Introduction
Semen Cuscutae is the seed of Cuscuta
chinensis Lam. (CCL, Cuscuta japonica Choisy), a
parasitic plant of the Convolvulaceae family that must germinate
close to its host plant (1). And
CCL contains 6 active candidate compounds. The candidate active
compound are rutin, hyperoside, astragalin, quercetin, kaempferol
and isorhamnetin. The activity and amount of each of these
compounds are being analyzed in another study. Thus we are unable
to provide information regarding these clearly yet. The mature seed
of CCL is harvested in the late fall and dried for use in
alternative medicine in Korea. CCL has been extensively used to
improve hepatic and renal functions, fatigue, high blood pressure,
and chronic diarrhea (1–3). Ancient documents such as the
Donguibogam, that describes principles and practices of eastern
medicine, state that CCL improves vitality, as well as reduces back
and knee pain, and regulates diabetes and sexual dysfunction when
consumed frequently.
In post-menopausal women, hormone replacement
therapy (HRT) with estradiol is the most effective remedy for
multi-symptoms of the menopause syndrome; however, it is associated
with several side effects including uterine bleeding, hyperplasia,
and the risk of breast cancer (4).
Previous studies have recommended that post-menopausal women with
non-hysterectomy should take the lowest effective dose of HRT for
the shortest amount of time possible to reduce the associated
risks. Phytoestrogens, natural herbal compounds that structurally
resemble estrogens and their metabolites, represent an alternative
to synthetic estrogen and/or progesterone therapy (4). The consumption of phytoestrogens has
been shown to improve menopausal symptoms such as hot flashes and
night sweats in women (4).
Menopause is associated with an increase in
low-density lipoprotein cholesterol concentration and subsequent
defects of circulation such as sluggish blood flow, and varicose
and spider veins (5). Moreover,
peripheral vascular disease may occur in response to changes in
serum lipid levels, shown to accelerate with age in menopausal
women (5,6). Moreover, estrogens can indirectly
and/or directly affect vascular dysfunctions during the mediation
of lipid profiles, as well as recovery from the ischemic damage
leading to atherosclerosis and embolus in limbs (6,7).
Regarding vascular functions, estrogens may also regulate
inflammatory responses and apoptosis under ischemic stress
(8).
Neovascularization occurs during pathological
conditions such as ischemic, immune, and inflammatory responses,
and seems to be driven by diverse cellular and molecular responses
(9). The process of
neovascularization includes vasculogenesis, angiogenesis, and
arteriogenesis, all of which contribute to the repair and
remodeling of damaged tissues during ischemia-related disease
(9). Ischemic tissue conditions
also regulate the expression of C-X-C motif chemokines (CXC) such
as CXCL-8, CXCL-9, CXCL-10 and CXCL-12, as well as the interaction
between these chemokines (CXCLs) and their receptors, CXCRs, in
angiogenesis (10). Conversely,
intracellular relations are mediated by endothelial cell adhesion
molecules including CD31, CD45, CD54 and CD106. Ischemic stress
also enhances the synthesis of pro-inflammatory cytokines such as
TNF-α, IL-6, and IL-1b in the muscle (9). Platelet activation also takes place
under ischemic conditions and modifies the expression of
inflammatory mediators, angiogenic (VEGF, ANG-1, PDGF and hFGF) and
anti-angiogenic (thrombospondin and endostatin, etc.) growth
factors, and that of receptors VEGFR and CXCR, in endothelial cells
(9).
In this study, we investigated whether CCL extract
modifies the expression of angiogenic or inflammatory factors, the
rate of the blood flow, and capillary density in an established
menopausal model of peripheral vascular disease. The
ischemic-/non-ischemic blood perfusion ratio was also evaluated
using a non-invasive laser Doppler perfusion imaging system,
following which the capillaries were visualized using anti-CD31
immunohistochemistry on endothelial cells in ischemic limb muscles
and counted. Additionally, the serum profiles of angiogenic or
inflammatory factors were evaluated using an antibody array kit,
and the effects of CCL extract on the expression of these factors
in the muscles of ischemic limbs were quantified.
Materials and methods
Preparation of CCL extract
Cuscuta chinensis Lamak (5 kg) was purchased
from a local traditional medicine store (Jechoen, Korea) in July of
2017. The authenticity of the plant species was confirmed by one of
the authors (Byoung Seob Ko; Korea Institute of Oriental Medicine),
after which the seeds were ground into powder. Next, powder (4 kg)
was extracted with 100% water (16 L) by heat reflux for 6 h, after
which the supernatant was collected and dried using a freeze-dryer
for 2 days. The extraction yield was of 0.005%, and 15 g of
CCL-extract was used for subsequent experiments. The plants we used
in the experiment were samples produced at GMP facilities in
accordance with the Korean Food and Drug Administration's standards
for manufacturing and quality control.
Experimental animals and
treatments
Female 6-week-old C57BL/6 mice were obtained from
Dahan Biolink (Eumseong, South Korea) and divided into five-groups
of seven. Mice were allowed to adapt to laboratory conditions
(temperature: 20±2°C, relative humidity: 45±5%, light/dark cycle:
12 h) for 1 week. All animal experimental procedures were approved
by the Ethics Committee of the Korea Institute of Oriental Medicine
(approved no. 17-028). Mice were anesthetized by an animal
anesthesia system (Vetequip, Abesko, Gyeonggi, Korea) using 2%
isoflurane (Choongwae Pharma Corp., Seoul, Korea). OVX surgery was
assessed according to the criteria of (11), and performed by liagation and
excision of ovaries along the upper horns, through aseptic
incisions of the dorsal skin and muscle layers. At 7 days after OVX
surgery, mice were anesthetized for hind-limb ischemia (HLI) after
which the vessels (arteries and veins) of the hind limb were
excised on day 0, and surgery was modified according to the
criteria of (12). After a 7 days
recover period, 17β-estradiol (E2; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and CCL extract were prepared in 0.2% CMC
(carboxymethyl cellulose) and administered for every 3-weeks by
oral injection (E2 for 100 µg/kg/day and CCL for 150 and 450
mg/kg/day, respectively). Crude extract doses according to criteria
of (13). Mice were euthanized 12
h after the final injection. And The liver, spleen, peritoneal fat,
etc., including tissues from the surgical region, were collected
and stored in 4% formalin or nitrogen immediately for long-term
storage at −80°C for the purpose of analysis.
Laser Doppler blood perfusion
analysis
The blood flow rates in ischemic/non-ischemic
(ISCH/nISCH) hind limbs were measured using a laser Doppler blood
perfusion imager (PeriScan-PIM3; Perimed AB, Järfälla, Sweden)
under anesthesia (Isoflurane; Choongwae Pharma Corp.) according to
the manufacturer's recommendations. The rate of ISCH/nISCH blood
flow was calculated at −7, 0, 7, 14, and 21 days after HLI by
colorimetric assay using PIMSoft (v1.5; Perimed AB).
Immunohistochemical stain
Tissues were fixed in 10% neutral buffered formalin,
embedded in paraffin, and cut into 5-µm-thick sections. Endogenous
peroxidase and non-specific reactions were blocked with BLOXALL
(for 30 min; Vector Lab. Inc., Burlingame, CA, USA) and CAS-BLOCK
(for 1 h; Thermo Fisher Scientific, Pittsburgh, PA, USA) at room
temperature (RT). Sections were subsequently incubated at RT for 4
h with an antibody against CD-31 (rabbit polyclonal, 1:250; Abcam).
The sections were incubated with Dako REAL™ Envision™/HRP
(Rabbit/Mouse) for 30 min at RT. Dako REAL™ DAB+ Chromogen (both
from Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) was
used as a chromogen, after which the sections were counterstained
with Mayer's hematoxylin (Sigma-Aldrich; Merck KGaA) and mounted
using Mounting Medium (Thermo Fisher Scientific). Tissue sample was
examined under a light microscope (BX43; Olympus, Tokyo, Japan).
And all images were captured using an Olympus DP-73 controller and
cellSens standard (both from Olympus) under a microscope.
Reverse transcription-quantitative
polymerase chain reaction
Total RNA was isolated using an RNeasy Mini Kit
(Qiagen, Hilden, Germany), and cDNA was synthesized from 1 µg of
total RNAs using the Thermo Scientific RevertAid First Strand cDNA
Synthesis Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturers' instructions. Briefly, extracted
RNA was used as a template for cDNA synthesis using Random Hexamer
primers and RevertAid M-MuL V RT, incubated 5 min at 25°C followed
by 60 min at 42°C. And termination of reaction by heating at 70°C
for 5 min. Real-time PCR was performed using SYBR Premix EX Taq
(Takara Bio Inc., Otsu, Japan) with 40 cycles for 30 sec at 95°C,
for 30 sec at 60°C, and for 30 sec at 72°C by ABI PRISM 7500HT
Sequence Detection System (Applied Biosystems, Foster City, CA,
USA). Quantification of target genes was accomplished by the
comparative Ct method, and the levels of mRNAs were normalized by
those of β-actin and presented as relative values. The primers used
for real-time PCR were synthesized by Macrogen Inc. (Seoul, Korea)
and are presented in Table I.
 | Table I.The list and sequence of primers used
for RT-qPCR analysis. |
Table I.
The list and sequence of primers used
for RT-qPCR analysis.
| No. | Gene | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) | Annealing
temperature (°C) |
|---|
| 1 | Angiopoietin-1 |
ATGCGGTTCAAAACCACACG |
AGCAGTTGGATTTCAAGACGG | 58 |
| 2 | Endothelin-1 |
AAGGAGTGTGTCTACTTCTGCC |
TTCCCTTGGTCTGTGGTCTT | 58 |
| 3 | CXCL12 |
GTTTGTCTCTTTGAGCTGAGGC |
TGAGACAGAGATGAGCATGGTG | 58 |
| 4 | ICAM-1 |
CGGAGCCAATTTCTCATGCTT |
ACCCTAGTCGGAAGATCGAA | 58 |
| 5 | IGFBP-3 |
GAAACACCACTGAGTCTGAGGA |
TGGCCTTTTATGATGACCTCCA | 58 |
| 6 | IL-1b |
AGGACCCAAGCACCTTCTTTT |
CAGACAGCACGAGGCATTTTT | 59 |
| 7 | IL-6 |
AACAGCGATGATGCACTGTCA |
TTGCTCTGAATGACTCTGGCT | 59 |
| 8 | TNF-α |
GCCAATGGCATGGATCTCAAA |
TGGTATGAAATGGCAAATCGGC | 60 |
| 9 | VEGF |
AACAAAGCCAGAAAAAAAATCA |
TCACCGCCTTGGCTTGTCACA | 58 |
| 10 | VEGFR2 |
CTCACACCAGTTTGCAAGAA |
AGTACAATGCCTAATCTTCT | 56 |
| 11 | Actin |
TACGTCGCCCTGGATTTT |
ATGAAAGAGGGCTGGAAGAG | 60 |
Analysis of angiogenesis related
protein and inflammatory cytokine levels in the serum
The levels of angiogenesis-related proteins and
inflammatory cytokines were analyzed using a Mouse Angiogenesis
Array Kit and Proteome Profiler Mouse Cytokine Array Kit (R&D
System, Minneapolis, MN, USA) according to the manufacturer's
instructions. Briefly, each membrane was incubated with the serum
of each group overnight at 4°C. Bound protein was then detected
with streptavidin conjugated to HRP using a digital image system
(Chemiluminescence Imaging-Fusion SL, Analis, The Netherlands).
Cell culture and cell viability
Human umbilical vein endothelial cells (HUVECs) were
obtained from Lonza (Walkersville, MD, USA) and maintained
according to the manufacturer's instructions. Cell viability was
detected using an EZ-Cytox kit (DoGenBio Co., Ltd., Seoul, Korea).
The HUVEC cells were seeded at a density of 3×104/well
in 96-well plates. High sensitivity water soluble tetrazolium (WST)
solution (10 µl per well in a 96-well plate) was added to the
culture fluid at 1:1 ratio, while WST solution without cells was
used as a blank control. Optical density was determined at various
time-points (after 4 h) on a microtiter plate reader at 450 nm,
after which the percentage viability was calculated using the
following formula: Optical density of treated sample/optical
density of untreated control ×100.
Migration assay
HUVECs migration was quantified using a corning
Transwell system (Corning, NY, USA). HUVECs were seeded
(2×105/well) into the upper chamber (8 mm) of a
Transwell plate in a serum-free medium. In the lower chamber, a
complete medium was placed as a chemoattractant. Following
incubation at 37°C under 5% CO2 for 6 h, the cells on
the top of the polycarbonate membrane were removed using cotton
swabs, and the migrating cells on the lower side of the membrane
were stained with hematoxylin and eosin (H&E) for 5 min. Four
random fields were counted under the microscope during each
assay.
Tube formation assay
HUVECs (5×104 cells/well) were seeded on
a Matrigel in 24-well plates supplemented with CCL (1, 10 and 100
µg/ml) and vascular endothelial growth factor (VEGF, 50 ng/ml),
17β-estradiol (E2, 1 nmol), and vinblasitne (1 pmol) were added to
the media without the addition of growth factors. After 24 h,
tubular structures of HUVECs were examined using an inverted
microscope (Nikon, Melville, NY, USA), and the total tube length
was measured in three fields (4×) using the ImageJ software version
1.51s (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All the results presented are representative of at
least 3 independent experiments. The results of the analyses of
blood flow, capillary density, and gene expression are presented as
the means ± SD. And the results of the analyses of cell viability,
migration, and tube formation are shown as mean ± SD. Paired
Student's t-tests were used to compare each group, while ANOVA with
Tukey's test was used for multiple comparison tests using the PRISM
software (v6.0; GraphPad, La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
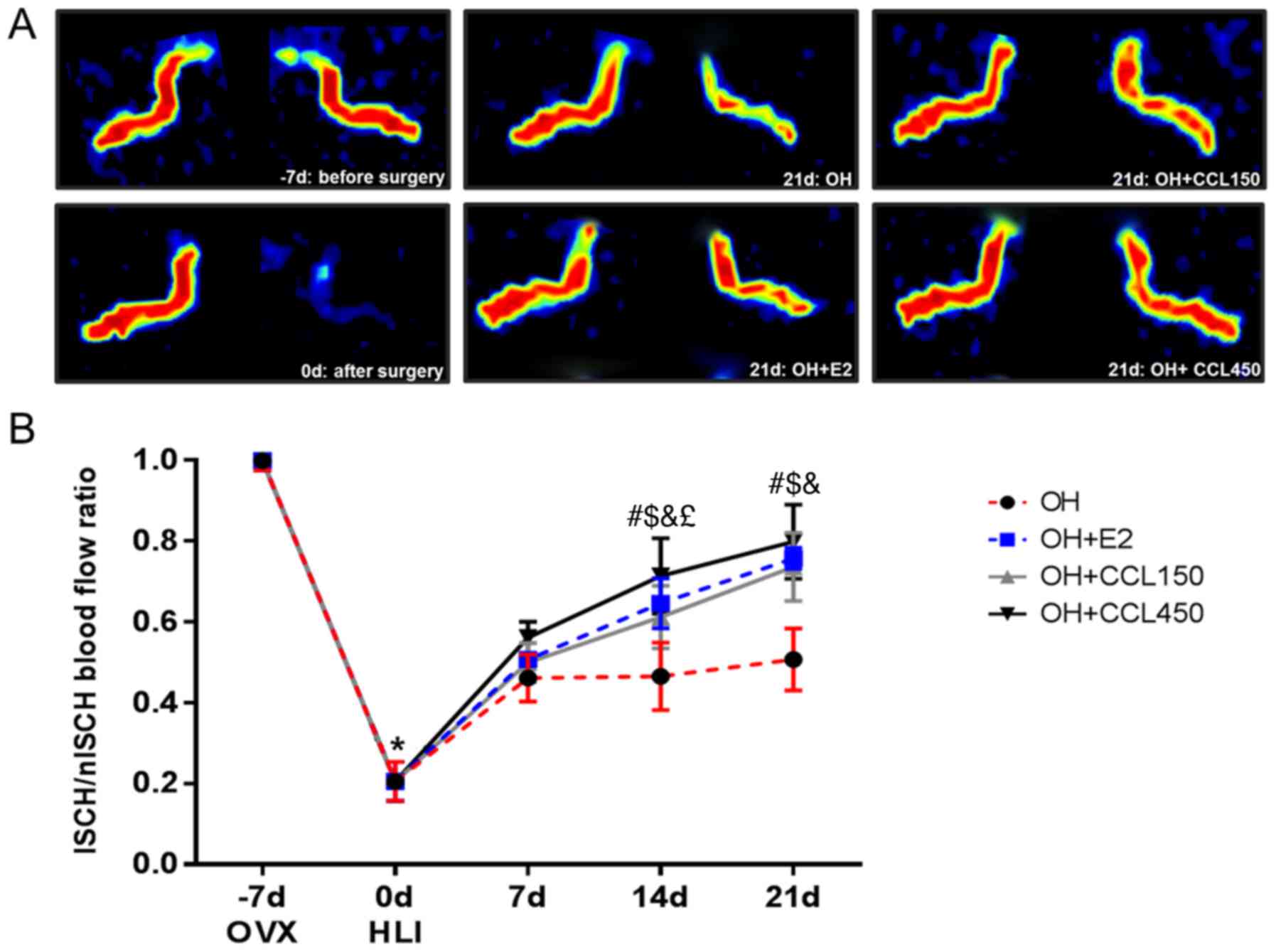
CCL improved blood perfusion
To determine whether CCL (150 or 450 mg/kg/day)
treatment stimulates blood reperfusion in hind-limb ischemia, mice
were treated with 17β-estradiol (E2) and CCL. As shown in Fig. 1, blood flow in the ischemic hind
limbs decreased equally in all surgery groups immediately following
ischemic surgery (0 day). At 14 and 21 days after ischemic surgery,
blood perfusion of the ischemic hind limb had improved
significantly in all treated groups compared with the vehicle
group. In particular, although no significant differences in blood
perfusion were detected between the CCL 150 and CCL 450 groups at
21 days after ischemic surgery, a high-dose of CCL (450 mg/kg/day)
significantly ameliorated the signs of hind-limb ischemia earlier
than a low-dose of CCL (150 mg/kg/day), at 14 days after surgical
induction of ischemia.
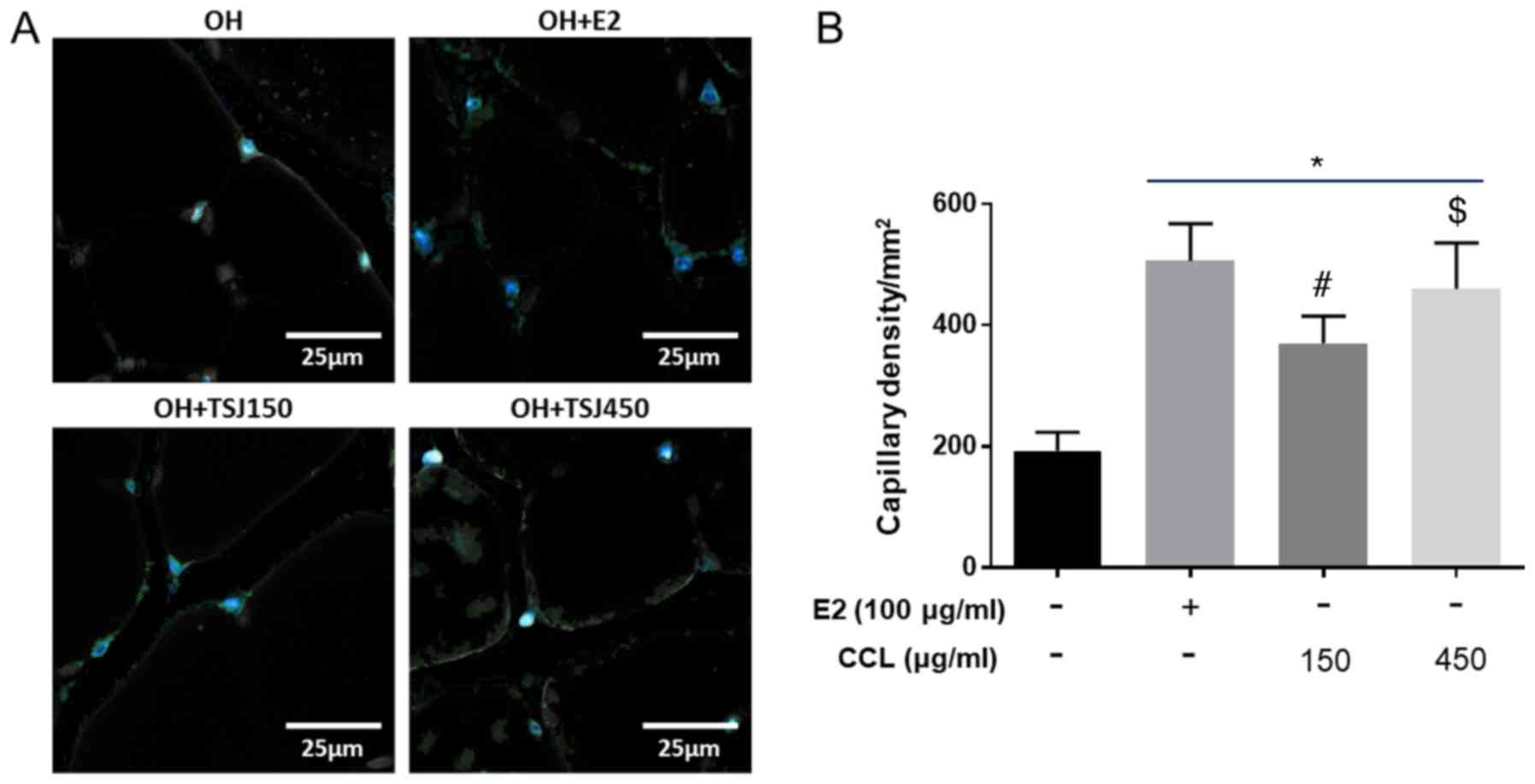
Capillary density, CD31
immunohistochemical staining
To determine the angiogenic effects of CCL on
microcirculation, we evaluated the capillary density in
immunostained (anti-CD31) tissue from ischemic thighs and calf
muscles. Positive structures were counted on five different
microscopic fields per section under 200× magnification to
determine capillary density (mean number of
capillaries/mm2). Immunohistochemistry showed that the
E2-treatment increased capillary density in ischemic muscles
compared to the vehicle control. In addition, the capillary density
significantly increased in both the low and high-dose CCL-treated
groups (Fig. 2).
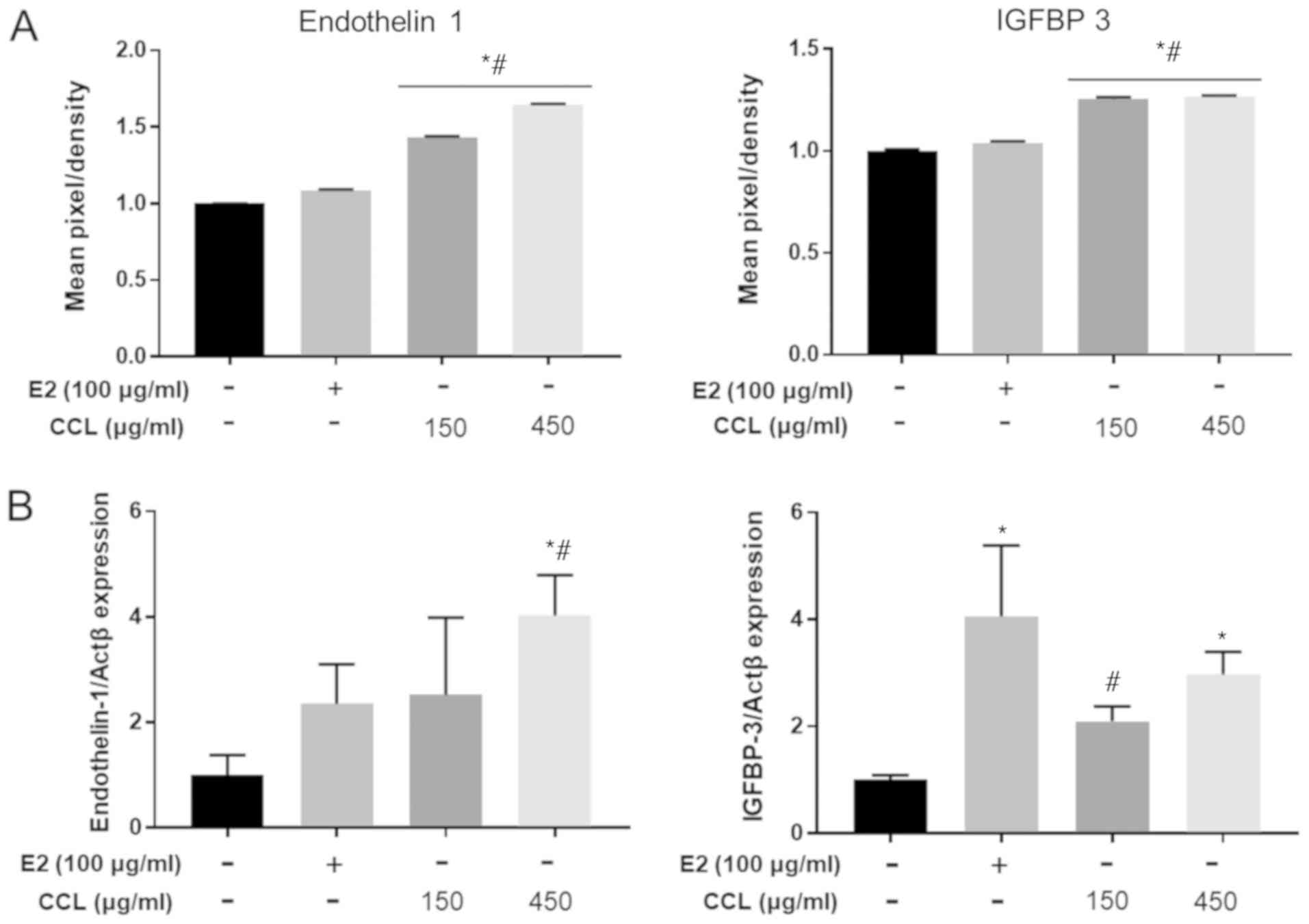
CCL increased angiogenesis related
proteins in serum and mRNA expression in surgical regions
We determined whether CCL treatment promoted the
expression of angiogenesis-related factors in serum and wound
region tissue of ovariectomized and hind-limb ischemia-induced
mice. The results revealed that the serum levels of the
endothelin-1 and IGFBP-3 gene expression were significantly
different between the treatment and vehicle groups. Moreover, the
treatment with CCL increased endothelin-1 protein concentration in
a dose-dependent manner (Fig. 3A).
Next, we confirmed these findings using RT-PCR. The assessment of
endothelin-1 and IGFBP-3 mRNA levels revealed a significant
increase by 1.2- to 1.5-fold (Fig.
3B).
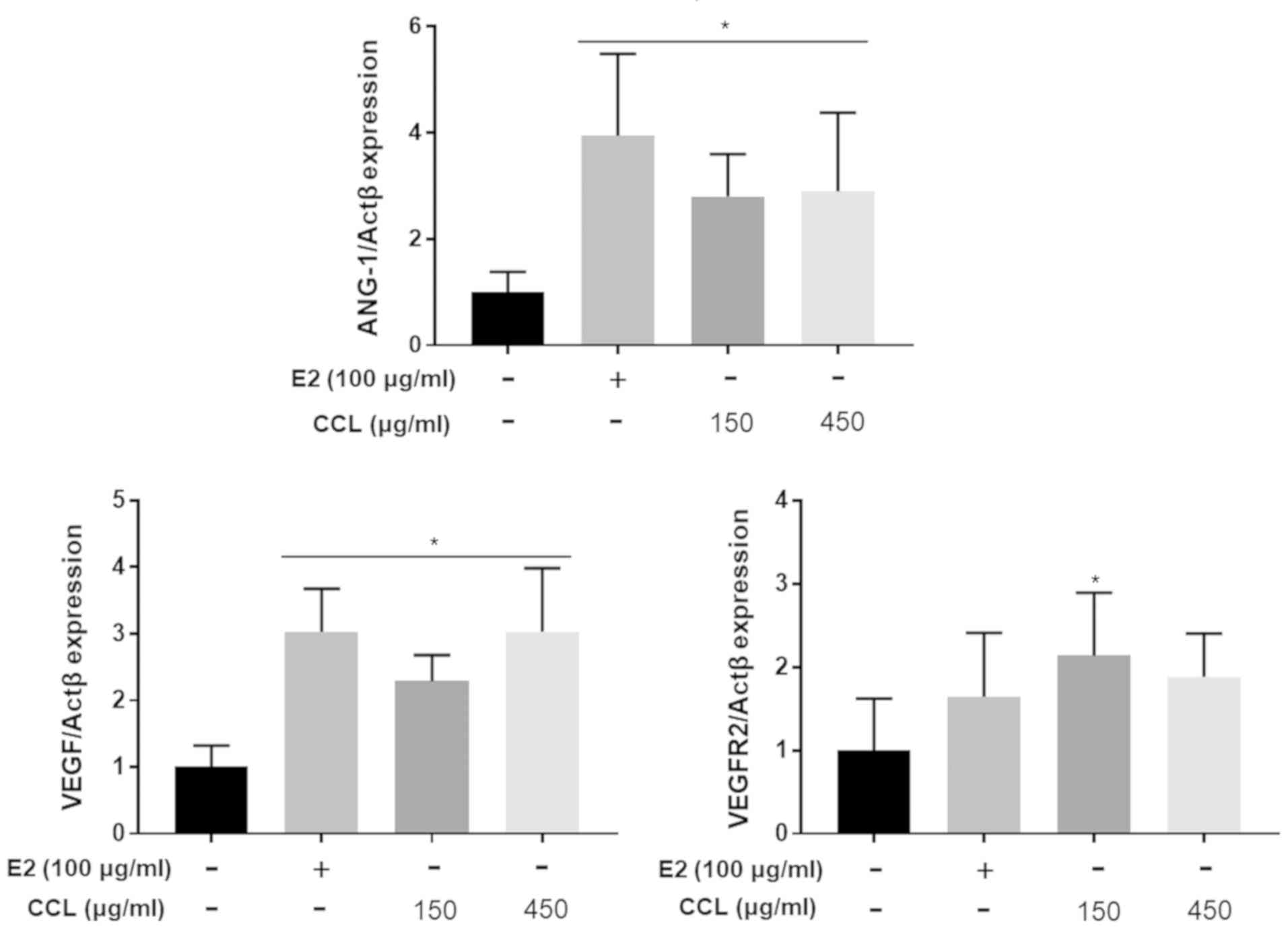
CCL induces wound healing via
upregulation of angiogenesis markers in hind-limb ischemic
lesions
In parallel with protein array, we confirmed the
effects of CCL on the mRNA expression of the angiogenesis-related
marker genes VEGF, angiopoetin-1, and VEGFR2 in ischemic lesions of
the hind limb. Tissue analyses revealed significant changes in the
genes investigated (transcript-level) in response to CCL treatment
(Fig. 4). Specifically, the
expression of all three genes was upregulated in response to 150
and 450 mg/ml of CCL, indicating that CCL treatment was effective
for wound healing and essential to accelerate angiogenesis.
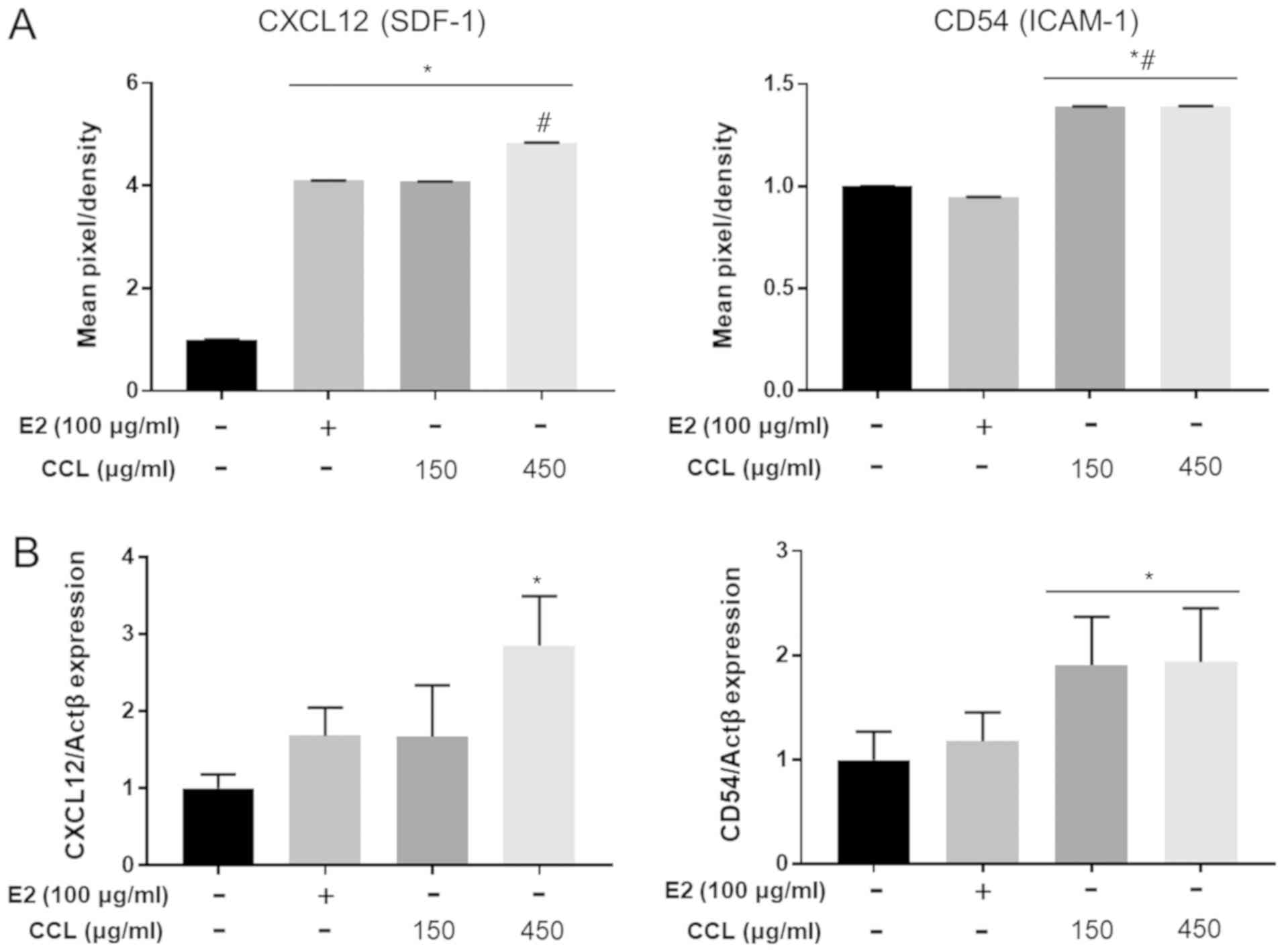
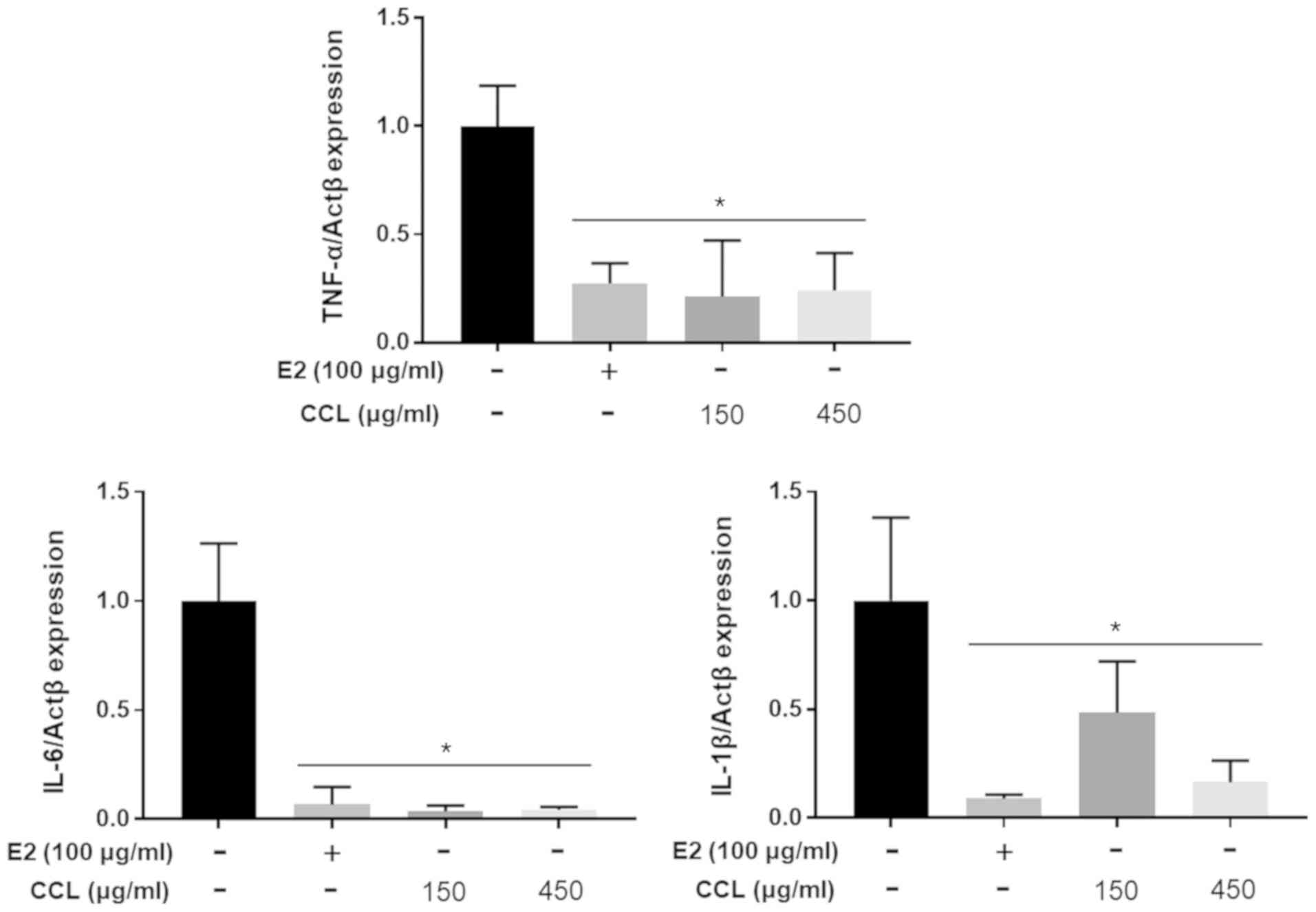
CCL regulates inflammatory or
pro-inflammatory cytokines in serum and mRNA expression in
hind-limb ischemic lesions
We conducted the analyses of mouse inflammatory or
pro-inflammatory cytokine expression to confirm the differences in
the E2- and CCL-treated groups. The treatment with E2 and CCL led
to secretion of high levels of CXCL12 (SDF-9) and CD54 (ICAM-1)
compared to the vehicle groups (Fig.
5A). Moreover, mRNA expression of CXCL12 and CD54 was
upregulated in the E2 and CCL groups (Fig. 5B). The results revealed that mRNA
expression of the pro-inflammatory genes, TNF-α, IL-6 and IL-1β,
was lower than that of the non-treatment group following the
treatment with E2 and CCL (Fig.
6).
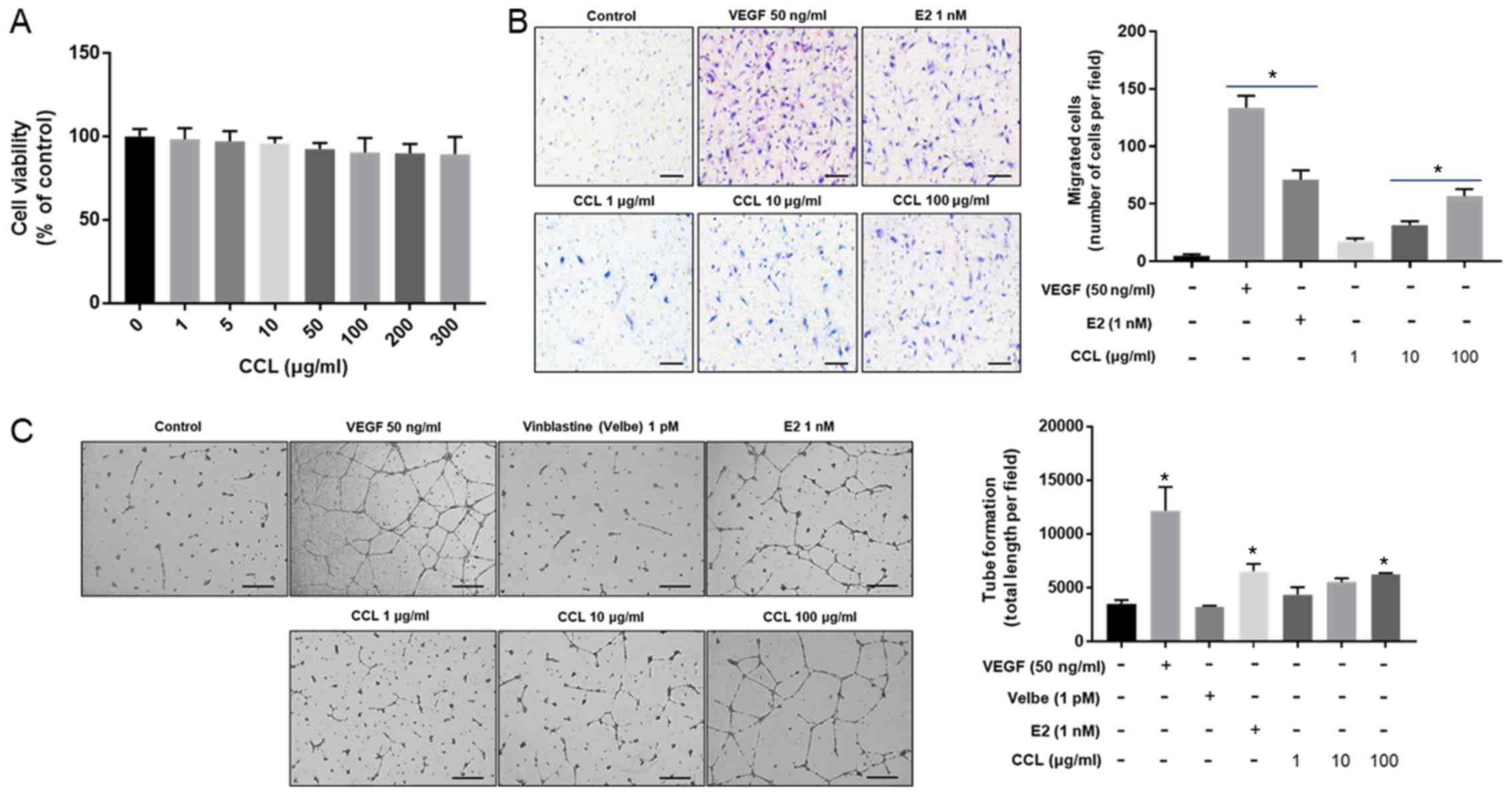
Evaluation of cell migration and tube
formation by CCL in HUVEC
We evaluated the effects of CCL at 0, 1, 5, 10, 50,
100, 200 and 300 µg/ml on the viability of HUVECs. As shown in
Fig. 7A, no significant
cytotoxicity was observed in response to the treatment with 300
µg/ml CCL. Furthermore, the number of cells that migrated was
significantly increased in VEGF and E2-treated groups, and this
increase was also induced in a dose-dependent fashion by CCL
treatment compared to the control (Fig. 7B). The tube formation increased in
the groups treated with VEGF and E2 compared to the control groups,
whereas tube organization was not induced by vinblastine. In
CCL-treated groups, the induction of tube formation was observed at
the dose of 100 µg/ml (Fig.
7C).
Discussion
Peripheral arterial disease is a serious medical
condition that develops in response to age and in the groups
showing common atherosclerosis risk factors (14). This condition is characterized by a
lower limb Doppler signal because of blood vessel blockage
associated with critical limb ischemia, intermittent claudication,
and rest pain (14,15). The present study was conducted to
investigate whether CCL extract alleviates the disturbance of the
blood flow rate caused by surgical damage in the hind limbs of
ovariectomized mice. To accomplish this, we surgically induced
estrogen deficiency and hind-limb ischemia in female mice, which
was expected to mimic the features of peripheral arterial disease
during menopause. We then measured the migration and tube formation
of vascular endothelial cells in vitro. In addition, we
examined the effects of CCL extract by measuring the changes in the
blood flow and capillary density, as well as the expression of
angiogenic or inflammatory factors in the muscles of the hind limb
in vivo.
The wound healing process involves neo-angiogenesis,
tissue formation and remodeling, as well as inflammatory response
(16–18). In the present study, the migration
and tube formation of HUVECs increased significantly following the
treatment with 10 and 100 µg/ml CCL. The increased movement of
endothelial cells during angiogenesis was previously found to be
closely linked to the formation of new blood vessels in wounds
(19,20). Ashcroft et al (21) reported accelerated wound healing
through a reduction of inflammation, and pro-inflammatory cytokines
were shown to play a crucial role during this healing response. In
our mouse model, we observed increased blood flow in response to
the treatment with CCL extract in the hind limbs of ischemic mice
compared to the non-treated group. Overall, these results
demonstrated a more pronounced angiogenic response, decreased
inflammatory factor expression, and increased capillary density of
the limb muscle in response to the treatment with CCL extract. The
expression of genes known as angiogenic factors (ANG-1, VEGF and
VEGFR) was upregulated by CCL extract treatment in the hind limbs
of mice. In contrast, the transcriptional levels of the
inflammatory factors TNF-α, IL-6 and IL-1b were reduced. ANG-1 and
VEGF are known to induce angiogenic effects and promote the
formation of mature neovessels and vascular stabilization in
ischemic lesions (22).
In addition, VEGF acts on two receptors, the
fms-like tyrosine kinase (flt-1; VEGFR1) and the kinase insert
domain-containing receptor, fetal liver kinase (KDR; VEGFR2)
(21). It has been previously
shown that VEGFR1 is abundantly expressed in both endothelial cells
and pericytes, while VEGFR2 is not expressed in pericytes (23). Moreover, VEGFR2 has been shown to
play a major role in VEGF-dependent angiogenesis (24). Additionally, IL-6 acts on a variety
of organs and cell types and promotes cell growth, and its
expression has been reported during wound healing (15,19,20).
Furthermore, TNF-α has been implicated as an indirect angiogenic
factor, and various cytokines including IL-1b have been shown to be
associated with VEGF-dependent angiogenesis (10,17,18).
In the present study, arrays for angiogenic or
inflammatory cytokines were conducted to evaluate the expression of
angiogenesis-mediated factors ET-1 and IGFBP-3, as well as the
inflammation-related factors, CXCL12 and CD54, and their
transcriptional expression in ischemic muscles. A previous study
has shown that endothelin-1 (ET-1) has important effects on
angiogenesis in wounds, and these are mediated by the stimulation
of proliferation and collagen production (25). Moreover, IGFBP-3 is known to play
an important role in wound healing through a direct binding to
fibrinogen (26). As in the case
of VEGF, increasing the movement of benign cells in ischemic
regions contributes to angiogenesis (23,24,27).
A critical role for CXCL12 in SDF1-induced angiogenesis in wounds
has been previously documented (28), and recent studies have shown that
CXCL12 promotes the synthesis and secretion of VEGF, which
contributes to VEGF-induced angiogenesis under ischemic conditions
(29,30). Also, an important part of the
Fig. 6 is the decrease in IL-1β
level compared to the level in a control group. Definitely, a
decrease with respect to E2, used as a positive control, would be a
better result; there is a possibility of a difference because the
amount of the expression is determined in the hind-limb ischemic
lesion, and not in the cell. However, the meaningful result is that
all three genes (TNF-α, IL-6, IL-1β) have decreased, dose
dependently, and that all have decreased significantly compared to
the level in the control group.
Several recent studies have been conducted to
mitigate the side effects of chemical drugs and hormone therapy for
the symptoms of menopause through treatment with natural
substances, and the interest for the use of herbal formulas to
treat symptoms in pre/post-menopausal women has increased (29,31).
One such formula is CCL, a medicinal herb known to protect the
liver and kidneys and potentially useful for treating bone
disorders such as rheumatoid arthritis and osteoporosis (32,33).
Moreover, prescriptions including CCL are known to reduce the
symptoms common to menopausal women, including obesity, metabolic
disorders, and colpoxerosis (vaginal dryness), as well as to
control the thickness of endothelial cells inside the vaginal wall
(34). Also, as shown in Fig. 1, the differences in concentration
seem insignificant over time. Therefore, it will not exhibit
significant effect of 450 mg/kg/day and we suggest it will not be
possible to expect further improved effects. Furthermore, we always
test the effects at low and high concentrations of a crude extract.
Natural products are administrated in dose as low as 100 to as high
as 500 (35), especially for crude
extracts such as CCL. Thus, we selected the lower-range
concentration of 150 mg/kg/day and the higher-range concentration
of 450 mg/kg/day. But, in the case of in vivo study, the
concentration is limited and it is necessary to experiment with a
wide range of concentrations including low concentrations. There is
also a need for higher concentration of experiments to obtain
IC50 values for in vitro study.
In conclusion, we demonstrated that CCL improves
blood perfusion and peripheral capillary density via regulation of
angiogenic and/or inflammatory responses in vivo and in
vitro. Based on the current findings, CCL may represent a
potent therapeutic agent for the treatment of ischemic injury.
Moreover, CCL shows a potential for use as a medicinal herb to
improve (post) menopausal women's health.
Acknowledgements
Not applicable.
Funding
This study was supported by the Korea Institute of
Oriental Medicine (grant no. KNS1515290).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Author's contributions
HJK, HY, DHJ, JTH and BSK performed the research,
analyzed the data, and wrote the manuscript; HJK and HY performed
in vivo experiments and data analysis; DHJ performed in
vitro experiments and data analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experiments Ethics Committee of the Korea Institute of Oriental
Medicine (17-028, Daejeon, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moon M, Jeong HU, Choi JG, Jeon SG, Song
EJ, Hong SP and Oh MS: Memory-enhancing effects of Cuscuta
japonica Choisy via enhancement of adult hippocampal
neurogenesis in mice. Behav Brain Res. 311:173–182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oh H, Kang DG, Lee S and Lee HS:
Angiotensin converting enzyme inhibitors from Cuscuta
japonica Choisy. J Ethnopharmacol. 83:105–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jang JY, Kim HN, Kim YR, Choi YH, Kim BW,
Shin HK and Choi BT: Aqueous fraction from Cuscuta japonica
seed suppresses melanin synthesis through inhibition of the p38
mitogen-activated protein kinase signaling pathway in B16F10 cells.
J Ethnopharmacol. 141:338–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patisaul HB and Jefferson W: The pros and
cons of phytoestrogens. Front Neuroendocrinol. 31:400–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stefanick ML, Mackey S, Sheehan M,
Ellsworth N, Haskell WL and Wood PD: Effects of diet and exercise
in men and postmenopausal women with low levels of HDL cholesterol
and high levels of LDL cholesterol. N Engl J Med. 339:12–20. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubanyi GM, Kauser K and Johns A: Role of
estrogen receptors in the vascular system. Vascul Pharmacol.
38:81–88. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cadenas C and Bolt HM: Estrogen receptors
in human disease. Arch Toxicol. 86:1489–1490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fagan SC, Hess DC, Hohnadel EJ, Pollock DM
and Ergul A: Targets for vascular protection after acute ischemic
stroke. Stroke. 35:2220–2225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silvestre JS, Mallat Z, Tedgui A and Lévy
BI: Post-ischaemic neovascularization and inflammation. Cardiovasc
Res. 78:242–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehrad B, Keane MP and Strieter RM:
Chemokines as mediators of angiogenesis. Thromb Haemost.
97:755–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khajuria DK, Razdan R and Mahapatra DR:
Description of a new method of ovariectomy in female rats. Rev Bras
Reumatol. 52:462–470. 2012.(In English, Portuguese). PubMed/NCBI
|
|
12
|
Hamada Y, Gonda K, Takeda M, Sato A,
Watanabe M, Yambe T, Satomi S and Ohuchi N: In vivo imaging of the
molecular distribution of the VEGF receptor during angiogenesis in
a mouse model of ischemia. Blood. 118:e93–e100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao JC, Chang WT, Lee MS, Chiu YJ, Chao
WK, Lin YC, Lin MK and Peng WH: Antinociceptive and
anti-inflammatory activities of Cuscuta chinensis seeds in
mice. Am J Chin Med. 42:223–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teodorescu VJ, Vavra AK and Kibbe MR:
Peripheral arterial disease in women. J Vasc Surg. 57 (Suppl
4):18S–26S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daskalopoulou SS, Daskalopoulos ME,
Mikhailidis DP and Liapis CD: Lipid management and peripheral
arterial disease. Curr Drug Targets. 8:561–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clement YN, Onakpoya I, Hung SK and Ernst
E: Effects of herbal and dietary supplements on cognition in
menopause: A systematic review. Maturitas. 68:256–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eming SA, Krieg T and Davidson JM:
Inflammation in wound repair: Molecular and cellular mechanisms. J
Invest Dermatol. 127:514–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong VW and Crawford JD: Vasculogenic
cytokines in wound healing. Biomed Res Int. 2013:1904862013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez AC, Costa TF, Andrade ZA and
Medrado AR: Wound healing-A literature review. An Bras Dermatol.
91:614–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang SH, Lee BH, Choi SH, Kim HJ, Won KJ,
Lee HM, Rhim H, Kim HC and Nah SY: Effects of gintonin on the
proliferation, migration, and tube formation of human
umbilical-vein endothelial cells: Involvement of
lysophosphatidic-acid receptors and
vascular-endothelial-growth-factor signaling. J Ginseng Res.
40:325–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashcroft GS, Greenwell-Wild T, Horan MA,
Wahl SM and Ferguson MW: Topical estrogen accelerates cutaneous
wound healing in aged humans associated with an altered
inflammatory response. Am J Pathol. 155:1137–1146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zacharek A, Chen J, Cui X, Li A, Li Y,
Roberts C, Feng Y, Gao Q and Chopp M: Angiopoietin1/Tie2 and
VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and
vascular stabilization after stroke. J Cereb Blood Flow Metab.
27:1684–1691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao R, Yan W and Rockey DC: Regulation of
endothelin-1 synthesis by endothelin-converting enzyme-1 during
wound healing. J Biol Chem. 274:3228–3234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campbell PG, Durham SK, Hayes JD,
Suwanichkul A and Powell DR: Insulin-like growth factor-binding
protein-3 binds fibrinogen and fibrin. J Biol Chem.
274:30215–30221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abbott JD, Huang Y, Liu D, Hickey R,
Krause DS and Giordano FJ: Stromal cell-derived factor-1alpha plays
a critical role in stem cell recruitment to the heart after
myocardial infarction but is not sufficient to induce homing in the
absence of injury. Circulation. 110:3300–3305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruiz de Almodovar C, Luttun A and
Carmeliet P: An SDF-1 trap for myeloid cells stimulates
angiogenesis. Cell. 124:18–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin DK, Shido K, Kopp HG, Petit I,
Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B,
et al: Cytokine-mediated deployment of SDF-1 induces
revascularization through recruitment of CXCR4+
hemangiocytes. Nat Med. 12:557–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Restivo TE, Mace KA, Harken AH and Young
DM: Application of the chemokine CXCL12 expression plasmid restores
wound healing to near normal in a diabetic mouse model. J Trauma.
69:392–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hidaka T, Yonezawa R and Saito S:
Kami-shoyo-san, Kampo (Japanese traditional medicine), is effective
for climacteric syndrome, especially in
hormone-replacement-therapy-resistant patients who strongly
complain of psychological symptoms. J Obstet Gynaecol Res.
39:223–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kijowski J, Baj-Krzyworzeka M, Majka M,
Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A
and Ratajczak MZ: The SDF-1-CXCR4 axis stimulates VEGF secretion
and activates integrins but does not affect proliferation and
survival in lymphohematopoietic cells. Stem Cells. 19:453–466.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin QZ, Lu H, Li LM, Yie SM, Hu X, Liu ZB,
Zheng X, Cao S and Yao ZY: Impacts of You Gui Wan on the expression
of estrogen receptors and angiogenic factors in OVXrat vagina: a
possible mechanism for the trophic effect of the formula on
OVX-induced vaginal atrophy. Mol Med Rep. 8:1329–1336. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
May MJ, D'Acquisto F, Madge LA, Glockner
J, Pober JS and Ghosh S: Selective inhibition of NF-kappaB
activation by a peptide that blocks the interaction of NEMO with
the IkappaB kinase complex. Science. 289:1550–1554. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang HM, Shin HK, Kang YH and Kim JK:
Cuscuta chinensis extract promotes osteoblast
differentiation and mineralization in human osteoblast-like MG-63
cells. J Med Food. 12:85–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akimova E, Lanzenberger R and Kasper S:
The serotonin-1A receptor in anxiety disorders. Biol Psychiatry.
66:627–635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pyun BJ, Yang H, Sohn E, Yu SY, Lee D,
Jung DH, Ko BS and Lee HW: Tetragonia tetragonioides (Pall.)
Kuntze regulates androgen production in a Letrozole-induced
polycystic ovary syndrome model. Molecules. 23:E11732018.
View Article : Google Scholar : PubMed/NCBI
|