Introduction
Preeclampsia (PE) is a pregnancy-specific syndrome
characterized by the new onset of hypertension and proteinuria
after 20 weeks of gestation. PE affects 3–5% pregnant women and
results in high maternal and neonatal morbidity and mortality
worldwide (1,2). Despite major efforts have been made
in past decades, the available therapeutic options to retard
disease progression remain limited. Thus, it is an urgent need to
search for a principal therapeutic target for PE.
Normal placentation is believed to play an important
role during pregnancy. The process of placentation involves cells
derived from the placenta, fetal extravillous trophoblasts (EVTs),
invading into the endometrium and one third of the myometrium, as
well as the associated spiral arteries under strict temporal and
spatial controls (3–5). Subsequently, the spiral arteries were
replaced and converted into highly dilated vessels that are able to
provide adequate placental perfusion to maintain the growth of
fetus (6). There is increasing
evidence to support that insufficient trophoblastic invasion is
central to the pathogenesis of PE (7,8).
However, the molecular mechanism for the regulation of trophoblast
invasion remains largely elusive.
MicroRNAs (miRNAs) are a class of small non-coding
RNAs which negatively regulate gene expression by binding to the
3′-untranslated regions (3′-UTR) of their target mRNAs for
transcript degradation or translational repression (9). miRNAs were recently reported to play
functional roles in various cellular processes in almost all
diseases, such as proliferation, apoptosis, invasion, and
differentiation (10,11). It has been found that the
expression profiles of miRNAs were significantly altered in
placenta tissues and maternal serum from PE pregnancies (12,13).
For example, by screening the expression profile of miRNAs in the
placentas of PE patients and healthy subjects, Enquobahrie et
al (14), identified that
eight miRNAs were differentially expressed in the PE placentas,
suggesting that aberrant expression of miRNAs may contribute to the
pathogenesis of PE.
In the present study, we focused the expression
level of miR-142-3p in the placentas of PE patients and studied its
role in the invasion and migration of trophoblast cells. Moreover,
we also studied the association between miR-142-3p and TGF-β1 in
the invasion ability of trophoblast cells, as well as the
downstream signaling pathway.
Materials and methods
Tissue samples
The placental tissues used in this study were
obtained from women with normal pregnancies (n=20) and patients
with PE (n=20) at the Department of Obstetrics and Gynecology,
Tangshan Worker Hospital. All experimental protocols were approved
by the Ethics Committee of the Tangshan Worker Hospital, He Bei
Medical University. All studies were performed in accordance with
the ethical guidelines of the Tangshan Worker Hospital, He Bei
Medical University. Written informed consent was obtained from all
patients.
miRNA expression profiling
Total RNA was isolated from frozen placentas tissue
by the miRNeasy mini kit (Qiagen, Ltd., West Sussex, UK) following
the manufacturer's protocol. After passing the RNA purity and
quantity measurement using the NanoDrop ND-1000 Spectrophotometry
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Agilent's
2100 Bioanalyzer, samples were labeled with miRCURY™ Hy3™/Hy5™
Power labeling kit and hybridized to the miRCURY LNA™ Array
(v.18.0; Agilent Technologies, Inc., Santa Clara, CA, USA),
incubated, washed and scanned in Agilent high resolution microarray
scanner. Data was analyzed using Genespring software (Agilent
Technologies, Inc.). The heat map of the 54 miRNAs most obvious
differences was created using a method of hierarchical clustering
by GeneSpring GX, v.7.3 (Agilent Technologies, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA and miRNA were isolated from placentas
specimens using the miRNeasy Mini kit (Qiagen GmbH, Hilden,
Germany) following the manufacturer's instructions. For the
detection of miR-142-3p, miR-142-3p was reverse transcribed using
the PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China) and quantified by RT-qPCR with the TaqMan MicroRNA
assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). For
detection of the TGF-β1 mRNA levels, 1 µg of total RNA was reverse
transcribed using Script™ cDNA Synthesis kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), RT-qPCR were performed using SYBR Premix
Ex Taq (Takara Biotechnology Co., Ltd., Dalian, China) on an ABI
PRISM 7500 Sequence Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). U6 and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) were used as normalization control in the
expression analysis of miR-142-3p and TGF-β1, respectively. The
sequences of the primers were as follows: MMP-2 sense
5′-GGCCTCGTATACCGCATCAATC-3′, anti-sense
5′-GGCCTCTCCTGACATTGACCTT-3′; MMP-9 sense 5′-CCCGGACCAAGGATACAG-3′,
anti-sense 5′-GGCTTTCTCTCGGTACTG-3′; GAPDH sense
5′-ATTGTTGCCATCAATGACCC-3′, anti-sense 5′-AGTAGAGGCAGGGATGATGT-3′.
Primers for miR-142-3p and U6 snRNA were obtained from GeneCopoeia.
The relative expression of RNAs was calculated using the
2−ΔΔCq method (15).
Each reaction was conducted in triplicate.
Cell culture
The extravillous trophoblast cell line HTR-8/SVneo
was obtained from ATCC and maintained in RPMI 1640 medium (Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.). Human 293T cells were purchased
from the American Type Culture Collection (Manassas, VA, USA) and
maintained in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1/100
streptomycin-penicillin mix (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The cells were incubated at 37°C with 5%
CO2.
Transfection
The miR-142-3p mimics, miR-142-3p inhibitor and
negative control (6) were
synthesized by GenePharma Biological Technology (Shanghai, China).
The TGF-β1 siRNA and NC siRNA were purchased from GenePharma
Biological Technology. Cell transfection was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The cells were
treated for further experiments 48 h after transfection.
In vitro invasion and migration
assay
For wound healing assay, HTR-8/SVneo cells were
transfected with miR-142-3p mimics, miR-142-3p inhibitor and
negative control (6) or si-TGF-β1
and seeded into 6-well plates at a density of 1,000 cells/well.
After 48 h, when the cells reached 80% confluence, scratch wounds
were wounded with a 10 µl pipette tip; cells were washed three
times with PBS to clear cell debris; and wound gaps were imaged and
calculated by Image J software (NIH, Bethesda, MD, USA) at 24 and
48 h. For invasion assay, the invasive ability of trophoblasts was
evaluated using a polycarbonate membrane cell culture insert
(Costar; Corning Incorporated, Corning, NY, USA) coated with growth
factor reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
as previously described (16). The
number of invaded cells was calculated by counting five random
views under the microscope at 24 and 48 h. The experiment was
performed in triplicate and repeated for three times.
Gelatin zymography
Gelatin zymography is an extremely sensitive and
useful technique for measuring the relative amounts of active and
inactive gelatinase (MMP-2 or MMP-9) in samples. Briefly, samples
(10 µl) were resuspended in 5× non-reducing sample buffer (4% SDS,
20% glycerol, 0.01% bromophenol blue and 125 mM Tris-HCl), and run
on a 10% SDS-PAGE gel containing 0.5 mg/ml gelatin without prior
denaturation. After electrophoresis, the gels were washed to remove
SDS and incubated for 5–10 min at room temperature (RT) in an
incubation buffer (50 mM Tris, 5 mM CaCl2, 1 µM
ZnCl2, and 1% Triton X-100). Next, replace with fresh
incubation buffer and incubate for 24 h at 37°C. The gels were
subsequently stained with Coomassie brilliant blue G-250, destained
in 30% methanol, and flooded with 10% acetic acid to detect
gelatinase secretion.
Western blot analysis
Total cell protein was abstracted from cells after
transfection using radioimmunoprecipitation (RIPA) lysis buffer
(Sigma-Aldrich; Merck KGaA) and the quantity of the protein was
determined using a Bicinchoninic Acid (BCA) protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). 40 µg of protein samples
were subjected to SDS-polyacrylamide gel electrophoresis and
transferred onto a PVDF membrane. The membrane was blocked with 5%
nonfat milk. The membrane was then incubated with primary
antibodies: Anti-TGF-β1 antibody (Rabbit polyclonal, 1:1,000) and
anti-β-actin antibody (Rabbit polyclonal, 1:2,000) (all antibodies
were purchased from Abcam, Cambridge, MA, USA), followed by
incubation with HRP-anti-rabbit secondary antibody at room
temperature for 1 h. Signals were detected using an ECL kit (GE
Healthcare, Chicago, IL, USA) according to the manufacturer's
protocol. The intensity of protein fragments was quantified with
the Quantity One software (v.4.5.0 basic, Bio-Rad Laboratories,
Inc.).
Luciferase assays
A cDNA fragment of the TGF-β1 3′-UTR mRNA containing
the seed sequence of the miR-142-3p-binding site or a mutated
binding site was cloned into the pmirGLO dual-luciferase vector
(Promega Corporation, Madison, WI, USA). The constructed
dual-luciferase vector was co-transfected with 10 pmol of
miR-142-3p mimics, miR-142-3p inhibitor or NC into 293T cells. The
cells were harvested and lysed 24 h later, and the luciferase
activity was measured by the Dual-Luciferase Assay System (Promega
Corporation) in accordance to the manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using GraphPad Prism v.5.0 (GraphPad Software, Inc., San
Diego, CA, USA). Differences were analyzed with the Student's
t-test between two groups or with one-way analysis of variance
followed by Tukey's multiple comparison tests between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
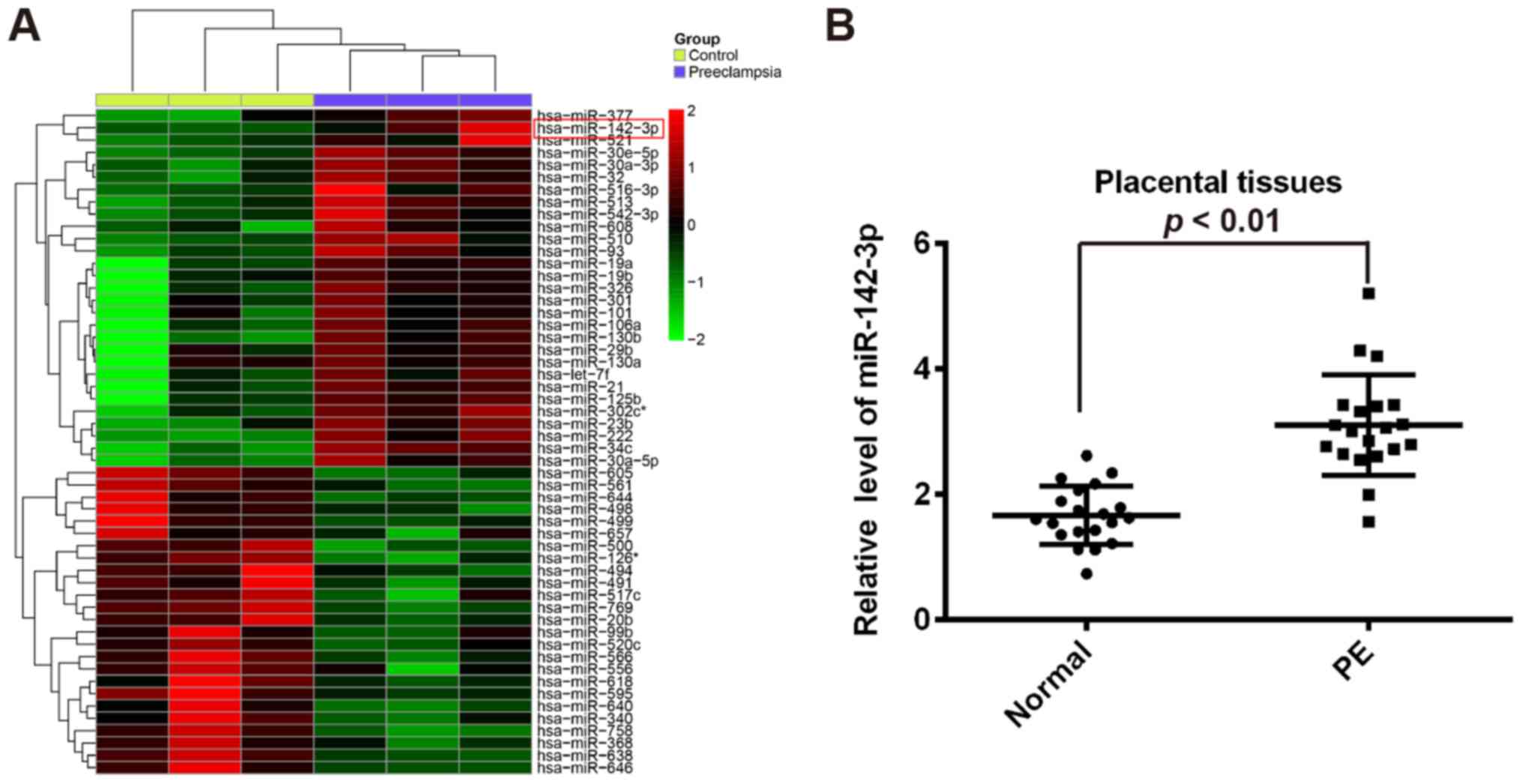
miR-142-3p was upregulated in
placentas from patients with PE
In order to identify the changes in miRNA expression
involved in the pathogenesis of PE, a miRNA microarray with a pool
of miRNAs of frozen placentas from 3 patients with PE and 3 normal
pregnancies was performed. Our data revealed that 29 miRNAs were
upregulated and 25 miRNAs were downregulated in the PE group
(Fig. 1A). Among the aberrantly
expressed miRNAs, miR-142-3p was one of the miRNAs being most
significantly upregulated. In previous studies, miR-142-3p has been
also found to be upregulated in the placenta tissues from PE
patients (17–19). Moreover, miR-142-3p expression
correlated with the invasiveness of different types of human cancer
cells (20–22). Therefore, we chose miR-142-3p for
further research. To validate the results of miRNA microarray, the
miR-142-3p expression in 20 pairs of placentas from patients with
PE and normal pregnancies was determined by RT-qPCR. The results
showed that miR-142-3p expression was significantly upregulated in
placentas compared with normal placentas tissues (Fig. 1B). These results suggested that
miR-142-3p may be involved in the pathogenesis of PE.
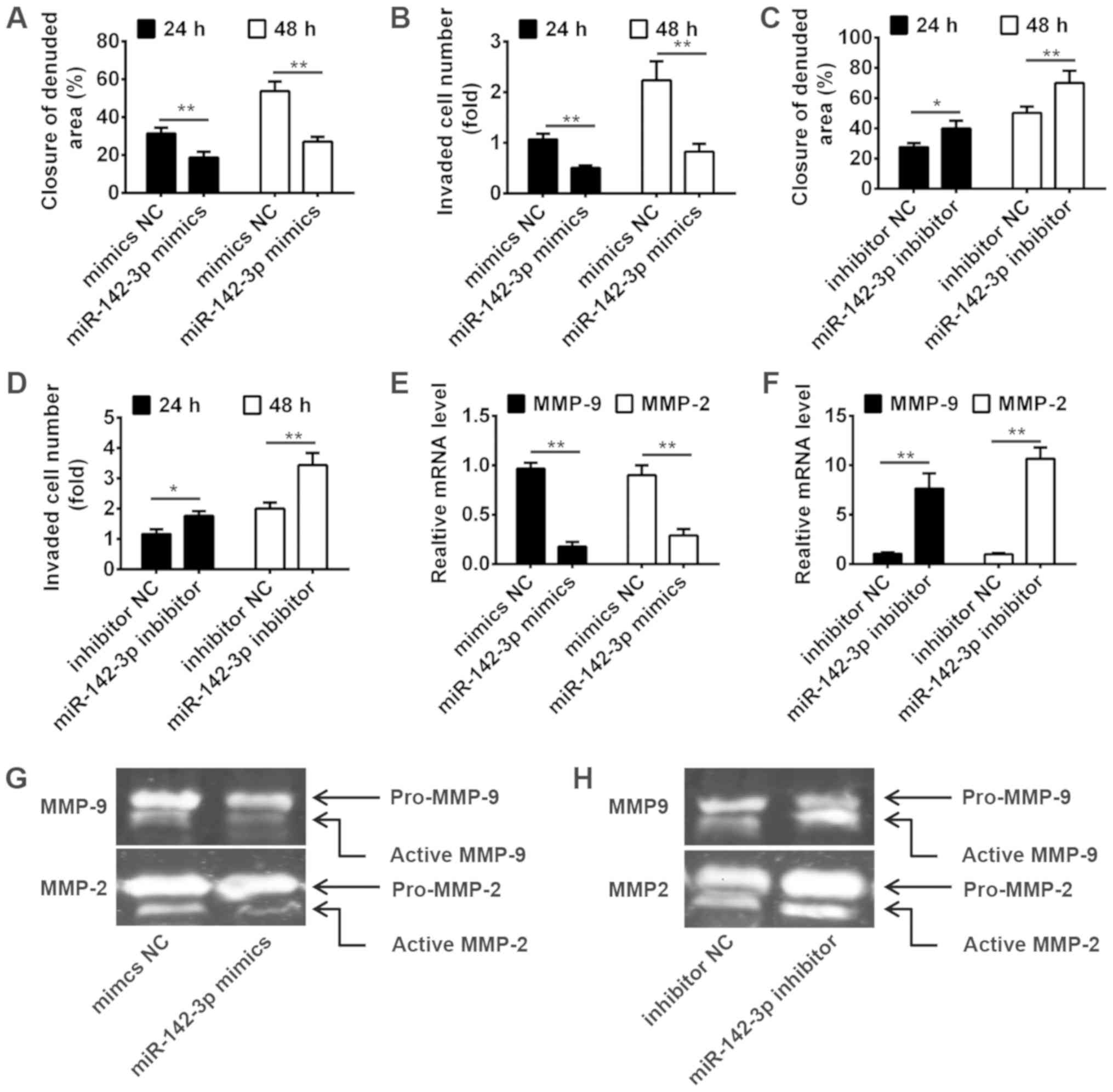
Knockdown of miR-142-3p promotes
invasiveness of trophoblast cells
To investigate the role of miR-142-3p on migration
and invasion of trophoblast cells, HTR-8/SVneo cells derived from
human extravillous trophoblast, which is widely used as in
vitro models for studies of trophoblast invasion (23), were transiently transfected with
miR-142-3p mimics, miR-142-3p inhibitor or negative control
(6), then the invasion and
migration were measured by wound healing assay and transwell
invasion assay at 24 and 48 h. As shown in Fig. 2A and B, miR-142-3p overexpression
significantly suppressed the migration and invasion of HTR-8/SVneo
cells. In contrast, knockdown of miR-142-3p significantly increased
the migration and invasion of HTR-8/SVneo cells (Fig. 2C and D). It is well known that the
MMPs activity plays an important role in trophoblast invasion
(24,25). For this reason, we measured the
effect of miR-142-3p on the mRNA levels of MMP2 and MMP9, which are
closely related to cell invasion. As shown in Fig. 2E and F, miR-142-3p overexpression
significantly inhibited the mRNA expressions of MMP2 and MMP9,
whereas knockdown of miR-142-3p significantly promoted the
expression of MMP2 and MMP9. We further detected the activity of
MMP2 and MMP9 by Gelatin zymography. The results showed that
miR-142-3p overexpression obviously reduced the gelatinolytic
activity of the active form of MMP2 and MMP9, while miR-142-3p
inhibition had an opposite result (Fig. 2G and H). These results indicated
that miR-142-3p can inhibit invasiveness of trophoblast cells via
increased secretion and activity of MMP2 and MMP9.
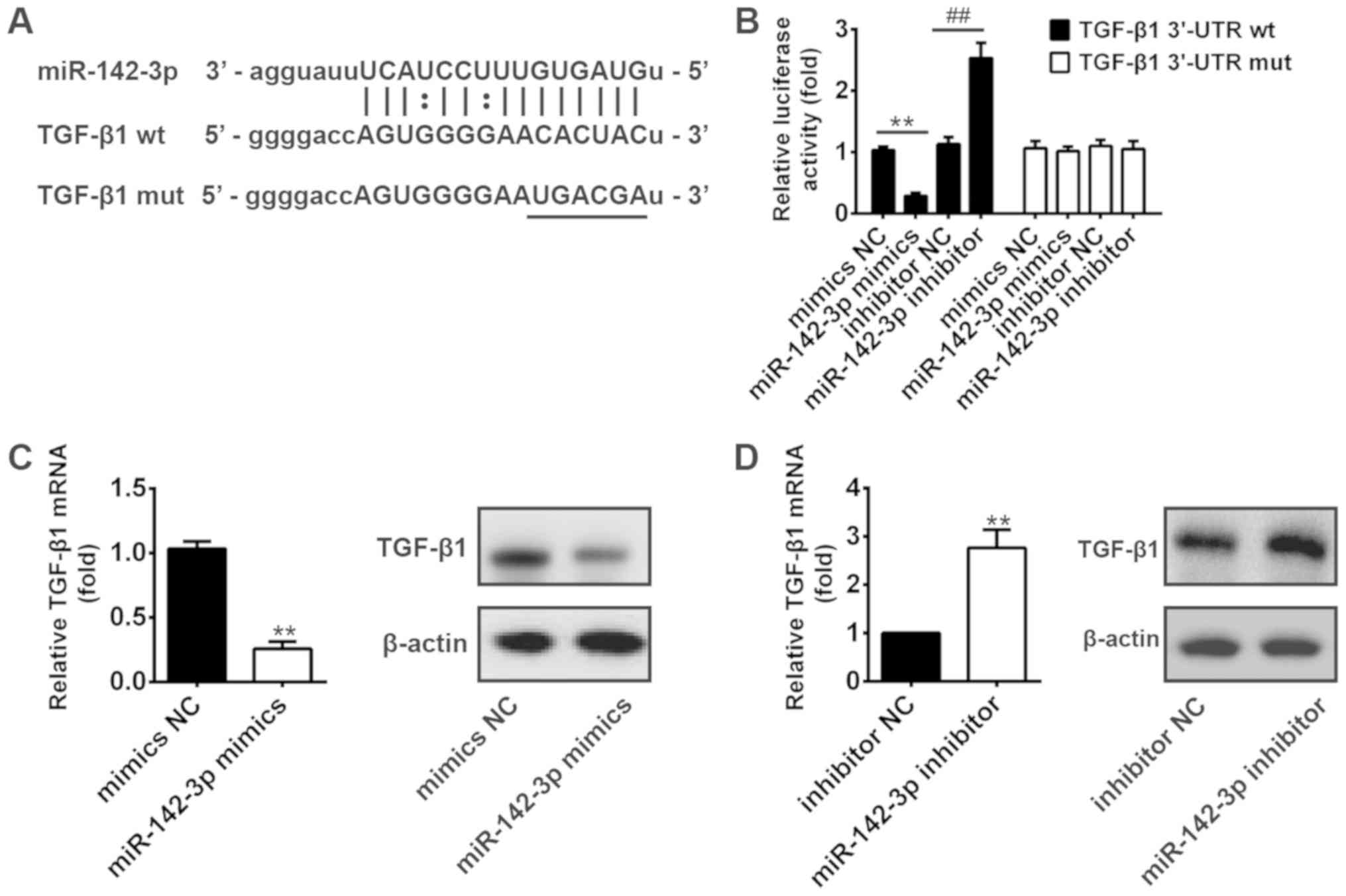
TGF-β1 is a direct target of
miR-142-3p
To further elucidate the underlying molecular
mechanisms by which miR-142-3p functions in HTR8/SVneo cells, we
used the bio-informatic tools (miRBase and TargetScan) to search
for the potential targets. To our interest, TGF-β1, which has been
found to be involved in the invasion of human trophoblast cells
(26–29), could bind to miR-142-3p (Fig. 3A). Thus, TGF-β1 was selected for
further investigation. To experimentally validate whether TGF-β1
was a direct target of miR-142-3p, a dual-luciferase reporter assay
was conducted. The results showed that overexpression of miR-142-3p
significantly decreased the luciferase activity of wt-TGF-β1-3′UTR,
whereas knockdown of miR-142-3p increased luciferase activity.
Likewise, cells co-transfected with miR-142-3p mimics, miR-142-3p
inhibitor, and TGF-β1-mut-3′UTR, showed no obvious change in their
luciferase activity (Fig. 3B). In
addition, we explored whether miR-142-3p could modulate the
expression of TGF-β1. As shown in Fig.
3C and D, the mRNA and protein levels of TGF-β1 was decreased
after overexpression of miR-142-3p, whereas it increased after
inhibition of miR-142-3p. These results suggest that miR-142-3p may
affect the invasion and migration of HTR8/SVneo cells partly at
least, by targeting TGF-β1.
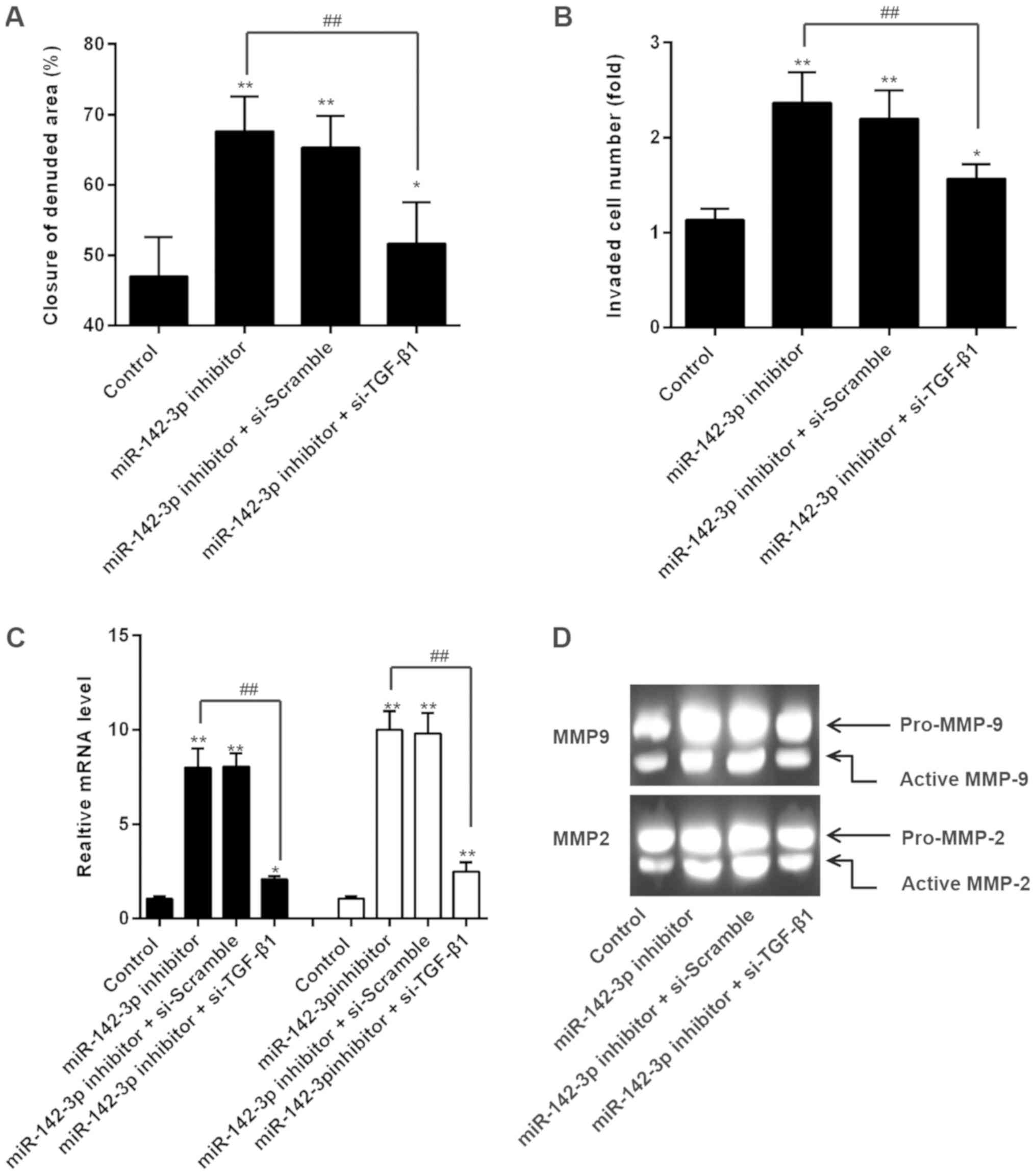
miR-142-3p inhibition promoted
invasiveness of trophoblast cells through the upregulation of
TGF-β1
Based on the findings above, we hypothesized that
miR-142-3p inhibitor might promote trophoblast cells invasion and
migration by upregulating TGF-β1 expression. Firstly, we
co-transfected miR-142-3p inhibitor and TGF-β1 siRNA into
HTR8/SVneo cells, and the invasion and migration were measured. As
shown in Fig. 4A and B, si-TGF-β1
reversed the promotive effects of miR-142-3p on HTR8/SVneo cell
invasion and migration. In addition, the increased mRNA expression
levels of MMP-2 and MMP-9 were markedly reduced when TGF-β1 siRNA
transfection (Fig. 4C). Similarly,
si-TGF-β1 markedly reduced the gelatinolytic activity of the active
form of MMP2 and MMP9 mediated by miR-142-3p inhibition (Fig. 4D). These data indicate that
miR-142-3p inhibitor promoted invasiveness of trophoblast cells
through the upregulation of TGF-β1.
Downregulation of miR-142-3p promoted
invasiveness of trophoblast cells via activation of TGF-β1/Smad3
signaling pathway
Previous studies showed that TGF-β1 stimulated
migration and invasion of various cell types via smad signaling
pathway (30–32). Therefore, we hypothesize that a
similar pathway might be involved in the action of miR-142-3p on
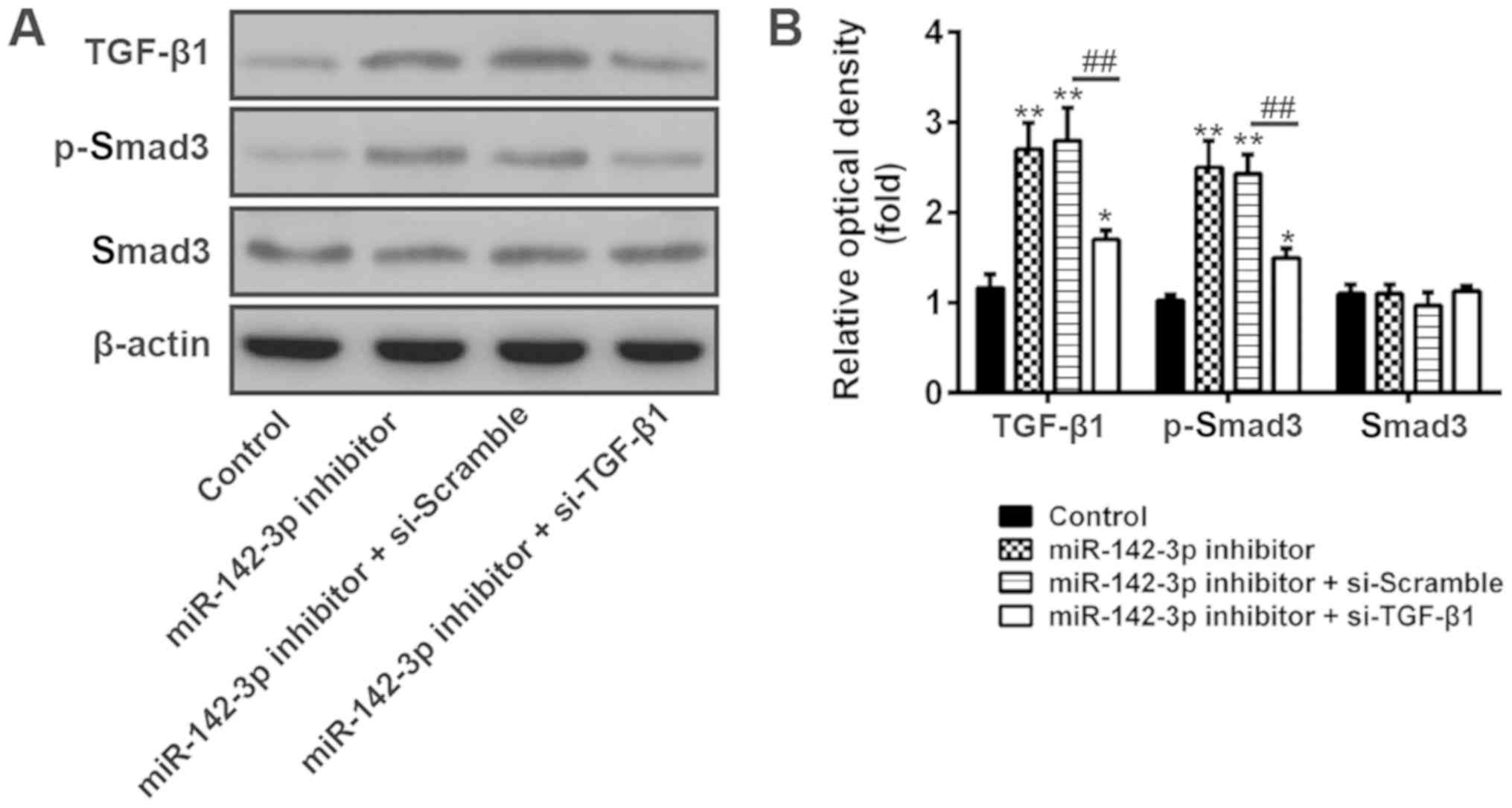
the invasion of trophoblast cells. As indicated in Fig. 5A and B, miR-142-3p inhibitor
promoted the phosphorylation level of smad3 (p-smad3) compared with
control group. In contrast, when si-TGF-β1 was transfected to the
miR-142-3p inhibitor group, the expression of p-smad3 became
significantly lower. In addition, the total protein of smad3 has
not been affected. This result indicates that miR-142-3p inhibitor
promotes the invasion and migration of trophoblast cells via the
TGF-β1/smad3 signaling pathway.
Discussion
In the present study, we found that miR-142-3p was
upregulated in placentas tissues. Our findings have also
demonstrated that miR-142-3p inhibition promoted the invasion and
migration of trophoblast cells by regulating the TGF-β1/Smad3
signaling pathway. Thus, miR-142-3p/TGF-β1/smad3 axis may be a
potential target for the prevention and treatment of PE.
Increasing evidence supports that miRNAs play
important roles in the development and progression of PE (7,33,34).
For example, Tamaru et al (35) found that miR-135b suppressed
HTR-8/SVneo cells invasion by directly down regulating chemokine
(C-X-C motif) ligand 12 (CXCL12) under low oxygen conditions.
Another study from Li et al (36), showed that miR-125b-1-3p could
suppress the invasiveness of trophoblast cells by targeting
sphingosine-1-phosphate receptor 1 (S1PR1) in PE. These inspired us
to investigate whether there may be other miRNAs involved in the
regulation of trophoblastic invasion. In this study, we conducted a
microarray miRNA expression analysis to identify miRNA(s)
associated with PE, and miR-142-3p was selected as one of miRNAs
being most significantly upregulated in placental tissues. Our data
imply miR-142-3p may be associated with the pathogenesis of PE.
miR-142-3p was originally identified as a tumor
suppressor, which played a crucial role in tumor cell proliferation
and invasion (37,38). For example, miR-142-3p was found to
be decreased in cervical cancer cells and overexpression of
miR-142-3p resulted in downregulation of frizzled7 receptor (FZD7)
and inhibited proliferation and invasion in HeLa and SiHa cells
(21). Furthermore, Schwickert
et al (20) found that
miR-142-3p inhibited breast cancer cell invasiveness by synchronous
targeting of wiskott-aldrich syndrome-like (WASL) and Integrin
Alpha V (ITGAV). However, whether miR-142-3p regulates trophoblast
cell function is largely unknown. In the present study, we proved
that the overexpression of miR-142-3p inhibited the invasion and
migration of HTR-8/SVneo cells, whereas the knockdown of miR-142-3p
led to increased invasive and migratory abilities of HTR-8/SVneo
cells. According to previous reports, the MMP family plays a
crucial role in the digestion of the extracellular matrix, MMP2 and
MMP9 are highly expressed in trophoblasts and crucial for
trophoblast invasion (24,25). Wang et al (39) showed that
Benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE) suppresses the
migration and invasion of human extravillous trophoblast
HTR-8/SVneo cells by downregulating MMP2. Deng et al
(40) found that
N-acetylglucosaminyltransferase V (MGAT5) inhibited the invasion of
trophoblast cells by attenuating MMP2/9 activity in early human
pregnancy. However, the significance of MMP2 and MMP9 for
miR-142-3p-mediated invasion of trophoblast cells has not been
elucidated. Here, we found that the mRNA expressions and the
activities of MMP2 and MMP9 was suppressed by overexpression of
miR-142-3p, but increased by miR-142-3p knockdown. These finding
indicate that miR-142-3p could suppress the invasiveness of
trophoblast cells via increased secretion and activity of MMP2 and
MMP9. However, the molecular mechanisms behind the regulation of
miR-142-3p expression and its role in the trophoblast invasion
remain unknown.
Transforming growth factor (TGF)-β1 is a member of
the TGF superfamily, which has been shown to be a multifunctional
cytokine required for embryonic development and regulation of
trophoblast cell behaviors (41).
Increasing evidence demonstrated that TGF-β1 could inhibit
trophoblast cell proliferation and invasion/migration in some of
the transformed trophoblast cell lines, including HRT-8/SVneo,
SGHP-4, and ED-27 (42–44). A recent research from Karmakar
et al (45), showed that
TGF-β1 mediated upregulation of cell-to-cell adhesion with reduced
cell-to-matrix interaction along with an increased ezrin and
E-cadherin expression, is associated with reduced invasiveness of
trophoblast cells. Interestingly, previous study reported that
miRNAs play important roles in the regulation of cancer metastasis
via regulating TGF-β1 directly or indirectly (46,47).
Thus, we speculated that whether the TGF-β1 is involved in
miR-142-3p mediated inhibiting effect on the invasion of
trophoblast cells. In the present study, bioinformatics was used to
predict the target genes that regulate by miR-142-3p and it was
determined that TGF-β1 may be closely associated with miR-142-3p.
Our study also revealed that the TGF-β/Smad3 signaling pathway was
modulated by miR-142-3p. TGF-β/Smad3 signaling pathway play a
crucial role in the invasion of human JEG-3 trophoblast cells
(48). It is well known that the
downstream effects of TGF-β1 are mediated by smads, a family of
intracellular transcription factors (49). We assumed that knockdown of
miR-142-3p can increase expression of TGF-β1, thus promoting smad3
changing to p-smad3 in trophoblast cells. In contrast, si-TGF-β1
reversed the promoting effect of miR-142-3p knockdown on the
activity of TGF-β/Smad3 signaling. Based on these findings, we
suggest that inhibition of miR-142-3p promotes the activation of
TGF-β1/Smad3 signaling, and subsequently enhances the invasion of
trophoblast cells.
In conclusion, we demonstrated that miR-142-3p is
highly expressed in preeclamptic placentas specimens. Furthermore,
our study provides preliminary evidence that inhibition of
miR-142-3p promotes the invasion of trophoblast cells via
TGF-β1/smad3 pathway. Therefore, miR-142-3p/TGF-β1/Smad3 axis may
be developed to be a potential therapeutic target for PE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
EL performed the experiments, contributed to data
analysis and wrote the paper. EL, ZL, YZ, MC, LW and JL analyzed
the data. ZL conceptualized the study design, and contributed to
the data analysis and experimental materials. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of the Tangshan Worker Hospital, He Bei Medical
University (Hebei, China). Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Redman CW: Current topic: Pre-eclampsia
and the placenta. Placenta. 12:301–308. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanasaki K and Kalluri R: The biology of
preeclampsia. Kidney Int. 76:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moffett A and Loke C: Immunology of
placentation in eutherian mammals. Nat Rev Immunol. 6:584–594.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noris M, Perico N and Remuzzi G:
Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol.
1:98–114; quiz 120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher SJ: The placental problem: Linking
abnormal cytotrophoblast differentiation to the maternal symptoms
of preeclampsia. Reprod Biol Endocrinol. 2:532004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang A, Rana S and Karumanchi SA:
Preeclampsia: The role of angiogenic factors in its pathogenesis.
Physiology (Bethesda). 24:147–158. 2009.PubMed/NCBI
|
|
7
|
Yu Y, Wang L, Liu T and Guan H:
MicroRNA-204 suppresses trophoblast-like cell invasion by targeting
matrix metalloproteinase-9. Biochem Biophys Res Commun.
463:285–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moindjie H, Santos ED, Loeuillet L,
Gronier H, de Mazancourt P, Barnea ER, Vialard F and Dieudonne MN:
Preimplantation factor (PIF) promotes human trophoblast invasion.
Biol Reprod. 91:1182014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tu K, Liu Z, Yao B, Han S and Yang W:
MicroRNA-519a promotes tumor growth by targeting PTEN/PI3K/AKT
signaling in hepatocellular carcinoma. Int J Oncol. 48:965–974.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Li P, Hao S, Liu L, Zhao J and Hou
Y: Differential expression of microRNAs in the placentae of Chinese
patients with severe pre-eclampsia. Clin Chem Lab Med. 47:923–929.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu XM, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:661.e1–7. 2009. View Article : Google Scholar
|
|
14
|
Enquobahrie DA, Abetew DF, Sorensen TK,
Willoughby D, Chidambaram K and Williams MA: Placental microRNA
expression in pregnancies complicated by preeclampsia. Am J Obstet
Gynecol. 204:178.e12–21. 2011. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nadeem L, Munir S, Fu G, Dunk C, Baczyk D,
Caniggia I, Lye S and Peng C: Nodal signals through activin
receptor-like kinase 7 to inhibit trophoblast migration and
invasion: Implication in the pathogenesis of preeclampsia. Am J
Pathol. 178:1177–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Li H, Ge Q, Guo L and Chen F:
Deregulated microRNA species in the plasma and placenta of patients
with preeclampsia. Mol Med Rep. 12:527–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Betoni JS, Derr K, Pahl MC, Rogers L,
Muller CL, Packard RE, Carey DJ, Kuivaniemi H and Tromp G: MicroRNA
analysis in placentas from patients with preeclampsia: Comparison
of new and published results. Hypertens Pregnancy. 32:321–339.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song J, Li Y and An RF: Identification of
early-onset preeclampsia-related genes and MicroRNAs by
bioinformatics approaches. Reprod Sci. 22:954–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwickert A, Weghake E, Brüggemann K,
Engbers A, Brinkmann BF, Kemper B, Seggewiß J, Stock C, Ebnet K,
Kiesel L, et al: microRNA miR-142-3p inhibits breast cancer cell
invasiveness by synchronous targeting of WASL, Integrin Alpha V and
additional cytoskeletal elements. PLoS One. 10:e01439932015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng B, Zhang Y, Zhang S, Wen F, Miao Y
and Guo K: MicroRNA-142-3p inhibits cell proliferation and invasion
of cervical cancer cells by targeting FZD7. Tumour Biol.
36:8065–8073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu G, Wang J, Jia Y, Shen F, Han W and
Kang Y: MiR-142-3p functions as a potential tumor suppressor in
human osteosarcoma by targeting HMGA1. Cell Physiol Biochem.
33:1329–1339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mandl M, Haas J, Bischof P, Nöhammer G and
Desoye G: Serum-dependent effects of IGF-I and insulin on
proliferation and invasion of human first trimester trophoblast
cell models. Histochem Cell Biol. 117:391–399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Staun-Ram E, Goldman S, Gabarin D and
Shalev E: Expression and importance of matrix metalloproteinase 2
and 9 (MMP-2 and −9) in human trophoblast invasion. Reprod Biol
Endocrinol. 2:592004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu P, Alfaidy N and Challis JR: Expression
of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and
fetal membranes in relation to preterm and term labor. J Clin
Endocrinol Metab. 87:1353–1361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhu H, Klausen C, Peng B and Leung
PC: Vascular endothelial growth factor-A (VEGF-A) mediates activin
A-induced human trophoblast endothelial-like tube formation.
Endocrinology. 156:4257–4268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belkacemi L, Lash GE, Macdonald-Goodfellow
SK, Caldwell JD and Graham CH: Inhibition of human trophoblast
invasiveness by high glucose concentrations. J Clin Endocrinol
Metab. 90:4846–4851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fitzgerald JS, Germeyer A, Huppertz B,
Jeschke U, Knöfler M, Moser G, Scholz C, Sonderegger S, Toth B and
Markert UR: Governing the invasive trophoblast: Current aspects on
intra- and extracellular regulation. Am J Reprod Immunol.
63:492–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knöfler M: Critical growth factors and
signalling pathways controlling human trophoblast invasion. Int J
Dev Biol. 54:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong S, Cheng JC, Klausen C, Zhao J and
Leung PC: TGF-β1 stimulates migration of type II endometrial cancer
cells by down-regulating PTEN via activation of SMAD and ERK1/2
signaling pathways. Oncotarget. 7:61262–61272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ungefroren H, Sebens S, Giehl K, Helm O,
Groth S, Fändrich F, Röcken C, Sipos B, Lehnert H and Gieseler F:
Rac1b negatively regulates TGF-β1-induced cell motility in
pancreatic ductal epithelial cells by suppressing Smad signalling.
Oncotarget. 5:277–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo L, Ye G, Nadeem L, Fu G, Yang BB,
Honarparvar E, Dunk C, Lye S and Peng C: MicroRNA-378a-5p promotes
trophoblast cell survival, migration and invasion by targeting
Nodal. J Cell Sci. 125:3124–3132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai Y, Yang W, Yang HX, Liao Q, Ye G, Fu
G, Ji L, Xu P, Wang H, Li YX, et al: Downregulated miR-195 detected
in preeclamptic placenta affects trophoblast cell invasion via
modulating ActRIIA expression. PLoS One. 7:e388752012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tamaru S, Mizuno Y, Tochigi H, Kajihara T,
Okazaki Y, Okagaki R, Kamei Y, Ishihara O and Itakura A:
MicroRNA-135b suppresses extravillous trophoblast-derived
HTR-8/SVneo cell invasion by directly down regulating CXCL12 under
low oxygen conditions. Biochem Biophys Res Commun. 461:421–426.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Q, Pan Z, Wang X, Gao Z, Ren C and Yang
W: miR-125b-1-3p inhibits trophoblast cell invasion by targeting
sphingosine-1-phosphate receptor 1 in preeclampsia. Biochem Biophys
Res Commun. 453:57–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12:2792014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chai S, Tong M, Ng KY, Kwan PS, Chan YP,
Fung TM, Lee TK, Wong N, Xie D, Yuan YF, et al: Regulatory role of
miR-142-3p on the functional hepatic cancer stem cell marker CD133.
Oncotarget. 5:5725–5735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang R, Wang W, Ao L, Wang Z, Hao X and
Zhang H: Benzo[a]pyrene-7,8-diol-9,10-epoxide suppresses the
migration and invasion of human extravillous trophoblast
HTR-8/SVneo cells by down-regulating MMP2 through inhibition of
FAK/SRC/PI3K/AKT pathway. Toxicology. 386:72–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng Q, Chen Y, Yin N, Shan N, Luo X, Tong
C, Zhang H, Baker PN, Liu X and Qi H:
N-acetylglucosaminyltransferase V inhibits the invasion of
trophoblast cells by attenuating MMP2/9 activity in early human
pregnancy. Placenta. 36:1291–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ayatollahi M, Geramizadeh B, Yazdani M and
Azarpira N: Effect of the immunoregulatory cytokines on successful
pregnancy depends upon the control of graft rejection mechanisms.
Transplant Proc. 39:244–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo S, Yu H, Wu D and Peng C: Transforming
growth factor-beta1 inhibits steroidogenesis in human trophoblast
cells. Mol Hum Reprod. 8:318–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma Y, Ryu JS, Dulay A, Segal M and Guller
S: Regulation of plasminogen activator inhibitor (PAI)-1 expression
in a human trophoblast cell line by glucocorticoid (GC) and
transforming growth factor (TGF)-beta. Placenta. 23:727–734. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tse WK, Whitley GS and Cartwright JE:
Transforming growth factor-beta1 regulates hepatocyte growth
factor-induced trophoblast motility and invasion. Placenta.
23:699–705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karmakar S and Das C: Regulation of
trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod
Immunol. 48:210–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Q, Cheng Q, Chen Z, Peng R, Chen R, Ma
Z, Wan X, Liu J, Meng M, Peng Z and Jiang B: MicroRNA-663 inhibits
the proliferation, migration and invasion of glioblastoma cells via
targeting TGF-β1. Oncol Rep. 35:1125–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu ZY, Zhang GL, Wang MM, Xiong YN and
Cui HQ: MicroRNA-663 targets TGFB1 and regulates lung cancer
proliferation. Asian Pac J Cancer Prev. 12:2819–2823.
2011.PubMed/NCBI
|
|
48
|
Huang Z, Li S, Fan W and Ma Q:
Transforming growth factor β1 promotes invasion of human JEG-3
trophoblast cells via TGF-β/Smad3 signaling pathway. Oncotarget.
8:33560–33570. 2017.PubMed/NCBI
|
|
49
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|