Introduction
Osteomyelitis is a bone infection, which is mainly
caused by microorganisms and accompanied by bone destruction.
Bacteria bind to the bone and induce acute inflammation, after
which, immune cells release cytokines and chemokines that regulate
bone metabolism (1).
Staphylococcus aureus (S. aureus) is the most common
bacterial species involved. This microorganism has several
characteristics that facilitate its role as a common pathogen in
human osteomyelitis (2), and there
are a series of extracellular and cell-associated factors
contributing to its virulence (3).
It has been reported that infection of cultured osteoblasts with
S. aureus prevents proliferation, induces apoptosis and
inhibits mineralization. In addition, S. aureus increases
receptor activator of nuclear factor (NF)-κB ligand (RANKL)
expression and decreases osteoprotegerin expression in osteoblasts;
these effects are likely to promote osteoclast formation and
function (4,5). Osteoclasts are generated from myeloid
progenitors produced in the bone marrow. They are released into the
vasculature and serve critical roles in the processes of bone
resorption and destruction, which occur in osteomyelitis (6–9).
Osteomyelitis requires multimodal, appropriate therapy. The goal is
to clear the infection thorough debridement and appropriate
antibiotic treatment. Recently, gene-targeted therapy has become
increasingly popular, encouraging investigation of the mechanisms
underlying this disease.
There are several important mechanisms involved in
the process of bone remodeling during bone-associated disease. In
particular, the NF-κB transcription factor has garnered increasing
attention (10–12). A recent study suggested that the
NF-κB pathway may be considered a pharmacological target for the
modulation of chronic inflammation-induced bone resorption
(13). The NF-κB family consists
of several members, including p50, p52, RelA/p65, RelB, c-Rel,
NF-κB1/p105 and NF-κB2/p100; as well as the inhibitory subunits
IκBα, IκBβ and IκBγ (14). NF-κB
dimers are activated via regulation of the inhibitory proteins, of
which IκBα is the most widely investigated (15). IκBα is phosphorylated at N-terminal
serine residues by a large IκB kinase (IKK) complex. The
predominant IKK complex is comprised of two catalytic subunits,
IKK1 (also known as IKKα) and IKK2 (also known as IKKβ), as well as
a regulatory subunit, IKKγ [also known as NF-κB essential modulator
(NEMO)] (14). The N-terminal,
α-helical region of NEMO associates with a hexapeptide sequence at
the distal carboxyl terminus of IKK2 and IKK1, termed the
NEMO-binding domain (NBD) (15).
It has been reported that a short cell-permeable peptide containing
the NBD sequence disrupts the association of NEMO with IKK2, blocks
NF-κB activation, and ameliorates bone resorption and inflammation
in several animal models (16). In
addition, disturbing the binding of NEMO to IKK2 with NBD peptides
inhibits osteoclastogenesis in vitro (16). NBD peptides can block NF-κB
activation in vivo, reduce osteoclast recruitment and bone
erosion, and ameliorate the pathological severity of inflammation
(17). However, to the best of our
knowledge, no previous studies have been conducted to investigate
the therapeutic potential of NBD peptides in S.
aureus-induced osteomyelitis.
Given the central role of the IKK complex in
osteoclastogenesis and inflammatory osteolysis, it was hypothesized
that the NBD peptide may prevent the absorption of bone by
arresting osteoclastogenesis in an osteomyelitis model. To test
this hypothesis, the NBD peptide was applied in vitro and
in vivo, the formation and function of osteoclasts cultured
on bone slices in the presence of the NBD peptide were studied, and
bone mineral density and morphology in a rabbit mandible S.
aureus osteomyelitis model were analyzed. This study was
conducted in order to investigate the potential role of the NBD
decoy peptide in an osteomyelitis model.
Materials and methods
NBD peptides
NBD peptides have been described previously
(16,18). A cell-permeable, wild type NBD
peptide (DRQIKIWFQNRRMKWKK-TALDWS-WLQTE) was provided by GL Biochem
(Shanghai) Ltd. (Shanghai, China) as lyophilized powder.
Immediately prior to use, 20 mM stock solutions of the NBD peptide
were prepared in dimethyl sulfoxide (DMSO). Stocks were diluted in
culture medium to the final concentration required.
Cell culture
RAW264.7 cells [American Type Culture Collection
(ATCC) no. TIB-71; Cell Bank of Chinese Academy of Sciences,
Shanghai, China] were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin
at 37°C in an atmosphere containing 5% CO2. Fresh media
were added every 2 days. To study osteoclast formation, RAW264.7
cells were cultured in complete α-minimum Eagle's medium (α-MEM;
Gibco; Thermo Fisher Scientific, Inc.) in the presence of NBD
peptide at concentrations of 0 or 20 µM (18), and simultaneously stimulated with
soluble recombinant mouse RANKL (100 ng/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) (19). The
media were replaced every 2 days.
Osteoclast formation
RAW264.7 cells grown as aforementioned were cultured
in the presence of soluble RANKL (100 ng/ml). After 4 days of
culture, cells were fixed with 4% formaldehyde at room temperature
for 10 min and histostained for tartrate-resistant acid phosphatase
(TRAP) using a TRAP staining kit (cat. no. 387A; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), according to the manufacturer's
protocol and as previously described (20,21).
Cells were also immunostained with fluorescein
isothiocyanate-labeled phalloidin (F-actin, 5 µg/ml; cat. no.
P5282-1MG; Sigma-Aldrich; Merck KGaA) for 60 min, and with
4′,6-diamidino-2-phenylindole (1 µg/ml; Sigma-Aldrich; Merck KGaA)
for 5 min at room temperature. Cells were analyzed using a Nikon
microscope (Nikon Corporation, Tokyo, Japan) under ×10
magnification, and images were captured and analyzed with
NIS-Elements F2.20 (Nikon Eclipse 80i; Nikon Corporation).
TRAP+ multinucleated cells, containing >3 nuclei,
were counted as osteoclasts. The average number of nuclei in
osteoclasts was detected from the immunostaining images.
Pit formation assay
Bovine femoral cortical bone (22), purchased fresh from a butcher's
shop, was cut into 0.1 cm slices, which were cleaned by
ultrasonication in sterile distilled water at 40 kHz for 30 min at
room temperature, autoclaved at 120°C and 0.4 MPa, and immersed in
DMEM. The medium was changed three times every 30 min, and the
slices were stored in DMEM at 4°C. Prior to each experiment, bone
slices were preheated at 37°C for 1 h in 48-well plates containing
1 ml α-MEM. Subsequently, RAW264.7 cells stimulated with RANKL were
added, with or without NBD peptide. After 4 days, the cells on the
slices were fixed with 4% formaldehyde at room temperature for 10
min and observed under scanning electron microscopy (SEM;
SU8010FE-SEM; Hitachi, Ltd., Tokyo, Japan). Subsequently, cells
were removed by ultrasonication at 40 kHz for 30 min at room
temperature and resorption lacunae were observed under the electron
microscope. The total area of bone lacunae on the slices was
quantified using NIS Elements-AR software (Nikon Corporation).
Chronic osteomyelitis model
New Zealand white rabbits were provided by the
Animal Experiment Center of Zhejiang University (Hangzhou, China).
All procedures were approved by the Institutional Animal Care and
Use Committee of Zhejiang University. Briefly, male rabbits
(n=6/group; age, 4 months; weight, 2.5–3.0 kg) were selected for
the osteomyelitis model (23).
Rabbits were housed in stainless steel cages under controlled
conditions (temperature, 23±2°C; relative humidity, 55±10%;
ventilation, >10 times/h; 12-h light/dark cycle). All animals
had free access to food and water throughout the acclimation and
experimentation periods, and were maintained according to the
Animal Experiment Center of Zhejiang University. Animals were
anesthetized with Sumianxin II (10 mg/kg, 0.2 ml/kg; Dunhua Shengda
Animal Medicine Co., Ltd., Dunhua, China; also known as xylazine
hydrochloride), and a bone defect (8×8×3 mm) was created at the
lateral inferior border of the mandible beside the middle joint.
Subsequently, 100 µl 5% sodium morrhuate containing
5.0×107 cfu/ml S. aureus (ATCC no. 25923;
Department of Microbiology of Zhejiang University) was injected
into the bone defect, which was then sealed with bone wax. After 6
weeks, rabbits were separated into the following three groups: i)
Untreated controls, ii) treated with debridement surgery, and iii)
treated with debridement surgery plus 500 µg/kg NBD peptide, which
was injected into the defect area. During debridement, dead,
damaged and infected tissue was removed from the bone defect. A
total of 4 and 8 weeks following surgery, rabbits were sacrificed
and specimens were harvested.
Dual energy X-ray and cone beam
computed tomography (CBCT)
A total of 4 and 8 weeks following surgery, rabbits
were euthanized and bone mineral density (BMD) was examined in the
surgical area by dual energy X-ray absorptiometry (24). In addition, bone morphology was
analyzed by radiographic analysis using CBCT (25) (NewTom 3G QR-DVT9000; NewTom,
Verona, Italy).
Statistical analysis
In vitro experiments were conducted three
times to obtain the mean and standard error from independent
experiments. In vivo experiments were conducted on six
animals/group. Statistical analysis was performed using SPSS 17.0
software package (SPSS, Inc., Chicago, IL, USA) by one-way analysis
of variance with Tukey's post hoc test for comparisons between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
NBD peptide inhibits RANKL-induced
osteoclast formation in vitro
RAW264.7 cells were treated with DMSO or the NBD
peptide and were stimulated with RANKL in vitro, in order to
induce osteoclastogenesis (Fig.
1A). Addition of the NBD peptide resulted in a 71±5.6%
reduction in the number of osteoclasts formed (P<0.05; Fig. 1B). In addition, there was a 55±1.5%
reduction in the average number of nuclei per osteoclast
(P<0.05; Fig. 1C). Osteoclast
morphology in bone slices was analyzed by electron microscopy.
Consistent with the TRAP staining results, the number and size of
osteoclasts were reduced in the NBD group compared with in the DMSO
group (Fig. 1A).
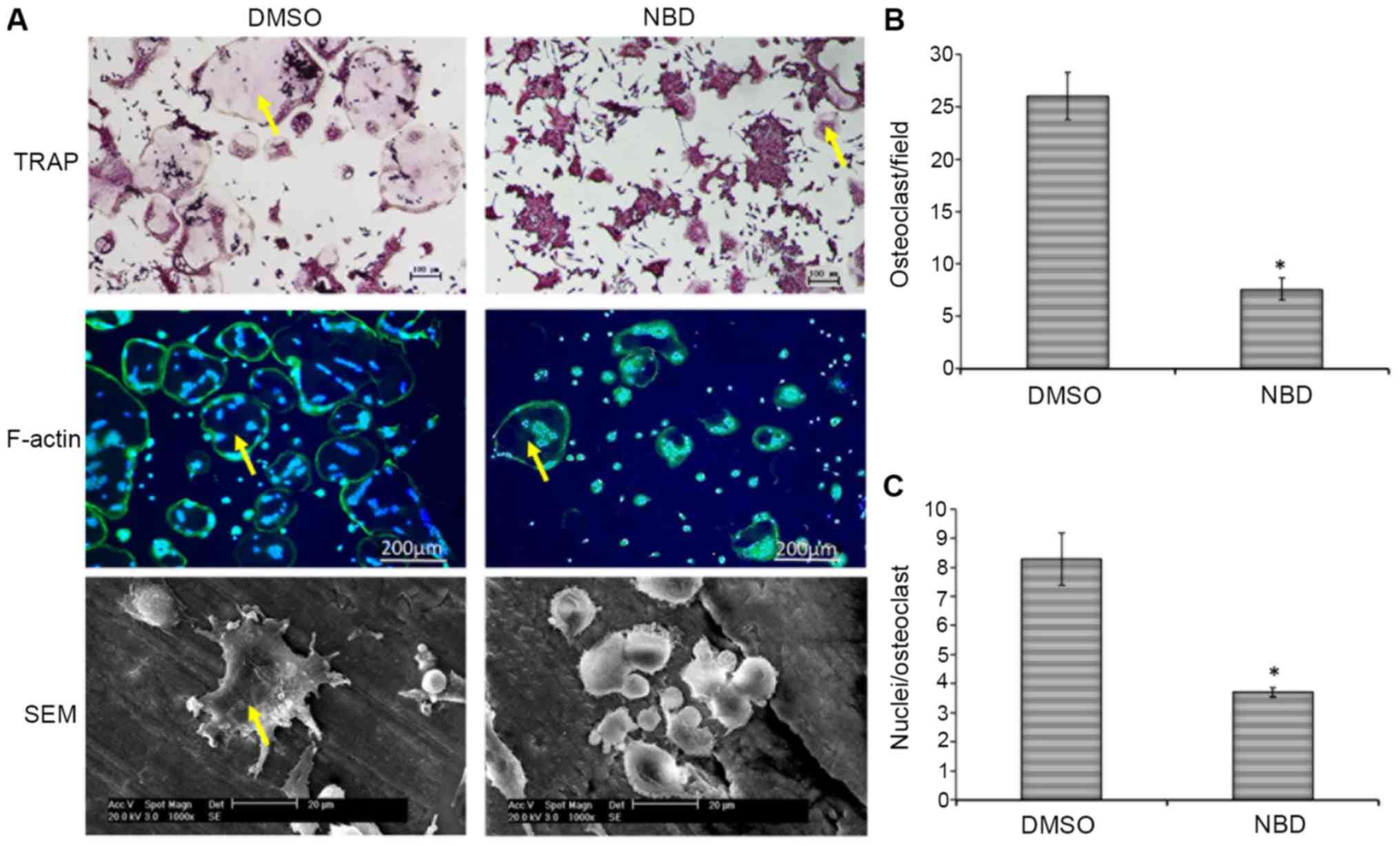 | Figure 1.NBD peptide inhibits RANKL-induced
osteoclast formation and function in vitro. (A) RAW 264.7
cells were incubated in the presence of RANKL (100 ng/ml) on plates
or bone slices. DMSO or NBD peptide (20 µM) were added
simultaneously with RANKL. After 4 days, cells were fixed and
stained for TRAP and F-actin (green), and counterstained with DAPI
(blue) (F-actin images, ×20 magnification; TRAP images, ×10
magnification). Yellow arrows indicate multinucleated osteoclasts
with >3 nuclei. In addition, bone slices were washed with PBS,
fixed and osteoclasts were visualized by scanning electron
microscopy. (B) Number of TRAP+ multinucleated
osteoclasts (>3 nuclei/osteoclast). (C) Mean number of nuclei
per osteoclast. Data are presented as the means ± standard error of
the mean of three independent experiments. *P<0.05 vs. the DMSO
group. DMSO, dimethyl sulfoxide; NBD, nuclear factor-κB essential
modulator-binding domain; SEM, scanning electron microscopy; TRAP,
tartrate-resistant acid phosphatase. |
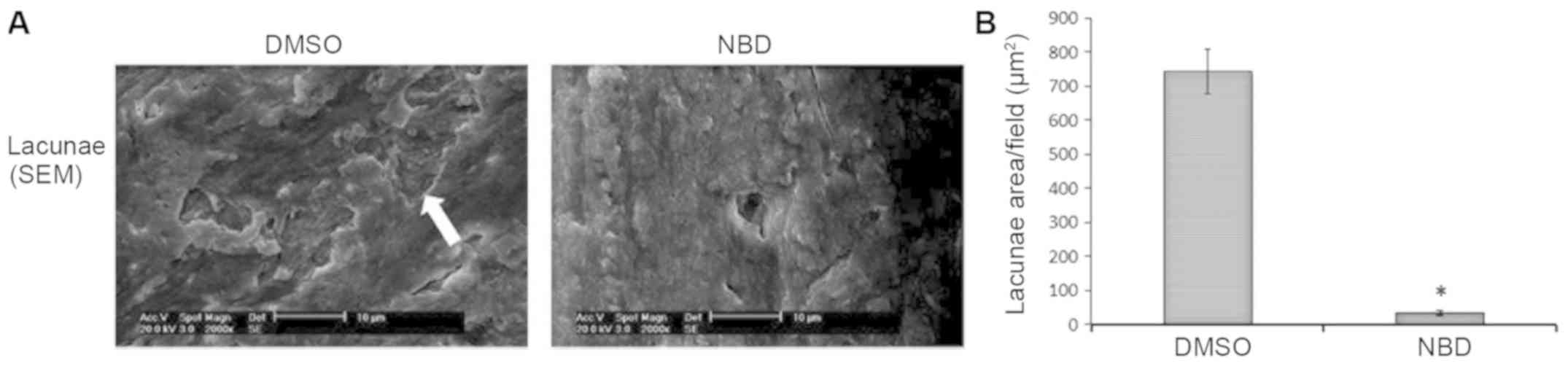
NBD peptide hinders bone erosion
caused by osteoclasts in vitro
Osteoclast function in vitro was measured
according to lacunae area on bone slices using scanning electron
microscopy (Fig. 2A). There was a
95±3.7% reduction in total lacunae area in the NBD group compared
with in the DMSO group (P<0.05; Fig. 2B).
NBD peptide ameliorates bone
resorption and reduces the size of the bone defect in an in vivo
model of mandibular osteomyelitis
A bone defect was created at the lateral inferior
border of the mandible beside the middle joint of laboratory
rabbits, and a 100-µl suspension of S. aureus
(5.0×108 cfu/ml) was injected into the bone defect.
After 6 weeks, redness and swelling in the surgical field of
rabbits were observed, thus confirming that osteomyelitis had been
established. Then animals were divided into three experimental
groups: Untreated, treated with debridement, and treated with
debridement plus NBD peptide. After another 4 and 8 weeks, BMD and
bone morphology of the surgical area were analyzed by dual energy
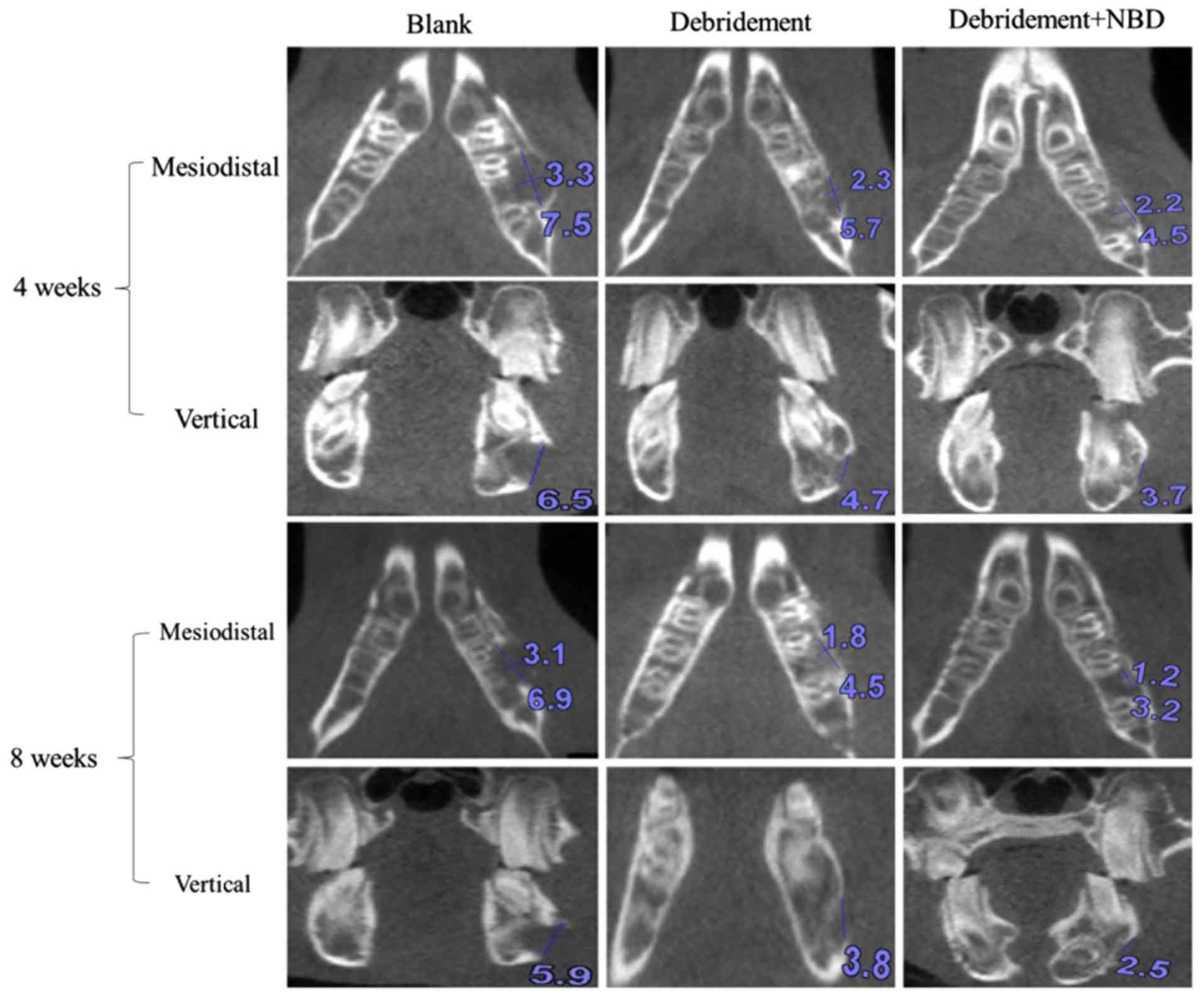
X-ray absorptiometry and CBCT. As shown in Fig. 3, the size of the bone defect was
gradually decreased in a 3-dimensional manner at 4 and 8 weeks in
the debridement plus NBD peptide group compared with in the
debridement group, as determined by CBCT.
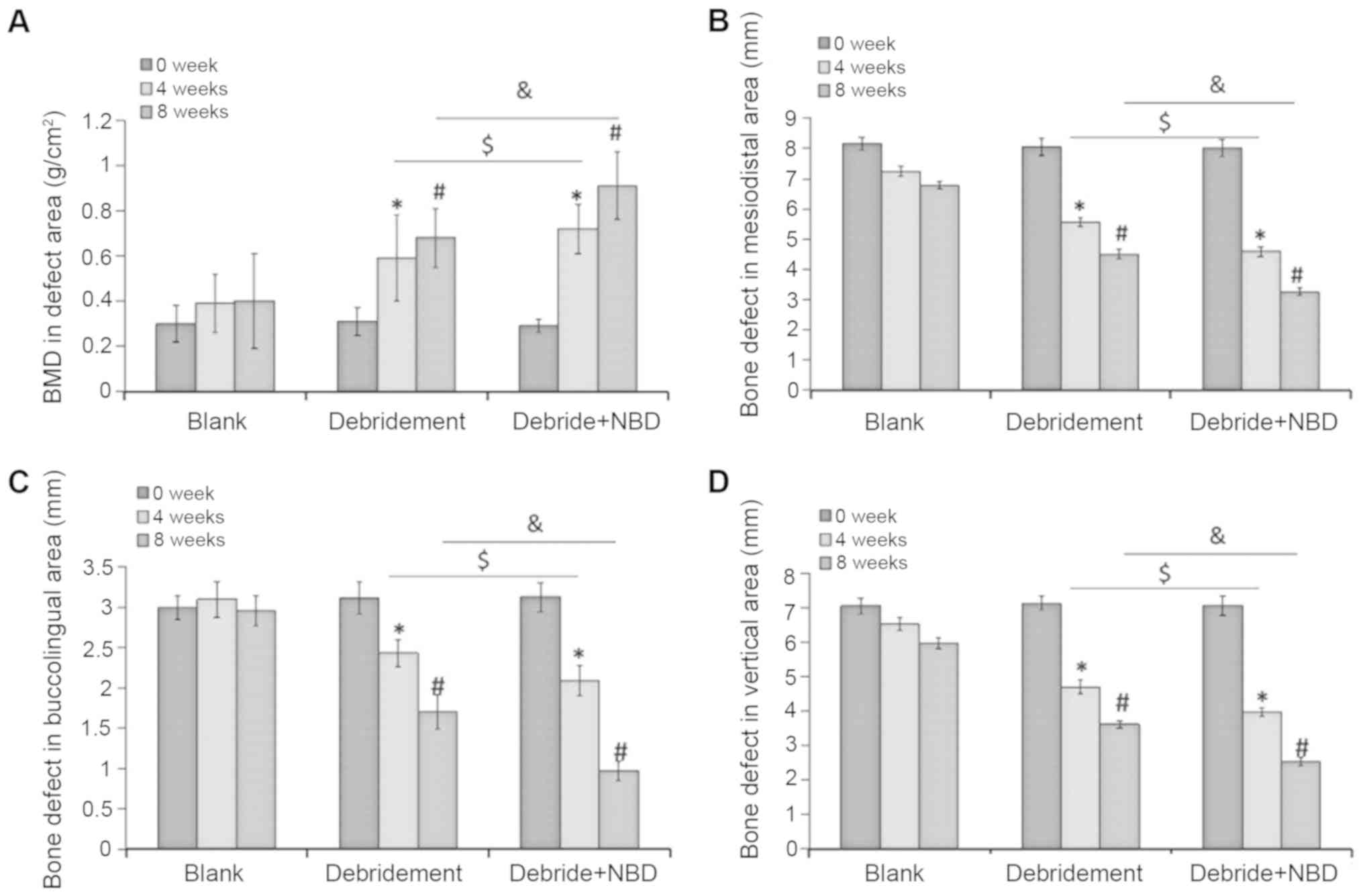
Dual energy X-ray absorptiometry revealed that there
were no significant differences in BMD between the three different
time-points in the control group (P>0.05; Fig. 4A). Conversely, BMD was increased by
51±6.1 and 85±2.2% at 4 and 8 weeks, respectively, in the
debridement group when compared with the baseline value at week 0
(P<0.05; Fig. 4A). Notably,
additional 18±4.5 and 42±5.1% increases in BMD were observed in the
debridement plus NBD peptide group when compared with the
debridement group at 4 and 8 weeks, respectively (P<0.05;
Fig. 4A).
The bone defects were also analyzed in three
different dimensions by CBCT. Similar to the BMD results, the
control group exhibited no significant differences in the
3-dimensional areas of the bone defect at the three time-points
analyzed (P>0.05 Fig. 4B-D).
Conversely, the bone defect area was decreased by 23.3±2.1,
21.6±2.5 and 28±2.6% in the mesiodistal, buccolingual and vertical
directions, respectively, at 4 weeks; and by 36.7±3.1, 32.6±2.5 and
39.2±2.9%, respectively, at 8 weeks in the debridement group
(P<0.05; Fig. 4B-D). Notably,
injection with the NBD peptide resulted in increased inhibition of
bone resorption, with an additional 10±1.1, 20±3.1 and 11.4±2.2%
reduction in bone defect areas at 4 weeks; and 15±3.6, 34.6±3.1 and
18.3±4.5% reductions at 8 weeks, when compared with debridement
alone (P<0.05) (Fig. 4B-D).
Discussion
The therapeutic potential of the NBD peptide in
osteomyelitis has received relatively little attention; however,
NBD has been reported to modulate inflammation and
osteoclastogenesis (18). The
present study demonstrated that the NBD peptide may ameliorate the
progression of osteomyelitis in an S. aureus-induced
mandibular osteomyelitis model. The results suggested that the NBD
peptide reduced RANKL-induced osteoclast formation and function
in vitro, and bone resorption in an in vivo model of
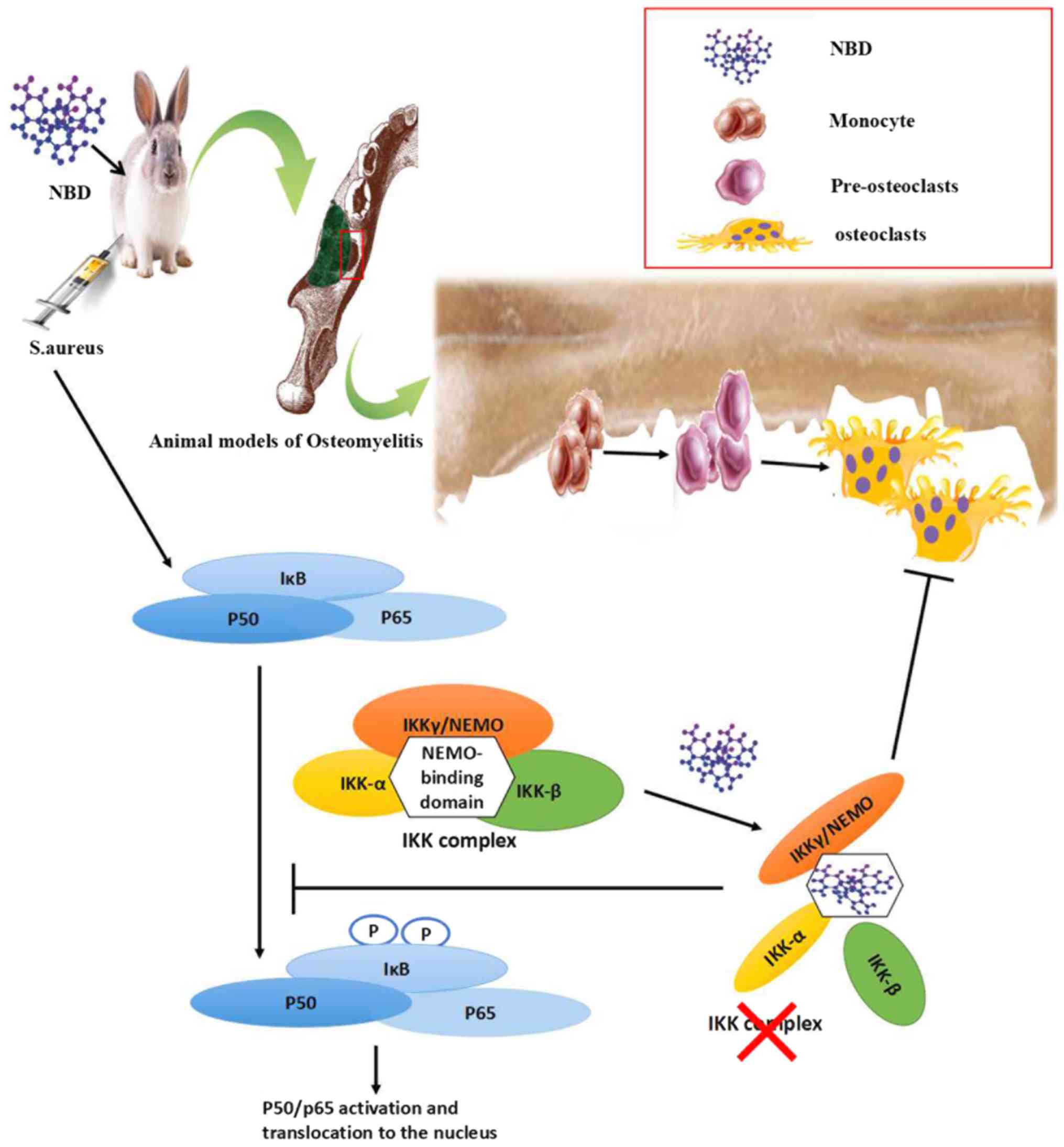
osteomyelitis. The possible mechanism underlying these effects is
that the NBD peptide suppresses synthesis of the IKK complex by
disrupting the association of IKKγ/NEMO with IKKα and IKKβ, and
further blocks the activation of NF-κB, which in turn inhibits the
formation of osteoclasts in an S. aureus-induced mandibular
osteomyelitis model (Fig. 5).
To investigate the role of NBD in RANKL-induced
osteoclastogenesis, the cell-permeable, wild type NBD peptide
(DRQIKIWFQNRRMKWKK-TALDWS-WLQTE) was synthesized as previously
reported (10). This study used
RAW264.7 cells, which can be induced to differentiate into mature
mouse osteoclasts (26), to study
the influence of the NBD peptide on osteoclastogenesis. Treatment
with the NBD peptide exhibited significant activity regulating the
effects of RANKL stimulation, as demonstrated by the significantly
reduced formation of osteoclasts revealed by TRAP staining and
F-actin immunostaining, and by the significantly smaller number of
osteoclasts and average number of nuclei observed per cell. The
area per osteoclast was also smaller in the NBD group when compared
with the control group, as determined by SEM. The osteoclast
bone-resorbing activity was also reduced when the NBD peptide was
tested in vitro, and the lacuna area measured by SEM was
smaller in the NBD group than in the control group. These results
demonstrated that the synthesized NBD peptide inhibited osteoclast
formation and resorption.
Several lines of evidence obtained through the
present study indicated that the NBD peptide may prevent the
progression of osteomyelitis in vivo. The osteomyelitis
model was established and animals were divided into three
experimental groups: Untreated, treated with debridement, and
treated with debridement plus NBD peptide. Treatment with the NBD
peptide increased BMD in the area of the bone defect, inhibited
bone resorption activity and restored bone tissue in a
3-dimensional manner when compared with the untreated and
debridement groups at 4 and 8 weeks. These findings suggested that
the NBD peptide ameliorated osteomyelitis induced by S.
aureus by modulating bone metabolism.
NF-κB is an inducible transcription factor that
regulates hundreds of genes involved in important physiological and
pathological processes. Although NF-κB can activate various genes,
those involved in inflammatory responses and osteoclastogenesis are
increasingly considered to be the most important activation targets
of this transcription factor. This study suggested that systemic
administration of the NBD peptide may not only inhibit the
formation of osteoclasts in S. aureus-induced osteomyelitis,
but may also modulate the function of osteoclasts. This is in
agreement with previous observations on osteolysis in an
osteoarthritis model (16).
Notably, the majority of original studies on osteomyelitis focused
on the effects of S. aureus on osteoblasts. However, the
interaction of S. aureus with osteoclasts, the only cells
known to degrade bone, has often been overlooked. As key cells
during bone infection, osteoclasts are not well equipped to kill
bacteria and can become a reservoir of bacterial pathogens; this
possibility requires further attention (27). The results of the present study
demonstrated that debridement alongside administration of the NBD
peptide into the bone defect area inhibited osteoclast activity and
bone destruction, thus indicating that it may be considered an
effective therapy that could be applied in osteomyelitis in the
future.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81771118, 81272157
and 81600909) and the Zhejiang Provincial Medical Science and
Technology Project of China (grant nos. 2018277520 and
2015PYA006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL performed the majority of the experiments. ZX and
YW designed the experiments. HX, YS and QJ prepared the figures and
conducted statistical analysis. XZ contributed to the animal
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Institutional Animal Care and Use Committee of Zhejiang
University. All surgical procedures and euthanasia were performed
in a manner that minimized animal suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hajishengallis G, Moutsopoulos NM,
Hajishengallis E and Chavakis T: Immune and regulatory functions of
neutrophils in inflammatory bone loss. Semin Immunol. 28:146–158.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inzana JA, Schwarz EM, Kates SL and Awad
HA: Biomaterials approaches to treating implant-associated
osteomyelitis. Biomaterials. 81:58–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang R, Braughton KR, Kretschmer D, Bach
TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel
A, et al: Identification of novel cytolytic peptides as key
virulence determinants for community-associated MRSA. Nat Med.
13:1510–1514. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park OJ, Kim J, Yang J, Yun CH and Han SH:
Muramyl dipeptide, a shared structural motif of peptidoglycans, is
a novel inducer of bone formation through induction of Runx2. J
Bone Miner Res. 32:1455–1468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verdrengh M, Bokarewa M, Ohlsson C,
Stolina M and Tarkowski A: RANKL-targeted therapy inhibits bone
resorption in experimental Staphylococcus aureus-induced arthritis.
Bone. 46:752–758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing L, Xiu Y and Boyce BF: Osteoclast
fusion and regulation by RANKL-dependent and independent factors.
World J Orthop. 3:212–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuo C, Huang Y, Bajis R, Sahih M, Li YP,
Dai K and Zhang X: Osteoblastogenesis regulation signals in bone
remodeling. Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gross C, Weber M, Creutzburg K, Möbius P,
Preidl R, Amann K and Wehrhan F: Osteoclast profile of
medication-related osteonecrosis of the jaw secondary to
bisphosphonate therapy: A comparison with osteoradionecrosis and
osteomyelitis. J Transl Med. 15:1282017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aurore V, Caldana F, Blanchard M, Kharoubi
Hess S, Lannes N, Mantel PY, Filgueira L and Walch M:
Silver-nanoparticles increase bactericidal activity and radical
oxygen responses against bacterial pathogens in human osteoclasts.
Nanomedicine. 14:601–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
May MJ, D'Acquisto F, Madge LA, Glöckner
J, Pober JS and Ghosh S: Selective inhibition of NF-kappaB
activation by a peptide that blocks the interaction of NEMO with
the IkappaB kinase complex. Science. 289:1550–1554. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
May MJ, Marienfeld RB and Ghosh S:
Characterization of the Ikappa B-kinase NEMO binding domain. J Biol
Chem. 277:45992–46000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleppe M, Koche R, Zou L, van Galen P,
Hill CE, Dong L, De Groote S, Papalexi E, Hanasoge Somasundara AV,
Cordner K, et al: Dual targeting of oncogenic activation and
inflammatory signaling increases therapeutic efficacy in
myeloproliferative neoplasms. Cancer Cell. 33:785–787. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo G, Li F, Li X, Wang ZG and Zhang B:
TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via
the NF-κB pathway. Mol Med Rep. 17:6605–6611. 2018.PubMed/NCBI
|
|
14
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vancurova I and Vancura A: Regulation and
function of nuclear IκBα in inflammation and cancer. Am J Clin Exp
Immunol. 1:56–66. 2012.PubMed/NCBI
|
|
16
|
Dai S, Hirayama T, Abbas S and Abu-Amer Y:
The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide,
blocks osteoclastogenesis and bone erosion in inflammatory
arthritis. J Biol Chem. 279:37219–37222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strickland I and Ghosh S: Use of cell
permeable NBD peptides for suppression of inflammation. Ann Rheum
Dis. 65 (Suppl 3):iii75–iii82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jimi E, Aoki K, Saito H, D'Acquisto F, May
MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, et al:
Selective inhibition of NF-kappa B blocks osteoclastogenesis and
prevents inflammatory bone destruction in vivo. Nat Med.
10:617–624. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Dong G, Jeon HH, Elazizi M, La LB,
Hameedaldeen A, Xiao E, Tian C, Alsadun S, Choi Y and Graves DT:
FOXO1 mediates RANKL-induced osteoclast formation and activity. J
Immunol. 194:2878–2887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swarnkar G and Abu-Amer Y: Regulation of
NF-κB signaling in osteoclasts and myeloid progenitors. Methods Mol
Biol. 1280:527–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Wu HF, Ang ES, Yip K, Woloszyn M,
Zheng MH and Tan RX: NF-kappaB modulators in osteolytic bone
diseases. Cytokine Growth Factor Rev. 20:7–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chacon GE, Bower DL, Larsen PE, McGlumphy
EA and Beck FM: Heat production by 3 implant drill systems after
repeated drilling and sterilization. J Oral Maxillofac Surg.
64:265–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moskowitz JS, Blaisse MR, Samuel RE, Hsu
HP, Harris MB, Martin SD, Lee JC, Spector M and Hammond PT: The
effectiveness of the controlled release of gentamicin from
polyelectrolyte multilayers in the treatment of Staphylococcus
aureus infection in a rabbit bone model. Biomaterials.
31:6019–6030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sánchez AR, Sheridan PJ, Lohse C and
Weaver A: Assessment of peripheral dual-energy X-ray absorptiometry
measurements in peri-implant bone defects in dogs. J Periodontol.
75:658–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kröpil P, Hakimi AR, Jungbluth P, Riegger
C, Rubbert C, Miese F, Lanzman RS, Wild M, Schek A, Scherer A, et
al: Cone beam CT in assessment of tibial bone defect healing: An
animal study. Acad Radiol. 19:320–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hotokezaka H, Sakai E, Kanaoka K, Saito K,
Matsuo K, Kitaura H, Yoshida N and Nakayama K: U0126 and PD98059,
specific inhibitors of MEK, accelerate differentiation of RAW264.7
cells into osteoclast-like cells. J Biol Chem. 277:47366–47372.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Liu X, Dou C, Cao Z, Liu C, Dong S
and Fei J: Staphylococcal protein A promotes osteoclastogenesis
through MAPK signaling during bone infection. J Cell Physiol.
232:2396–2406. 2017. View Article : Google Scholar : PubMed/NCBI
|