Introduction
Osteoarthritis (OA) is a common and progressive
joint disease associated with aging, obesity, joint injury and
heredity (1). OA may affect the
quality of life of patients and result in socioeconomic burden to
families, communities and nations (2). Accumulating evidence suggests that
chronic inflammation serves an important role in the pathogenesis
and prognosis of OA (2). Of note,
persistent infiltration and proinflammatory differentiation of
monocytes may induce chronic inflammation in OA (3). Therefore, suppression of inflammation
and prevention of articular cartilage degeneration are important
factors in the treatment of OA.
Puerarin is a phytoestrogen with potential
beneficial effects in attenuating neuronal injury, diabetic kidney
disease and heart failure (4–7). The
herbal compound modulates oxidative stress and inflammatory
responses in cardiovascular diseases (8). Furthermore, it has been reported that
puerarin may be beneficial in treating diabetes mellitus (5). Additionally, the protective roles of
puerarin have been reported in a number of cell lineages, including
endothelial progenitor cells, cardiac muscle cells and neuronal
cells (9,10). Puerarin has been hypothesized to
act in an arginase 2-dependent manner, a mitochondrial enzyme, via
modulation of nicotinamide adenine dinucleotide phosphate (NADPH)
oxidase-associated oxidative stress (6,11–13).
In addition, puerarin alters the production of innate immune cells
by regulating the differentiation of progenitor cells via estrogen
receptors (14). Previous studies
reported the protective effects of puerarin on the viability and
function of endothelial progenitor cells derived from bone borrow
and peripheral blood (10,14). Furthermore, puerarin exhibits
ameliorative effects in various inflammatory disorders via
inhibiting the degranulation of mast cells, reducing monocyte
adhesion to endothelial cells and modifying chemotactic agent
expression in cardiac fibrotic tissue (15–17).
Innate immune signal transduction pathways, including nuclear
factor κ-light-chain-enhancer of activated B cells (NF-κB),
mitogen-activated protein kinase and extracellular signal-regulated
kinase signaling may be affected by treatment with puerarin
(14,18,19).
It has been hypothesized that puerarin exerts potent
anti-inflammatory and chondroprotective effects by inhibiting
osteoclast formation, bone loss and collagen degradation (20–22);
however, its regulatory effects on monocyte/macrophage function
remain unclear. Therefore, the present study aimed to investigate
the therapeutic effects of puerarin on inflammatory responses and
monocyte recruitment within in vitro and in vivo
models of OA.
Materials and methods
Reagents
Puerarin was purchased from PI & PI Biotech Inc.
(Guangzhou, China). Cell Counting Kit-8 (CCK-8) reagent was
obtained from MedChemExpress LLC (Monmouth Junction, NJ, USA).
Fetal bovine serum (FBS) was purchased from BBI Solutions (Cardiff,
UK). Dulbecco's modified Eagle's medium (DMEM)/F12 and RPMI-1640
media were purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA).
Cell isolation and culture
Primary human chondrocytes were isolated from
cartilage tissues collected from 6 patients with OA (aged 60–63
years old; female to male ratio, 2:1) that underwent knee
arthroplasty between March 2016 and January 2018 using enzyme
extraction. The study was approved by the Medical Ethics Committee
of Changzhou Traditional Chinese Medicine Hospital (Changzhou,
China), and patients provided written informed consent. Briefly,
cartilage tissue was minced into ~1 mm3 pieces and
digested in 1 mg/ml collagenase B (Roche Diagnostics, Basel,
Switzerland) and 200 U/ml DNase I in a CO2 incubator at
37°C for 3 h. The resulting suspension was diluted with 20 ml of
DMEM/F12 medium containing 10% FBS and filtered via a 70-µm cell
strainer. Cells were washed with DMEM/F12/10% FBS, seeded in 6-well
cell culture plates at a density of 5×105 cells/well and
cultured overnight at 37°C. Additionally, a human monocytic
leukemia cell line (THP-1) was obtained from the American Type
Culture Collection (Manassas, VA, USA). THP-1 cells were cultured
in RPMI-1640 containing 10% FBS at 37°C and underwent
differentiation with 50 ng/ml phorbol myristate acetate overnight.
Then, the two cell lines were stimulated with 10 ng/ml IL-1β
(Prospec-Tany TechnoGene, Ltd., East Brunswick, NJ, USA) for 12 h
in the absence or presence of puerarin (25, 50 and 100 nM).
Cell proliferation assays
CCK-8 assays were performed to determine cell
proliferation according to the manufacturer's protocols. Briefly,
primary chondrocytes were plated at 1×104 cells/well in
a 96-well cell culture plate. Serial concentrations of puerarin (0,
25, 50 and 100 nM) were applied to the culture medium for 24 h at
37°C. The working concentration selected was 50, and 50 nM puerarin
was applied to the culture medium for 0, 6, 12, 24 and 48 h. CCK-8
reagent was added prior to 2 h of further incubation at 37°C. The
proliferation of cells was determined by the absorbance 450 nm as
detected by a microplate reader.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) flow cytometry assay
An Annexin V-FITC/PI detection kit (Yeasen
Biotechnology Co., Ltd., Shanghai, China) was used to determine
cell apoptosis according to the manufacturer's protocols. Briefly,
primary chondrocytes were plated at 1×106 cells/well in
6-well cell culture plates. Serial concentrations of puerarin (0,
25, 50 and 100 nM) were applied to the culture for 24 h. The cells
were collected, stained with Annexin V-FITC/PI and measured using a
flow cytometer. Data were analyzed using FlowJo software 7.0 (Tree
Star, Inc., Ashland, OR, USA).
ELISA
Cell culture supernatants were collected and stored
at −80°C for cytokine assays. Human PGE2 (cat. no. KHL1701), IL-6
(cat. no. 88-7066-22), TNF-α (cat. no. 88-7346-22), IL-12 (cat. no.
BMS2013), TGF-β1 (cat. no. BMS249-4) and IL-10 (cat. no.
88-7105-22) ELISA kits were purchased from BioSource International
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). All procedures
were performed according to the manufacturer's protocols.
Animal experiment
Male B6 mice (age, 8 weeks; weight, 20–22 g) were
purchased from the Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China) and housed in standard conditions of
humidity and temperature (temperature, 23±2°C; humidity, 50–80%),
with free access to food and water under a 12-h light/dark cycle in
the Animal Center of the Nanjing University of Chinese Medicine
(Nanjing, China). The animal experimental procedures were approved
by the Committee on Laboratory Animal Care of the Nanjing
University of Chinese Medicine (license no. 20170621-X). To
investigate the effects of puerarin following induction of OA, the
mice were randomly divided into five groups, four of which were
injected intra-articularly with 5 µl mono-iodoacetate (MIA; 20
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as described
previously (23). Puerarin (0, 10,
25 or 50 mg/kg) was injected intraperitoneally at a 3-day interval
starting from the day prior to OA induction from day-1 to day 14.
To examine the effect of puerarin on blood monocytes/macrophages
migration, the mice were randomly divided into four groups, OA was
induced in three groups and mice were treated with puerarin (25 or
50 mg/kg) at a 3-day interval starting, and sacrificed at day 10.
The mice in the control group (NC) were not treated with MIA and OA
was not induced. All mice were sacrificed under general anesthesia
and their joints were collected for RNA extraction and histological
analysis.
Histology
Joints were fixed with 10% neutral buffered
formalin, decalcified in 10% EDTA for 1 month at room temperature
and subsequently embedded in paraffin. Sections (5-µm) were stained
with H&E (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) to detect inflammation or with Safranin O/Fast
green (Beijing Solarbio Science & Technology Co., Ltd.) to
detect cartilage alterations. For hematoxylin and eosin staining,
the sections were stained with hematoxylin at 25°C for 15 min.
Subsequently, the sections were washed with purified water,
dehydrated in ethyl alcohol series and stained with eosin solution
for 5 min at 25°C. The samples were washed and dehydrated in ethyl
alcohol, and the sections were visualized using a light microscope
(DMI4000B; Leica Microsystems GmbH, Wetzlar, Germany;
magnification, ×200). For Safranin O/Fast green staining, the
sections were washed twice for 10 min in Xylene at 25°C, rehydrated
in ascending ethyl alcohol series and stained at 25°C for 12 h in a
1% Safranin O solution. Subsequently, the sections were washed in
purified water twice for 10 min, dehydrated in ascending ethyl
alcohol series, and stained at 25°C for 10 sec in Fast Green
solution. Following rinsing and removal of the Fast Green solution,
the samples were imaged using a light microscope (DMI4000B; Leica
Microsystems GmbH; magnification, ×200).
Flow cytometric assay of blood
monocytes
In total, 500 µl blood was collected from the
angular vein of mice using EDTA-Na2 anticoagulant tubes.
Following dilution with an equivalent volume of PBS, the blood
samples were blocked using the purified anti-mouse CD16/32 antibody
(dilution, 1:100; clone, 93; cat. no. 101301; BioLegend, Inc., San
Diego, CA, USA) for 15 min on ice and subsequently stained with
fluorescein isothiocyanate-labeled cluster of differentiation 11b
(CD11b; dilution 1:200; clone, M1/70; cat. no. 11-0112-82;
eBioscience; Thermo Fisher Scientific, Inc.), phyllochlorin-labeled
CD115 (dilution, 1:100; clone, AFS98; cat. no. 25-1152-80;
eBioscience; Thermo Fisher Scientific, Inc.),
phycoerythrobilin-labeled lymphocyte Ag 6G (Ly6G; dilution, 1:100;
clone, 1A8-Ly6g; cat. no. 17-9668-80; eBioscience; Thermo Fisher
Scientific, Inc.), phycoerythrin-cyanin 7-labeled Ly6C (dilution,
1:100; clone, HK1.4; cat. no. 12-5932-80; eBioscience; Thermo
Fisher Scientific, Inc.) and allophycocyanin-labeled C-C chemokine
receptor 2 (CCR2; dilution, 1:100; clone, SA203G11; cat. no.
150621; BioLegend, Inc.) antibodies for 40 min on ice.
Subsequently, red blood cells (RBCs) were removed using the RBC
lysis buffer (BioLegend, Inc.). Following a centrifugation at 500 ×
g for 10 min at 4°C, the remaining cell pellets were washed three
times with PBS and resuspended in 300 µl fluorescent-activated cell
sorting (FACS) buffer (PBS/0.5% FBS/0.06% NaN3) for
assays. Finally, data were acquired using a BD FACS Aria II flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data were
analyzed using the FlowJo software 7.0 (Tree Star, Inc., Ashland,
OR, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse knee cartilage
and synovium using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
RT of RNA was conducted using a Moloney Murine Leukemia Virus
reverse transcriptase PCR Kit (Vazyme, Piscataway, NJ, USA)
according to the manufacturer's protocol. qPCR was performed using
a SYBR® Green Kit (Vazyme) with the following primers:
C-C chemokine ligand 2 (CCL2), forward 5′-AGGTGTCCCAAAGAAGCTGTA-3′,
reverse, 5′-ATGTCTGGACCCATTCCTTCT-3′; CCL5, forward
5′-ATATGGCTCGGACACCACTC-3′, reverse, 5′-GTGACAAACACGACTGCAAGA-3′;
and GADPH, forward 5′-CATGGCCTTCCGTGTTCCTA-3′ and reverse,
5′-GCGGCACGTCAGATCCA-3′.
The thermocycling conditions were as follows: 30
cycles of 95°C for 5 min, 95°C for 30 sec, 63°C for 30 sec and 72°C
for 30 sec. The mRNA expression levels were normalized to GADPH
using the 2−ΔΔCq quantification method as previously
described (24).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism v5.0 software (GraphPad Software, Inc., La Jolla,
CA, USA). All experiments were repeated at least three times. The
data are presented as the mean ± standard deviation. Significant
differences between groups were determined using the Kruskal-Wallis
test followed by Tukey's multiple comparisons test. P<0.05 was
considered to represent a statistically significant difference.
Results
Effects of puerarin on the cell
proliferation of chondrocytes
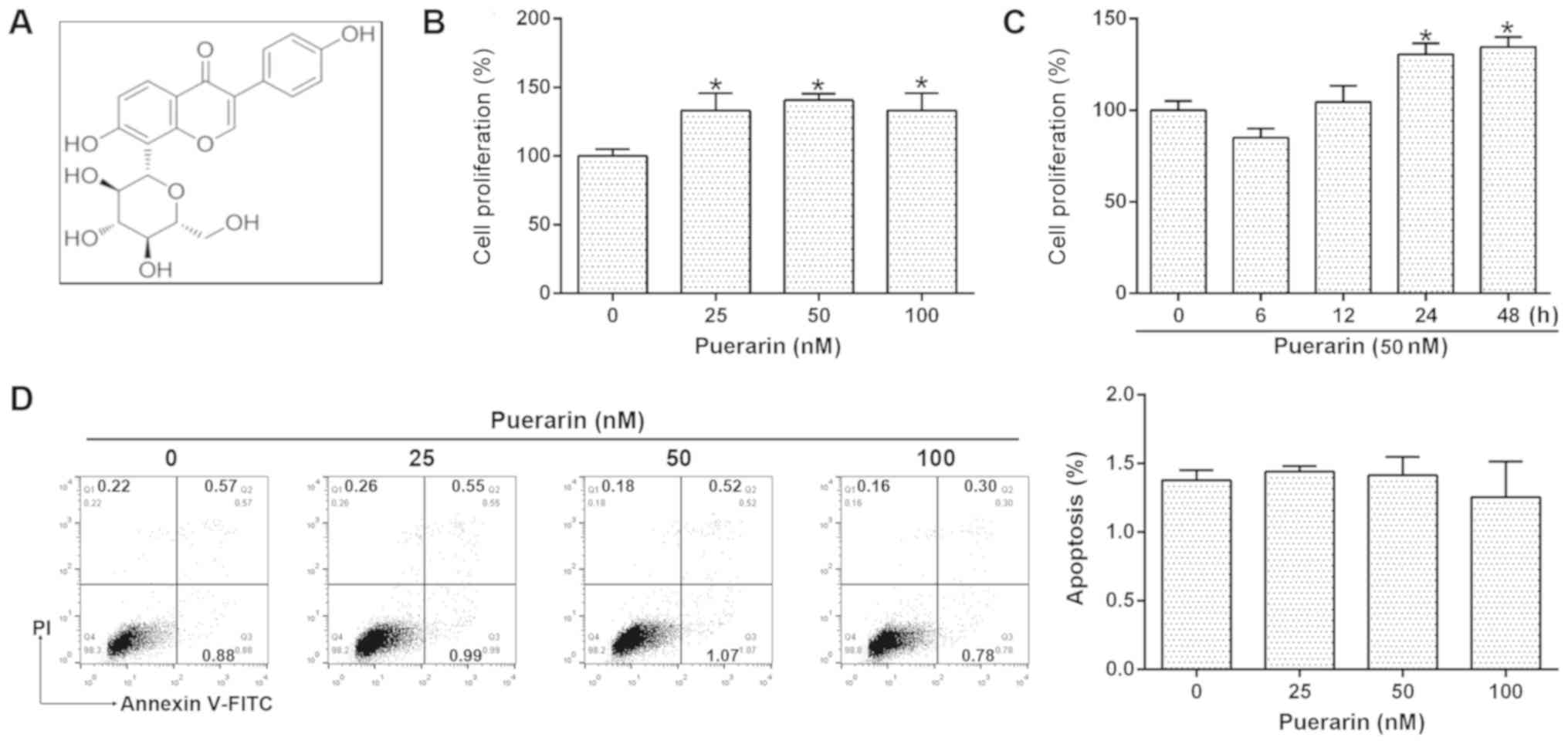
To investigate the effects of puerarin (Fig. 1A) on human chondrocytes, the
proliferation of chondrocytes was determined following treatment
with various concentrations (0, 25, 50 or 100 nM) of puerarin for
24 h. As presented in Fig. 1B, 25,
50 or 100 nM puerarin significantly increased the proliferation of
cells compared with the control; thus, 50 nM puerarin was selected
to determine the effects of puerarin in the further analyses.
Compared with baseline proliferation prior to treatment, 50 nM
puerarin induced a marked decrease in chondrocyte proliferation at
6 h, but significantly promoted cell proliferation at 24 and 48 h
(Fig. 1C). These results were
supported by Annexin V-FITC/PI flow cytometry; no significant
alterations in the apoptosis of cells were observed following
puerarin treatment (Fig. 1D).
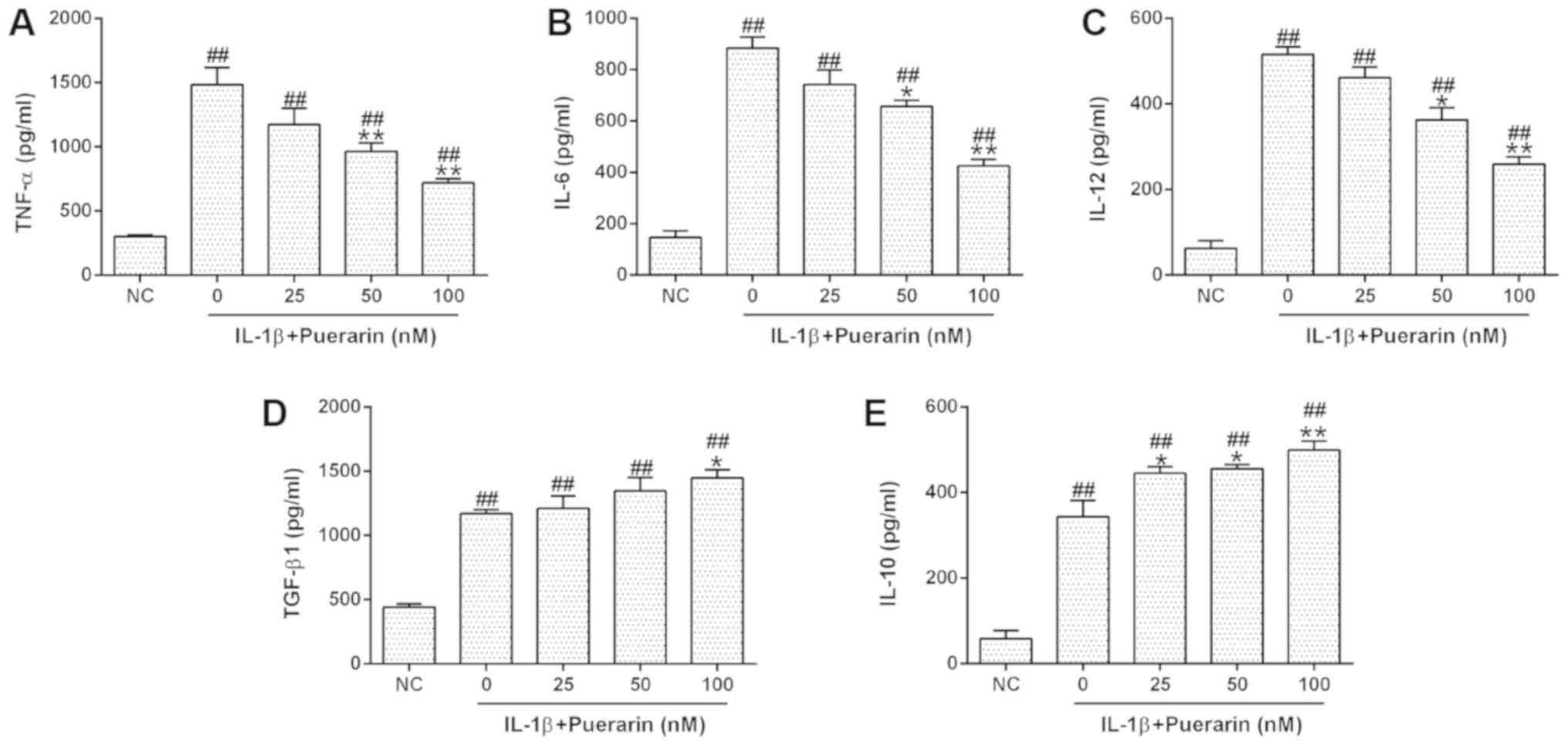
Puerarin inhibits the production of
IL-1β-induced inflammatory cytokines
The effects of puerarin on IL-1β-induced
inflammatory responses were subsequently investigated in OA
chondrocytes. The levels of TNF-α, IL-6 and IL-12 expression were
measured via an ELISA (Fig. 2A-C).
It was revealed that puerarin significantly reduced the
IL-1β-induced upregulation of PGE2, IL-6 and TNF-α expression
levels in a dose-dependent manner.
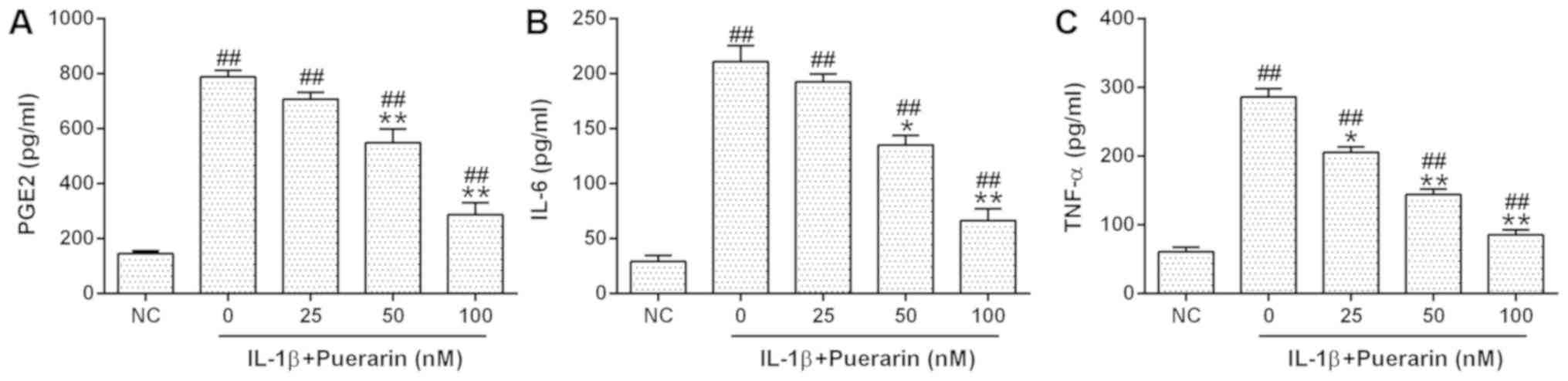
The effects of puerarin on the function of human
monocytes/macrophages were subsequently investigated. THP-1
macrophages were treated with puerarin for 24 h, followed by
stimulation with IL-1β. The expression levels of TNF-α, IL-6 and
IL-12 were measured by ELISA. Puerarin treatment significantly
downregulated IL-1β-induced expression of TNF-α, IL-6 and IL-12 in
THP-1 macrophages (Fig. 3A-C);
however, the levels of anti-inflammatory cytokine expression,
including TGF-β1 and IL-10, were increased in IL-1β-treated cells
following puerarin exposure (Fig. 3D
and E), suggesting that puerarin treatment altered the function
of monocytes/macrophages.
 | Figure 3.Puerarin treatment reduces the release
of inflammatory mediators from monocytes/macrophages following
IL-1β stimulation. THP-1 cells were differentiated using 50 nM
phorbol myristate acetate, and then stimulated with 10 ng/ml IL-1β
in the presence of 0, 25, 50 or 100 nM puerarin. Following culture
for 48 h, the levels of (A) TNF-α, (B) IL-6, (C) IL-12, (D) TGF-β1
and (E) IL-10 in the culture supernatant were determined by ELISA.
Experiments were performed at least two times. Data are presented
as the mean ± standard deviation. ##P<0.01 vs. NC.
*P<0.05, **P<0.01 vs. 0 nM. IL, interleukin; NC, negative
control; TGF-β1, transforming growth factor β1; TNF-α, tumor
necrosis factor α. |
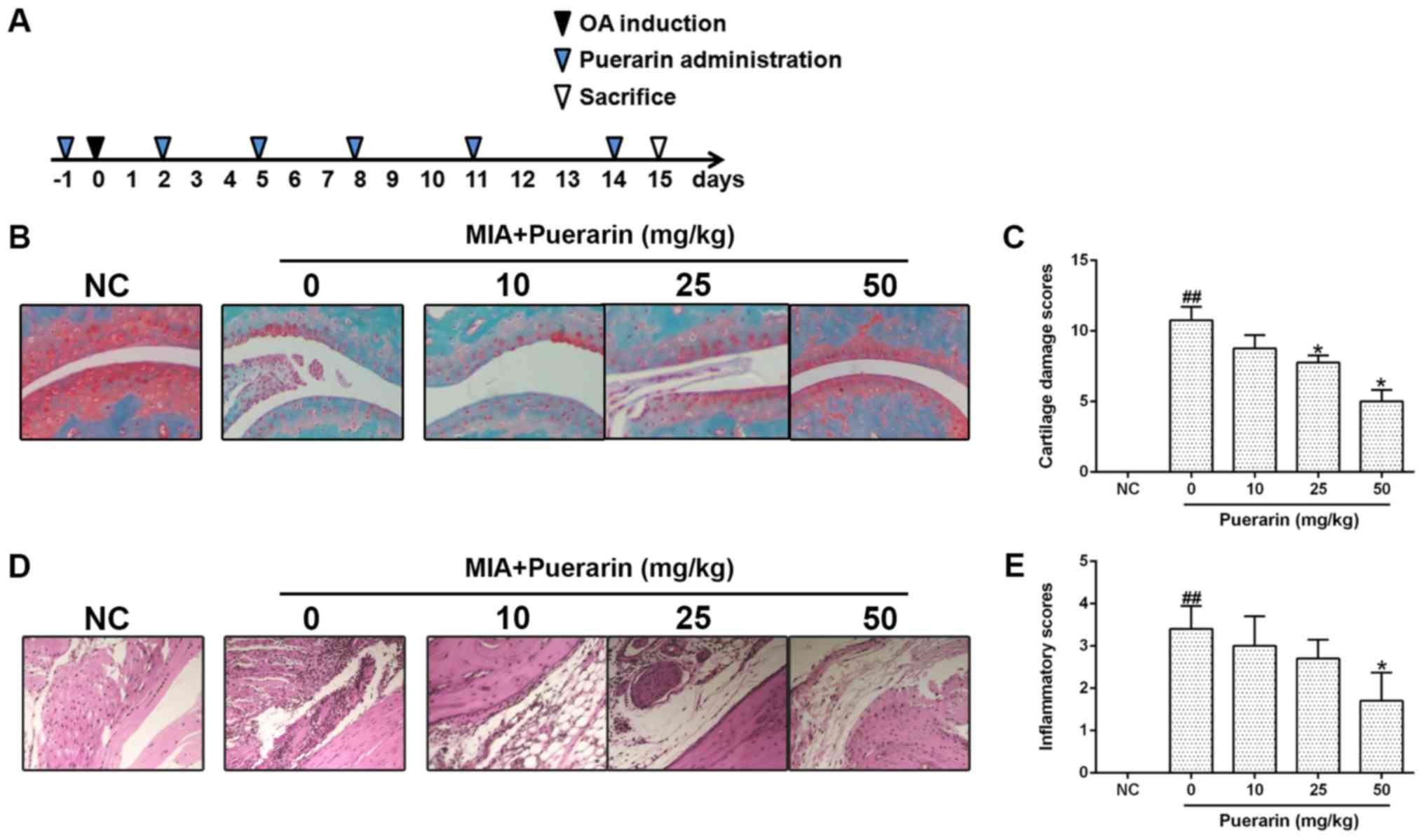
Effects of puerarin on degenerative
cartilage destruction
To evaluate the effects of puerarin on OA, a mouse
model of OA was generated using a method of intra-articular
injection of MIA as previously described (23). Treatment with puerarin was
initiated the day prior to MIA injection, and repeated at 3-day
intervals at a range of concentrations (0, 10, 25 or 50 mg/kg;
Fig. 4A); a normal control (NC)
group did not receive MIA. Following puerarin treatment, the degree
of cartilage destruction and synovitis score were determined by
Safranin O and H&E staining, respectively. MIA treatment
induced a significant increase in cartilage damage compared with
the NC group, which reduced in a dose-dependent manner following
puerarin treatment (Fig. 4B and
C). Similarly, the severity of synovitis was significantly
increased following MIA treatment compared with the NC group, but
was reduced in MIA-injected mice following treatment with puerarin,
suggesting that 50 mg/kg of puerarin attenuated the inflammatory
responses (Fig. 4D and E).
Collectively, the results indicate that puerarin may ameliorate
cartilage damage and synovitis-associated pathological alterations
in a mouse model of MIA-induced OA.
Effects of puerarin on blood
monocytes/macrophages in vivo
The effects of puerarin on monocyte recruitment were
investigated by measuring the number of monocytes in the blood and
the levels of CCL2 and CCL5 expression in synovial tissues 10 days
post-OA induction. All mice in the experimental groups underwent
MIA-injection to induce OA on day 0; the mice in the control group
were not treated with MIA. It was revealed that the number of
CD11b+/Ly6C− cells, characterized by low
expression levels of CCR2 (25),
was notably increased in OA mice following treatment with 50 mg/kg
puerarin. Conversely, puerarin treatment significantly reduced the
number of CD11b+/Ly6C+ cells, the numbers of
which were increased significantly following MIA injection compared
with the NC group (Fig. 5A-C). Of
note, although MIA injection induced an increase in the expression
levels of CCL2 and CCL5 (Fig. 5D and
E), 50 mg/kg puerarin-treated mice demonstrated significantly
reduced expression levels of CCL2 mRNA, suggesting that puerarin
reduced the infiltration of monocytes in the mouse model of OA
(Fig. 5D). Collectively, the
results indicate that puerarin may suppress proinflammatory
monocyte recruitment during OA.
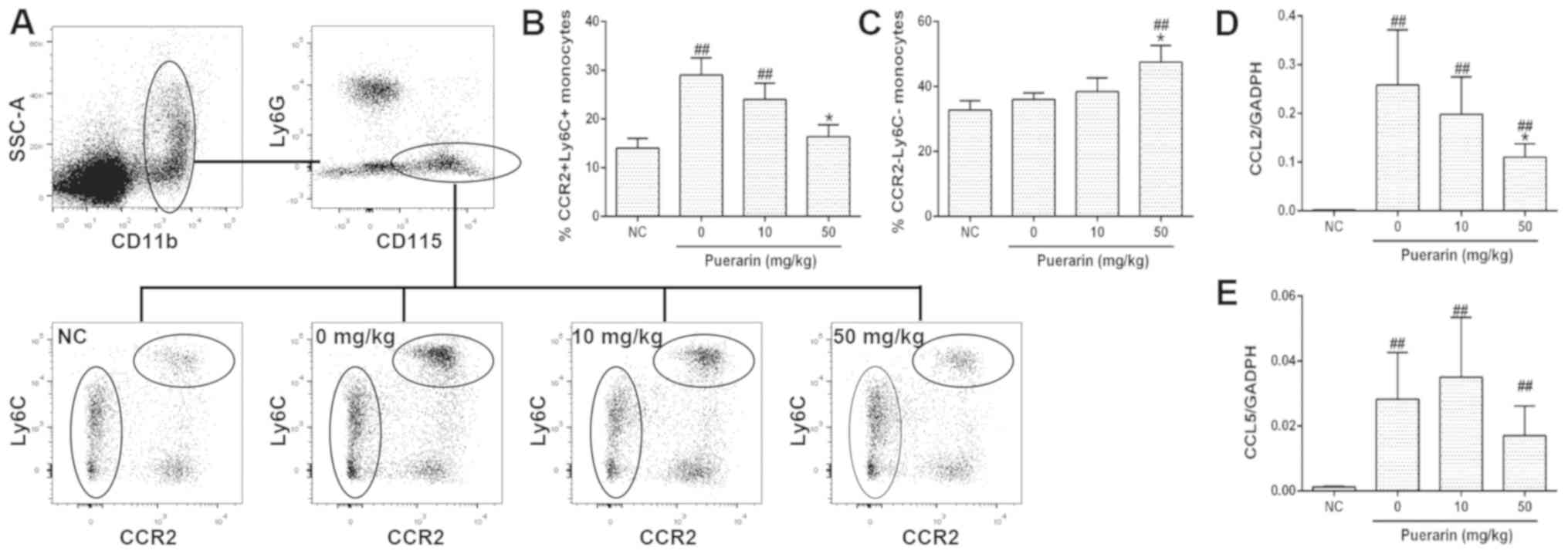 | Figure 5.Effects of puerarin on blood
monocytes/macrophages in vivo. Blood was collected on day 10
post-OA induction, stained with CD11b, CD115, Ly6G, Ly6C and CCR2
antibodies and assayed by fluorescent-activated cell sorting. (A)
Gating strategy of monocytes and representative images. (B) Ratio
of CCR2+/Ly6C+ cells in the
CD11b+/CD115+/Ly6G− cells. (C)
Ratio of CCR2−/Ly6C− cells in the
CD11b+/CD115+/Ly6G− cells. mRNA
was extracted from the knee joints of mice with OA on day 14, and
the levels of (D) CCL2 and (E) CCL5 expression were determined
using reverse transcription-quantitative polymerase chain reaction.
N=6–8 animals/group. Data are presented as the mean ± standard
deviation. ##P<0.01 vs. NC. *P<0.05 vs. 0 nM. CCL,
C-C chemokine ligand; CCR, C-C chemokine receptor; CD, cluster of
differentiation; Ly, lymphocyte Ag; NC, negative control; OA,
osteoarthritis. |
Discussion
Puerarin is a versatile compound extracted from the
root of Pueraria (Radix puerariae) (26). The therapeutic potential of
puerarin has been reported in vivo and in vitro; for
instance, puerarin protects retinal pericytes from apoptosis in a
rat model with retinal pericyte loss (13). The molecular mechanisms underlying
this effect may be associated with the inhibition of NADPH
oxidase-associated reactive oxygen species (ROS) pathways and the
suppression of NF-κB activation. The present study revealed that
puerarin treatment suppressed the production of numerous
proinflammatory cytokines from activated human chondrocytes, which
may be associated with the inhibition of the ROS and NF-κB
pathways. Additionally, puerarin ameliorates nerve injury-induced
depression and pain following spared nerve injury in an arginase
2-dependent manner (27).
Furthermore, puerarin attenuates mechanical and chemical nerve
injuries in experimental models of cerebral ischemia, diabetic
complication and cardiac diseases (6,8,13).
These findings indicated the antioxidative, anti-inflammatory and
antiapoptotic activities of puerarin in vivo and in
vitro. In the present study, the potential of puerarin in the
treatment of OA, and the anti-inflammatory effects of puerarin on
in vitro and in vivo models of OA were investigated.
Human chondrocytes exhibited increased cell proliferation following
treatment with puerarin without a significant alteration in
apoptosis, suggesting chondroprotective activity in vitro.
Future experiments should aim to verify these findings and
investigate the underling mechanisms using western blotting and
lactate dehydrogenase release assays. Additionally, high
concentrations of puerarin were used to demonstrate the effects of
puerarin in vitro; however, further study is required to
determine suitable doses and routes of administration for use in
humans.
OA is a common joint disease with persistent,
low-degree inflammation in the synovium (3). Puerarin has been reported to exhibit
anti-inflammatory effects in various mouse models of inflammatory
disease, including ischemia/reperfusion, atopic dermatitis,
mastitis and collagen antibody-induced arthritis (11,16,28,29).
Puerarin treatment inhibits chronic inflammation induced by
lipopolysaccharide or pro-inflammatory mediators, such as IL-1β, in
various types of cells (9,19,30).
The present study revealed that puerarin not only inhibited
inflammatory responses in IL-1β-stimulated OA chondrocytes, but may
have also altered the function of monocytes/macrophages in
vitro. A recent study identified the role of blood-derived
monocytes and the CCL2/CCR2 axis in the establishment of chronic
inflammation (25); however, the
CCL5/CCR5 axis may be responsible for the aggravation of OA
(25,31). In the present study, puerarin was
administrated intraperitoneally to mice, and may therefore affect
monocytes in the blood prior to migrating to joints.
In conclusion, it was revealed that puerarin
protected human chondrocytes and reduced the production of
inflammatory mediators. In vivo, puerarin ameliorated the
progression of MIA-induced OA in mice and altered blood monocyte
development. Further investigation is required to determine the
specific mechanisms by which puerarin interacts with blood
monocytes and whether puerarin alters monocyte infiltration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LP, ZX and JP performed the animal experiments, and
collected and analyzed data. LP, BW, YG and YQ contributed to the
conception and design of the present study, and interpreted the
data. LP and YQ drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Changzhou Traditional Chinese Medicine Hospital
(Changzhou, China), and patients provided written informed
consent.
Patient consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poole AR, Rizkalla G, Ionescu M, Reiner A,
Brooks E, Rorabeck C, Bourne R and Bogoch E: Osteoarthritis in the
human knee: A dynamic process of cartilage matrix degradation,
synthesis and reorganization. Agents Actions Suppl. 39:3–13.
1993.PubMed/NCBI
|
|
2
|
Messier SP, Mihalko SL, Legault C, Miller
GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein
F, et al: Effects of intensive diet and exercise on knee joint
loads, inflammation, and clinical outcomes among overweight and
obese adults with knee osteoarthritis: The IDEA randomized clinical
trial. JAMA. 310:1263–1273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abramson SB: Inflammation in
osteoarthritis. J Rheumatol Suppl. 70:70–76. 2004.PubMed/NCBI
|
|
4
|
Tao J, Cui Y, Duan Y, Zhang N, Wang C and
Zhang F: Puerarin attenuates locomotor and cognitive deficits as
well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β
signaling pathway in an in vivo model of cerebral ischemia.
Oncotarget. 8:106283–106295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Cai W, Lee K, Liu B, Deng Y, Chen Y,
Zhang X, He JC and Zhong Y: Puerarin attenuates diabetic kidney
injury through the suppression of NOX4 expression in podocytes. Sci
Rep. 7:146032017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Cheng Y, Yang C, Lau S, Lao L,
Shuai B, Cai J and Rong J: Botanical drug puerarin attenuates
6-hydroxydopamine (6-OHDA)-induced neurotoxicity via upregulating
mitochondrial enzyme arginase-2. Mol Neurobiol. 53:2200–2211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu B, Zhao C, Li H, Chen X, Ding Y and Xu
S: Puerarin protects against heart failure induced by pressure
overload through mitigation of ferroptosis. Biochem Biophys Res
Commun. 497:233–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Zhao M, Liang S, Huang Q, Xiao Y, Ye
L, Wang Q, He L, Ma L, Zhang H, et al: The effects of puerarin on
rat ventricular myocytes and the potential mechanism. Sci Rep.
6:354752016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Zhai Z, Zhou H, Li Y, Li X, Lin
Y, Li W, Shi Y and Zhou MS: Puerarin inhibits oxLDL-induced
macrophage activation and foam cell formation in human THP1
macrophage. Biomed Res Int. 2015:4036162015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu J, Wang X, Shang Y, Xie X, Zhang F,
Chen J and Fu G: Puerarin reduces endothelial progenitor cells
senescence through augmentation of telomerase activity. Vascul
Pharmacol. 49:106–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo BQ, Xu JB, Xiao M, Ding M and Duan LJ:
Puerarin reduces ischemia/reperfusion-induced myocardial injury in
diabetic rats via upregulation of vascular endothelial growth
factor A/angiotensin-1 and suppression of apoptosis. Mol Med Rep.
17:7421–7427. 2018.PubMed/NCBI
|
|
12
|
Mercer LD, Kelly BL, Horne MK and Beart
PM: Dietary polyphenols protect dopamine neurons from oxidative
insults and apoptosis: Investigations in primary rat mesencephalic
cultures. Biochem Pharmacol. 69:339–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim J, Kim KM, Kim CS, Sohn E, Lee YM, Jo
K and Kim JS: Puerarin inhibits the retinal pericyte apoptosis
induced by advanced glycation end products in vitro and in vivo by
inhibiting NADPH oxidase-related oxidative stress. Free Radic Biol
Med. 53:357–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu C, Chen B, Jin X, Liu X, Wang F, Guo R,
Chen Z, Zheng H, Wang L and Zhang Y: Puerarin protects endothelial
progenitor cells from damage of angiotensin II via activation of
ERK1/2Nrf2 signaling pathway. Mol Med Rep. 17:3877–3883.
2018.PubMed/NCBI
|
|
15
|
Deng Y, Lei T, Li H, Mo X, Wang Z and Ou
H: ERK5/KLF2 activation is involved in the reducing effects of
puerarin on monocyte adhesion to endothelial cells and
atherosclerotic lesion in apolipoprotein E-deficient mice. Biochim
Biophys Acta Mol Basis Dis. 1864:2590–2599. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Jeon YD, Lee YM and Kim DK: The
suppressive effect of puerarin on atopic dermatitis-like skin
lesions through regulation of inflammatory mediators in vitro and
in vivo. Biochem Biophys Res Commun. 498:707–714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao Z, Ge Y, Zhou N, Wang Y, Cheng W and
Yang Z: Puerarin inhibits cardiac fibrosis via monocyte
chemoattractant protein (MCP)-1 and the transforming growth
factor-β1 (TGF-β1) pathway in myocardial infarction mice. Am J
Transl Res. 8:4425–4433. 2016.PubMed/NCBI
|
|
18
|
Wei HY, Zhang YJ and Zhao SZ: Puerarin
regulates neovascular glaucoma through pigment epitheliumderived
growth factorinduced NFkappaB signaling pathway. Mol Med Rep.
17:7866–7874. 2018.PubMed/NCBI
|
|
19
|
Liu X, Zhao W, Wang W, Lin S and Yang L:
Puerarin suppresses LPS-induced breast cancer cell migration,
invasion and adhesion by blockage NF-κB and Erk pathway. Biomed
Pharmacother. 92:429–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amzaleg Y, Ji J, Kittivanichkul D, E
Törnqvist A, Windahl S, Sabag E, Khalid AB, Sternberg H, West M,
Katzenellenbogen JA, et al: Estrogens and selective estrogen
receptor modulators differentially antagonize Runx2 in ST2
mesenchymal progenitor cells. J Steroid Biochem Mol Biol.
183:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan SY, Sheng T, Liu LQ, Zhang YL, Liu
XM, Ma T, Zheng H, Yan Y, Ishimi Y and Wang XX: Puerarin prevents
bone loss in ovariectomized mice and inhibits osteoclast formation
in vitro. Chin J Nat Med. 14:265–269. 2016.PubMed/NCBI
|
|
22
|
Yang X, Zhang H, Wang J, Zhang Z and Li C:
Puerarin decreases bone loss and collagen destruction in rats with
ligature-induced periodontitis. J Periodontal Res. 50:748–757.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guingamp C, Gegout-Pottie P, Philippe L,
Terlain B, Netter P and Gillet P: Mono-iodoacetate-induced
experimental osteoarthritis: A dose-response study of loss of
mobility, morphology, and biochemistry. Arthritis Rheum.
40:1670–1679. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ueda MY, Alvarenga PG, Real JM, Moreira
Ede S, Watanabe A, Passos-Castilho AM, Vescovi M, Novis Y, Rocha V,
Seber A, et al: Optimisation of a quantitative polymerase chain
reaction-based strategy for the detection and quantification of
human herpesvirus 6 DNA in patients undergoing allogeneic
haematopoietic stem cell transplantation. Mem Inst Oswaldo Cruz.
110:461–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raghu H, Lepus CM, Wang Q, Wong HH,
Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR,
et al: CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment,
inflammation and cartilage destruction in osteoarthritis. Ann Rheum
Dis. 76:914–922. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeung DK, Leung SW, Xu YC, Vanhoutte PM
and Man RY: Puerarin, an isoflavonoid derived from Radix puerariae,
potentiates endothelium-independent relaxation via the cyclic AMP
pathway in porcine coronary artery. Eur J Pharmacol. 552:105–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Luo D, Liang Z, Lao L and Rong J:
Plant natural product puerarin ameliorates depressive behaviors and
chronic pain in mice with spared nerve injury (SNI). Mol Neurobiol.
54:2801–2812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Yan J, Xu X, Duan C, Xie Z, Su Z,
Ma H, Ma H, Wei X and Du X: Puerarin prevents LPS-induced acute
lung injury via inhibiting inflammatory response. Microb Pathog.
118:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu
C and Deng G: Puerarin exerts an antiinflammatory effect by
inhibiting NF-kB and MAPK activation in staphylococcus
aureus-induced mastitis. Phytother Res. 30:1658–1664. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng HF, Wang S, Li L, Zhou Q, Guo WB,
Wang XL, Liu MD, Liu K and Xiao XZ: Puerarin prevents vascular
endothelial injury through suppression of NF-κB activation in
LPS-challenged human umbilical vein endothelial cells. Biomed
Pharmacother. 104:261–267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tall AR and Yvan-Charvet L: Cholesterol,
inflammation and innate immunity. Nat Rev Immunol. 15:104–116.
2015. View
Article : Google Scholar : PubMed/NCBI
|