Introduction
Chemerin is a novel 16-kDa adipokine that is
implicated in the regulation of innate and adaptive immunity,
adipocyte differentiation and metabolism (1,2). It
can act as a chemoattractant agent promoting the recruitment of
immune cells to lymphoid organs and sites of tissue damage
(1). Knockdown of chemerin
expression impaired differentiation of 3T3-L1 cells into
adipocytes, reduces the expression of genes involved in glucose and
lipid homeostasis, and alters metabolic functions in mature
adipocytes (2). It is expressed in
numerous types of tissues, including white adipose tissue, liver,
and lung (3,4), and it is secreted as an inactive
prochemerin that, following activation, binds to the chemerin
chemokine-like receptor 1 (CMKLR1) to exert its biological
functions (5). CMKLR1 is coupled G
proteins, and the interaction between chemerin and CMKLR1 inhibits
cAMP production and promotes phospholipase C activation, IP3
release, and activation of PI3K and mitogen-activated protein
kinase pathways (6). It has been
demonstrated that the genetic knockdown of chemerin or CMKLR1 in
preadipocytes results in the downregulation of genes controlling
glucose and lipid metabolism (2),
and chemerin can induce insulin resistance (IR) in cardiomyocytes
by modulating the ERK1/2 pathway (7). Furthermore, chemerin reportedly
promotes insulin signaling in 3T3-L1 adipocytes and enhances
glucose uptake (8) and circulating
chemerin is associated with inflammation and metabolic syndrome
(9,10). The baseline levels of chemerin in
Type 2 diabetes mellitus (T2DM) group were significantly higher
compared with the normal control group (10). However, the role of chemerin in
regulating IR remains unclear.
DM is a metabolic disease characterized by the
presence of chronic hyperglycemia (11). The diagnosis of DM is based on the
glucose criteria including fasting plasma glucose levels,
postprandial plasma glucose levels and hemoglobin A1C (12). The mechanisms underlying different
types of DM include impaired insulin secretion, IR, or a
combination of the two (13). Type
1 DM is associated with absolute insulin deficiency. T2DM is
characterized by insulin resistance and relative insulin deficiency
(12). Pancreatogenic DM (PDM) is
recognized as DM occurring secondary to chronic pancreatitis or
pancreatic resection due to the loss of the loss of islet cell
mass. A retrospective study enrolling nearly 1,900 patients
indicated that PDM accounted for approximately 9% of all diabetics
(14). Exocrine pancreatic
diseases underlying PDM include benign and malign conditions such
as acute or chronic pancreatitis of any etiology, cystic fibrosis,
fibrocalculous pancreatopathy, hemochromatosis, pancreatectomy,
pancreatic agenesis and pancreatic cancer (15). However, the relationship between
chemerin and IR in PDM remains to be investigated. The aim of the
present study was to determine the association between chemerin
levels and PDM in patients and a mouse PDM model; which was
characterized as the simultaneous presence of impaired glucose
tolerance and IR. In addition, the efficacy of the CMKLR1 agonist,
chemerin-9, in alleviating the impaired glucose tolerance and IR
was investigated in the PDM mouse model.
Materials and methods
Patient studies
Patients with T2DM (n=110) or PDM (n=113) were
recruited between January 2016 and December 2019 in the Department
of Endocrinology and the Center for Severe Acute Pancreatitis (SAP)
at the Jinling Hospital, Medical school of Nanjing University.
Blood samples from healthy populations, which are difficult to
collect, were not included since the aim of this study was to
investigate the role of chemerin-9 in DM. T2DM patients were 69
male and 41 female with a mean age of 48.6±2.1 years. PDM patients
were 82 male and 31 female with a mean age of 45.3±1.8 years. The
diagnosis of T2DM or PDM was verified according to the Expert
Committee on Diagnosis and Classification of Diabetes Mellitus
(12). The underlying cause of PDM
was either acute or chronic pancreatitis. To evaluate the
association between chemerin levels and IR status, the patients
with T2DM or PDM were further divided into two groups, with
(IR>1) and without (IR≤1) IR, and serum samples (10 ml) were
collected for subsequent ELISA analysis. Written informed consent
for the use of serum samples was obtained from all patients
enrolled in this study. The study was approved by The Ethics
Committee of the Jinling Hospital (Nanjing, China).
ELISA assay
Serum levels of chemerin (ml063020 for mice,
ml058526 for human) and serum glucose (ml057865 for mice, ml063205
for human) and insulin (DCM076-8) were analyzed using ELISA kit
from Shanghai Enzyme-linked Biotechnology Co., Ltd Serum levels of
IL-1 (SEA057Mu) and TNF-α (SEA133Mu) were analyzed ELISA kits from
Cloud-Clone Corp.
Animal studies
C57BJ/6J mice (age, 8 weeks; weight, 18–22 g;
female; n=24) obtained from the Experimental Animal Institute of
Jinling Hospital were used to establish a model of PDM. Mice were
housed under a 12-h light/dark cycle at 23±1°C with a relative
humidity of 50±5%. Mice in the PDM model group (n=8) were injected
peritoneally with arginine (16)
(350 µg/g/day) for 42 days. Mice in PDM group or the control diet
group (n=8) were fed with a normal diet for 42 days. Mice in the
high-fat diet group (n=8) to model T2DM were fed with a high-fat
diet, for 42 days; the composition of the control diet and high-fat
diet is listed in Table I. The
health and behavior of mice were checked every 3 days. Mice were
anesthetized with an intraperitoneal injection of chloral hydrate
(250 mg/kg) prior to collecting blood (200 µl/mouse) through the
tail vein at day 0 and at 21 and 42 days post-modeling to measure
fasting serum glucose (FPG) and postprandial serum glucose (PPG),
the levels of chemerin, interleukin (IL)-1, and tumor necrosis
factor (TNF)-α, as well as the homeostatic model assessment of
insulin resistance (HOMA-IR). Following blood collection at 42
days, the mice were euthanized by 100% CO2 inhalation
administered at 30% volume/minute. Death was verified by cervical
dislocation and the end-point weight of the mice was 25–27 g.
Subsequently, pancreatic head tissue was collected for RNA
extraction and hematoxylin and eosin (H&E) staining.
 | Table I.Composition of the normal (control)
and high-fat diet for C57BL/6 mice. |
Table I.
Composition of the normal (control)
and high-fat diet for C57BL/6 mice.
| Composition | Normal food (%) | High-fat food
(%) |
|---|
| Starch | 52.4 | 0 |
| Sucrose |
4.9 | 45.0 |
| Protein | 18.9 | 23.0 |
| Fat |
6.0 | 20.0 |
| Cellulose |
3.8 |
5.0 |
| Vitamins |
5.8 |
1.5 |
| Minerals |
8.2 |
5.5 |
To evaluate the impact of the CMKLR1 agonist,
chemerin-9, on PDM, peritoneal injections of phosphate buffer
solution (PBS, 200 µl, Control group, n=8) or chemerin-9 (4 µg in
200 µl PBS, n=8) or were performed every day for 42 days, as
previously described (17). Blood
samples were collected at day 0 and 42. The in vivo animal
experimental protocols were approved by the Ethics Committee of
Jinling Hospital (Nanjing, China).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from pancreatic head tissue samples (40
mg) was extracted using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. qPCR was subsequently performed using the SYBR Green PCR
Master mix including reverse transcriptase, buffer, dNTPs (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Thermal cycling of RT-qPCR was set as:
95°C for 5 min, 95°C for 10 sec and 60°C for 20 sec, repeated for
40 cycles. Primers were obtained from Tiangen Biotech Company. The
following primer pairs were used for qPCR: PDX1 forward,
5′-GCGAGATGCTGGCAGACCTCT-3′ and reverse,
5′-GGCAGACCTGGCGGTTCACAT-3′; GLUT2 forward,
5′-CAATTTCATCATCGCCCTCT-3′ and reverse, 5′-TGCAGCAATTTCGTCAAAAG-3′;
and β-actin forward, 5′-TCACTGAGGATGAGGTGGAAC-3′ and reverse,
5′-TCAGTCGCTCCAGGTCTTCACG-3′. The 2−ΔΔCq method was used
to analyze the relative expression of mRNAs (18). β-actin was used as an internal
reference control. The relative levels of mRNAs was normalized to
the internal reference gene β-actin.
H&E staining
The pancreatic tissues were fixed in 10% formalin
solution for 24 h at room temperature, and were processed by
routine histological tissue preparation. All specimens were
embedded in wax and sectioned at 4 µm. The sections were stained
with hematoxylin (0.5%, 5 mins at room temperature) and eosin
(0.5%, 1 min at room temperature) for pathological histological
examination.
FPG and insulin levels, postprandial
glucose and homeostatic model assessment of insulin resistance
(HOMA-IR) calculation
Glucose and insulin levels were measured in the mice
on day 21 and 42 following fasting for 16 h. For postprandial
glucose, fasting mice were injected with 1–1.5 mg/g of glucose and
blood samples were collected after 120 min. The concentrations of
serum glucose and insulin were determined using ELISA assays.
Following obtaining the fasting glucose and insulin levels, the
HOMA-IR was calculated according to the following formula:
HOMA-IR=[fasting glucose (nmol/l) × fasting insulin
(µU/l)]/22.5.
Statistical analysis
Statistical analyses were performed using the SPSS
version 22.0 (IBM Corp.) and GraphPad Prism version 5.0 (GraphPad
Software, Inc.) software. Quantitative data are presented as the
mean ± SEM with at least three independent repeats. Statistical
significance between groups was determined using the Student's
t-test or one-way ANOVA with post hoc Tukey HSD (Honestly
Significant Difference) Test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Chemerin levels are low in the serum
of patients with PDM and is negatively associated with the IR
status of DM patients
The role of chemerin in IR in patients with T2DM or
PDM was explored by determining whether an association between the
level of chemerin and IR status is present in these subjects.
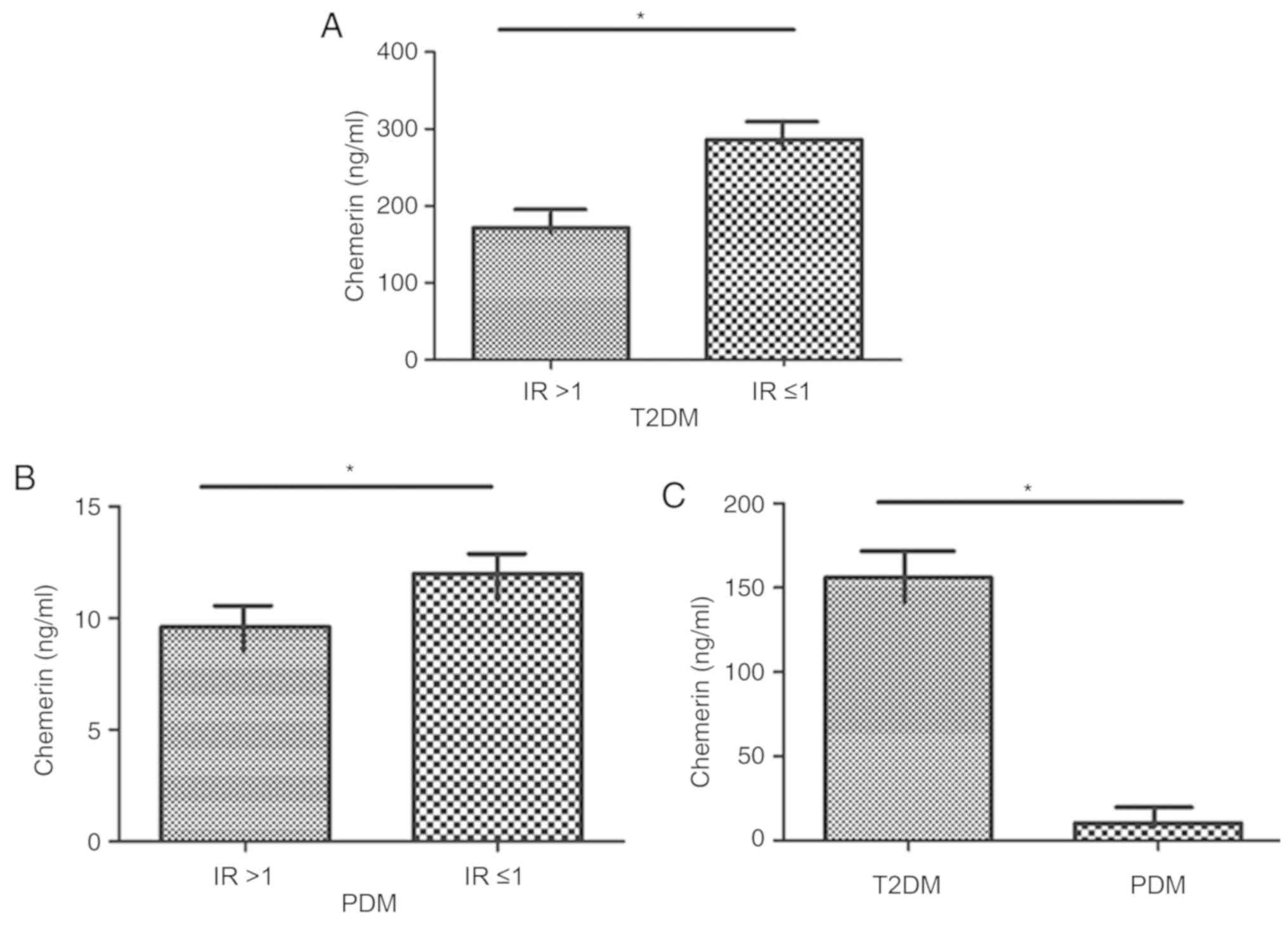
Compared with patients with T2DM with an IR≤1, patients with an
IR>1 exhibited a significantly decreased level of chemerin
(P<0.05; Fig. 1A). Patients
with PDM with an IR>1 had a significantly lower level of
chemerin compared with those with IR ≤1 (P<0.05; Fig. 1B). The level of chemerin in
patients with PDM was significantly lower compared with levels in
patients with T2DM (P<0.05; Fig.
1C). Together, these data suggested a close association of
chemerin levels and IR in patients with DM (T1/2), and suggested
that chemerin may serve a crucial role in the pathogenesis of PDM,
which is characterized by impaired glucose tolerance and IR.
Successful establishment of a mouse
PDM model
A mouse model of PDM was generated to further
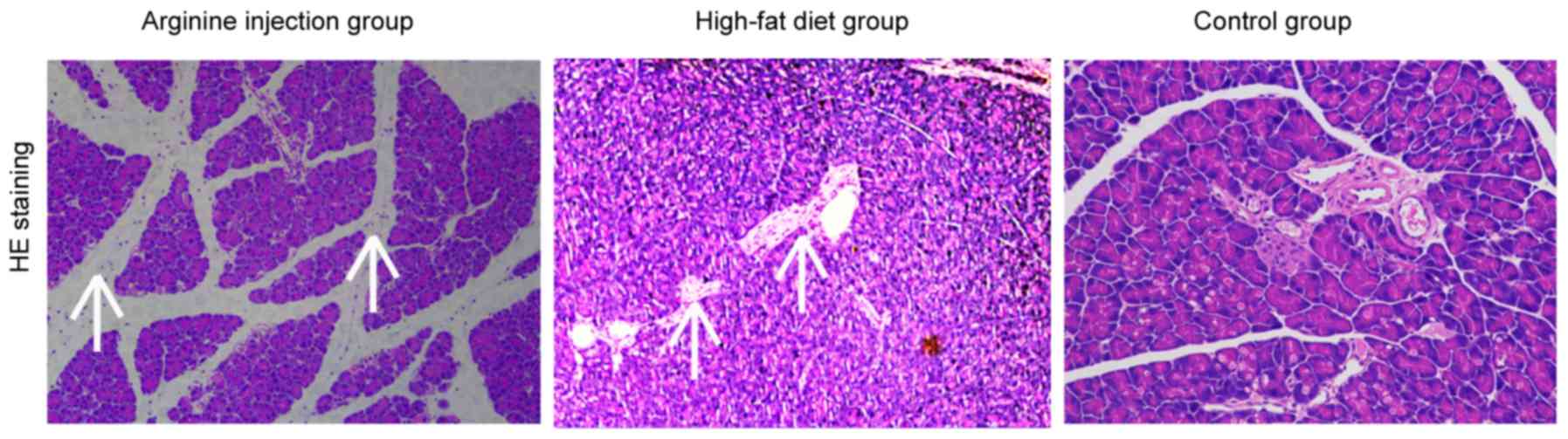
evaluate the role between chemerin and IR. Subsequent H&E
staining revealed that compared with mice fed the high-fat diet or
the control group, mice in the arginine group exhibited a focal
enlargement of the interlobular septum in the pancreatic head and a
minor increase in the number of white blood cells in, or around,
the pancreatic lobules (data not shown). Compared with control
group, the high-fat diet group showed pancreatic alveolar atrophy
and interstitial fibrosis. Necrosis of glandular cells and bleeding
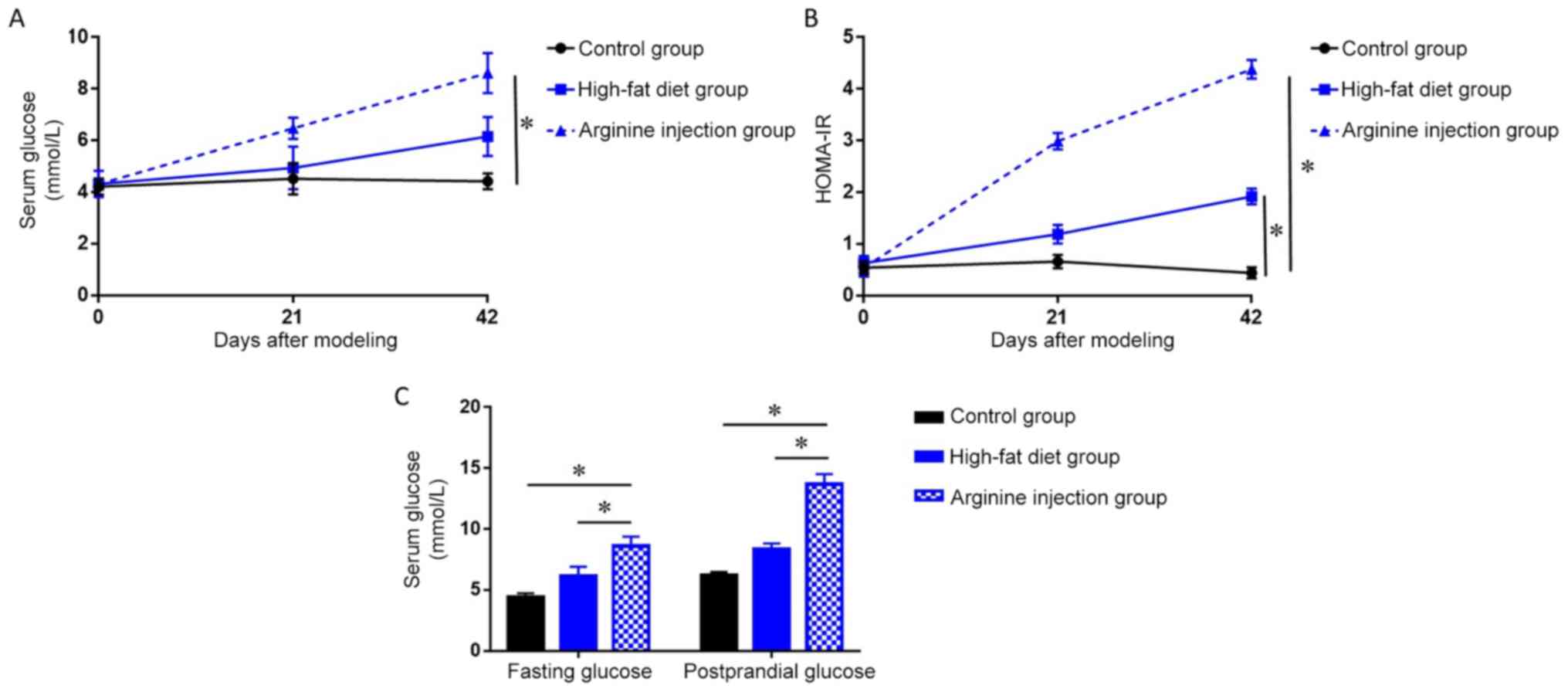
were also absent in the pancreatic head region (Fig. 2). PDM mice had significantly higher
fasting blood glucose levels and HOMA-IR at 42 days post-modeling
compared to the high-fat diet and control group (P<0.05;
Fig. 3A and B). Notably, following
days, PDM model mice demonstrated a significant increase in the
levels of FPG and PPG (P<0.05; Fig.
3C) compared with the high-fat diet or control group. Together,
these data indicated that arginine injection for 42 days
successfully induced PDM in mice, resulting in higher FPG levels,
impaired glucose tolerance, and enhanced IR.
Chemerin levels decrease in PDM
mice
The levels of circulating chemerin, IL-1, and TNF-α
in PDM mice, high-fat diet mice and the control group were measured
by ELISA. PDM mice exhibited significantly reduced levels of
chemerin at 42 days compared with mice fed a high-fat or control
diet (P<0.05; Fig. 4A), in
addition to significantly elevated levels of IL-1 and TNF-α
compared with the high-fat diet and control group at 42 days (both
P<0.05; Fig. 4B and C).
Compared with control group, mice in high-fat diet group also
showed decreased level of chemerin, as well as increased level of
IL-1 and TNF-a (P<0.05, Fig.
4A-C). Subsequent RT-qPCR analysis of GLUT2 and PDX1 mRNA
expression levels in the pancreatic head tissues collected from
each group revealed that PDM mice exhibited significantly lower
levels of both genes compared with mice in the high-fat diet and
control group (P<0.05; Fig. 4D and
E). These results indicated chemerin may exert protective role
in the pathogenic process of PDM.
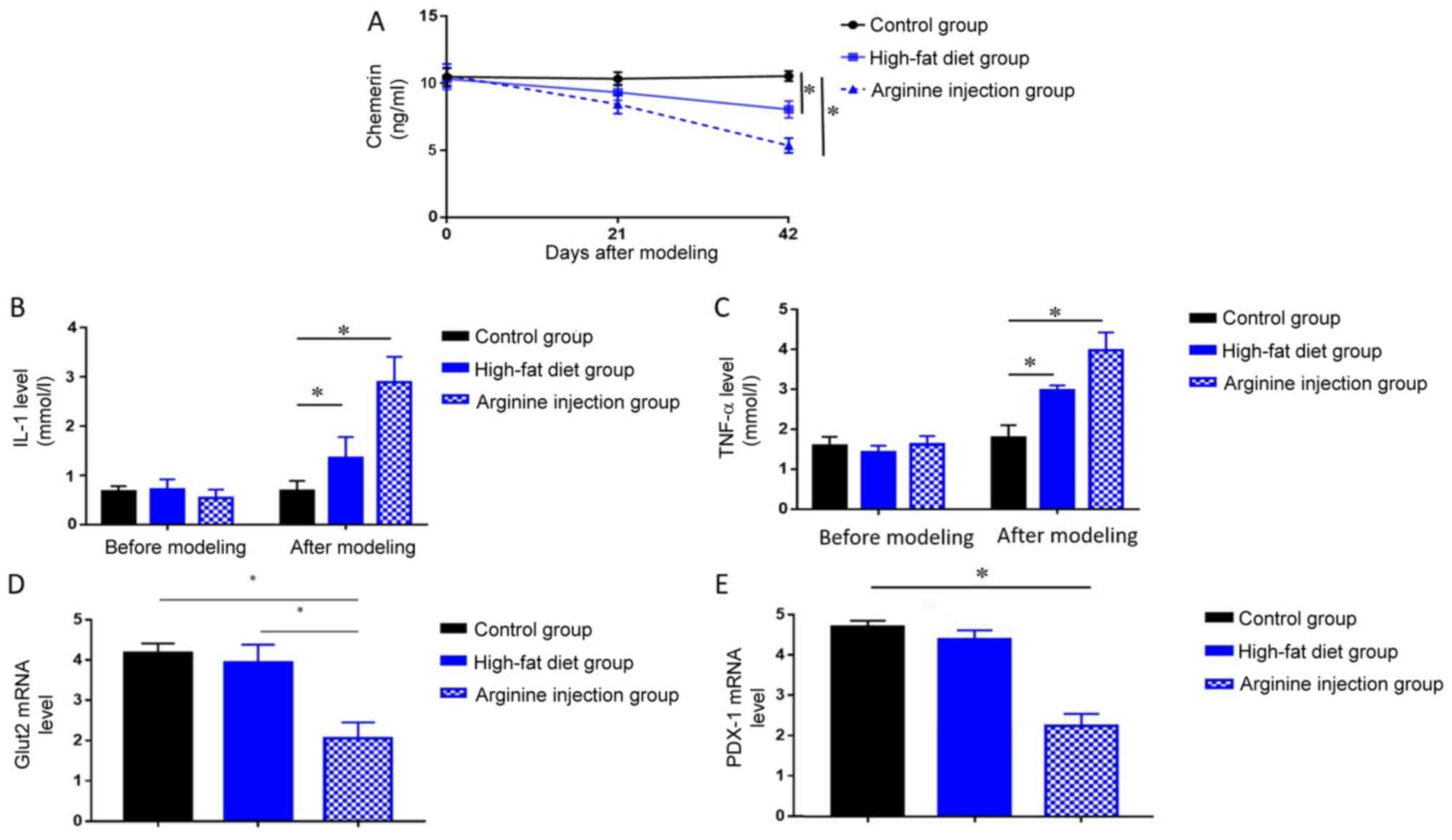 | Figure 4.Levels of chemerin, IL-1, TNF-α,
GLUT2, and PDX1 in mice. Changes in serum levels of (A) chemerin,
(B) IL-1, (C) TNF-α were detected in the PDM, high-fat diet and
control groups after modeling at 42 days using ELISA. Expression
levels of (D) GLUT2 or (E) PDX1 were detected at 42 days in the PDM
group, high-fat diet or control group by reverse
transcription-quantitative PCR. *P<0.05. GLUT2, glucose
transporter 2; IL, interleukin; PDX1, pancreatic and duodenal
homeobox; PDM, pancreatogenic diabetes mellitus; TNF, tumor
necrosis factor. |
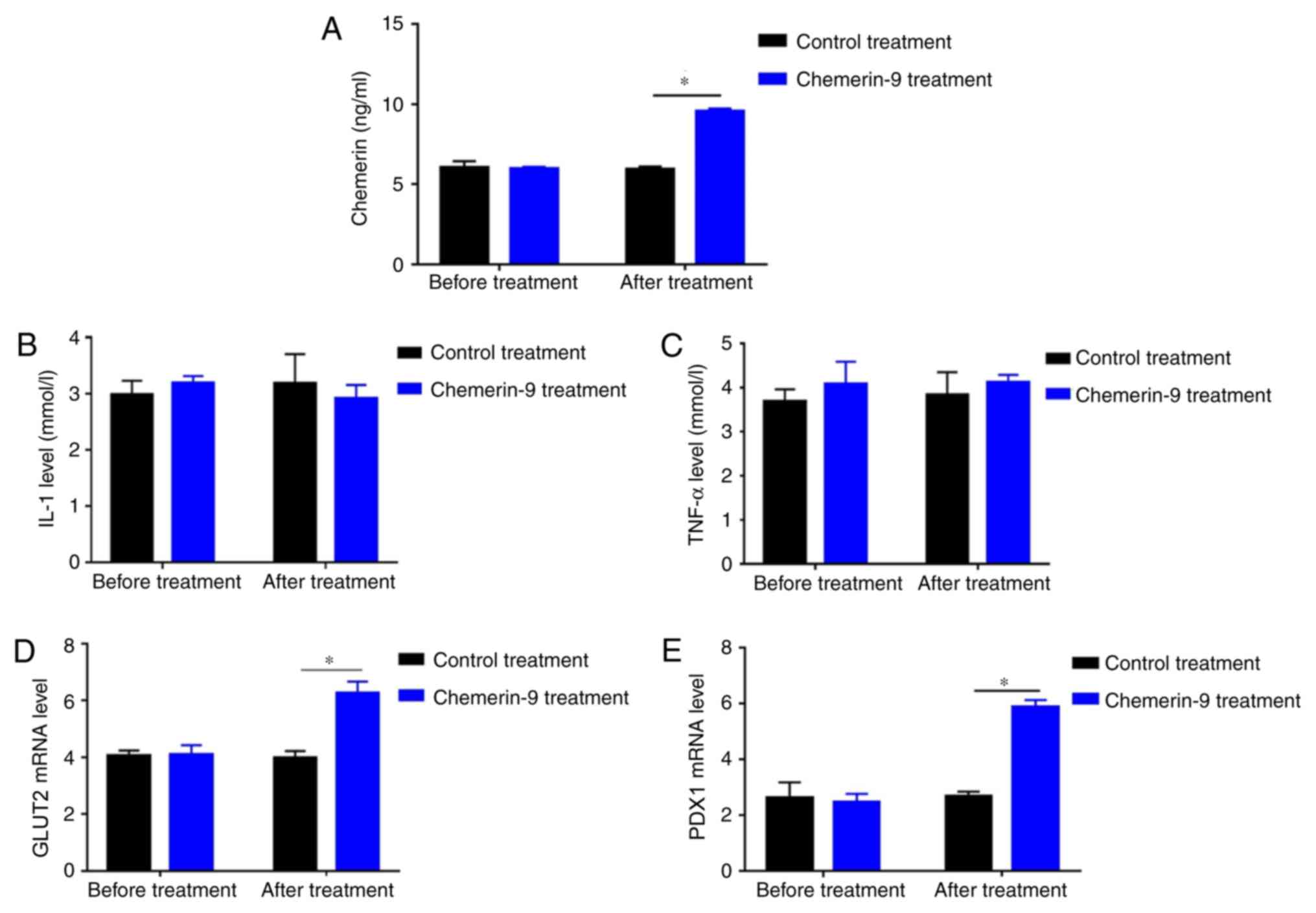
CMKLR1 agonist chemerin-9 alleviates
glucose intolerance and IR in PDM mice
To further clarify the role of chemerin in PDM, the
model mice were treated with chemerin-9, a classical agonist of
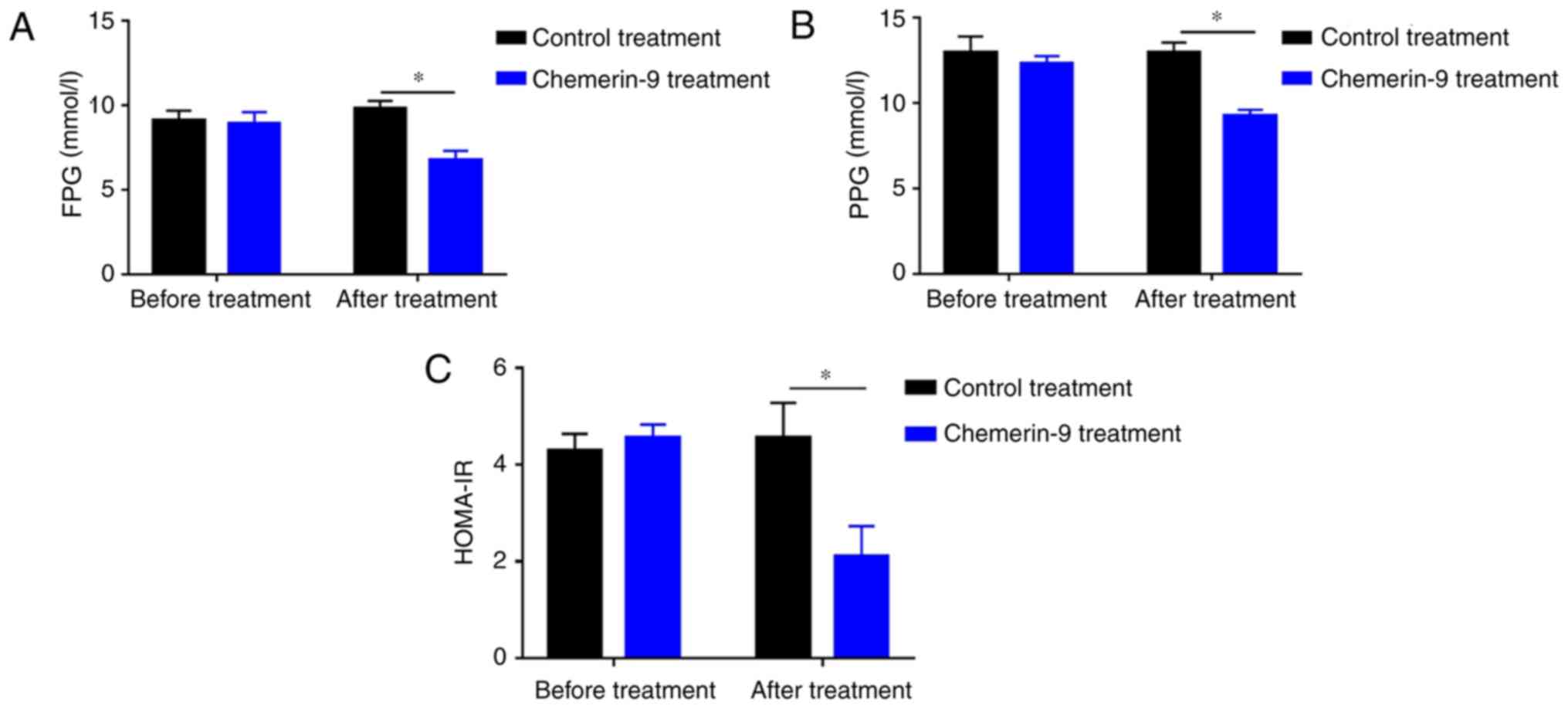
CMKLR1. Before treatment, mice in control and chemerin-9 group
showed similar level in fasting glucose, postprandial glucose and
HOMA-IR (Fig. 5A-C). The treatment
significantly decreased the FPG levels, PPG levels and HOMA-IR
compared with the mice in the control group (all P<0.05;
Fig. 5A-C). In addition,
chemerin-9 treatment significantly increased the levels of chemerin
compared with the control treatment group (P<0.05; Fig. 6A). No measurable change was
reported in the serum levels of IL-1 or TNF-α between the
chemerin-9 treatment and the control before or after treatment
(Fig. 6B and C). In addition, the
mRNA expression levels of GLUT2 and PDX1 were significantly
increased following chemerin-9 treatment PDM mice compared with the
control treatment (both P<0.05; Fig. 6D and E). These results indicate
targeting chemerin may represent a novel therapeutic strategy for
PDM.
Discussion
Chemerin, a novel adipokine, regulates innate and
adaptive immunity, adipocyte differentiation and metabolism
(9). The role of chemerin in T2DM
and IR has gained increasing attention (19,20).
Chemerin levels were found to be markedly increased in patients
with T2DM with hypertension compared with patients with T2DM and
normal controls (19). In
gestational DM, chemerin significantly and positively correlated
with HOMA-IR (20). One previous
study reported that chemerin levels in T2DM were significantly
higher compared with the expression in the control group, and the
level of chemerin positively correlated with HOMA-IR (10). However, another study of T2DM
indicated that chemerin levels were not significantly different
between subjects with T2DM and normal controls (9). These indicate that the role of
chemerin in DM remains controversial. In addition, the function of
chemerin in PDM remains unknown. The present study demonstrated
that serum levels of chemerin in patients with PDM were
significantly lower compared with patients with T2DM, and chemerin
levels were negatively associated with the HOMA-IR status of
patients with T2DM or PDM. These findings indicated that the
function of chemerin, and its underlying mechanisms, may be
different in PDM compared with T2DM, and it may affect the
pathogenesis of PDM by serving a protective role through
alleviating IR, thus, revealing a potential novel molecular
mechanism and therapeutic strategy for PDM. It is worth mentioning
here that the data from the present study differs from that in T2DM
and gestational DM (10,20), indicating that the molecular
mechanism underlying different types of DM is different.
To further validate the role of chemerin in PDM, a
mouse model of the disease was established using the arginine
injection method (16). Following
42 days of arginine administration, the treated mice exhibited mild
inflammatory changes in the pancreas, whereas the animals in the
control and high-fat groups failed to exhibit this pathological
feature. The inflammatory changes were not so clear in the high-fat
group. Notably, arginine injections resulted in an increased
concentration of FPG, PPG, and HOMA-IR, demonstrating the
successful establishment of the mouse model of PDM (21,22).
The levels of chemerin were significantly decreased in PDM mice,
which was consistent with the patient data. The administration of
chemerin-9, a classical agonist of CMKLR1, resulted in increased
levels of chemerin in the PDM mice, decreased the concentrations of
FPG and PPG, and alleviated the IR of these animals. Thus,
increasing the level of chemerin may represent a novel therapeutic
strategy for PDM. However, the molecular mechanism underlying these
biological functions of chemerin in PDM remains to be
elucidated.
In the present study, the concentrations of two
inflammatory mediators, IL-1 and TNF-α, were found to be increased
during PDM development. These data were consistent with previous
studies reporting that elevated TNF-α, IL-1β, and IL-6 in type 1 DM
and T2DM (23,24) and indicated that chronic
inflammation may be involved in PDM and may be a common underlying
mechanism for different types of DM. However, the administration of
chemerin-9 did not have an effect on the level of IL-1 and TNF-α,
which suggested that chronic inflammation may not be mediating the
therapeutic effect of chermin-9 in PDM.
GLUT2, the major mediator of glucose uptake by
hepatocytes and pancreatic β-cells is decreased in patients with DM
(25–27). The present study demonstrated that
PDM mice had decreased levels of GLUT2 mRNA, which led to the
impaired uptake of glucose and secretion of insulin. Upon
administration of chemerin-9, GLUT2 expression was increased, which
was accompanied by decreased IR in the PDM mice. Thus, decreased
GLUT2 levels may be implicated in the pathogenesis of PDM, and
chemerin-9 may alleviate the IR associated with PDM by increasing
the expression of GLUT2 (28).
PDX1 is a crucial transcription factor regulating
the transcription of the insulin gene (29). The present study revealed that PDX1
mRNA levels decreased during the development of PDM but
significantly increased following the administration of chemerin-9,
which alleviated IR. This is consistent with previously published
studies reporting that PDX1 expression was decreased in patients
with T2DM (30,31) and that the activation of the
PDX1/JAK signal transduction cascade in C57BL/6 mice ameliorates
the IR of DM (32). Thus, this
supports the notion that the impaired proliferation of pancreatic
β-cells resulting from decreased PDX1 expression may be causally
related to the pathogenesis of PDM, and that restoring PDX1
signaling using chemerin-9 may explain the protective role against
IR in PDM.
One limitation of the present study is a lack of a
healthy cohort. Including healthy individuals will be beneficial
for better interpretation of chemerin levels and IR. Another
limitation of this study is that the mechanism underlying the
function of chemerin in PDM remains unknown. In conclusion, the
present study demonstrated that chemerin levels are decreased in
the serum of patients with PDM, and are negatively associated with
IR in this population. In vivo experiments utilizing a mouse
model of PDM revealed that chemerin levels decreased during the
development of the disease, together with a concomitant increase in
the levels of IL-1 and TNF-α, and decreased mRNA expression levels
of GLUT2 and PDX1. Administration of the CMKLR1 agonist,
chemerin-9, caused an increased expression of chemerin, GLUT2, and
PDX1, which led to the alleviation of glucose intolerance and IR in
PDM model mice. Together, these data indicated that chemerin may
exert a protective function against PDM, and the restoration of the
chemerin/CMKLR1 pathway may represent a novel therapeutic strategy
for the treatment of PDM.
Acknowledgements
Not applicable.
Funding
This study was supported by The Natural Science
Foundation of Zhejiang Provincial (grant. no. LY18H150005), The
Science and Technology Foundation of Zhejiang Province (grant. no.
2013C37022) and The National Natural Science Foundation of China
(grant. nos. 81670588 and 81570584).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT, WL and QX designed the study. YY, JZ, GL, LK and
ZT performed the in vivo experiments and collected the data.
MK, DH and QX performed the in vivo experiments and the
statistical analysis. QX and JT wrote the manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the Ethics
Committee of the Jinling Hospital (Nanjing, China). Written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wittamer V, Franssen JD, Vulcano M,
Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R,
Blanpain C, Detheux M, et al: Specific recruitment of
antigen-presenting cells by chemerin, a novel processed ligand from
human inflammatory fluids. J Exp Med. 198:977–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goralski KB, McCarthy TC, Hanniman EA,
Zabel BA, Butcher EC, Parlee SD, Muruganandan S and Sinal CJ:
Chemerin, a novel adipokine that regulates adipogenesis and
adipocyte metabolism. J Biol Chem. 282:28175–28188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conde J, Scotece M, Gómez R, López V,
Gómez-Reino JJ, Lago F and Gualillo O: Adipokines: Biofactors from
white adipose tissue. A complex hub among inflammation, metabolism,
and immunity. Biofactors. 37:413–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ouchi N, Parker JL, Lugus JJ and Walsh K:
Adipokines in inflammation and metabolic disease. Nat Rev Immunol.
11:85–97. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ernst MC and Sinal CJ: Chemerin: At the
crossroads of inflammation and obesity. Trends Endocrinol Metab.
21:660–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bondue B, Wittamer V and Parmentier M:
Chemerin and its receptors in leukocyte trafficking, inflammation
and metabolism. Cytokine Growth Factor Rev. 22:331–338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang R, Liu S, Guo B, Chang L and Li Y:
Chemerin induces insulin resistance in rat cardiomyocytes in part
through the ERK1/2 signaling pathway. Pharmacology. 94:259–264.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi M, Takahashi Y, Takahashi K,
Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H,
Kitazawa S, et al: Chemerin enhances insulin signaling and
potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes.
FEBS Lett. 582:573–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bozaoglu K, Bolton K, McMillan J, Zimmet
P, Jowett J, Collier G, Walder K and Segal D: Chemerin is a novel
adipokine associated with obesity and metabolic syndrome.
Endocrinology. 148:4687–4694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu S, Zhang Y, Li MZ, Xu H, Wang Q, Song
J, Lin P, Zhang L, Liu Q, Huang QX, et al: Chemerin and apelin are
positively correlated with inflammation in obese type 2 diabetic
patients. Chin Med J (Engl). 125:3440–3444. 2012.PubMed/NCBI
|
|
11
|
Chatterjee S and Davies MJ: Accurate
diagnosis of diabetes mellitus and new paradigms of classification.
Nat Rev Endocrinol. 14:386–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33 (Suppl
1):S62–S69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Expert Committee on the Diagnosis and
Classification of Diabetes Mellitus: Report of the expert committee
on the diagnosis and classification of diabetes mellitus. Diabetes
Care. 26 (Suppl 1):S5–S20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ewald N, Kaufmann C, Raspe A, Kloer HU,
Bretzel RG and Hardt PD: Prevalence of diabetes mellitus secondary
to pancreatic diseases (type 3c). Diabetes Metab Res Rev.
28:338–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ewald N and Bretzel RG: Diabetes mellitus
secondary to pancreatic diseases (Type 3c)-are we neglecting an
important disease? Eur J Intern Med. 24:203–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weaver C, Bishop AE and Polak JM:
Pancreatic changes elicited by chronic administration of excess
L-arginine. Exp Mol Pathol. 60:71–87. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kennedy AJ, Yang P, Read C, Kuc RE, Yang
L, Taylor EJ, Taylor CW, Maguire JJ and Davenport AP: Chemerin
elicits potent constrictor actions via chemokine-like receptor 1
(CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat
vasculature. J Am Heart Assoc. 5(pii): e0044212016.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang M, Yang G, Dong J, Liu Y, Zong H, Liu
H, Boden G and Li L: Elevated plasma levels of chemerin in newly
diagnosed type 2 diabetes mellitus with hypertension. J Investig
Med. 58:883–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfau D, Stepan H, Kratzsch J, Verlohren M,
Verlohren HJ, Drynda K, Lössner U, Blüher M, Stumvoll M and
Fasshauer M: Circulating levels of the adipokine chemerin in
gestational diabetes mellitus. Horm Res Paediatr. 74:56–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hart PA, Bellin MD, Andersen DK, Bradley
D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc
M, Kudva YC, et al: Type 3c (pancreatogenic) diabetes mellitus
secondary to chronic pancreatitis and pancreatic cancer. Lancet
Gastroenterol Hepatol. 1:226–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andersen DK: The practical importance of
recognizing pancreatogenic or type 3c diabetes. Diabetes Metab Res
Rev. 28:326–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Araya AV, Pavez V, Perez C, Gonzalez F,
Columbo A, Aguirre A, Schiattino I and Aguillón JC: Ex vivo
lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2
secretion in whole blood from Type 1 diabetes mellitus patients
with or without aggressive periodontitis. Eur Cytokine Netw.
14:128–133. 2003.PubMed/NCBI
|
|
24
|
Saxena M, Srivastava N and Banerjee M:
Association of IL-6, TNF-α and IL-10 gene polymorphisms with type 2
diabetes mellitus. Mol Biol Rep. 40:6271–6279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hassani-Nezhad-Gashti F, Rysa J, Kummu O,
Näpänkangas J, Buler M, Karpale M, Hukkanen J and Hakkola J:
Activation of nuclear receptor PXR impairs glucose tolerance and
dysregulates GLUT2 expression and subcellular localization in
liver. Biochem Pharmacol. 148:253–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beamish CA, Zhang L, Szlapinski SK, Strutt
BJ and Hill DJ: An increase in immature β-cells lacking Glut2
precedes the expansion of β-cell mass in the pregnant mouse. PLoS
One. 12:e01822562017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khandelwal P, Sinha A, Jain V, Houghton J,
Hari P and Bagga A: Fanconi syndrome and neonatal diabetes:
Phenotypic heterogeneity in patients with GLUT2 defects. CEN Case
Rep. 7:1–4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rathinam A and Pari L: Myrtenal
ameliorates hyperglycemia by enhancing GLUT2 through Akt in the
skeletal muscle and liver of diabetic rats. Chem Biol Interact.
256:161–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei J, Ding D, Wang T, Liu Q and Lin Y:
MiR-338 controls BPA-triggered pancreatic islet insulin secretory
dysfunction from compensation to decompensation by targeting Pdx-1.
FASEB J. 31:5184–5195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi S, Zhao L and Zheng L: NSD2 is
downregulated in T2DM and promotes β cell proliferation and insulin
secretion through the transcriptionally regulation of PDX1. Mol Med
Rep. 18:3513–3520. 2018.PubMed/NCBI
|
|
31
|
Yang BT, Dayeh TA, Volkov PA, Kirkpatrick
CL, Malmgren S, Jing X, Renström E, Wollheim CB, Nitert MD and Ling
C: Increased DNA methylation and decreased expression of PDX-1 in
pancreatic islets from patients with type 2 diabetes. Mol
Endocrinol. 26:1203–1212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hao T, Zhang H, Li S and Tian H:
Glucagon-like peptide 1 receptor agonist ameliorates the insulin
resistance function of islet β cells via the activation of
PDX-1/JAK signaling transduction in C57/BL6 mice with high-fat
diet-induced diabetes. Int J Mol Med. 39:1029–1036. 2017.
View Article : Google Scholar : PubMed/NCBI
|