Introduction
Liver transplantation (LT) is the most effective
treatment for end-stage liver diseases; however, donor shortage has
confined the application of LT (1). Donation after brain death (DBD) may
possibly overcome the severe imbalance between organ transplant
demand and recipients. On the contrary, BD is a complex
pathophysiologic process which may affects donor organs. Compared
with living donors, there is an approximate graft survival rate in
adult liver transplantation while in pediatric liver
transplantation the graft survival rate of DBD donor was
significantly lower than a living donor or split-liver
transplantation (2). It has been
demonstrated that the primary factor of BD-induced liver damage was
the Cushing reaction, and the subsequent severe changes in the
hemodynamic and oxygenation state (3). Previously, it was indicated that BD
was associated with the endoplasmic reticulum (ER) (4,5). The
ER is one of the most important organelles involved in protein
synthesis and folding (6). While
many adverse factors can obstruct the physiological function of the
ER, leading to the damage this organelle, ER stress (ERS) can occur
through protein kinase RNA-like ER kinase (PERK),
inositol-requiring enzyme 1α (IRE1α) and activating transcription
factor 6 (ATF6) pathways, which would finally induce apoptosis
(7,8). Protein phosphatase 2A (PP2A) is a
major serine/threonine phosphatase in cells. It plays critical
roles in many physiological processes such as cell proliferation,
migration, cell cycle control and death (9). Currently, several studies have
revealed that ERS could induce activation of PP2A resulting in
apoptosis (10–12). The present study aimed to
investigate the relationship between ERS and PP2A in liver cell
damage after BD.
Materials and methods
Animals and grouping
Thirty male adult Sprague-Dawley rats, weighing
300±20 g, ~8 weeks of age were obtained from the Centers for
Disease Control and Prevention of Wuhan (Wuhan, China), and were
maintained in the Animal Experimental Center of Zhongnan Hospital,
Wuhan University (Wuhan China). Prior to the experiments, standard
chow and water were given to the rats ad libitum, and they
were housed at 20–25°C and 50–70% humidity with a 12 h light/dark
cycle. All the rats were randomized into three groups equally:
Sham-operation group (S group), BD group, and 4-phenylbutyric acid
group (4-PBA group), with 10 rats in each group. All animals were
fed in the Animal Experiment Center of Zhongnan Hospital of Wuhan
University 7 days before the experimental operation. All experiment
operations were performed in accordance with the Experimental
Animal Regulations of the People's Republic of China and the Guide
for the Care and Use of Laboratory Animals (13). Prior to the establishment of animal
model all animals were fasted overnight but had free access to
water.
Establishment of animal model and
specimens harvesting
The BD model was established by intracranial
inflating (14). All animals were
anesthetized with pentobarbital solution (50 mg/kg) via an
intraperitoneal injection; atropine solution (0.03 mg/kg) was used
to decrease secretion of glands. Following anesthesia, rats were
fixed on a homeothermic warming blanket linking to an Intelligent
Temperature Controller (Taimeng Biological Technology Co., Ltd.)
with the temperature probe placed into the anus to maintain
temperature at ~38±1°C. Skin of calva was prepared. The scalp and
the epicranial muscles and periosteum were excised. A 2-mm diameter
hole was drilled on the skull at the intersection of sagittal line
and the midpoint of two eyes. A 3F Fogarty catheter balloon
(Fogarty Arterial Embolectomy Catheter, 3F, Edwards Lifesciences
Co.) was put into the epidural space completely through the burr
hole. Then, the catheter was gradually inflated to a final volume
of ~2,000 ml. The femoral artery was catheterized to monitor blood
pressure. The femoral vein was catheterized to prepare for fluid
administration. An electrocardiogram and electroencephalogram were
monitored using the Biological Function Date Acquisition and
Monitoring System (Taimeng Biological Technology Co., Ltd.);
tracheal intubation was also performed. The criterions of BD were
referred to China Adult Brain Death Determination Technology
Specifications and USA Adult Brain Death Diagnostics Guide
(15). Rats of the BD group had
underwent BD which was maintained for 6 h. Sham-operation rats were
anesthetized, skull-drilled and intracranial catheterized but
without inflating; anesthesia was maintained for 6 h. 4-PBA was
administered 300 mg/kg via an intraperitoneal injection when the BD
model was established; liver specimens were harvested 6 h after
BD.
Exposure of the liver
After 6 h of BD, the peritoneal cavity was opened.
The falciform ligament and bilateral deltoid ligament were moved to
carefully expose the liver. The liver was gently moved with a
sterile swab and then placed on a piece of sterile gauze. The color
and appearance of the liver was analyzed under a fine-light
view.
Histology
Following reperfusion, about 0.25 g liver samples
were fixed with 10% buffered formalin for 24 h at room temperature
(pH=7.2; cat. no. G2161; Beijing Solarbio Science & Technology,
Co., Ltd.), then put into 75, 80, 85, 90, 95 and 100% alcohol for 1
h respectively, so as to fully dehydrate the tissues. Following
dehydration, the tissues were soaked in 50% alcohol + 50% xylene
solution for 30 min, and then soaked in 100% xylene until they
became transparent. The transparent samples were soaked in liquid
paraffin at 60°C twice (1 h each) until the paraffin had completely
infiltrated. The samples after paraffin immersion were embedded in
paraffin wax and cut into 4-µm sections for histological analysis
via hematoxylin-eosin staining (H&E; hematoxylin staining for
5–15 min and eosin staining for 1–3 min; all performed at room
temperature). Sections were analyzed under a confocal microscope
(magnification, ×200; Nikon A1R/A1; Nikon Corporation) and images
were obtained. A total of 6 fields of view per section were
randomly selected for the assessment of liver damage. Numerical
assessment of liver damage was conducted according to the
histological criteria for assessment of liver damage.
Western blot analyses
Protein expression of glucose-regulated protein 78
(Grp78), ATF6, PP2A and phosphorylated PP2A (PP2A Y307) were
examined by western blotting. Frozen liver tissues were cut into
pieces and solubilized in radioimmunoprecipitation assay buffer
(cat. no. G2002; Wuhan Servicebio Technology Co., Ltd.) with
phenylmethanesulfonyl fluoride 10 µl/ml. The turbid liquid was
centrifuged for 15 min at 12,000 × g at 4°C. Protein concentration
of the supernatant was determined by the bicinchoninic acid kit
(cat. no. G2026; Wuhan Servicebio Technology Co., Ltd.). Then, the
extracted protein was mixed with 5X loading buffer containing
bromophenol blue as an indicator, which was then heated to 100°C
for 10 min. 10% SDS-PAGE was performed with equal protein quantity
each group using a constant voltage of 80 V in spacer gel and 120 V
in separation gel. After electrophoresis, proteins were transferred
to a polyvinylidenedifluoride filter membrane with a constant
electric current of 278 mA in a mixture of ice and water. The
membrane was blocked in 5% nonfat milk for 1 h at room temperature
and incubated overnight at 4°C with anti-Grp78 (1:1,000, bs-1219R,
Beijing Biosynthesis Biotechnology Co., Ltd., China), anti-ATF6
(1:1,000, bs-1634R, Beijing Biosynthesis Biotechnology Co., Ltd.,
China), anti-total PP2A (1:1,000, ab32104, Abcam), anti-PP2A Y307
(1:1,000, AF1756, Beyotime Institute of Biotechnology) and
anti-β-actin (1:3,000, AF0003, Beyotime Institute of
Biotechnology). Following washing with TBS-T, the membranes were
then incubated for 2 h in anti-rabbit IgG (1:3,000, GB23303, Wuhan
Goodbio Technology Co. Ltd.) at 37°C. Finally, the bands were
visualized using an enhanced chemiluminescence reagent (cat. no.
G2020; Wuhan Servicebio Co., Ltd.). Then, the bands were scanned
and analyzed with Image-Pro Plus v6.0 software (Media Cybernetics,
Inc.).
Activation of PP2A
The activation of PP2A was detected with a
quantitative detection kit (GMS50042.3, Genmed Scientific Inc.) by
reactive colorimetry. Washing buffer (3 ml) was added to 500 mg
liver tissue of each group; following washing they were placed in
liquid nitrogen overnight. The next day 500 µl lysate buffer was
added to each group and fully lysed with vortex oscillation. Then
all the lysate were incubated for 30 min at 4°C and centrifuged for
5 min at 16,000 × g. Protein concentration of each group was
detected by the bicinchoninic acid kit (cat. no. G2026; Wuhan
Servicebio Technology Co., Ltd.) for later use. According to the
protocol, the standard curves were drawn with negative and standard
solutions of different concentrations. Then, 20 µl protein lysates
of each group were taken to measure the background phosphorus
concentration, total active phosphorus concentration and
non-specific active phosphorus concentration by spectrophotometer
(Shanghai CHOIF Co., Ltd.) and the specific phosphorus
concentration of each sample was calculated according to the
manufacturer's protocol.
Terminal deoxynucleotidyl
transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end
labeling (TUNEL) assay
Cell apoptosis of each group was detected by TUNEL
assay. Detection was performed with the One Step TUNEL Apoptosis
Assay Kit (Beyotime Institute of Biotechnology); 4-µm paraffin
sections were deparaffinized in xylene (Sinopharm Chemical Reagent
Co., Ltd.) three times (10 min each) and rehydrated in alcohol
(anhydrous, 95, 80, 75%) and double distilled water successively, 5
min each. Then, the sections were treated with proteinase K for 20
min at room temperature and subsequently incubated at room
temperature with a mixture of fluorescent labeling solution and TdT
enzyme for 1 h in a the dark. After washing in PBS and drying,
sections were incubated with DNase I for 10 min in the dark at room
temperature. Then the sections were gently shaken to dry, DAPI
staining solution was added to the tissue, incubated in dark for 10
min at room temperature. Finally the sections were washed with PBS
for 3 times, 5 min each time, and sealed with anti-fluorescence
quenching agent. The fluorescein isothiocyanate-labeled
TUNEL-positive cells were imaged using fluorescent microscopy with
488 nm excitation and 530 nm emission wavelengths, and DAPI with
364 nm excitation and 454 nm emission wavelengths. The cells
labeled with green fluorescence were deemed apoptotic cells.
Statistical analysis
Statistical analysis was performed with SPSS for
windows version 17.0 (SPSS, Inc.). Continuous variables were
presented as the mean ± standard deviation unless explicitly noted
otherwise. Statistical significance between groups was tested by
one-way ANOVA with Tukey's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
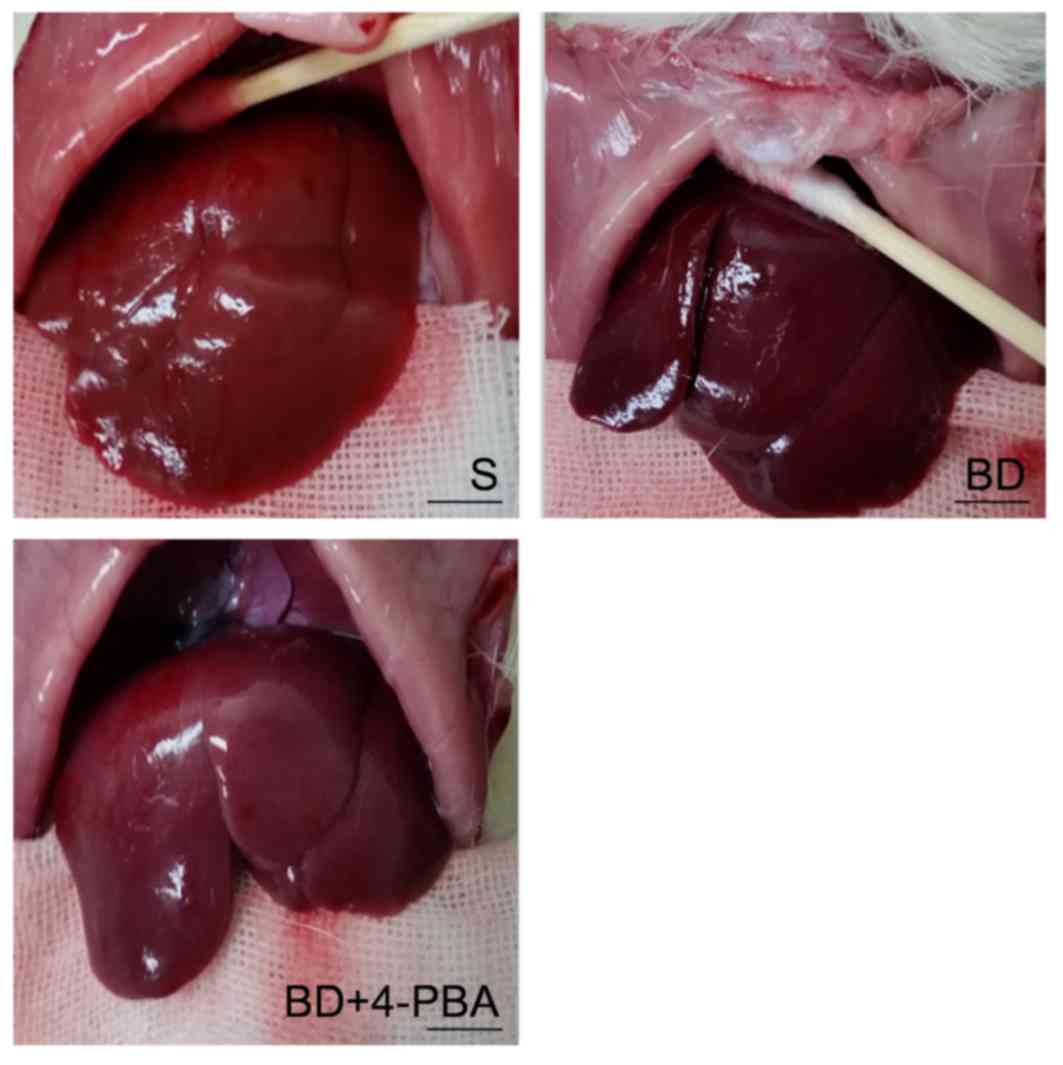
Macroscopic appearance of livers
As presented in Fig.
1, variations in the macroscopic appearance of livers were
observed. Livers of the S group were normally red, with a clear
edge and smooth surface. BD livers were aubergine in color and the
edge of liver was blunt with notable edema. 4-PBA livers had a more
pronounced shape and were brighter in color than the BD group, but
less so than the S group.
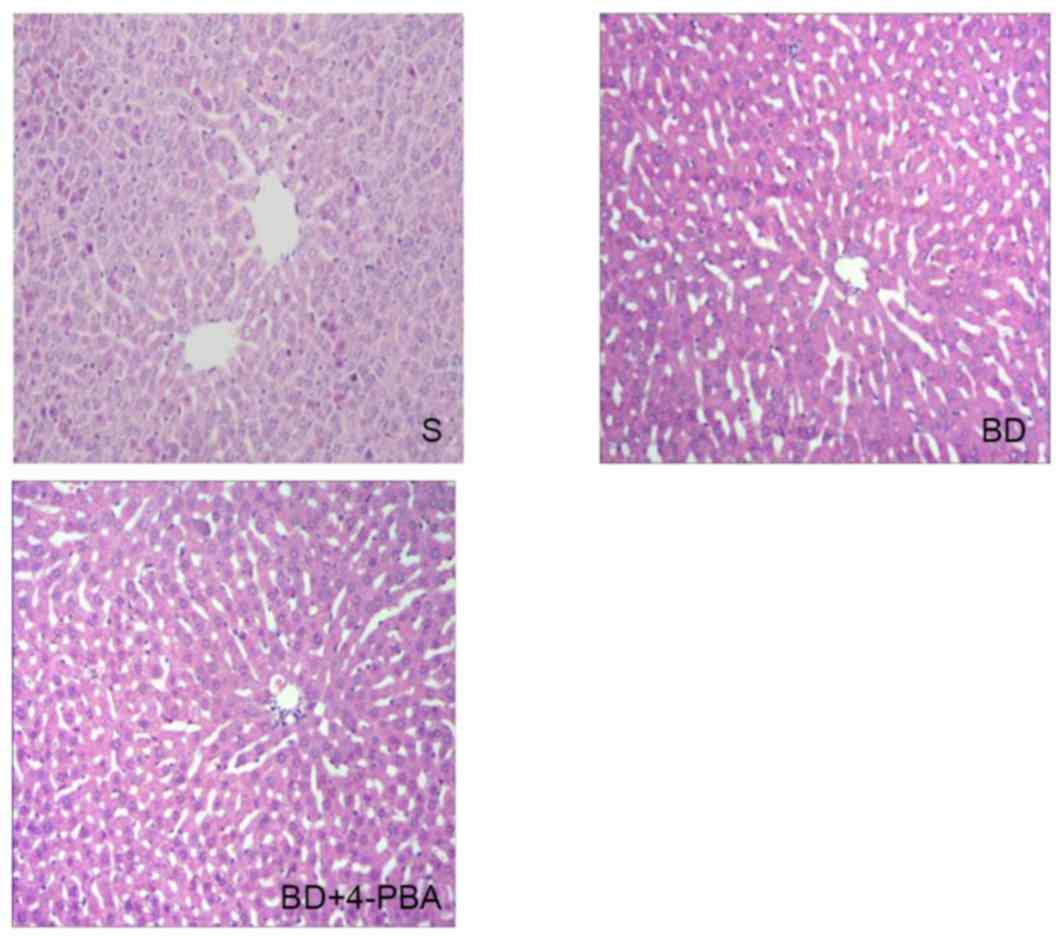
Histological changes
Hematoxylin-eosin staining was performed using the
liver sections of the three groups, which were then observed under
an optical microscope; representative images of analysis were
presented in Fig. 2. In the S
group, livers were histologically normal, as depicted by an orderly
matrix of the hepatic sinusoid and an integrated hepatic lobule. In
the BD group the hepatic sinusoid was misaligned; the hepatic
lobule was malformed, while central venous endothelial injury,
swelled liver cells and apoptosis were observed. Treatment with
4-PBA lessened the injury of to the hepatic lobule associated with
BD.
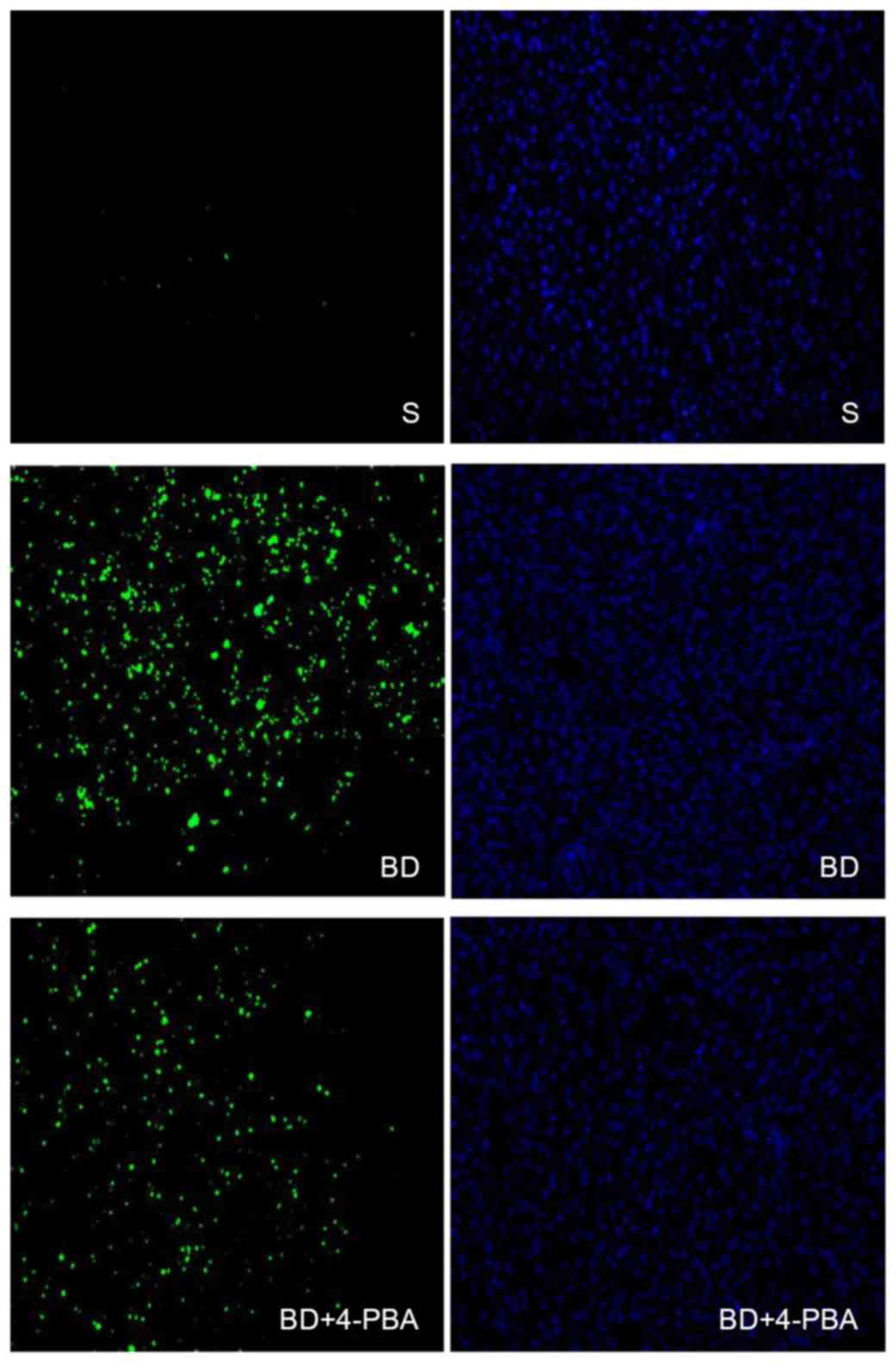
BD promotes the apoptosis of liver
cells
The extent of liver cell apoptosis was presented in
Fig. 3. Liver specimens after
TUNEL straining were observed with an inverted fluorescence
microscope. Green fluorescence was positive a result of the TUNEL
assay, which indicated cell death, while blue fluorescence
indicated positive DAPI staining for the total number of cells. The
results showed that the apoptosis of the S group was only 10±1%,
which is significantly lower than the BD (80±2%, P<0.05) and
4-PBA (68±2%, P<0.05) groups, while differences between the
4-PBA and BD groups were statistically significant (P<0.05;
Table I).
 | Table I.Quantification of TUNEL assay. |
Table I.
Quantification of TUNEL assay.
|
| S (%) | BD (%) | BD+4-PBA (%) |
|---|
| TUNEL positive | 10±1 |
8±2 | 68±2 |
| DAPI positive | 93±2 | 93±2 | 92±1 |
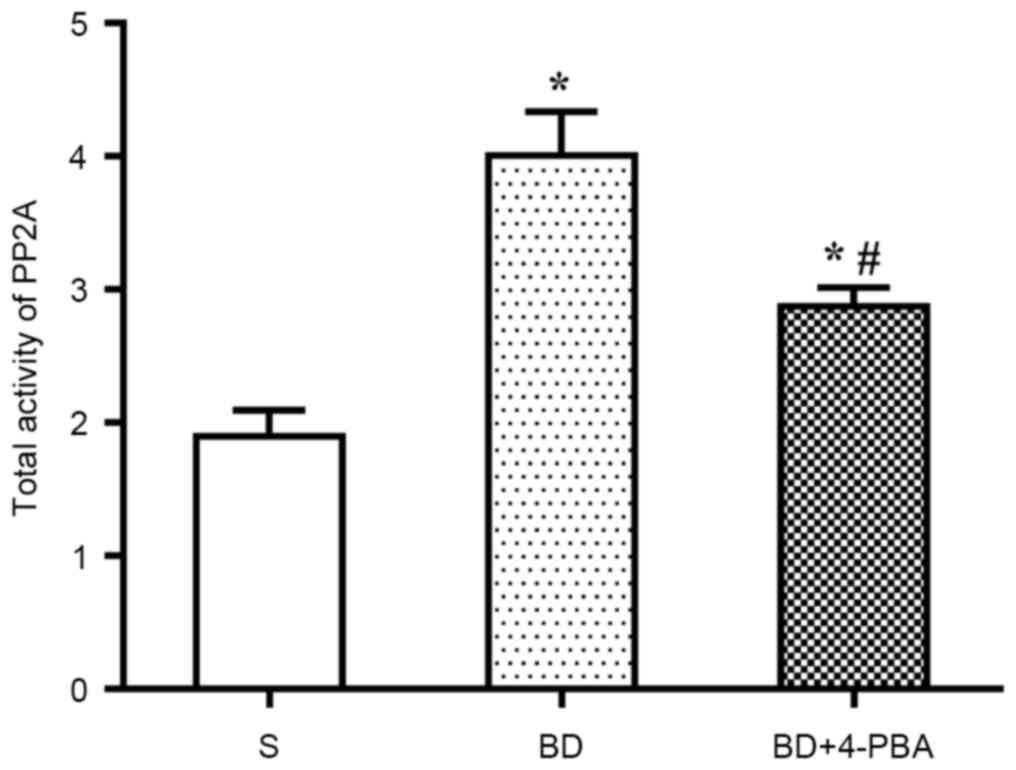
BD increases the activation of
PP2A
Activation of PP2A was detected via colorimetry. As
shown in Fig. 4, compared with the
S group (1.898±0.337 nmolP/mg/min), the PP2A activity of the BD
(4.008±0.566 nmolP/mg/min, P=0.005) and 4-PBA (2.874±0.241
nmolP/mg/min, P=0.015) groups were significantly increased. In
addition, PP2A activation in the BD group was significantly higher
than in the 4-PBA group (P=0.033).
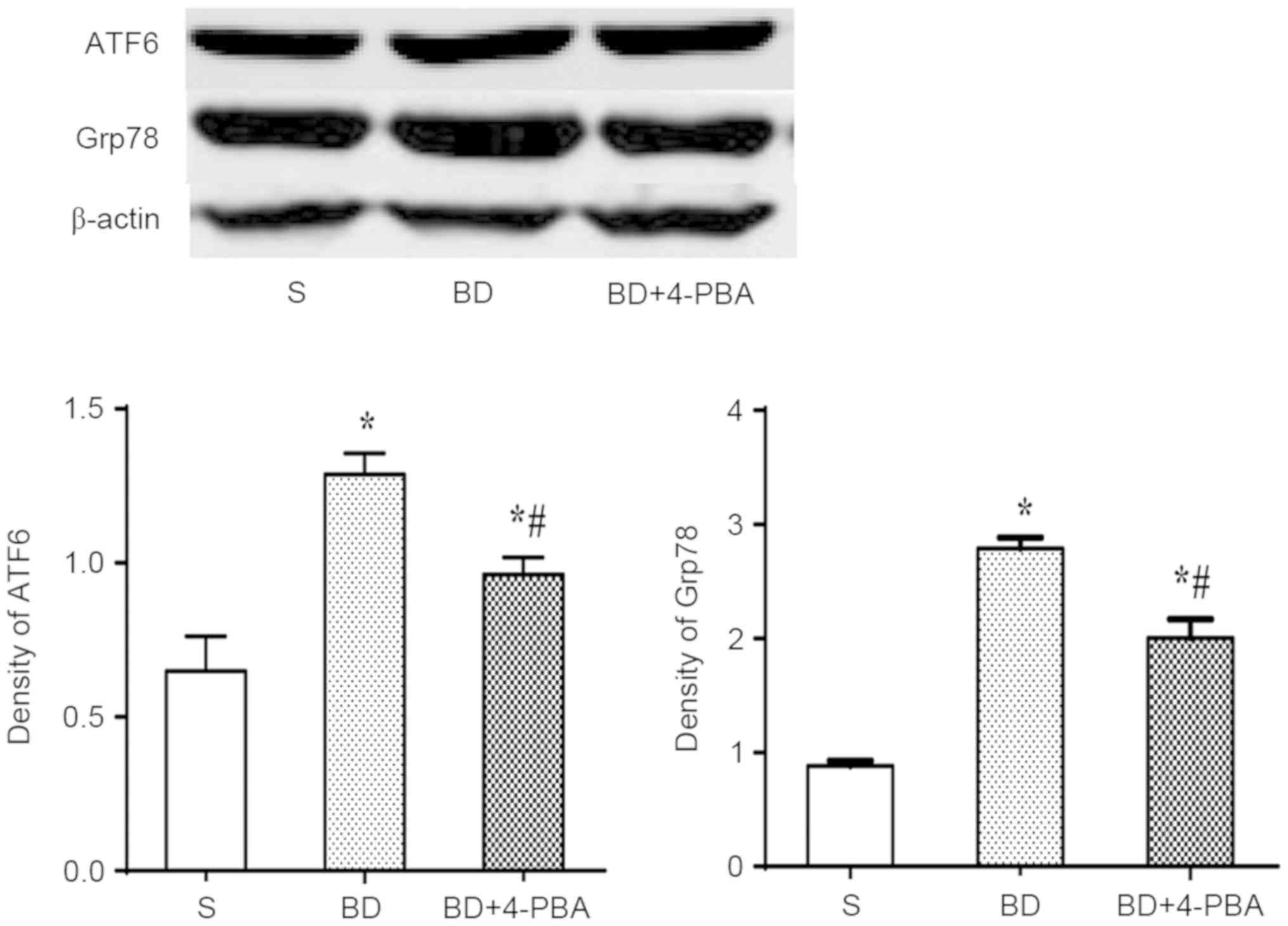
BD promotes the expression of Grp78
and ATF6
The expression of Grp78 and ATF6 was detected by
western blotting to assess the activation of ERS (Fig. 5). The results showed that the
expression of Grp78 was significantly increased in the BD
(2.792±0.151, P<0.001) and 4-PBA (2.011±0.174, P=0.003) group
compared with the S group (0.881±0.014). Consistent with the
increase Grp78 expression, ATF6 expression was also increased
significantly in the BD (1.287±0.116, P<0.01) and 4-PBA
(0.961±0.108, P=0.014) groups than the S group (0.692±0.155). The
expression of Grp78 and ATF6 after 4-PBAtreatment were
significantly lower than in the BD group (P=0.015 and 0.003,
respectively).
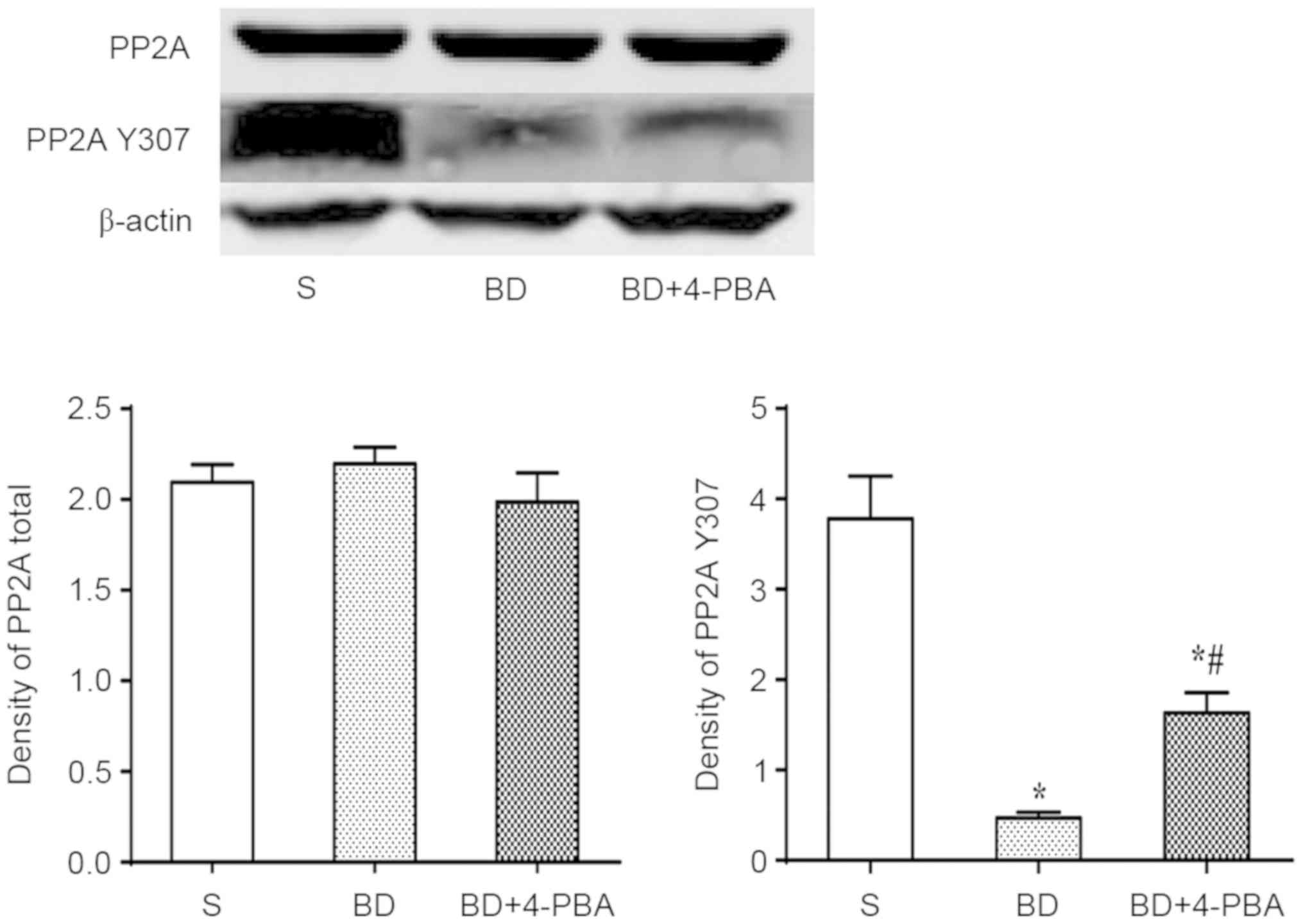
BD suppresses the phosphorylation of
PP2A, which is reversed by4-PBA
Total expression of total PP2A and phosphorylated
PP2A (PP2A Y307) were detected by western blotting. The results
indicated no significant differences in total PP2A between the S
and BD (2.096±0.101 vs. 2.197±0.109, P=0.586; Fig. 6). The expression of PP2A Y307 was
significantly decreased after BD than in the S group (0.467±0.066
vs. 3.780±0.473, P=0.002), indicating that BD suppressed the
phosphorylation of PP2A, which promoted the activation of PP2A. In
addition, the expression of PP2A Y307 of the 4-PBA (1.632±0.222)
group was significantly higher than the BD group (P=0.007) but
lower than the S group (P=0.015; Fig.
6).
Discussion
Since its clinical application, LT has saved the
lives of thousands of patients with end-stage liver diseases
globally. The majority of donations in China are post
mortem. The use of DBD organs has expanded the donor pool
effectively, yet BD has been confirmed to be involved in a series
of pathophysiological processes associated with liver damage,
including hemodynamic instability, inflammatory responses,
immunological changes and cytokine-induced liver cell apoptosis
(16). A previous study showed
that ERS may play a role in BD-induced cell damage, but the
underlying mechanisms remain unclear (17). To reveal the possible relationship
between ERS and liver damage, a rat BD model was established by
gradual intracranial inflating. The present study demonstrated that
the expression of Grp78 and ATF6 were increased significantly under
conditions of BD. Grp78, also known as immunoglobulin heavy chain
binding protein, is a member of the heat shock protein 70 protein
family (18). GRP78 is an ER
chaperone protein that primarily resides in the lumen of the ER and
is the master regulator of the unfolded protein response (UPR)
(19), binding to all 3 ERS
sensors (PERK, ATF6 and IRE1) and inhibiting their function under
normal physiological condition (20). ER stress could increase the
expression of Grp78 and promote Grp78 translocation to the cell
surface, causing activation of the ERS sensors (21). A previous study showed that Grp78
could inhibit breast cancer cell apoptosis via AKT/mitochondrial
pathway (22). Additionally,
reducing the expression of Grp78 resulted in the poor prognosis of
patients with advanced head cancer (23). ATF6 is one member of the ATF/CREB
family and serves as a transmembrane protein of ER (24). It is a membrane-bound transcription
factor in ER and regulates many pathophysiological processes
including apoptosis (25),
pediatric inflammatory bowel disease (26) and odontoblastic differentiation
(27). ATF6 is localized at the ER
under basal conditions (24). In
cells undergoing ERS, ATF6 translocates to the Golgi apparatus and
is processed by site 1 protease and site 2 protease releasing
ATF6f, which controls the upregulation of select UPR target genes
(28). Cleaved ATF6 could also
increase the expression of Grp78 (29), and Grp78 could induce apoptosis
through the mitochondrial and non-mitochondrial pathway (30). Therefore, ATF6 and Grp78 could be
considered as biomarkers of ERS.
PP2A is a major serine/threonine phosphatase in
cells. Only two forms have been observed in cells until now. The
holoenzyme of PP2A contains a structural A subunit, a regulatory B
subunit and a catalytic C subunit (9). The core enzyme is a dimer consisting
of an A subunit and a C subunit. The C subunit alone was not found
in vivo (9). It has been
shown that A subunits regulate PP2A holoenzyme composition, and
catalytic activity of PP2A is mainly depend on C subunits (31). B subunits exert a wide variety of
effects. Different B subunits have different structures,
localization and functions to the PP2A homoenzyme (32,33).
A previous study showed that activation of PP2A induces apoptosis
by dephosphorylating Bad and Bcl-2 directly (34). In addition, PP2A could also induce
apoptosis through the MAPK and AKT/PKB pathways (35,36).
Phosphorylation decreased the catalytic activation of PP2A
(37). There have been many
studies that have revealed that activation of ERS and PP2A may have
a synergistic effect on cell damage under different pathological
states (38–40). According to a study on
neurodegenerative disorders, after rat brain endothelial cells were
exposed to okadaic acid, a well-known inhibitor of PP2A, inhibition
of PP2A and an increase in ERS markers expression were observed,
which suggests that inhibition of PP2A affected ERS (41). Furthermore, research in acute
pancreatitis indicated that disulfide stress as a novel type of
oxidative stress could activate ERS, and the expression of
catalytic subunit of protein phosphatase 2A was increased as well
(42). ERS could trigger apoptosis
by activating Bim in melanoma cancer cells, while suppression of
PP2A could reduce apoptosis induced by ERS, which suggested that
ERS may cause cell death via the PP2A pathway (43). In addition, a previous study showed
that in liver cells that underwent oxidative stress, the expression
of ceramide was increased (44);
in an alcoholic liver disease animal model, activation of ERS and
upregulation of ceramide, as well as PP2A activation were observed
(45). However, further
investigation into ERS and PP2A under BD are required.
The present study established a BD model in
Sprague-Dawley rats. The apoptosis of liver cells was detected by a
TUNEL assay, which suggested that there was a significant increase
in apoptosis after BD. The expression of biomarkers of ERS in liver
tissues were detected by western blotting. The results showed that
expression of Grp78 and ATF6 were increased significantly after BD,
indicating that BD could induce ERS in donor livers. Meanwhile,
activation of PP2A and the expression of total PP2A and PP2A Y307
were examined. Activation of PP2A significantly increased after BD,
but the expression of total PP2A showed no notable differences
between the BD and S groups. Furthermore, the expression of PP2A
Y307 was decreased significantly after BD, indicating that BD may
increase the activation of PP2A by suppressing phosphorylation of
PP2A. To investigate the possible relationship between ERS and
PP2A, 4-PBA was applied via an intraperitoneal injection when BD
was established to suppress the activation of ERS. It was found
that 4-PBA decreased the expression of Grp78 and ATF6, while
activation of PP2A was reduced. In addition, the expression of PP2A
Y307 was increased following 4-PBA treatment. In conclusion, BD was
determined to induce the activation of ERS, subsequently activating
PP2A through suppressing its phosphorylation, which leads to liver
cell apoptosis, and finally donor liver damage.
Acknowledgements
Not applicable.
Funding
The research was supported by the State Key Program
of National Natural Science of China (grant no. U1403222); Natural
Science Foundation of Hubei province (grant no. 2015CFB208).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and ZZ performed the research, established the
rat brain death model, collected liver specimens, analyzed the
data, and wrote the article. YX and YW designed the experiment and
helped experiment operations. QY designed the experiments, provided
overall guidance, and helped with the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Wuhan
University for Animal Experiment/ABSL-III Laboratory.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BD
|
brain death
|
|
DBD
|
donation after brain death
|
|
4-PBA
|
phenylbutyric acid
|
|
ERS
|
endoplasmic reticulum stress
|
|
PP2A
|
protein phosphatase 2A
|
|
Grp78
|
glucose-regulated protein 78
|
|
PERK
|
protein kinase RNA-like ER kinase
|
|
IRE1α
|
inositol-requiring enzyme 1α
|
|
ATF6
|
activating transcription factor 6
|
References
|
1
|
Wertheim JA, Petrowsky H, Saab S,
Kupiec-Weglinski JW and Busuttil RW: Major challenges limiting
liver transplantation in the United States. Am J Transplant.
11:1773–1184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adam R, Karam V, Cailliez V, O Grady JG,
Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J,
Jamieson N, et al: 2018 annual report of the European Liver
Transplant Registry (ELTR)-50-year evolution of liver
transplantation. Transpl Int. 31:1293–1317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belzberg H, Shoemaker WC, Wo CC, Nicholls
TP, Dang AB, Zelman V, Gruen JP, Berne TV and Demetriades D:
Hemodynamic and oxygen transport patterns after head trauma and
brain death: Implications for management of the organ donor. J
Trauma. 63:1032–1042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carlessi R, Lemos NE, Dias AL, Oliveira
FS, Brondani LA, Canani LH, Bauer AC, Leitão CB and Crispim D:
Exendin-4 protects rat islets against loss of viability and
function induced by brain death. Mol Cell Endocrinol. 412:239–250.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao S, Wang T, Yan B, Lu Y, Zhao Y and
Zhang S: Brain death is associated with endoplasmic reticulum
stress and apoptosis in rat liver. Transplant Proc. 46:3297–3302.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salvador-Gallego R, Hoyer MJ and Voeltz
GK: SnapShot: Functions of endoplasmic reticulum membrane contact
sites. Cell. 171:1224.e12017. View Article : Google Scholar
|
|
7
|
Wang S and Kaufman RJ: The impact of the
unfolded protein response on human disease. J Cell Biol.
197:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janssens V and Goris J: Protein
phosphatase 2A: A highly regulated family of serine/threonine
phosphatases implicated in cell growth and signalling. Biochem J.
353:417–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guichard C, Pedruzzi E, Fay M, Marie JC,
Braut-Boucher F, Daniel F, Grodet A, Gougerot-Pocidalo MA, Chastre
E, Kotelevets L, et al: Dihydroxyphenylethanol induces apoptosis by
activating serine/threonine protein phosphatase PP2A and promotes
the endoplasmic reticulum stress response in human colon carcinoma
cells. Carcinogenesis. 27:1812–1827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christen V, Treves S, Duong FH and Heim
MH: Activation of endoplasmic reticulum stress response by
hepatitis viruses up-regulates protein phosphatase 2A. Hepatology.
46:558–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu Y, Mao T, Zhang Y, Shao M, You J, Ding
Q, Chen Y, Wu D, Xie D, Lin X, et al: A crucial role for RACK1 in
the regulation of glucose-stimulated IRE1alpha activation in
pancreatic beta-cells. Sci Signal. 3:ra72010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council: Guide for the
care and use of laboratory animals. 8th. The National Academies
press; Washington, DC: 2011
|
|
14
|
Pratschke J, Wilhelm MJ, Kusaka M,
Laskowski I and Tilney NL: A model of gradual onset brain death for
transplant-associated studies in rats. Transplantation. 69:427–430.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wijdicks EF: Brain death guidelines
explained. Semin Neurol. 35:105–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Der Hoeven JA, Moshage H, Schuurs T,
Nijboer M, Van Schilfgaarde R and Ploeg RJ: Brain death induces
apoptosis in donor liver of the rat. Transplantation. 76:1150–1154.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao S, Yan B, Lu Y, Zhang G, Li J, Guo W,
Zhao Y and Zhang S: C/EBP homologous protein-mediated endoplasmic
reticulum stress-related renal apoptosis is involved in rats with
brain death. Transplant Proc. 47:354–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rose MD, Misra LM and Vogel JP: KAR2, a
karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78
gene. Cell. 57:1211–1221. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gifford JB, Huang W, Zeleniak AE, Hindoyan
A, Wu H, Donahue TR and Hill R: Expression of GRP78, master
regulator of the unfolded protein response, increases
chemoresistance in pancreatic ductal adenocarcinoma. Mol Cancer
Ther. 15:1043–1052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida H, Matsui T, Hosokawa N, Kaufman
RJ, Nagata K and Mori K: A time-dependent phase shift in the
mammalian unfolded protein response. Dev Cell. 4:265–271. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee AS: The ER chaperone and signaling
regulator GRP78/BiP as a monitor of endoplasmic reticulum stress.
Methods. 35:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie J, Tao ZH, Zhao J, Li T, Wu ZH, Zhang
JF, Zhang J and Hu XC: Glucose regulated protein 78 (GRP78)
inhibits apoptosis and attentinutes chemosensitivity of gemcitabine
in breast cancer cell via AKT/mitochondrial apoptotic pathway.
Biochem Biophys Res Commun. 474:612–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaira K, Toyoda M, Shimizu A, Imai H,
Sakakura K, Nikkuni O, Suzuki M, Iijima M, Asao T and Chikamatsu K:
Prognostic significance of GRP78/BiP expression in patients with
Stage III/IV hypopharyngeal squamous cell carcinoma. Neoplasma.
63:477–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. NatRev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar
|
|
25
|
Morishima N, Nakanishi K and Nakano A:
Activating transcription factor-6 (ATF6) mediates apoptosis with
reduction of myeloid cell leukemia sequence 1 (Mcl-1) protein via
induction of WW domain binding protein 1. J Biol Chem.
286:35227–35235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Negroni A, Prete E, Vitali R, Cesi V, Aloi
M, Civitelli F, Cucchiara S and Stronati L: Endoplasmic reticulum
stress and unfolded protein response are involved in paediatric
inflammatory bowel disease. Dig Liver Dis. 46:788–794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JW, Choi H, Jeong BC, Oh SH, Hur SW,
Lee BN, Kim SH, Nör JE, Koh JT and Hwang YC: Transcriptional factor
ATF6 is involved in odontoblastic differentiation. J Dent Res.
93:483–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hetz C, Chevet E and Harding HP: Targeting
the unfolded protein response in disease. Nat Rev Drug Discov.
12:703–719. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuda M, Kondoh N, Imazeki N, Tanaka K,
Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al:
Activation of the ATF6, XBP1 and grp78 genes in human
hepatocellular carcinoma: A possible involvement of the ER stress
pathway in hepatocarcinogenesis. J Hepatol. 38:604–614. 2003.
View Article : Google Scholar
|
|
30
|
Grkovic S, O'Reilly VC, Han S, Hong M,
Baxter RC and Firth SM: IGFBP-3 binds GRP78, stimulates autophagy
and promotes the survival of breast cancer cells exposed to adverse
microenvironments. Oncogene. 32:2412–2420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruediger R, Fields K and Walter G: Binding
specificity of protein phosphatase 2A core enzyme for regulatory B
subunits and T antigens. J Virol. 73:839–842. 1999.PubMed/NCBI
|
|
32
|
Virshup DM and Shenolikar S: From
promiscuity to precision: Protein phosphatases get a makeover. Mol
Cell. 33:537–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seshacharyulu P, Pandey P, Datta K and
Batra SK: Phosphatase: PP2A structural importance, regulation and
its aberrant expression in cancer. Cancer Lett. 335:9–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruvolo PP, Deng X, Ito T, Carr BK and May
WS: Ceramide induces Bcl2 dephosphorylation via a mechanism
involving mitochondrial PP2A. J Biol Chem. 274:20296–20300. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang H, Lin H, Yi L, Zhu J, Zhou Y, Mi M
and Zhang Q: 3,6-Dihydroxyflavone induces apoptosis in leukemia
HL-60 cell via reactive oxygen species-mediated p38 MAPK/JNK
pathway. Eur J Pharmacol. 648:31–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu GP, Wei W, Zhou X, Zhang Y, Shi HH,
Yin J, Yao XQ, Peng CX, Hu J, Wang Q, et al: I(2)(PP2A) regulates
p53 and Akt correlatively and leads the neurons to abort apoptosis.
Neurobiol Aging. 33:254–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takai A and Mieskes G: Inhibitory effect
of okadaic acid on the p-nitrophenyl phosphate phosphatase activity
of protein phosphatases. Biochem J. 275:233–239. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Viner RI, Hühmer AF, Bigelow DJ and
Schöneich C: The oxidative inactivation of sarcoplasmic reticulum
Ca(2+)-ATPase by peroxynitrite. Free Radic Res. 24:243–259. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Naing C, Mak JW, Wai N and Maung M:
Diabetes and infections-hepatitis C: Is there type 2 diabetes
excess in hepatitis C infection? Curr Diab Rep. 13:428–434. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Plácido AI, Pereira CM, Correira SC,
Carvalho C, Oliveira CR and Moreira PI: Phosphatase 2A inhibition
affects endoplasmic reticulum and mitochondria homeostasis via
cytoskeletal alterations in brain endothelial cells. Mol Neurobiol.
54:154–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moreno ML, Escobar J, Izquierdo-Álvarez,
Gil A, Pérez S, Pereda J, Zapico I, Vento M, Sabater L, Marina A,
et al: Disulfide stress: A novel type of oxidative stress in acute
pancreatitis. Free Radic Biol Med. 70:265–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tay KH, Jin L, Tseng HY, Jiang CC, Ye Y,
Thorne RF, Liu T, Guo ST, Verrills NM, Hersey P and Zhang XD:
Suppression of PP2A is critical for protection of melanoma cells
upon endoplasmic reticulum stress. Cell Death Dis. 28:e3372012.
View Article : Google Scholar
|
|
44
|
Heinrich M, Wickel M, Schneider-Brachert
W, Sandberg C, Gahr J, Schwandner R, Weber T, Saftig P, Peters C,
Brunner J, et al: Cathepsin D targeted by acid
sphingomyelinase-derived ceramide. EMBO J. 18:5252–5263. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang L, Jin GH and Zhou JY: The role of
ceramide in the pathogenesis of alcoholic liver disease. Alcohol
Alcohol. 51:251–257. 2016. View Article : Google Scholar : PubMed/NCBI
|