Introduction
Despite numerous advances in biotechnology, cancer
is still the leading cause of death worldwide, responsible for ~1/4
of mortality in the USA (1).
Several nanoscale drug delivery systems, including polymeric
nanoparticles, liposomes and nanogels, have been developed for
modern cancer therapy (1,2). Compared with other traditional
approaches, such as injection of free drug solution or drug loaded
microparticles, these nanoscale drug formulations have demonstrated
several advantages, including decreased adverse effects, improved
pharmacological profiles and drug tolerance and prolonged
circulation (3). Despite their
merits, the majority of nanoscale drug delivery systems exhibit
inefficient drug release and cellular uptake, which largely
compromises the drug treatment benefits in the clinic (3). To elicit their therapeutic effects,
many drugs, including small molecule drugs, proteins and small
interfering RNAs, have to be delivered and released into specific
cellular compartments, such as the cytoplasm of pathological cells.
Therefore, to ensure sufficient drug levels in the pathological
cells, drug delivery systems have to enter the target cells and
efficiently release the drug molecules into the targeted cell
compartment (4). In the past
decade, a number of studies have investigated methods of enhancing
cellular uptake and achieving rapid intracellular release; however,
only a few approaches were able to achieve both of these effects
(5–7).
Multifunctional hybrid drug delivery systems,
including polymer or nanoparticle modified liposomes, exhibit the
advantages of two or more delivery systems and have become one of
the most popular delivery systems (8–10). A
previous study revealed that light-responsive core shell
nanoparticles, i.e., gold nanoparticle-coated liposomes, achieved
rapid intracellular drug release in MDA-MB-231-R cancer cells
(11). Under short pulsed laser
irradiation (532 nm; 6 ns), highly localized heat was generated
owing to the surface plasmon resonance of the gold nanoparticle
shell, and the encapsulated cargo was rapidly released due to
increased membrane permeability of the liposomal core. Although the
developed system was effective for intracellular drug release and
enhancing drug therapeutic efficacy, the trigger used was green
light (532 nm), which exhibits poor tissue penetration and
photo-damage to biological tissues and cells. The deep tissue
penetration and reduced photo-damage of near-infrared light (NIR)
make it a particularly attractive option for biomolecule release
(12–16). Therefore, further optimization of
the light-triggered release system by switching the trigger from
green light to NIR light is required.
In the present study, to further enhance the
cellular uptake and achieve a high intracellular drug
concentration, the core shell nanoparticles described by Jiang
et al (11) were further
modified by hyaluronic acid (HA), forming corona core-shell
nanoparticles. HA, a naturally occurring high-molecular weight
glycosaminoglycan, is a major component of the extracellular matrix
(17). As a natural excipient, HA
has several advantages, such as preventing protein absorption due
to its hydrophilic and polyanionic characteristics in a
physiological environment (18).
Notably, HA has demonstrated high efficiency in targeting to
tissues with HA receptors, such as classification determinant 44
(CD44) and hyaluronan receptor for endocytosis (HARE) (19). A number of cancer cells upregulate
the expression of the HA receptor CD44, which has been proposed as
a marker of breast cancer stem cells (20). On the basis of these findings, the
present study developed an HA-modified hybrid nanoparticle (CCS) as
an alternative to the unmodified CS to achieve higher intracellular
drug concentration upon light activation and to further enhance
therapeutic effects.
In the present study, CCS were developed and
characterized by transmission electron microscopy (TEM), dynamic
laser light scattering (DLS) and ultraviolet-visible (UV–Vis)
spectroscopy. The results revealed that HA modification enhanced
cellular uptake in MDA-MB-231 cells 1.9-fold. Pulsed laser
irradiation rapidly released encapsulated 6-carboxyfluorescein
(6-CF) from CCS and CS trapped in endosomes and lysosomes into the
cytosol and nucleus. Furthermore, the cytotoxicity of doxorubicin
was significantly enhanced by CCS delivery upon laser irradiation.
The results showed that HA-modified light responsive nanoparticles
were able to achieve high intracellular drug concentration by
enhancing cellular uptake and had rapid intracellular drug release
upon light activation, suggesting that this approach may be a
promising intracellular drug delivery system.
Materials and methods
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)
and cholesterol were purchased from Avanti Polar Lipids; Merck
KGaA. Sodium hyaluronate, cystamine hydrochloride, hyaluronidase
(type I-S), NaBH3CN, gold (III) chloride trihydrate and 6-CF were
purchased from Sigma-Aldrich; Merck KGaA. Ascorbic acid, borate
buffer were obtained from Thermo Fisher Scientific, Inc. DMEM and
fetal bovine serum were purchased from Gibco; Thermo Fisher
Scientific, Inc. 4′,6-Diamidino-2-phenylindole (DAPI) and
LysoTracker Red DND-99 were purchased from Thermo Fisher
Scientific, Inc. Doxorubicin sodium salt was purchased from Alfa
Aesar. All other reagents were of analytical grade and used without
further purification.
Synthesis of thiol end-functionalized
HA
A low-molecular weight HA was obtained via digestion
of HA with hyaluronidase (type I-S) following a previously reported
method (21). Briefly,
hyaluronidase was added into the HA solution (10 mg/ml; pH 7.0) at
a ratio of 100 units of hyaluronidase per mg of HA. The mixture was
incubated at 37°C for 20 h, followed by boiling (100°C) for 10 min
to inactivate the hyaluronidase. An Amicon ultrafiltration kit
[molecular weight cut-off (MWCO); 1,000 Da; Amicon Corporation] was
used to remove aggregated hyaluronidase and high molecular weight
HA. The filtrated solution was then dialyzed (MWCO, 1,000 Da) for
24 h under 25°C to remove 1, 2 or 3 mer HA. Dry oligo-HA was
obtained after the lyophilization of the HA solution following
dialysis. For lypholiziation, HA solutions were placed into 25 ml
glass vials and kept at −80°C for 2 h, followed by freeze-drying
(Lio-Labor®; Telstar) for 48 h to reach a freezing
temperature (−45°C), a sublimation temperature (from −45-25°C) and
a sublimation pressure (4.54×10−4 atmosphere). For thiol
modification at the reducing end, 50 mg of oligo-HA and 60 mg of
cystamine hydrochloride were dissolved in borate buffer (pH 8.5;
0.4 M NaCl). Sodium borohydride (NaBH3CN) was added to the reaction
mixture at a final concentration of 200 mM and incubated at 40°C
for 5 days. Free thiol groups were obtained by incubation of the
resulting mixture with 100 mM dithiothreitol (DTT) at 25°C for 12
h. The mixture was further dialyzed (MWCO: 1,000 Da) for 24 h at
25°C to remove unreacted chemicals and was subsequently lyophilized
using the same protocol as HA lyophilization.
Preparation of CCS and CS. Gold nanoparticle coated
liposomes (CS) were prepared following a previously reported method
(8). Briefly, liposomes were
prepared with DPPC and cholesterol at a molar ratio of 70:30. Lipid
powders were dissolved in chloroform and dried under nitrogen
stream, and then placed in a vacuum overnight to completely remove
chloroform. For the encapsulation of doxorubicin, the lipid film
was hydrated with 300 mM ammonium sulfate (pH=7.5), followed by
extrusion for 21 times through a 200 nm polycarbonate membrane
using an Avanti mini-extruder (Sigma-Aldrich; Merck KGaA). Empty
liposomes were then passed through the S1000 column (GE Healthcare)
pre-equilibrated with isotonic
N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES) buffer
(140 mM NaCl; 10 mM HEPES; pH=7.4) to remove the extra ammonium
sulfate solution. Subsequently, doxorubicin hydrochloride was added
to the liposome suspension to achieve a drug to lipid ratio of 1:3
(mol/mol). The loading process was carried out at 37°C for 2 h. For
the encapsulation of 6-CF, the drug-lipid film was hydrated with
6-CF solution (0.1 or 100 mM) at 55°C for 1 h. The free doxorubicin
or 6-CF was removed by size extrusion chromatography eluted with
HEPES buffer with a flow rate of 1 ml/min. The samples were
detected at 280 nm.
For gold coating, liposomes were diluted to 1 mM
using HEPES buffer. Gold chloride solution at a concentration of 20
mM was added and mixed with liposomes, followed by addition of
ascorbic acid solution (40 mM). Following reduction, gold liposomes
were dialyzed against HEPES buffer for 24 h at 4°C to remove
unreacted gold chloride and ascorbic acid. The thiol
end-functionalized HA (HA-SH) was then immobilized onto the surface
of CS via gold-thiol chemistry (21). Briefly, HA-SH was incubated with CS
solutions at room temperature for 2 h, and then the unreacted HA-SH
was removed by dialysis using a dialysis tubing (MWCO, 12,000 Da;
Sigma-Aldrich; Merck KGaA). The resulting CCS samples were stored
at 4°C until further use.
The hydrodynamic radius and polydispersity of
liposomes, CS and CCS were determined by diffraction light
scattering (DLS) method using a Zetasizer (Nano ZS; Malvern
Panalytical). The UV–Vis spectra in the range of 400–1,100 nm were
recorded using a spectrometer. The morphology of the CCS samples
was observed by TEM operating at a voltage of 200 kV. CCS samples
were diluted with HEPES buffer and placed onto a carbon film coated
copper grid. The samples were air-dried for 2 h at room temperature
and subsequently imaged using a 125K magnification.
Cytotoxicity and cellular uptake of CS
and CCS
The cytotoxicity of CS and CCS on MDA-MB-231 cells
was evaluated using the XTT method following a previously reported
method (22). MDA-MB-231 cells
from the American Type Culture Collection were cultured in a
96-well plate (1×104 cells/well) using DMEM with 10% FBS
and incubated for 24 h at 37°C. The cells were treated with blank
CS and CCS (0.1, 0.5, 1.0 mg/ml) for 4 h at 37°C. After aspirating
the treatment media, 100 µl of DMEM and 25 µl of XTT solution were
added to the cells. The control cells were treated with medium
alone without CS or CCS. After a 4-h incubation at 37°C, the
absorbance was measured at a wavelength of 450 nm using a Multiskan
GO microplate reader (Thermo Fisher Scientific, Inc.). Cell
viability was calculated as a percentage of control cells.
Flow cytometry was used to quantify the endocytosed
nanoparticles and the effects of free HA on the endocytosis
efficiency of nanoparticles. MDA-MB-231 cells were seeded into a
24-well plate at a density of 2×105 cells/ml and cells
in the preconfluent state were used for uptake studies. The cells
were incubated with 6-CF (0.1 mM) encapsulated CS and CCS for 4 h
at 37°C, washed three times with ice-cold PBS, and subsequently
harvested and analyzed using a ZE5 cell analyzer (Everest software
2.2; Bio-Rad Laboratories, Inc.). Cells incubated with medium alone
without any nanoparticles were used as the negative control. A
total of 10,000 cells were analyzed in each group. To investigate
whether the cellular uptake of CCS was mediated by the HA
receptors, the cells were pretreated with free HA (10 mg/ml,
hydrated overnight in serum-free DMEM) for 1 h at 37°C before the
addition of nanoparticles.
To investigate the intracellular distribution of CS
and CCS, cells with endocytosed nanoparticles were observed by
confocal laser scanning microscopy (CLSM; FV1000; Olympus
Corporation). Briefly, MDA-MB-231 cells were seeded in 25-mm glass
bottom dishes at a density of 1×105 cells/plate and
cultured at 37°C for 24 h. The DMEM was then replaced with 1 ml of
medium containing CCS or CS and incubated at 37°C for 4 h. After
the nanoparticle containing medium was removed, late endosomes and
lysosomes were stained with LysoTracker Red DND-99 at 37°C for 30
min. Cells were then supplied with fresh medium and immediately
observed under a confocal laser microscope using ×100
magnification.
Light-triggered intracellular
release
To monitor pulsed laser-triggered release, CS and
CCS were encapsulated with 100 mM 6-CF, a concentration at which
its fluorescence is self-quenched (16). The cell nuclei of MDA-MB-231 cells
cultured in 25-mm glass bottom dishes at a density of
1×105 cells/plate were stained with 5 µg/ml Hoechst
33342 at 37°C for 5 min, and then cells were treated with 6-CF
encapsulated CS and CCS (100 mM) 37°C for 4 h. Cells were
subsequently washed with PBS and supplied with fresh DMEM prior to
laser irradiation (700 nm; 100 mJ/cm2; 5 pulses), and
then immediately observed by CLSM using ×100 magnification.
Cytotoxicity of doxorubicin-containing
CS and CCS
The effects of CCS and pulsed laser irradiation on
the cytotoxicity of doxorubicin on MDA-MB 231-R cells were
investigated using the XTT method. MDA-MB 231-R cells were cultured
overnight in 96-well plates at a density of 1×104/well
at 37°C. The control group consisted of free doxorubicin-treated
cells, and the treatment groups were as follows: i)
Doxorubicin-encapsulated CS with or without laser irradiation; and
ii) doxorubicin-encapsulated CCS with or without laser irradiation.
The cells were washed with PBS and incubated with fresh DMEM that
contained doxorubicin encapsulated in CS or CCS for 3 h at 37°C.
The cells were washed 3 times with PBS, and the laser-treated
groups were irradiated with nanosecond (ns) laser pulse (700 nm; 5
pulses; 100 mJ/cm2), followed by the addition of fresh
DMEM and incubation for 24 h at 37°C. Subsequently, the cell
viability was determined by the XTT assay as previously mentioned.
The drug concentration causing 50% inhibition (IC50) was
calculated.
Statistical analysis
All data are presented as the mean ± standard
deviation of three independent experiments. The unpaired Student's
t-test was used to compare two groups. Three or more groups were
analyzed using the one-way ANOVA followed by the Dunnett's post
hoc. P<0.05 was considered to indicate a statistically
significant difference.
Results
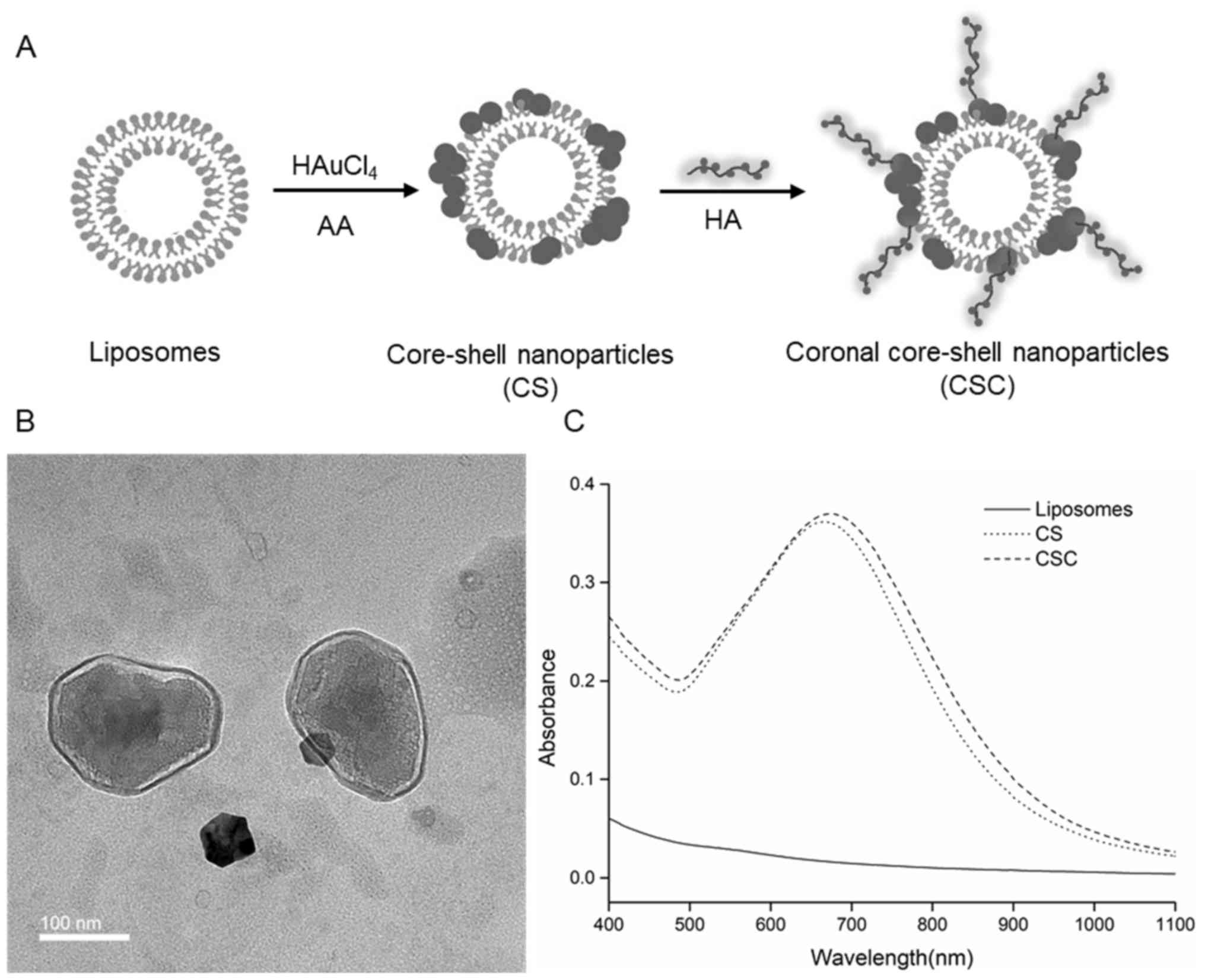
Preparation and characterization of
CCS
CCS nanoparticles were prepared using a three step
method as shown in Fig. 1A. The
CCS had a hydrodynamic size of ~227 nm, while uncoated liposomes
and CS measured ~185 and 210 nm, respectively (Table I). CCS is monodispersed with
Polydispersity index of 0.163. TEM observations revealed that the
CCS exhibited a typical coronal core shell structure as expected.
Gold nanoparticles were distributed on the surface of the liposomal
core, while HA formed a polymer corona on the outer surface
(Fig. 1B). The TEM results
suggested that HA had attached onto the gold nanoparticles on the
surface. The UV–Vis spectra showed that CCS and CS exhibited an
absorption peak at ~673 and 667 nm, respectively, while uncoated
liposomes did not show any resonance peak in the wavelength range
of 400–1,100 nm (Fig. 1C). Gold
liposomes presented high absorbance in the wavelength range 500–800
nm, suggesting that gold liposomes could be activated by light in
the range of 500–800 nm. CCS and CS were activated at 700 nm in
subsequent experiments to ensure maximum absorbance in the NIR
range.
 | Table I.Hydrodynamic diameter and
polydispersity of uncoated liposomes, CS and CCS. |
Table I.
Hydrodynamic diameter and
polydispersity of uncoated liposomes, CS and CCS.
| Sample | Diameter (nm) | Polydispersity
index |
|---|
| Uncoated
liposomes | 185±10 | 0.051±0.012 |
| CS | 210±18 | 0.138±0.039 |
| CCS | 227±21 | 0.163±0.031 |
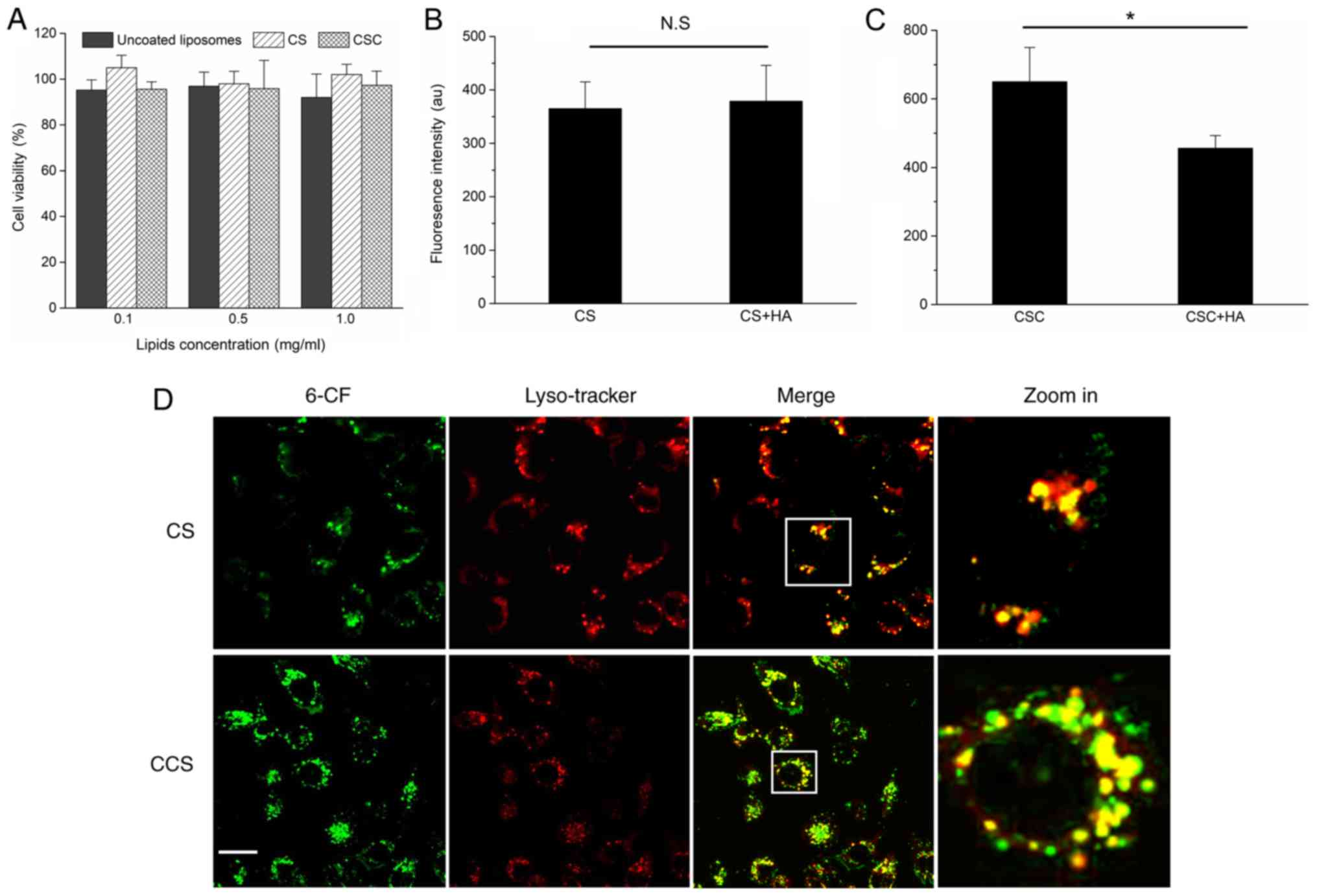
Characterization of cellular uptake,
cytotoxicity and intracellular distribution of CCS
The cytotoxicity of blank CS and CCS are shown in
Fig. 2A. No significant
cytotoxicity was observed for CS and CCS at the tested
concentrations (0.1, 0.5 and 1.0 mg/ml). Quantitative analysis by
flow cytometry revealed that HA modification significantly enhanced
the cellular uptake of CS (Fig. 2B and
C). The cellular uptake of CCS was ~1.9-fold higher than CS in
the absence of HA modification on the surface. To further verify
whether the uptake of CCS was specific to HA receptors, competitive
binding experiments were performed by pretreating MDA-MB-231 cells
with excess free HA (200–400 kDa) before incubation. As shown in
Figs. 2B and C, ligand
pretreatment did not change the cellular uptakes of CS, while the
cellular uptake of CCS was significantly reduced. These results
suggested that the free HA competed with CCS for receptor binding
sites. Thus, cell surface HA receptors, mainly CD44 and HARE, may
have mediated the cellular uptake process. Late endosomes and
lysosomes were labeled with LysoTracker Red DND-99 to investigate
the co-localization of 6-CF encapsulated CS and CCS with
endolysosomes. As shown in Fig.
2D, following 4 h of incubation, the green fluorescence from
6-CF was highly co-localized with LysoTracker Red DND-99 (red
fluorescence). The results indicated that endocytosed CCS and CS
were largely trapped in endolysosomes and may have failed to
release their cargo into the cytosol.
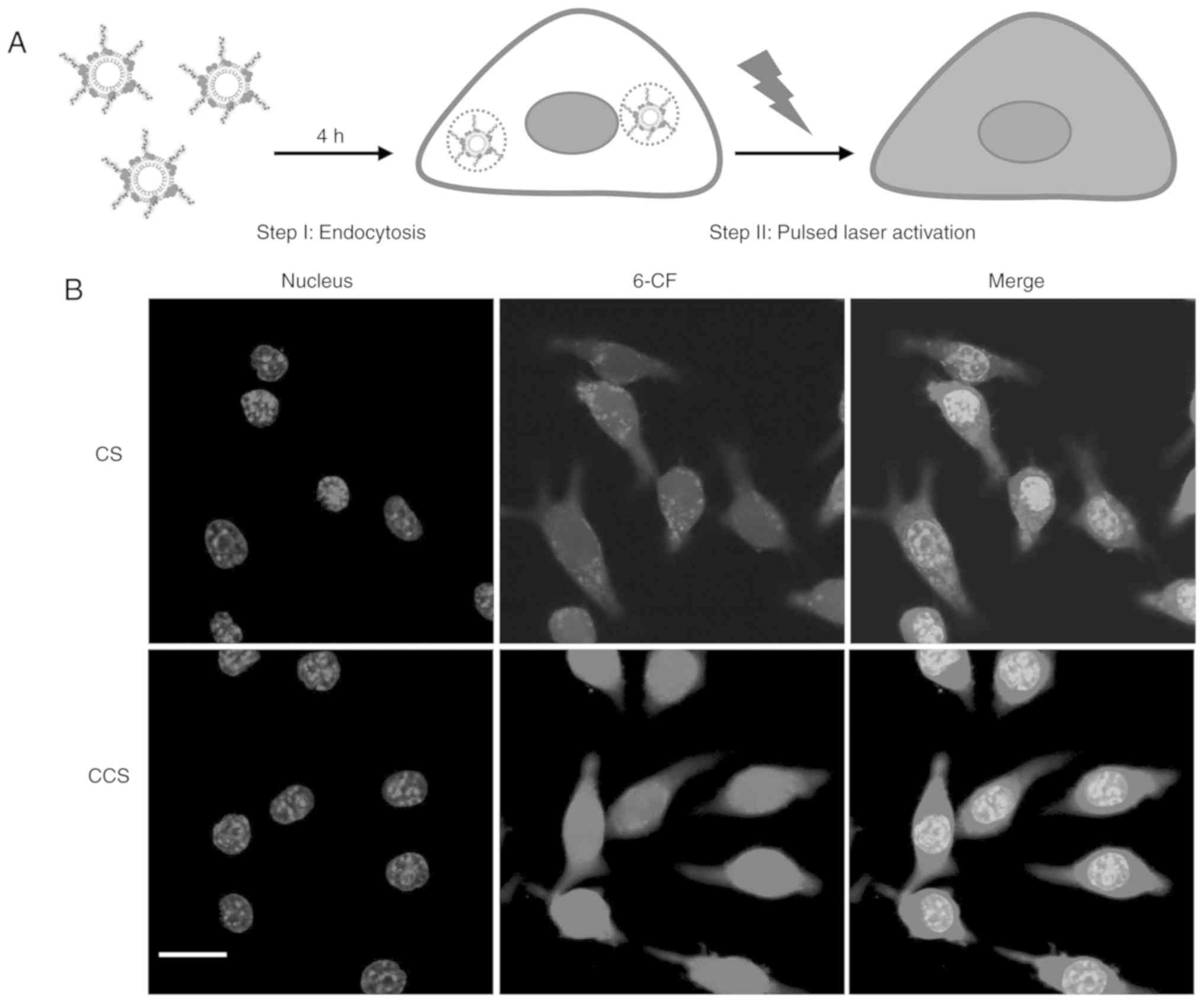
Pulse laser-triggered intracellular
release
Upon activation by the ns pulsed laser,
intracellular doxorubicin release was observed by CLSM imaging. The
fluorescence of 6-CF at a high concentration was quenched due to
its self-association. Release of 6-CF from liposomal particles and
dilution by the surrounding medium de-quench 6-CF and increase its
fluorescence intensity (16). The
results demonstrated that upon short-pulsed laser irradiation (700
nm; 100 mJ/cm2; 5 pulses), 6-CF was rapidly released
from CS and CCS trapped in endolysosomes and evenly distributed in
the cytosol and nucleus (Fig. 3).
This observation was in agreement with a previous study where gold
nanoparticle liposomes were triggered at a different wavelength
(532 nm; 100 mJ/cm2; 5 pulses) (8). CCS exhibited a stronger green
fluorescence intensity after laser activation, suggesting that
increased cellular uptake by HA modification may have contributed
to higher intracellular free drug concentration following
activation.
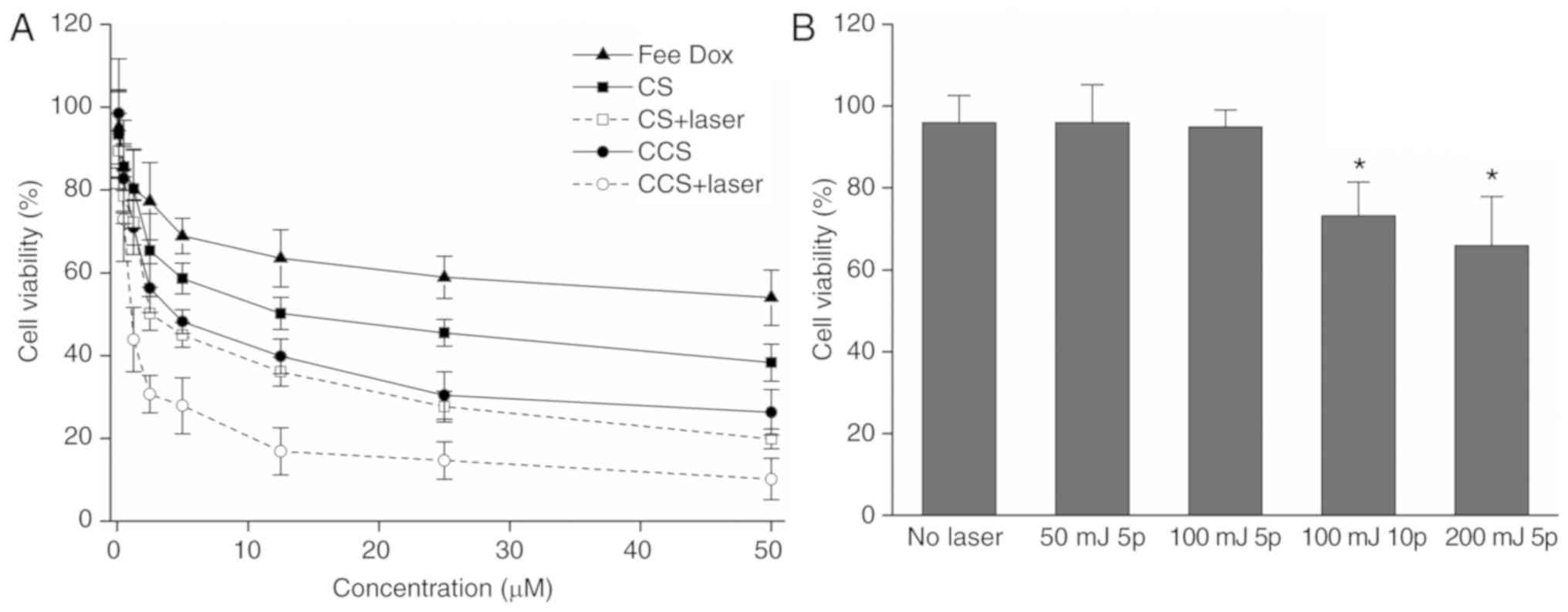
Cytotoxicity of doxorubicin on
MDA-MB-231 cells
To test the ability of CCS to enhance the
therapeutic effects of doxorubicin, the cytotoxicity of
doxorubicin-encapsulated CS and CCS with or without laser
irradiation was measured by the XTT assay. The results demonstrated
that laser irradiation markedly increased the cytotoxicity of
doxorubicin in both CS and CCS (Fig.
4A). HA modification also enhanced the cytotoxicity of
doxorubicin, as the IC50 of doxorubicin encapsulated in
CCS decreased to 3.0 µM, while the IC50 of doxorubicin
encapsulated in CS was 5.8 µM. Cytotoxicity enhancement was the
most significant when doxorubicin was incorporated into CCS and
activated by ns pulsed laser, and the IC50 was further
decreased to 1.5 µM. Under the current experimental conditions,
laser irradiation alone (5 pulses; 100 mJ/cm2) did not
result in significant cell death, as indicated in Fig. 4B.
Discussion
While several cancer drug delivery systems have been
developed, limited curative effects in patients have been observed
(2). Enhancing drug release from
endocytosed drug carriers is of great significance since the
majority of therapeutic agents have to be released into the cytosol
or nucleus to elicit their therapeutic effects (3). A number of controlled drug release
strategies have been reported in recent years, including
diffusion-based and biologically-activated drug release systems
(3,23–24).
However, drug release based on these mechanisms is usually slow,
cannot be precisely controlled and does not amplify the site
selectivity of drug delivery (25). Strategies that enable fast drug
release upon activation remain desirable to enhance intracellular
cancer drug delivery. A previous study reported that burst release
resulted in instant high intracellular drug concentration, which
significantly enhanced doxorubicin cytotoxicity (11). In the present study, a
light-triggered delivery system was optimized by using NIR laser
pulses as the drug release trigger. The results demonstrated that
similar to green light, ns NIR pulses triggered rapid intracellular
drug release from CS and CCS trapped in endolysosomes.
In addition to increasing intracellular drug
release, enhancing the cellular uptake of drug carriers is an
important step to ensure a high intracellular concentration of free
drug (26). Therefore, in the
present study, the light responsive nanoparticles, i.e., gold
nanoparticle coated liposomes, were further modified by HA to
increase the endocytosis efficiency and maximize the triggering
effects. The results revealed that HA modification significantly
increased the cellular uptake of nanoparticles, resulting in a
greater intracellular drug concentration. HA modification alone
significantly enhanced the cytotoxicity of doxorubicin as the
IC50 in MDA-MB-231R cells decreased from 5.8 to 3.0 µM.
When activated by short-pulsed laser (700 nm; 100
mJ/cm2; 5 pulses), the IC50 of doxorubicin
was further decreased by ~2-fold. The results suggested that
ligand-modified light responsive nanoparticles may be a promising
drug delivery system for intracellular drug delivery by combining
enhanced cellular uptake and rapid light-triggered intracellular
release.
The advantages of using NIR as the light trigger
include deep tissue penetration, minimum auto-fluorescence and
tissue scattering as well as high biosafety (16). While CCS has demonstrated its
potential as an intracellular drug delivery carrier at the cellular
level, further in vivo studies are required to confirm its
ability to enhance the therapeutic efficiency of drugs. Although
NIR increases tissue penetration compared to green light (16), in vivo single site light
delivery remains a challenge. Therefore, approaches that deliver a
light trigger to target tissue are required prior to applying this
technique in vivo.
In conclusion, the present study developed a dual
functional CCS nanoparticle for intracellular drug delivery. Light
responsive nanoparticles were further modified by HA, which
enhanced the cellular uptake by 1.9-fold. In MDA-MB 231-R cancer
cells, short pulsed laser (700 nm; 100 mJ/cm2; 5 pulses)
liberated 6-CF from endocytosed CCS into the cytosol and nucleus.
Furthermore, laser irradiation significantly increased the
cytotoxicity of doxorubicin encapsulated in CCS in MDA-MB 231-R
cells. The results suggested that a combination of active targeting
and laser triggered on-demand release of chemotherapeutic agents
may be a promising approach for intracellular drug delivery and may
enhance therapeutic effects.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Province
Medical and Health Technology Program (grant no. 2017KY235).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ, XZ and CW designed the study. JJ and HZ
performed the experiments. XZ and CW analyzed the results and wrote
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dreaden EC, Austin LA, Mackey MA and
El-Sayed MA: Size matters: Gold nanoparticles in targeted cancer
drug delivery. Ther Deliv. 3:457–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lukianova-Hleb EY, Ren X, Sawant RR, Wu X,
Torchilin VP and Lapotko DO: On-demand intracellular amplification
of chemoradiation with cancer-specific plasmonic nanobubbles. Nat
Med. 20:778–784. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng FH, Cheng R, Deng C and Zhong ZY:
Intracellular drug release nanosystems. Mater Today. 15:436–442.
2012. View Article : Google Scholar
|
|
4
|
Li X, Kang P, Chen Z, Lal S, Zhang L,
Gassensmith JJ and Qin Z: Rock the nucleus: Significantly enhanced
nuclear membrane permeability and gene transfection by plasmonic
nanobubble induced nanomechanical transduction. Chem Commun (Camb).
54:2479–2482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun H, Guo B, Cheng R, Meng F, Liu H and
Zhong Z: Biodegradable micelles with sheddable poly(ethylene
glycol) shells for triggered intracellular release of doxorubicin.
Biomaterials. 30:6358–6366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin AL, Bernas LM, Rutt BK, Foster PJ
and Gillies ER: Enhanced cell uptake of superparamagnetic iron
oxide nanoparticles functionalized with dendritic guanidines.
Bioconjug Chem. 19:2375–2384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komin A, Russell LM, Hristova KA and
Searson PC: Peptide-based strategies for enhanced cell uptake,
transcellular transport, and circulation: Mechanisms and
challenges. Adv Drug Deliv Rev. 110-111:52–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XY, Guo SY, Zhu CL, Zhu QL, Gan Y,
Rantanen J, Rahbek UL, Hovgaard L and Yang MS: Intestinal mucosa
permeability following oral insulin delivery using core shell
corona nanolipoparticles. Biomaterials. 34:9678–9687. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohanraj VJ, Barnes TJ and Prestidge CA:
Silica nanoparticle coated liposomes: A new type of hybrid
nanocapsule for proteins. Int J Pharm. 392:285–293. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Chen D, Le C, Zhu C, Gan Y, Hovgaard
L and Yang M: Novel mucus-penetrating liposomes as a potential oral
drug delivery system: Preparation, in vitro characterization and
enhanced cellular uptake. Int J Nanomedicine. 6:3151–3162.
2011.PubMed/NCBI
|
|
11
|
Jiang J, Liu S, Wang C and Zhang H:
Overcoming multidrug resistance by on-demand int racellular release
of doxorubicin and verapamil. J Nanomater. 2018:72018. View Article : Google Scholar
|
|
12
|
Zhang Y, Huang L, Li Z, Ma G, Zhou Y and
Han G: Illuminating cell signaling with near-infrared
light-responsive nanomaterials. ACS Nano. 10:3881–3885. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Yu ZZ, Pan W, Han YY, Zhang TT and
Tang B: A near-infrared light-triggered nanocarrier with reversible
DNA valves for intracellular controlled release. Adv Funct Mater.
23:2255–2262. 2013. View Article : Google Scholar
|
|
14
|
Yavuz MS, Cheng Y, Chen J, Cobley CM,
Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, et al: Gold
nanocages covered by smart polymers for controlled release with
near-infrared light. Nat Mater. 8:935–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weissleder R: A clearer vision for in vivo
imaging. Nat Biotechnol. 19:316–317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Che Z, Mazhar K, Price T and Qin Z:
Ultrafast near-infrared light-triggered intracellular uncaging to
probe cell signaling. Adv Funct Mater. 27:16057782017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bignami A, Hosley M and Dahl D: Hyaluronic
acid and hyaluronic acid-binding proteins in brain extracellular
matrix. Anat Embryol (Berl). 188:419–433. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surace C, Arpicco S, Dufay-Wojcicki A,
Marsaud V, Bouclier C, Clay D, Cattel L, Renoir JM and Fattal E:
Lipoplexes targeting the CD44 hyaluronic acid receptor for
efficient transfection of breast cancer cells. Mol Pharm.
6:1062–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh EJ, Park K, Kim KS, Kim J, Yang JA,
Kong JH, Lee MY, Hoffman AS and Hahn SK: Target specific and
long-acting delivery of protein, peptide, and nucleotide
therapeutics using hyaluronic acid derivatives. J Control Release.
141:2–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee H, Lee K, Kim IK and Park TG:
Synthesis, characterization, and in vivo diagnostic applications of
hyaluronic acid immobilized gold nanoprobes. Biomaterials.
29:4709–4718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang M, Gan L, Zhu C, Dong Y, Liu J and
Gan Y: Cationic core-shell liponanoparticles for ocular gene
delivery. Biomaterials. 33:7621–7630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fomina N, Sankaranarayanan J and Almutairi
A: Photochemical mechanisms of light-triggered release from
nanocarriers. Adv Drug Deliv Rev. 64:1005–1020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng R, Feng F, Meng F, Deng C, Feijen J
and Zhong Z: Glutathione-responsive nano-vehicles as a promising
platform for targeted intracellular drug and gene delivery. J
Control Release. 152:2–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anderson LJ, Hansen E, Lukianova-Hleb EY,
Hafner JH and Lapotko DO: Optically guided controlled release from
liposomes with tunable plasmonic nanobubbles. J Control Release.
144:151–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuhrmann G, Serio A, Mazo M, Nair R and
Stevens MM: Active loading into extracellular vesicles
significantly improves the cellular uptake and photodynamic effect
of porphyrins. J Control Release. 205:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|