Introduction
Atherosclerosis, a chronic disease characterized by
the accumulation of lipids and fibrous elements in the large
arteries, is the principal cause of CAD, a leading cause of
morbidity and mortality worldwide (1). Plasma high-density lipoprotein (HDL)
is thought to be a sterol transporter which is a protective factor
against atherosclerosis. Meanwhile, the inverse correlation between
plasma HDL-C and the incidence of atherosclerosis is well
established (2,3).
The formation of HDL occurs in the liver and
intestine. The interaction between lipid poor apolipoprotein A1
(ApoA1) with the ATP binding cassette A1 (ABCA1) mediates this
first step in HDL formation (4).
ABCA1 is a member of the ABC family of membrane transporters that
promotes phospholipid and cholesterol transfer from cells to poorly
lapidated ApoA1. Recently, genetic association study and functional
study in mice have indicate an important role of ABCA1 during the
pathogenesis of atherosclerosis (5–8).
In cardiovascular pathologies circulating miRNAs
have been described as disease-specific biomarkers and various
animal models and clinical studies have proven miRNAs suitable for
diagnostic purposes in CAD and myocardial infarction (MI) (9,10).
Exosomes ranging in size from 40–100 nm in diameter, secreted by
cells are proposed to be mechanism through which secreted cells
pass signals to targeted cells. Meanwhile, altered exosomal miRNAs
in serum has been found existed in the patients with CAD (11,12).
In the present study, we detected 9 candidate miRNAs
in the plasma exosome from 42 patients with coronary
atherosclerosis. The function of disturbed miRNA was examined by
dual-luciferase assay and immunoblotting.
Materials and methods
Clinical samples
This study includes 42 consecutive patients with
coronary atherosclerosis and 42 age and sex paired healthy
controls. All the participants were collected from Beijing
Institute of Heart Lung and Blood Vessel Diseases between September
2014 and November 2015. Clinical diagnosis of coronary
atherosclerosis was evaluated by percutaneous coronary angiography,
reviewed by two experienced cardiologists. Healthy control
subjects, without atherosclerosis, were selected in the same
period. Written informed consent was obtained from all participants
and this study was approved by the Ethics Committee of Beijing
Institute of Heart Lung and Blood Vessel Diseases.
A total of 10 ml peripheral venous blood was
collected from each participant. Portion of the blood samples were
processed for total cholesterol, HDL-C and LDL-C detection. Portion
of blood sample was processed for plasma separation and exosomes
extracted subsequently. The processing of these blood samples was
started within 30 min after collection.
Plasma exosome extraction
Exosomes were extracted from plasma using ExoQuick
Exosome Precipitation Solution (System Biosciences, Mountain View,
CA, USA). Plasma was obtained by centrifugation at ×3,000 g for 15
min to remove cells and cellular fragments, and subsequent
filtration of the supernatant was accomplished through a 0.45-µm
pore polyvinylidene fluoride filter (Millipore, Billerica, MA,
USA). Add 100 µl Thromboplastin D reagent rapidly into 100 µl
plasma sample to mix thoroughly and then Incubate at 37°C for 15
min. Spin at 10,000 rpm at RT for 5 min. ExoQuick was added to the
supernatants, and exosomes were precipitated by refrigeration at
4°C for 12 h. Exosome pellets collected by centrifugation at ×1,500
g for 30 min were dissolved in 20 µl PBS.
Cell culture
HepG2 and HEK293T cells were purchased from China
Infrastructure of Cell Line Resources and cultured in Dulbecco's
Modified Eagle Medium containing 10% fetal bovine serum (Hyclone,
Logan, UT, USA), 100 IU/ml penicillin and 10 mg/ml streptomycin.
THP1 cells were cultured in RPMI 1640 supplemented with 10% (v/v)
fetal bovine serum (Hyclone). All cells were maintained at 37°C
under an atmosphere of 5% CO2.
For macrophages differentiation, THP1 cells (1×106
cells/ml) were transferred into 100 mm-dishes by the addition of
100 ng/ml phorbol 12-myristate 13-acetate for a 72-h period.
RNA isolation and qRT-PCR
Total RNA was extracted from exosomes or from cell
samples by using Trizol Reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. The expression level
of miRNAs was detected by TaqMan miRNA RT-Real Time PCR.
Single-stranded cDNA was synthesized by using TaqMan MicroRNA
Reverse Transcription Kit (Applied Biosystems, Foster City, CA,
USA) and then amplified by using TaqMan Universal PCR Master Mix
(Applied Biosystems) together with miRNA-specific TaqMan MGB probes
(Applied Biosystems). U6 level was quantified for normalization.
Each sample in each group was measured in triplicate and the
experiment was repeated at least three times for the detection of
miRNAs.
Dual luciferase assay
A segment of 956 bp ABCA1 3′UTR segment containing
the potential target sites of miR-92a and miR-30a was cloned into
downstream of firefly luciferase coding region in pmirGLO plasmid
(Promega, Madison, WI, USA) to generate luciferase reporter vector.
For luciferase reporter assays, HEK293T cells were seeded in
48-well plates. 20 nM miRNAs mimic or inhibitor and luciferase
reporter vector (200 ng/well) were co-transfected by using
lipofectamine 2000 (Invitrogen). Two days later, cells were
harvested and assayed with the Dual-Luciferase Assay kit (Promega).
Each treatment was performed in triplicate in three independent
experiments. The results were expressed as relative luciferase
activity (Firefly LUC/Renilla LUC).
Western blot analysis
Protein extracts were boiled in
SDS/β-mercaptoethanol sample buffer, and 30 µg samples were loaded
into each lane of 10% polyacrylamide gels. The proteins were
separated by electrophoresis, and the proteins in the gels were
blotted onto PVDF membranes (Amersham Pharmacia Biotech, St.
Albans, Herts, UK) by electrophoretic transfer. The membrane was
incubated with mouse anti-ABCA1 monoclonal antibody (Abcam,
Cambridge, MA, USA) or mouse anti-β-actin monoclonal antibody
(Santa Cruz Biotechnology Inc.) over night at 4°C. The specific
protein-antibody complex was detected by using horseradish
peroxidase conjugated goat anti-rabbit or rabbit anti-mouse IgG.
Detection by the chemiluminescence reaction was carried using the
ECL kit (Pierce, Appleton, WI, USA). The β-actin signal was used as
a loading control.
Enzyme linked immunosorbent assay
(ELISA) for estimating ABCA1 protein
Serum ABCA1 level was estimated by using sandwich
ELISA method and rabbit and mouse anti-ABCA1 antibodies (Abcam).
Briefly, the 96-well plates were coated by mouse anti-ABCA1
(1:1,000 diluted) antibody and then incubated with 1:100 diluted
serum samples for 2 h at room temperature. After washed by PBST,
the plates were incubated using rabbit anti-ABCA1 antibody (1:1,000
diluted) followed by HRP labeled goat anti-rabbit secondary
antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). TMB
solution (Abcam) was added into each well, and incubated for 15–30
min. After adding equal volume of stopping solution the optical
density was read at 450 nm. The relative concentrations were
compared using OD value directly.
Blood biochemical indexes
Blood samples, from the same participants, were
drawn for measurement of serum levels of TC, HDL-C, LDL-C after a
12-h overnight fast. Serum levels of TC (mmol/l), HDL-C (mmol/l),
and LDL-C (mmol/l) were determined by colorimetric enzymatic assays
with use of an Auto-Analyzer.
Cell proliferation assay
THP1 cells were seeded in 96-well plates at low
density (5×103) and then transfected with miR-30e or
miR-92a mimic or inhibitor. Twenty microliters MTT (5 mg/ml)
(Sigma, St. Louis, MO, USA) were added into each well 48 h after
transfection, and the cells were incubated for further 4 h. The
absorbance was recorded at A570 nm with a 96-well plate reader
after the DMSO addition.
Apoptosis analysis
Cell apoptosis was performed using Annexin V-FITC
and propidium iodide (PI) staining and analyzed by flow
cytometry.
Cholesterol efflux assessment
The cholesterol efflux of differentiated THP1
macrophages was examined using cholesterol efflux assay kit (Abcam)
following the manufacture's instruction. Briefly, differentiated
THP1 cells were transfected with miR-30e or miR-92a mimic or
inhibitor for 6 h and then incubated with Labeling Reagent for 16
h. Wash cells by RPMI1640 medium, the cells were cultured at 37°C.
Transfer the supernatant to 96-well plates at different time points
to measure the fluorescence (Ex/Em=482/515 nm).
Statistical analysis
All the results were analyzed by using SPSS
Statistical Package version 16. The data of two groups were
analyzed by student's t-test and the correlation analysis was
processed by χ2-analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Atherosclerosis is a chronic inflammatory disease of
the vascular wall which leads to cardiovascular pathologies such as
myocardial infarction, ischemic stroke and peripheral arterial
disease. To find a new marker for atherosclerosis diagnosis and
unveil the pathogenesis of atherosclerosis related CAD, we detected
the expression of 9 candidate miRNAs expression in the exosome from
serum sample of 42 patients with coronary atherosclerosis and age,
sex paired healthy controls (Table
I). These candidate miRNAs were reported to be related to the
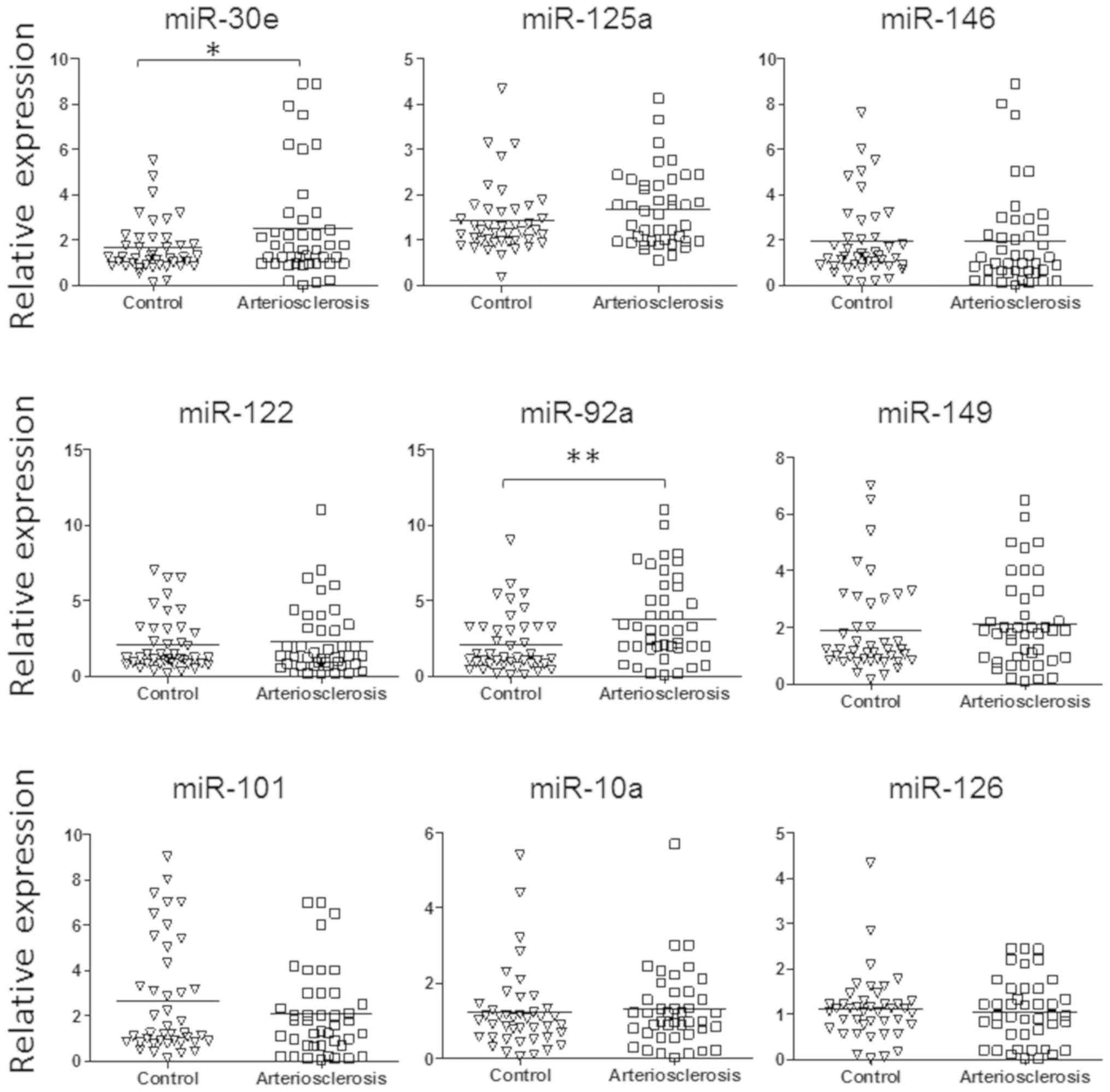
pathogenesis of CAD (13–15). As shown in Fig. 1, the level of miR-30e and miR-92a
was significantly upregulated in the plasma exosome of patients
with atherosclerosis.
 | Table I.Characteristics of cases and
controls. |
Table I.
Characteristics of cases and
controls.
| Characteristics | Cases (n=42) | Controls | P-value |
|---|
| Age | 63 (42) | 63 (42) | 1 |
| Sex
(male/female) | 22/20 | 22/20 | 1 |
| Diabetes | 7 (42) | 5 (42) | 0.041 |
| Hypertension
(yes/no) | 29 (42) | 21(42) | 0.0035 |
| Total cholesterol
(mmol/l) | 4.64 (4.24–5.48) | 4.01 (3.28–5.23) | <0.001 |
| HDL-C (mmol/l) | 1.19 (1.05–1.43) | 1.25 (1.03–1.64) | <0.001 |
| LDL-C (mmol/l) | 3.09 (2.70–3.45) | 2.42 (2.04–3.01) | <0.001 |
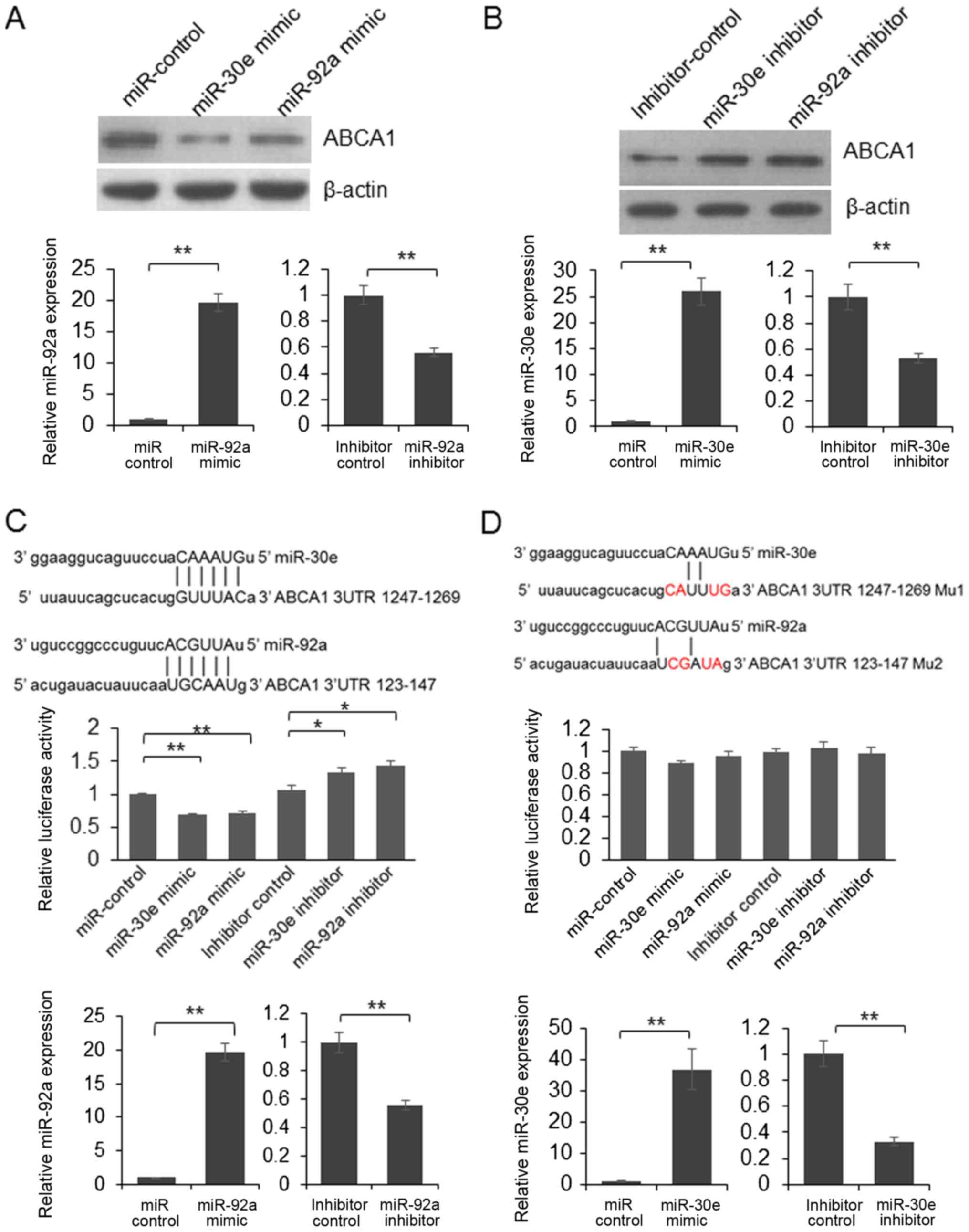
To further understand the biological function of
miR-30e and miR-92a, the potential direct targets of miR-30e and
miR-92a were predicted using online bioinformatics tool: miRanda
(http://www.microrna.org). We found that ABCA1 is
a potential target of miR-30e and miR-92a. To understand whether
the expression of endogenous ABCA1 is repressed by miR-30e and
miR-92a, HepG2 cells were transfected with mimic or inhibitor of
miR-30e or miR-92a. 48 h after transfection, the cells were lysed
and the expression of ABCA1 was examined by immunoblotting. As
shown in Fig. 2A, the protein
level was reduced in miR-30e or miR-92a mimic transfected cells and
up-regulated in the cells transfected with miR-30e or miR-92a
inhibitor (Fig. 2B).
To confirm the direct interaction between ABCA1 and
miRNAs, we constructed a reporter vector through inserting ABCA1
3′UTR into pmirGLO vector, following the stop codon of firefly
luciferase. Subsequently, dual luciferase assay was processed. As
shown in Fig. 2C, the relative
luciferase activity was decreased significantly in miR-30e or
miR-92a mimic transfected cells. Meanwhile, the luciferase activity
was up-regulated by inhibitors of miR-30e and miR-92a (Fig. 2C). When 4 nucleotides in the
predicted target regions altered, the luciferase activity was not
changed significantly by the mimic of miR-30e or miR-92a (Fig. 2D). These results indicated that
miR-30e and miR-92a can repress the expression of luciferase by
targeting 3′UTR of ABCA1. These results indicated that ABCA1 is a
direct target of miR-30e and miR-92a.
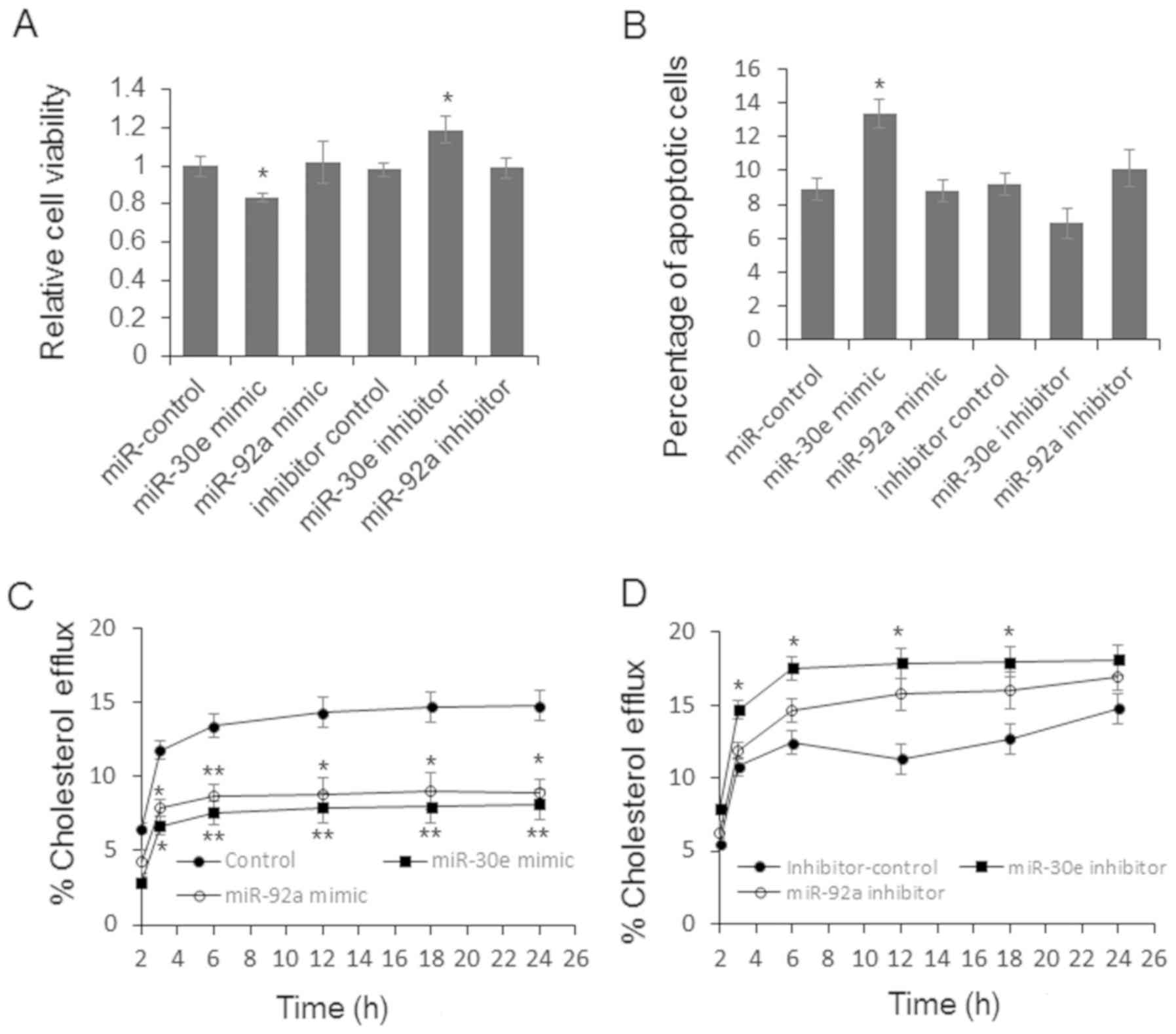
ABCA1, also known as the cholesterol efflux
regulatory protein (CERP) is a major regulator of cellular
cholesterol and phospholipid homeostasis. Meanwhile, disturbed
miRNA level also has the potential of altered cell proliferation
and apoptosis. To further explore the function of miR-30e and
miR-92a during the pathogenesis of CAD, we first examined the cell
viability and apoptosis by MTT assay and flow cytometry. As shown
in Fig. 3A, the relative cell
viability was significantly repressed by miR-30e and up-regulated
by miR-30e inhibitor. Meanwhile, the apoptotic cell number was
increased in the cells transfected with miR-30e mimic (Fig. 3B). Subsequent cholesterol efflux
assay results indicated that miR-30e and miR-92a mimic can repress
the cholesterol efflux significantly (Fig. 3C). miR-30e inhibitor treatment
relates to increased cholesterol efflux (Fig. 3D). miR-92a inhibitor up-regulated
the cholesterol efflux slightly, but the difference was not
significant (Fig. 3D).
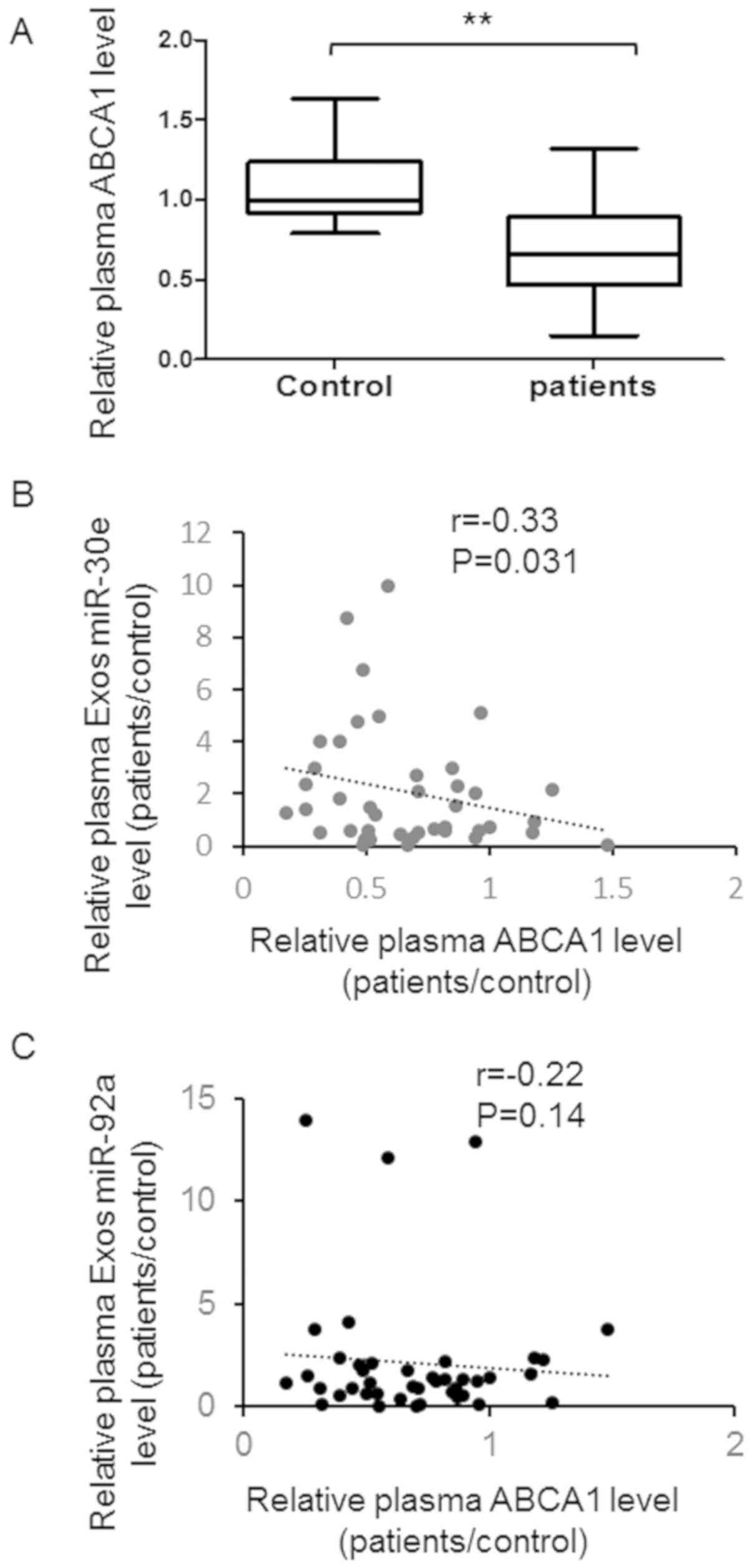
To understand the correlation between the expression
of miR-30e, miR-92a in the plasma and clinical characters of
patients, we detected the plasma level of ABCA1 and cholesterol in
the patients and relative controls. As shown in Fig. 4A, a higher plasma ABCA1 exists in
the patients with atherosclerosis compared with healthy control.
Meanwhile, a significant negative correlation was found between
plasma ABCA1 level and plasma exosomal miR-30e level (Fig. 4B). However, no significant
correlation was found between plasma ABCA1 level and exosomal
miR-92a level (Fig. 4C).
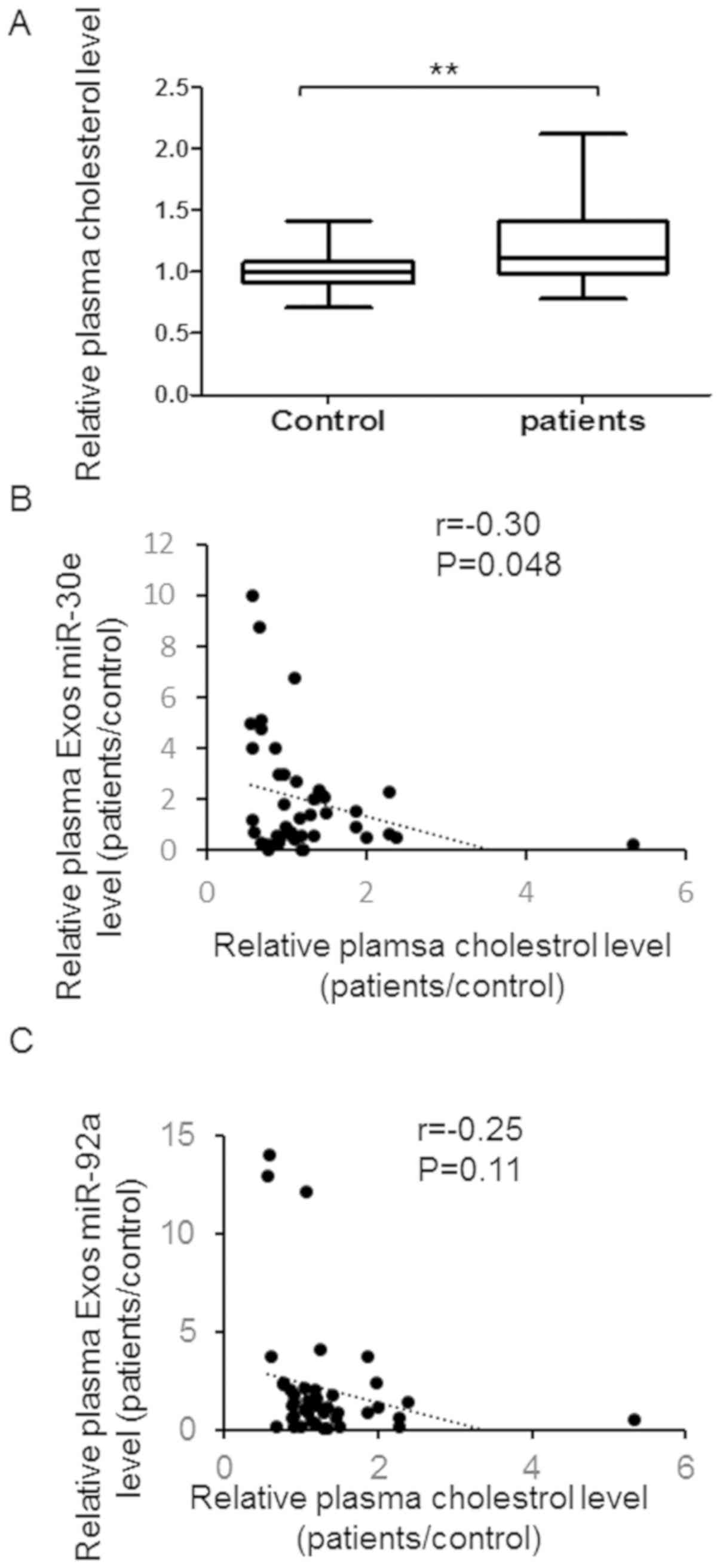
Furthermore, plasma cholesterol level was up-regulated in patients
with atherosclerosis and negatively correlate with plasma exosomes
miR-30e level instead of miR-92a (Fig.
5A-C).
Discussion
Atherosclerosis is a chronic disease characterized
by the accumulation of lipids and fibrous elements in the large
arteries which is the principal cause of CAD. Disturbed exosomal
miRNAs in serum have been found in patients with a lot of kinds of
diseases including CAD. In this study, we detected 9 candidate
miRNAs in the plasma exosome from 42 patients with coronary
atherosclerosis and found a higher expression of miR-30e and
miR-92a in patients. Analyzed by bioinformatics tools and confirmed
by immunoblotting, we found that ABCA1 is a direct target of
miR-30e and miR-92a. Furthermore, a negative correlation was found
between plasma miR-30e and ABCA1, or miR-30e and cholesterol. So
miR-30e may have the potential to be a new biomarker for coronary
atherosclerosis.
Exosomes are shed by cells under both normal and
pathological conditions, and they carry nucleic acids and proteins
from their host cells that are indicative of pathophysiological
conditions. Meanwhile, under the protection of lipid bilayer, the
bio-functional molecules are more stable than that exposed in the
biofluids (16). So, they are
widely considered to be crucial for biomarker discovery for
clinical diagnostics, and that is why we choose miRNAs in exosomes
to screen biomarkers for atherosclerosis. We chose 9 candidate
miRNAs that were reported to be related to the pathogenesis of CAD
(13–15,17).
We find an increased exosomal miR-92a expression in patients with
coronary atherosclerosis, which is in line with the report of
Niculescu LS et al (14).
Meanwhile, we report for the first time that overexpressed miR-30e
relates to coronary atherosclerosis, which was first reported in a
mouse atherosclerosis model (15).
However, we could not verify abnormal level of the other 7 miRNAs,
the altered level of which was reported in the peripheral blood of
patients with CAD. These results suggesting that exosomes may
provide more specific biomarkers for atherosclerosis clinical
diagnosis.
Exosomes are full of common constituents which are
fusion of multivesicular bodies attachment to target cells. In this
study, we reported that miR-30e and miR-92a were up-regulated in
the serum exosomes of patients with coronary atherosclerosis and
ABCA1 is a direct target of miR-30e and miR-92a. These two exosomal
miRNAs may have the potential to be a biomarker for atherosclerosis
diagnosis. Meanwhile, miRNAs can be delivered from the donor cells
to the recipient cells by exosomes (18). So whether overexpressed miR-92a and
miR-30e can be functionally delivered to target cells or not need
to be further examined. Meanwhile, our study partially explained
the function of disturbed miR-30e and miR-92a in the cell
proliferation, apoptosis and cholesterol efflux, however, the role
of miR-30e and miR-92a in the pathogenesis of atherosclerosis needs
to be further unveiled.
The ABCA1 is a member of the ABC1 subfamily that
moves phospholipids and cholesterol across the cell membrane to
HDL-C and has an important role in the pathogenesis of
atherosclerotic vascular diseases due to their involvement in
cholesterol homeostasis, blood pressure regulation, endothelial
function, vascular inflammation, as well as platelet production and
aggregation (19). It is confirmed
the more than XX miRNAs modulate ABCA1 expression directly
including miR-33a, miR-122, miR-467b, miR-183, miR-28 and so on
(20,21). In the present study, we confirm
ABCA1 is a direct target of miR-92a and miR-30e for the first time,
which is an import supplement of our knowledge of ABCA1 modulation
system.
In conclusion, the level of plasma exosomal miR-30e
and miR-92a was up-regulated in patients with atherosclerosis and
negative correlate with the plasma cholesterol and ABCA1 level,
which may provide a new biomarker for clinical diagnosis and
treatment of coronary atherosclerosis.
References
|
1
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller GJ and Miller NE:
Plasma-high-density-lipoprotein concentration and development of
ischaemic heart-disease. Lancet. 1:16–19. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ and Yellon DM: Targeting
residual cardiovascular risk: Raising high-density lipoprotein
cholesterol levels. Heart. 94:706–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duong PT, Collins HL, Nickel M, Lund-Katz
S, Rothblat GH and Phillips MC: Characterization of nascent HDL
particles and microparticles formed by ABCA1-mediated efflux of
cellular lipids to apoA-I. J Lipid Res. 47:832–843. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abd El-Aziz TA, Mohamed RH and Hagrass HA:
Increased risk of premature coronary artery disease in egyptians
with ABCA1 (R219K), CETP (TaqIB), and LCAT (4886C/T) genes
polymorphism. J Clin Lipidol. 8:381–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koopal C, Visseren FL, Kastelein JJ and
Westerink J: Premature atherosclerosis, extremely low
HDL-cholesterol and concurrent defects in APOA1 and ABCA1 genes: A
family case report. Int J Cardiol. 177:e19–e21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv YC, Tang YY, Peng J, Zhao GJ, Yang J,
Yao F, Ouyang XP, He PP, Xie W, Tan YL, et al: MicroRNA-19b
promotes macrophage cholesterol accumulation and aortic
atherosclerosis by targeting ATP-binding cassette transporter A1.
Atherosclerosis. 236:215–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X,
Zhu N, Liu J, Wu Y, Li Y, et al: Rutaecarpine suppresses
atherosclerosis in ApoE-/- mice through upregulating ABCA1 and
SR-BI within RCT. J Lipid Res. 55:1634–1647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maegdefessel L: The emerging role of
microRNAs in cardiovascular disease. J Intern Med. 276:633–644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fichtlscherer S, De Rosa S, Fox H,
Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T,
Müller-Ardogan M, et al: Circulating microRNAs in patients with
coronary artery disease. Circ Res. 107:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Cardiac extracellular vesicles in normal and infarcted heart.
Int J Mol Sci. 17(pii): E632016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huber HJ and Holvoet P: Exosomes: Emerging
roles in communication between blood cells and vascular tissues
during atherosclerosis. Curr Opin Lipidol. 26:412–419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sayed AS, Xia K, Li F, Deng X, Salma U, Li
T, Deng H, Yang D, Haoyang Z, Yang T and Peng J: The diagnostic
value of circulating microRNAs for middle-aged (40-60-year-old)
coronary artery disease patients. Clinics (Sao Paulo). 70:257–263.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niculescu LS, Simionescu N, Sanda GM,
Carnuta MG, Stancu CS, Popescu AC, Popescu MR, Vlad A, Dimulescu
DR, Simionescu M and Sima AV: MiR-486 and miR-92a identified in
circulating HDL discriminate between stable and vulnerable coronary
artery disease patients. PLoS One. 10:e01409582015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang T, Tian F, Wang J, Jing J, Zhou SS
and Chen YD: Endothelial cell autophagy in atherosclerosis is
regulated by miR-30-mediated translational control of ATG6. Cell
Physiol Biochem. 37:1369–1378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki E, Fujita D, Takahashi M, Oba S and
Nishimatsu H: Stem cell-derived exosomes as a therapeutic tool for
cardiovascular disease. World J Stem Cells. 8:297–305. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raitoharju E, Oksala N and Lehtimäki T:
MicroRNAs in the atherosclerotic plaque. Clin Chem. 59:1708–1721.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Narahari A, Hussain M and Sreeram V:
MicroRNAs as biomarkers for psychiatric conditions: A review of
current research. Innov Clin Neurosci. 14:53–55. 2017.PubMed/NCBI
|
|
19
|
Schumacher T and Benndorf RA: ABC
transport proteins in cardiovascular disease-A brief summary.
Molecules. 22(pii): E5892017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rotllan N, Price N, Pati P, Goedeke L and
Fernández-Hernando C: microRNAs in lipoprotein metabolism and
cardiometabolic disorders. Atherosclerosis. 246:352–360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Z, Cappello T and Wang L: Emerging
role of microRNAs in lipid metabolism. Acta Pharm Sin B. 5:145–150.
2015. View Article : Google Scholar : PubMed/NCBI
|