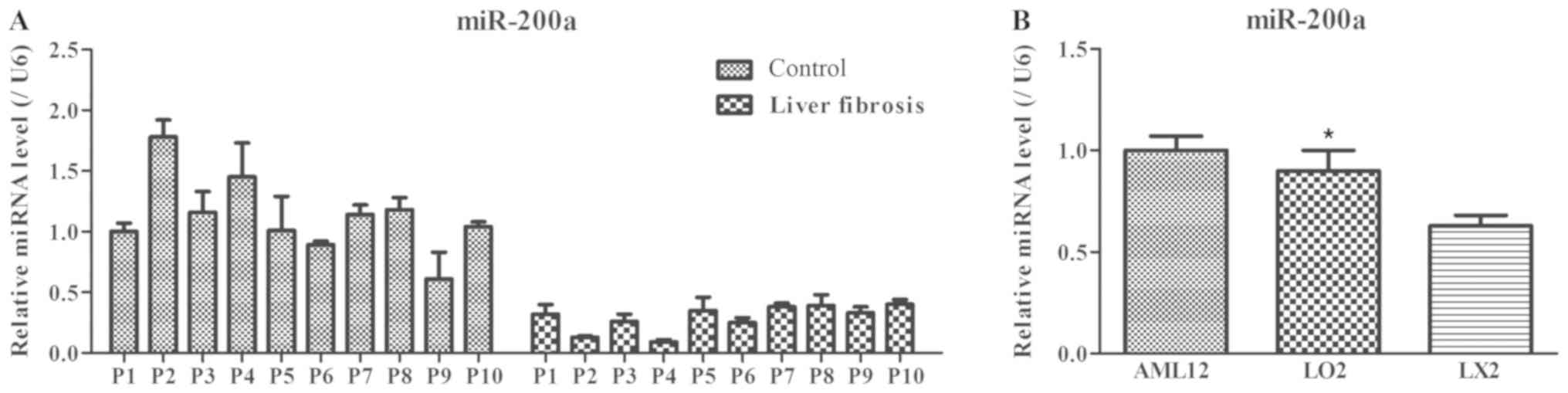
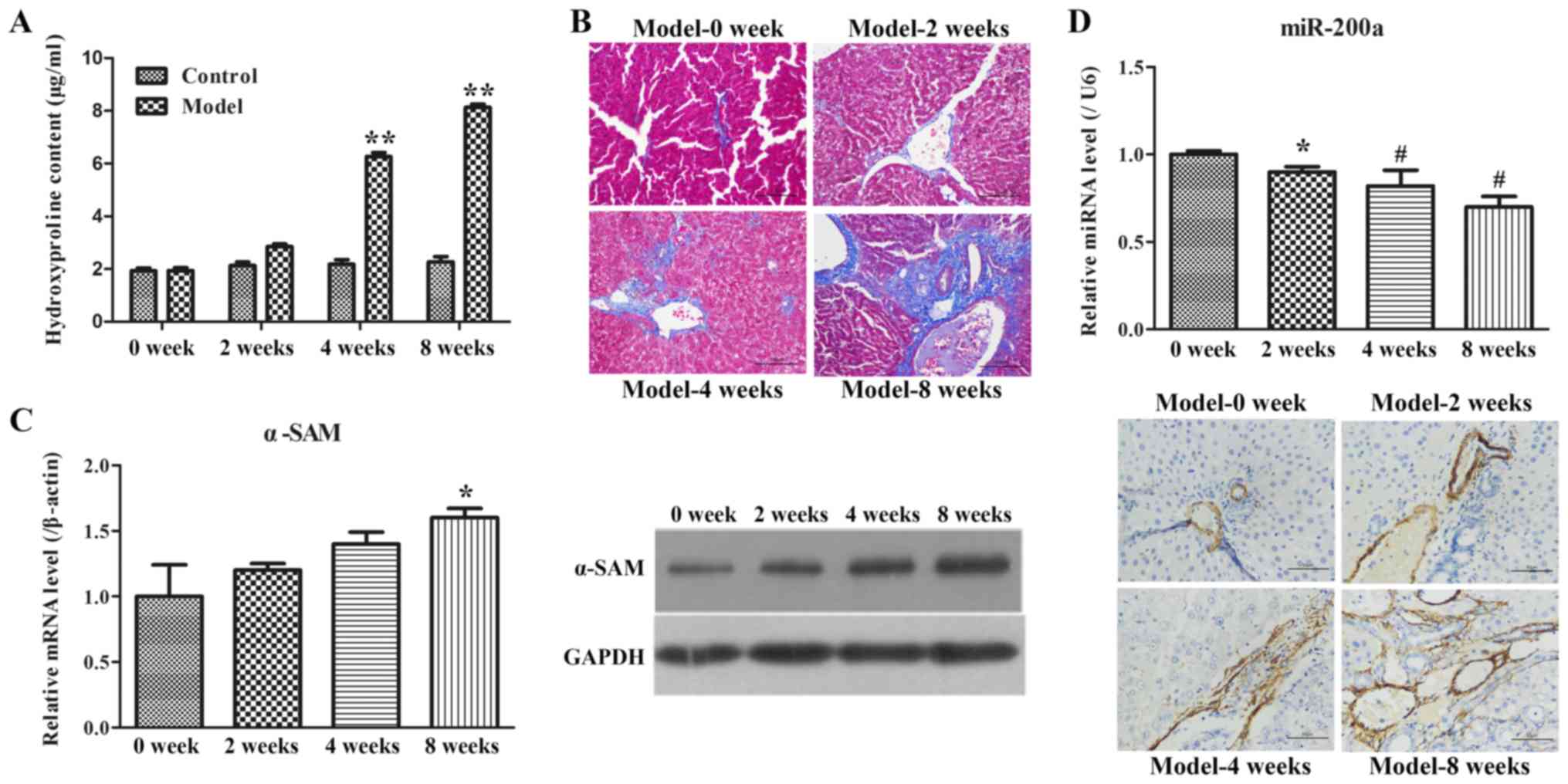
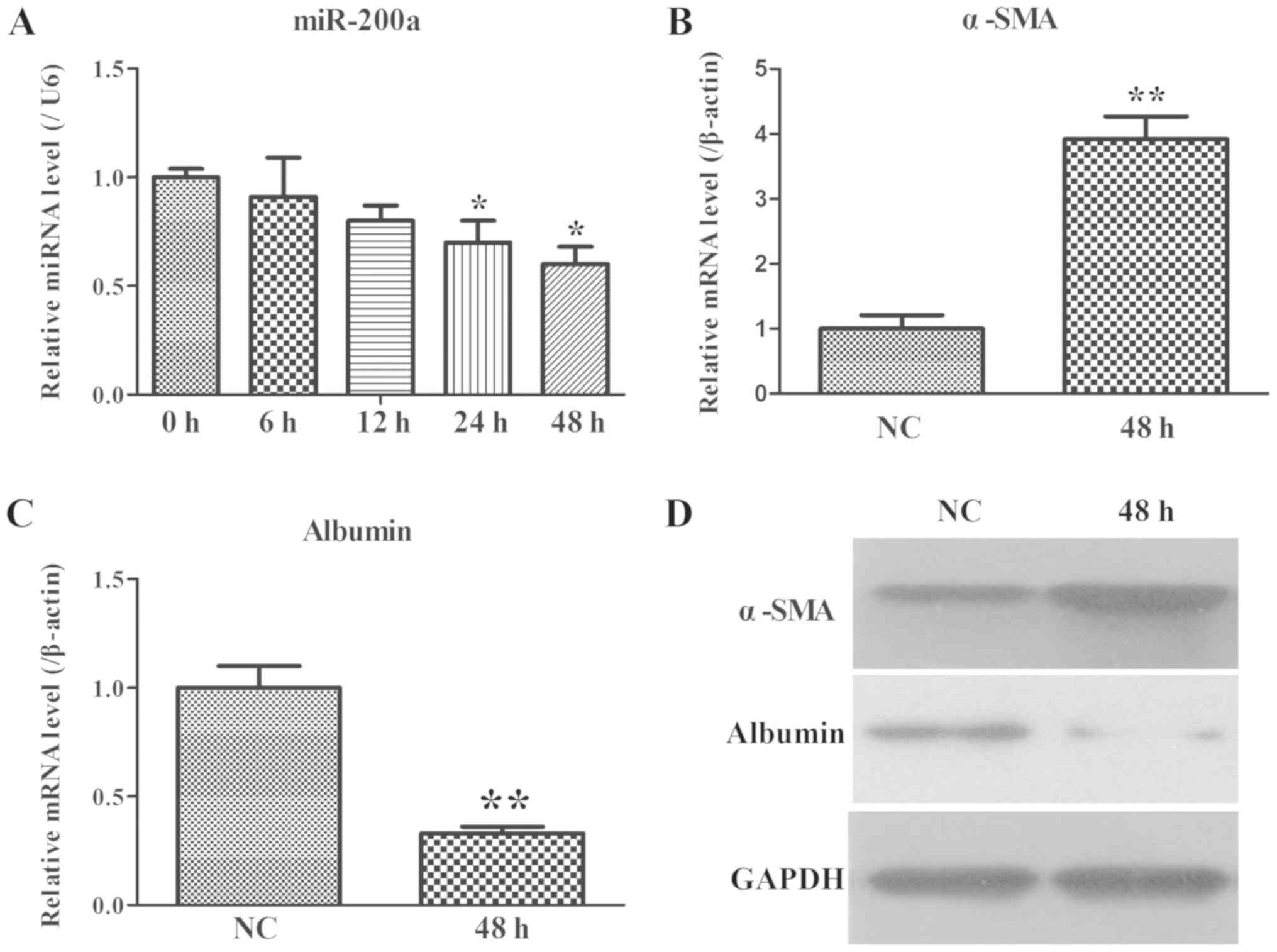
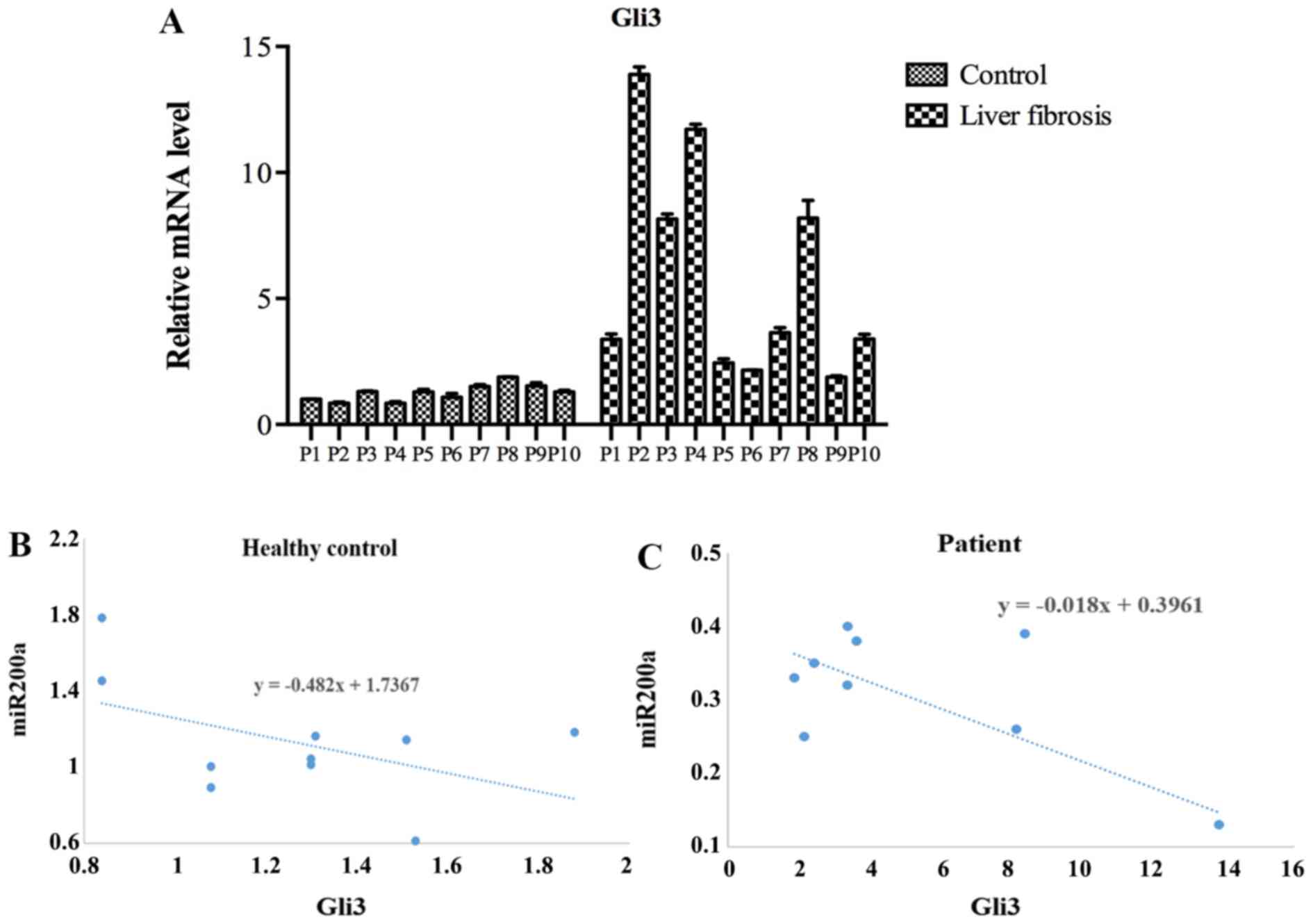
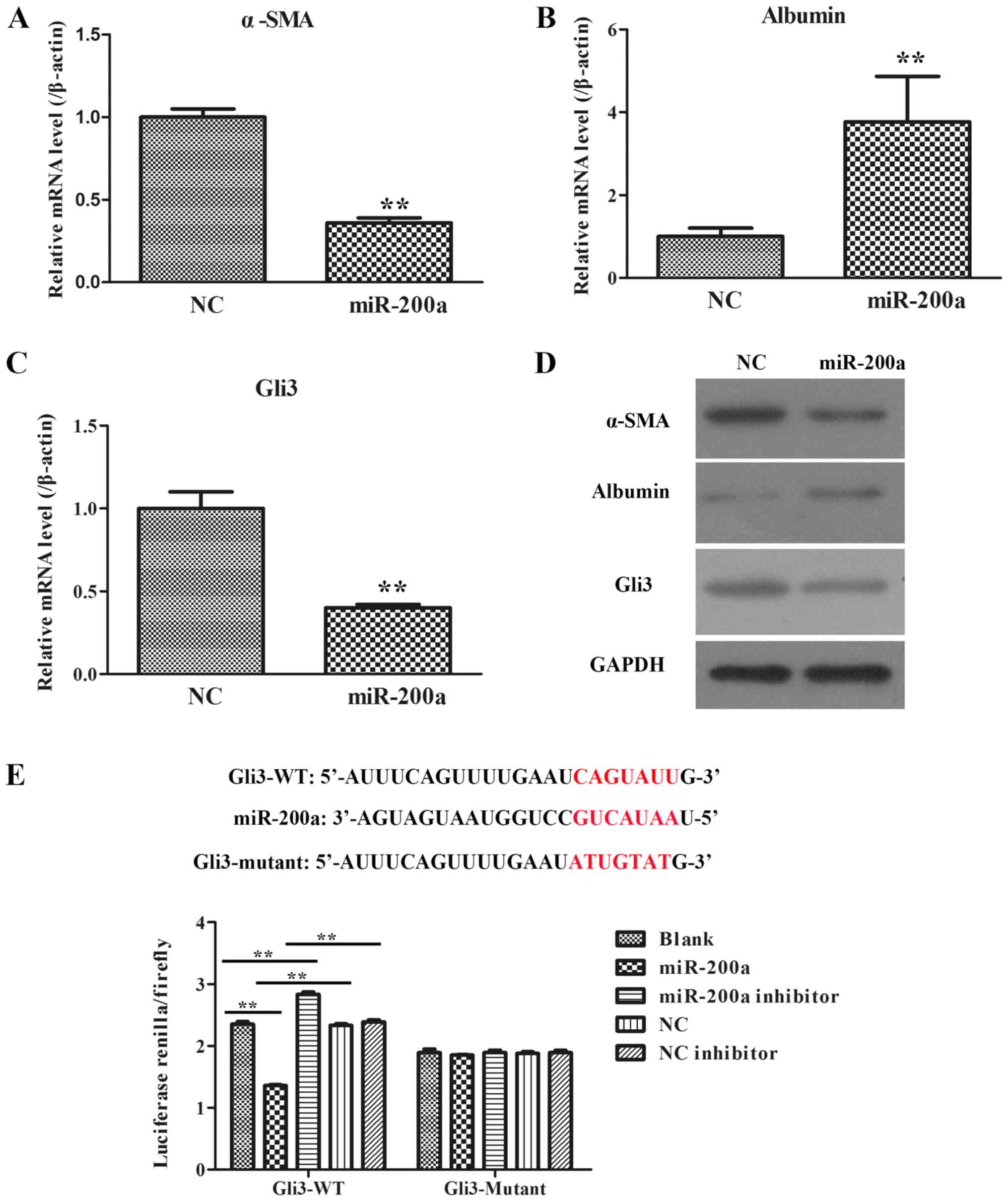
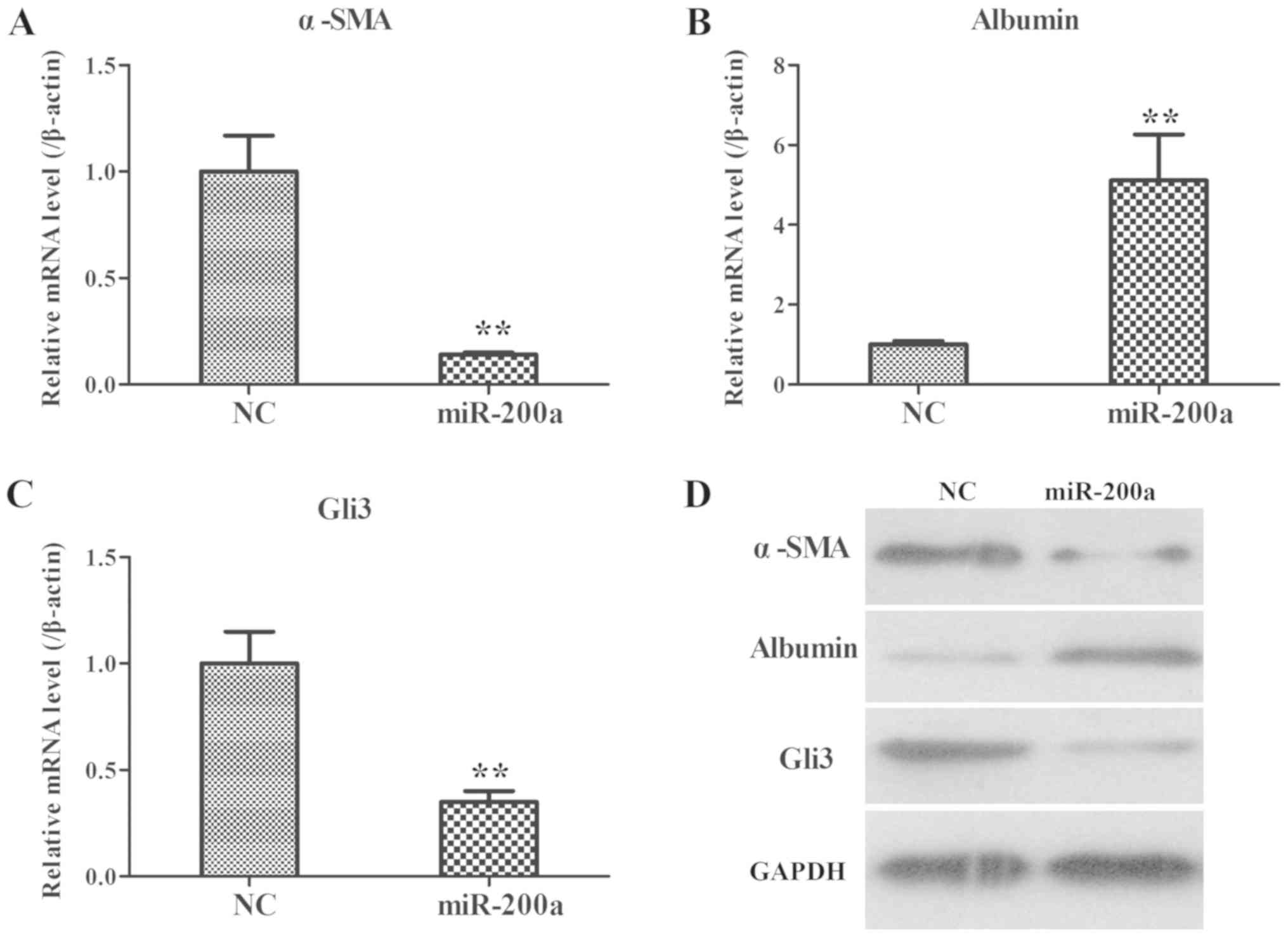
|
1
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elpek GÖ: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman SL: Mechanisms of disease:
Mechanisms of hepatic fibrosis and therapeutic implications. Nat
Clin Pract Gastroenterol Hepatol. 1:98–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coletta M, Nicolini D, Benedetti
Cacciaguerra A, Mazzocato S, Rossi R and Vivarelli M: Bridging
patients with hepatocellular cancer waiting for liver transplant:
All the patients are the same? Transl Gastroenterol Hepatol.
2:782017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim R, Ricardo SD and Sievert W:
Cell-based therapies for tissue fibrosis. Front Pharmacol.
8:6332017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Treiber T, Treiber N, Plessmann U,
Harlander S, Daiß JL, Eichner N, Lehmann G, Schall K, Urlaub H and
Meister G: A compendium of RNA-binding proteins that regulate
MicroRNA biogenesis. Mol Cell. 66:270–284 e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Fortin K and Mourelatos Z:
MicroRNAs: Biogenesis and molecular functions. Brain Pathol.
18:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami Y and Kawada N: MicroRNAs in
hepatic pathophysiology. Hepatol Res. 47:60–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roderburg C, Urban GW, Bettermann K,
Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C,
Knolle P, et al: Micro-RNA profiling reveals a role for miR-29 in
human and murine liver fibrosis. Hepatology. 53:209–218. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sekiya Y, Ogawa T, Yoshizato K, Ikeda K
and Kawada N: Suppression of hepatic stellate cell activation by
microRNA-29b. Biochem Biophys Res Commun. 412:74–79. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa T, Iizuka M, Sekiya Y, Yoshizato K,
Ikeda K and Kawada N: Suppression of type I collagen production by
microRNA-29b in cultured human stellate cells. Biochem Biophys Res
Commun. 391:316–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Ghazwani M, Zhang Y, Lu J, Li J, Fan
J, Gandhi CR and Li S: miR-122 regulates collagen production via
targeting hepatic stellate cells and suppressing P4HA1 expression.
J Hepatol. 58:522–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao J, Tang N, Wu K, Dai W, Ye C, Shi J,
Zhang J, Ning B, Zeng X and Lin Y: MiR-21 simultaneously regulates
ERK1 signaling in HSC activation and hepatocyte EMT in hepatic
fibrosis. PLoS On. 9:e1080052014. View Article : Google Scholar
|
|
14
|
He Y, Huang C, Zhang SP, Sun X, Long XR
and Li J: The potential of microRNAs in liver fibrosis. Cell
Signal. 24:2268–2272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang S, Banerjee S, de Freitas A, Sanders
YY, Ding Q, Matalon S, Thannickal VJ, Abraham E and Liu G:
Participation of miR-200 in pulmonary fibrosis. Am J Pathol.
180:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Wang G, Zhou H, Cai J, Li P, Zhou M,
Lu Y, Jiang X, Huang H, Zhang Y and Gong A: TGF-β1-miR-200a-PTEN
induces epithelial-mesenchymal transition and fibrosis of
pancreatic stellate cells. Mol Cell Biochem. 431:161–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JJ, Tao H, Hu W, Liu LP, Shi KH, Deng
ZY and Li J: MicroRNA-200a controls Nrf2 activation by target Keap1
in hepatic stellate cell proliferation and fibrosis. Cell Signal.
26:2381–2389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun X, He Y, Ma TT, Huang C, Zhang L and
Li J: Participation of miR-200a in TGF-β1-mediated hepatic stellate
cell activation. Mol Cell Biochem. 388:11–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riobo NA and Manning DR: Pathways of
signal transduction employed by vertebrate Hedgehogs. Biochem J.
403:369–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bloomsmith MA, Perlman JE, Hutchinson E
and Sharpless M: Behavioral management programs to promote
laboratory animal welfare. Weichbrod RH, Thompson GAH and Norton
JN: Management of animal care and use programs in research,
education, and testing. 2nd. Boca Raton (FL): CRC Press/Taylor
& Francis. Chapter 5. 2018
|
|
21
|
Prestigiacomo V, Weston A, Messner S,
Lampart F and Suter-Dick L: Pro-fibrotic compounds induce stellate
cell activation, ECM-remodelling and Nrf2 activation in a human
3D-multicellular model of liver fibrosis. PLoS One.
12:e01799952017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park EK, Jung HS, Yang HI, Yoo MC, Kim C
and Kim KS: Optimized THP-1 differentiation is required for the
detection of responses to weak stimuli. Inflamm Res. 56:45–50.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou L, Dong X, Wang L, Shan L, Li T, Xu
W, Ding Y, Lai M, Lin X, Dai M, et al: Casticin attenuates liver
fibrosis and hepatic stellate cell activation by blocking
TGF-β/Smad signaling pathway. Oncotarget. 8:56267–56280.
2017.PubMed/NCBI
|
|
24
|
Knight V, Lourensz D, Tchongue J, Correia
J, Tipping P and Sievert W: Cytoplasmic domain of tissue factor
promotes liver fibrosis in rat. World J Gastroenterol.
23:5692–5699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishak K, Baptista A, Bianchi L, Callea F,
De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve
PM and Casey PJ: MicroRNA-182 and microRNA-200a control G-protein
subunit α-13 (GNA13) expression and cell invasion synergistically
in prostate cancer cells. J Biol Chem. 288:7986–7995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JY, An HJ, Kim WH, Gwon MG, Gu H, Park
YY and Park KK: Anti-fibrotic effects of synthetic
oligodeoxynucleotide for TGF-β1 and smad in an animal model of
liver cirrhosis. Mol Ther Nucleic Acids. 8:250–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
King A, Houlihan DD, Kavanagh D, Haldar D,
Luu N, Owen A, Suresh S, Than NN, Reynolds G, Penny J, et al:
Sphingosine-1-phosphate prevents egress of hematopoietic stem cells
from liver to reduce fibrosis. Gastroenterology. 153:233–248.e16.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu F, Han Y, Zhu D, Tian H, Zhu H, Ren J,
Gu D and Duan Y: Construction of a recombinant pIRES2-EGFP-ARTS
plasmid and its effect on LX-2 cells. Mol Med Rep. 16:4737–4743.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang XP, Ai WB, Wan LY, Zhang YQ and Wu
JF: The roles of microRNA families in hepatic fibrosis. Cell
Biosci. 7:342017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou DD, Wang X, Wang Y, Xiang XJ, Liang
ZC, Zhou Y, Xu A, Bi CH and Zhang L: MicroRNA-145 inhibits hepatic
stellate cell activation and proliferation by targeting ZEB2
through Wnt/β-catenin pathway. Mol Immunol. 75:151–160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong R, Zheng Y, Chen G, Zhao R, Zhou Z
and Zheng S: miR-222 overexpression may contribute to liver
fibrosis in biliary atresia by targeting PPP2R2A. J Pediatr
Gastroenterol Nutr. 60:84–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia H, Ng SS, Jiang S, Cheung WK, Sze J,
Bian XW, Kung HF and Lin MC: miR-200a-mediated downregulation of
ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma
cell growth, migration and invasion. Biochem Biophys Res Commun.
391:535–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding XQ, Wu WY, Jiao RQ, Gu TT, Xu Q, Pan
Y and Kong LD: Curcumin and allopurinol ameliorate fructose-induced
hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3
inflammasome. Pharmacol Res. 137:64–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saha S, Choudhury J and Ain R:
MicroRNA-141-3p and miR-200a-3p regulate insulin-like growth factor
2 during mouse placental development. Mol Cell Endocrinol.
414:186–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hyun J, Wang S, Kim J, Rao KM, Park SY,
Chung I, Ha CS, Kim SW, Yun YH and Jung Y: MicroRNA-378 limits
activation of hepatic stellate cells and liver fibrosis by
suppressing Gli3 expression. Nat Commun. 7:109932016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Trnski D, Sabol M, Gojević A, Martinić M,
Ozretić P, Musani V, Ramić S and Levanat S: GSK3β and Gli3 play a
role in activation of Hedgehog-Gli pathway in human colon
cancer-Targeting GSK3β downregulates the signaling pathway and
reduces cell proliferation. Biochim Biophys Acta. 1852:2574–2584.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen X, Peng Y and Li H: The
injury-related activation of hedgehog signaling pathway modulates
the repair-associated inflammation in liver fibrosis. Front
Immunol. 8:14502017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Machado MV and Diehl AM: Hedgehog
signalling in liver pathophysiology. J Hepatol. 68:550–562. 2018.
View Article : Google Scholar : PubMed/NCBI
|