Introduction
Osteoclasts (OCs) are primarily derived from
mononuclear macrophages that arise from pluripotent hematopoietic
stem cells; as the only cell with bone resorption function, OCs
coordinate the dynamic balance between bone resorption and
osteoblast formation (1). Numerous
cytokines are involved in osteoclastogenesis from OC precursors.
Macrophage colony-stimulating factor (M-CSF) and receptor activator
of NF-κB ligand (RANKL) are produced by activated T cells and
osteoblasts, and are essential cytokines for osteoclastogenesis
(2). M-CSF regulates the
proliferation and differentiation of mononuclear macrophages,
binding to the cell surface expression receptor, c-Fms, whereas
RANKL binds to the OC membrane receptor RANK and induces the
expression of downstream signaling molecules, such as nuclear
factor of activated T-cells cytoplasmic (NFATc1), NF-κB and c-Fos,
in order to regulate osteoclastogenesis (3,4).
Numerous studies have reported that microRNAs
(miRNAs/miRs) serve important roles during the process of
osteoclastogenesis, including miR-503 (5), miR-148 (6) and miR-3 (7,8), and
are involved in this process via different signaling pathways. As
an oncogene, miR-21 promotes the occurrence and progression of
multiple myeloma and other solid tumors by inhibiting the
expression of numerous tumor suppressor genes (9). Notably, the role of miR-21 in bone
metabolism is gaining an increasing amount of interest. miR-21
inhibitor regulates the RANKL/osteoprotegerin (OPG) balance to
decrease the bone resorption of mature OCs (10), and estrogen can downregulate
miR-21, and upregulate Fas ligand (FasL) expression, inducing
osteoclastic apoptosis (11).
Therefore, it was hypothesized that miR-21 may be an important
regulator associated with osteoclastogenesis and bone resorption.
However, the molecular mechanism underlying the effects of miR-21
on OCs remains unclear.
In the present study, bioinformatics analysis
predicted that Pten was a target gene of miR-21. Pten is a negative
regulator of PI3K; Pten dephosphorylates PI3K, resulting in reduced
PI3K/Akt signaling activity (12).
Previous research has indicated that the PI3K/Akt signaling pathway
has an important role in OC differentiation (13). Therefore, the present study aimed
to investigate the effects of miR-21 on osteoclastogenesis and bone
resorption, as well as the potential molecular mechanisms
underlying the Pten-PI3K/Akt signaling pathway in OCs.
Materials and methods
Cell culture
The murine macrophage cell line RAW264.7 was
purchased from the American Type Culture Collection and was
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin (100 U/ml)/streptomycin (100
µg/ml) (Hyclone; GE Healthcare Life Sciences) in an incubator
containing 5% CO2 at 37°C. RAW264.7 cells were cultured
with 50 ng/ml RANKL (Thermo Fisher Scientific, Inc.) for 3–5 days
to induce osteoclastogenesis.
Dual-luciferase reporter gene
assay
TargetScan (version 7.2; www.targetscan.org/vert_72) and miRTarBase (version
7.0; mirtarbase.mbc.nctu.edu.tw/php/index.php) were used to
predict the potential target genes of miR-21. Wild-type (WT) and
mutant (MT) binding sites of miR-21 in the 3′-untranslated region
(UTR) of Pten were subcloned into pGL3 basic vectors (Promega
Corporation), to generate pGL3-Pten-WT and pGL3-Pten-MT,
respectively. RAW264.7 cells (1×106 cells/well) were
seeded into 6-well plates and were transfected with 10 ng
Renilla luciferase plasmid (pRL-TK; Promega Corporation), 10
ng pGL3-Pten-WT or pGL3-Pten-MT, and 25 µM miR-21 mimic (Guangzhou
RiboBio Co., Ltd.) or miR-negative control (NC;
5′-CAGUACUUUUGUGUAGUACAA-3′; Guangzhou RiboBio Co., Ltd.) using
Lipofectamine® RNAi Max (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. After
transfection, the cell were cultured at 37°C with 5% CO2
for 48 h, a Dual-Luciferase Reporter Gene Analysis system (Promega
Corporation) was used to detect the luciferase activity, according
to the manufacturer's protocol. With Renilla luciferase used
as an internal control, luciferase activity was calculated as
firefly fluorescence/Renilla fluorescence ratio.
Cell transfection
miR-NC (5′-CAGUACUUUUGUGUAGUACAA-3), miR-21 mimic
and miR-21 inhibitor (5′-UCAACAUCAGUCUGAUAAGCUA-3′) were
synthesized and obtained from Guangzhou RiboBio Co., Ltd. RAW264.7
cells at the logarithmic phase were seeded into 6-well plates and
separated into the following groups: MiR-NC (cells transfected with
25 µM miR-NC), miR-21 mimic (cells transfected with 25 µM miR-21
mimic), miR-21 inhibitor (cells transfected with 25 µM miR-21
inhibitor) and miR-21 mimic + LY294002 [cells transfected with
miR-21 mimic and subsequently treated with the PI3K inhibitor
LY294002 (10 µM; APExBio Technology) and incubated at 37°C for 48
h]. At 70% confluence, the cells were transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. At 48
h post-transfection at 37°C with 5% CO2, >60% cells
were successfully transfected. Prior to subsequent reverse
transcription-quantitative PCR (RT-qPCR), TRAP staining and western
blotting experiments, the cells were cultured with 50 ng/ml RANKL
for 3 days at 37°C with 5% CO2 to induce
osteoclastogenesis.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol and was reverse
transcribed using the SuperScript First Strand cDNA system
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. PCR amplification was performed using the
SYBR Green PCR master mix (Thermo Fisher Scientific, Inc.).
Stem-loop RT-qPCR and conventional RT-qPCR were used for
quantification of miR-21, and tartrate-resistant acid phosphatase
(TRAP; a specific marker of OCs and bone resorption) and Pten,
respectively. The primers used in the present study were
synthesized by Sangon Biotech Co., Ltd. (Table I). The reaction conditions were as
follows: 95°C for 10 min; 40 cycles of 95°C for 15 sec and 58°C for
30 sec; 72°C for 40 sec; and final extension at 72°C for 8 min. The
2−ΔΔCq method (14) was
used to calculate the relative expression levels of miR-21, TRAP
and Pten. miRNA and mRNA levels were normalized to the internal
reference genes U6 and GAPDH, respectively.
 | Table I.Primers sequence for reverse
transcription-quantitative PCR. |
Table I.
Primers sequence for reverse
transcription-quantitative PCR.
|
| Sequence (5′→3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| miR-21 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-GCCGCTAGCTTATCAGACTCAACA-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
| TRAP |
5′-ACACAGTGATGCTGTGTGGCAACTC-3′ |
5′-CCAGAGGCTTCCACATATATGATGG-3′ |
| Pten |
5′-AATTCCCAGTCAGAGGCGCTATGT-3′ |
5′-GATTGCAAGTTCCGCCACTGAACA-3′ |
| GAPDH |
5′-AACATCAAATGGGGTGAGGCC-3′ |
5′-GTTGTCATGGATGACCTTGGC-3′ |
TRAP staining
RANKL-induced RAW264.7 cells were seeded at a
density of 5×105 cells/well into 24-well plates and
cultured for 24 h at 37°C with 5% CO2. Subsequently, the
cells were fixed with 4% paraformaldehyde for 20 min at room
temperature. TRAP staining solution was added and incubated at 37°C
for 1 h in the dark. The procedure was performed according to the
TRAP staining kit protocol (Sigma Aldrich; Merck KGaA).
TRAP-positive multi-nucleated cells (TRAP+ MNCs)
containing >3 nuclei were considered OCs and were observed under
an inverted light microscope (magnification, ×200). The number of
TRAP+ MNCs/well was independently counted under the
inverted microscope by two individuals. The average of the three
experiments was taken as the number of OCs present.
Bone resorption assay
A pit assay was performed to observe the bone
resorption of OCs. Bovine bone slices were generated in the
laboratory and were obtained from fresh bovine cortical bone. Bone
was purchased from cows that were sacrificed for
commercial/consumer purposes. Notably, cows are not an endangered
species, and the bones were obtained from a licensed source and did
not contain potentially harmful agents (biological and chemical or
genetically modified material). Bovine cortical bone was dried,
rough-cut and sliced using a low speed diamond saw into pieces ~50
µm thick and 6 mm in diameter. Subsequently, the bone slices were
treated in distilled water for ultrasonic cleaning prior to use. At
48 h post-transfection and prior to treatment with RANKL, RAW264.7
cells were seeded onto the bovine bone slices in 24-well plates at
5×105 cells/well and were cultured in routine medium for
24 h at 37°C with 5% CO2. Subsequently, the medium was
changed to routine medium supplemented with 50 ng/ml RANKL for 3
days, and then the bovine bone slices were ultrasonicated at 30 Hz
for 10 min at room temperature in 1 mol/l NH4OH to
remove adherent cells. Bone slices were stained with 0.1% toluidine
blue at room temperature for 3–5 min and the bone resorption pits
were subsequently observed under a light microscope (magnification,
×400). Bone resorption was calculated as pit area/total bone area
of each slice, and was calculated using ImageJ software (version
1.8.0; National Institutes of Health).
Western blot analysis
Total protein was extracted from cells using a Total
Protein Extraction kit (Beiing Solarbio Science and Technology Co.,
Ltd.), according to the manufacturer's protocol. The bicinchoninic
acid method was used to measure protein concentration and 40 µg
protein/lane was separated by SDS-PAGE on 10% gels and transferred
to PVDF membranes (EMD Millipore). PVDF membranes were blocked in
TBS containing 0.25% Tween and 5% skimmed milk for 1 h at room
temperature. Membranes were incubated with primary antibodies
targeted against: Pten (cat. no. ab32199; 1:1,000), Akt (cat. no.
ab18785; 1:5,000), phosphorylated (p)-Akt (cat. no. ab38449;
1:1,000), NFATc1 (cat. no. ab25916; 1:500) and GAPDH (cat. no.
ab9485; 1:5,000) at 4°C overnight. All primary antibodies were
purchased from Abcam. Membranes were then incubated with
horseradish peroxidase-conjugated IgG secondary antibodies (cat.
no. ab6721; 1:10,000; Abcam) for 1 h at room temperature. ECL
detection reagent (Beiing Solarbio Science and Technology Co.,
Ltd.) was used to observe protein bands and relative protein
expression levels were calculated using ImageJ software (version
1.8.0; National Institutes of Health) with GAPDH as an internal
reference.
Statistical analysis
Each experiment was repeated at least 3 times. SPSS
software (version 19.0; IBM Corp.) was used to analyze all data.
Kolmogorov-Smirnov test was used to confirm that data were normally
distributed and data are presented as the mean ± SD. Comparisons
among multiple groups were assessed by one-way ANOVA followed by
Tukey's post hoc test. An unpaired Student's t-test was used to
assess the differences between the two groups in the
dual-luciferase assay. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-21 is upregulated
during osteoclastogenesis
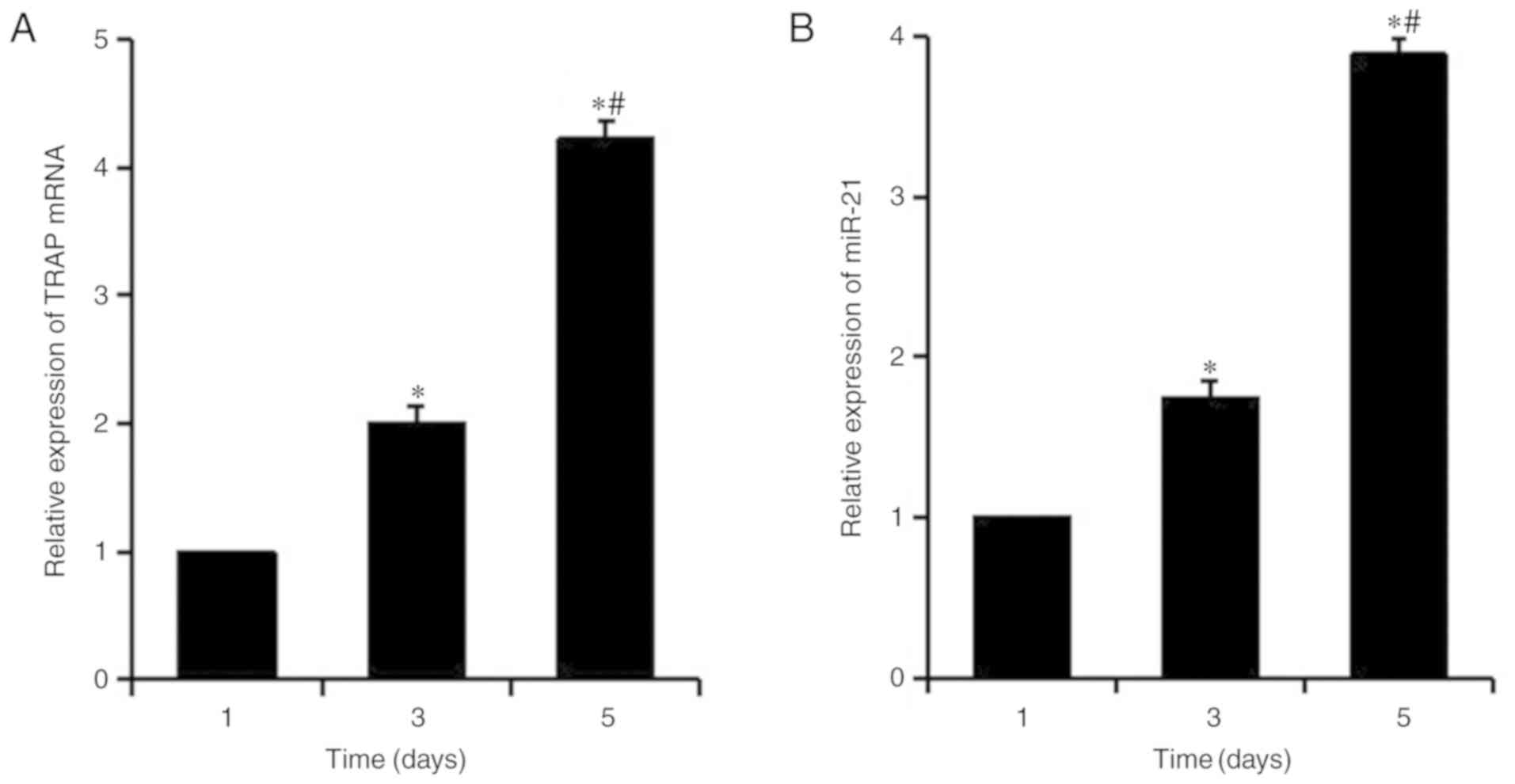
The mRNA expression levels of the OC marker TRAP
were detected in RANKL-induced macrophage RAW264.7 cells at
different time points by RT-qPCR, in order to analyze
osteoclastogenesis (Fig. 1A).
After 3 days of induction, the expression levels of TRAP were
significantly increased, and the expression of TRAP was
significantly higher on the fifth day compared with on the first
and third days. Our preliminary studies detected the background
levels of TRAP expression at 0 h and confirmed that they are lower
than at the first day of RANKL induction; therefore, the expression
of TRAP at 0 h was not recorded in the present study. As presented
in Fig. 1B, the expression levels
of miR-21 increased progressively during the process of
osteoclastogenesis and the change in miR-21 was consistent with the
upregulation of TRAP mRNA, indicating that miR-21 was upregulated
during osteoclastogenesis in vitro.
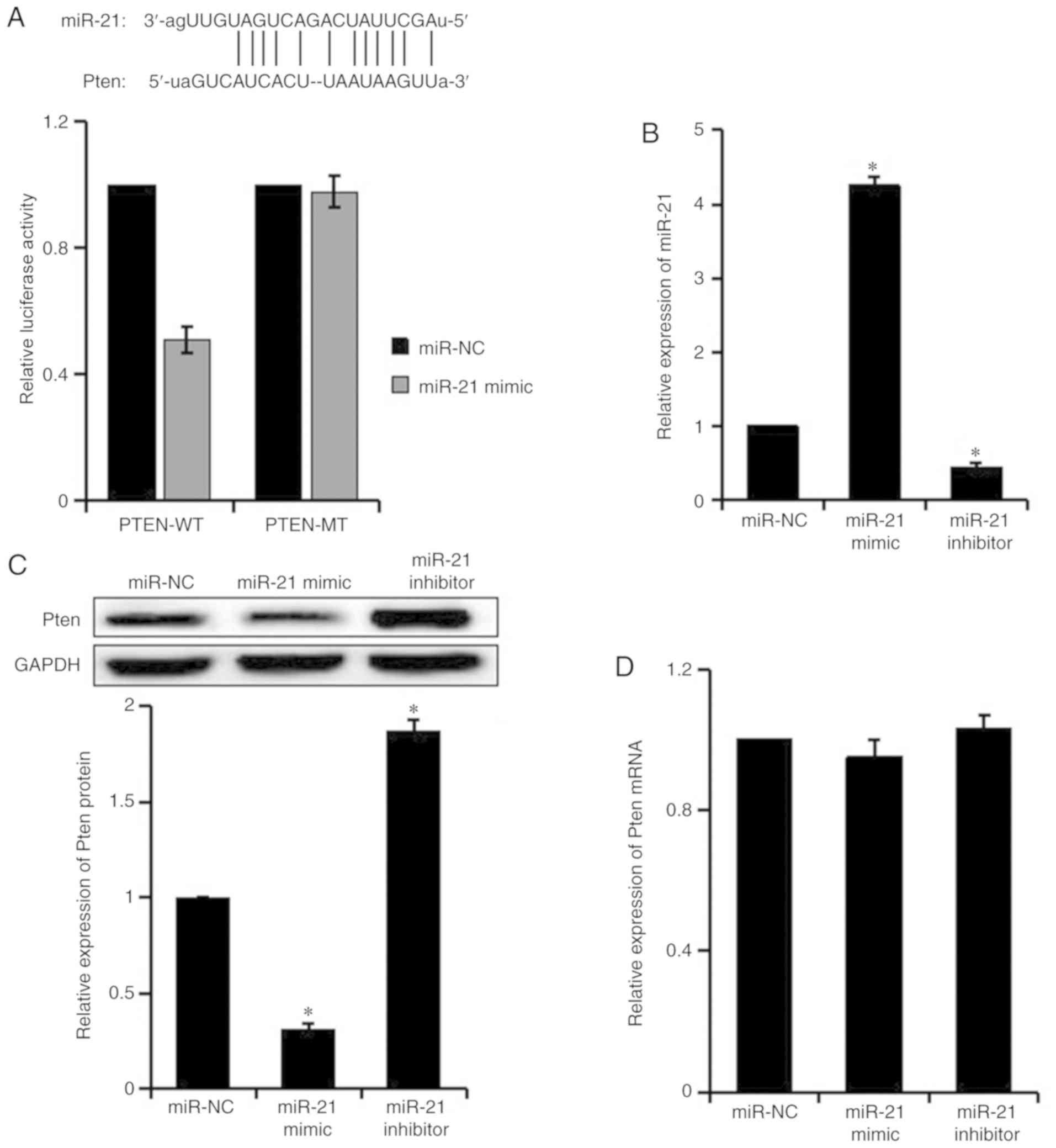
miR-21 targets Pten
Pten promotes the proliferation of T cells and tumor
cells, and has a critical role in RANKL-induced OC differentiation
(15,16). In the present study, target genes
that were potentially regulated by miR-21 were predicted by
TargetScan and miRTarBase; Pten, which contains miR-21 binding
sites in its 3′UTR, was selected as one of the target genes of
miR-21. The results of a dual luciferase reporter gene assay
revealed that the luciferase activity of Pten-WT was inhibited by
the miR-21 mimic (P<0.05), whereas the luciferase activity of
Pten-MT was not affected, thus suggesting that miR-21 directly
targeted Pten (Fig. 2A).
In order to verify the effects of miR-21 on Pten,
the expression levels of miR-21 and Pten were detected in
RANKL-induced RAW264.7 cells post-transfection by RT-qPCR and
western blotting (Fig. 2B-D).
Compared with the miR-NC group, miR-21 expression was increased,
whereas Pten protein expression was decreased in the miR-21 mimic
group. Furthermore, the expression levels of miR-21 were
downregulated, whereas the protein expression levels of Pten were
upregulated in the miR-21 inhibitor group. There was no difference
in Pten mRNA expression among the groups. These results suggested
that miR-21 negatively regulated Pten at the translational
level.
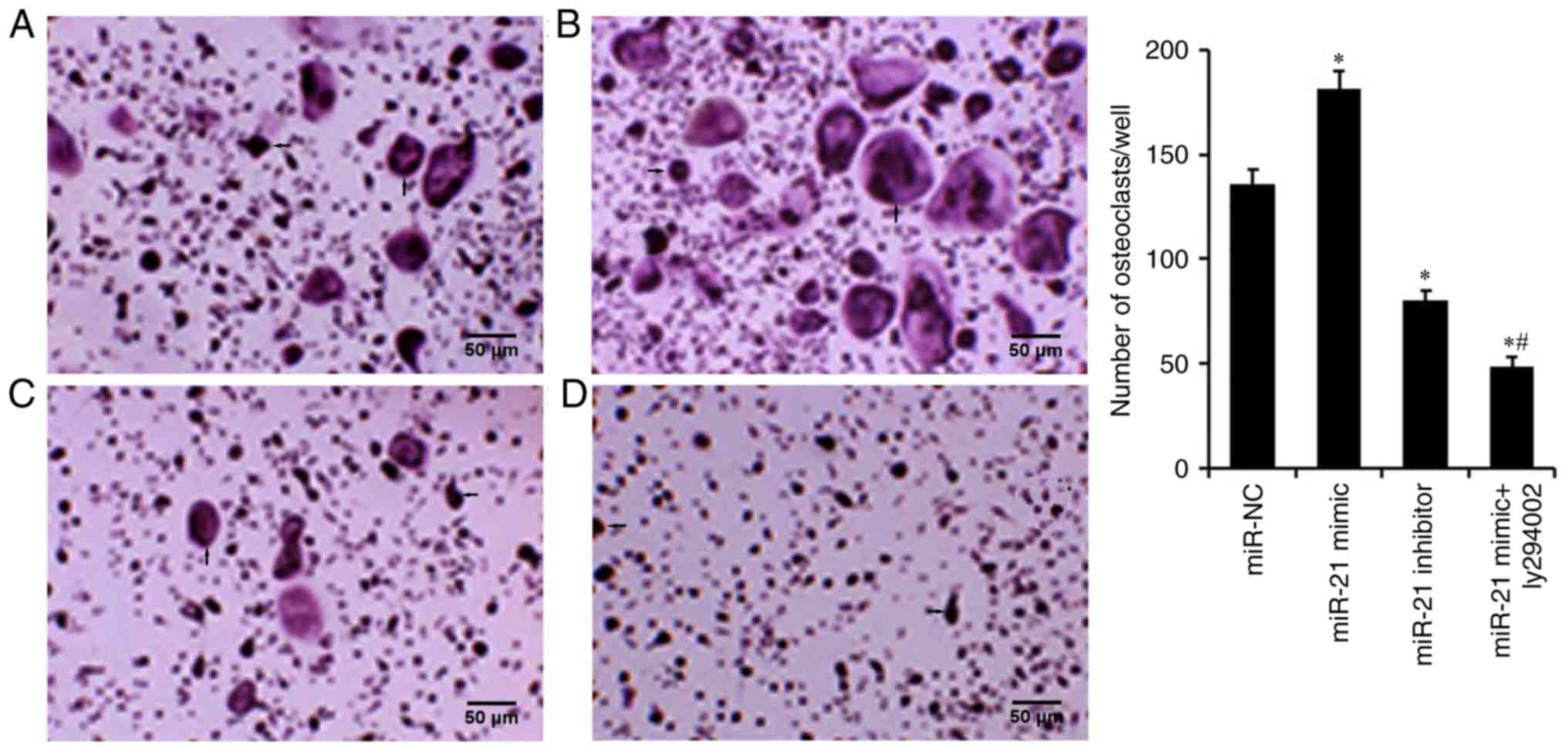
miR-21 promotes
osteoclastogenesis
TRAP staining was used to detect osteoclastogenesis
of RAW264.7 cells transfected with miR-21 mimic and miR-21
inhibitor, in order to assess the effect of miR-21 on OC
differentiation (Fig. 3). The
results revealed that the number of OCs was increased in the miR-21
mimic group and markedly decreased in the miR-21 inhibitor group,
when compared with the miR-NC group, thus indicating that miR-21
may be crucial for osteoclastogenesis.
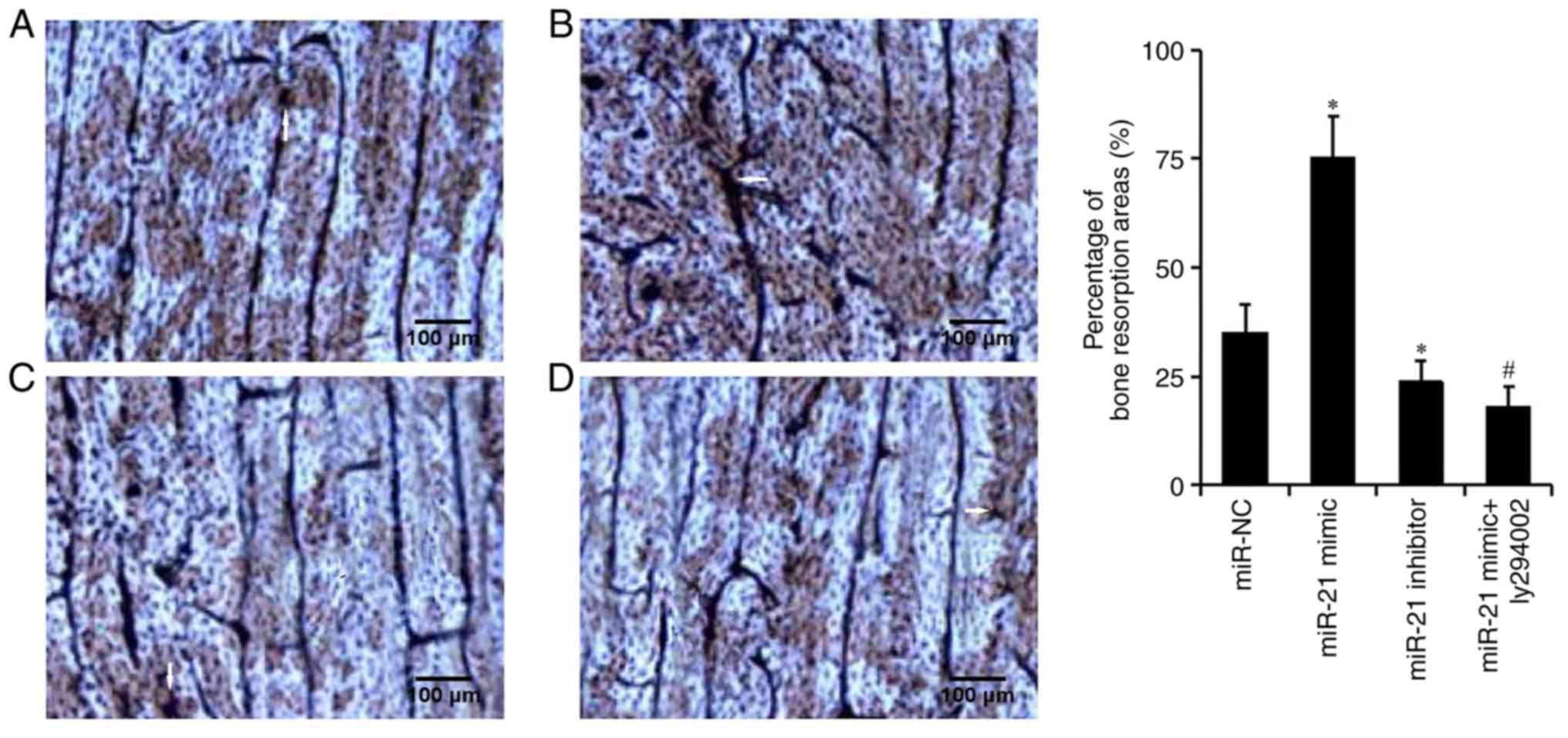
miR-21 enhances bone resorption of
OCs
The bone resorption assay was used to detect the
percentage of bone resorption (Fig.
4). The percentage of bone resorption was significantly higher
in the miR-21 mimic group than in the miR-NC group (75.4±10.2 vs.
35.1±7.3%, respectively), and was significantly higher than that in
the miR-21 inhibitor group (23.8±5.6%). These results suggested
that miR-21 could enhance the bone resorption of OCs.
miR-21 promotes osteoclastogenesis and
bone resorption via regulation of the Pten-PI3K/Akt signaling
pathway
It has previously been indicated that Pten regulates
osteoclastogenesis in RANKL-induced RAW264.7 OC precursors via
activating the PI3K/Akt signaling pathway (16). In the present study, the results of
TRAP staining and the bone resorption assay revealed that the
number of OCs and the percentage of bone resorption in the
miR-21mimic + LY294002 group were lower than those in the miR-21
mimic group (Figs. 3 and 4). These findings indicated that miR-21
may regulate osteoclastogenesis and bone resorption via regulating
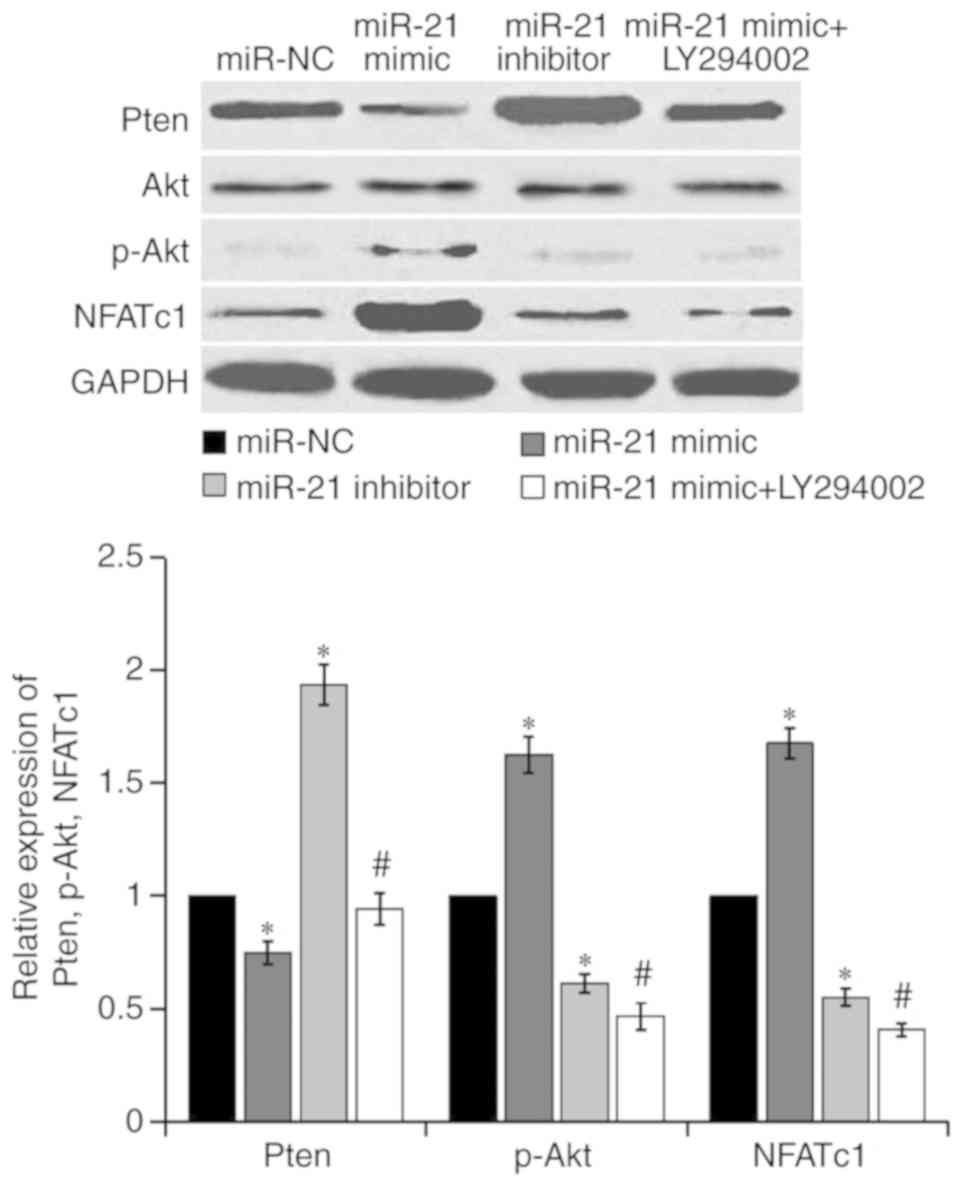
the PI3K/Akt signaling pathway. The present study further detected
Pten, Akt, p-Akt and NFATc1 expression in RAW264.7 cells
post-transfection, as presented in Fig. 5. The results demonstrated that,
compared with the miR-NC group, the protein expression levels of
Pten were decreased, and p-Akt and NFATc1 expression was increased
in the miR-21 mimic group. Conversely, the protein expression
levels of Pten were increased, whereas p-Akt and NFATc1 expression
levels were decreased in the miR-21 inhibitor group. Compared with
the miR-21 mimic group, the protein expression levels of Pten were
upregulated, whereas p-Akt and NFATc1 expression were reduced in
the miR-21 mimic + LY294002 group, thus suggesting that the PI3K
inhibitor LY294002 strongly reversed the effects induced by the
miR-21 mimic. These results indicated that miR-21 may promote
osteoclastogenesis and bone resorption via activation of the
PI3K/Akt signaling pathway by targeting Pten.
Discussion
Kagiya and Nakamura (17) reported that >50 miRNAs,
including miR-21, miR-146a and miR-155, are abnormally expressed
during TNF-α/RANKL-regulated osteoclastogenesis, thus suggesting
that these miRNAs may be essential in the regulation of OC
differentiation. Emerging evidence has indicated that miRNAs serve
important roles in osteoblastogenesis, chondrocyte proliferation
and differentiation, and osteoclastogenesis (18). However, the role of miRNAs in the
process of OC differentiation and its underlying molecular
mechanism remain to be elucidated; identifying these roles may
provide a novel direction for the clinical treatment of diseases
associated with bone metabolism.
As a carcinogenic miRNA, miR-21 is upregulated in
almost all types of cancer, and is responsible for promoting their
occurrence and development, including gastric cancer (19), colorectal cancer (20) and non-small cell lung cancer
(21). In recent years, the effect
of miR-21 on bone metabolism has received extensive attention:
Upregulation of miR-21 has been reported to not only increase the
expression of osteopontin and alkaline phosphatase in osteogenesis,
but may also promote mineralization during osteogenic induction
(22). Furthermore, it was
verified that miR-21 acts as a regulator of osteoclastogenesis and
a promoter of OC differentiation in vitro and in vivo
(16,23). Xu et al (24) also confirmed that miR-21 was
upregulated in A549 cells and overexpression of miR-21 facilitated
osteoclastogenesis by increasing the levels of miR-21 in exosomes.
In the present study, miR-21 was upregulated during
osteoclastogenesis in RANKL-induced RAW264.7 cells, and it was
revealed that upregulation of miR-21 could promote OC
differentiation and bone resorption, whereas downregulation of
miR-21 could inhibit OC differentiation and bone resorption. These
findings indicated that miR-21 was crucial to
osteoclastogenesis.
At present, the molecular mechanism underlying the
effects of miR-21 on the regulation of osteoclastogenesis remains
unclear. Sugatani et al (25) demonstrated that the transcription
factor c-Fos upregulated miR-21 expression, and miR-21
downregulated programmed cell death (PDCD4) protein expression,
whereas diminished PDCD4 removed the suppressive effects of c-Fos
in the process of RANKL-induced osteoclastogenesis; therefore, a
positive feedback loop of c-Fos/miR-21/PDCD4 may be a potential
novel molecular mechanism underlying the regulation of
osteoclastogenesis. Fujita et al (26) also confirmed that c-Fos promoted OC
differentiation by binding to miR-21 through activation protein 1,
and that miR-21 served a central role during estrogen-controlled
osteoclastogenesis: Estrogen induced osteoclast apoptosis by
downregulating miR-21 expression and by increasing the target gene
of miR-21, FasL. Pitari et al (10) reported that miR-21 expression was
markedly enhanced, whereas OPG was strongly reduced in bone marrow
stromal cell (BMSCs) adherent multiple myeloma cells and OPG was
targeted by miR-21. Furthermore, treatment of BMSCs with miR-21
inhibitors restored RANKL/OPG balance and markedly impaired the
resorptive activity of mature OCs (10). However, a number of target genes of
miR-21 have been revealed via bioinformatics analysis. In the
present study, bioinformatics and dual luciferase reporter assays
verified that Pten was a target gene of miR-21, and Pten was
negatively regulated by miR-21. Pten is a tumor suppressor and as a
dual-specificity phosphatase, it regulates the cell cycle, inhibits
cell proliferation and promotes apoptosis by blocking the PI3K/Akt
signaling pathway. Conversely, miR-21 promotes tumor cell growth,
proliferation, invasion and metastasis by inhibiting Pten, which
leads to abnormal activation of the PI3K/Akt pathway; this is
particularly associated with an increase in p-Akt (27,28).
The present study demonstrated that the number of OCs and the
percentage of bone resorption were decreased in the miR-21 mimic +
LY294002 group compared with in the miR-21 mimic group. Compared
with the miR-21 mimic group, the protein expression levels of Pten
were increased, and p-Akt and NFATc1 expression was decreased in
the miR-21 mimic + LY294002 group, indicating that LY294002
reversed the mir-21 mimic-induced decreases in Pten, and increases
in p-Akt and NFATc1. It has therefore been hypothesized that miR-21
promoted osteoclastogenesis and bone resorption through activation
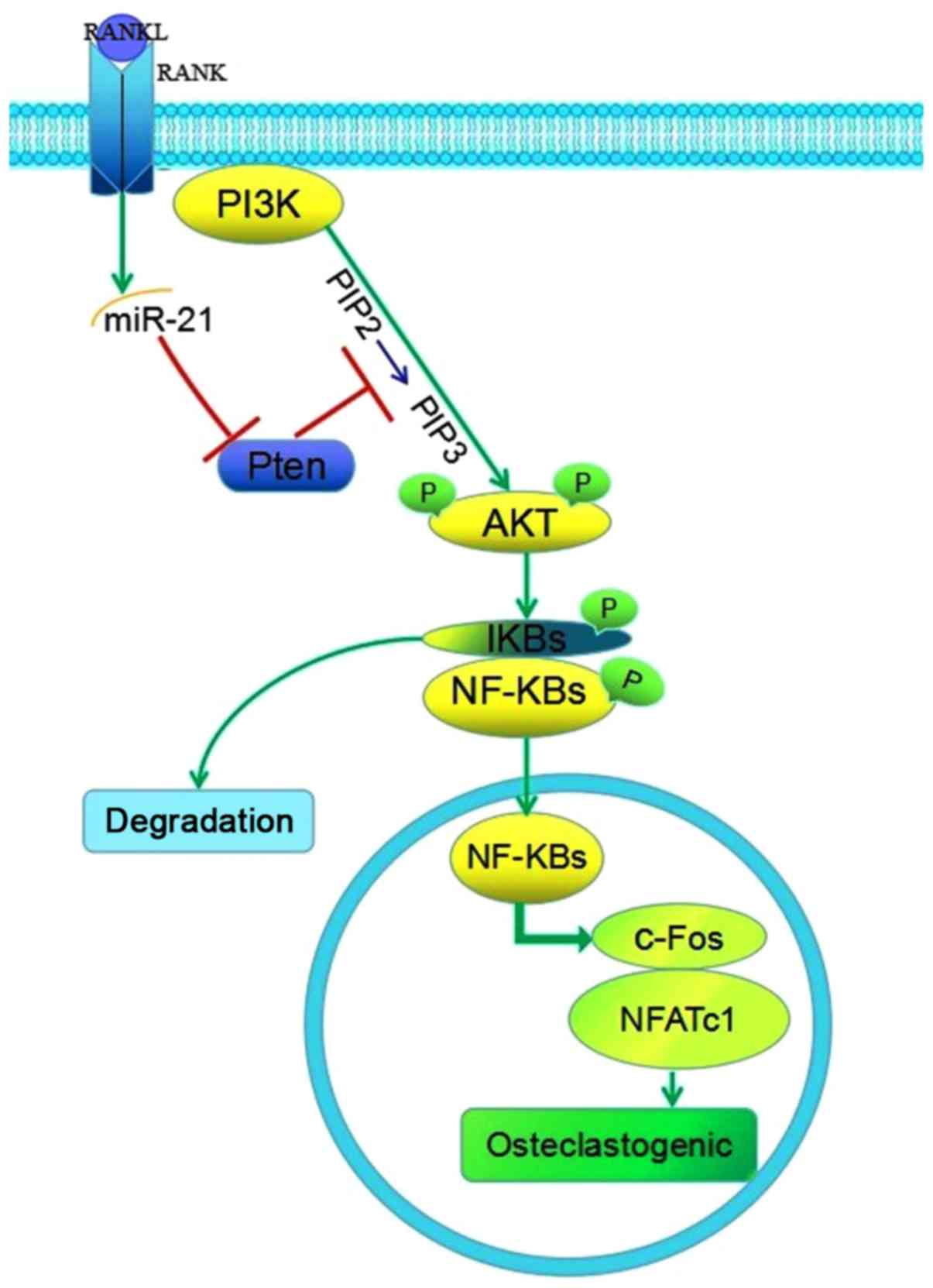
of the PI3K/Akt signaling pathway by targeting Pten (Fig. 6).
In conclusion, the present study revealed that
miR-21 was upregulated in osteoclastogenesis, and promoted
osteoclastogenesis and bone resorption via activating the PI3K/Akt
signaling pathway through targeting Pten. These results provided
preliminary evidence towards elucidating the molecular mechanism
underlying how miR-21 promotes osteoclastogenesis, laying a
theoretical foundation for the clinical treatment of diseases
associated with bone metabolism, including osteoporosis and
osteomalacia.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of China (grant. no. 81471558).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request, and in the TargetScan (www.targetscan.org/vert_72) and miRTarBase (mirtarbase.mbc.nctu.edu.tw/php/index.php)
databases.
Authors' contributions
SW and XW designed the study. SW drafted the
manuscript. ZL performed TRAP staining and the bone resorption
assay. JW analyzed and verified the target gene of miR-21. XJ
performed western blotting. ZY acquired, analyzed and interpreted
the data. XW revised the manuscript. All authors approved the final
published version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Drissi H and Sanjay A: The multifaceted
osteoclast; far and beyond bone resorption. J Cell Biochem.
117:1753–1756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyce BF: Advances in the regulation of
osteoclasts and osteoclast functions. J Dent Res. 92:860–867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodge JM, Collier FM, Pavlos NJ, Kirkland
MA and Nicholson GC: M-CSF potently augments RANKL-induced
resorption activation in mature human osteoclasts. PLoS One.
6:e214622011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen C, Cheng P, Xie H, Zhou HD, Wu XP,
Liao EY and Luo XH: MiR-503 regulates osteoclastogenesis via
targeting RANK. J Bone Miner Res. 29:338–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie
H, Zhu W, Dai RC, Wu XP, Liao EY and Luo XH: miR-148a regulates
osteoclastogenesis by targeting V-maf musculoaponeurotic
fibrosarcoma oncogene homolog B. J Bone Miner Res. 28:1180–1190.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizoguchi F, Murakami Y, Saito T, Miyasaka
N and Kohsaka H: miR-31 controls osteoclast formation and bone
resorption by targeting RhoA. Arthritis Res Ther. 15:R1022013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge J, Guo S, Fu Y, Zhou P, Zhang P, Du Y,
Li M, Cheng J and Jiang H: Dental follicle cells participate in
tooth eruption via the RUNX2-MiR-31-SATB2 loop. J Dent Res.
94:936–944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JH, Zheng WW, Cheng ST, Liu BX, Liu
FR and Song JQ: Correlation between microRNA21 and sprouty homolog
2 gene expression in multiple myeloma. Mol Med Rep. 11:4220–4224.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pitari MR, Rossi M, Amodio N, Botta C,
Morelli E, Federico C, Gullà A, Caracciolo D, Di Martino MT,
Arbitrio M, et al: Inhibition of miR-21 restores RANKL/OPG ratio in
multiple myeloma-derived bone marrow stromal cells and impairs the
resorbing activity of mature osteoclasts. Oncotarget.
6:27343–27358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugatani T and Hruska KA: Down-regulation
of miR-21 biogenesis by estrogen action contributes to osteoclastic
apoptosis. J Cell Biochem. 114:1217–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ocana A, Vera-Badillo F, Al-Mubarak M,
Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L, Cuenca-Lopez MD,
Seruga B, Pandiella A and Amir E: Activation of the PI3K/mTOR/AKT
pathway and survival in solid tumors: Systematic review and
meta-analysis. PLoS One. 9:e952192014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L and Guo X: Research advance in
signal pathways and signal factors during the process of osteoclast
differentiation. Chin J Osteoporo. 21:742–748. 2015.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan J, Ling X, Peng B and Ding G:
MiR-142-5p regulates CD4+ T cells in human non-small cell lung
cancer through PD-L1 expression via the PTEN pathway. Oncol Rep.
40:272–282. 2018.PubMed/NCBI
|
|
16
|
Jang HD, Noh JY, Shin JH, Lin JJ and Lee
SY: PTEN regulation by the Akt/GSK-3β axis during RANKL signaling.
Bone. 55:126–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kagiya T and Nakamura S: Expression
profiling of microRNAs in RAW264.7 cells treated with a combination
of tumor necrosis factor alpha and RANKL during osteoclast
differentiation. J Periodontal Res. 48:373–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng Z and Jin J: MicroRNAs in regulation
of bone metabolism: A literature review. Orthop J China.
24:1019–1022. 2016.
|
|
19
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peacock O, Lee AC, Cameron F, Tarbox R,
Vafadar-Isfahani N, Tufarelli C and Lund JN: Inflammation and
MiR-21 pathways functionally interact to downregulate PDCD4 in
colorectal cancer. PLoS One. 9:e1102672014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Xu L, Huang S, Hou Y, Liu Y, Chan
KM, Pan XH and Li G: mir-21 overexpressing mesenchymal stem cells
accelerate fracture healing in a rat closed femur fracture model.
Biomed Res Int. 2015:4123272015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu CH, Sui BD, Du FY, Shuai Y, Zheng CX,
Zhao P, Yu XR and Jin Y: miR-21 deficiency inhibits osteoclast
function and prevents bone loss in mice. Sci Rep. 7:431912017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Z, Liu X, Wang H, Li J, Dai L, Li J and
Dong C: Lung adenocarcinoma cell-derived exosomal miR-21
facilitates osteoclastogenesis. Gene. 666:116–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugatani T, Vacher J and Hruska KA: A
microRNA expression signature of osteoclastogenesis. Blood.
117:3648–3657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujita S, Ito T Mizutani T, Minoguchi S,
Yamamichi N, Sakurai K and Iba H: miR-21 gene expression triggered
by AP-1 is sustained through a double-negative feedback mechanism.
J Mol Biol. 378:492–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang P, Guan Q, Zhou D, Yu Z, Song Y and
Qiu W: miR-21 inhibitors modulate biological functions of gastric
cancer cells via PTEN/PI3K/mTOR pathway. Dna Cell Biol. 37:38–45.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiong B, Cheng Y, Ma L and Zhang C: MiR-21
regulates biological behavior through the PTEN/PI-3 K/Akt signaling
pathway in human colorectal cancer cells. Int J Oncol. 42:219–228.
2013. View Article : Google Scholar : PubMed/NCBI
|