Introduction
Colon cancer is one of the leading causes of
cancer-associated mortality worldwide and its incidence has
increased in recent years. In China, colon cancer is the fifth most
common type of cancer and has the fifth highest mortality rate
(1). It has been estimated that
>370,000 cases of colon cancer and 190,000 colon
cancer-associated fatalities occur annually in China. Despite
advances in conventional cancer therapies, including surgery,
radiotherapy and chemotherapy, the 5-year relative survival rate of
colon cancer remains poor, with lymphatic and distant metastasis
being the main causes of colon cancer-associated mortality
(2). Therefore, there is an urgent
need to determine the molecular basis of colon cancer and to
discover novel therapeutic targets for its treatment.
MicroRNAs (miRNAs/miRs) are endogenous non-coding
RNAs with a length of ~22 nucleotides that can regulate the
expression of target genes at the post-transcriptional level
(3). It has been well established
that miRNAs are involved in numerous physiological processes by
regulating the expression of different target genes. In addition,
abnormal expression and function of miRNAs are associated with a
number of human diseases, including cancer (4). miR-192 has been reported to be
abnormally expressed in numerous malignant tumors, including lung,
gastric and bladder cancer (5–7).
However, the role of miR-192 in colon cancer and the underlying
molecular mechanisms remain unclear.
Elucidating the roles of specific miRNAs in cancer
may enable the development of novel treatments via the regulation
of these miRNAs. Small molecules may regulate endogenous miRNAs,
thereby affecting therapeutic outcomes (8,9). For
example, enoxacin has been demonstrated to be a universal miRNA
activator and to inhibit cancer growth (10,11).
Polylysine and trypaflavine have been found to be universal
inhibitors of miRNAs that may reverse the occurrence of tumors via
modulation of endogenous miRNA expression (12). Therefore, the aim of the present
study was to investigate the association between miR-192 and colon
cancer, and identify a potential small molecule that regulates
miR-192 expression and subsequently inhibits cancer growth.
Materials and methods
Cells and reagents
The HCT-116, HT-29, SW480 and RKO human colon cancer
cell lines, as well as the FHC normal colon epithelial cell line
and the 293T cell line, were acquired from the Shanghai Institute
for Biological Sciences (Shanghai, China). The cells were cultured
in McCoy's 5A (modified) medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C
in 5% CO2. Cells in exponential growth phase (~80%
confluence) were used for subsequent experiments. Statins
(pravastatin, simvastatin, fluvastatin, compactin, lovastatin,
rosuvastatin, atorvastatin and pitavastatin) were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and dissolved in
dimethyl sulfoxide (DMSO) to establish 10 mM stock solutions.
Bioinformatics analysis
TargetScan was used to predict target mRNAs for
miR-192 (13).
Cell proliferation assay
Post-digestion, HCT-116 cells in the logarithmic
growth phase were counted and plated in 96-well plates
(5×103 cells/well per 100 µl medium). Following
incubation overnight, HCT-116 cells were treated with various
statins concentrations (0, 1, 2, 4, 8, 16, 32, 64, 128 and 256 µM;
5 wells per dosage group) for 48 h. A total of 100 µl MTT solution
(1 mg/ml; Sigma-Aldrich; Merck KGaA) was then added and the
resultant solution was incubated for a further 4 h at 37°C.
Following removal of the culture medium, 100 µl DMSO
(Sigma-Aldrich; Merck KGaA) was added to each well. The absorbance
values were then measured at a wavelength of 560 nm using a
Multiskan Spectrum (Molecular Devices, LLC, Sunnyvale, CA, USA).
Half-maximal inhibitory concentration (IC50) values were
determined using Graphpad Prism 5.01 software (GraphPad Software,
Inc., La Jolla, CA. USA).
To determine the effect of miR-192 on cell activity,
HCT-116 cells (100 µl) were seeded into 96-well plates at a density
of 5×104/ml per well. Following incubation overnight,
the cells were transfected with either miR-192 mimics (sense
strand, 5′-cugaccuaugaauugacagcc-3′; passenger strand,
5′-cugccaauuccauaggucacag-3′) or miR-negative control mimics (sense
strand, 5′-uucuccgaacgugucacguuu-3′; passenger strand,
5′-aaacgugacacguucggagaa-3′) (Synthgene Biotech, Nanjing, China)
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. 10 µl MTT (5 mg/ml)
was added to the medium for 0, 12, 24 and 48 h post-transfection,
in accordance with the manufacturer's protocol. After 4 h of
incubation, medium was removed and 100 µl DMSO was added to each
well for 30 min at 37°C. The absorption of each well at 490 nm was
collected on using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) to assess cell viability.
Wound-healing assay
Wound-healing assays were performed to evaluate
cancer cell migration as previously described by Liu et al
(14). Briefly, HCT-116 cells were
plated in a 12-well plate at 2×104 cells/well. When the
cells reached confluence, a horizontal scratch was created using a
10-µl pipette tip. At 0 and 48 h post-wound infliction, the cell
migration status was determined using a light microscope (scale
bar, 500 µM).
Transwell assay
Cell migration and invasion abilities were
investigated using specialized Transwell chambers (8-µm pore size;
BD Biosciences, Franklin Lakes, NJ, USA). For migration assays,
HCT-116 cells (2×105/ml) and 4 µM simvastatin suspension
were added to the upper chamber and 450 µl culture medium
supplemented with 15% FBS was added to the lower chamber. Following
incubation for 48 h at 37°C in 5% CO2, any non-migrating
or non-invading cells on the upper surface were removed. The cells
in the lower chamber were then fixed with methanol and stained with
hematoxylin for 30 min at 37°C. The number of invading cells was
counted under a light microscope (magnification, ×200; three visual
fields/well).
In order to perform invasion assays, Matrigel (BD
Biosciences) kept in a −20°C refrigerator was defrosted on ice and
the pipette tips, Eppendorf tubes and medium were precooled at 4°C.
Subsequently, Matrigel and medium were mixed at a ratio of 1:8. A
total of 40 µl mixed medium was then added to the upper chamber and
the chamber was incubated at 37°C for 4 h. The cell suspension (150
ml; 3×105 cells/ml) and the drug suspension were added
to the upper chamber, and 600 µl complete medium containing 15% FBS
(Gibco; Thermo Fisher Scientific, Inc.) was added to the lower
chamber. A total of three replicates were performed per group.
Following 48 h of incubation, Matrigel and non-invading cells were
removed using cotton swabs, fixed with methanol at 37°C for 30 min
and then stained with crystal violet solution for 30 min at room
temperature. The number of invading cells was counted under a light
microscope.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The analysis was conducted with the
2−ΔΔCq quantification method as described by Livak and
Schmittgen (15). Total RNA was
isolated from HCT-116 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to a
previously published protocol by Beekman et al (16). To quantify miRNAs, TaqMan probes
(Thermo Fisher Scientific, Inc.) were used in accordance with the
manufacturer's protocol. Briefly, 1 µg total RNA was
reverse-transcribed to cDNA using AMV reverse transcriptase (Takara
Biotechnology Co., Ltd., Dalian, China) and an RT primer. The
reaction conditions were 16°C for 30 min, 42°C for 30 min and 85°C
for 5 min. qPCR was performed using TaqMan QPCR Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The reactions were performed in a 96-well
plate at 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec
and 60°C for 1 min. U6 was used as an internal control. The primers
for U6 were 5′-CGCTTCGGCAGCACATATACTA-3′ (forward) and
5′-CGCTTCACGAATTTGCGTGTCA-3′ (reverse).
The expression levels of Ras-related protein Rab-2A
(RAB2A), epithelial (E)-cadherin, β-catenin and twist mRNA were
detected using a SYBR QPCR kit (Synthgene Biotech) according to the
manufacturer's protocol. The reactions were performed in a 96-well
plate at 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec,
60°C for 30 sec and 72°C for 30 sec. GAPDH was used as an internal
control. The primers used in this experiment were as follows: RAB2A
5′-GGCGACACAGGTGTAGAGTT-3′ (forward) and 5′-TGATTGCCTGCATGTGTTGC-3′
(reverse); E-cadherin 5′-TGCCCAGAAAATGAAAAAGG-3′ (forward) and
5′-GTGTATGTGGCAATGCGTTC-3′ (reverse); β-catenin
5′-GAAACGGCTTTCAGTTGAGC-3′ (forward) and 5′-CTGGCCATATCCACCAGAGT-3′
(reverse); twist 5′-GGAGTCCGCAGTCTTACGAG-3′ (forward) and
5′-TCTGGAGGACCTGGTAGAGG-3′ (reverse); and GAPDH
5′-TGTTGCCATCAATGACCCCTT-3′ (forward) and 5′-CTCCACGACGTACTCAGCG-3′
(reverse).
Luciferase reporter assay
The entire 3′-untranslated region (UTR) of RAB2A was
inserted into a luciferase reporter plasmid named
pMIR-REPORT™ Luciferase (Synthgene Biotech). To
investigate the binding specificity, sequences that interacted with
miR-192 were mutated and mutant RAB2A 3′-UTRs were then inserted
into an equivalent luciferase reporter plasmid. In order to perform
the luciferase reporter assay, 293T cells (2×104
cells/ml) were plated in 24-well plates and 0.3 µg luciferase
reporter plasmid and 0.2 µg β-galactosidase plasmid (internal
control) were added to each well. After 4 h, 15 pmol of miR-192
mimics and negative control (NC) mimics were transfected into 293T
cells using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following 48 h of incubation, firefly and Renilla luciferase
activities were measured using a Dual-Luciferase®
Reporter Assay System in accordance with the manufacturer's
protocol (Promega Corporation, Madison, WI, USA).
Western blotting
HCT-116 cells were treated with either 4, 8 or 16
µmol/l, respectively. The HCT-116 cells were washed twice with
ice-cold PBS and centrifuged at 12,000 × g for 10 min at 4°C, lysed
using radioimmunoprecipitation assay buffer (Beijing Solarbio
Science & Technology, Co., Ltd., Beijing, China) and incubated
on ice for 20 min. The protein concentration of the supernatant was
determined with a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Extracted proteins of 20 µg/lane were
diluted in 1X SDS loading buffer, pre-denatured and then resolved
via 10% SDS-PAGE. Subsequently, proteins were transferred to a
polyvinylidene difluoride membrane, blocked using 5% non-fat milk
with Tris-buffered saline containing Tween-20 [TBST; 20 mM
Tris-HCl, 150 mM NaCl and 0.1% (v/v) Tween-20; pH 7.4] at room
temperature for 1 h, and then incubated with the following primary
antibodies at 4°C overnight: Anti-E-cadherin (1:1,000; cat. no.
ab1416), anti-β-catenin (1:1,000; cat. no. ab16051), anti-twist
(1:1,000; cat. no. ab50581), anti-RAB2A (1:1,000; cat. no.
ab154729), anti-phosphatidylinositol 3-kinase (PI3K; 1:1,000; cat.
no. ab32089), anti-extracellular signal-regulated kinase (ERK;
1:1,000; cat. no. ab166847) and anti-GAPDH (1:2,000; cat. no.
ab8245; all Abcam, Cambridge, MA, USA). After washing, the membrane
was further incubated with HRP-conjugated secondary antibodies
(1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) for
1 h at room temperature and proteins were then visualized using
electrochemiluminescence reagents (Bio-Rad Laboratories, Inc.) by
ImageJ Software version 1.6 (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All experiments were performed in triplicate and the
data are expressed as the mean ± standard error of the mean. Unless
stated otherwise, statistical analysis was performed using GraphPad
Prism 5.01 software (GraphPad Software, Inc.). The statistical
significance of the differences between groups was assessed using
one-way analysis of variance followed by Tukey's post-hoc test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-192 is downregulated in colon
cancer cells
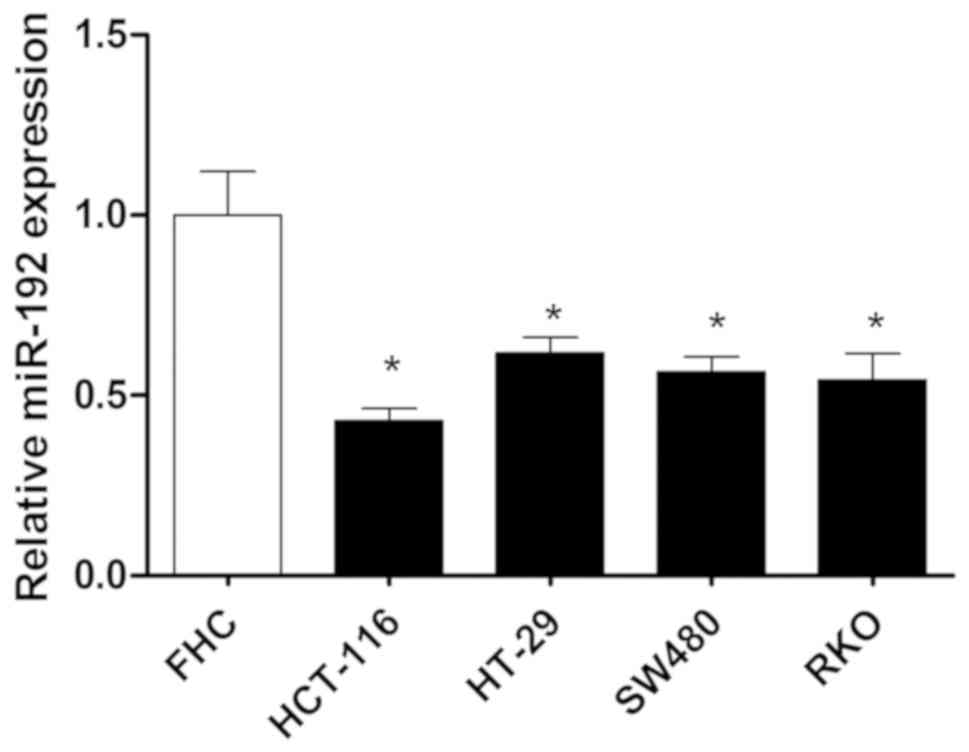
RT-qPCR was performed to investigate the expression
of miR-192 in colon cancer cells. The results revealed that the
expression of miR-192 in colon cancer cells was significantly
decreased compared with normal colon cells and this effect was most
prominent in HCT-116 cells (P<0.05; Fig. 1). Therefore, it may be suggested
that downregulation of miR-192 is associated with the occurrence
and progression of colon cancer.
miR-192 inhibits the proliferation,
migration and invasion of HCT-116 cells
To determine the association between miR-192
expression and the occurrence and metastasis of colon cancer, the
effects induced by transfection with miR-192 mimics and miR-192
inhibitors on the proliferation, migration and invasion of HCT-116
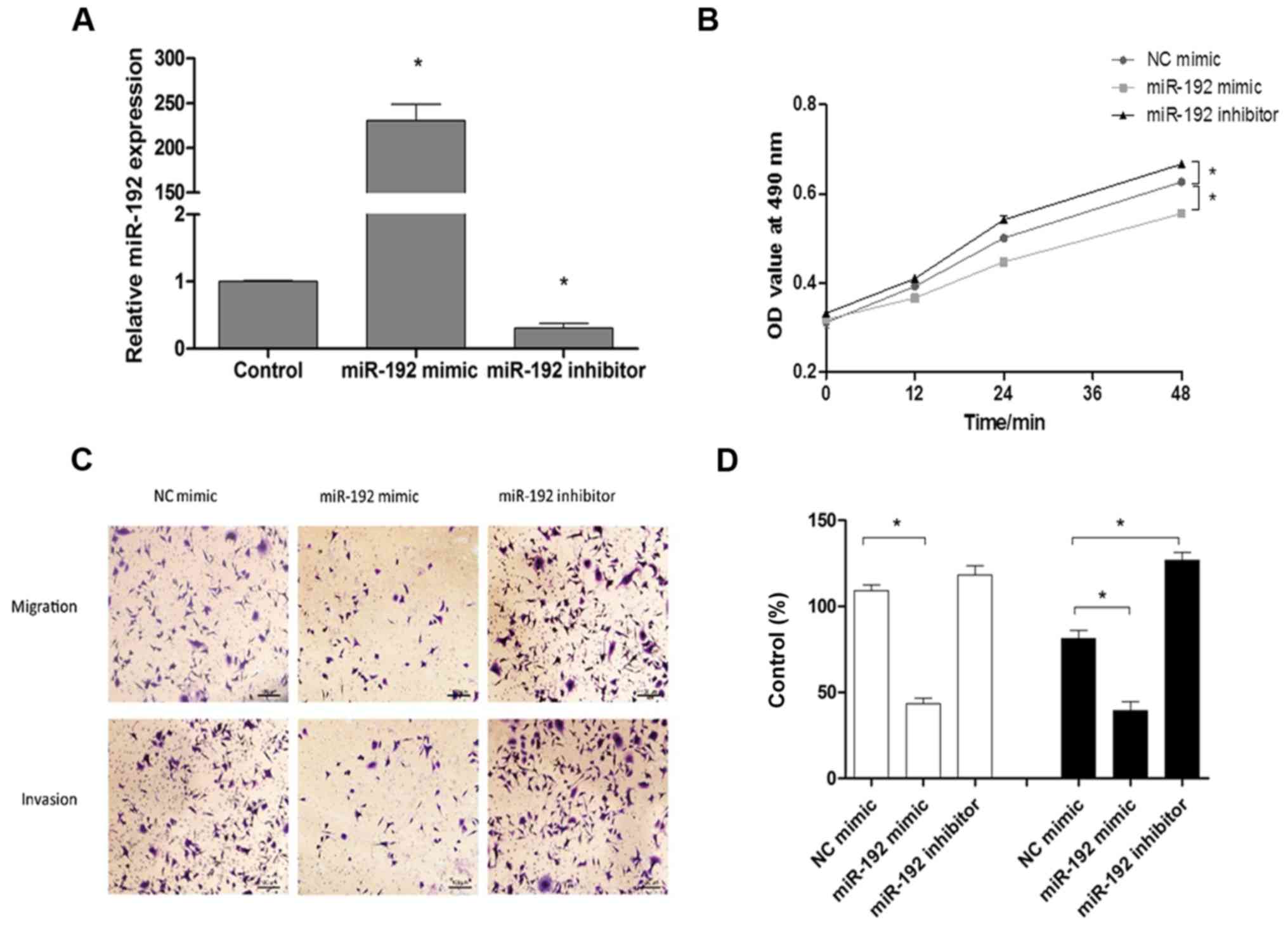
cells were observed. As presented in Fig. 2A, the relative expression of
miR-192 significantly increased following transfection with the
miR-192 mimic (P<0.05), while the relative expression of miR-192
significantly decreased following transfection with the miR-192
inhibitor (P<0.05). As determined by MTT assays, upregulation of
miR-192 expression significantly inhibited the proliferation of
HCT-116 cells, while downregulation of miR-192 expression
significantly enhanced the proliferation of HCT-116 cells
(P<0.05; Fig. 2B).
Subsequently, the migration and invasion of HCT-116 cells were
investigated, and the results revealed that expression of miR-192
mimics significantly decreased cell migration and invasion
(P<0.05), whereas the miR-192 inhibitors exerted the opposite
effect compared with the control (Fig.
2C and D).
RAB2A is a direct target of miR-192 in
HCT-116 cells
To identify genes regulated by miR-192, TargetScan
(13) was used to predict
potential targets. RAB2A, a member of the RAS family and a
previously determined oncogene, was demonstrated to be a potential
target gene of miR-192. It has been reported that an abnormal
increase in RAB2A expression is associated with carcinogenesis and
the expression of RAB2A in cancer cells is enhanced compared with
that in normal cells (17).
Following prediction by TargetScan, the results demonstrated that
miR-192-5p may bind with the 3′-UTR of RAB2A mRNA and that the
predicted binding site was close to the 218–225 bp region.
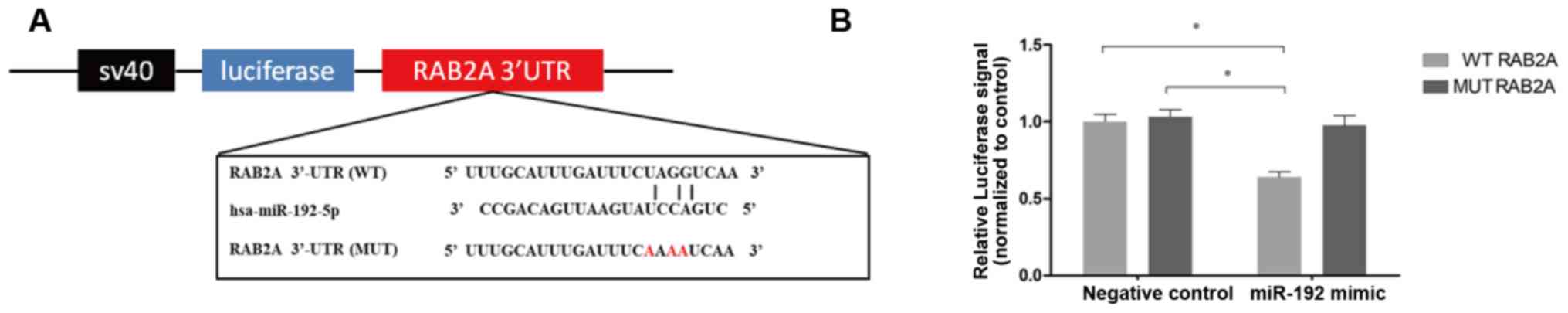
To determine whether the regulatory effect of
miR-192 on RAB2A expression is due to the binding of miR-192-5p to
the predicted sites in the 3′-UTR of RAB2A (Fig. 3A), full-length RAB2A 3′-UTRs were
inserted into the firefly luciferase gene. The resultant plasmid
was then co-transfected with miR-192 mimics into 293T cells and
luciferase signals were then detected to determine whether
miR-192-5p can bind to the 3′-UTR of RAB2A. As presented in
Fig. 3B, luciferase signals were
significantly decreased in 293T cells transfected with miR-192
mimics, but not in those transfected with NC mimics (P<0.05).
Furthermore, to confirm that miR-192-5p can bind to the predicted
sites, the predicted miR-192-5p binding sites were mutated in the
3′-UTR of RAB2A (Fig. 3A) and
subsequently inserted into the luciferase gene. The results
demonstrated that luciferase signals were not markedly decreased in
these cells; therefore, RAB2A appears to be a target gene of
miR-192.
Screening of small molecules that
activate miR-192 in HCT-116 cells
Statins are associated with the prevention and
treatment of colon cancer. It has been previously demonstrated that
statins may serve a protective role against the development of
adenomatous polyps and are used during the early treatment stages
of colonic polypoid tumors (18).
It has also been reported that the regulation of miR-92a expression
may represent a novel clinical target of statins when used to treat
endothelial cell dysfunction in patients with coronary heart
disease (19). In the present
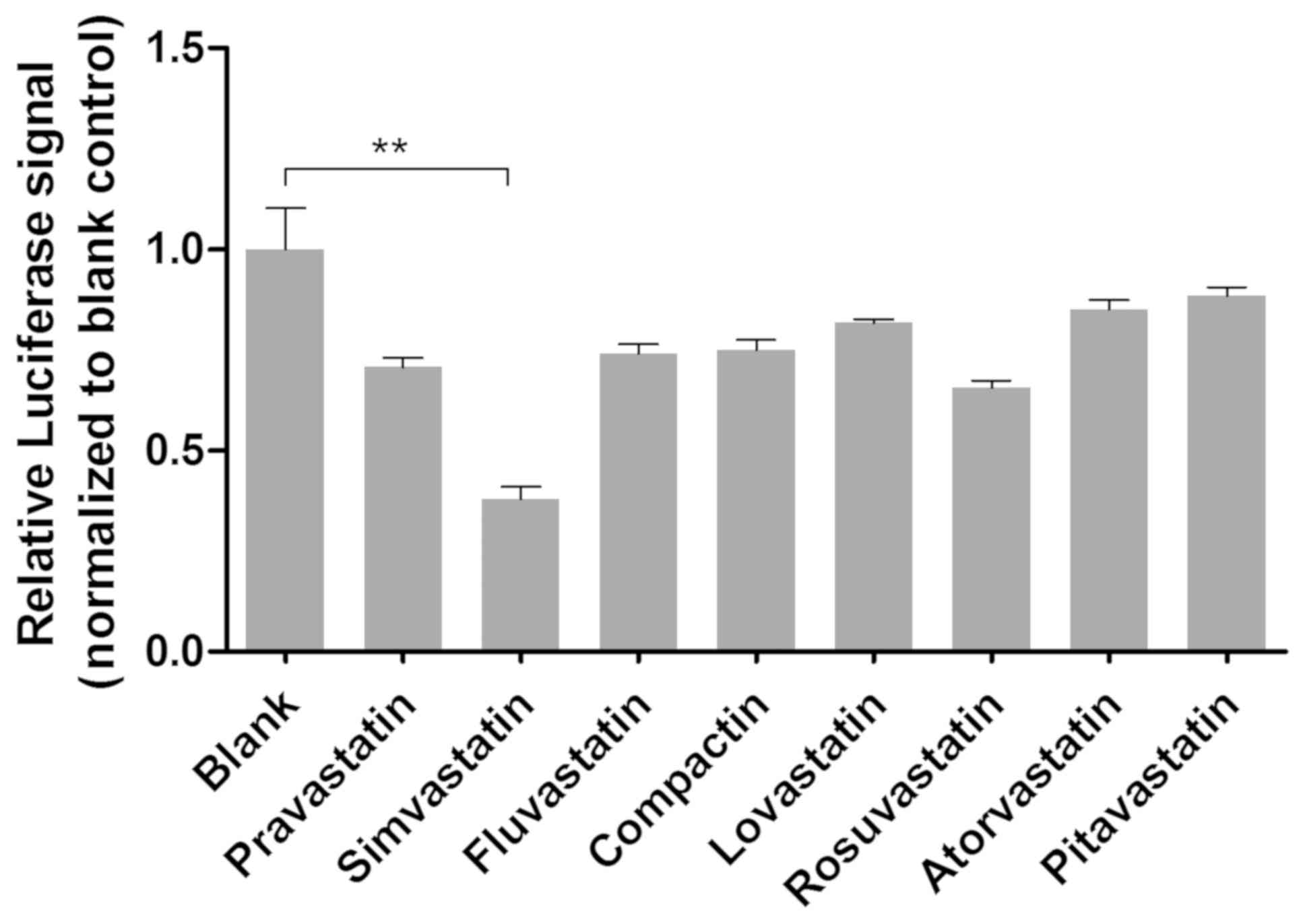
study, eight variants of small molecules were screened using the
RAB2A 3′-UTR luciferase system. Following treatment with statins
for 48 h, the relative luciferase signals exhibited by cells were
determined (Fig. 4). The results
revealed that the relative luciferase signals in cells treated with
simvastatin were significantly decreased compared with the other
small molecules investigated (P<0.01), indicating that
simvastatin markedly upregulated miR-192 expression.
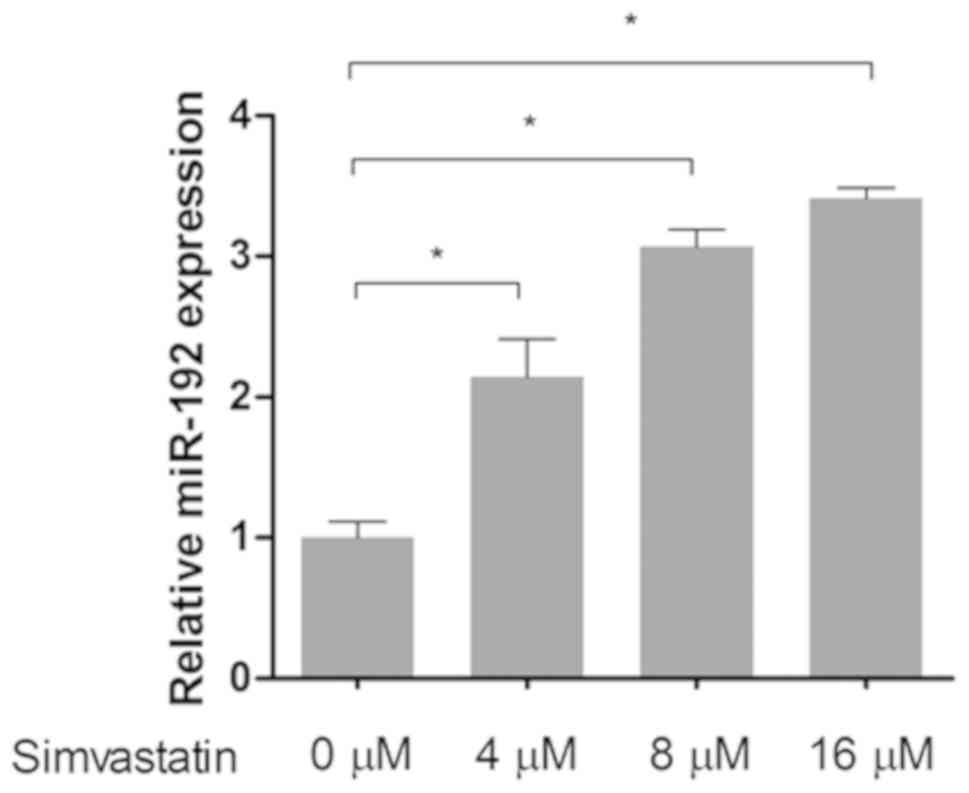
Simvastatin affects the expression of
miR-192 and associated downstream pathway proteins in HCT-116 colon
cancer cells
Epithelial-to-mesenchymal transition (EMT) is
required for the migration and invasion of epithelium-derived
malignant tumor cells and is considered to represent an important
regulatory mechanism of tumor growth, invasion and metastasis.
During the process of EMT, the expression of the epithelial marker
E-cadherin decreased and then the contact between the tumor cells
and the surrounding cells decreased. The decrease or loss of
intercellular adhesion leads to the increase of movement and
invasion ability of tumor cells, therefore infiltrating and
transferring to the surrounding tissues. As a marker of
interstitial cells, the elevated expression level of β-catenin
indicates the transformation of epithelial cells into mesenchymal
cells. In contrast, the reduced level of β-catenin expression is a
sign that the EMT process is suppressed (20–22).
In the present study, the effect of simvastatin on the mRNA levels
of downstream pathway proteins was investigated. Cells were treated
with 4, 8 and 16 µM of simvastatin according to the IC50
value previously determined by MTT assays. The results of RT-qPCR
revealed that following treatment with simvastatin the level of
E-cadherin mRNA significantly increased and the levels of β-catenin
and twist mRNA decreased in a dose-dependent manner, indicating
that the EMT process was inhibited (P<0.05; Fig. 5A-C). Subsequently, the protein
expression levels of E-cadherin, β-catenin, twist, RAB2A, PI3K and
ERK were determined via western blot analysis. As presented in
Fig. 5D and E, the protein
expression levels of β-catenin, twist, RAB2A, PI3K and ERK were
significantly decreased (P<0.05), and the protein expression of
E-cadherin was markedly increased in a dose-dependent manner
following treatment with simvastatin. These results suggested that
simvastatin inhibited the EMT process by regulating the expression
of EMT-associated proteins in the downstream pathway of
miR-192.
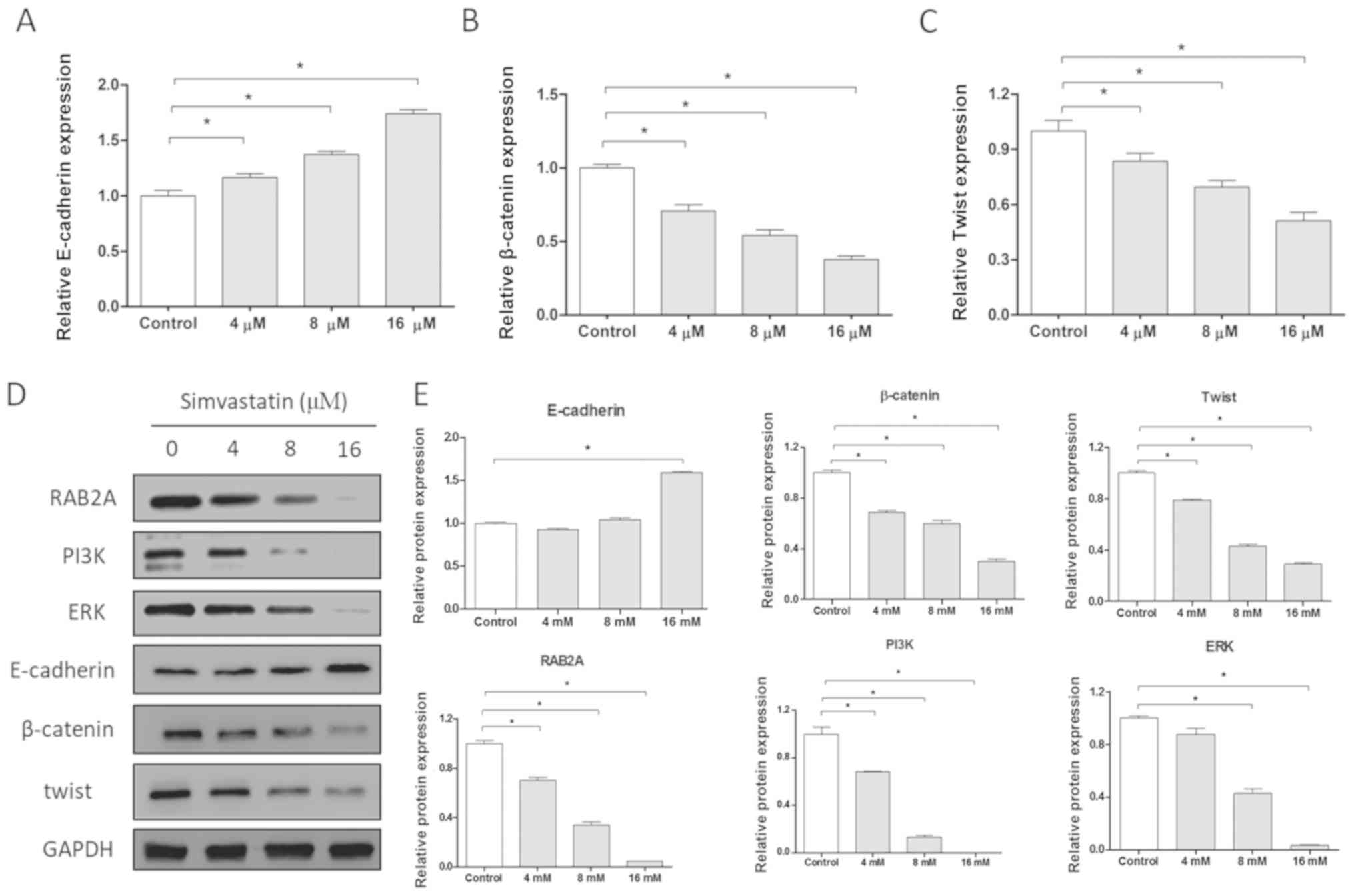 | Figure 5.Simvastatin inhibits EMT and the
PI3K/Akt pathways in HCT-116 cells. mRNA expression levels of
EMT-associated proteins (A) E-cadherin, (B) β-catenin and (C) twist
following treatment with various concentrations of simvastatin.
Protein expression levels of E-cadherin, β-catenin, twist, RAB2A,
PI3K and ERK in HCT-116 cells, presented as a (D) representative
image and (E) quantitative analysis. *P<0.05. RAB2A, Ras-related
protein Rab-2A; PI3K, phosphatidylinositol 3-kinase; EMT,
epithelial-to-mesenchymal transition; Akt, protein kinase B; ERK,
extracellular signal-regulated kinase; E, epithelial. |
Effect of statins on the proliferation
of HCT-116 cells
Various concentrations of statins were established
(0, 1, 2, 4, 8, 16, 32, 64, 128 and 256 µM) and added to HCT-116
cells for 48 h. Subsequently, the effect of statins on cell
activity was investigated using MTT assays and the results revealed
that cell activity was markedly decreased following treatment with
statins (Table I). Using the
growth inhibition curve of compound concentration as well as cell
viability, the IC50 values of numerous drugs were
determined (Table I). The results
demonstrated that simvastatin inhibited the growth of HCT-116 cells
to a greater extent compared with the other statins, which is in
agreement with the small molecule screening results presented in
Fig. 4.
 | Table I.HCT-116 colon cancer cells were
treated with different statins. |
Table I.
HCT-116 colon cancer cells were
treated with different statins.
| Compound | IC50
(µM) |
|---|
| Pravastatin | 81 |
| Simvastatin | 16 |
| Fluvastatin | 93 |
| Campactin | 82 |
| Lovavastatin | 109 |
| Rosuvastatin | 80 |
| Atorvastatin | 100 |
| Pitavastatin | 120 |
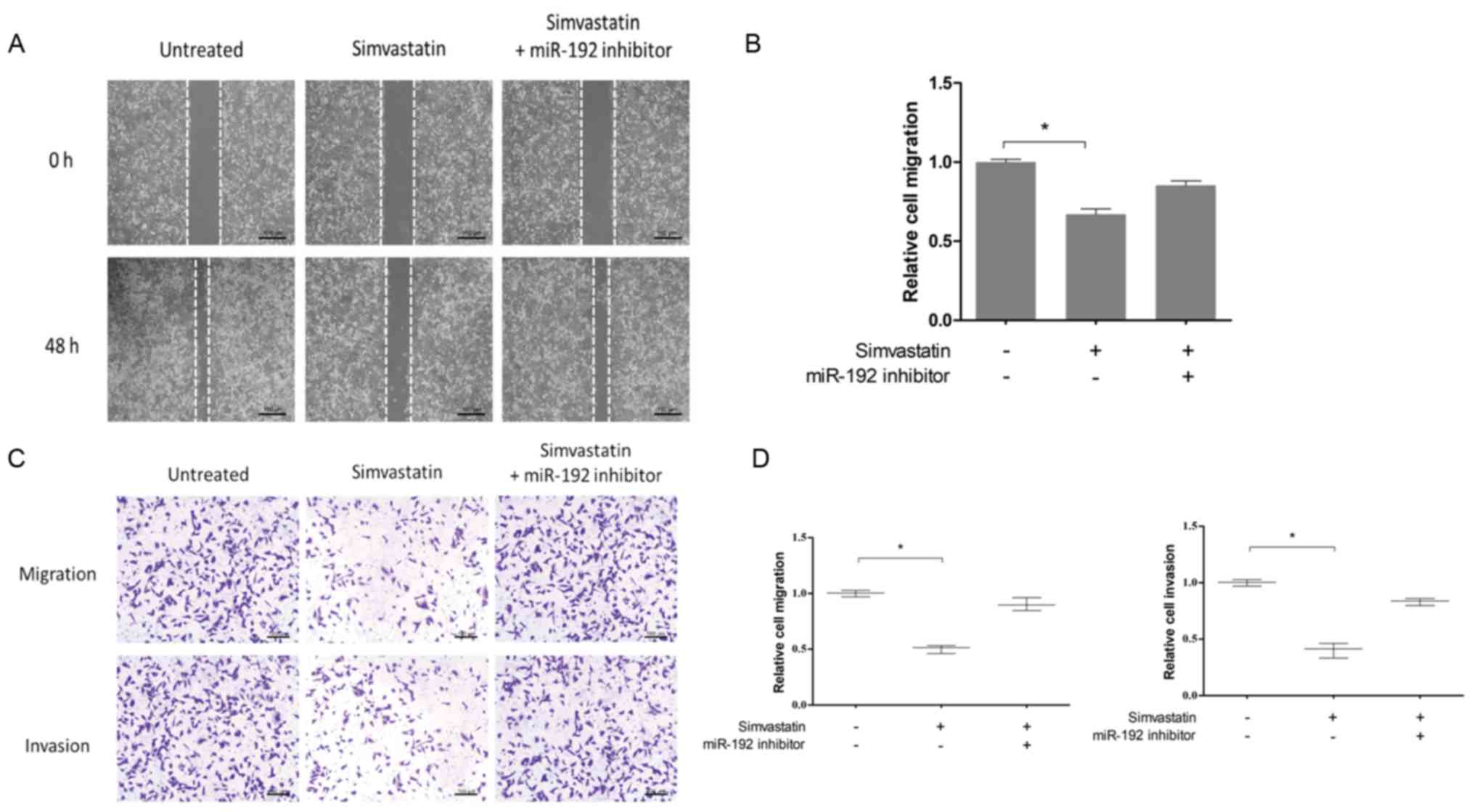
Simvastatin inhibits the migration and
invasion of HCT-116 cells by upregulating miR-192-5p
expression
The role of simvastatin on miR-192-5p expression in
HCT-116 colon cancer cells was investigated. As presented in
Fig. 6, the expression of
miR-192-5p in HCT-116 cells was significantly increased following
treatment with simvastatin (P<0.05). To determine the effects of
simvastatin on the migration and invasion of HCT-116 cells, the
migration and invasion rates of the untreated group, the
simvastatin group, and the simvastatin + miR-192 inhibitor group
were investigated, and the results demonstrated that inhibition of
miR-192-5p attenuated the effects of simvastatin on the migration
and invasion of HCT-116 cells (Fig.
7). These results indicated that simvastatin may inhibit the
migration and invasion of colon cancer via upregulation of
miR-192-5p.
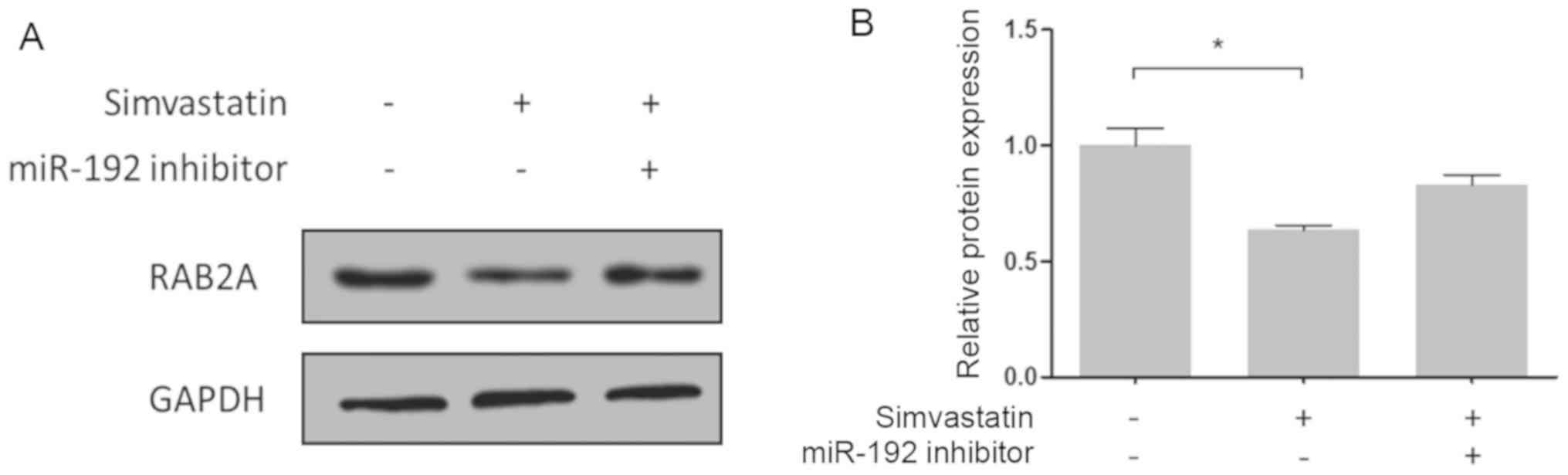
The expression levels of the RAB2A protein in
HCT-116 cells were then evaluated by western blotting. As presented
in Fig. 8, simvastatin inhibited
RAB2A protein expression via regulation of miR-192. Therefore,
miR-192 appears to regulate RAB2A expression in colon cancer cells
by directly binding to its 3′-UTR.
Discussion
Colon cancer is a common malignancy of the digestive
tract and one of the leading causes of cancer-associated mortality.
Elucidation of the molecular mechanism underlying colon cancer
development is urgently required, as is the discovery of novel
therapeutic targets for the treatment of colon cancer patients.
miRNAs regulate protein expression and serve an important role in
tumor progression by mediating the occurrence and development of
tumors via regulation of downstream target genes. miR-192 has been
demonstrated to be abnormally expressed in various tumors. For
example, miR-192 was demonstrated to be downregulated in lung
cancer tissues compared with normal lung tissues. Previous in
vitro analyses have demonstrated that overexpression of miR-192
may inhibit the proliferation and apoptosis of lung cancer cells
via regulation of the RB transcriptional corepressor 1 gene
(5). In addition, overexpression
of miR-192 may inhibit the migration and invasion of renal cell
carcinoma cells (23). In the
present study, the expression of miR-192 in colon cancer cells was
demonstrated to be markedly decreased compared with that in normal
colon cells and this result was most prominent in HCT-116 cells.
Furthermore, overexpression of miR-192 was demonstrated to inhibit
the proliferation, migration and invasion of HCT-116 cells. In
addition, the present study identified RAB2A as a novel oncogene in
colon cancer, which is regulated by miR-192 via direct binding with
its 3′-UTR. These results suggested that miR-192 acts as a tumor
suppressor gene in colon cancer cells.
As important endogenous biomolecules, the regulation
of miRNAs is becoming increasingly important. The regulation of
miRNAs by small molecules has been extensively investigated.
Statins are lipid-modifying drugs that inhibit the synthesis of
cholesterol by selectively and competitively inhibiting
hydroxymethylpentacyl-coenzyme A reductase expression, and are
predominantly used as lipid-lowering drugs for the prevention of
cerebrovascular and cardiovascular diseases. Recent studies have
demonstrated that statins are highly effective in the treatment of
colon, lung, pancreatic and breast cancer, as well as other solid
tumors (24,25). It has also been reported that
different doses of statins may reduce the risk of colon cancer by
94% (26). In the present study,
small molecules that regulate miR-192 were screened and simvastatin
was found to represent a novel activator of miR-192. Furthermore,
it was demonstrated that simvastatin upregulated miR-192 and
inhibited the expression of the downstream targets of miR-192,
which subsequently led to suppressed proliferation, migration and
invasion of colon cancer cells. In addition, it was observed that
simvastatin inhibited the growth of colon cancer cells in
vitro and exerted the most potent inhibitory effect among all
the small molecules investigated. Furthermore, the results of the
present study indicated that simvastatin may inhibit the migration
and invasion of colon cancer cells. Therefore, simvastatin appears
to upregulate miR-192, thereby inhibiting cancer growth.
In conclusion, miR-192 was identified as a tumor
suppressor in colon cancer and simvastatin was found to be an
activator of miR-192, which may represent a novel therapeutic
approach to the treatment of patients with colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and KL designed the experiments. XZ, XW, RZ and
XL performed the experiments. XZ, XW, RZ and XL analyzed the data.
XZ and KL wrote the manuscript. XZ and KL revised the manuscript.
All authors reviewed the revised manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van CE, Sagaert X, Topal B, Haustermans K
and Prenen H: Gastric cancer. Lancet. 388:2654–2664. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elias D, Faron M, Iuga BS, Honoré C,
Dumont F, Bourgain JL, Dartigues P, Ducreux M and Goéré D:
Prognostic similarities and differences in optimally resected liver
metastases and peritoneal metastases from colorectal cancers. Ann
Surg. 261:157–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin H and Hannon GJ: Micro RNAs: Small
RNAs with a big role in gene regulation. Nat Rev Genet. 5:522–531.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uchino K, Takeshita F, Takahashi RU,
Kosaka N, Fujiwara K, Naruoka H, Sonoke S, Yano J, Sasaki H, Nozawa
S, et al: Therapeutic effects of MicroRNA-582-5p and −3p on the
inhibition of bladder cancer progression. Mol Ther. 21:610–619.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng S, Cong S, Zhang X, Bao X, Wang W, Li
H, Wang Z, Wang G, Xu J, Du B, et al: MicroRNA-192 targeting
retinoblastoma 1 inhibits cell proliferation and induces cell
apoptosis in lung cancer cells. Nucleic Acids Res. 39:6669–6678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin Z, Selaru FM, Cheng Y, Kan T, Agarwal
R, Mori Y, Olaru AV, Yang J, David S, Hamilton JP, et al:
MicroRNA-192 and −215 are upregulated in human gastric cancer in
vivo and suppress ALCAM expression in vitro. Oncogene.
30:1577–1585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin Y, Lu J, Wen J, Shen Y and Wen X:
Regulation of growth of human bladder cancer by miR-192. Tumour
Biol. 36:3791–3797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Tan S, Kooger R, Zhang C and Zhang
Y: MicroRNAs as novel biological targets for detection and
regulation. Chem Soc Rev. 43:506–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zhang W, Zhou M, Kooger R and Zhang
Y: Small molecules modulating biogenesis or processing of microRNAs
with therapeutic potentials. Curr Med Chem. 20:3604–3612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shan G, Li Y, Zhang J, Li W, Szulwach KE,
Duan R, Faghihi MA, Khalil AM, Lu L, Paroo Z, et al: A small
molecule enhances RNA interference and promotes microRNA
processing. Nat Biotechnol. 26:933–940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melo S, Villanueva A, Moutinho C, Davalos
V, Spizzo R, Ivan C, Rossi S, Setien F, Casanovas O, Simo-Riudalbas
L, et al: Small molecule enoxacin is a cancer-specific growth
inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated
microRNA processing. Proc Natl Acad Sci USA. 108:4394–4399. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watashi K, Yeung ML, Starost MF, Hosmane
RS and Jeang KT: Identification of small molecules that suppress
microRNA function and reverse tumorigenesis. J Biol Chem.
285:24707–24716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
TargetScan, . http://www.targetscan.org/vert_71/
|
|
14
|
Liu JJ, Liu JY, Chen J, Wu YX, Yan P, Ji
CD, Wang YX, Xiang DF, Zhang X, Zhang P, et al: Scinderin promotes
the invasion and metastasis of gastric cancer cells and predicts
the outcome of patients. Cancer Lett. 376:110–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beekman JM, Reischl J, Henderson D, Bauer
D, Ternes R, Peña C, Lathia C and Heubach JF: Recovery of
microarray-quality RNA from frozen EDTA blood samples. J Pharmacol
Toxicol Methods. 59:44–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kajiho H, Kajiho Y, Frittoli E,
Confalonieri S, Bertalot G, Viale G, Di Fiore PP, Oldani A, Garre
M, Beznoussenko GV, et al: RAB2A controls MT1-MMP endocytic and
E-cadherin polarized Golgi trafficking to promote invasive breast
cancer programs. EMBO Rep. 17:1061–1080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Broughton T, Sington J and Beales IL:
Statin use is associated with a reduced incidence of colorectal
adenomatous polyps. Int J Colorectal Dis. 28:469–476. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Lu HM, Yang WH, Luo C, Lu SH, Zhou
Y and Lin YZ: The influence of statin therapy on circulating
microRNA-92a expression in patients with coronary heart disease.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 24:215–218. 2012.(In
Chinese). PubMed/NCBI
|
|
20
|
Rodriguez JA, Huerta-Yepez S, Law IK,
Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, Iliopoulos D,
Hommes DW, Verspaget HW, Chang L, et al: Diminished expression of
CRHR2 in human colon cancer promotes tumor growth and EMT via
persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol.
1:610–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uwafuji S, Goi T, Naruse T, Kurebayashi H,
Nakazawa T, Hirono Y and Yamaguchi A: Protein-bound polysaccharide
K reduced the invasive ability of colon cancer cell lines.
Anticancer Res. 33:4841–4845. 2013.PubMed/NCBI
|
|
22
|
Bao H, Zhang Q, Zhu Z, Xu H, Ding F, Wang
M, Du S, Du Y and Yan Z: BHX, a novel pyrazoline derivative,
inhibits breast cancer cell invasion by reversing the
epithelial-mesenchymal transition and down-regulating Wnt/β-catenin
signalling. Sci Rep. 7:91532017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khella HW, Bakhet M, Allo G, Jewett MA,
Girgis AH, Latif A, Girgis H, Von Both I, Bjarnason GA and Yousef
GM: miR-192, miR-194 and miR-215: A convergent microRNA network
suppressing tumor progression in renal cell carcinoma.
Carcinogenesis. 34:2231–2239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li L and Zhuang Z: Anticancer effects of
statins and application in prevention and treatment of esophageal
cancer. Chin J Gastroenterol. 18:493–496. 2013.(In Chinese).
|
|
25
|
Yu X, Pan Y, Ma H and Li W: Simvastatin
inhibits proliferation and induces apoptosis in human lung cancer
cells. Oncol Res. 20:351–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poynter JN, Gruber SB, Higgins PD, Almog
R, Bonner JD, Rennert HS, Low M, Greenson JK and Rennert G: Statins
and the risk of colorectal cancer. N Engl J Med. 352:2184–2192.
2005. View Article : Google Scholar : PubMed/NCBI
|