Introduction
Ovarian cancer (OC) is the most lethal form of
gynecological malignancy and the fifth most common cause of
cancer-associated mortality among women worldwide (1,2). The
etiology of OC remains unclear and may be related to the following
factors: Genetics, early menarche, ovulation abnormalities,
nulliparity or not breastfeeding. Despite improvements in the
diagnosis, surgery and chemotherapy treatments available for OC
over the past few decades, the overall survival (OS) of patients
with OC has not changed, with a 5-year survival rate for all stages
of 35–38% (3,4). One of the most prominent factors
contributing to this poor outcome is the fact that the majority of
patients with OC are diagnosed at a late stage (stage III–IV),
which is closely associated with tumor recurrence and metastasis
(5). A number of previous studies
have reported that the initiation and development of OC is a
multi-step pathological process involving a variety of alterations
in gene expression and gene variants (6,7).
Therefore, an enhanced knowledge of the mechanism, and the
identification of novel prognostic and therapeutic targets is
crucial to devising novel, effective therapies for patients with
OC.
Long non-coding RNAs (lncRNAs) are a group of
non-coding RNAs>200 nucleotides in length that do not encode
proteins (8). The majority of
lncRNAs are evolutionarily conserved and serve important functions
in the modulation of gene expression at the post-transcriptional
level (9,10); previous studies have observed that
lncRNAs are involved in regulating various cellular processes, such
as cell proliferation, cell cycle, cell apoptosis,
epithelial-mesenchymal transition, metastasis and chemosensitivity
(11,12). To date, accumulating evidence has
suggested that the aberrant expression of lncRNAs is related to the
initiation and development of various types of human malignancy,
and that lncRNAs may be considered potential candidate prognostic
biomarkers (13). For example, Liu
et al (14) demonstrated
that long intergenic non-protein coding RNA 460 served a role in OC
progression and suggested that it could be used as a novel
therapeutic strategy for OC. Li et al (15) observed that the upregulation of
SPRY4 intronic transcript 1 accelerated tumorigenesis in patients
with OC and its upregulation was associated with a poor prognosis.
In addition, Luo and Liu (16)
reported that TTN antisense RNA 1 (TTN-AS1) may have a carcinogenic
role in lung adenocarcinoma through destabilizing the PTEN gene to
activate the PI3K/AKT signaling pathway, thus suggesting that
TTN-AS1 may be a potential biomarker for lung adenocarcinoma
treatment. However, the lncRNA-mediated regulatory mechanism
driving the initiation and development of OC remains largely
unknown.
Cervical carcinoma expressed PCNA regulatory lncRNA
(CCEPR) has been reported to serve an oncogenic role in cervical
cancer and urothelial bladder carcinoma (17,18);
however, the clinical significance and biological function of CCEPR
in OC has not yet been illustrated. In the present study, the
oncogenic function of CCEPR in OC and its prognostic value in
patients with OC was confirmed. CCEPR knockdown suppressed the
progression of OC by decreasing cell proliferation and invasion,
and inducing cell apoptosis. In addition, CCEPR inhibition was
observed to decrease the expression levels of proteins involved in
the Wnt/β-catenin signaling pathway, including cyclin D1,
β-catenin, Myc and matrix metallopeptidase-7 (MMP-7). In
conclusion, the present study provided a novel insight into the
biological role and regulatory pathways of CCEPR in OC progression,
and identified CCEPR as a promising prognostic and therapeutic
biomarker for OC intervention.
Materials and methods
Patient studies
The present study was conducted in accordance with
the Declaration of Helsinki 1991 and was approved by the
Institutional Review Board of Tianjin Central Hospital of
Gynecology and Obstetrics (Tianjin, China). Written informed
consent was obtained from all patients. A total of 70 paired OC
tissues and corresponding normal tissues were collected from
patients (aged 28–72 years) at Tianjin Central Hospital of
Gynecology and Obstetrics between February 2007 and August 2015.
The following inclusion criteria were required: i) Good indication
of suitability for surgical intervention; ii) must not have
received radiotherapy or chemotherapy prior to surgery; and iii)
complete clinical history. The following exclusion criteria were
required: i) Previous surgical intervention; ii) severe liver
and/or kidney dysfunction; iii) abnormal coagulation; and iv)
incomplete clinical history. All clinical and pathological
information was obtained from each patient's history record. An
experienced gynecological pathologist at the hospital assessed all
tissue specimens. After surgery, each patient was followed up every
3 months for a period of 60 months. The OS time was defined as the
time between the date of surgery and the date of death or last
follow-up. All clinical specimens were immediately snap-frozen in
liquid nitrogen and subsequently stored at −80°C until required for
RNA extraction.
Cell lines and reagents
The two human OC cell lines, SK-OV-3 (cat. no.
HTB-77) and OVCAR-3 (cat. no. HTB-161), were purchased from the
American Type Culture Collection. The human ovarian surface
epithelial cell line HOSEpiC (cat. no. 7310) was obtained from
ScienCell Research Laboratories, Inc. All cells were cultured in
RPMI-1640 medium (HyClone; GE Healthcare Life Sciences),
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences)
and 100 µg/ml penicillin/streptomycin. All cells were cultured at
37°C in a humidified atmosphere containing 5% CO2.
Cell transfection
Two short hairpin (sh)RNAs targeting CCEPR, shCCERP1
(cat. no. H-4861, 5′-ATGTTATAGCTAAATGGATGTGACTAG-3′) and shCCEPR2
(cat. no. H-4862, 5′-CATTTTATGTCTTGACAATGCCTCGATTTG-3′), and the
corresponding scrambled shRNAs [negative control (NC)], shNC1 (cat.
no. H-4861-1, 5′-ACTGGCTCATTACCTGAGGAAATGTGT-3′) and shNC2 (cat.
no. H-4862-1, 5′-AATGTTGGACCCTTTACAGTTGGAAATC-3′), were designed
and cloned into psi-H1 vector from GeneCopoeia, Inc (Guangzhou,
China). Sequences of all oligonucleotides were confirmed by Sanger
sequencing (Thermo Fisher Scientific, Inc.). Upon reaching 80%
confluence, cells in 6-well plates at 8×106 cells/well
were transfected with 2 µg/well shRNA or NC using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) for 6 h at 37°C, according to the manufacturer's
protocol. Cells were incubated with RPMI-1640 at 37°C supplemented
with 10% FBS for 48 h prior to subsequent experimentation. The
efficiency of cell transfection was analyzed using reverse
transcription-quantitative PCR (RT-qPCR) analysis.
Cell proliferation assays
To measure the cell proliferation rate, a total of
3×103 transfected OC cells/well were plated into 96-well
plates and cultured overnight. Subsequently, 10 µl Cell Counting
Kit-8 (CCK-8) solution (Dojindo Molecular Technologies, Inc.) was
added to each well at the at 0, 24, 48 and 72 h. Following
incubation for 3 h at 37°C, the absorbance was measured at 450 nm
using an automatic microplate reader (Bio-Rad Laboratories,
Inc.).
Western blotting
Total protein was extracted from cells with ice-cold
mammalian cell total protein lysis buffer (Sangon Biotech Co.,
Ltd.) and centrifuged at 13,000 × g for 30 min to obtain the
protein samples at 4°C. Total protein was quantified using a
bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.) and 25 µg protein/lane was separated via SDS-PAGE on a 12%
gel. The separated proteins were subsequently transferred onto PVDF
membranes (EMD Millipore) and blocked with 5% non-fat milk in
TBS-0.1% Tween 20 for 1 h at 37°C. The membranes were incubated
with the following primary antibodies overnight at 4°C: Anti-Myc
(1:1,000; cat. no. D199941; Sangon Biotech Co., Ltd.), anti-MMP-7
(1:2,000; cat. no. MAB9071-100; R&D Systems, Inc.), anti-cyclin
D1 (1:1,000; cat. no. D198702; Sangon Biotech Co., Ltd.),
anti-β-catenin (1:500; cat. no. D199519; Sangon Biotech Co., Ltd.)
and anti-GAPDH (1:2,500; cat. no. D190090; Sangon Biotech Co.,
Ltd.). Following the primary antibody incubation, membranes were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:2,000; cat. no. D110098; Sangon Biotech Co., Ltd.)
for 1 h at 37°C. Protein bands were visualized using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.) and protein
expression was semi-quantified using a v4.6.6 Quantity One software
(Bio-Rad Laboratories, Inc.), with GAPDH as the loading
control.
Bioinformatics analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway online database (https://www.genome.jp/kegg/pathway.html) was used to
predict the downstream signaling pathways of CCEPR.
ELISA
Human Bcl-2 (cat. no. CSB-E08853h), Bax (cat. no.
CSB-E09344h) and caspase-3 (cat. no. CSB-E08856h) ELISA kits
(Cusabio Technology LLC) were used to analyze the expression levels
of Bcl-2, Bax and caspase-3 in transfected cells. Briefly, a total
of 1×106 transfected OC cells were harvested and lysed
with lysis buffer (Cusabio Technology LLC) provided by the kits on
ice for 30 min at 4°C and centrifuged at 13,000 × g for 30 min to
obtain the supernatants at 4°C. A total of 100 µl/well supernatant
was added to the 96-well ELISA plates and incubated at 37°C for 30
min. Subsequently, 100 µl 1X HRP-conjugate solution was added to
each well and incubated for 30 min at 37°C. Then, 90 µl
tetramethylbenzidine substrate solution/well was added to the
plates and incubated for 20 min at 37°C in the dark. Finally, 50 µl
stop solution was added to each well to terminate the reaction and
the optical density value at 570 nm was measured using an automatic
microplate reader (Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNA was extracted from OC cell lines and
cancer/normal tissues using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The quality was assessed using a SmartSpec Plus
spectrophotometer (Bio-Rad Laboratories, Inc.) and 1 µg RNA was
reverse transcribed into cDNA using a cDNA Synthesis kit (Toyobo
Life Science), according to the manufacturer's protocol. qPCR was
subsequently performed using the SYBR® qPCR mix (Toyobo
Life Science) and a 7900HT Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The following primer pairs (Sangon Biotech
Co., Ltd.) were used for qPCR: CCEPR, forward,
5′-AAGGTCCCAGGATACTCGC-3′, reverse, 5′-GTGTCGTGGACTGGCAAAAT-3′; and
GAPDH, forward, 5′-CGCTCTCTGCTCCTCCTGTTC-3′ and reverse,
5′-ATCCGTTGACTCCGACCTTCAC-3′. The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 60
sec; followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec and
72°C for 45 sec, finally at 4°C for 30 min. Expression levels were
quantified using the 2−ΔΔCq method (19) and normalized to the internal
reference gene GAPDH.
Flow cytometry
A total of 7×104 transfected cells/well
were plated into 6-well plates and cultured for 48 h in 5%
CO2, prior to being harvested. Subsequently, cells were
centrifuged at 1,000 × g for 10 min at 37°C and 1×106
cells were fixed overnight with 70% ice-cold ethanol at 4°C. The
cells were resuspended in PBS containing 50 mg/ml RNase A (cat. no.
ST576; Beyotime Institute of Biotechnology) for 30 min at 37°C, and
blocked with 10% goat serum (Solarbio Science & Technology Co.,
Ltd., Beijing, China) for 2 h at 37°C. Next, the cells were stained
with 3 µl propidium iodide and 5 µl Annexin V-FITC (Thermo Fisher
Scientific, Inc.) in the dark for 1 h at 37°C. The apoptotic cells
were visualized using a BD FACSCalibur™ flow cytometer
(BD Biosciences) and subsequently analyzed using BD
CellQuest™ Pro version 5.1 software (BD
Biosciences).
Transwell invasion assay
The invasion assay was performed using a 24-well
Boyden chamber (pore size, 8 µm; Corning, Inc.). A total of
5×104 transfected cells/well were resuspended in 200 µl
serum-free RPMI-1640 medium and were plated in the upper chambers,
which were precoated with Matrigel. A total of 500 µl RPMI-1640
medium supplemented with 10% FBS was plated in the lower chambers
as a chemoattractant. After 48 h, the invasive cells were fixed
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) at 37°C for 30
min and subsequently stained with 0.5% crystal violet
(Sigma-Aldrich; Merck KGaA) at 37°C for 15 min. Stained cells were
counted in five randomly selected fields using an Olympus light
microscope at ×200 magnification (Olympus Corporation).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc.). Data are presented as
the mean ± SD and are representative of ≥3 experimental repeats.
The association between CCEPR expression and clinicopathological
variables was analyzed using a χ2 test; Kaplan-Meier
curves and the log-rank test were used to analyze the association
between CCEPR expression levels and OS in patients with OC;
Student's t-tests were used to identify statistical differences
between two groups; and differences among >2 groups were
analyzed using one-way ANOVA followed by a Bonferroni's correction
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CCEPR is highly expressed in OC
tissues and cell lines
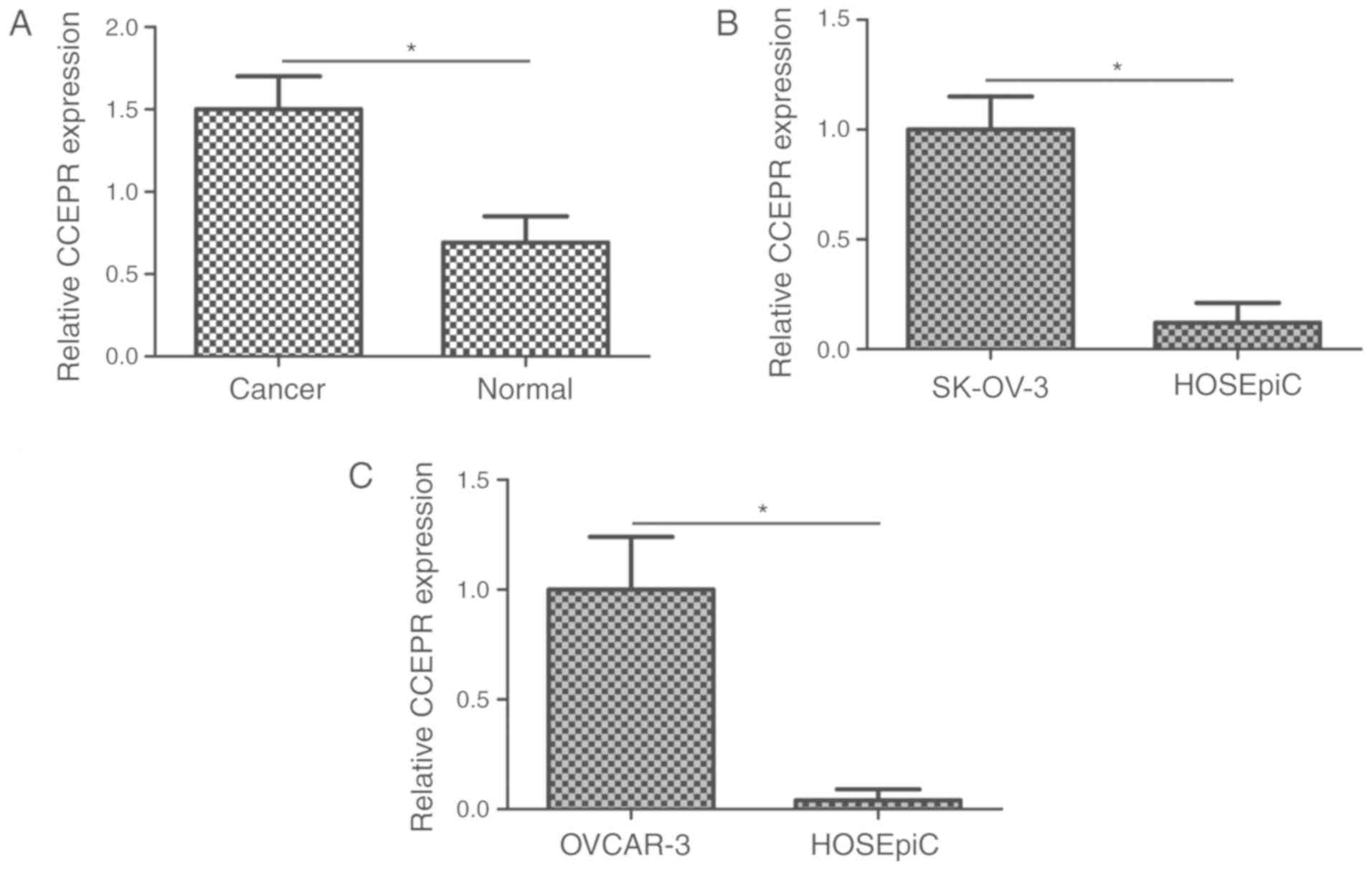
To determine the potential role of CCEPR in the
progression of OC, RT-qPCR was performed to analyze the expression
levels of CCEPR in 70 OC and matched normal tissues. The expression
levels of CCEPR were significantly increased in OC tissues compared
with the matched normal tissues (Fig.
1A; P<0.05). Furthermore, CCEPR expression was also examined
in two OC cell lines, SK-OV-3 and OVCAR-3. Compared with the
HOSEpiC cell line, CCEPR expression levels were significantly
increased in the two OC cell lines (Fig. 1B and C; P<0.05).
CCEPR expression is associated with
the clinicopathological features and prognosis of patients with
OC
To determine the clinical value of CCEPR in OC, the
relationship between the expression levels of CCEPR and
clinicopathological variables was analyzed. Based on the median
expression levels of CCEPR in OC tissues, patients with OC were
divided into two groups: High expression group (n=35) and low
expression group (n=35). Increased CCEPR expression levels were
associated with an increased invasive ability and a higher
International Federation of Gynecology and Obstetrics (FIGO) stage
(Table I; P<0.05), but not with
patients' age, pathological type, differentiation, tumor size,
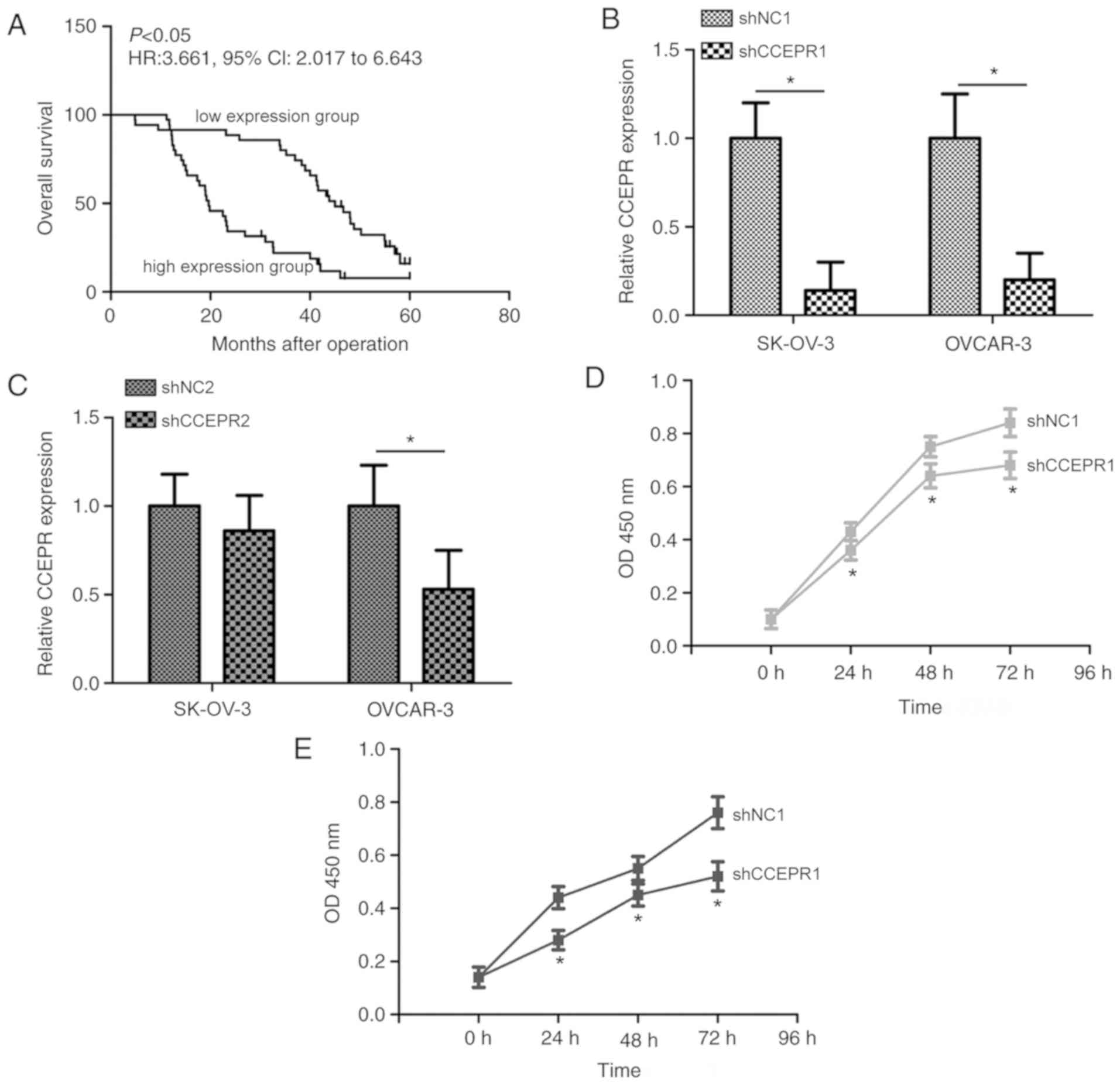
CA125 levels or lymph node metastasis. Kaplan-Meier curves revealed
that the 5-year OS rate was significantly lower in patients within
the high expression group (11.43%) compared with patients in the
low expression group (25.71%; Fig.
2A; P<0.05). The median survival time of patients with OC in
the low expression group was 45.0 months, whereas the median
survival time of patients with OC in the high expression group was
19.6 months. These data suggested that increased CCEPR expression
levels may be related to aggressive clinicopathological behaviors
and may predict an unfavorable prognosis in patients with OC.
 | Table I.Association between CCEPR expression
levels and clinicopathological variables in patients with ovarian
cancer. |
Table I.
Association between CCEPR expression
levels and clinicopathological variables in patients with ovarian
cancer.
|
|
| CCEPR expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | N | Low | High | χ2 | P-value |
|---|
| Age (years) |
|
|
| 2.809 | 0.094 |
|
<50 | 37 | 15 | 22 |
|
|
| ≥50 | 33 | 20 | 13 |
|
|
|
Differentiation |
|
|
| 2.885 | 0.089 |
|
Well | 29 | 18 | 11 |
|
|
|
Poor | 41 | 17 | 24 |
|
|
| Tumor size
(cm) |
|
|
| 2.692 | 0.101 |
|
<4 | 52 | 29 | 23 |
|
|
| ≥4 | 18 | 6 | 12 |
|
|
| CA125 levels
(U/ml) |
|
|
| 3.049 | 0.081 |
|
<5,000 | 25 | 16 | 9 |
|
|
|
≥5,000 | 45 | 19 | 26 |
|
|
| Pathological
type |
|
|
| 0.402 | 0.526 |
|
Serous | 58 | 30 | 28 |
|
|
|
Mucinous | 12 | 5 | 7 |
|
|
| Invasion |
|
|
| 4.242 | 0.039 |
| T1 +
T2 | 22 | 15 | 7 |
|
|
| T3 +
T4 | 48 | 20 | 28 |
|
|
| Lymph node
metastasis |
|
|
| 1.296 | 0.255 |
| Absent
(N0) | 16 | 10 | 6 |
|
|
| Present
(N1-N3) | 54 | 25 | 29 |
|
|
| International
federation of gynecology and obstetrics stage |
|
|
| 17.425 | <0.001 |
|
I–II | 27 | 22 | 5 |
|
|
|
III–IV | 43 | 13 | 30 |
|
|
CCEPR knockdown suppresses the
proliferation and invasion of OC cells
Given the association of CCEPR expression with
invasion and FIGO stage in OC, the biological effects of CCEPR on
the proliferation rate and invasive ability of OC cells were
further investigated by loss-off function assays. CCEPR expression
levels were significantly decreased in SK-OV-3 and OVCAR-3 cells
following transfection with shCCEPR1 compared with
shNC1-transfected cells (Fig. 2B;
P<0.05); however, transfection with shCCEPR2 only significantly
decreased expression levels in OVCAR-3 cells compared with
shNC2-transfected cells (Fig. 2C).
Therefore, the shCCEPR1 shRNA was chosen for subsequent
experiments. The CCK-8 assay revealed that the proliferative
ability of cells was markedly suppressed in shCCEPR-1-transfected
SK-OV-3 and OVCAR-3 cells compared with the shNC1-transfected group
(Fig. 2D and E; P<0.05). In
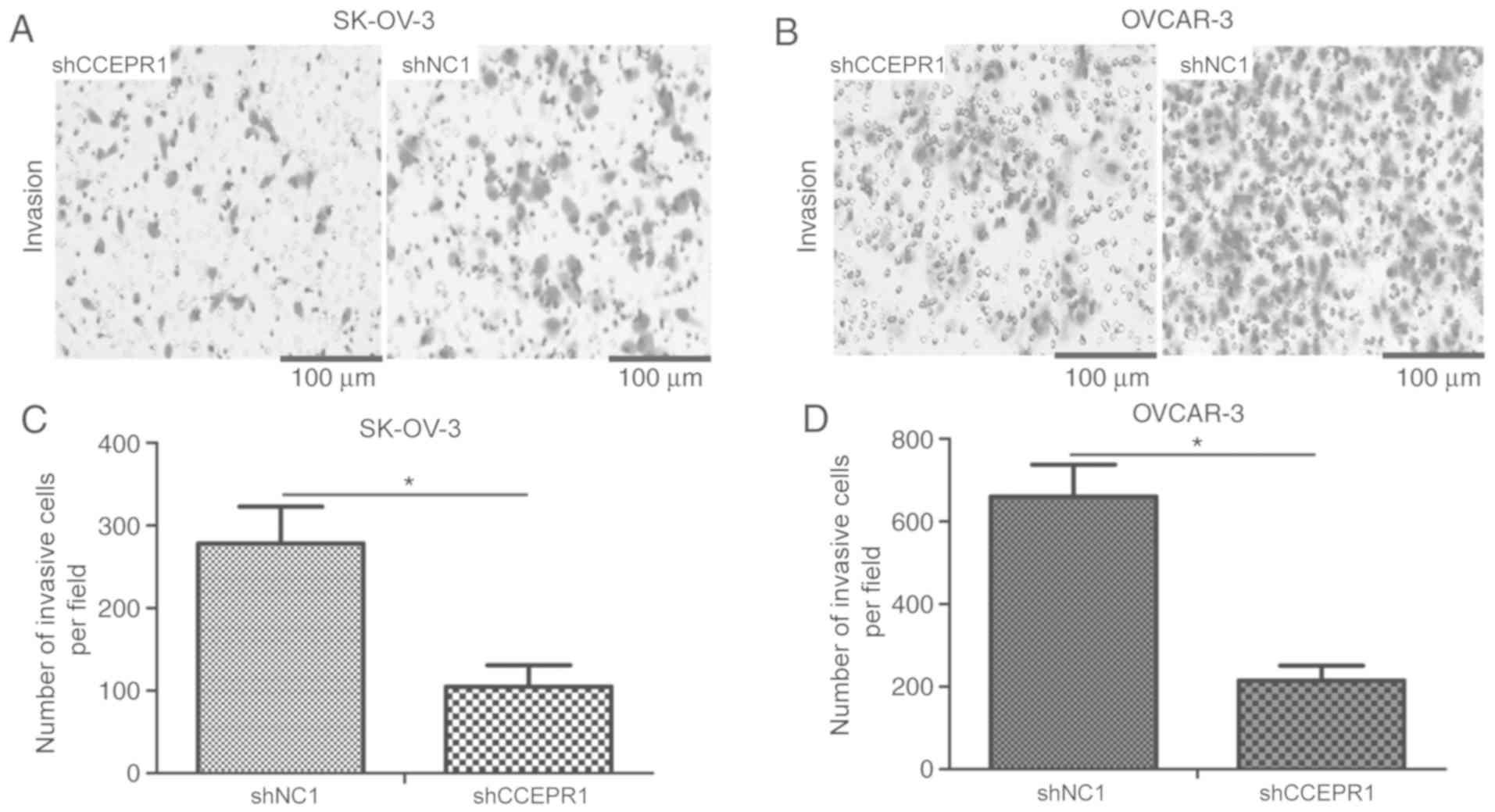
addition, a Transwell invasion assay was performed to investigate
the effect of CCEPR expression on tumor cell invasion. CCEPR
knockdown significantly decreased the invasive abilities of SK-OV-3
and OVCAR-3 cells (Fig. 3A-D;
P<0.05) compared with the cells transfected with the shNC1.
These findings suggested that the knockdown of CCEPR may impede
cell growth and invasion in vitro.
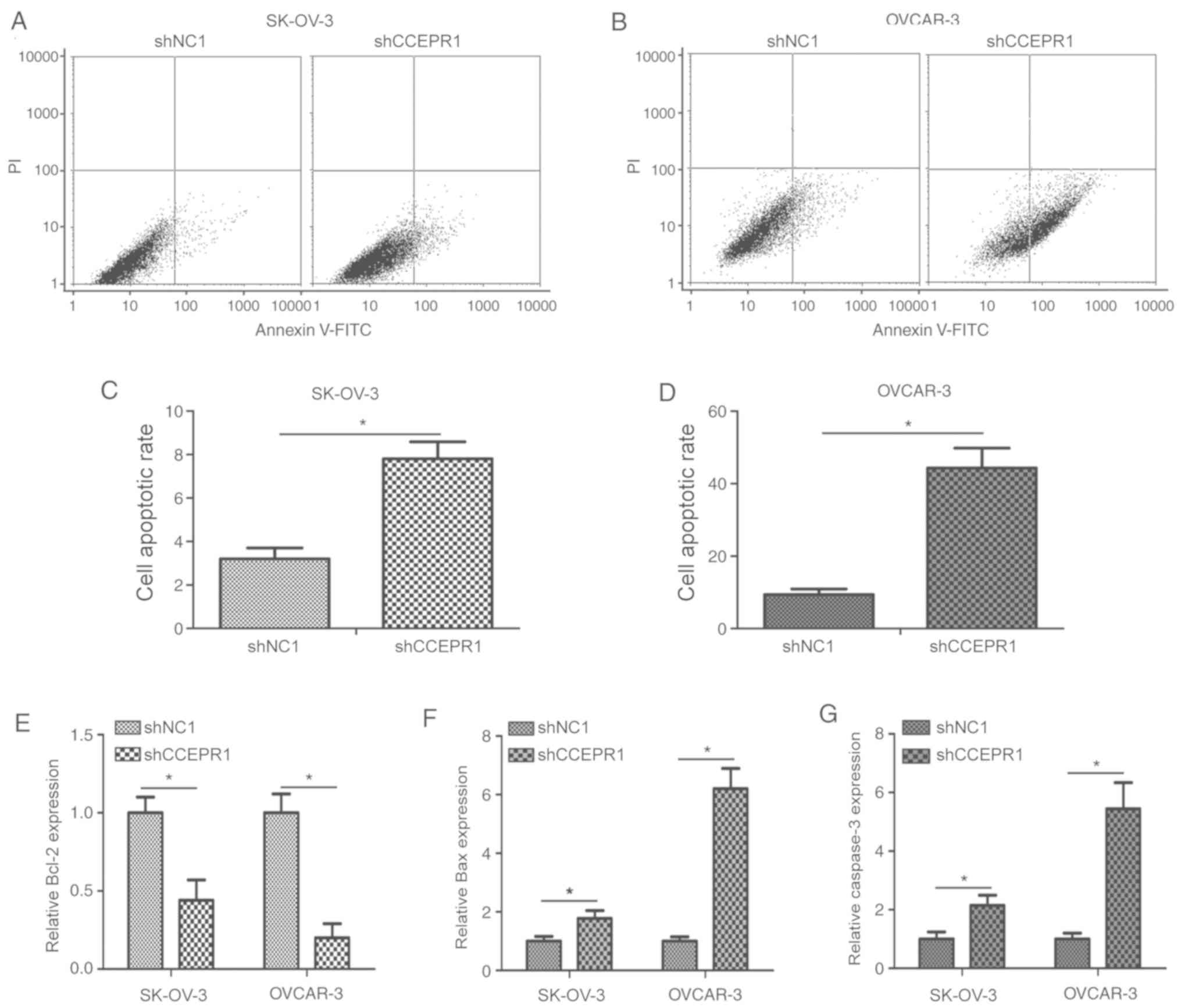
CCEPR knockdown induces apoptosis of
OC cells
To further confirm whether CCEPR knockdown
suppressed OC cell proliferation through regulating cell apoptosis,
flow cytometric analysis was performed. The results revealed that
the apoptotic rate in the shCCEPR1-transfected cells was
significantly increased compared with the shNC1-transfected cells
(Fig. 4A-D; P<0.05).
Furthermore, the expression levels of cell apoptosis-related
proteins, including Bcl-2, Bax and caspase-3 were analyzed using
ELISA following CCEPR knockdown. The protein expression levels of
Bcl-2 were significantly decreased in OC cells post-transfection
with shCCEPR1 compared with the shNC1-transfected cells (Fig. 4E; P<0.05), whereas the protein
expression levels of Bax and caspase-3 were significantly increased
in shCCEPR1-transfected cells compared with shNC1-transfected cells
(Fig. 4F and G; P<0.05). These
results suggested that the knockdown of CCEPR may induce apoptosis
of OC cells in vitro.
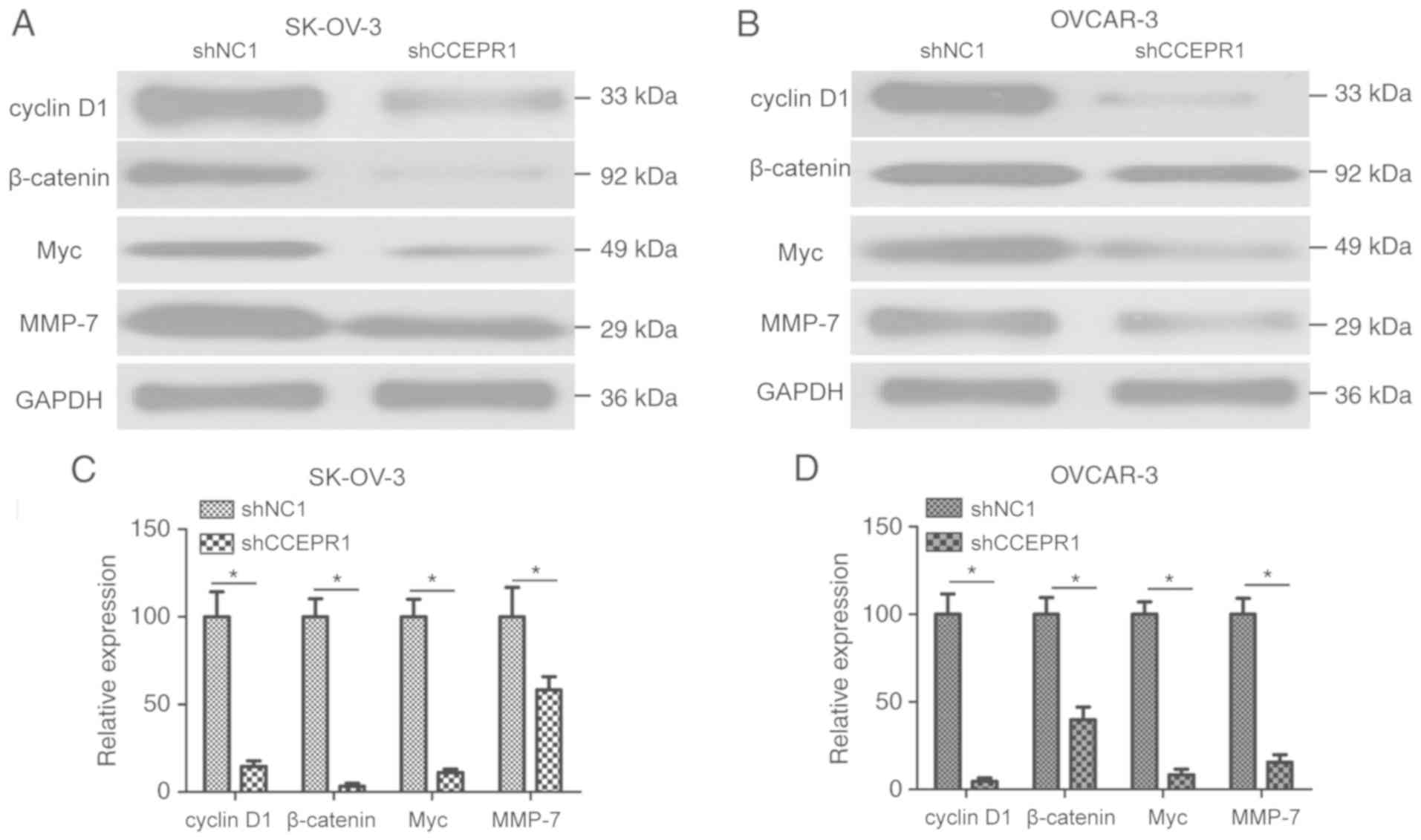
Wnt/β-catenin signaling pathway is
associated with the role of CCEPR in OC cells
The potential mechanism through which CCEPR
regulates OC progression was subsequently investigated. KEGG
analysis was used to predict the downstream signaling pathways of
CCEPR and the Wnt/β-catenin signaling pathway was highly enriched;
thus, western blotting was used to verify this. Significantly
decreased expression levels of proteins involved in the
Wnt/β-catenin signaling pathway, including cyclin D1, β-catenin,
Myc and MMP-7, were observed in shCCEPR1-transfected OC cells
compared with the shNC1-transfected cells (Fig. 5A-D; P<0.05). These results
suggested that CCEPR may be involved in the progression of OC
through regulating the Wnt/β-catenin signaling pathway.
Discussion
Accumulating evidence has demonstrated that lncRNAs
are frequently dysregulated in OC and serve as important regulators
of numerous cellular processes (20,21).
For example, TP73-AS1 was highly expressed in OC tissues and cells,
whereas the knockdown of TP73-AS1 expression significantly
suppressed proliferation, and the migratory and invasive ability of
SK-OV-3 cells (22). Growth arrest
specific 5 has been reported to serve a tumor suppressive role
towards the proliferation of OC cells through suppressing microRNA
(miR)-21 expression and increasing sprouty RTK signaling antagonist
2 expression (23). Nuclear
paraspeckle assembly transcript 1 promoted OC cell metastasis via
regulating the miR-382-3p/ROCK1 signaling pathway (24); and lncRNA SRY-box transcription
factor 4 has been reported to exert an oncogenic effect on the
development of OC by increasing cell proliferation and reducing
cell apoptosis (25). These
findings indicated that lncRNAs may demonstrate potential as novel
biomarkers and therapeutic targets for the treatment of OC. In the
present study, increased CCEPR expression levels were associated
with a poor prognosis in patients with OC, and contributed to the
progression of OC through regulating the Wnt/β-catenin signaling
pathway.
CCEPR is a newly identified lncRNA 2,502 nucleotides
in length, which is localized on the human chromosome 10q21.1
region (26). A previous study
demonstrated that increased CCEPR expression was significantly
correlated with a higher TNM stage and histological grade in
urothelial bladder carcinoma, in addition to predicting a poorer
prognosis (17). Consistent with
these findings, in the present study it was demonstrated that the
expression levels of CCEPR were significantly increased in OC
tissues and cell lines. Furthermore, higher CCEPR expression levels
were significantly associated with poor OS and unfavorable
clinicopathological behaviors, including an increased invasive
ability and an advanced FIGO stage. Notably, the present results
revealed that the 5-year OS rate was significantly lower in
patients with OS that demonstrated high expression levels of CCEPR
compared with those patients with low expression levels of CCEPR.
These results suggested that increased CCEPR expression may be used
as a promising prognostic biomarker for patients with OC.
There is increasing interest regarding the roles of
CCEPR in the proliferation and apoptosis of human cancer (17,18).
Thus, to evaluate the function of CCEPR, SK-OV-3 and OVCAR-3 cells
were used to detect the effect of CCEPR knockdown on cell behaviors
in vitro. The results indicated that CCEPR expression levels
were significantly decreased in SK-OV-3 and OVCAR-3 cells following
transfection with shCCEPR1. Using the CCK-8 assay, Transwell
invasion assay and flow cytometric analysis, it was observed that
transfection of OC cell lines with shCCEPR1 significantly
suppressed proliferation and invasion, whilst promoting cell
apoptosis. On account of these observations, it was hypothesized
that CCEPR may serve an oncogenic role in OC cells. These data were
consistent with previous studies, in which CCEPR was demonstrated
to be an oncogene in cervical cancer and urothelial bladder
carcinoma (17,18).
The Wnt/β-catenin signaling transduction pathway
regulates development and homeostasis, but is also tightly
associated with the development of cancer (27,28).
Activation of the Wnt/β-catenin signaling pathway has been
demonstrated to mediate the initiation and progression of OC
through regulating cell proliferation, apoptosis and metastasis
(29). In addition, the
Wnt/β-catenin signaling pathway has been reported to be an
important modulator of cell invasion, and it has been observed to
participate in cisplatin-induced chemoresistance of OC (30). In the Wnt/β-catenin signaling
pathway, the β-catenin and T-cell factor complex translocates to
the nucleus, where it drives the transcription of downstream genes,
such as MYC, cyclin D1 and MMP-7, which promotes the transformation
of a normal cell into a tumor cell (31,32).
Using KEGG pathway analysis, the Wnt/β-catenin signaling pathway
was observed to be highly enriched among the downstream signaling
pathways of CCEPR. In order to confirm whether CCEPR exerted a
biological role in the Wnt/β-catenin signaling pathway, western
blotting revealed that the knockdown of CCEPR significantly
decreased the expression levels of protein involved in the
Wnt/β-catenin signaling pathway, including cyclin D1, β-catenin,
Myc and MMP-7. Thus, it was suggested that CCEPR may contribute to
the progression of OC via regulating the Wnt/β-catenin signaling
pathway. However, one limitation of the study was that only 70
cases of OC were analyzed; hence, in vivo experiments and
further investigations are required in the future to explore the
oncogenic mechanism of CCEPR in OC.
In conclusion, to the best of our knowledge, the
present study was the first to identify that high CCEPR expression
may be related to unfavorable clinicopathological behaviors and may
predict a worse prognosis in patients with OC. In addition, the
data suggested that CCEPR may contribute to the progression of OC
through regulating the Wnt/β-catenin signaling pathway. These
findings suggested a novel direction for the potential therapeutic
value of CCEPR in OC treatment in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC and YZ designed the study, performed the
experiments, analyzed the data and wrote the manuscript. XF, YL and
QF performed the experiments and analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of Tianjin Central Hospital of Gynecology and Obstetrics
(Tianjin, China). Written informed consent was obtained from all
patients.
Patient consent for publication
Patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naumann RW, Coleman RL, Brown J and Moore
KN: Phase III trials in ovarian cancer: The evolving landscape of
front line therapy. Gynecol Oncol. 153:436–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paik ES, Shim M, Choi HJ, Lee YY, Kim TJ,
Lee JW, Kim BG, Bae DS and Choi CH: Impact of lymphadenectomy on
survival after recurrence in patients with advanced ovarian cancer
without suspected lymph node metastasis. Gynecol Oncol.
143:252–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Worzfeld T, Pogge von Strandmann E, Huber
M, Adhikary T, Wagner U, Reinartz S and Müller R: The unique
molecular and cellular microenvironment of ovarian cancer. Front
Oncol. 7:242017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeda T, Banno K, Okawa R, Yanokura M,
Iijima M, Irie-Kunitomi H, Nakamura K, Iida M, Adachi M, Umene K,
et al: ARID1A gene mutation in ovarian and endometrial cancers
(Review). Oncol Rep. 35:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Expósito-Villén A, E Aránega A and Franco
D: Functional role of non-coding RNAs during
epithelial-to-mesenchymal transition. Noncoding RNA.
4:E142018.PubMed/NCBI
|
|
13
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Wen J, Wang H and Wang Y: Long
non-coding RNA LINC00460 promotes epithelial ovarian cancer
progression by regulating microRNA-338-3p. Biomed Pharmacother.
108:1022–1028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Liu C, Lu Z, Chen L, Wang J, Li Y
and Ma H: Upregulation of the long non-coding RNA SPRY4-IT1
indicates a poor prognosis and promotes tumorigenesis in ovarian
cancer. Biomed Pharmacother. 88:529–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo J and Liu Z: Long non-coding RNA
TTN-AS1 promotes the progression of lung adenocarcinoma by
regulating PTEN/PI3K/AKT signaling pathway. Biochem Biophys Res
Commun. 514:140–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhan Y, Li Y, Guan B, Chen X, Chen Z, He
A, He S, Gong Y, Peng D, Liu Y, et al: Increased expression of long
non-coding RNA CCEPR is associated with poor prognosis and promotes
tumorigenesis in urothelial bladder carcinoma. Oncotarget.
8:44326–44334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang M, Zhai X, Xia B, Wang Y and Lou G:
Long noncoding RNA CCHE1 promotes cervical cancer cell
proliferation via upregulating PCNA. Tumour Biol. 36:7615–7622.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhan L, Li J and Wei B: Long non-coding
RNAs in ovarian cancer. J Exp Clin Cancer Res. 37:1202018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong Y, Gao D, He S, Shuai C and Peng S:
Dysregulated expression of long noncoding RNAs in ovarian cancer.
Int J Gynecol Cancer. 26:1564–1570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Yang B, She Y and Ye Y: The lncRNA
TP73-AS1 promotes ovarian cancer cell proliferation and metastasis
via modulation of MMP2 and MMP9. J Cell Biochem. 119:7790–7799.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma N, Li S, Zhang Q, Wang H, Qin H and
Wang S: Long non-coding RNA GAS5 inhibits ovarian cancer cell
proliferation via the control of microRNA-21 and SPRY2 expression.
Exp Ther Med. 16:73–82. 2018.PubMed/NCBI
|
|
24
|
Liu Y, Wang Y, Fu X and Lu Z: Long
non-coding RNA NEAT1 promoted ovarian cancer cells' metastasis
through regulation of miR-382-3p/ROCK1 axial. Cancer Sci.
109:2188–2198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Wang Y, Yao D and Cui D: LncSOX4
serves an oncogenic role in the tumorigenesis of epithelial ovarian
cancer by promoting cell proliferation and inhibiting apoptosis.
Mol Med Rep. 17:8282–8288. 2018.PubMed/NCBI
|
|
26
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu XY, Hou PF, Li TT, Quan HY, Li ML, Lin
T, Liu JJ, Bai J and Zheng JN: The roles of Wnt/β-catenin signaling
pathway related lncRNAs in cancer. Int J Biol Sci. 14:2003–2011.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng X, Xu X, Chen D, Zhao F and Wang W:
Therapeutic potential of targeting the Wnt/β-catenin signaling
pathway in colorectal cancer. Biomed Pharmacother. 110:473–481.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arend RC, Londono-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang L, Jin Y, Feng S, Zou Y, Xu S, Qiu
S, Li L and Zheng J: Role of Wnt/β-catenin, Wnt/c-Jun N-terminal
kinase and Wnt/Ca2+ pathways in cisplatin-induced
chemoresistance in ovarian cancer. Exp Ther Med. 12:3851–3858.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mei Y, Liu YB, Cao S, Tian ZW and Zhou HH:
RIF1 promotes tumor growth and cancer stem cell-like traits in
NSCLC by protein phosphatase 1-mediated activation of Wnt/β-catenin
signaling. Cell Death Dis. 9:9422018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng S, Seifert AM, Zhang JQ, Cavnar MJ,
Kim TS, Balachandran VP, Santamaria-Barria JA, Cohen NA, Beckman
MJ, Medina BD, et al: Wnt/β-catenin signaling contributes to tumor
malignancy and is targetable in gastrointestinal stromal tumor. Mol
Cancer Ther. 16:1954–1966. 2017. View Article : Google Scholar : PubMed/NCBI
|