ventricle fractional
shortening; ROS, reactive oxygen species; GRP78, glucose-regulated
protein 78; CHOP, C/EBP homologous protein; PKG, protein kinase G;
PDE5, cGMP-specific phosphodiesterase type 5; E, exercise; Sil,
sildenafil; TM, tunicamycin. |
Discussion
The present study identified that swimming exercise
significantly attenuated the negative effects of aging on cardiac
structure and myocardial function by suppressing the oxidative
stress and ER stress responses. In addition, it was found that the
cGMP-PKG cascade may serve an important role in the
exercise-induced decrease in ER stress in aged hearts. The present
results not only provide additional evidence supporting the
cardioprotective effects of swimming exercise on reducing cardiac
injuries in aged mice, but also emphasize the importance of the ER
stress-dependent cGMP-PKG cascade in the protective effects of
swimming exercise on the heart.
Cardiac aging alters cardiac filling function,
compliance and ventricular pump capacity, thus contributing to a
decrease in cardiac function (19). In the present study, the cardiac
structure and function were found to be impaired in aged mice. The
aging-induced deterioration of the cardiac structure and decreased
cardiac function were attenuated in mice performing swimming
exercise. Therefore, swimming exercise improved aging-induced
impairments in cardiac performance. Specific increases in oxidative
stress have been reported to represent potential factors that
determine the induction and maintenance of cellular senescence, and
the aging process (8,20,21).
An imbalance in ROS generation triggers oxidative stress, and ROS
play an important role in many physiological and pathological
processes (22). Additionally, SOD
and MDA are involved in oxidative stress defense (23). Regular physical exercise delays the
accumulation of ROS-mediated cell damage by improving antioxidative
protective mechanisms in the myocardium (24). Exercise has been reported to
increase SOD mRNA expression (25). As shown in the present study, aging
decreased SOD activity and increased MDA levels. However, swimming
exercise exerted beneficial effects by increasing the activity of
the antioxidant enzyme SOD, and by decreasing MDA and ROS
production.
Accumulating evidence demonstrated that oxidative
stress and the ER stress are associated events (17,18).
The ER-localized transmembrane kinase PERK is a major transducer of
ER stress (26). Normally, PERK is
maintained in an inactive, monomeric state by binding to GRP78.
When this binding is disrupted, PERK homodimerizes, undergoes
autophosphorylation, becomes active and initiates downstream
signaling (27). Protein
accumulation in the ER results in ER stress and ultimately
activates apoptosis. This pathway involves the upregulation of CHOP
(28). CHOP levels are elevated in
the liver in response to aging (29). Consistent with this previous study,
increased levels of CHOP were observed in aged hearts in the
present study. Swimming exercise reduced CHOP activity. Moreover,
in the present study, swimming exercise inhibited the increase in
GRP78 expression in aged hearts. The present results suggested that
swimming exercise protected the hearts of aged mice from oxidative
stimulation and ER stress.
The primary mediator of cGMP signaling is PKG, which
in turn phosphorylates multiple intracellular proteins in the
cardiovascular system (30). Both
cGMP and its downstream effector, the PKG signaling pathway, are
associated with markedly reduced infarct sizes (15) and regulate cardiac function
(13). Previously, aging has been
reported to be associated with the downregulation of cGMP-PKG
signaling in vascular smooth muscle cells (31) and postmenopausal women (32). The previous studies provided
evidence supporting the relationship between aging and the
increased risk of cardiovascular disease. The levels of cGMP and
PKG activity were decreased in aged hearts in the present study,
indicating that aging impaired myocardial cGMP-PKG signaling.
Consistent with the present results, previous studies reported a
significant downregulation of the cGMP-PKG axis in diabetic animals
(33) and in response to
myocardial ischemia/reperfusion injury (14). Therefore, decreased cGMP-PKG
signaling may be associated with aggravated damage induced by a
myocardial insult. The present study suggested that the reduced
levels of cGMP and PKG activity in the heart were ameliorated in
aged mice following swimming exercise. Taken together, the findings
of the present study indicated that the cGMP-PKG signaling pathway
may be involved in the protective effects of swimming exercise
against the aging-induced decline in cardiac function.
According to previous in vitro and in
vivo studies, cardiac cGMP levels are increased following
inhibition of the cGMP-degrading enzyme PDE5 (33–35).
PDE5 is expressed at low levels in the heart under normal
physiological conditions (36).
PDE5 expression is upregulated in myocardial samples from patients
with different heart diseases (37–39).
Theoretically, the blockade of the pathological effects of PDE5
should exert cytoprotective effects against different
cardiovascular diseases. Similarly, PDE5 inhibitors are useful
treatments that improve cGMP signaling to reduce cardiomyocyte
stiffness (34,35). In the present study, PDE5
expression was increased in aged hearts. Furthermore, PDE5
expression was decreased in aged mice after swimming exercise or
sildenafil treatment. The results of the present study further
support the hypothesis that the effects of swimming exercise on
aged hearts are potentiated via the activation of the cGMP-PKG
signaling pathway.
ER stress is increased in response to aging and
cardiovascular disease (40). The
cGMP-PKG signaling pathway has been reported to contribute to
cardioprotective mechanisms (14).
Gong et al (41) reported a
significant correlation between increased PDE5 expression and the
activation of ER stress in failing hearts. However, few studies
have examined the effects of the cGMP-PKG pathway on ER stress in
aged hearts. In the present study, treatment with the PDE5
inhibitor sildenafil or swimming exercise attenuated myocardial
injury and levels of ER stress-related proteins. A specific inducer
of ER stress, TM, was used to determine whether the activity of the
cGMP signaling pathway was suppressed by ER stress. TM abolished
the beneficial effects of swimming exercise on the aged hearts.
Based on the results from the present study, age-induced cardiac
dysfunction is associated with enhanced oxidative stress and ER
stress. By contrast, swimming exercise improved cardiac function by
inhibiting the oxidative stress and ER stress responses, which were
mediated through activation of the cGMP-PKG signaling pathway.
Collectively, the present study suggested that
swimming exercise improved myocardial function and exerted
beneficial effects on aged hearts. The underlying mechanism may be
explained by a swimming exercise-mediated increase in cGMP-PKG
signaling, causing an increase in the antioxidant response and
reductions in ER stress. The present study provided new insights
into the myocardial effects of physical activity, which may
facilitate the identification of novel therapeutic regimens for
age-related cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of China (grant nos. 81870172, 81670365,
81470438, 81601306 and 81671648) and the Key Problems of Social
Development Science and Technology of Shaanxi Province (grant nos.
2018SF-129, 2018ZDXM-SF-068 and 2018SF-114).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC, XMZ, MYZ, XLZ, MZZ and JY conceived and designed
the research. PC, XMZ, MYZ, GL, LH, HZ, JW, XW, KW, JZ, MR, BC,
XXZ, XLZ, MZZ and JY performed experiments. PC, XMZ, MYZ, GL, LH,
HZ, JW, XW, MZZ, JY and KW analyzed data. PC, XMZ, MYZ, JZ, BC,
XXZ, XLZ, MZZ, JY and MR drafted the manuscript. PC, XMZ, MYZ and
MZZ and JY edited and revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experimental procedures and protocols
were approved by The Ethics Committee of The Fourth Military
Medical University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
endoplasmic reticulum
|
|
TM
|
tunicamycin
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Yang X, Sreejayan N and Ren J: Views from
within and beyond: Narratives of cardiac contractile dysfunction
under senescence. Endocrine. 26:127–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu J, Xia S, Kalionis B, Wan W and Sun T:
The role of oxidative stress and inflammation in cardiovascular
aging. Biomed Res Int. 2014:6153122014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lakatta EG and Levy D: Arterial and
cardiac aging: Major shareholders in cardiovascular disease
enterprises: Part II: The aging heart in health: Links to heart
disease. Circulation. 107:346–354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozturk N, Olgar Y, Er H, Kucuk M and
Ozdemir S: Swimming exercise reverses aging-related contractile
abnormalities of female heart by improving structural alterations.
Cardiol J. 24:85–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bachschmid MM, Schildknecht S, Matsui R,
Zee R, Haeussler D, Cohen RA, Pimental D and Loo B: Vascular aging:
Chronic oxidative stress and impairment of redox
signaling-consequences for vascular homeostasis and disease. Ann
Med. 45:17–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belaya I, Suwa M, Chen T, Giniatullin R,
Kanninen KM, Atalay M and Kumagai S: Long-term exercise protects
against cellular stresses in aged mice. Oxid Med Cell Longev.
2018:28942472018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SR and Lee YC: Endoplasmic reticulum
stress and the related signaling networks in severe asthma. Allergy
Asthma Immunol Res. 7:106–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lesnefsky EJ, Chen Q and Hoppel CL:
Mitochondrial metabolism in aging heart. Circ Res. 118:1593–1611.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
da Luz G, Frederico MJ, da Silva S, Vitto
MF, Cesconetto PA, de Pinho RA, Pauli JR, Silva AS, Cintra DE,
Ropelle ER and De Souza CT: Endurance exercise training ameliorates
insulin resistance and reticulum stress in adipose and hepatic
tissue in obese rats. Eur J Appl Physiol. 111:2015–2023. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Wang S, Wier WG, Zhang Q, Jiang H,
Li Q, Chen S, Tian Z, Li Y, Yu X, et al: Exercise improves the
dilatation function of mesenteric arteries in postmyocardial
infarction rats via a PI3K/Akt/eNOS pathway-mediated mechanism. Am
J Physiol Heart Circ Physiol. 299:H2097–H2106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang QJ, Li QX, Zhang HF, Zhang KR, Guo
WY, Wang HC, Zhou Z, Cheng HP, Ren J and Gao F: Swim training
sensitizes myocardial response to insulin: Role of Akt-dependent
eNOS activation. Cardiovasc Res. 75:369–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puzzo D, Loreto C, Giunta S, Musumeci G,
Frasca G, Podda MV, Arancio O and Palmeri A: Effect of
phosphodiesterase-5 inhibition on apoptosis and beta amyloid load
in aged mice. Neurobiol Aging. 35:520–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takimoto E, Champion HC, Li M, Belardi D,
Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y and Kass DA:
Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and
reverses cardiac hypertrophy. Nat Med. 11:214–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inserte J and Garcia-Dorado D: The
cGMP/PKG pathway as a common mediator of cardioprotection:
Translatability and mechanism. Br J Pharmacol. 172:1996–2009. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu LM, Di WC, Dong X, Li Z, Zhang Y, Xue
XD, Xu YL, Zhang J, Xiao X, Han JS, et al: Melatonin protects
diabetic heart against ischemia-reperfusion injury, role of
membrane receptor-dependent cGMP-PKG activation. Biochim Biophys
Acta Mol Basis Dis. 1864:563–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prola A, Pires Da Silva J, Guilbert A,
Lecru L, Piquereau J, Ribeiro M, Mateo P, Gressette M, Fortin D,
Boursier C, et al: SIRT1 protects the heart from ER stress-induced
cell death through eIF2α deacetylation. Cell Death Differ.
24:343–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang P, Zhang M, Zhang X, Li G, Hu H, Wu
J, Wang X, Yang Z, Zhang J, Chen W, et al: B-type natriuretic
peptide attenuates endoplasmic reticulum stress in H9c2
cardiomyocytes underwent hypoxia/reoxygenation injury under high
glucose/high fat conditions. Peptides. 111:103–111. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Wang C, Zhou J, Sun A,
Hueckstaedt LK, Ge J and Ren J: Complex inhibition of autophagy by
mitochondrial aldehyde dehydrogenase shortens lifespan and
exacerbates cardiac aging. Biochim Biophys Acta Mol Basis Dis.
1863:1919–1932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gambino V, De Michele G, Venezia O,
Migliaccio P, Dall'Olio V, Bernard L, Minardi SP, Della Fazia MA,
Bartoli D, Servillo G, et al: Oxidative stress activates a specific
p53 transcriptional response that regulates cellular senescence and
aging. Aging Cell. 12:435–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vigneron A and Vousden KH: p53, ROS and
senescence in the control of aging. Aging (Albany NY). 2:471–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sikka SC and Hellstrom WJ: Role of
oxidative stress and antioxidants in Peyronie's disease. Int J
Impot Res. 14:353–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amin MM, Rafiei N, Poursafa P, Ebrahimpour
K, Mozafarian N, Shoshtari-Yeganeh B, Hashemi M and Kelishadi R:
Association of benzene exposure with insulin resistance, SOD, and
MDA as markers of oxidative stress in children and adolescents.
Environ Sci Pollut Res Int. 25:34046–34052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Golbidi S and Laher I: Exercise and the
cardiovascular system. Cardiol Res Pract. 2012:2108522012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao H, Liu J, Pan S, Sun Y, Li Q, Li F,
Ma L and Guo Q: SOD mRNA and MDA expression in rectus femoris
muscle of rats with different eccentric exercise programs and time
points. PLoS One. 8:e736342013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui W, Li J, Ron D and Sha B: The
structure of the PERK kinase domain suggests the mechanism for its
activation. Acta Crystallogr D Biol Crystallogr. 67:423–428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanderson TH, Deogracias MP, Nangia KK,
Wang J, Krause GS and Kumar R: PKR-like endoplasmic reticulum
kinase (PERK) activation following brain ischemia is independent of
unfolded nascent proteins. Neuroscience. 169:1307–1314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delbrel E, Soumare A, Naguez A, Label R,
Bernard O, Bruhat A, Fafournoux P, Tremblais G, Marchant D, Gille
T, et al: HIF-1α triggers ER stress and CHOP-mediated apoptosis in
alveolar epithelial cells, a key event in pulmonary fibrosis. Sci
Rep. 8:179392018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikeyama S, Wang XT, Li J, Podlutsky A,
Martindale JL, Kokkonen G, van Huizen R, Gorospe M and Holbrook NJ:
Expression of the pro-apoptotic gene gadd153/chop is elevated in
liver with aging and sensitizes cells to oxidant injury. J Biol
Chem. 278:16726–16731. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Breivik L, Jensen A, Guvåg S, Aarnes EK,
Aspevik A, Helgeland E, Hovland S, Brattelid T and Jonassen AK:
B-type natriuretic peptide expression and cardioprotection is
regulated by Akt dependent signaling at early reperfusion.
Peptides. 66:43–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin CS, Liu X, Tu R, Chow S and Lue TF:
Age-related decrease of protein kinase G activation in vascular
smooth muscle cells. Biochem Biophys Res Commun. 287:244–248. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cui R, Iso H, Yamagishi K, Ohira T,
Tanigawa T, Kitamura A, Kiyama M, Imano H, Konishi M and Shimamoto
T: Relationship of urinary cGMP excretion with aging and menopausal
status in a general population. J Atheroscler Thromb. 16:457–462.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mátyás C, Németh BT, Oláh A, Török M,
Ruppert M, Kellermayer D, Barta BA, Szabó G, Kökény G, Horváth EM,
et al: Prevention of the development of heart failure with
preserved ejection fraction by the phosphodiesterase-5A inhibitor
vardenafil in rats with type 2 diabetes. Eur J Heart Fail.
19:326–336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Chopp M, Szalad A, Liu Z, Bolz M,
Alvarez FM, Lu M, Zhang L, Cui Y, Zhang RL and Zhang ZG:
Phosphodiesterase-5 is a therapeutic target for peripheral
neuropathy in diabetic mice. Neuroscience. 193:399–410. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang M and Kass DA: Phosphodiesterases
and cardiac cGMP: Evolving roles and controversies. Trends
Pharmacol Sci. 32:360–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheitlin MD, Hutter AM Jr, Brindis RG,
Ganz P, Kaul S, Russell RO Jr and Zusman RM: ACC/AHA expert
consensus document. Use of sildenafil (Viagra) in patients with
cardiovascular disease. American College of Cardiology/American
Heart Association. J Am Coll Cardiol. 33:273–282. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rashid M, Kotwani A and Fahim M:
Long-acting phosphodiesterase 5 inhibitor, tadalafil, and
superoxide dismutase mimetic, tempol, protect against acute
hypoxia-induced pulmonary hypertension in rats. Hum Exp Toxicol.
31:626–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu
G, Fassett J, Tao Y, Zhang P, dos Remedios C, et al: Oxidative
stress regulates left ventricular PDE5 expression in the failing
heart. Circulation. 121:1474–1483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kukreja RC, Salloum FN and Das A: Cyclic
guanosine monophosphate signaling and phosphodiesterase-5
inhibitors in cardioprotection. J Am Coll Cardiol. 59:1921–1927.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang C, Syed TW, Liu R and Yu J: Role of
endoplasmic reticulum stress, autophagy, and inflammation in
cardiovascular disease. Front Cardiovasc Med. 4:292017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong W, Duan Q, Cai Z, Chen C, Ni L, Yan
M, Wang X, Cianflone K and Wang DW: Chronic inhibition of
cGMP-specific phosphodiesterase 5 suppresses endoplasmic reticulum
stress in heart failure. Br J Pharmacol. 170:1396–1409. 2013.
View Article : Google Scholar : PubMed/NCBI
|