Introduction
Obesity is defined as abnormal or excessive fat
accumulation in adipose tissue, and results from an imbalance
between energy intake and expenditure. The excess dietary free
fatty acids are esterified to inert triglycerides, which are stored
in lipid droplets in adipose tissue. When energy demand is
increased, triglycerides are hydrolyzed to free fatty acids and
glycerol. This process is called lipolysis. Lipolysis is regulated
by specific hydrolases and its activators. Adipose triglyceride
lipase (ATGL) and hormone-sensitive lipase (HSL) are the major
lipases. ATGL deficiency in mice is associated with severely
reduced lipolysis, resulting in increased fat deposition (1), suggesting the important role of ATGL
in triglyceride catabolism. Furthermore, deficiency or dysfunction
of a potent ATGL coactivator, comparative gene identification-58
(CGI-58), also causes severe systemic triglyceride accumulation in
mice and human patients (2,3).
Perilipin A coats lipid droplets and regulates triglyceride
hydrolysis by inhibiting the access of lipases into lipid droplets.
Obesity is associated with reduced lipolytic activity and
lipolysis-related proteins in adipose tissue (4,5).
Thus, understanding the regulatory mechanisms of lipolytic activity
and lipolysis-related proteins is important to develop strategies
against metabolic disorders.
Dysregulation of iron status has been shown to be
linked to the development of obesity-related metabolic disorders,
such as type 2 diabetes. The potential links between iron and
metabolic disorders are hypothesized to an induction of
inflammation and oxidative stress due to the iron overload
(6,7). In contrast, previous studies have
reported that the etiology of obesity-associated iron deficiency
includes inadequate dietary iron intake, increased iron
requirements, and menstrual irregularities (8–12);
however, the precise mechanisms are unclear. Hemoglobin
concentration or liver iron storage have been reported to be
inversely related to body fat in a rodent model and humans
(13,14). As lipolytic activity is
dysregulated in adipose tissue by obesity as mentioned above, it is
possible that not only accumulation of iron, but iron deficiency
affects lipolytic activity in adipose tissue. Yamagishi et
al (15) reported that rats
fed iron deficient diet for 1 week shows the increased
catecholamine-stimulated lipolytic activity in epididymal
adipocytes. However, the elevation of lipolytic activity by iron
deficient diet was not observed rats fed the diet for 5 weeks.
Furthermore, the influence of iron availability in the regulation
of lipolysis-related proteins, such as ATGL, HSL or perilipin A in
adipocytes were not evaluated in that study.
Recent evidence obtained from 3T3-L1 adipocytes
revealed that micro-lipid droplets (mLDs) play an important role in
active lipolysis (16,17). During mLD formation, glycerol is
derived from glucose through glycolysis to synthesize
triglycerides, because of the lack of glycerol kinase in
adipocytes. It is well known that glucose is preferred over lipid
as a substrate under iron deficiency (18,19).
Therefore, changes in glucose utilization induced by iron
deficiency may influence lipolytic activity in adipocytes.
To evaluate these possibilities, we treated 3T3-L1
adipocytes with a hydrophilic chelator, deferoxamine (DFO) used in
clinical practice to remove excess iron. Here, we report that
DFO-induced iron deficiency reduces lipolytic activity through
downregulation of lipolysis-related proteins and glucose
utilization in adipocytes.
Materials and methods
Cell culture
All reagents for cell culture were obtained from
Nacalai Tesque, unless otherwise indicated. 3T3-L1 cells were
purchased from JCRB Cell Bank. Cells were cultured as previously
reported (20).
Experimental design
3T3-L1 cells were maintained in growth medium (GM)
containing DMEM, 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. For differentiation, confluent cells were treated
with a hormone mixture containing 1 µM dexamethasone, 0.5 mM
3-isobutyl-1-methylxanthine (IBMX) and 1 µg/ml insulin in GM. After
48 h, the hormone mixture was removed and cells were further
cultured in GM supplemented with 1 µg/ml insulin. At 7 days after
differentiation induction, 3T3-L1 adipocytes were treated with
deferoxamine mesylate (DFO) (Sigma-Aldrich; Merck KGaA) at the
indicated concentrations for 48 h. At day 9, the cells in all
groups were analyzed.
Lipolytic stimulation
Lipolytic stimulation was applied to differentiated
3T3-L1 adipocytes as follows. After washing twice with PBS, the
cells were incubated with phenol red-free DMEM containing 2% BSA in
presence or absence of 1 µM isoproterenol at 37°C for up to 4 h. To
inhibit glucose uptake, the cells were treated as above, except
that 500 µM cytochalasin B (Sigma-Aldrich; Merck KGaA) was added to
the medium 30 min before lipolysis stimulation. Aliquots of the
medium were collected as specified time points, and glycerol and
glucose concentrations were measured using a glycerol assay kit
(Sigma-Aldrich; Merck KGaA) and a Glucose CII test wako (Wako),
respectively.
Oil Red O staining
3T3-L1 adipocytes were grown in 24-well plates.
Differentiated cells at day 9 were washed twice with PBS and fixed
with 4% PFA for 30 min. Oil Red O stain stock solution was diluted
at a 6:4 ratio with distilled water and then filtered. Cells were
stained with the Oil Red O solution for 30 min at room temperature.
The stained cells were washed with distilled water three times and
observed under a microscope (Keyence). To quantify the lipid
content, the Oil Red O was eluted with 100% isopropanol for 10 min,
and the absorbance at 490 nm was measured using a microplate reader
(Bio-Rad Laboratories, Inc.).
Cellular iron concentration
measurement
3T3-L1 adipocytes were grown in 6-well plates as
described above. Differentiated cells at day 9 were washed with PBS
twice and lysed in protein precipitation solution containing 0.53 N
HCl, 5.3% trichloroacetic acid. The crude lysate was boiled at
100°C for 30 min, then the sample was allowed to slowly cool down
to room temperature. The lysate was centrifuged at 15,000 × g for 5
min, and the supernatant was used to measure iron concentration.
The Iron Assay kit (Metallogenics) was used to measure cellular
iron content according to the manufacturer's protocol
Western blotting
3T3-L1 adipocytes were grown in 12-well plates as
described above, washed with PBS twice, and then harvested in
ice-cold radioimmunoprecipitation assay buffer containing 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1
mM EDTA, and protease/phosphatase inhibitor cocktail. Cell lysates
were kept on ice for 10 min, then centrifuged at 15,000 × g for 10
min. The supernatants were collected and protein concentrations
were measured using a bicinchoninic acid assay kit. Samples were
prepared in 4X Laemmli sample buffer. Equal amounts of sample
protein were separated by 7.5 or 12.5% SDS-PAGE, transferred to a
PVDF membrane (#88518; Pierce; Thermo Fisher Scientific, Inc.), and
incubated overnight at 4°C with primary antibodies. Primary
antibodies are listed in Table
SI. Enhanced chemiluminescence (Merck KGaA) was used to
facilitate the detection of protein bands. Images were scanned
using a chemiluminescence detector (LAS500; GE Healthcare
Bio-Sciences AB). Band intensities were quantified using ImageJ
1.52a (National institutes of Health). Equal loading was checked by
staining the blot with Coomassie Brilliant Blue (#296-21541; Wako)
(16,17).
Statistical analysis
Data are expressed as means ± standard errors of the
mean. Statistical analyses were carried out using BellCurve for
Excel version 3.10 (Social Survey Research Information, Tokyo,
Japan). Differences between groups were assessed by one- or two-way
analysis of variance (ANOVA) followed by Bonferroni's post hoc
test, or unpaired t-tests, as indicated. P<0.05 was considered
to indicate a statistically significant difference.
Results
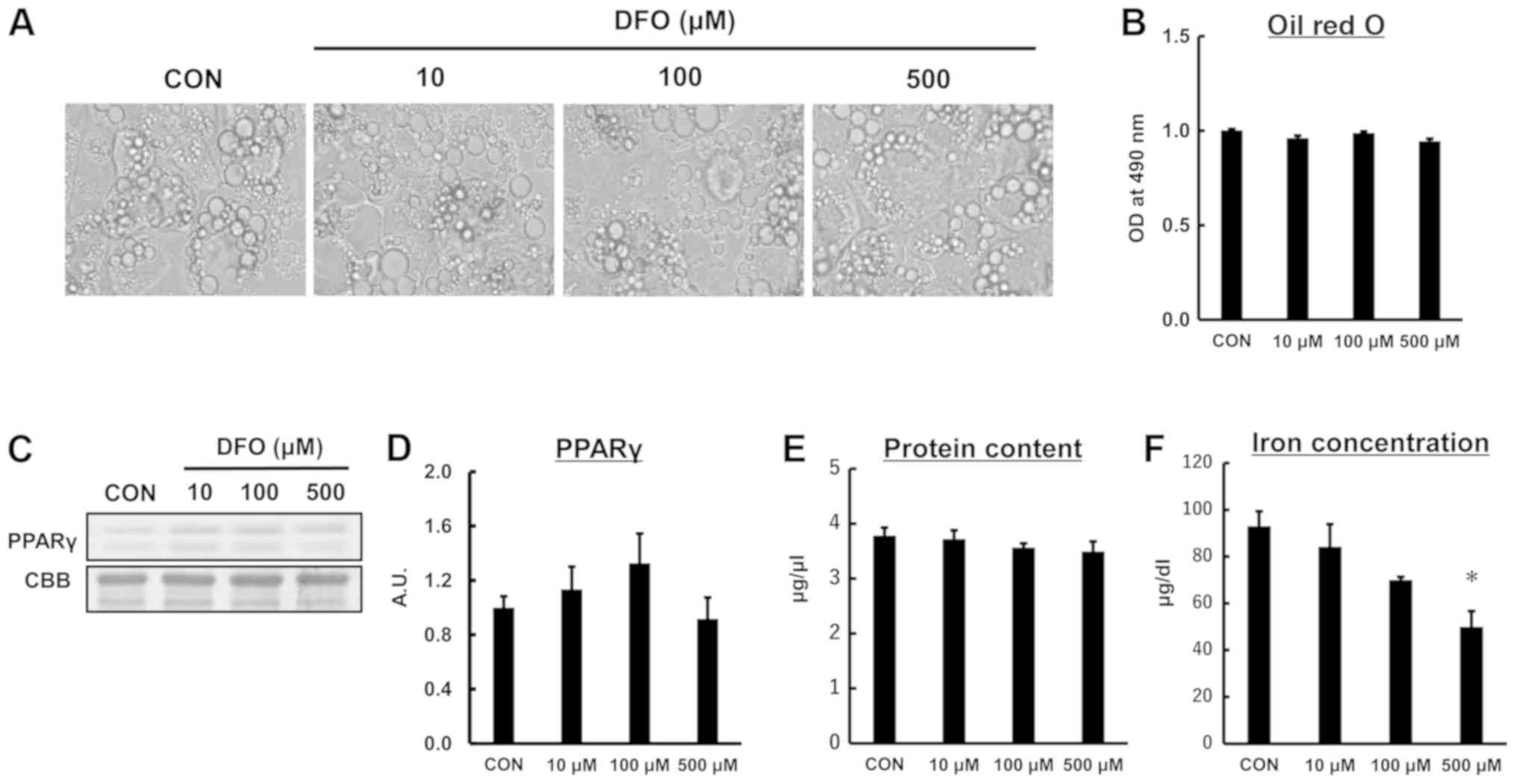
DFO treatment does not affect lipid
and protein contents in 3T3-L1 adipocytes
We first examined whether iron deficiency caused by
the iron chelator DFO affects lipid storage in 3T3-L1 adipocytes.
As shown in Fig. 1A and B, lipid
content in 3T3-L1 adipocytes did not differ between the treatment
groups (P=0.674). The protein content of PPARγ, a marker of
adipocyte differentiation, was not different among the groups
(P=0.176; Fig. 1C and D). In
addition, there was no difference in total protein content between
the groups (P=0.531; Fig. 1E). DFO
treatment at 500 µM significantly decreased cellular iron
concentration compared to the control group (P<0.05; Fig. 1F). These results suggested that the
protocols used in the present study reduces the iron concentration
in adipocytes, but did not affect lipid content or cause
maladaptive responses.
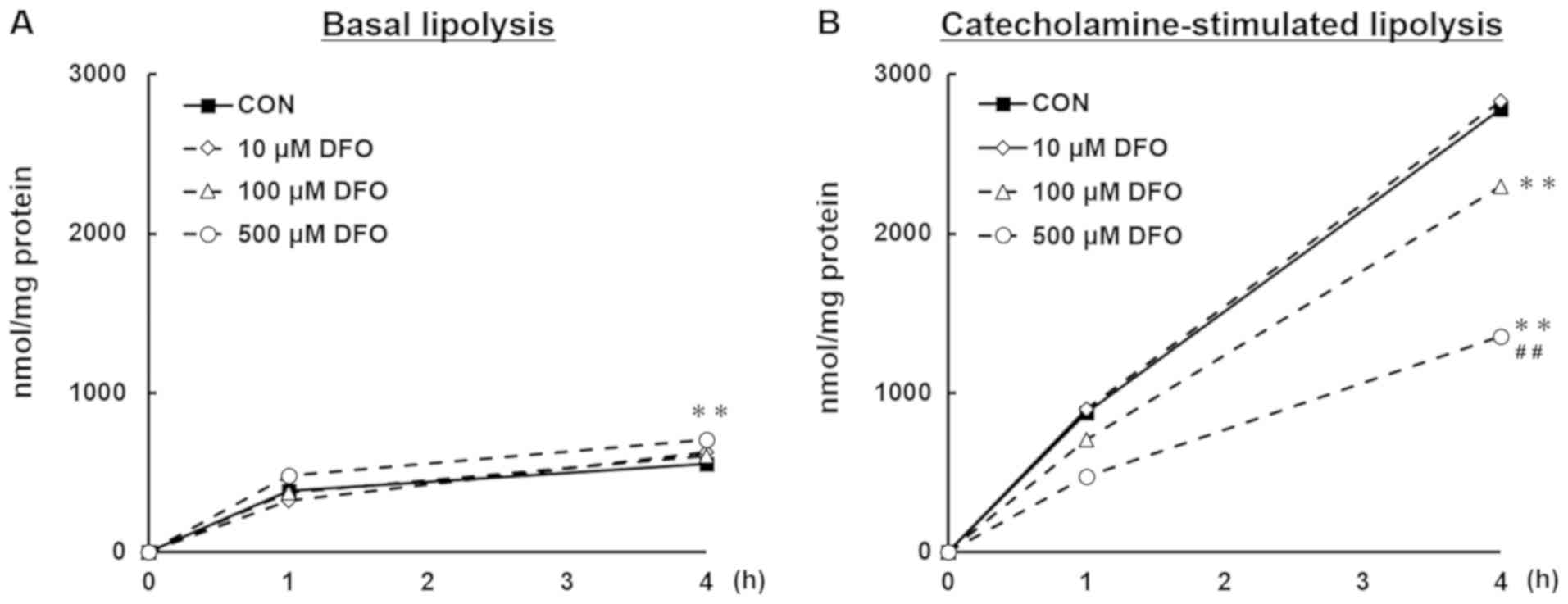
Iron deficiency reduces
catecholamine-stimulated, but not basal lipolytic activity in
3T3-L1 adipocytes
Next, we investigated the basal and
catecholamine-stimulated lipolytic activities in DFO-treated
adipocytes. Glycerol release into the medium during lipolysis in
the presence or absence of catecholamine was measured for 4 h. The
amount of glycerol, which represents total lipolytic activity in
adipocytes, was increased in cells treated with 500 µM DFO at basal
state compared to the CON group (P<0.01; Fig. 2A). In contrast, under
catecholamine-stimulated lipolysis, DFO treatment at 100 µM
significantly decreased glycerol release when compared to the
control group (P<0.01), and a further decrease in glycerol
release was observed in the 500 µM group (P<0.01; Fig. 2B).
Iron deficiency decreases
lipolysis-related proteins in 3T3-L1 adipocytes
Lipolysis-related proteins, including ATGL, HSL,
perilipin A and CGI-58, play important roles in regulating fat
storage and breakdown (16,21–23).
Given the decrease in catecholamine-stimulated lipolysis activity
due to 2-day iron deficiency, we hypothesized that iron deficiency
would suppress lipolysis-related proteins. Therefore, we
investigated the effect of iron deficiency on lipolysis-related
protein contents. As shown in Fig.
3, DFO treatment decreased the levels of ATGL, HSL, CGI-58, and
perilipin A proteins in a concentration-dependent manner. Hypoxia
inducible factor (HIF)-1α was induced by DFO treatment (Fig. 3F).
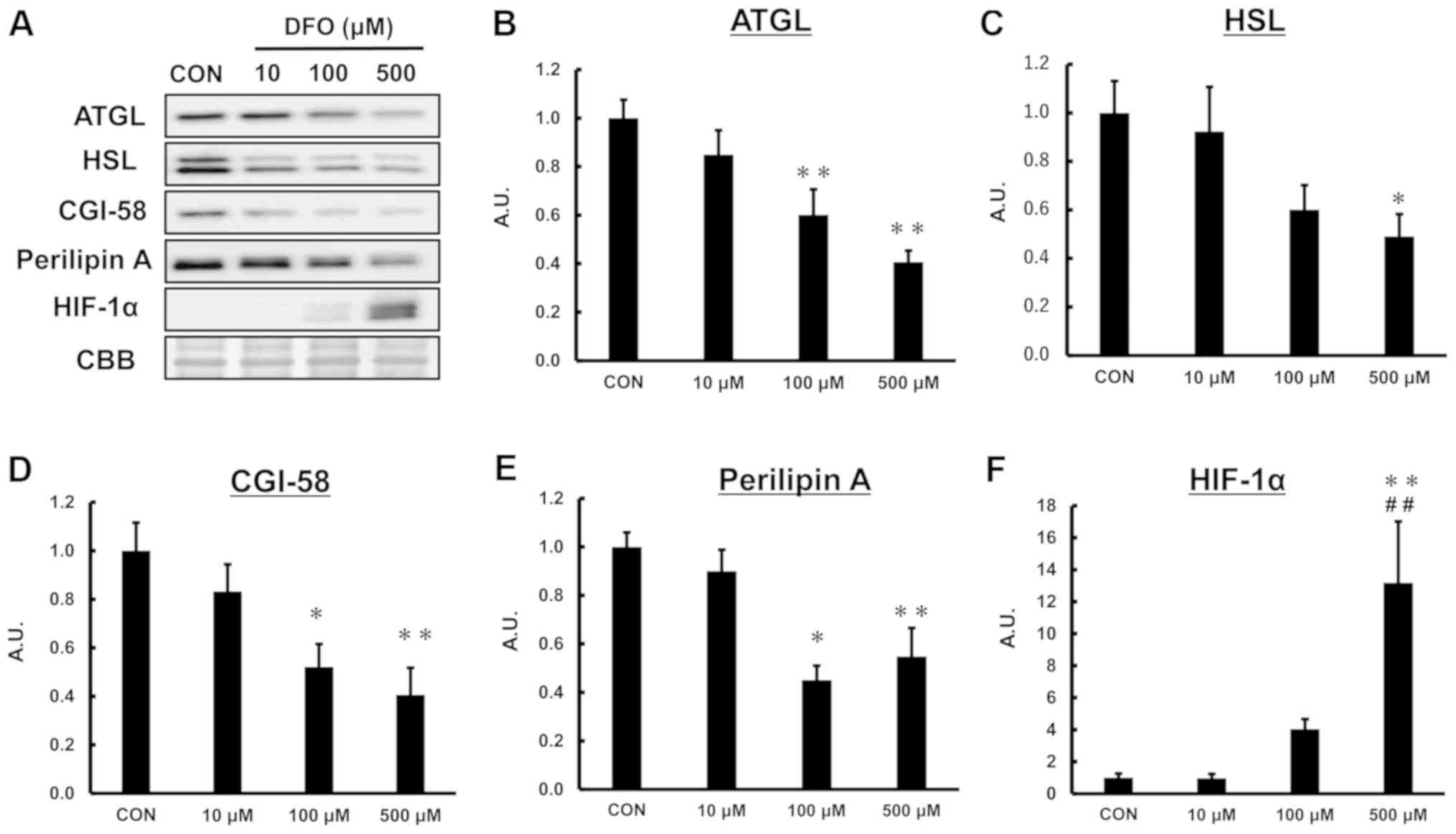 | Figure 3.Effect of iron deficiency on
adipocyte lipid-related proteins. (A) Representative western blot
analysis for (B) ATGL, (C) HSL, (D) CGI-58, (E) perilipin A and (F)
HIF-1α. CBB was used as a loading control. One-way ANOVA was used
for statistical analysis (n=5–6). *P<0.05 and **P<0.01 vs.
CON. ##P<0.01 vs. 10 µM DFO. ATGL, adipose
triglyceride lipase; HSL, hormone sensitive lipase; CGI-58,
comparative gene identification-58; HIF-1α, hypoxia inducible
factor-1α; CON, control; DFO, deferoxamine mesylate; CBB, coomassie
brilliant blue; A.U., arbitrary units. |
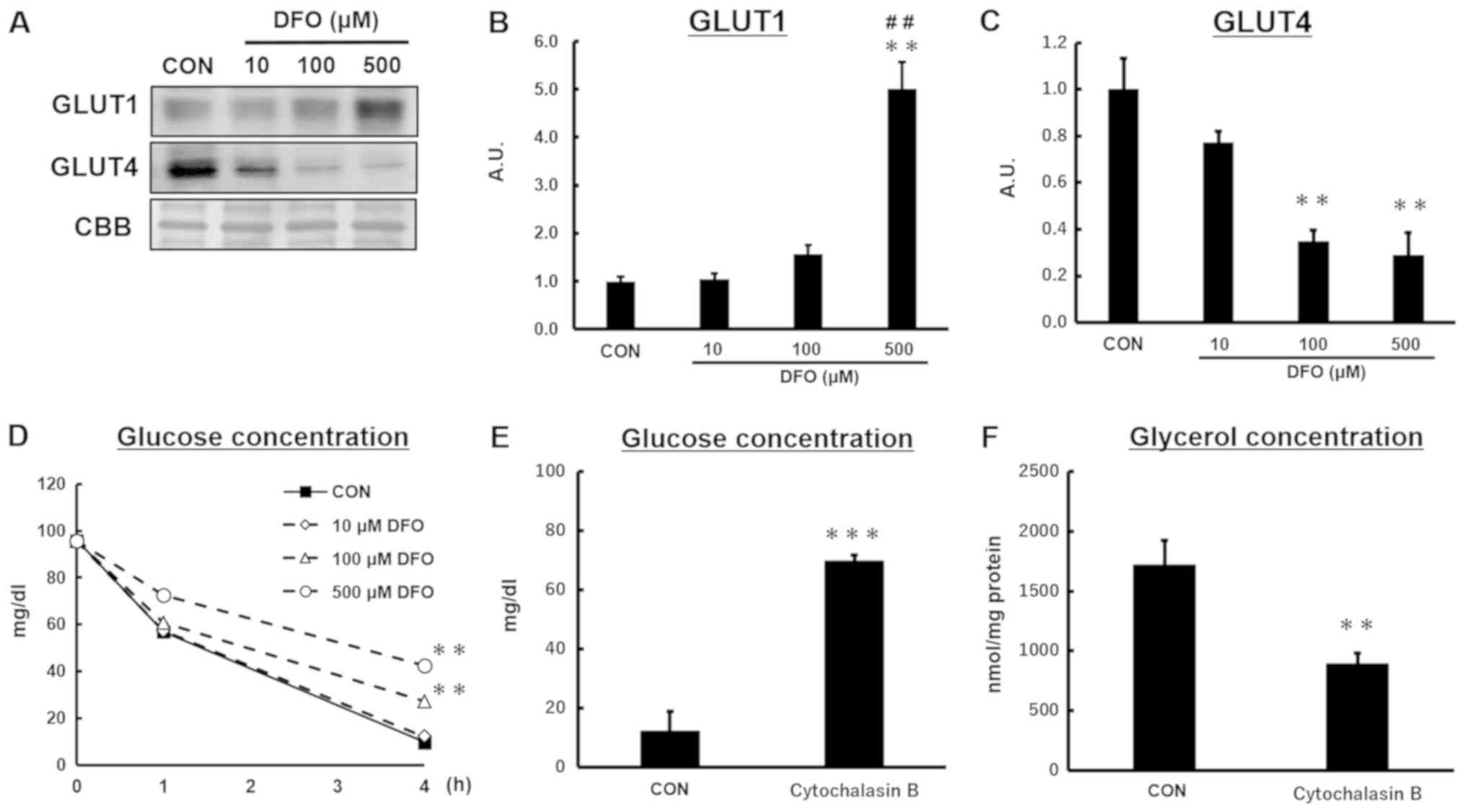
Iron deficiency modulates glucose
transporters and glucose utilization during lipolysis in 3T3-L1
adipocytes
GLUT1, a transporter that plays an important role in
basal glucose uptake, was strongly induced by iron deficiency
(Fig. 4B). However, the protein
content of the insulin-responsive glucose transporter GLUT4 was
decreased by DFO treatment in a dose-dependent manner (Fig. 4C). The decrease rate of the glucose
concentration in the medium, which represents glucose utilization
of adipocytes, was lower in DFO-treated than control cells
(Fig. 4D).
To evaluate the effect of glucose availability on
lipolytic capacity in adipocytes, the cells were pretreated with
cytochalasin B before lipolysis stimulation. Pretreatment with
cytochalasin B resulted in higher glucose concentrations in the
culture medium 4 h after lipolysis stimulation, indicating low
glucose utilization (Fig. 4E). The
glycerol concentration in the medium was lower in cytochalasin
B-treated adipocytes (Fig. 4F),
implying that inhibition of glucose utilization reduces
catecholamine-stimulated lipolysis.
Short-term iron deficiency does not
affect mitochondrial proteins
The protein content of NDUFB8, a subunit of
mitochondrial complex I containing iron, did not differ between the
treatment groups (CON, 1.00±0.15 arbitrary unit; 10 µM DFO,
0.92±0.13; 100 µM DFO, 0.91±0.23; 500 µM DFO, 0.91±0.21). The
non-iron-containing mitochondrial proteins citrate synthase (CON,
1.00±0.10 arbitrary unit; 10 µM DFO, 1.06±0.11; 100 µM DFO,
1.13±0.1; 500 µM DFO, 0.95±0.01) and long-chain acyl-CoA
dehydrogenase (CON, 1.00±0.03 arbitrary unit; 10 µM DFO, 0.90±0.10;
100 µM DFO, 0.88±008; 500 µM DFO, 0.84±0.11) were also unaffected
by DFO treatment.
Discussion
Although iron deficiency is frequently found in
advanced stages of obesity, the precise mechanisms by which obesity
status causes iron deficiency are unclear (24). It was reported that
catecholamine-stimulated lipolysis increased in epididymal white
adipocytes isolated from rats fed iron deficient diet for 1 week.
However, in that study, the effect of iron deficient diet on
lipolytic activity wore off when the diet was continued for 5 weeks
(15), and effect of iron
deficiency on lipolysis-related proteins were not evaluated. In
this study, we used 3T3-L1 adipocytes to investigate the molecular
regulation of lipolysis in iron deficient state in 3T3-L1
adipocytes and found that DFO-induced iron deficiency results in
decreases in lipolysis-related proteins, including perilipin A,
ATGL, HSL, and CGI-58. Consequently, catecholamine-stimulated
lipolytic activity was reduced in 3T3-L1 adipocytes treated with
DFO in a dose-dependent manner. These results suggested that low
iron availability attenuates lipolytic activity through the
reduction of lipolysis-related proteins in adipocytes.
During active lipolysis, numerous mLDs, which play
an important role in lipolysis, appear in all areas of the
cytoplasm (16). For the formation
of mLDs, glucose utilization becomes active to provide
glycerol-3-phosphate, a partner of fatty acid, to form triglyceride
during lipolysis. Our results demonstrated that iron deficiency
causes a decrease in GLUT4 protein content, concomitant to reduced
glucose utilization. These results indicated that not only
lipolytic enzymes, but also glucose utilization capacity
contributes to the reduction in catecholamine-stimulated lipolytic
activity in DFO-treated cells.
Previous reports have shown that GLUT1 and GLUT4
respond differently to physiological stimuli in 3T3-L1 adipocytes
and human adipose tissue (25–27).
We observed an induction of GLUT1 and HIF-1α protein contents by
DFO treatment, which is consistent with a previous study showing
that hypoxic stimulation induces GLUT1 in 3T3-L1 adipocytes
(28). In contrast, the protein
content of the insulin-responsive glucose transporter, GLUT4, was
decreased in DFO-treated cells. These results indicated that GLUT4
and glucose play a critical role in catecholamine-stimulated
lipolytic activity, and induction of GLUT1 by iron deficiency is
not sufficient to compensate the reduction in GLUT4. Because there
is a hypoxia-responsive element in the GLUT1 promoter, the
induction of GLUT1 by DFO is reasonable. It is difficult to explain
the mechanisms underlying the reduction in GLUT4 expression in this
study, although some other studies have also reported such a
decrease in GLUT4 in response to hypoxia (26–28).
In this study, we measured the total protein content of GLUT1 and
GLUT4 in adipocytes. It is well known that GLUTs translocate from
the intracellular pool to plasma membranes and elevate glucose
transport activity by physiological stimuli. Therefore, the
distribution of GLUTs in response to catecholamine stimulation and
iron deficiency should be examined in future studies.
When adipocytes become hypertrophic during the
development of obesity, oxygen cannot diffuse into cells, which
results in hypoxia. As shown in Fig.
3F, DFO treatment induced HIF-1α, indicating that low iron
availability causes a hypoxic state in adipocytes. Iron deficiency
has been shown to induce hypoxia in various cell types. As
carbohydrate is preferred as an energy source over fat under
hypoxia, the hypoxic state is one possible mechanism explaining the
reduction in lipolysis-related proteins in DFO-treated adipocytes.
However, the molecular mechanisms by which an iron
deficiency-induced hypoxic state downregulates lipolysis-related
proteins remains unknown. In addition, there is no direct evidence
that the hypoxic state observed in adipose tissue from obese
subjects is due to iron deficiency. Further in vivo study is
needed to determine whether iron deficiency is responsible for the
hypoxic state and the reduction in lipolytic capacity in obese
adipose tissue. In addition, the responses to physiological stimuli
that activate lipolysis, such as catecholamine or starvation,
should be examined in vivo.
There are conflicting reports showing that hypoxia
increases or decreases lipid accumulation in adipocytes (26,29–33).
Although HIF-1α was elevated in DFO-treated adipocytes in this
study, there was no difference in lipid concentration between the
groups. This might be due to the short-term treatment with DFO. For
example, Marques et al (26) reported an increment in lipid
accumulation in adipocytes treated with CoCl2, a hypoxia
mimetic, or DFO for 7 days. In contrast, we treated adipocytes with
DFO for 2 days, which is a shorter treatment time than that in
previous studies, because it has been reported that hypoxia
attenuates adipocyte differentiation via suppression of PPARγ
(34). To exclude the effect of
iron deficiency on adipocyte differentiation, we started DFO
treatment at day 7 after differentiation initiation, when the
formation of large lipid droplets was visible. DFO treatment had no
effect on PPARγ protein content, suggesting that DFO-induced
reduction in lipolysis-related proteins was not due to inhibition
of adipocyte differentiation.
To support our results, we further incubated
differentiated 3T3-L1 adipocytes with iron sulfate, and found that
iron overload causes a significant reduction of lipid droplet size
(data not shown). This could be because of oxidative stress caused
by excess free iron. It was reported that iron overload induces a
decrease in fat mass in C57BL/6 mice fed a high-iron diet for 8
weeks (35). These results led us
to consider that iron overload model is suitable for evaluation of
adipocyte differentiation, but not for lipolysis. Establishment of
a suitable model for iron overload is needed to evaluate the effect
of iron accumulation on lipolysis in adipocytes.
Mitochondrial dysfunction attenuates lipolysis in
adipocytes (36). Inhibition of
the electron transport chain abolishes lipolysis stimulated by
catecholamine (37). Iron
deficiency has been shown to reduce mitochondrial proteins that
contain iron as a cofactor in several cell types (19,38,39).
Based on these findings, we asked whether mitochondrial dysfunction
is a primary cause of reduced catecholamine-stimulated lipolysis in
adipocytes treated with DFO. However, contents of both
iron-containing (complex I subunit) and non-iron-containing protein
(citrate synthase and LCAD) did not differ between treatment
groups. These results suggest that the iron deficiency-induced
decrease in catecholamine-stimulated lipolysis occurs independently
of or prior to mitochondrial dysfunction in adipocytes. The
molecular mechanism by which iron deficiency attenuates lipolysis
without a reduction in mitochondria proteins remains unknown.
In conclusion, our results suggest that low iron
availability attenuates catecholamine-stimulated lipolysis in
3T3-L1 adipocytes. This reduction is due to decreases in
lipolysis-related proteins and glucose utilization. Taking these
results together, the present study extends our understanding of
the importance of iron in adipocyte biology and lipid metabolism.
Further studies are required to reveal how iron deficiency causes
hypoxia in adipose tissue and stimulates the metabolic programing
observed in this study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by a Grant-in-Aid
for Young Scientists (grant no. 17K13188 to HK) and a Grant-in-Aid
for Scientific Research (grant no. 16K01727 to NN) from the Japan
Society for the Promotion of Science.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH and NN designed and performed the experiments,
analyzed the data and wrote the manuscript. KH, NT and SI performed
the experiments and analyzed the data. TH designed the study,
analyzed the data and wrote the manuscript. KH, NT, SI, TH and NN
discussed the results, commented on the manuscript and approved the
manuscript for submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DFO
|
deferoxamine mesylate
|
|
GLUT
|
glucose transporter
|
|
mLD
|
micro-lipid droplet
|
References
|
1
|
Haemmerle G, Lass A, Zimmermann R,
Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C,
Eder S, et al: Defective lipolysis and altered energy metabolism in
mice lacking adipose triglyceride lipase. Science. 312:734–737.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lefèvre C, Jobard F, Caux F, Bouadjar B,
Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL,
Weissenbach J, et al: Mutations in CGI-58, the gene encoding a new
protein of the esterase/lipase/thioesterase subfamily, in
Chanarin-Dorfman syndrome. Am J Hum Genet. 69:1002–1012. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Radner FP, Streith IE, Schoiswohl G,
Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder
S, Schauer S, et al: Growth retardation, impaired triacylglycerol
catabolism, hepatic steatosis, and lethal skin barrier defect in
mice lacking comparative gene identification-58 (CGI-58). J Biol
Chem. 285:7300–7311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langin D, Dicker A, Tavernier G, Hoffstedt
J, Mairal A, Rydén M, Arner E, Sicard A, Jenkins CM, Viguerie N, et
al: Adipocyte lipases and defect of lipolysis in human obesity.
Diabetes. 54:3190–3197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinberg GR, Kemp BE and Watt MJ:
Adipocyte triglyceride lipase expression in human obesity. Am J
Physiol Endocrinol Metab. 293:E958–E964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cighetti G, Duca L, Bortone L, Sala S,
Nava I, Fiorelli G and Cappellini MD: Oxidative status and
malondialdehyde in beta-thalassaemia patients. Eur J Clin Invest.
32 (Suppl 1):55–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan HF, Liu ZY, Guan ZA and Guo C:
Deferoxamine ameliorates adipocyte dysfunction by modulating iron
metabolism in ob/ob mice. Endocr Connect. 7:604–616. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Zhang X, Shen Y, Fang X, Wang Y
and Wang F: Obesity and iron deficiency: A quantitative
meta-analysis. Obes Rev. 16:1081–1093. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moayeri H, Bidad K, Zadhoush S, Gholami N
and Anari S: Increasing prevalence of iron deficiency in overweight
and obese children and adolescents (Tehran Adolescent Obesity
Study). Eur J Pediatr. 165:813–814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nead KG, Halterman JS, Kaczorowski JM,
Auinger P and Weitzman M: Overweight children and adolescents: A
risk group for iron deficiency. Pediatrics. 114:104–108. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinhas-Hamiel O, Newfield RS, Koren I,
Agmon A, Lilos P and Phillip M: Greater prevalence of iron
deficiency in overweight and obese children and adolescents. Int J
Obes Relat Metab Disord. 27:416–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wenzel BJ, Stults HB and Mayer J:
Hypoferraemia in obese adolescents. Lancet. 2:327–328. 1962.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagni UV, Luiz RR and Veiga GV: Overweight
is associated with low hemoglobin levels in adolescent girls. Obes
Res Clin Pract. 7:e218–e229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park CY, Chung J, Koo KO, Kim MS and Han
SN: Hepatic iron storage is related to body adiposity and hepatic
inflammation. Nutr Metab (Lond). 14:142017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamagishi H, Okazaki H, Shimizu M, Izawa T
and Komabayashi T: Relationships among serum triacylglycerol, fat
pad weight, and lipolysis in iron-deficient rats. J Nutr Biochem.
11:455–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashimoto T, Segawa H, Okuno M, Kano H,
Hamaguchi HO, Haraguchi T, Hiraoka Y, Hasui S, Yamaguchi T, Hirose
F, et al: Active involvement of micro-lipid droplets and
lipid-droplet-associated proteins in hormone-stimulated lipolysis
in adipocytes. J Cell Sci. 125:6127–6136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi T, Omatsu N, Morimoto E,
Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F and Osumi T:
CGI-58 facilitates lipolysis on lipid droplets but is not involved
in the vesiculation of lipid droplets caused by hormonal
stimulation. J Lipid Res. 48:1078–1089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borel MJ, Beard JL and Farrell PA: Hepatic
glucose production and insulin sensitivity and responsiveness in
iron-deficient anemic rats. Am J Physiol. 264:E380–E390.
1993.PubMed/NCBI
|
|
19
|
Han DH, Hancock CR, Jung SR, Higashida K,
Kim SH and Holloszy JO: Deficiency of the mitochondrial electron
transport chain in muscle does not cause insulin resistance. PLoS
One. 6:e197392011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hashimoto T, Yokokawa T, Endo Y, Iwanaka
N, Higashida K and Taguchi S: Modest hypoxia significantly reduces
triglyceride content and lipid droplet size in 3T3-L1 adipocytes.
Biochem Biophys Res Commun. 440:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granneman JG, Moore HP, Krishnamoorthy R
and Rathod M: Perilipin controls lipolysis by regulating the
interactions of AB-hydrolase containing 5 (Abhd5) and adipose
triglyceride lipase (Atgl). J Biol Chem. 284:34538–34544. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Granneman JG and Moore H-PH: Location,
location: Protein trafficking and lipolysis in adipocytes. Trends
Endocrinol Metab. 19:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zechner R, Kienesberger PC, Haemmerle G,
Zimmermann R and Lass A: Adipose triglyceride lipase and the
lipolytic catabolism of cellular fat stores. J Lipid Res. 50:3–21.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Datz C, Felder TK, Niederseer D and Aigner
E: Iron homeostasis in the metabolic syndrome. Eur J Clin Invest.
43:215–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bakar MH, Sarmidi MR, Kai CK, Huri HZ and
Yaakob H: Amelioration of mitochondrial dysfunction-induced insulin
resistance in differentiated 3T3-L1 adipocytes via inhibition of
NF-κB pathways. Int J Mol Sci. 15:22227–22257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marques AP, Rosmaninho-Salgado J, Estrada
M, Cortez V, Nobre RJ and Cavadas C: Hypoxia mimetic induces lipid
accumulation through mitochondrial dysfunction and stimulates
autophagy in murine preadipocyte cell line. Biochim Biophys Acta
Gen Subj. 1861:673–682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wood IS, Wang B, Lorente-Cebrián S and
Trayhurn P: Hypoxia increases expression of selective facilitative
glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human
adipocytes. Biochem Biophys Res Commun. 361:468–473. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varela-Guruceaga M, Milagro FI, Martínez
JA and de Miguel C: Effect of hypoxia on caveolae-related protein
expression and insulin signaling in adipocytes. Mol Cell
Endocrinol. 473:257–267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weiszenstein M, Musutova M, Plihalova A,
Westlake K, Elkalaf M, Koc M, Prochazka A, Pala J, Gulati S, Trnka
J, et al: Adipogenesis, lipogenesis and lipolysis is stimulated by
mild but not severe hypoxia in 3T3-L1 cells. Biochem Biophys Res
Commun. 478:727–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fink T, Abildtrup L, Fogd K, Abdallah BM,
Kassem M, Ebbesen P and Zachar V: Induction of adipocyte-like
phenotype in human mesenchymal stem cells by hypoxia. Stem Cells.
22:1346–1355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Q, Lee YJ and Yun Z: Differentiation
arrest by hypoxia. J Biol Chem. 281:30678–30683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park YS, Huang Y, Park YJ, David AE, White
L, He H, Chung HS and Yang VC: Specific down regulation of 3T3-L1
adipocyte differentiation by cell-permeable antisense
HIF1alpha-oligonucleotide. Control Release. 144:82–90. 2010.
View Article : Google Scholar
|
|
33
|
Yun Z, Maecker HL, Johnson RS and Giaccia
AJ: Inhibition of PPAR gamma 2 gene expression by the
HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of
adipogenesis by hypoxia. Dev Cell. 2:331–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim KH, Song MJ, Chung J, Park H and Kim
JB: Hypoxia inhibits adipocyte differentiation in a
HDAC-independent manner. Biochem Biophys Res Commun. 333:1178–1184.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gabrielsen JS, Gao Y, Simcox JA, Huang J,
Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, et
al: Adipocyte iron regulates adiponectin and insulin sensitivity. J
Clin Invest. 122:3529–3540. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boudina S and Graham TE: Mitochondrial
function/dysfunction in white adipose tissue. Exp Physiol.
99:1168–1178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fassina G, Dorigo P and Gaion RM:
Equilibrium between metabolic pathways producing energy: A key
factor in regulating lipolysis. Pharmacol Res Commun. 6:1–21. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rensvold JW, Krautkramer KA, Dowell JA,
Denu JM and Pagliarini DJ: Iron Deprivation Induces Transcriptional
Regulation of Mitochondrial Biogenesis. J Biol Chem.
291:20827–20837. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rensvold JW, Ong SE, Jeevananthan A, Carr
SA, Mootha VK and Pagliarini DJ: Complementary RNA and protein
profiling identifies iron as a key regulator of mitochondrial
biogenesis. Cell Rep. 3:237–245. 2013. View Article : Google Scholar : PubMed/NCBI
|