Introduction
Osteoarthritis (OA) is the most prevalent joint
disorder in the United States. For instance, OA in the knees occurs
in ~10% of males and 13% of females above the age of 60 (1). This number is likely to increase due
to the aging population and the obesity epidemic (2). The most common modalities of
treatment for OA include surgery and pharmaceutical intervention.
For example, acetaminophen and non-steroidal anti-inflammatory
drugs are commonly prescribed for pain relief in patients with OA.
In more severe cases, steroid injections and surgical interventions
are required (3).
Adipose tissue-derived stem cells (ADSCs) have been
characterized as having the ability to self-renew and differentiate
into different connective tissue cells, including osteoblasts,
adipocytes, chondrocytes and myocytes, under specific inductive
stimuli (4). ADSCs are abundant
and can be easily acquired by liposuction with minimal donor
morbidity (5). In total, 1–10% of
nucleated cells in adipose tissue are ADSCs, whereas only
0.0001–0.01% of nucleated cells in bone marrow are stem cells
(5). A previous study suggested
that the age of the donor does not have a significant role in the
phenotype or function of ADSCs (4). Most clinical trials which utilize
ADSCs for OA treatment have been based on the autologous cells from
the stromal vascular fraction, as have most of the in vivo
studies (6,7). Although the use of ADSCs for treating
OA has been gaining attention clinically and experimentally, the
underlying mechanisms by which ADSCs attenuate OA have not been
fully elucidated.
Exosomes are small, membrane-bound extracellular
vesicles that have been shown to serve a role in intercellular
communications; they are derived from the cell membrane during
endocytic internalization. Exosomes are present and stable in the
blood and in synovial fluids (7).
Emerging evidence shows that exosomes are involved in the
development of joint diseases, such as OA and rheumatoid arthritis
(8). The dysregulation of exosome
secretion and/or uptake can lead to acute and chronic inflammation,
followed by the degeneration of cartilage and the destruction of
joints (9). Exosomes in the blood
have been shown to possess both diagnostic and therapeutic values
for joint disorders, such as OA (10–12).
In the present study, the function and the mechanisms of exosomes
released from ADSCs (ADSC-Exos) were investigated, in order to
assess their therapeutic potential in the treatment of OA. ADSCs
were isolated from an obese patient diagnosed with OA in order to
establish a source of exosomes for further experiments. It was
found that ADSC-Exos effectively promoted chondrocyte proliferation
and migration. Furthermore, ADSC-Exos prevented the
H2O2-induced apoptosis of chondrocytes and
suppressed inflammatory markers in activated synovial fibroblasts
(SFs). Mechanistically, it was demonstrated that ADSC-Exo treatment
led to increased levels of chondrogenic microRNA (miR)-145 and
miR-221, as well as chondrogenic markers, in periosteal cells. The
present study provided evidence and a mechanistic explanation for
the therapeutic applications of ADSC-derived exosomes in the
treatment of OA.
Materials and methods
Isolation and characterization of
ADSCs
The present study was conducted in compliance with
the Declaration of Helsinki. The clinical specimens were obtained
between July and October 2017 in Zhejiang Provincial People'
Hospital, People's Hospital of Hangzhou Medical College. Informed
written consent from all the participants was obtained. ADSCs were
collected and isolated from adipose tissue during elective
liposuction surgery of a healthy donor. The activated SFs were
isolated from an obese patient diagnosed with OA in middle-aged
male subjects (55–70 yrs). Adipose tissue (~1.5 g) was harvested
from the subcutaneous adipose tissue and washed with PBS. The
tissue was cut into strips and digested with collagenase (final
concentration 1 mg/ml in 25 ml PBS) at 37°C for 45 min, after which
25 ml of DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) was
added to neutralize collagenase activity. The digested tissues were
then filtered with a 0.22 µm filter and centrifuged at 800 × g for
6 min at 25°C and the supernatant was discarded. The resulting
pellet contained ADSCs. ADSCs were seeded at 5×104
cells/cm2 in 60 cm2 tissue culture dishes and
cultured with DMEM containing 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.), 100 units/ml penicillin and 100 µg/ml
streptomycin. After 1 week of culture, the cells were harvested
with 0.25% trypsin-EDTA, centrifuged 800 × g at 25°C for 6 min n
and washed twice with PBS. The ADSCs were then used for co-culture
assays. To evaluate the multipotent potential of the ADSCs, the
ADSCs were cultured under inductive conditions, where osteogenesis
and chondrogenesis were promoted, according to an established
protocol (13). The cell lineage
and differentiation state were evaluated using immunohistochemical
staining and quantitative PCR reactions.
Isolation and culture of primary
synovial fibroblasts and periosteal cells
The isolation and culture of synovial tissues was
performed using a previously established method (14). In brief, synovial tissues were
minced, digested for 30 min at 37°C in PBS containing 0.1% trypsin,
followed by digestion with 0.1% collagenase in DMEM with 10% FBS
for 1 h. The suspension was filtered and centrifuged 800 × g at
25°C for 10 min. The pellet collected contained the cells of
interest. The cells were cultured for 7 days in DMEM supplemented
with 10% FBS, 25 mM HEPES, 100 U/ml penicillin, 100 µg/ml
streptomycin and 2.5 µg/ml amphotericin B (Gibco; Thermo Fisher
Scientific, Inc.). The non-adherent cells were carefully removed.
Subsequently, the cells were harvested, resuspended in DMEM/Ham's
F12 medium containing 10% human allogenic serum, plated in cell
culture dishes (diameter = 15 cm), and allowed to attach for about
4–6 days. Moreover, primary periosteal cells were isolated from
four independent donors. In brief, the periosteal flap was rinsed
with Hanks solution (Biochrom) three times, minced and digested for
3 h in Dulbecco's modified eagle medium (DMEM)/Ham's F12 medium
(Biochrom) containing 10,000 U/ml collagenase II (Biochrom), 10%
human allogenic serum (German Red Cross), 2.5% Hepes (Biochrom) and
1% penicillin/streptomycin solution (Biochrom). Subsequently, the
cells were harvested, resuspended in DMEM/Ham'sF12 medium
containing 10% human allogenic serum, plated in cell culture dishes
(diameter = 15 cm), and allowed to attach for about 4–6 days.
Phenotype analysis of the expression of synovial fibroblasts
markers, as well as that of synovial fibroblasts features
previously reported at a tissue level, was conducted by flow
cytometry in synovial fibroblasts, either negatively isolated from
primary culture or obtained from conventional fourth passage.
Isolation of mesenchymal stem cells
(MSCs) from adipose tissues
Briefly, adipose tissues were digested by
collagenase using the EpiQuik Whole Cell Extraction kit
(Invitrogen; Thermo Fisher Scientific, Inc.). The stromal vascular
fractions (SVFs) were then resuspended into MSCs medium
(Invitrogen; Thermo Fisher Scientific, Inc.). These cells were then
sub-cultured to the 5th passage and used for experiments.
Exosome isolation and
characterization
For exosome isolation, ADSCs between passage 4 and 8
were expanded in traditional 2D adherent cell culture and then
seeded on BioNOC II micro carriers (Sigma-Aldrich, Merck KGaA). For
seeding, 8×106 cells resuspended in 50 ml of DMEM with
10% FBS, 1% L-glutamine and 1% P/S and layered on top of 2 g of
sterilized BioNOC II in a 250 ml Erlenmeyer flask (CLS431144,
Sigma-Aldrich, Merck KGaA). The cells were maintained in static
incubation during the first 16 h and were then supplemented with
additional 200 ml of medium. The supernatant was ultracentrifuged
using a W32Ti rotor (L-80XP; Beckman Coulter, Inc.) at 110,000 × g
for 70 min to pellet the exosomes. The pellet was washed in PBS and
centrifuged for a second time at 110,000 × g for 70 min. The PBS
was removed and the exosomes were re-suspended in 100 µl
nuclease-free water. All centrifugation key steps were performed at
4°C.
Measurement of TNF-α and IL-10
levels
Adipose-derived stem cell-derived Exos suppress
inflammatory markers in activated SFs. The concentration of TNF-α
and IL-10 levels in chondrocytes was detected with an ELISA kit
(DTA00C and D1000B, R&D Systems) according to the
manufacturer's instructions.
Induction of apoptosis
Patient-derived articular chondrocytes were isolated
and seeded at a density of the 5,000 cells/well in 12-well plate
and cultured for 5–7 days until confluency was reached.
Chondrocytes were exposed to 100 µM H2O2 with
or without exosomes (1, 5 and 10×1010 particles/ml) for
1 h at 37°C. The resultant cells were then subjected to further
analysis. The effect of chondrocytes and exosomes on cell apoptosis
was determined using an Annexin V-FITC Apoptosis Detection kit
[cat. no. 1035-100, Hangzhou Multisciences (Lianke) Biotech Co.,
Ltd.] and flow cytometry. Annexin V-FITC binding and PI staining
[cat. no. 1035-100, Hangzhou Multisciences (Lianke) Biotech Co.,
Ltd.] were detected using a flow cytometer and analyzed with
FACSDiva v8.0.3 (Becton-Dickinson) software. The experiments were
performed independently three times.
Reverse transcription-quantitative
(RT-q)PCR
RNA samples were extracted and quantified using the
Qubit HS RNA kit (cat. no. 2019-0023, Thermo Fisher Scientific,
Inc.) with a Qubit 3.0 Fluorometer (v2.0.3 software, Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions. The
synthesis of complementary DNA was performed using the PrimeScript™
RT reagent kit [cat. no. 2312, Hangzhou Multisciences (Lianke)
Biotech Co., Ltd.]. RT-qPCR was performed using a human miRNA
RT-qPCR detection kit specifically for Homo sapiens
(hsa)-miR-145, hsa-miR-221, hsa-miR-194, hsa-miR-101 and RNA U6
small nuclear 6 pseudo-genes (RNU6B; cat. no. 33456, Guangzhou
RiboBio Co., Ltd.). RNU6B was used for normalization of miRNA
expression levels. qPCR was performed using SYBR® and
GAPDH was used for normalization of the results. The sequences of
target gene were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and miRBase
(http://www.mirbase.org/). The primers were
designed using Primer Designer 2.0, and the sequences are shown
below. The miR-specific primers were: hsa-miR-145
AGCGAGTGCAGTGGTGAAA, hsa-miR-221GAGTGGGGGTGGGACATAAA, hsa-miR-3188
GTGCGGATACGGGGAAAA, hsa-miR-194 TGGGCTGGGTTGGGAAA and hsa-miR-101
GGCCCAGTGGGGGGAA. The PCR conditions: 95°C pre-denaturation for 15
min, followed by 40 cycles of denaturation at 94°C for 15 sec,
annealing at 55°C for 30 sec and extension at 70°C for 30 sec.
Relative gene expression was analyzed using the 2−ΔΔCq
method. U6 small nuclear RNA was used as a miRNA internal control.
miRNA and U6 (cat. no. HmiRQP9001) primers were purchased from
iGeneBio (GeneCopoeia, Inc.). All primers were synthesized by
Beijing Liu He Synthetic Genomics Ltd. U6 was used as the internal
control.
Western blotting
All western blotting analyses were performed
according to established protocols (13). The following primary antibodies
were obtained from Cell Signaling Technology, Inc.: Interleukin
(IL)-6 (cat. no. 8904, dilution, 1:1,000), nuclear factor-κB
(NF-κB) (cat. no. D14E12, dilution, 1:2,000), tumor necrosis
factor-α (TNF-α) (cat. no. D2D4, dilution, 1:1,500), IL-10 (cat.
no. D13A11, dilution, 1:2,000) and β-actin (cat. no. 12E5,
dilution, 1:5,000). The transcription factor SRY-box transcription
factor 9 (Sox9) primary antibody was obtained from Abcam (cat. no.
D8G8H, dilution, 1:1,000). Collagen type II (Col II cat. no. E819H,
Abcam, dilution, 1:1,000) was analyzed using 5% SDS-PAGE gels with
an anti-Col II primary antibody (cat. no. E819H, Abcam, dilution,
1:1,000). Next, the membrane was washed and incubated with goat
anti-rabbit secondary antibody (cat. no. 33456, Abcam, dilution
1:5,000). Finally, an enhanced chemiluminescence (ECL, Biovision)
kit was used to observe protein bands in a ChemiDoc XRS Plus
luminescent image analyzer (Bio-Rad Laboratories, Inc.). β-actin
was used as the internal control in the experiment, and therefore,
the ratio of the integrated optical density of the targeted protein
bands to that of β-actin in every group was determined as the final
relative protein expression intensity. All experiments were
replicated 3 times.
Alcian blue staining
Osteogenic differentiation was assessed using alcian
blue staining. Briefly, tissue was embedded in paraffin and
sections (4.0 µm thick) were mounted onto glass slides, cleared
using xylene, and gradually rehydrated. Antigen retrieval was
performed on slides using sodium citrate buffer (10 mM sodium
citrate and pH 6) at 100°C for 20 min. Slides were cooled and
rinsed in distilled water to remove citrate solution. Slides were
blocked using Super Block (Thermo Fisher Scientific, Inc.) for 1 h
at room temperature. For slides stained with alcian blue staining,
sections were stained with alcian blue solution (Sigma-Aldrich,
Merck KGaA) and Weigert's Iron Hematoxylin (Sigma-Aldrich, Merck
KGaA) according to the manufacturer's protocol. Alcian blue Stain
kit (Connective Tissue Stain; Abcam) was also used to stain
sections according to the manufacturer. For slides stained with
hematoxylin and eosin (H&E), sections were cleared and
rehydrated followed by staining with H&E, according to standard
protocols. Sections were then dehydrated gradually and mounted for
imaging. All images were acquired using a Nikon Digital Imaging
System.
Von Kossa staining intensity
analysis
After removing the culture medium, the cells were
rinsed twice with PBS and fixed with 95% ethyl alcohol and 5%
isopropyl alcohol for 2 h at 4°C. Subsequently, the cells were
rinsed twice with deionized water. The cells were treated with 2.5%
silver nitrate solution in deionized water at room temperature for
24 h, rinsed twice with deionized water, and 1 ml freshly prepared
0.5% hydroquinone solution in deionized water was applied to the
cell layers on microscope slides and incubated for 2 min in the
dark at room temperature. The hydroquinone solution was removed and
1 ml freshly prepared 5% sodium thiosulfate solution in deionized
water was added for 2 min at room temperature. The cells were
washed again with deionized water. The staining was
semi-quantitatively evaluated under a light microscope using the
color intensity of the Von Kossa staining. Under a
microscope, measurements were made in 10 consecutive visual areas
at 400× magnification.
Proliferation of chondrocytes
The effect of exosome stimulation on chondrocytes
was measured using the 5-ethynyl-2′-deoxyuridine (EdU)-488 Cell
Proliferation kit (Guangzhou RiboBio Co. Ltd.) and flow cytometry,
according to the manufacturer's instructions. Briefly, normal
chondrocytes were seeded into 48-well plates and cultured with
exosomes (400 µg/ml) for 48 h. EdU working solution, consisting of
150 µl complete culture medium for chondrocytes supplemented with
0.15 µl EdU, was added per well and incubated at 37°C for 3 h. The
cells were then trypsinized, washed with PBS, fixed in 4%
paraformaldehyde (PFA) for 15 min at room temperature, neutralized
with 2 mg/ml glycine for 2 h and washed again with PBS.
Permeabilization was performed using 0.4% Triton X-100 for 5 min at
room temperature and the cells were washed twice with PBS. The
labelled chondrocytes were resuspended in the staining solution
from the kit, incubated for 10 min at room temperature in the dark,
washed twice with 0.4% Triton X-100 and then resuspended in PBS for
flow cytometry analysis and analyzed with FACSDiva v8.0.3 software
(Becton-Dickinson).
Migration of chondrocytes
A chondrocyte migration assay was performed using
Transwell inserts. In total, 5×104 chondrocytes were
seeded into the upper chambers of the Transwell (8 µm pores) in 10%
FBS chondrocyte culture DMEM media, while the lower chamber was
filled with 600 µl chondrocyte culture medium containing exosomes
(400 µg/ml). The chondrocytes were allowed to migrate at 37°C for
12 h. The non-migrated chondrocytes remaining on the upper surface
of the insert were carefully removed. The migrated chondrocytes on
the bottom surface of the insert were fixed with 4% PFA for 15 min
at room temperature and stained with 0.5% crystal violet for 10 min
at room temperature, before washing with PBS. In total, five fields
were randomly selected and photographed (magnification, ×100) using
a Leica light microscope (Leica Microsystems, Inc.).
Statistical analysis
Statistical analysis was performed with SPSS v11.0
(SPSS, Inc.). Data represents assays performed a minimum of three
times in triplicates. Data are presented as the mean ± SD.
Comparisons of macroscopic and histological scores were performed
using the Mann-Whitney U test. Comparison made among multiple
groups were performed using ANOVA and Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
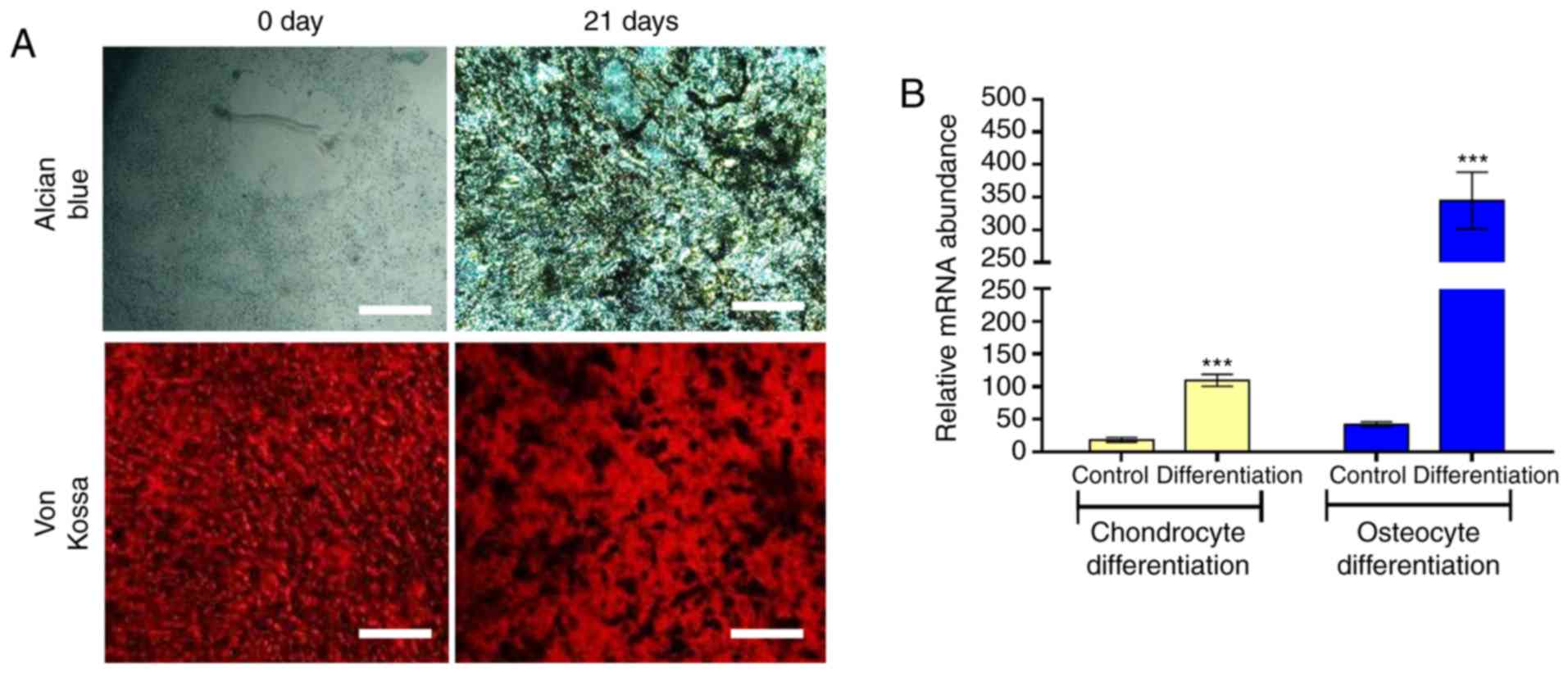
Characterization of ADSCs
The multipotency of ADSCs obtained from an obese
patient with OA was assessed. ADSCs were cultured under different
inductive conditions. For example, under osteogenic conditions,
ADSCs differentiated along the osteogenic lineage, as shown by the
increased expression of runt-related transcription factor 2 (Runx2)
mRNA (~1,000-fold higher than the control) and the von Kossa
staining intensity of deposited calcium phosphate. (Fig. 1A and B). The increased Col II mRNA
(~250-fold higher than the control) and Alcian Blue staining was a
method to detect the glycosaminoglycans in cells that were
indicators of chondrogenic differentiation (Fig. 1A and B). These data provided ex
vivo evidence that the primary ADSCs obtained in the present
study were multipotent.
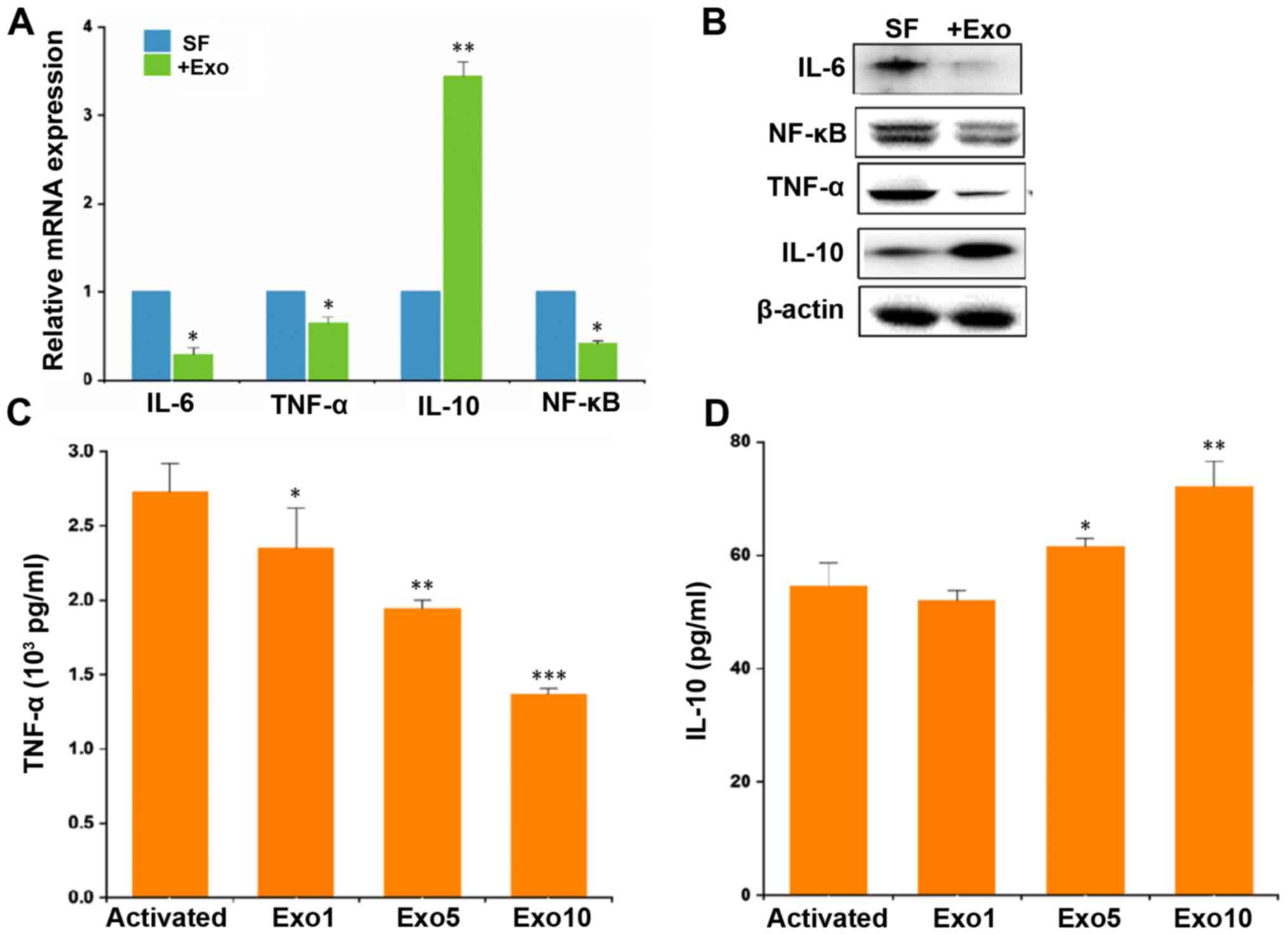
ADSC-derived exosomes (ADSC-Exos)
decrease inflammation in activated SFs and macrophages
Secreted exosomes have been reported to be involved
in communication among cells and shown to participate in a wide
spectrum of cellular processes, including the pathological
development and progression of arthritis (5,6). In
the present study, secreted exosomes were isolated from ADSCs and
incubated with activated SFs obtained from an obese patient with
OA. The results demonstrated that exosome-treated SFs exhibited
significantly reduced levels of inflammatory biomarkers, namely
IL-6, TNF-α and NF-κB, while there were increased levels of IL-10
(Fig. 2A and B). The
microenvironment was also assessed by examining the synovial
macrophages. It was found that ADSC-Exos had immune-suppressive
effects on the microphages; the M1 type macrophage marker TNF-α was
significantly downregulated by treatment with ~1010
exosomes particles/ml, while IL-10 was upregulated (Fig. 2C and D).
ADSC-Exos protect articular
chondrocytes and promote mesenchymal differentiation and
migration
After establishing that exosomes from ADSCs reduced
inflammation in SFs, their actions on articular chondrocytes were
investigated. Primary articular chondrocytes were cultured and
subjected to H2O2 insults in order to mimic
the oxidative stress induced by M1 macrophages in the arthritic
synovium (9,10). H2O2 treatment
induced apoptosis in the articular chondrocytes. When a sufficient
number of exosomes were added to the articular chondrocytes treated
with H2O2, significantly less apoptotic
chondrocytes were detected (Fig.
3A). For example, ~37% of chondrocytes treated with exosomes
(Exo 10 group; 10×1010 particles/ml) survived
H2O2 treatment (Fig. 3A). In addition, the effects of
ADSC-derived exosomes on the migration of MSCs was assessed. The
migratory ability of MSCs appeared to increase in a dose-dependent
manner with increasing exosome treatment (Fig. 3B). The morphogenic effects of
ADSC-Exos on MSCs were then investigated. It was found that MSCs
incubated with exosomes had a higher differentiation potential,
including osteogenesis, adipogenesis and chondrogenesis. This was
reflected by the increased mRNA expression of peroxisome
proliferator-activated receptor-γ (PPARγ; a transcription factor
involved in adipocyte cells differentiation), Col II (a marker of
chondrocyte differentiation) and Runx2 (an osteogenesis marker).
Runx2 was the most elevated in the MSCs (~1,000-fold higher)
following incubation with exosomes (Fig. 3C).
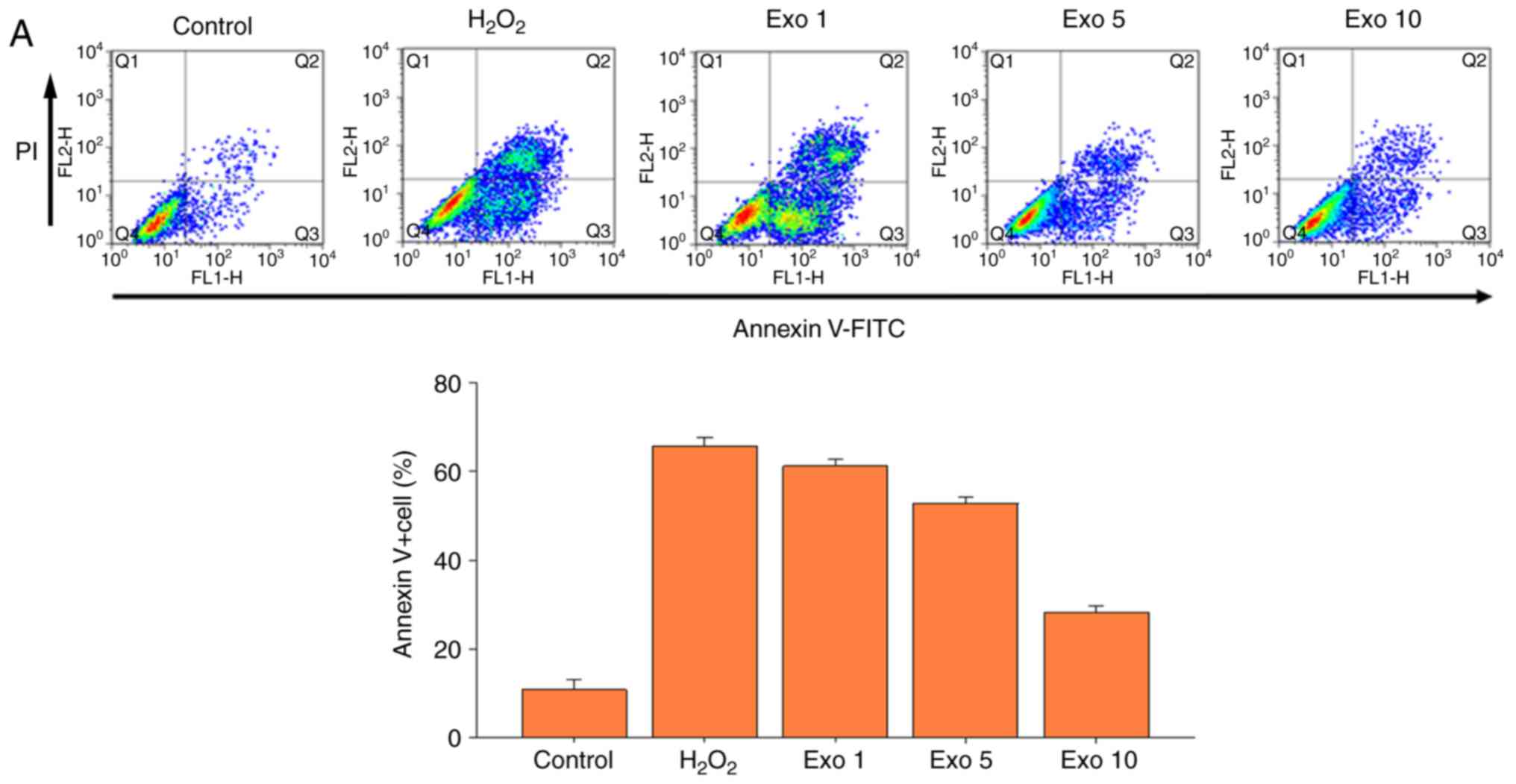 | Figure 3.ADSC-derived Exos suppress apoptosis
and promote migration and differentiation of MSCs. (A) Articular
chondrocytes were subjected to H2O2 treatment
to simulate oxidative stress in the arthritic microenvironment.
Different doses of Exos (Exo1, 1×105 particles/ml; Exo5,
5×105 particles/ml; Exo10, 10×105
particles/ml) were used to investigate their effect against
H2O2-induced oxidative stress and apoptosis.
The upper panel shows representative flow cytometry plots of
Annexin V-FITC/PI labeling; the lower panel shows the
quantification of Annexin V-positive cells. The control group
represents the baseline apoptotic status of the articular
chondrocytes without H2O2 treatment. (B)
Transwell chamber migration assay. Exo treatment increased the
migratory ability of MSCs in a dose-dependent manner. The upper
panels show representative images of the inserts and the lower
panels show representative images of the migrated MSCs.
Quantification is shown from three independent repeats. (C)
ADSC-derived Exos increased the mRNA expression levels of PPARγ,
Col II and Runx2. The data is represented as a ratio to the control
group (set as 1). ***P<0.001 vs. untreated. ADSC,
adipose-derived stem cell; Exo, exosome; MSCs, mesenchymal stem
cells; PI, propidium iodide; PPAR-γ, peroxisome
proliferator-activated receptor-γ; Col II, collagen type 2; Runx2,
runt-related transcription factor 2. |
ADSC-Exos promote chondrogenesis in
vitro
As ADSC-Exos induced Runx2 mRNA expression in MSCs,
the chondrogenesis-promoting effect of exosomes on periosteal cells
(precursor cells) was investigated. ADSC-Exo incubation led to
increased Alcian Blue staining in a time-dependent manner (Fig. 4A). Periosteal cells exhibited
chondrocyte morphology, with a substantial Alcian Blue staining
intensity, after 10 days of incubation (Fig. 4A). The increased Alcian Blue
staining was accompanied by the increased mRNA expression of
chondrogenesis markers, namely Sox9, Col II and β-catenin (Fig. 4b). The increased β-catenin mRNA in
the exosome-treated periosteal cells was consistent with previous
studies that indicated that the Wnt/β-catenin/T cell
factor-mediated transcription process served an important role in
chondrocytic differentiation and proliferation (11,12).
To test this hypothesis, ICG-001, an antagonist of Wnt/β-catenin
signaling, was introduced along with the exosome treatment. The
presence of ICG-001 appeared to completely prevent the periosteal
cells from differentiating into chondrocytes, as reflected by the
absence of Alcian Blue stain (Fig.
4C). Consistently, the RT-qPCR analysis of the
ICG-001+exosome-treated periosteal cells revealed that the
expression of markers of chondrogenesis (Sox9, Col II and
β-catenin) was effectively reversed (Fig. 4D).
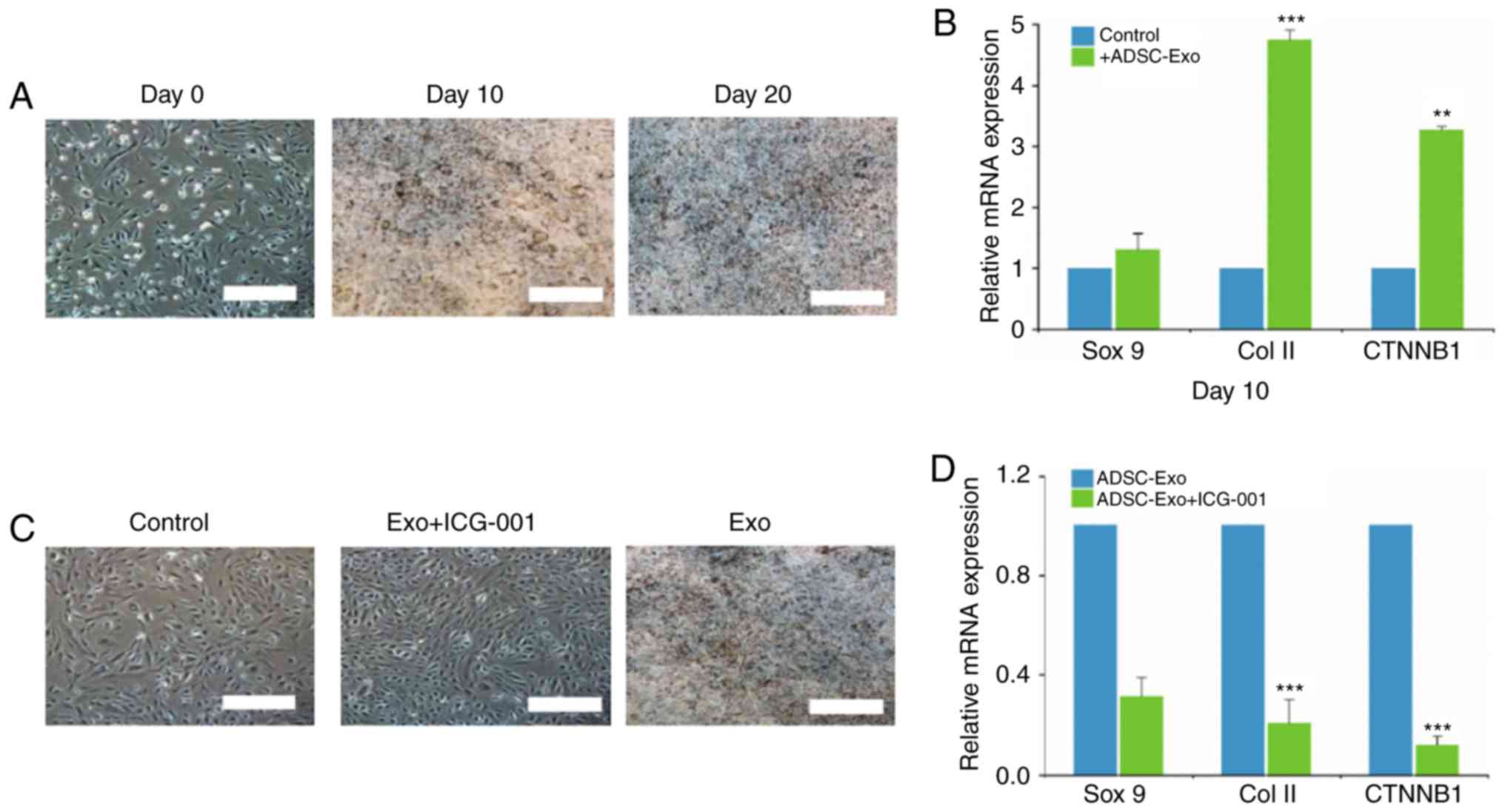 | Figure 4.ADSC-derived Exos induce
chondrogenesis in periosteal cells. (A) Periosteal cells cultured
with ADSC-derived Exos (10×1010 particles/ml) exhibited
increased Alcian Blue staining over time, indicative of
chondrocytic differentiation. (B) RT-qPCR analysis of Sox9, Col II
and CTNNB1 expression in periosteal cells treated with Exos. (C)
The addition of ICG-001, a Wnt/β-catenin antagonist, abolished the
chondrogenic effects of ADSC-derived Exos, as evidenced by Alcian
Blue staining. (D) RT-qPCR analysis of Sox9, Col II and CTNNB1
expression in periosteal cells cultured with ADSC-derived Exos, in
the presence or absence of ICG-001. **P<0.01, ***P<0.001.
ADSC, adipose-derived stem cell; Exo, exosome; RT-qPCR, reverse
transcription-quantitative PCR; Sox9, SRY-box transcription factor
9; Col II, collagen type 2; CTNNB1, β-catenin. |
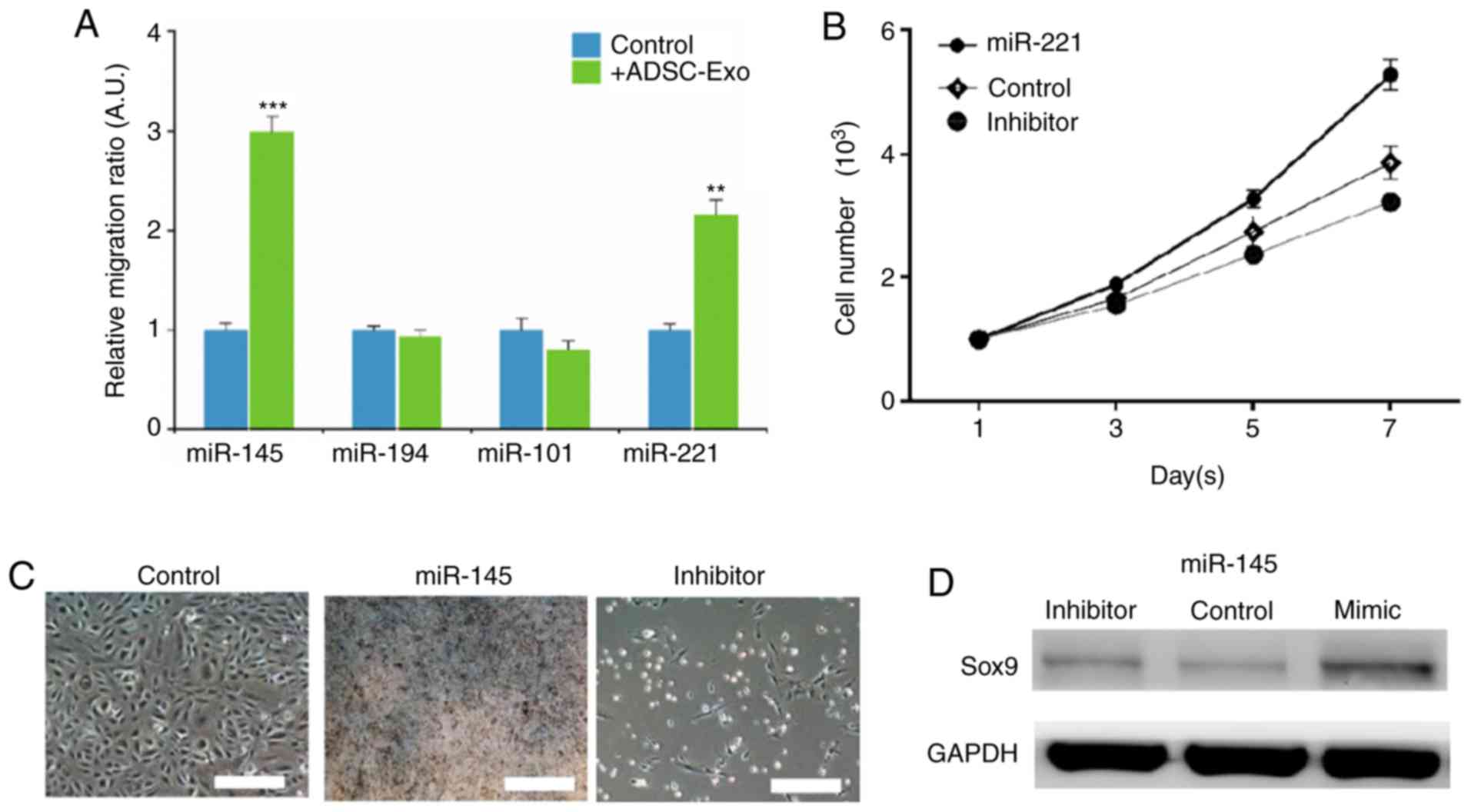
ADSC-derived exosomes promote
chondrogenesis by upregulating miR-145 and miR-221
After observing that the ADSC-derived exosomes
induced the expression of pro-chondrogenic markers, the underlying
mechanism of action was investigated. Several established miRNAs
responsible for promoting chondrocyte differentiation and
proliferation were screened. Among these miRNAs, miR-145 and
miR-221 were increased following ADSC-Exo treatment, while no
significant changes were identified in the levels of miR-101 and
miR-194 (Fig. 5A). The effects of
miR-221 and miR-145 on periosteal cells were examined using
transient transfections of their respective mimics and inhibitors
into periosteal cells (Fig. S1).
The mimic-transfected periosteal cells had increased viability,
while the opposite was found in the cells transfected with the
miR-221 inhibitor (Fig. 5B). In
addition, periosteal cells transfected with the miR-145 mimic had a
marked increase in alcian Blue staining compared with almost no
staining in the inhibitor-transfected cells (Fig. 5C); this result was supported by
western blotting, which showed that the expression of Sox9 was
reduced in the inhibitor-transfected periosteal cells compared with
the mimic-transfected cells (Fig.
5D).
Discussion
OA is a common joint disorder that affects millions
of patients globally (1).
Inflammation has been shown to be a major underlying mechanism of
OA, which not only initiates but also propagates the degradation of
the chondrocytes in the joints of patients with OA (14). In the present study, the potential
of exosomes isolated from ADCS in reducing inflammation and
promoting chondrogenesis was investigated in vitro. The
present findings suggested that ADSC-Exos may be beneficial as a
therapeutic tool in patients with OA.
Adipose tissue contains a subset of cells with the
ability to differentiate into multiple lineages. ADSCs have been
demonstrated to differentiate into multiple different cell types,
including adipocytes, osteoblasts, chondrocytes and myocytes, in
specific inductive conditions (15). Due to the multipotent nature of
ADSCs, researchers and clinicians have been exploring their
therapeutic potential in various joint disorders, including OA
(16). Another advantage of ADSCs
is the avoidance of rejection. Autologous ADSCs can now be easily
isolated in substantial quantities from subcutaneous adipose
tissues and cultured ex vivo (17). In addition, ADSCs can be harvested
in a much less invasive manner compared with bone marrow-derived
MSCs (4). Previous studies have
shed light on the underlying mechanism of stem cell proliferation
and communication within the stem cell niche. One such mechanism
involves the secretion and uptake of exosomes (18). Exosomes have been shown to
participate in stem cell- or progenitor cell-mediated tissue
regeneration. In the bones, exosomes regulate multiple signaling
processes involved in the differentiation of osteocytes and bone
architecture maintenance in a paracrine manner (6). For instance, exosomes were detected
in the bone microenvironment, where they modulated intracellular or
intercellular signaling, by targeting the same cells, adjacent
cells or distant organs (16).
Exosomes have been considered to be an important component in the
delivery of chondrogenic signaling factors for bone regeneration
(19).
The present study demonstrated that exosomes
isolated from ADSCs activated SFs and downregulated the
pro-inflammatory markers IL-6 and NF-κB; without exosomes, SFs had
significantly higher expression levels of IL-6. Previous studies
indicated that the synovial inflammation and cartilage destruction
characteristic of patients with OA were largely influenced by IL-6
and NF-κB (1,2); therefore, the ability of ADSC-Exos to
suppress these pro-inflammatory cytokines suggested they have the
potential to ameliorate OA. Treatment with exosomes suppressed
apoptosis and promoted chondrogenesis in periosteal cells. Several
miRNAs responsible for promoting chondrocyte differentiation and
proliferation were screened. Among these miRNAs, miR-145 and
miR-221 were elevated after exosome treatment, while no significant
changes were identified in the levels of miR-101 and miR-194.
Previous studies indicated that exosomes contain regulatory
molecules, including RNAs, peptides, miRNAs and proteins, all of
which participate in altering cellular signaling (20,21).
One such example is the modulation of inflammation. Either
intrinsic or exogenous exosomes have been shown to modulate
inflammation and tissue repair in OA (21). In agreement with this, miR-145 has
been implicated in several previous studies to have a role in
suppressing TNF-α mediated inflammation and attenuating the
degradation of cartilage (22).
The reduced level of miR-221 has been associated with osteoporosis
and cartilage degradation (23);
the increased expression of miR-221 in exosome-treated periosteal
cells promoted the proliferation of periosteal cells, which have
the potential to differentiate along the chondrogenic or osteogenic
lineages (24).
In conclusion, the present study demonstrated that
ADSC-Exos may have a therapeutic value. ADSC-Exos exerted a strong
stimulatory effect on chondrocyte migration and proliferation. As
ADSCs can be obtained in a patient-specific manner and are
theoretically inexhaustible, ADSC-Exos may represent a novel
therapeutic approach for the treatment of OA in future clinical
settings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science
Technology Department of Zhejiang Province (grant no.
2016F81G1360053) and the Zhejiang Province Bureau of Health (grant
no. 2017208160).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ was responsible for the conception and design of
the study. WP and BY performed the experiments and analyzed and
interpreted the data. QB was responsible for the collection and
assembly of data. JC and YX performed data analysis and
interpretation. The final version of the manuscript has been read
and approved by all authors.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Zhejiang Provincial People's Hospital, and informed consents were
obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xia B, Di Chen, Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felson DT, Lawrence RC, Dieppe PA, Hirsch
R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y,
et al: Osteoarthritis: New insights. Part 1: The disease and its
risk factors. Ann Intern Med. 133:635–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashford S and Williard J: Osteoarthritis:
A review. Nurse Pract. 39:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gokce A, Peak TC, Abdel-Mageed AB and
Hellstrom WJ: Adipose Tissue-derived stem cells for the treatment
of erectile dysfunction. Curr Urol Rep. 17:142016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sabol RA, Bowles AC, Cote A, Wise R,
Pashos N and Bunnell BA: Therapeutic potential of adipose stem
cells. Adv Exp Med Biol. Jul 27–2018.doi: 10.1007/5584_2018_248
(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pak J, Lee JH, Pak N, Pak Y, Park KS, Jeon
JH, Jeong BC and Lee SH: Cartilage regeneration in humans with
adipose tissue-derived stem cells and adipose stromal vascular
fraction cells: Updated status. Int J Mol Sci. 19(pii): E21462018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simpson RJ, Lim JW, Moritz RL and
Mathivanan S: Exosomes: Proteomic insights and diagnostic
potential. Expert Rev Proteomics. 6:267–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tran TH, Mattheolabakis G, Aldawsari H and
Amiji M: Exosomes as nanocarriers for immunotherapy of cancer and
inflammatory diseases. Clin Immunol. 160:46–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barile L and Vassalli G: Exosomes: Therapy
delivery tools and biomarkers of diseases. Pharmacol Ther.
174:63–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Wang Y, Xiao K, Xiang S, Li Z and
Weng X: Emerging role of exosomes in the joint diseases. Cell
Physiol Biochem. 47:2008–2017. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arai Y, Park S, Choi B, Ko KW, Choi WC,
Lee JM, Han DW, Park HK, Han I, Lee JH and Lee SH: Enhancement of
Matrix Metalloproteinase-2 (MMP-2) as a potential chondrogenic
marker during chondrogenic differentiation of human adipose-derived
stem cells. Int J Mol Sci. 17(pii): E9632016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zimmermann T, Kunisch E, Pfeiffer R, Hirth
A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E,
et al: Isolation and characterization of rheumatoid arthritis
synovial fibroblasts from primary culture-primary culture cells
markedly differ from fourth-passage cells. Arthritis Res. 3:72–76.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai M, Sui B, Xue Y, Liu X and Sun J:
Cartilage repair in degenerative osteoarthritis mediated by squid
type II collagen via immunomodulating activation of M2 macrophages,
inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials.
180:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldring MB: The role of cytokines as
inflammatory mediators in osteoarthritis: Lessons from animal
models. Connect Tissue Res. 40:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naito M, Ohashi A and Takahashi T:
Dexamethasone inhibits chondrocyte differentiation by suppression
of Wnt/β-catenin signaling in the chondrogenic cell line ATDC5.
Histochem Cell Biol. 144:261–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan X, Liu H, Huang H, Liu H, Li L, Yang
J, Shi W, Liu W and Wu L: The key role of canonical Wnt/β-catenin
signaling in cartilage chondrocytes. Curr Drug Targets. 17:475–484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldring MB: Anticytokine therapy for
osteoarthritis. Expert Opin Biol Ther. 1:817–829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sutton S, Clutterbuck A, Harris P, Gent T,
Freeman S, Foster N, Barrett-Jolley R and Mobasheri A: The
contribution of the synovium, synovial derived inflammatory
cytokines and neuropeptides to the pathogenesis of osteoarthritis.
Vet J. 179:10–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miana VV and Gonzalez EAP: Adipose tissue
stem cells in regenerative medicine. Ecancermedicalscience.
12:8222018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eirin A, Riester SM, Zhu XY, Tang H, Evans
JM, O'Brien D, van Wijnen AJ and Lerman LO: MicroRNA and mRNA cargo
of extracellular vesicles from porcine adipose tissue-derived
mesenchymal stem cells. Gene. 551:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baglio SR, Rooijers K, Koppers-Lalic D,
Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen
HW, Baldini N and Pegtel DM: Human bone marrow- and
adipose-mesenchymal stem cells secrete exosomes enriched in
distinctive miRNA and tRNA species. Stem Cell Res Ther. 6:1272015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu
J, Zuo B, Zhao C, Wang C and Zhang X: MicroRNA-145 attenuates
TNF-α-driven cartilage matrix degradation in osteoarthritis via
direct suppression of MKK4. Cell Death Dis. 8:e31402017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng X, Zhao FC, Pang Y, Li DY, Yao SC,
Sun SS and Guo KJ: Downregulation of miR-221-3p contributes to
IL-1β-induced cartilage degradation by directly targeting the
SDF1/CXCR4 signaling pathway. J Mol Med (Berl). 95:615–627. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Gao Y, Cai L, Li F, Lou Y, Xu N,
Kang Y and Yang H: MicroRNA-221 is involved in the regulation of
osteoporosis through regulates RUNX2 protein expression and
osteoblast differentiation. Am J Transl Res. 9:126–135.
2017.PubMed/NCBI
|