Introduction
Glial cells are immune cells that reside in the
central nervous system, and they have an important role in numerous
neurodegenerative diseases, including Alzheimer's (AD), Parkinson's
and Huntington's disease (1,2).
Glial cells are normally in a resting state; however, they are
activated in response to an inflammatory stimulus. Despite
comprising <10% of the cells in the brain, the microglia
regulate the neuroimmune system (3). Reactive microglia are observed in
several neuroinflammatory conditions, including ischemia, brain
injury and infection (4). These
glial cells aggravate neurodegenerative diseases by secreting
inflammatory mediators or proinflammatory cytokines that induce
neurotoxicity (5–7). In addition, they release soluble
toxins, which induce reactive A1 astrocytes that are neurotoxic
(8). Similarly, astrocytes, which
are the most abundant brain cell types, cause several brain
inflammatory conditions by secreting proinflammatory mediators in
response to a neuroinflammatory stimulus (9,10).
Elevated nitric oxide (NO) levels have been observed in glial cells
of patients with AD or ischemia (11,12).
In addition, reactive glial cells promote the production of tumor
necrosis factor (TNF) α, interleukin (IL) 1β, and IL-6, which
contribute to the development of neuroinflammatory conditions and
brain damage (13–15). Therefore, reactive glial cell
regulation is crucial for the initiation and progression of
neurodegenerative diseases and neuronal cell death (4,16,17).
Plant-based anti-inflammatory compounds may be
potential sources of safe and effective drugs (18,19).
Traditional herbal medicines derived from natural products and
their active constituents have been extensively studied, and
several ingredients from natural products have been found to be
beneficial for the treatment of neuroinflammatory and
neurodegenerative diseases, given their ability to suppress glial
activation (20–22). Panax ginseng (23), Curcuma longa (24), and Camellia sinensis
(25) display neuroprotective
effects by suppressing neuroinflammatory cytokine secretion via
glial cell activation. In addition, natural products are safe,
inexpensive and easy to obtain. Therefore, the discovery of a novel
natural product that can regulate glial activation is crucial for
the development of neurodegenerative disease treatments (26).
The peach tree (Prunus persica L. Batsch) was
initially reported as a deciduous tree native to the northwest
region of China. It is now grown worldwide, including the temperate
regions of eastern Asia, including Vietnam, China, Japan and Korea
(27). Various parts of P.
persica have therapeutic effects. The dried seeds of ripened
P. persica fruit are widely used in traditional medicine
(Persicae Semen) in Korea and China, and persicaside, an alkaloid
compound derived from P. persica seeds, inhibits the
production of NO (28). P.
persica flowers are used for the treatment of rashes and eczema
(29). The flavonoid compounds
4-O-caffeoylquinic acid, quercetin-3-O-rhamnoside and kaempferol
glycoside, which are P. persica flower derivatives, have
anti-inflammatory properties (30). In addition, P. persica fruit
has anti-allergenic properties (31). P. persica roots can suppress
the growth of liver cancer cells (27), and its leaves have antioxidant and
antibacterial properties (32–34).
However, the antioxidant properties of P. persica leaves
have only been evaluated with in vitro analysis (e.g.,
2,2-diphenyl-1-picrylhydrazyl assay) (32,33).
Therefore, in vivo biological evidence is required.
The present study aimed to investigate the
anti-inflammatory effects of P. persica aerial parts
(leaves, fruits and twigs) on glial cells. P. persica
methanol extract (PPB) was applied to BV2 cells and primary
astrocytes. PPB inhibited the production of proinflammatory
mediators and cytokines in lipopolysaccharide (LPS)-stimulated BV2
cells by suppressing NF-κB translocation and mitogen-activated
protein kinase (MAPK) signaling pathways. Furthermore, PPB also
inhibited NO production and NF-κB translocation in cultured primary
astrocytes.
Materials and methods
Materials
DMEM and FBS were purchased from Gibco (Thermo
Fisher Scientific, Inc.). Chlorogenic acid, catechin, quercetin,
quercetin-3-O-glucoside, formic acid, diclofenac, and LPS
(Escherichia coli O55:B5) were purchased from Sigma-Aldrich
(Merck KGaA). Antibodies against inducible nitric oxide synthase
(iNOS; cat. no. 610431; BD Biosciences), cyclooxygenase-2 (COX-2;
1:2,000; cat. no. sc-376861; Santa Cruz Biotechnology, Inc.), ERK
[1:2,000; cat. no. 9102S; Cell Signaling Technology, Inc. (CST)],
phosphorylated (p)-ERK (1:1,000; cat. no. 9101S; CST), JNK
(1:2,000; cat. no. 9258S; CST), p-JNK (1:1,000; cat. no. 9251S;
CST), p38 (1:2,000; cat. no. 9212S; CST), p-p38 (1:1,000; cat. no.
9211S; CST), inhibitor of (I)κBα (1:2,000; cat. no. 9242S; CST),
p-IκBα (1:1,000; cat. no. 2859S; CST), and β-actin (1:5,000; cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.) were used for western
blot analysis. Horseradish peroxidase (HRP)-conjugated secondary
antibodies for western blots, goat anti-mouse IgG (1:5,000; cat.
no. 1706516; Bio-Rad Laboratories, Inc.) and goat anti-rabbit IgG
(1:5,000; cat. no. 1706515; Bio-Rad Laboratories, Inc.) were used.
For immunocytochemistry, p65 (1:500; cat. no. 8242S; CST Biological
Reagents Co., Ltd.) and glial fibrillary acidic protein (GFAP;
1:500; cat. no. MAB3402; Merck KGaA) were used as primary
antibodies, and Alexa Fluor 488 donkey anti-mouse IgG (1:500; cat.
no. ab150105; Abcam) and Alexa Fluor 594 donkey anti-rabbit IgG
(1:500; cat. no. ab150076; Abcam) were used as secondary
antibodies.
Preparation of PPB
In 2008, P. persica was obtained from various
regions in Vietnam (Bằng Lũng, Chợ Đồn, and Bắc Kạn). The samples
were authenticated by the chief executive of the Institute of
Ecology and Biological Resources. It is characterized by 4–7 m
tall, dark green, deciduous leaves and flowers in single,
semi-double and double form in colors from white to deep red. A
voucher specimen (KRIB0018669, accession number of KRIBB) was
deposited in the herbarium of the Korea Research Institute of
Bioscience and Biotechnology. P. persica dried and refined
aerial parts (leaves, fruits and twigs; total weight, 63 g) were
extracted with 600 ml of 99.9% (v/v) methanol and were repeatedly
sonicated with 1,500 W input and 40 kHz frequency for 15 min at 2-h
intervals for 3 days. All procedures were performed at 45°C. The
product was filtered through non-fluorescent cotton and
concentrated by rotary evaporation using an N-1000SWD device (EYELA
Co., Ltd.) under reduced pressure at 45°C. Finally, 8.05 g of PPB
was obtained by freeze-drying and then dissolved in DMSO
(Sigma-Aldrich; Merck KGaA).
Contents of the marker substances in
PPB
Chlorogenic acid, catechin, quercetin and
quercetin-3-O-glucoside were selected as marker substances
in PPB based on a previous study (35). The extract contents were measured
using high-performance liquid chromatography coupled with tandem
mass spectrometry (HPLC-MS/MS). Briefly, to identify the product
ions of each substance, the marker substances (100 ng/ml) were
individually infused into the mass spectrometer at a flow rate of
10 µl/min. Precursor ions and fragmentation patterns were monitored
in a negative-ion mode. Using an API 4000 LC/MS/MS system (AB SCIEX
Analytical Instrument Trading Co.) equipped with an electrospray
ionization interface, major peaks in the MS/MS scan were used to
quantify the substances. The compounds were separated on a
reversed-phase column (Kinetex® C18, 2.6-µm particle
size, 100×2.1 mm internal diameter; Phenomenex Ltd.) in the mobile
phase of a water:acetonitrile mixture at a 3:7 (v/v) ratio
including 0.1% formic acid. The column was heated at 30°C, and the
mobile phase was delivered at a flow rate of 0.2 ml/min using an HP
1100 series pump (Agilent Technologies, Inc.). The injection volume
was 5 µl. The turbo ion spray interface was operated at −4,200 V at
450°C. Nitrogen was used as a curtain gas at 35 psi, and air dried
was nebulized as a collision gas at 4 psi and ion source gases 1
(45 psi) and 2 (55 psi). The ion transitions of the precursor to
the product ion were monitored in negative mode as deprotonated
ions [M-H]− at m/z 353.0 → 190.8 [declustering potential
(DP), −60 eV; collision energy (CE), −22 eV] for chlorogenic acid,
289.0 → 244.8 (DP, −90 eV; CE, −20 eV) for catechin, 301.0 → 151.0
(DP, −100 eV, CE, −32 eV) for quercetin, 463.1 → 300.0 (DP, −120
eV, CE, −36 eV) for quercetin-3-O-glucoside, and 296.1 →
251.7 (DP, −100 eV, CE, −32 eV) for diclofenac (internal standard).
Quantification was performed by selective reaction monitoring of
deprotonated precursor ions and related product ions using the
ratio of the area under the peak for each solution. All analytical
data were processed using Analyst software (version 1.5.2; Applied
Biosystems; Thermo Fisher Scientific, Inc.). Standard marker
substance solutions were serially diluted with methanol including
the internal standard (IS, 100 ng/ml) to obtain 10–1,000 ng/ml
concentrations. Using linear regression, two calibration graphs
were derived from the ratios between the areas under the peaks for
each substance and those of the IS. PPB (20 mg/vial) was dissolved
in 1 ml of methanol and was serially diluted with methanol
including the IS by 1,000-fold. The concentrations of each marker
substance were predicted using the calibration graph, and their
contents within the extracts were calculated.
Cell culture and treatment
The BV2 cells (CLC catalog code, ATL03001) were
provided by Dr Dong-Kug Choi (Konkuk University, Chungju-si,
Chungcheongbuk-do, Republic of Korea) and were maintained in DMEM
containing 5% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin
(Welgene, Inc.) in a humidified incubator with air containing 5%
CO2. In all of the experiments, 500,000 cells were
plated and treated with PPB (20, 100 and 200 µg/ml) for 1 h prior
to LPS treatment (200 ng/ml, cat. no. L2880; Sigma-Aldrich; Merck
KGaA), and the vehicle was a DMSO only treatment (0.1%). BV2 cells
were treated with LPS for 6 and 24 h for reverse transcriptase
polymerase chain reaction (RT-PCR) and western blots,
respectively.
Hydrogen peroxide (H2O2, 250
µM) was applied to BV2 cells for 24 h to measure cytotoxicity.
Primary astrocytes were prepared from the cortices
of postnatal day 2 Sprague-Dawley rats (7–9 g), as previously
described by McCarthy and de Villis (36). Postnatal day 1 Sprague-Dawley rats
were purchased (Young Bio) and were maintained for 24 h in
controlled temperature (20–25°C), humidity (50–55%) and 12-h
light/dark cycle with their mother. A total of 6 animals (3 males
and 3 females) were used for this study. All animals were handled
in accordance with the Principle of Laboratory Animal Care
(37) and the study was approved
by the Institutional Animal Care and Use Committee of Chung-Ang
University (2017-00093).
Briefly, the astrocytes were plated in poly-d-lysine
(PDL)-coated T-75-cm2 flasks in DMEM/F12 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin. Microglia were removed at 7
days in vitro (DIV), and subcultured astrocytes at 15 DIV
were used for further experiments. Primary astrocytes were treated
with PPB (20, 100 and 200 µg/ml) for 1 h prior to LPS treatment (10
ng/ml), and the control group was treated with vehicle (DMSO;
0.1%).
Cell viability
Cell viability was determined using the MTT assay.
Cells were treated with PPB for 24 h and incubated with MTT (100
µg/ml) for 2 h. DMSO was added to dissolve the insoluble formazan
crystals that had formed within viable cells, and the absorbance
was measured at 540 nm.
Propidium iodide (PI) staining
BV2 cells, which were protected from the light, were
fixed with 70% ethanol at for 30 min at 20–25°C and stained using
FxCycle PI/RNase staining solution (cat. no. F10797; Thermo Fisher,
Inc.) for 30 min at 20–25°C. Nuclei were stained with DAPI (cat.
no. D1306; Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min
at 20–25°C. Cell images were visualized using a confocal microscope
(Zeiss GmbH).
Nitrite measurement
NO release was measured from nitrite levels using a
Griess reagent (0.1% naphthylethylenediamine and 1% sulfanilamide
in 5% H3PO4). The supernatant was mixed with
an equal volume of Griess reagent for 5 min at 20–25°C. Mixture
absorbance was measured at 540 nm using a microplate reader (Tecan
Group, Ltd.).
Isolation of total RNA and RT-PCR
Total RNA was isolated from LPS-stimulated cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Reverse transcription
was conducted for 5 min at 70°C and 5 min at 4°C with total RNA (1
µg) and GoScript Reverse Transcriptase (Promega Corporation). cDNA
was subsequently amplified by RT-PCR with specific primers and
GoTaq DNA polymerase (Promega Corporation). The PCR primer sets
were as follows: iNOS (sense, 5′-GAGGTACTCAGCGTGCTCCA-3′;
antisense, 5′-AGGGAGGAAAGGGAGAGAGG-3′), COX-2 (sense,
5′-TGAGTGGTAGCCAGCAAAGC-3′; antisense, 5′-CTGCAGTCCAGGTTCAATGG-3′),
TNF-α (sense, 5′-AGGGAGAGTGGTCAGGTTGC-3′; antisense,
5′-CAGCCTGGTCACCAAATCAG-3′), IL-1β (sense,
5′-CAAGGAGAACCAAGCAACGA-3′; antisense, 5′-TTGGCCGAGGACTAAGGAGT-3′),
IL-6 (sense, 5′-GGAGGCTTAATTACACATGTT-3′; antisense,
5′-TGATTTCAAGATGAATTGGAT-3′), and GAPDH (sense,
5′-CCAGTAGACTCCACTCACG-3′; antisense, 5′-CCTTCCACAATGCCAAAGTT-3′).
Thermal cycling was performed as follows: Initial denaturation
(95°C for 2 min), amplification (95°C for 1 min; 55°C for 1 min and
72°C for 1 min, 35 cycles), and final extension (72°C for 5 min).
After amplification, the PCR products were resolved by agarose gel
electrophoresis on 1% agarose gel containing 1X TAE (Biosesang,
Inc.) and 1X Midori green advance (Nippon Genetics Europe GmbH),
and visualized using a LAS-3000 device (FujiFilm). The band
intensity was determined using ImageJ software (version 1.38;
National Institutes of Health).
Western blot analysis
Lysates were extracted from cells using RIPA buffer
(Biosesang, Inc.) and total protein was determined by BCA assay. A
total amount of 30 µg of proteins were loaded. Proteins were
separated using 10% SDS-PAGE and transferred to nitrocellulose
membranes (Whatman; GE Healthcare Life Sciences). The membranes
were blocked with 5% skimmed milk (Biosesang, Inc.) in TBS-Tween
(0.1% Tween-20) for 1 h at 20–25°C. The membranes were incubated
with primary antibodies for overnight at 4°C. Then, the membranes
were incubated with HRP-conjugated secondary antibodies for 1 h at
20–25°C. The bands were developed using enhanced chemiluminescence
solution (WEST-ZOL Plus, iNtRON Biotechnology) and visualized with
the LAS-3000 device (FujiFilm). The band intensity was analyzed
with ImageJ software (version 1.38; National Institutes of
Health).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 15 min
at 20–25°C and then permeabilized with 0.1% Triton X-100 for 10
min. Coverslips were incubated with blocking buffer (1% BSA in PBS)
for 1 h at 20–25°C and then incubated with primary antibodies for
overnight at 4°C. Coverslips were incubated with secondary
antibodies for 1 h at 20–25°C, and images were visualized using a
confocal microscopy (400× magnification; Carl Zeiss).
Statistical analysis
Data were expressed as mean ± SEM from ≥3
independent experiments. Statistical analyses were performed using
GraphPad Prism 5.0 software (version 5.01; GraphPad Software,
Inc.). The significance of each group was determined using one-way
ANOVA followed by Turkey's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Contents of the marker substances in
PPB
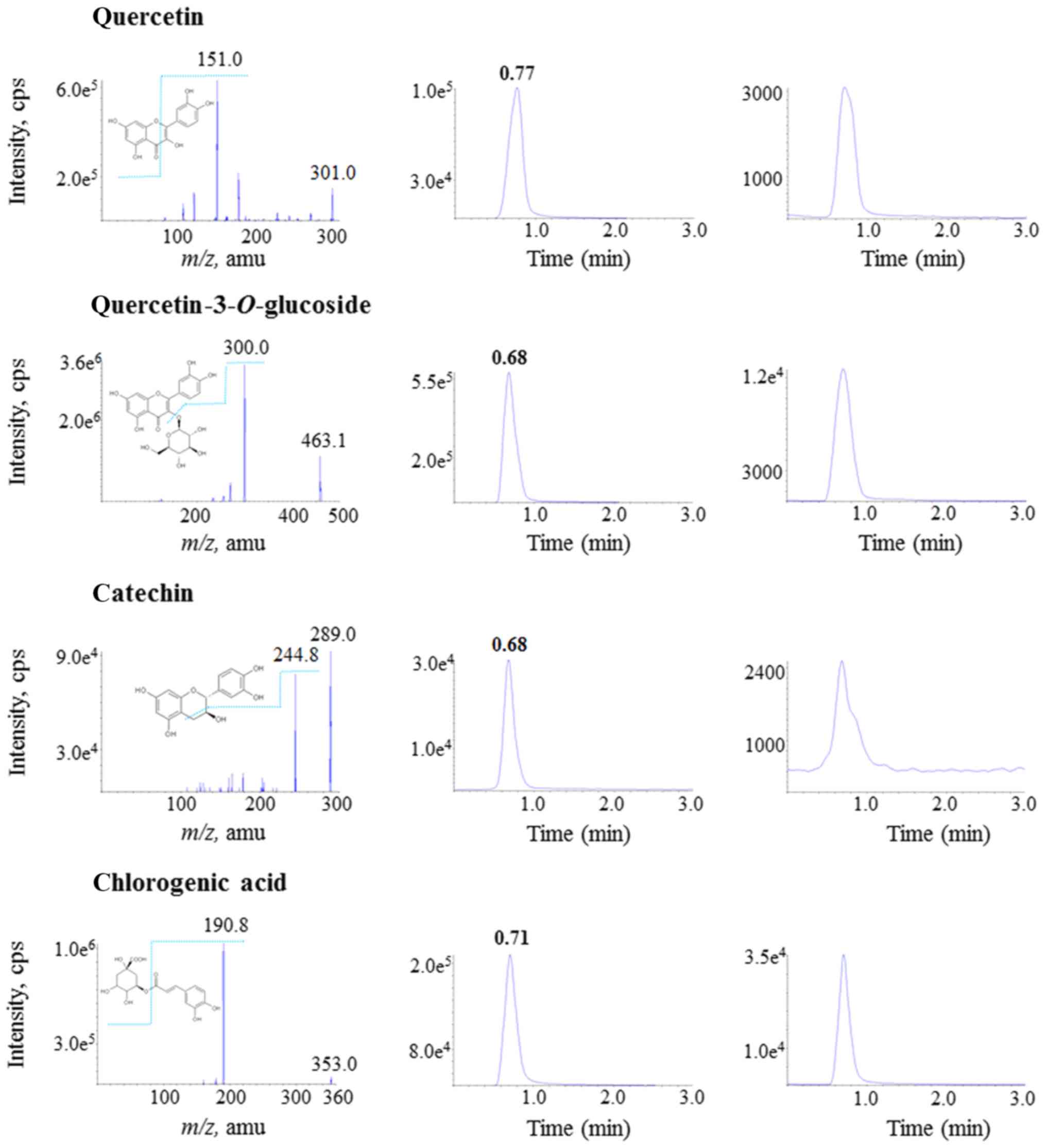
Fig. 1 illustrates
the mass spectra and proposed fragment ions of the four marker
substances (left column), chromatograms of the standard solution
(250 ng/ml) of each substance (middle column), and PPB solution
that was diluted 1,000-fold (right column). PPB contained 0.193%
chlorogenic acid, 0.097% catechin, 0.040% quercetin and 0.035%
quercetin-3-O-glucoside.
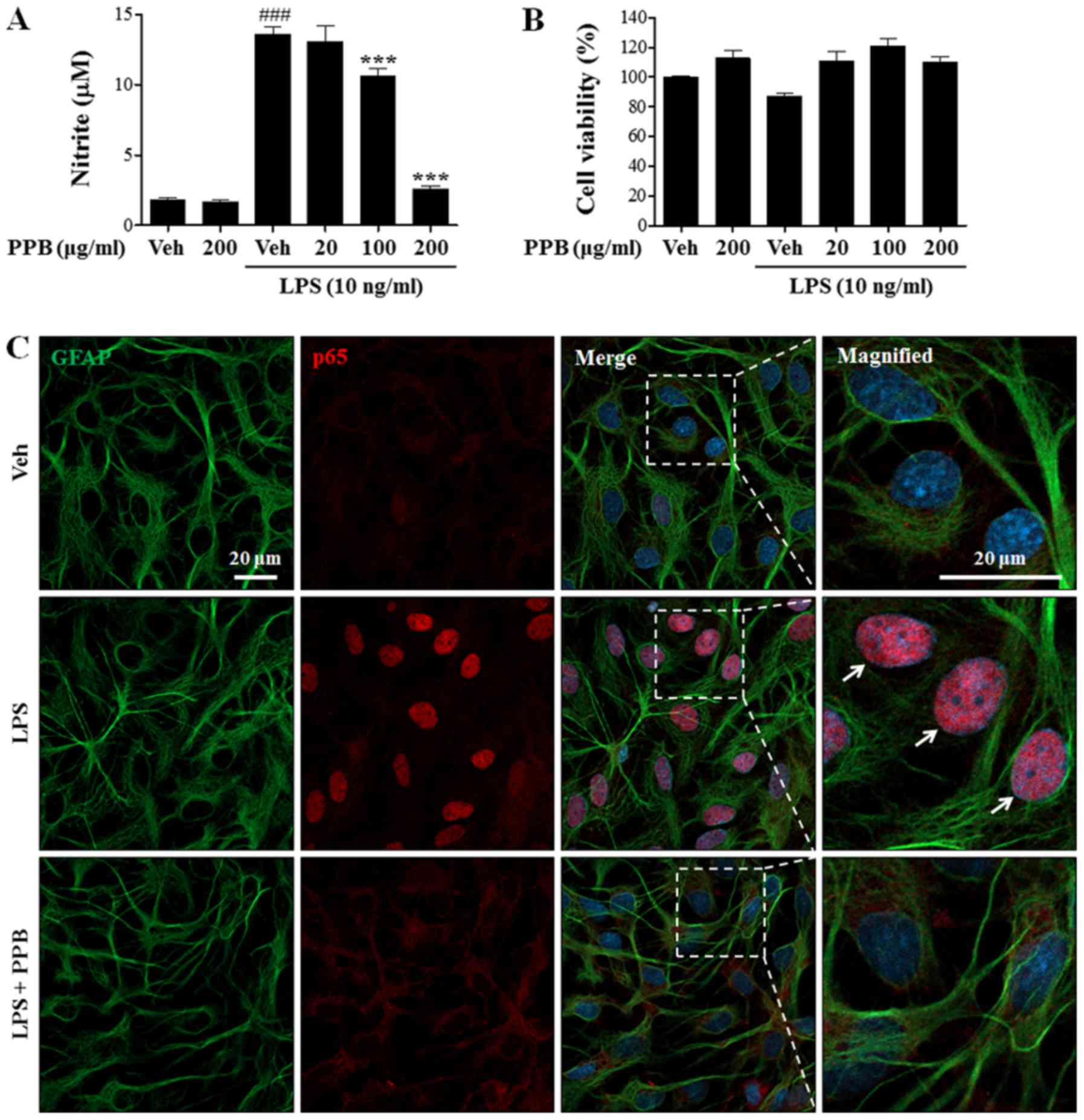
Effect of PPB on NO release in
LPS-stimulated BV2 cells
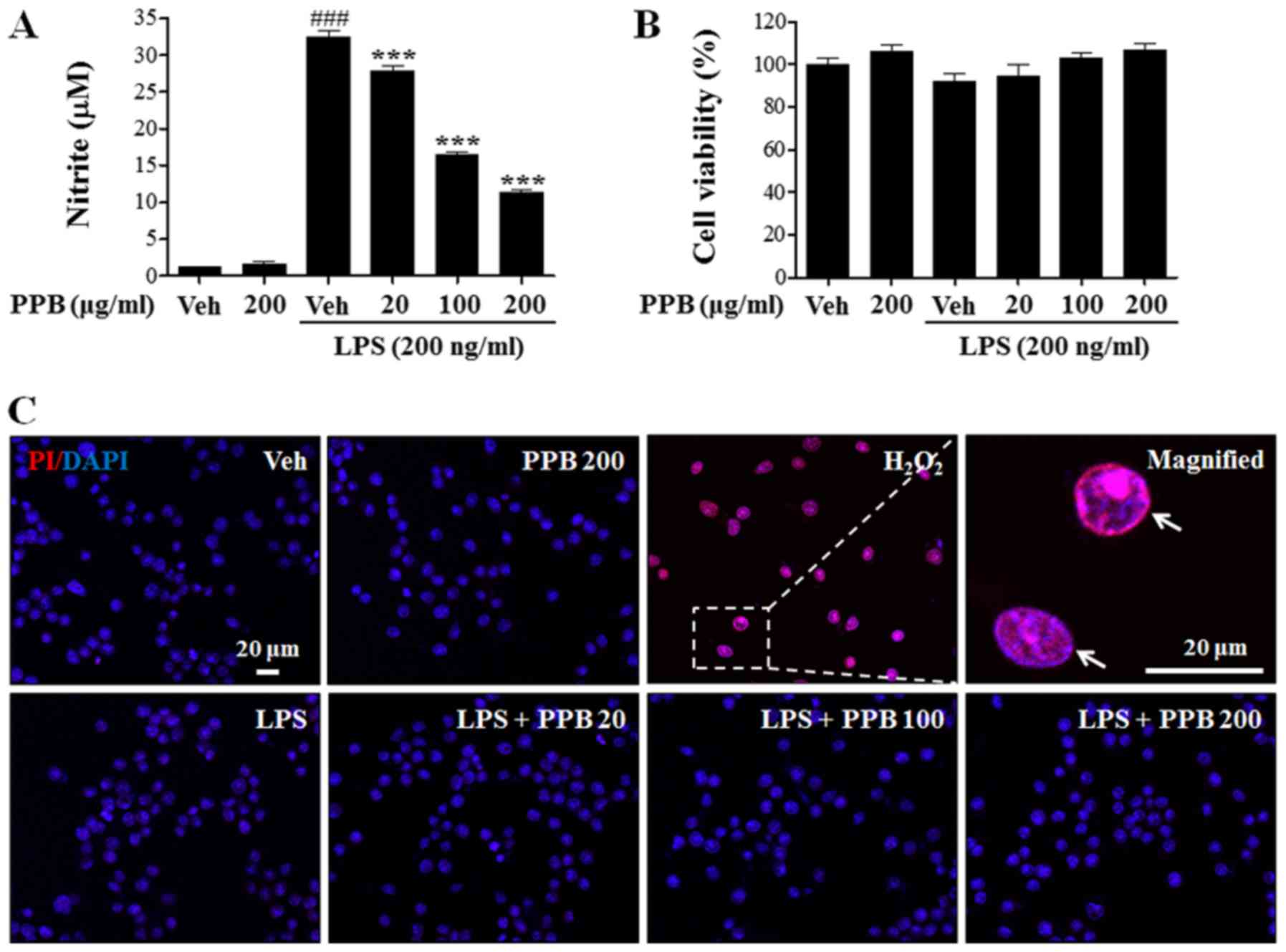
To examine the anti-inflammatory role of PPB, the
nitrite levels in LPS-stimulated BV2 cells was measured.
LPS-induced upregulation of NO production was reduced by PPB
treatment in a dose-dependent manner (Fig. 2A). The MTT assay showed that PPB
did not have a significant effect on cell viability (Fig. 2B). To further examine the presence
of cytotoxicity, PI staining was performed as previously described
(38) and quantitatively showed
that H2O2, but not PPB, induced cellular
toxicity (Fig. 2C). These results
suggested that PPB suppressed NO release in LPS-stimulated BV2
cells without inducing cellular toxicity.
Effect of PPB on iNOS, COX-2 and
proinflammatory cytokine expression
LPS promoted iNOS and COX-2 protein expression,
which was inhibited by PPB (Fig.
3A). PPB attenuated LPS-induced elevation of iNOS and COX-2
mRNA (Fig. 3B). Therefore, PPB
inhibits iNOS and COX-2 expression in LPS-stimulated BV2 cells. To
further investigate the anti-inflammatory role of PPB, TNF-α, IL-1β
and IL-6 mRNA expression levels were investigated. PPB alleviated
LPS-induced enhancement of proinflammatory cytokines (Fig. 3C). Notably, PPB significantly
inhibited iNOS and COX-2 expression at a lower concentration (100
µg/ml) than the concentration that affected TNF-α, IL-1β, and IL-6
expression (200 µg/ml). The present results may indicate that PPB
had preferential inhibitory effects on iNOS and COX-2 compared with
TNF-α, IL-1β and IL-6 in LPS-stimulated BV2 cells.
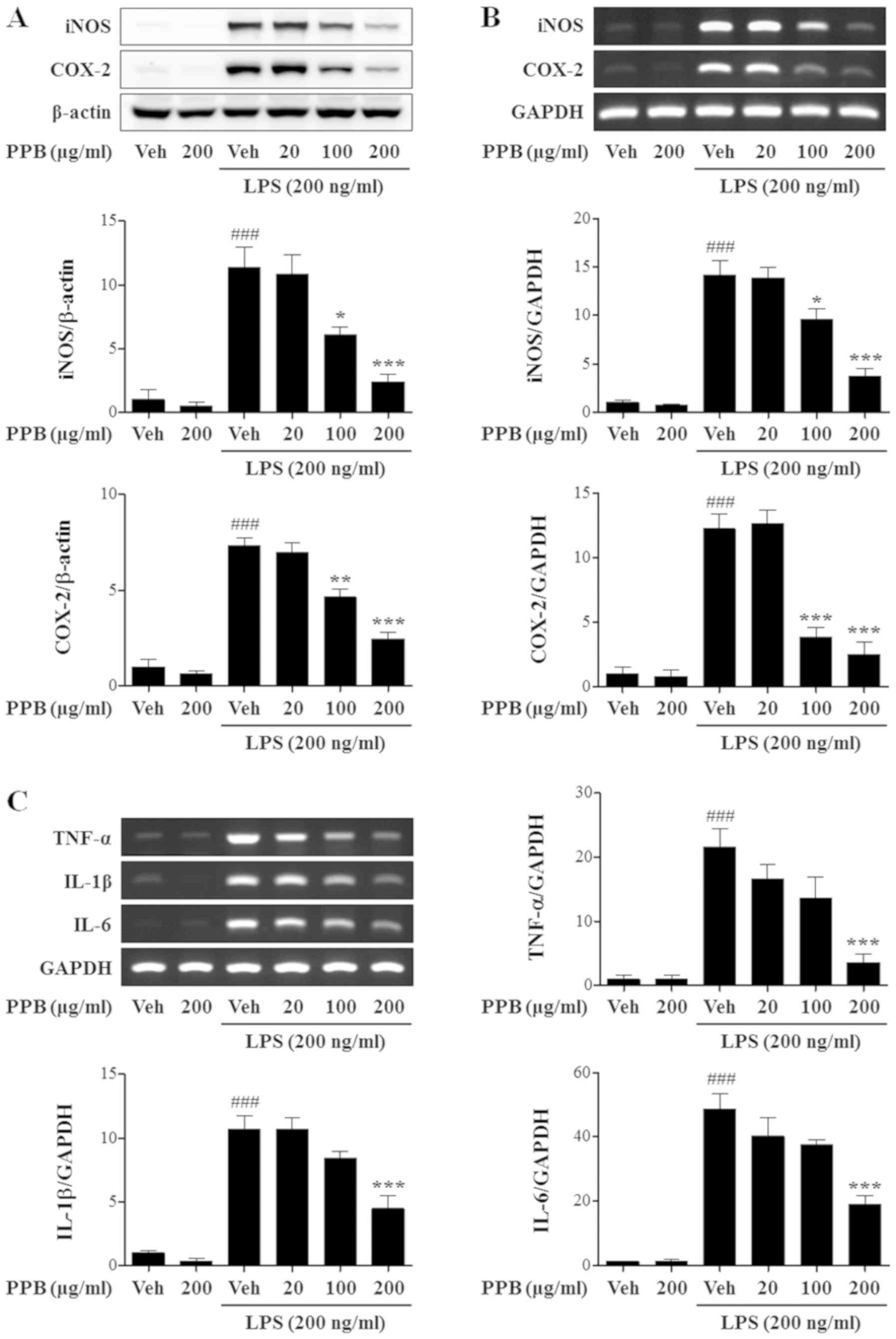 | Figure 3.PPB inhibits proinflammatory mediator
expression in LPS-stimulated BV2 cells. (A) iNOS and COX-2 protein
expression is normalized with β-actin. (B) mRNA expression levels
of iNOS and COX-2. iNOS and COX-2 mRNA expression was normalized
with GAPDH. (C) TNF-α, IL-1β and IL-6 protein expression was
normalized with GAPDH. ###P<0.001 vs. Veh; *P<0.05,
**P<0.01, and ***P<0.001 vs. Veh + LPS. PPB, Prunus persica
methanol extract; LPS, lipopolysaccharide; iNOS, inducible nitric
oxide synthase; COX-2, cyclooxygenase-2; TNF, tumor necrosis
factor; IL, interleukin; Veh, vehicle. |
Effect of PPB on LPS-induced NF-κB
pathways
NF-κB is a major transcription factor that regulates
the expression of proinflammatory cytokines during inflammation. In
response to inflammatory stimuli, IκB is rapidly phosphorylated and
degraded, and subsequently dissociates from NF-κB, which
translocates to the nucleus, leading to the transcription of
proinflammatory mediators and cytokines (39,40).
To clarify the underlying mechanism of the anti-inflammatory
effects of PPB, the NF-κB signaling pathway was investigated.
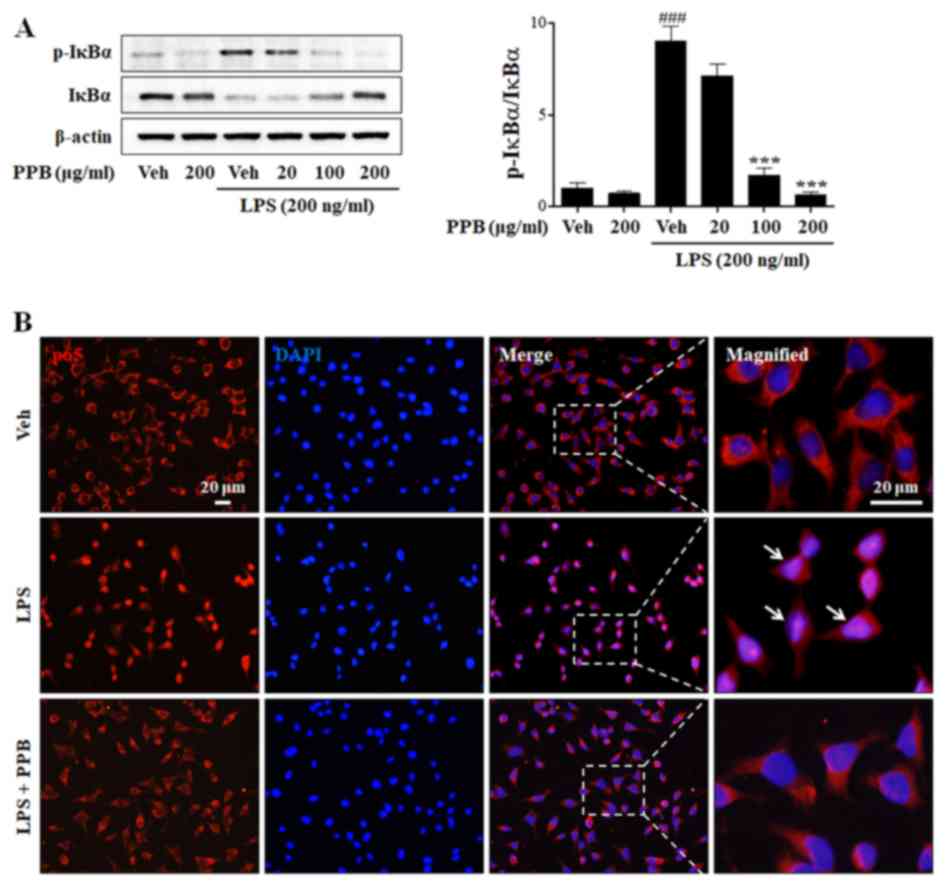
Normally, the level of IκBα expression is high, whereas that of
p-IκBα expression is low. In the present study, LPS exposure
decreased IκBα expression and enhanced p-IκBα expression (Fig. 4A). PPB ameliorated the LPS-induced
alteration of IκBα and p-IκBα expression. To further examine the
effect of PPB on p65 translocation, the expression of p65 was
investigated by immunostaining. As expected, PPB suppressed
LPS-induced p65 translocation into the nucleus (Fig. 4B). The present results indicated
that PPB regulates proinflammatory cytokine expression by
suppressing the NF-κB signaling pathway.
Effect of PPB on LPS-induced MAPK
activation
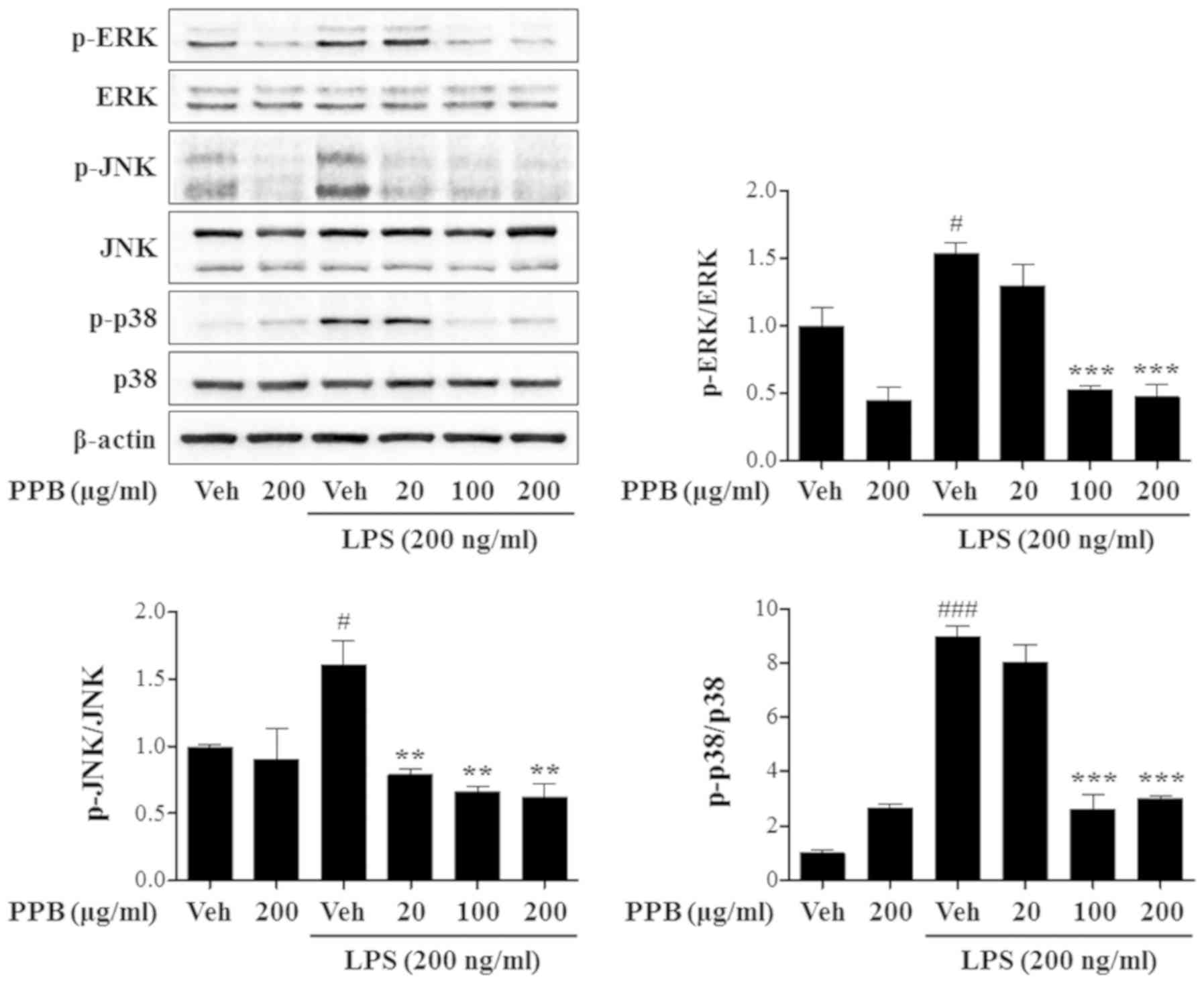
In microglia, LPS exposure enhances iNOS and TNF-α
expression, which is accompanied by the activation of MAPK
signaling pathways, including the ERK, JNK and p38 pathways
(16). In addition, MAPK is
involved in COX-2 and iNOS production in LPS-stimulated microglial
cells (41). To further examine
the underlying mechanism of PPB, MAPK activation was investigated.
PPB repressed LPS-induced phosphorylation of ERK, JN and p38
(Fig. 5). Notably, PPB
significantly suppressed the JNK phosphorylation at a lower
concentration (20 µg/ml) than that for ERK or p38 (100 µg/ml). The
present results indicated that PPB preferentially inhibited JNK in
LPS-stimulated BV2 cells. Taken together, the present results
demonstrated that PPB exerts an anti-inflammatory effect in
LPS-stimulated BV2 cells through the inhibition of NF-κB and MAPK
pathway activation.
Effect of PPB on NO release and p65
translocation in LPS-stimulated primary astrocytes
To further evaluate the suppressive effect of PPB on
neuroinflammation, NO production in primary cultured astrocytes was
investigated. Consistent with the BV2 cell results, PPB also
suppressed LPS-induced NO production without inducing cellular
toxicity (Fig. 6A and B). In
addition, immunostaining results indicated that PPB successfully
alleviated LPS-induced p65 translocation into the nucleus (Fig. 6C). The present results indicated
the anti-inflammatory role of PPB in glial cell activation by
repressing repressed NO production and p65 translocation in primary
astrocytes (Fig. S1).
Discussion
The present study indicated that PPB presents
anti-inflammatory effects on LPS-stimulated glial cells. PPB
significantly decreased pro-inflammatory mediators and cytokines at
the transcriptional level, which was accompanied by NF-κB and MAPK
activation suppression in LPS-stimulated microglial cells. The
present in vitro findings suggested PPB repressed NO
production and p65 translocation in primary astrocytes.
Although iNOS is rarely expressed under normal
conditions, it is highly expressed in activated glial cells under
neuroinflammatory conditions (42). In contrast to other NOS enzymes in
the brain, iNOS exerts neurotoxic effects through continuous NO
production due to its calcium independence (43). Therefore, iNOS regulation is key to
suppressing NO production in the presence of a neuroinflammatory
stimulus. The inhibitory effect of PPB on NO release was examined
via the suppression of iNOS expression. In addition, the expression
levels of COX-2, which is the most representative proinflammatory
mediator during an inflammatory response, was examined (44).
To clarify the protective role of PPB in glial
cells, the LPS-induced neuroinflammation model was used. Peripheral
LPS administration has not been effective in the brain because LPS
could not penetrate the blood-brain barrier (BBB) (45). However, previous studies have
indicated that LPS can induce neuroinflammation in the brain with
minimal penetration (46) or BBB
disruption (47). In previous
studies, LPS treatment was considered a representative
neuroinflammation model for several brain inflammatory cells, such
as primary cultured astrocytes (48), microglia (49), BV2 cells (16) and C6 glioma cells (50). Therefore, the results of the
present study suggested that PPB has suppressive effects in
response to neuroinflammation.
Microglial cell activation increases the levels of
certain neurotoxic and proinflammatory mediators and induces
neuronal cell death, leading to various neuroinflammatory diseases
(9,16,17).
Thus, the regulation of microglial cell activity alleviates
neuroinflammation, which may further inhibit neuronal cell death.
NF-κB, a transcription factor, regulates the production of multiple
genes related to immunity and inflammation (51). NF-κB activation induces the
transcription of proinflammatory genes through a variety of
intracellular signaling pathways (52). In the cytoplasm, NF-κB is a
heterotrimeric complex consisting of p50, p65, and IκB subunits
(39). Activation of this complex
results in IκB phosphorylation and degradation, which leads to the
translocation of NF-κB dimers (p50/p65 complex) to the nucleus as
well as the activation of target genes, including proinflammatory
cytokines (40). Thus, regulating
NF-κB translocation is important in inflammatory diseases. The
inhibitory activity of PPB may affect this signaling by acting as a
suppressor of inflammatory mediators. Indeed, PPB inhibits IκB
phosphorylation and p65 expression in the nucleus of LPS-stimulated
BV2 cells and astrocytes. These findings suggest that the
repression of inflammatory mediators by PPB is caused by NF-κB
signaling pathway inhibition.
MAPK signaling pathways (i.e., ERK, JNK and p38
MAPK) can regulate NF-κB activity in LPS-stimulated cells and
participate in COX-2 and iNOS production (53). In in vivo and in
vitro experiments, MAPK signaling pathways induce the
activation of transcription factors and proinflammatory cytokines
(54,55). Activated ERK pathways induce COX-2
expression and PGE2 production in microglia (56). In primary astrocytes and microglia,
LPS not only increases iNOS and TNF-α expression, but also induces
MAPK activation (57). Consistent
with these studies, the results of the present study showed that
PPB inhibited MAPK activation in LPS-stimulated BV2 microglial
cells, suggesting that its anti-inflammatory effects were also due
to MAPK signaling pathway inhibition.
Epidemiological studies have suggested that phenolic
compounds and carotenoid from peach pulps and peel decrease the
risk of cardiac disorders, brain disorders and cancer because of
their antioxidant and anti-inflammatory effect (58,59).
In addition, P. persica flower extract suppressed
inflammation in LPS-simulated macrophages (60). It was hypothesized that the
antioxidant effect of P. persica is derived from phenolics,
flavonoids, and anthocyanins (58). Previous studies evaluating P.
persica phenolic and flavonol content showed that chlorogenic
acid and catechin were enriched in the peels and pulps of the fruit
from P. persica cultivars (35). Likewise, PPB contained chlorogenic
acid, catechin, quercetin, and quercetin-3-O-glucoside. This
finding suggests that the anti-inflammatory effect of PPB in BV2
cells was due to the presence of phenolics and flavonols. In a
previous study, chlorogenic acid suppressed glucose-induced glial
cell activation (61). Moreover,
neochlorogenic acid, an isomer of chlorogenic acid, inhibited the
activation of LPS-induced microglia and p38 MAPK (62). Catechin also inhibited LPS-induced
NO production in BV2 cells (63).
In addition, the catechin flavonoid, epigallocatechin gallate,
exerts a neuroprotective effect by suppressing glial cell
activation induced by LPS (25) or
β-amyloid (64). The present
results indicated that the application of chlorogenic acid,
catechin, or their combination may suppress the activation of glial
cells. To further confirm whether the anti-inflammatory effect of
PPB is due to the presence of chlorogenic acid or catechin, each
bioactive molecule should be explored in future studies. In
addition, future studies should also investigate whether PPB has a
greater anti-inflammatory effect than chlorogenic acid or catechin
alone due to the synergistic effect of bioactive molecules.
Although results from BV2 cells or primary astrocytes may not
reflect in vivo conditions, these findings suggest that PPB
is a potential therapeutic agent for neuroinflammatory and
neurodegenerative disease treatment via the repression of glial
cell activation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a National
Research Foundation of Korea grant (no. 2017R1D1A1B03031920) funded
by the Korean government and Chung-Ang University Research
Scholarship Grants in 2018.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KHS performed the western blot analysis and
maintained the cells. SYC designed the experiments and performed
the immunocytochemistry analysis. YJ and YIK performed the RT-PCR.
SE and TTB performed the nitrite and MTT assays. SHJ and HMY
performed the western blot analysis for the translocation of NF-κB.
YSK supervised and performed p65 immunostaining experiments. WKW
critically revised manuscript and designed astrocytes experiments
for revision. SYJ performed PI staining and was involved in
drafting the manuscript. HS performed the HPLC-MS/MS. WK supervised
and resolved the PPB analysis. HMK designed and supervised the
experiments. SHL designed overall experiments and drafted the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
IL-1β
|
interleukin-1 β
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor κB
|
|
NO
|
nitric oxide
|
|
PPB
|
Prunus persica methanol extract
|
|
TNF-α
|
tumor necrosis factor α
|
References
|
1
|
González-Scarano F and Baltuch G:
Microglia as mediators of inflammatory and degenerative diseases.
Annu Rev Neurosci. 22:219–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maragakis NJ and Rothstein JD: Mechanisms
of disease: Astrocytes in neurodegenerative disease. Nat Clin Pract
Neurol. 2:679–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prinz M and Priller J: Microglia and brain
macrophages in the molecular age: From origin to neuropsychiatric
disease. Nat Rev Neurosci. 15:300–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu B and Hong JS: Role of microglia in
inflammation-mediated neurodegenerative diseases: Mechanisms and
strategies for therapeutic intervention. J Pharmacol Exp Ther.
304:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanchez Guajardo V, Tentillier N and
Romero Ramos M: The relation between α-synuclein and microglia in
Parkinson's disease: Recent developments. Neuroscience. 302:47–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keren Shaul H, Spinrad A, Weiner A,
Matcovitch Natan O, Dvir Szternfeld R, Ulland TK, David E, Baruch
K, Lara Astaiso D, Toth B, et al: A unique microglia type
associated with restricting development of Alzheimer's disease.
Cell. 169:1276–1290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DC, Lee DS, Ko W, Kim KW, Kim HJ, Yoon
CS, Oh H and Kim YC: Heme oxygenase-1-inducing activity of
4-methoxydalbergione and 4′-hydroxy-4-methoxydalbergione from
Dalbergia odorifera and their anti-inflammatory and cytoprotective
effects in murine hippocampal and BV2 microglial cell line and
primary rat microglial cells. Neurotox Res. 33:337–352. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liddelow SA, Guttenplan KA, Clarke LE,
Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS,
Peterson TC, et al: Neurotoxic reactive astrocytes are induced by
activated microglia. Nature. 541:481–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Y and Benveniste EN: Immune function
of astrocytes. Glia. 36:180–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Yang L, Yang L, Xing F, Yang H,
Qin L, Lan Y, Wu H, Zhang B, Shi H, et al: Gypenoside IX suppresses
p38 MAPK/Akt/NFκB signaling pathway activation and inflammatory
responses in astrocytes stimulated by proinflammatory mediators.
Inflammation. 40:2137–2150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Benveniste H, Klitzman B and
Piantadosi CA: Nitric oxide synthase inhibition and extracellular
glutamate concentration after cerebral ischemia/reperfusion.
Stroke. 26:298–304. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Law A, Gauthier S and Quirion R: Say NO to
Alzheimer's disease: The putative links between nitric oxide and
dementia of the Alzheimer's type. Brain Res Brain Res Rev.
35:73–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung WK, Lee DY, Park C, Choi YH, Choi I,
Park SG, Seo SK, Lee SW, Yea SS, Ahn SC, et al: Cilostazol is
anti-inflammatory in BV2 microglial cells by inactivating nuclear
factor-kappaB and inhibiting mitogen-activated protein kinases. Br
J Pharmacol. 159:1274–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilms H, Sievers J, Rickert U,
Rostami-Yazdi M, Mrowietz U and Lucius R: Dimethylfumarate inhibits
microglial and astrocytic inflammation by suppressing the synthesis
of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model
of brain inflammation. J Neuroinflammation. 7:302010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu D, Wang Z, Liu S, Wang F, Zhao S and
Hao A: Anti-inflammatory effects of fluoxetine in
lipopolysaccharide (LPS)-stimulated microglial cells.
Neuropharmacology. 61:592–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boche D, Perry VH and Nicoll JA: Review:
Activation patterns of microglia and their identification in the
human brain. Neuropathol Appl Neurobiol. 39:3–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Recio MC, Andujar I and Rios JL:
Anti-inflammatory agents from plants: Progress and potential. Curr
Med Chem. 19:2088–2103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: Leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang
SS, Cho GJ, Choi WS and Suk K: Flavonoid wogonin from medicinal
herb is neuroprotective by inhibiting inflammatory activation of
microglia. FASEB J. 17:1943–1944. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li FQ, Wang T, Pei Z, Liu B and Hong JS:
Inhibition of microglial activation by the herbal flavonoid
baicalein attenuates inflammation-mediated degeneration of
dopaminergic neurons. J Neural Transm (Vienna). 112:331–347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suk K, Lee H, Kang SS, Cho GJ and Choi WS:
Flavonoid baicalein attenuates activation-induced cell death of
brain microglia. J Pharmacol Exp Ther. 305:638–645. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JS, Park EM, Kim DH, Jung K, Jung JS,
Lee EJ, Hyun JW, Kang JL and Kim HS: Anti-inflammatory mechanism of
ginseng saponins in activated microglia. J Neuroimmunol. 209:40–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He LF, Chen HJ, Qian LH, Chen GY and Buzby
JS: Curcumin protects pre-oligodendrocytes from activated microglia
in vitro and in vivo. Brain Res. 1339:60–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li R, Huang YG, Fang D and Le WD:
(−)-Epigallocatechin gallate inhibits lipopolysaccharide-induced
microglial activation and protects against inflammation-mediated
dopaminergic neuronal injury. J Neurosci Res. 78:723–731. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi DK, Koppula S and Suk K: Inhibitors
of microglial neurotoxicity: Focus on natural products. Molecules.
16:1021–1043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen H, Wang H, Wang L, Wang L, Zhu M,
Ming Y, Zhao S, Fan J and Lai EY: Ethanol extract of root of Prunus
persica inhibited the growth of liver cancer cell HepG2 by inducing
cell cycle arrest and migration suppression. Evid Based Complement
Alternat Med. 2017:82319362017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rho JR, Jun CS, Ha Y, Yoo MJ, Cui MX, Baek
HS, Lim JA, Lee YH and Chai KY: Isolation and characterization of a
new alkaloid from the seed of Prunus persica L. and its
anti-inflammatory activity. Bull Korean Chem Soc. 28:1289–1293.
2007. View Article : Google Scholar
|
|
29
|
Lee JY and An BJ: Anti-oxidant and
anti-inflammation activities of prunus persica flos. J Appl Biol
Chem. 53:162–169. 2010. View Article : Google Scholar
|
|
30
|
Kwak CS, Yang J, Shin CY and Chung JH:
Topical or oral treatment of peach flower extract attenuates
UV-induced epidermal thickening, matrix metalloproteinase-13
expression and pro-inflammatory cytokine production in hairless
mice skin. Nutr Res Pract. 12:29–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin TY, Park SB, Yoo JS, Kim IK, Lee HS,
Kwon TK, Kim MK, Kim JC and Kim SH: Anti-allergic inflammatory
activity of the fruit of prunus persica: Role of calcium and
NF-kappaB. Food Chem Toxicol. 48:2797–2802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benmehdi H, Fellah K, Amrouche A, Memmou
F, Malainine H, Dalile H and Siata W: Phytochemical study,
antioxidant activity and kinetic behaviour of flavonoids fractions
isolated from prunus persica L. Leaves. Asian J Chem. 29:132017.
View Article : Google Scholar
|
|
33
|
Deb L, Gupta R, Dutta A, Yadav A, Bhowmik
D and Kumar KS: Evaluation of antioxidant activity of aqueous
fraction of Prunus persica L. aqueous extract. Der Pharmacia
Sinica. 1:157–164. 2010.
|
|
34
|
Prakash V, Rana S and Sagar A: Studies on
analysis of antibacterial and antioxidant activity of Prunus
persica (L.) Batsch. Int J Sci Nat. 8:54–58. 2017.
|
|
35
|
Zhao X, Zhang W, Yin X, Su M, Sun C, Li X
and Chen K: Phenolic composition and antioxidant properties of
different peach [Prunus persica (L.) Batsch] cultivars in China.
Int J Mol Sci. 16:5762–5778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McCarthy KD and de Vellis J: Preparation
of separate astroglial and oligodendroglial cell cultures from rat
cerebral tissue. J Cell Biol. 85:890–902. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
NIH, . Laboratory animal welfare. Special
Edition of the NIH Guide for Grants and Contracts. NIH; Bethesda,
MD, USA: pp. 85–23. 1985
|
|
38
|
Wrobel K, Claudio E, Segade F, Ramos S and
Lazo PS: Measurement of cytotoxicity by propidium iodide staining
of target cell DNA: Application to the quantification of murine
TNF-alpha. J Immunol Methods. 189:243–249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baldwin AS Jr: The NF-kappaB and I kappaB
proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF- kappaB regulation: The nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oh YT, Lee JY, Lee J, Kim H, Yoon KS, Choe
W and Kang I: Oleic acid reduces lipopolysaccharide-induced
expression of iNOS and COX-2 in BV2 murine microglial cells:
Possible involvement of reactive oxygen species, p38 MAPK, and
IKK/NF-kappaB signaling pathways. Neurosci Lett. 464:93–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mander P and Brown GC: Nitric oxide,
hypoxia and brain inflammation. Biochem Soc Trans. 32:1068–1069.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pautz A, Art J, Hahn S, Nowag S, Voss C
and Kleinert H: Regulation of the expression of inducible nitric
oxide synthase. Nitric Oxide. 23:75–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bishop Bailey D, Calatayud S, Warner TD,
Hla T and Mitchell JA: Prostaglandins and the regulation of tumor
growth. J Environ Pathol Toxicol Oncol. 21:93–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singh AK and Jiang Y: How does peripheral
lipopolysaccharide induce gene expression in the brain of rats?
Toxicology. 201:197–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banks WA and Robinson SM: Minimal
penetration of lipopolysaccharide across the murine blood-brain
barrier. Brain Behav Immun. 24:102–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Banks WA, Gray AM, Erickson MA, Salameh
TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y,
Cook DG and Reed MJ: Lipopolysaccharide-induced blood-brain barrier
disruption: Roles of cyclooxygenase, oxidative stress,
neuroinflammation, and elements of the neurovascular unit. J
Neuroinflammation. 12:2232015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tarassishin L, Suh HS and Lee SC: LPS and
IL-1 differentially activate mouse and human astrocytes: Role of
CD14. Glia. 62:999–1013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Panicker N, Saminathan H, Jin H, Neal M,
Harischandra DS, Gordon R, Kanthasamy K, Lawana V, Sarkar S, Luo J,
et al: Fyn kinase regulates microglial neuroinflammatory responses
in cell culture and animal models of Parkinson's disease. J
Neurosci. 35:10058–10077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim YJ, Hwang SY and Han IO: Insoluble
matrix components of glioma cells suppress LPS-mediated iNOS/NO
induction in microglia. Biochem Biophys Res Commun. 347:731–738.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bhatt D and Ghosh S: Regulation of the
NF-κB-mediated transcription of inflammatory genes. Front Immunol.
5:712014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Korcheva V, Wong J, Corless C, Iordanov M
and Magun B: Administration of ricin induces a severe inflammatory
response via nonredundant stimulation of ERK, JNK, and P38 MAPK and
provides a mouse model of hemolytic uremic syndrome. Am J Pathol.
166:323–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Da Silva J, Pierrat B, Mary JL and
Lesslauer W: Blockade of p38 mitogen-activated protein kinase
pathway inhibits inducible nitric-oxide synthase expression in
mouse astrocytes. J Biol Chem. 272:28373–28380. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H,
Chen D, Fu C, Zheng L, Zhen X, et al: Induction of COX-2-PGE2
synthesis by activation of the MAPK/ERK pathway contributes to
neuronal death triggered by TDP-43-depleted microglia. Cell Death
Dis. 6:e17022015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bhat NR, Zhang P, Lee JC and Hogan EL:
Extracellular signal-regulated kinase and p38 subgroups of
mitogen-activated protein kinases regulate inducible nitric oxide
synthase and tumor necrosis factor-α gene expression in
endotoxin-stimulated primary glial cultures. J Neurosci.
18:1633–1641. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abidi W, Jiménez S, Moreno MÁ and
Gogorcena Y: Evaluation of antioxidant compounds and total sugar
content in a nectarine [Prunus persica (L.) Batsch] progeny. Int J
Mol Sci. 12:6919–6935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gasparotto J, Somensi N, Bortolin RC,
Moresco KS, Girardi CS, Klafke K, Rabelo TK, Morrone Mda S,
Vizzotto M and Raseira Mdo C: Effects of different products of
peach (Prunus persica L. Batsch) from a variety developed in
southern Brazil on oxidative stress and inflammatory parameters in
vitro and ex vivo. J Clin Biochem Nutr. 55:110–119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee JY and An BJ: Antioxidant and
anti-inflammatory effects of fractions from Pruni persicae Flos.
Korea J Herbol. 27:55–63. 2012. View Article : Google Scholar
|
|
61
|
Mei X, Zhou L, Zhang T, Lu B, Sheng Y and
Ji L: Chlorogenic acid attenuates diabetic retinopathy by reducing
VEGF expression and inhibiting VEGF-mediated retinal
neoangiogenesis. Vascul Pharmacol. 101:29–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim M, Choi SY, Lee P and Hur J:
Neochlorogenic acid inhibits lipopolysaccharide-induced activation
and pro-inflammatory responses in BV2 microglial cells. Neurochem
Res. 40:1792–1798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li N, Wang Y, Li X, Zhang H, Zhou D, Wang
W, Li W, Zhang X, Li X, Hou Y and Meng D: Bioactive phenols as
potential neuroinflammation inhibitors from the leaves of
Xanthoceras sorbifolia Bunge. Bioorg Med Chem Lett. 26:5018–5023.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim CY, Lee C, Park GH and Jang JH:
Neuroprotective effect of epigallocatechin-3-gallate against
β-amyloid-induced oxidative and nitrosative cell death via
augmentation of antioxidant defense capacity. Arch Pharm Res.
32:869–881. 2009. View Article : Google Scholar : PubMed/NCBI
|