Introduction
Idiopathic pulmonary fibrosis (IPF) is a severe and
lethal interstitial lung disease, which is characterized by
progressive dyspnea and distorted lung function (1). The incidence of IPF gradually
increases with age, and primarily occurs in individuals between
60–70 years (2,3). A study from the United States
estimated that the prevalence rate of IPF varied from 14–43 per
100,000 persons (4). However,
despite widespread efforts, the etiology and pathogenesis of the
disease remain unclear, and effective treatments with limited side
effects are urgently required. Currently, novel treatment
strategies should aim to combine multiple therapeutic targets to
prevent the pathogenesis of IPF (5,6).
Due to its complexity, the pathogenic mechanism of
IPF are not fully understood. The underlying mechanisms of IPF
involve multiple pathways, such as the inflammatory response,
epithelial-mesenchymal transition (EMT), apoptosis, oxidative
stress (7–9), and developmental processes, which
leading to alveolar epithelial cell injury and fibroblast
proliferation that consequently leads to excessive deposition of
extracellular collagen (10). It
has been reported that inflammatory cell accumulation,
pro-inflammatory cytokine release, including interleukin-6, tumor
necrosis factor-α and transforming growth factor-β, and oxidative
stress disorder exacerbate lung injury in bleomycin-induced
pulmonary fibrosis (11). The
primary pathological features of pulmonary fibrosis are
characterized by the damage and apoptosis of pulmonary tissues,
excessive proliferation of pulmonary fibroblasts, and mass
secretion and accumulation of collagen matrix (12). Although glucocorticoids,
colchicines and cyclophosphamides are currently used to treat
pulmonary fibrosis, their poor curative effect and high incidence
of side effects makes them less desirable (13). This highlights the importance of
further study into the pathogenesis of pulmonary fibrosis and the
examination of multi-target therapeutic drugs, which may offer a
new developmental direction for the treatment of IPF. Traditional
Chinese medicine compounds are characterized by their
multi-targeting nature and low side effects, which allows for the
investigation of more suitable drugs for IPF treatment (14).
Baicalin is the biologically active form of the
dried roots of Scutellaria baicalensis Georgi, and the major
bioactive ingredient of which is flavinoid. The main chemical
constituent flavonoid of baicalin has a variety of biological
activities, such as including anti-inflammatory (15), antioxidative (16), antithrombotic (17), anti-proliferative (18,19)
and regulation of apoptosis (20–22).
The multiple functions of baicalin have been well-studied in
different fields. Moreover, previous studies have shown that
baicalin has protective effects on liver fibrosis induced by
CCl4 (23), and it can
inhibit the formation of renal fibrosis (24). Baicalin alleviates silica-induced
lung inflammation by suppressing IL-6 and IL-23, which reflect Th17
response and lung fibrosis (25).
Baicalin also inhibited BLM-induced pulmonary fibrosis by
upregulating adenosine A2a receptor and downregulating transforming
growth factor (TGF)-β1 and phosphorylated (p)-ERK1/2 expression
(26). These results indicate that
baicalin is may be a potential treatment for IPF. Thus, it is of
great significance to investigate the specific mechanism and
signaling pathway via which baicalin inhibits pulmonary fibrosis,
as this may be a theoretical basis for the use of baicalin in the
treatment of IPF.
AKT is a serine/threonine protein kinase that is
activated by a number of growth factors and cytokines such as TGF-β
and IL-6, in a phosphatidylinositol-3 kinase (PI3K)-dependent
manner (27). The PI3K/AKT
signaling pathway is involved in numerous cellular processes, such
as cell differentiation, proliferation, apoptosis and angiogenesis
(28–30), and is stimulated by the activation
of receptor tyrosine kinases, which leads to the translocation of
PI3K from the cytoplasm to the plasma membrane (31). Leptin accelerates EMT of human lung
carcinoma A549 cell and promotes pulmonary fibrosis by inhibiting
autophagy via the PI3K/AKT/mTOR pathway (32). Furthermore, syndecan-2 attenuates
radiation-induced pulmonary fibrosis, and inhibits mouse lung
fibroblast migration and proliferation by suppressing
PI3K/AKT/Rho-associated protein kinase signaling via CD148
(33). Moreover, the PI3K/AKT
pathway activates endoplasmic reticulum (ER) stress and influences
lung fibroblast proliferation in BLM-induced pulmonary fibrosis
(34).
Calcium/calmodulin-dependent kinase II (CaMKII),
which is known as a general integrator of Ca2+
signaling, is activated upon binding to Ca2+/calmodulin
(CaM), which subsequently undergoes autophosphorylation (35). Previous studies have revealed that
CaM KII is involved in the pathological course of lung diseases via
the modulation of intracellular Ca2+ concentrations
(36,37). It has also been shown that puerarin
stimulates endothelial nitric oxide synthase (eNOS) activation,
which is mediated by PI3K/AKT and 5′-AMP-activated protein kinase
(AMPK) activation via an ER- and CaMKII-dependent pathway (38). Moreover, prostaglandin
E2 was found to interfere with Ca2+ signaling
and prevent the activation of AKT and CaMKII in human pulmonary
fibroblasts isolated from patients with IPF (39). Ca2+ signaling and
calmodulin-dependent protein kinase IIβ and IIδ are also involved
in TGF-β-induced extracellular matrix gene expression in human
pulmonary fibroblasts (6).
Additionally, insulin stimulates the proliferation of dermal
fibroblasts via activated CaMKII, and induces Raf-1 and ERK
expression (40). However, the
impact of the PI3K/AKT and CaMKII pathways in baicalin-regulated
pulmonary fibrosis and pulmonary fibroblast proliferation is not
fully understood, Thus the present study aimed to investigate its
role and identify its underlying mechanism. It was hypothesized
that CaMKII and PI3K/AKT may be involved in the activation of
fibroblast proliferation in the pulmonary fibrosis. In the present
study, the protective function of adenosine baicalin and lung
fibroblast proliferation were investigated in a BLM-induced
pulmonary fibrosis model, to determine whether baicalin suppressed
lung fibroblasts proliferation via the inhibition of the CaMKII and
AKT signaling pathways.
Materials and methods
Materials
BLM was purchased from Nippon Kayaku Co., Ltd., and
dissolved in saline to a final concentration of 5 mg/ml. Baicalin
(Sigma-Aldrich; Merck KGaA; purify, >95%) was prepared in
physiological saline at a 2% concentration. The superoxide
dismutase (SOD), glutathione peroxidase (GSH-px), malondialdehyde
(MDA) and hydroxyproline (Hyp) kits were purchased from the Nanjing
Jiancheng Bioengineering Institute. The TUNEL Apoptosis Assay kit
was purchased from Roche Diagnostics. Antibodies against Bcl-2 and
cyclin A were acquired from Beyotime Institute of Biotechnology.
Antibodies against caspase-3, Bax, cyclin D, cyclin E,
proliferating cell nuclear antigen (PCNA), phosphorylated (p)-AKT,
total (t)-AKT, p-CaMKII, t-CaMKII and β-actin were acquired from
Cell Signaling Technology, Inc. The Cell Cycle and Apoptosis
Analysis kit (cat. no. C1052) was purchased from the Beyotime
Institute of Biotechnology, and ECL reagents were obtained from GE
Healthcare.
Animal model and experimental
protocol
Adult female Wistar rats (age, 42–49 days; weight,
160–200 g) used in this study were purchased from the Animal
Research Center of Harbin Medical University, which is fully
accredited by the Institutional Animal Care and Use Committee. All
animals were housed in cages, in a 12:12h light/dark cycle, the
temperature at 24°C, and humidity of 50–70%, allowed free access to
food and water. All experiments were approved by the Animal Care
Committee of China Medical University. The animals were randomized
into the following four groups (n=6/group): i) The sham group; ii)
the sham + baicalin group; iii) the BLM group; and iv) the BLM +
baicalin group. The pulmonary fibrosis model was prepared as
previously reported (41).
Briefly, the rats were anesthetized with an intraperitoneal
injection of 1% pentobarbital sodium (50 mg/kg), and then treated
with a single intratracheal instillation of 5 mg/kg BLM solution.
The sham group rats received intratracheal injection of an equal
volume of the sterilized saline. The following day, 50 mg/kg
baicalin was intraperitoneally administered, which was continued
once a day for 28 days, and six animals per group were sacrificed
on day 29. The rats were anesthetized with 1% pentobarbital sodium
(50 mg/kg), the blood and bronchial alveolar lavage fluid were
collected, and the lungs were removed for subsequent
experimentation. After sampling, the rats were euthanized by
overdose anesthesia. Blood was collected from the abdominal aorta
under anesthesia and centrifuged at 2,411 × g for 5 min at room
temperature, and the serum was then stored at −20°C until use. The
left lung of each animal was morphologically assessed, the right
lung was stored at −80°C and the Hyp content was determined using a
commercial assay kit, according to the manufacturer's
instructions.
Histology
The left lung was perfused with 4% paraformaldehyde
(PFA) at a pressure of 25 cm H2O via the left principal
bronchus at room temperature, and immersed in PFA solution
overnight following the tracheal ligation. Subsequently, the lung
tissues were embedded in paraffin, and cut into 4-µm thick serial
sections. Then, 2 min hematoxylin and eosin (H&E) staining, as
well as 8 min Masson staining were performed at room temperature to
determine the degree of alveolitis and fibrosis, and the sections
were observed by light microscopy (magnification, ×10).
Measurement of Hyp content
Frozen lungs (~100 mg) were cut into fragments and
then ground using a glass homogenizer on ice. The Hyp content of
the lung tissue was assessed using the Hyp ELISA kit (cat. no.
A030-3-1; Nanjing Jiancheng Bioengineering Institute), according to
the manufacturer's protocol. Lung tissues were hydrolyzed at 100°C
for 20 min, and adjusted to a preset pH. The mixture was incubated
at 60°C for 15 min and centrifuged at 2,813 × g for 10 min in room
temperature after cooling. The absorbance of each sample at 550 nm
was determined, and the data were calculated as µg Hyp per mg wet
lung weight.
Bronchial alveolar lavage fluid (BALF)
cell count
The right lung of each animal was ligated at the
hilus, and an 18-gauge catheter was inserted into the trachea and
placed in the left main bronchus. BALF was obtained by cannulating
the trachea and injecting and retrieving three 3 ml aliquots of
sterile saline, the fluid was then centrifuged at 1,205 × g for 15
min at 4°C, and the supernatants were discarded. The cell pellets
were resuspended in PBS and the total number of cells was counted
using a hemocytometer.
Measurement of MDA content, total-SOD
(T-SOD), GSH-px and GSH activities in serum
The enzymatic activities of SOD and GSH, and the MDA
content were assessed using different commercial assay kits in
accordance with the manufacturer's instructions. The serum content
of T-SOD was determined using the T-SOD assay kit (cat. no.
A001-1-2; Nanjing Jiancheng Bioengineering Institute), according to
the manufacturers instructions. SOD activity was detected in serum
by measuring the ability to inhibit the photochemical reduction of
nitro blue tetrazolium (NBT); the optical density was measured at
550 nm and the data were expressed as µ/ml.
GSH and GSH-px activity were analyzed using
commercial kits per the manufacturer's protocols (GSH, cat. no.
A006-2-1; GSH-px, cat. no. A005-1-2; Nanjing Jiancheng
Bioengineering Institute), which were based on the consumption of
reduced glutathione. Absorbance was determined at 412 nm and the
levels of GSH and GSH-px were defined as µ/ml.
The serum MDA concentration was detected by
measuring the degradation product of thiobarbituric acid at a
maximum wavelength of 532 nm, according to the manufacturer's
instructions (MDA, cat. no. A003-1-2; Nanjing Jiancheng
Bioengineering Institute). Serum samples (100 µl) were supplemented
with 5 mg/ml butylated hydroxytoluene to prevent artificial lipid
peroxidation. The samples were then centrifuged (1,600 × g, 10 min,
4°C) to remove insoluble material, and 100 µl supernatants were
transferred to a microcentrifuge tube and supplemented with 800 µl
thiobarbituric acid (TBA) to generate an MDA-TBA adduct. To
accelerate the process, samples were incubated at 95°C for 60 min,
placed on an ice bath for 10 min to inhibit the reaction and
centrifuged (1,600 × g, 10 min, 4°C). The final product was
measured colorimetrically at 532 nm, and the absorbance values were
compared to a calibration curve prepared using the MDA standard;
data were expressed as nmol/ml.
Measurement of TGF-β1 and TNF-α
detection in BALF
The concentrations of TGF-β1 and TNF-α in the BALF
were determined using the Rat TGF-β1 and TNF-α ELISA kits (Rat
TGF-β1 ELISA kit, cat. no. PT878; Rat TNF-α ELISA kit, cat. no.
PT516) according to the manufacturer's instructions. Samples or
reference standards (100 µl) were added to each well of a
microplate precoated with biotinylated antibody 100 µl specific to
TGF-β1 or TNF-α and incubated at room temperature for 60 min. After
washing out unbounded proteins, a horseradish peroxidase
(HRP)-conjugated polyclonal secondary antibody was added to the
wells (100 µl/well) and incubated for 30 min at room temperature in
the dark. After washing with the washing reagent from the kit and
100 µl developer 3,3′,5,5′-Tetramethylbenzidine solution was added
at room temperature for 20 min in the dark, as well as 50 µl
termination solution, optical density was determined at 450 nm. The
results were quantified using the linear regression equation of the
standard curve.
TUNEL assay
The detection procedure was performed according to
the manufacturer's instructions, but with modifications.
Deparaffinized 4-µm-thick tissue sections were treated with 20
µg/ml proteinase K for 15 min at room temperature. Subsequently,
the TUNEL reagent containing dUTP and TdT (Roche Diagnostics) was
added to each section, and incubated in a humidified chamber at
37°C for 60 min. The sections were rinsed with PBS and the sections
were incubated with 0.3% converter-peroxidase for 30 min at 37°C,
then the sections rinsed with PBS prior to the addition of DAB
substrate 10 min at room temperature and color rendering was
observed under the microscope for 3–8 min. After the color
development with tap water, sections were rinsed for 15 min and
hematoxylin staining was performed for 2 min at room temperature,
and subsequent steps were performed as follows. At the room
temperature, the sections rinsed with tap water and differentiated
with hydrochloric acid alcohol for 3–8 sec, then the slices were
immersed in 70% alcohol at 5 min, 80% alcohol at 5 min, 90% alcohol
at 5 min and 100% alcohol at 5 min twice, soaked in xylene at 10
min twice, and sealed with neutral balsam. All specimens were
examined by two pathologists who were blinded to the experimental
protocol. The sections were observed by light microscopy
(magnification, ×10).
Isolation of rat primary lung
fibroblasts
Normal rat primary fibroblasts from female Wistar
rats were prepared and cultured as previously described (42). The cells were cultured in DMEM
containing 15% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a
5% CO2 incubator. Upon reaching confluence, the cells
were trypsinized, counted, seeded into culture flasks at a density
of 104/ml and used in the following experiments. All
experiments were performed using cells between passages 2 and
6.
Western blotting
Lung tissues and fibroblasts were homogenized in
cold RIPA lysis buffer, which contained 50 mM Tris (pH 7.4),150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM
sodium orthovanadate, 1 mM sodium fluoride, 1 mM EDTA and 1 µg/ml
leupeptin. Total protein was quantified using a bicinchoninic acid
assay kit. The western blot protocol was an adaptation of a
previously described (43).
Briefly, equal amounts of protein (50 µg per lane) were separated
on 10% SDS-PAGE gels and electro-blotted onto nitrocellulose
membranes (EMD Millipore). The membranes were then blocked with 5%
non-fat milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T) for
1 h at room temperature, and incubated at 4°C overnight with the
following primary antibodies: Anti-Bcl-2 (1:500; cat. no. AB112),
anti-Bax (1:500; cat. no. 2772), anti-caspase-3 (1:200; cat. no.
9661), anti-cyclin A (1:500; cat. no. AF2524), anti-cyclin D
(1:500; cat. no. 2922), anti-cyclin E (1:500; cat. no. 20808),
anti-PCNA (1:500; cat. no. 2586), anti-p-AKT (1:500; cat. no.
9271), anti-t-AKT (1:500; cat. no. 9272), anti-p-CaMKII (1:500;
cat. no. 12716) and anti-t-CaMKII (1:500; cat. no. 3362). The
primary antibodies were purchased from Cell Signaling Technology,
Inc and the Beyotime Institute of Biotechnology. After three rinses
with TBS-T for 10 min each, the membranes were incubated with
HRP-conjugated secondary antibodies (mouse; 1:5,000; cat. no.
ZB2305; rabbit; 1:5,000; cat. no. ZB2301; Zhongshan Golden Bridge
Bio-technology) for 1 h at room temperature. For immunodetection,
the membranes were incubated with ECL reagents (Applygen
Technologies, Inc.). Protein expression was semi-quantified using
Quantity One software (version 4.6.2; Bio-Rad Laboratories, Inc.)
with β-actin and GAPDH as the loading controls.
MTT assay
Fibroblasts (1×104 cells/well) were
cultured in 96-well microtiter plates with serum-free DMEM for 24 h
to induce growth arrest at 37°C in a 5% CO2 incubator.
BLM and baicalin were diluted in PBS and then purified using a
filter with a 0.22-µm pore size (Millex-GS; EMD Millipore). Then,
190 µl filtered PBS was added to DMEM in each control group. The
cells were incubated with 190 µl different concentrations of BLM
(0.1, 1.0, 10, 20 or 40 µg/ml) and baicalin (20, 40, 60 or 80
µg/ml) for 24 h at 37°C in a 5% CO2 incubator and
treated for 4 h in medium containing 0.5% MTT 10 µl (at 37°C).
After removing the supernatant, 150 µl dimethyl sulfoxide was added
to each well and the plates were mixed on a plate shaker for 10 min
at room temperature. The absorbance of each sample was determined
using a microplate reader at 490 nm.
Cell cycle analysis
To study alterations to the cell cycle induced by
BLM, the proportion of fibroblasts in the
G0/G1, S and G2/M phases was
analyzed by flow cytometry, as previously reported (44). The detection procedure was
performed according to the manufacturer's instructions, using a
Cell cycle and apoptosis detection kit (Beyotime Institute of
Biotechnology; cat. no. C1052). Following drug treatment at special
time intervals, the cells were subjected with harvested by
trypsinization, centrifuged at 300 × g for 5 min at room
temperature and then resuspended in 1 ml cold PBS. Following two
washes with PBS, the cells were resuspended and fixed using 70%
cold ethanol and stored at 4°C for ≥2 h until use. Prior to cell
cycle analysis, the fixed cells were centrifuged at 300 × g for 5
min at room temperature, and the supernatant was discarded. Each
sample was resuspended in a mixed solution at room temperature,
which contained 500 µl staining buffer, 10 µl RNase A and 25 µl
propidium iodide. Then, the suspension was mixed and incubated at
37°C for 30 min in the dark. Cells were filtered using 400-mesh
sieves prior to analysis using a flow cytometer (C6 type; BD
Biosciences). The data of the experiment was exported to Excel 2007
(Microsoft Corporation) and SPSS 19.0 (SPSS, Inc.) was used to
analyze the statistical significance.
Measurement of
[Ca2+]i
Fibroblasts at a density of 104/ml were
incubated with a working solution containing 10 mM Fluo-3/AM
(acetoxymethyl ester; Molecular Probes; Thermo Fisher Scientific,
Inc.) and 0.03% PluronicF-127, at 37°C for 40 min. The cells were
then rinsed twice with Tyrode solution to move any remaining dye.
Changes in [Ca2+]i levels were indicated by
fluorescence intensity (FI). The cellular FI of Fluo-3/AM was
detected for 5 min using a laser scanning confocal microscope
(magnification, ×10; Olympus Corporation) with an emission
wavelength of 530 nm and excitation wavelength of 488 nm. FI was
assessed in 10 randomly selected cells, and the average intensity
was determined.
Statistical analysis
Data were analyzed using SPSS 19.0 (45) and are presented as the mean ± SEM.
Statistical analysis was performed using one-way ANOVA followed by
Tukey's test, which was used for the comparison of >3 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
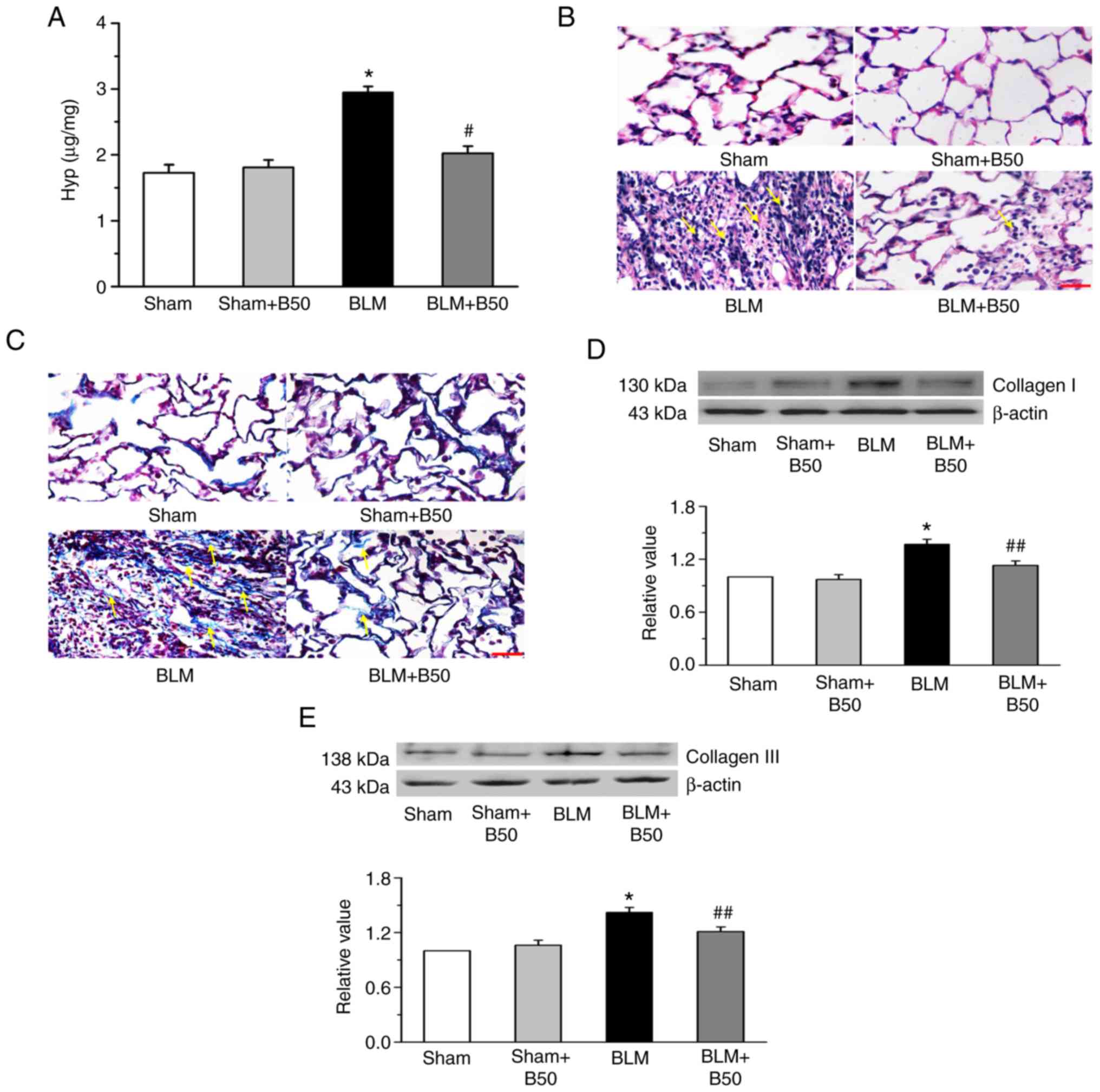
Baicalin attenuates lung fibrosis, and
reduces Hyp content and collagen protein expression in a
BLM-induced rat model of pulmonary fibrosis
A significant increase in the Hyp content was
observed following BLM administration in the model group, compared
with the sham group (Fig. 1A).
However, this was significantly inhibited by treatment with 50
mg/kg baicalin, which had no significant effect on the sham group.
To further investigate the effects of baicalin in the pathogenesis
of BLM-induced lung fibrosis, lung tissue morphology was examined
by H&E and Masson staining. At day 28, the lung tissues of BLM
group exhibited severe fibrosis compared with the sham group
(Fig. 1B), which included
inflammatory cell infiltration and alveolar hemorrhage. It was also
found that the alveoli were enlarged and the alveolar interstitium
was thickened, as indicated by yellow arrows. Furthermore, Masson's
staining demonstrated abundant collagen deposition (yellow arrows;
Fig. 1C). Treatment with 50 mg/kg
baicalin significantly reduced BLM-induced pathological changes,
which included collagen deposition, and damage to the lung
structure and interstitium. However baicalin treatment did not have
a significant effect on the sham group (Fig. 1B and C). Baicalin also suppressed
the BLM-induced expression of collagen I and III (Fig. 1D and E). The results suggested that
baicalin had a protective role during BLM-induced pulmonary
fibrosis and lung tissue apoptosis.
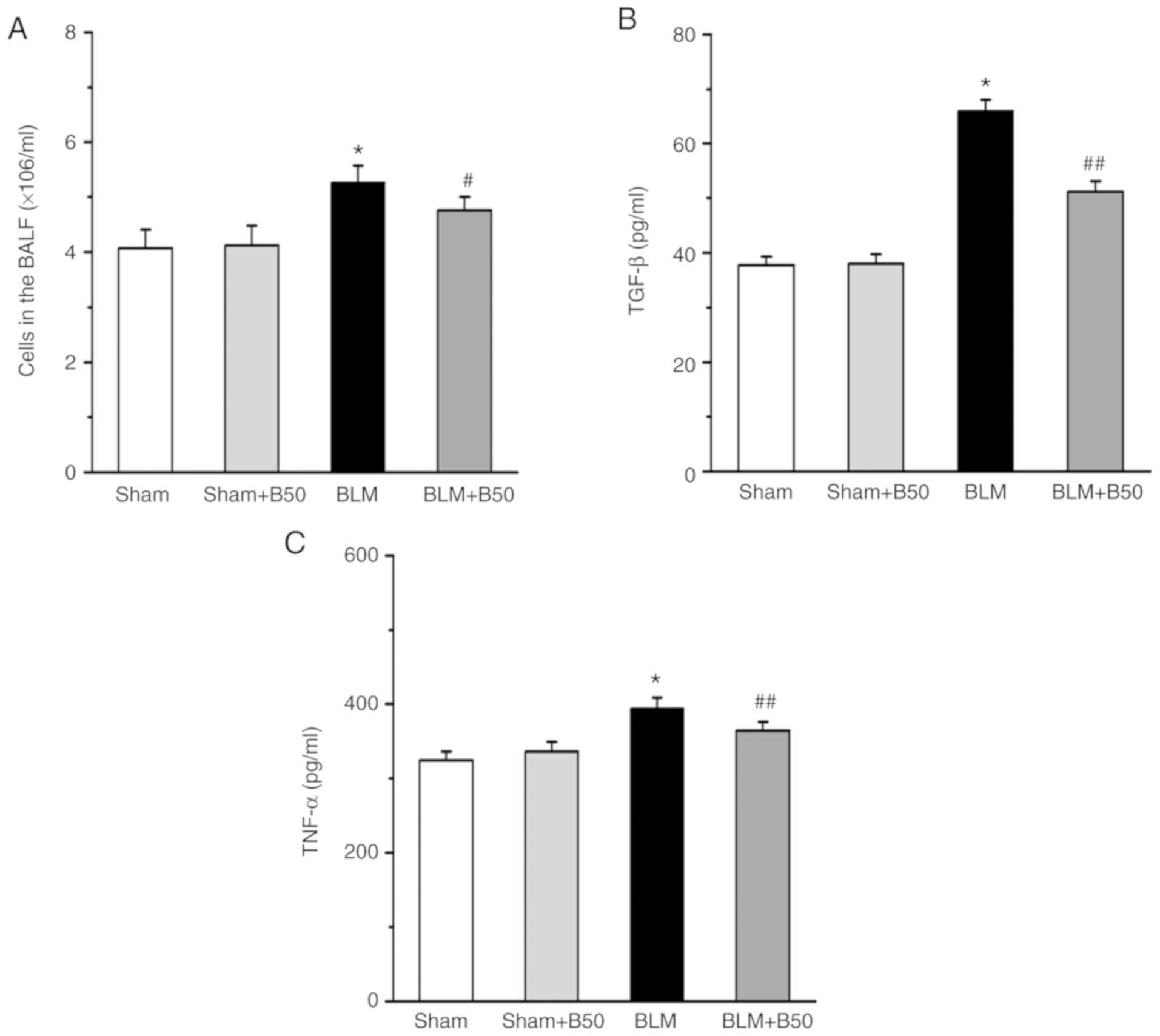
Baicalin attenuates the inflammatory
response, and decreases TGF-β1 and TNF-α expression levels in a rat
model of BLM-induced pulmonary fibrosis
At day 28, the total number of cells in the BALF of
rats treated with BLM (5 mg/kg) and baicalin (B50 mg/kg) was
determined. The total number of cells in the BLM-treated group was
significantly increased (5.26±0.31) compared with the sham group
(4.07±0.34; Fig. 2A). However, the
increase in the total cell number was decreased by the application
of 50 mg/kg baicalin (4.76±0.24), while there was no significant
difference between the sham group and the sham + baicalin 50 mg/kg
group (4.12±0.36). The expression levels of TGF-β1 and TNF-α in the
BALF were quantified by ELISA, which revealed that both TGF-β1 and
TNF-α were significantly upregulated in the BALF of the BLM group
(65.92±2.1 and 394±15, respectively) compared with the sham group
(37.68±1.6 and 324±12, respectively). However, baicalin
significantly decreased TGF-β1 and TNF-α expression (51.2±1.9 and
364±12, respectively) in BLM-treated rats. Moreover, baicalin did
not affect the expression of TGF-β1 and TNF-α under sham conditions
(38.01±1.7 and 336±13, respectively; Fig. 2B and C).
Baicalin attenuates oxidative
stress-associated damage
To investigate whether baicalin contributes to
oxidative stress, the activities of GSH-px, T-SOD and GSH, as well
as the serum MDA content were determined. It was demonstrated that
GSH-px, T-SOD and GSH activities were decreased significantly in
the serum of the 5 mg/kg BLM group (738±13, 186±11 and 795±26,
respectively) compared with the sham group (809±14, 224.7±13 and
945±25, respectively; Fig. 3A-C).
However, treatment with 50 mg/kg baicalin significantly increased
the activities of GSH-px, T-SOD and GSH (769±18, 212±12 and 838±23,
respectively). Furthermore, the level of serum MDA, an index of
lipid peroxidation (46) was
significantly increased in the 5 mg/kg BLM group (5.39±0.47)
compared with the sham group (4.33±0.33; Fig. 3D), after the administration of 50
mg/kg baicalin, BLM-mediated lipid peroxidation was significantly
decreased (4.69±0.39). However, 50 mg/kg baicalin had no obvious
effect on oxidative damage in the sham group (4.29±0.36).
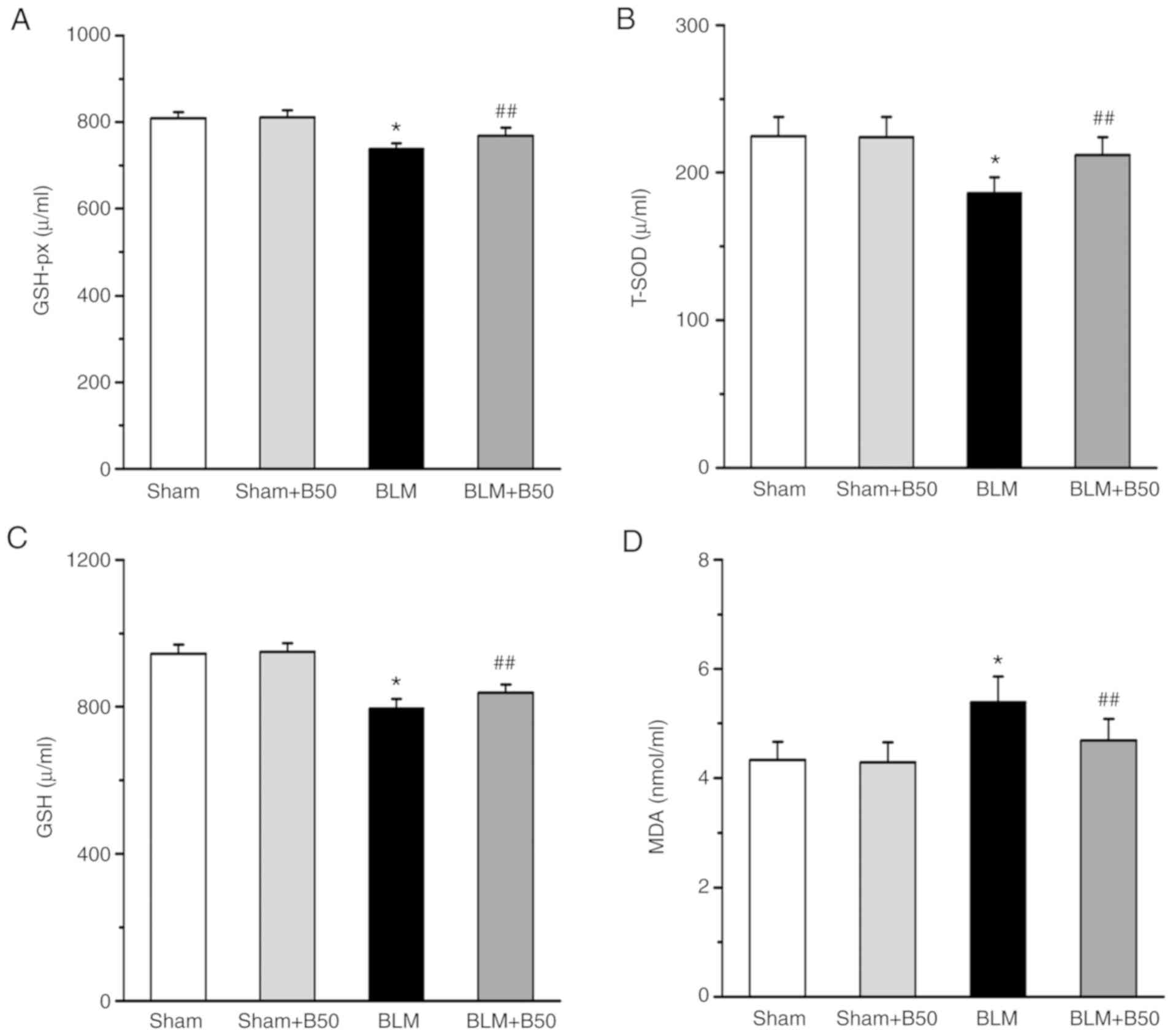 | Figure 3.Baicalin decreases BLM-induced
oxidative stress damage. The effect of 50 mg/kg baicalin on (A)
GSH-px, (B) T-SOD and (C) GSH activities, and (D) the MDA content
of lung tissues. Data are presented as the mean ± SEM from ≥3
separate experiments. *P<0.05 vs. the sham group.
##P<0.01 vs. the BLM group. BLM, bleomycin; GSH-px,
glutathione peroxidase; T-SOD, total superoxide dismutase; GSH,
glutathione; MDA, malondialdehyde; Sham, sham group; BLM,
bleomycin; B50, 50 mg/kg baicalin. |
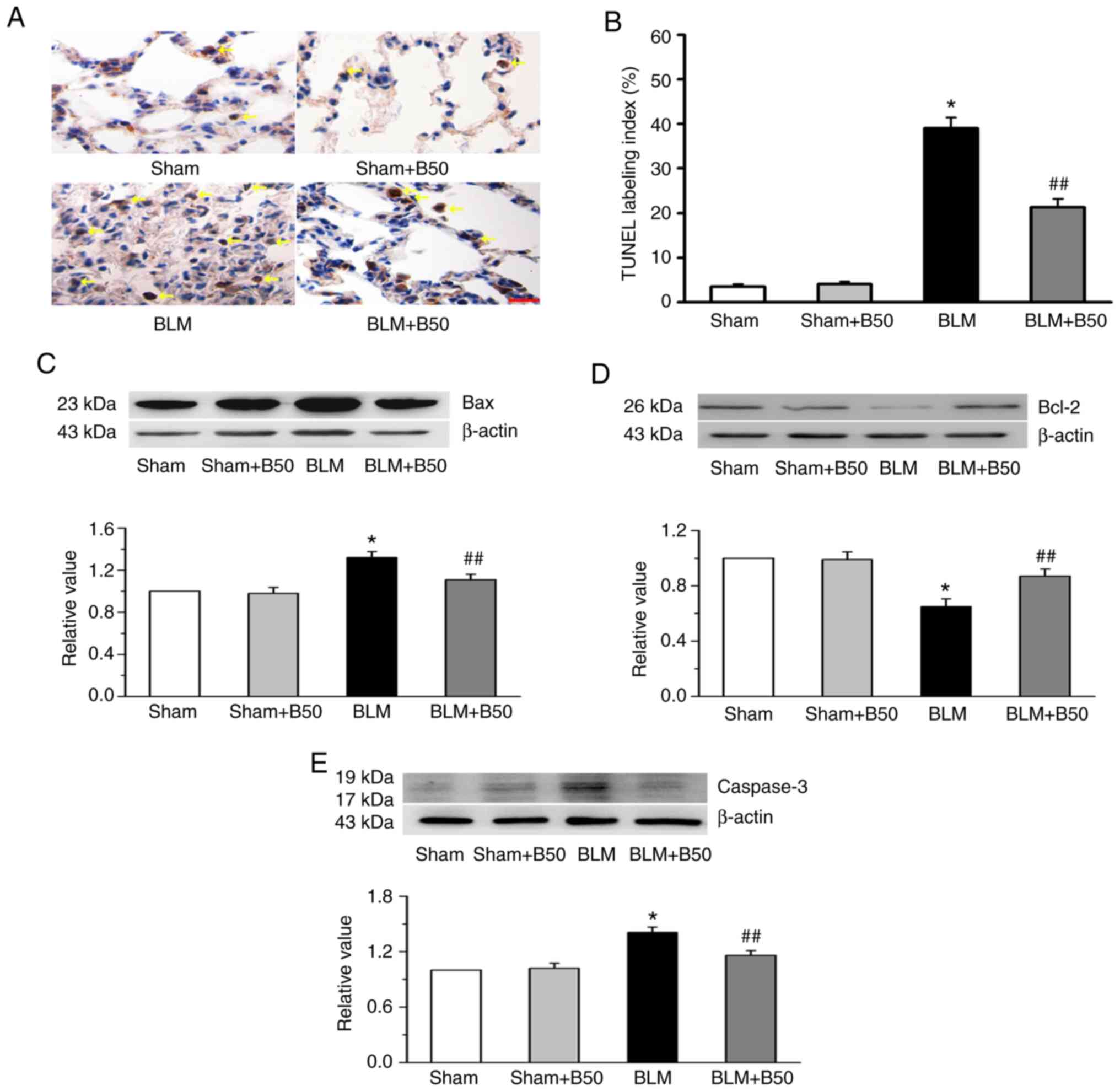
Baicalin inhibits BLM-induced
apoptotic protein expression in rat lung tissues
To investigate whether baicalin is involved in lung
tissue apoptosis, TUNEL staining was conducted. Compared with the
sham group, a significant increase in TUNEL-positive cells (yellow
arrows) was observed in the BLM group (Fig. 4A and B). Furthermore, the
administration of 50 mg/kg baicalin inhibited the appearance of
TUNEL-positive cells in the BLM group, which was indicated in the
yellow arrows, but not the sham group. In addition, the mechanism
of baicalin-induced lung tissue apoptosis was investigated. Bcl-2
and Bax are localized on the mitochondrial membrane and play
important roles in apoptosis (47). Bcl-2 is known to be anti-apoptotic,
whereas Bax is a pro-apoptotic protein (47). Therefore, in the present study, the
effect of baicalin on the protein expression levels of Bcl-2 and
Bax were investigated. The data showed that the expression levels
of Bcl-2 were downregulated, while that of Bax was upregulated by
BLM. Moreover, baicalin (50 mg/kg) increased Bcl-2, and suppressed
Bax protein expression in rat lung tissues (Fig. 4C and D). Pro-caspase-3 is cleaved
and activated to form caspase-3 by originator caspases (including
caspase 2, 8, 9, 10, 11 and 12) in response to apoptotic stimuli
(48). In addition, BLM promoted
the protein expression of caspase-3, an effect which was primarily
blocked by 50 mg/kg baicalin treatment (Fig. 4E). However, baicalin had no
significant effect on the expression of Bcl-2, Bax and caspase-3 in
the sham group.
Baicalin reverses BLM-induced
fibroblast proliferation, cyclin protein expression and cell cycle
progression in vivo
Following BLM treatment, MTT was used to examine the
effect of baicalin on the viability of rat lung fibroblasts. After
a 24-h treatment period (1.0, 10, 20 or 40 µg/ml BLM) caused a
significant dose-dependent increase in lung fibroblast viability,
compared with that of the control group (Fig. 5A). With regards to the safety of
the drug, 20 µg/ml BLM was selected for the following experiments.
To demonstrate the effect of baicalin on lung fibroblasts, the
dose-dependent effect was examined. Treatment with 40, 60 or 80
µg/ml baicalin reduced cell viability at 24 h, while 20 µg/ml
baicalin failed to reduce cell viability under 20 µg/ml BLM
treatment. Among the different treatment doses, 80 µg/ml baicalin
exerted the most significant inhibitory effect (Fig. 5B). Therefore, unless otherwise
stated, all subsequent experiments were performed under these
conditions (24 h, 20 µg/ml BLM and 80 µg/ml baicalin). Western blot
analysis was conducted to observe the expression levels of cyclin
proteins in lung fibroblasts treated with 80 µg/ml baicalin. The
results indicated that 20 µg/ml BLM significantly increased the
expression of cyclin A, cyclin D, cyclin E, as well as PCNA in rat
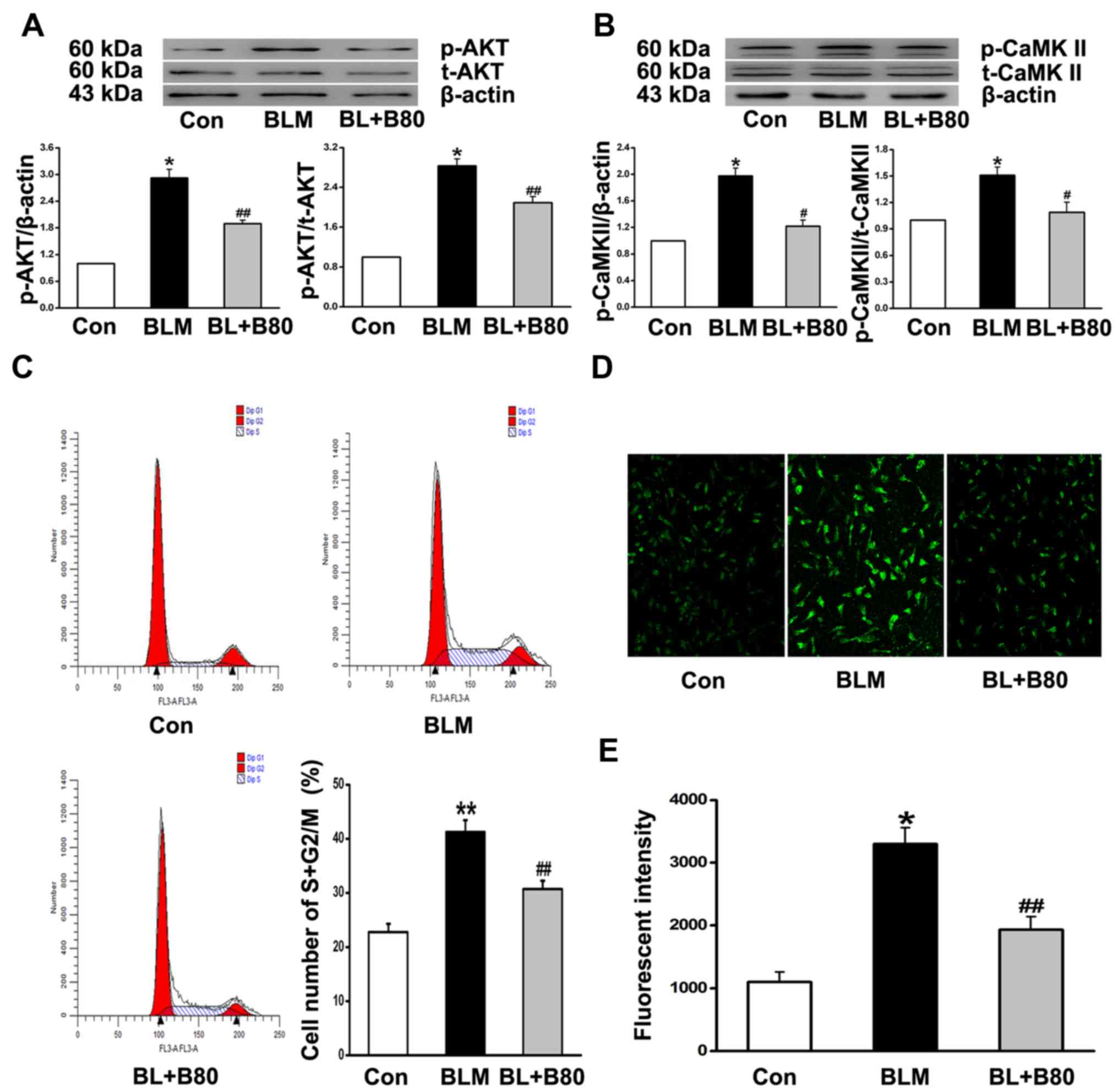
fibroblasts (Fig. 5C-F). CaMKII is
reported to be activated by intracellular Ca2+ (35), and the western blot results
indicated a significant increase in p-AKT and p-CaMKII expression
levels in the BLM group (Fig. 6A and
B). However, 80 µg/ml baicalin suppressed the expression of
cyclin A, D and E, PCNA, p-AKT and p-CaMKII (Figs. 5C-F and 6A and B).
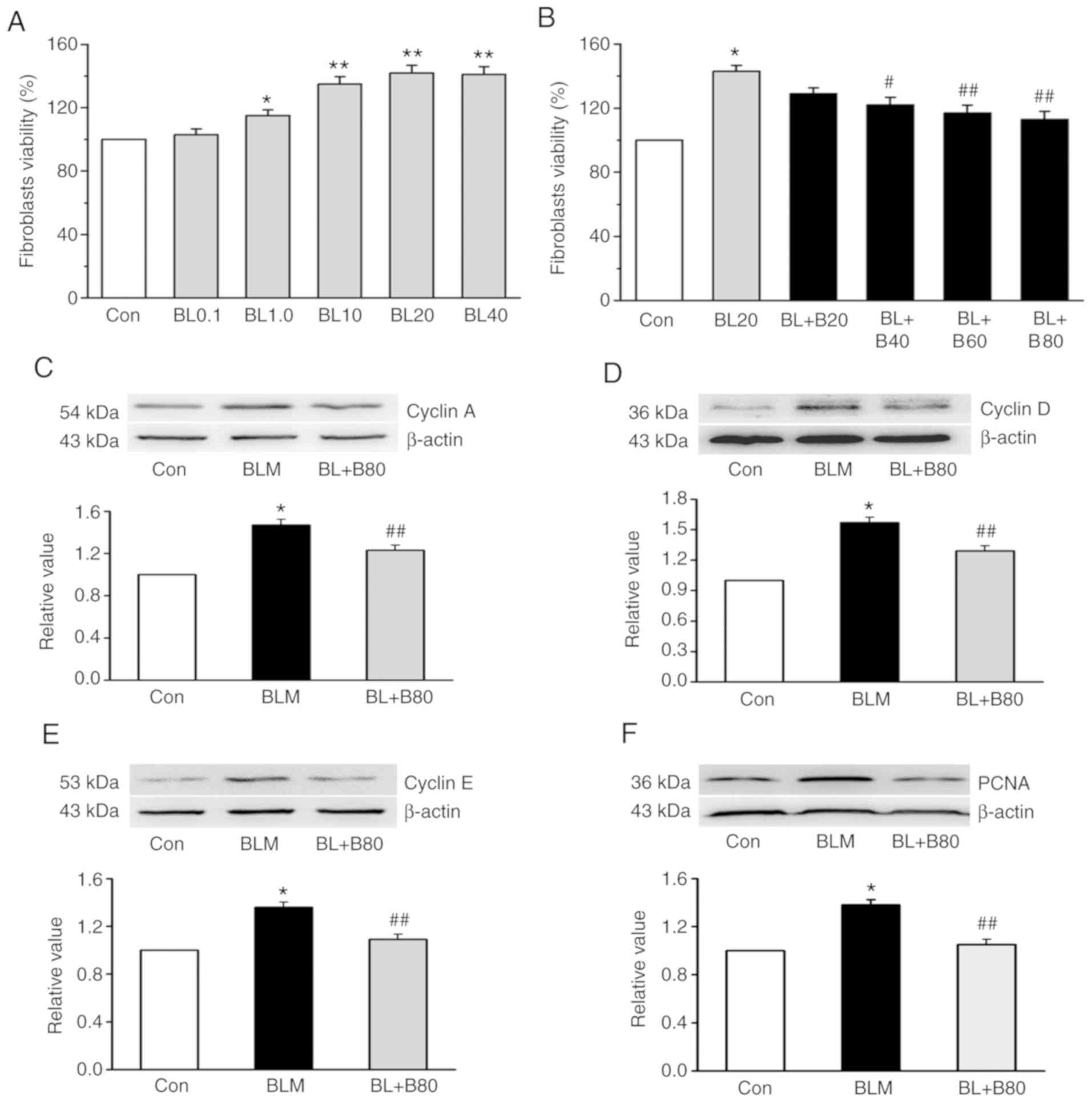 | Figure 5.Baicalin reverses BLM-induced
fibroblast proliferation, and the expression of PI3K/AKT and CaMKII
in vivo. Lung fibroblasts were growth-arrested for 24 h and
subsequently treated with different concentrations of BLM (0.1,
1.0, 10, 20 and 40 µg/ml). (A) Effect of BLM on lung fibroblast
viability. (B) Dose-dependent effects of baicalin (20, 40, 60 and
80 µg/ml) on lung fibroblast viability under the treatment of BLM
(20 µg/ml). BLM-induced (20 µg/ml) upregulation of (C) cyclin A,
(D) cyclin D, (E) cyclin E and (F) PCNA protein expression was
reduced by baicalin (80 µg/ml) in lung fibroblasts. Data are
presented as the mean ± SEM from ≥3 separate experiments.
*P<0.05 and **P<0.01 vs. the Con group. #P<0.05
and ##P<0.01 vs. the BLM group. Con, control; BLM,
bleomycin; BL, bleomycin; CaMKII, calcium/calmodulin-dependent
kinase II; PCNA, proliferating cell nuclear antigen; BL0.1, 0.1
µg/ml bleomycin; BL1.0, 1.0 µg/ml bleomycin; BL10, 10 µg/ml
bleomycin; BL20, 20 µg/ml bleomycin; BL40, 40 µg/ml bleomycin; B20,
20 mg/kg baicalin; B40, 40 mg/kg baicalin; B60, 60 mg/kg baicalin;
B80, 80 mg/kg baicalin. |
Additionally, BLM increased the percentage of cells
in the S and G2/M phases of the cell cycle. Baicalin
inhibited fibroblast cell cycle progression and promoted arrest at
the G0/G1 phase (Fig. 6C). Moreover, laser scanning
confocal microscopy indicated that the increase in
[Ca2+]i in BLM-treated fibroblasts was
significantly attenuated by baicalin treatment (Fig. 6D and E). The results suggested that
baicalin protected cell cycle activity and inhibited fibroblast
proliferation, ultimately contributing to pulmonary fibrosis.
Discussion
Pulmonary fibrosis is the final stage of several
diffuse parenchymal lung diseases, and is characterized by
excessive matrix deposition and destruction of the lung
architecture, eventually resulting in respiratory insufficiency
(49). IPF is a progressive, end
stage disease, and the pathophysiological basis of the disease has
been a topic of debate over the last few decades (8). Animal models of BLM-induced lung
fibrosis are widely used to study IPF (50), though the precise mechanism of IPF
is still unclear. Previous studies have demonstrated that baicalin
exhibits strong antitumor activity and inhibits apoptosis of tumor
cells (51–53). Therefore, in the present study, the
mechanisms underlying the effect of baicalin on pulmonary fibrosis
was investigated, demonstrating that baicalin reversed BLM-induced
lung tissue apoptosis and pulmonary fibrosis, attenuated oxidative
stress and inhibited rat lung fibroblast proliferation, at least
partly via the CaMKII and AKT pathways.
In a short period of time, BLM can induce
inflammatory and fibrotic aberrations in the lung tissue, and
intratracheal administration of BLM has been shown to increase the
expression of fibrogenic cytokines such as TNF-α, TGF-β, IL-1β and
IL-6 (50). Pathological changes
due to lung tissue inflammation were also observed in the present
study, after exposure to BLM, a rapid increase in Hyp content and
the infiltration of inflammatory cells was apparent, and alveolar
septal thickening was indicated by H&E and Masson staining.
Conversely, rats in the BLM + baicalin group exhibited reduced
inflammatory cell infiltration and collagen deposition in the
lungs. In addition to a reduction in collagen I and III protein
expression following baicalin treatment, these results indicated
that baicalin attenuated BLM-induced pulmonary fibrosis.
Following administration of 5 mg/kg BLM, which
emulates inflammatory stimulation (54), the total number of cells in the
BALF was significantly increased, which was subsequently reduced by
baicalin treatment (5 mg/kg). Previous studies have revealed that
TNF-α is overexpressed in the fibrotic lung tissues of animal
models of BLM-induced pulmonary fibrosis (55–57),
which serves a vital role in fibrotic progression (58). TGF-β1 is also a crucial profibrotic
cytokine (59,60) and plays a central role in the
mechanism of systemic sclerosis-associated pulmonary fibrosis
(61,62). In the present study, baicalin was
identified to inhibit the expression of TGF-β1 and TNF-α in the
BALF of the BLM group. These results suggested that baicalin
attenuates the pulmonary inflammation induced by BLM. Additionally,
Moreover, the present results indicated that baicalin can
significantly alleviated pulmonary fibrosis and inflammatory
responses induced by BLM in rats. However, the anti-pulmonary
fibrosis effect of baicalin has also been reported in other species
and strains. Liu et al reported that baicalin alleviated
silica-induced lung inflammation by suppressing IL-6 and IL-23,
which reflects Th17 response and lung fibrosis in C57BL/6 mice
(25). Baicalin inhibited
BLM-induced pulmonary fibrosis in mice by upregulating Adenosine
A2a receptor and downregulating TGF-β1 and p-ERK1/2 expression
(26). The cross-species effect of
baicalin also suggested that future studies should investigate the
specific mechanism and underlying signaling pathways of baicalin in
inhibiting pulmonary fibrosis in a murine model.
Previous studies have suggested that oxidative
stress contributes to fibrosis in a variety of organs (63,64),
and that there is an imbalance between the generation of ROS
(65–67). Therefore, in the present study,
oxidative stress was assessed by detecting GSH-px, T-SOD and GSH
activity, as well as the levels of MDA in the serum of fibrotic
rats. It was demonstrated that in the BLM group, MDA levels were
significantly increased, while GSH-px, T-SOD and GSH levels were
reduced. By contrast, baicalin significantly reduced the MDA
content, and simultaneously increased the levels of GSH-px, T-SOD
and GSH. The results implied that baicalin significantly suppressed
BLM-induced fibrosis and oxidative stress injury, which may explain
the protective effects against BLM-induced pulmonary fibrosis.
The mechanisms of baicalin-associated lung tissue
apoptosis were also investigated following BLM administration.
Morphological evaluation demonstrated that 50 mg/kg baicalin
decreased the number of TUNEL-positive cells in the lung tissue.
Furthermore, the down-regulation of BLM-induced Bcl-2 expression
was significantly reversed by baicalin treatment. Baicalin also
reversed the BLM-associated increase in Bax and caspase-3
expression levels, thus demonstrating that baicalin protects
against apoptosis in pulmonary tissues.
Fibroblasts are critical cells for the pathogenesis
of pulmonary fibrosis (68). In
the present study, it was demonstrated that BLM promoted rat lung
fibroblast viability in dose-dependent manner. Similar results have
been reported in other studies. F2-isoprostanes mediated BLM (25,
50 and 100 µg/ml) induced rat lung fibroblast proliferation and
collagen synthesis, and the incubation time of bleomycin was 48 h
(69). Additionally, NLRP3
participates in the regulation of epithelial-mesenchymal transition
(EMT) in BLM-induced pulmonary fibrosis, the concentrations of
bleomycin was treated to A549 cells were 0, 40, 80, 120, 160 and
200 µM for 24 h to study cell viability, and those added to RLE-6TN
cells were 0, 10, 20, 30, 40, 50 and 60 µM for 24 h (70). It has been shown that different
cell types may be differentially effected by the same drug. In the
present study, cell viability assays revealed that fibroblast
proliferation was consistent at 20 and 40 µg/ml BLM, thus, 20 µg/ml
BLM treatment for 24 h was utilized for subsequent experimentation.
Following the administration of different concentrations of
baicalin (40, 60 and 80 µg/ml), the results indicated that 40, 60
and 80 µg/ml baicalin significantly reduced lung fibroblast
viability in the 20 µg/ml BLM group, however, no significant effect
was observed following treatment with 20 µg/ml baicalin. It has
been reported that baicalin treatment (50 or 100 µM for 24 h)
promoted the repair of DNA single-strand breaks and improved cell
viability in H2O2-treated NIH3T3 mouse
fibroblasts (71). Furthermore,
baicalin (25, 50 and 100 µg/ml) reduced the colistin
sulfate-induced apoptosis of PC12 cells in a dose-dependent manner
(72). Additionally, baicalin (200
µM) activated caspase-3 expression to induce apoptosis in SW620
human colorectal carcinoma cells (73). In clinical practice, baicalin
displays good drug safety, and the present study further suggested
that 80 µg/ml baicalin significantly reduced the viability of lung
fibroblasts.
The cell cycle plays an important role in cell
proliferation (74), but how the
cell cycle progression responds to the baicalin remains unknown.
The cell cycle is subdivided into four phases: DNA replication
occurs during S phase, and chromosome segregation occurs during M
phase. The S and M phases are separated by G1 (before
DNA replication) and G2 (before mitosis) (75). Cyclin A is essential for
progression through the S phase, and cyclin D and E, are necessary
for G1/S transition (76–78).
The results of the present study demonstrated that BLM increased
the expression of cyclin A, D and E, and PCNA in vitro, and
increased the proportion of cells in the S and G2/M
phases, suggesting that BLM induces proliferation via the cell
cycle. In vitro, baicalin (20 µg/ml) decreased the
BLM-induced protein expression levels of cyclin A, D and E, and
PCNA, and the proportion of cells in the S and G2/M
phases. The PI3K/AKT pathway is involved in the regulation of
several target proteins such as NF-κB, p53, glycogen synthase
kinase-3β, Bad and caspase-9 that regulate cellular proliferation
and apoptosis (43).
Immunohistochemical analysis of lung biopsy specimens from patients
with IPF revealed that fibroblasts within fibrotic foci expressed
low levels of PTEN and upregulated levels of AKT (79). Fucoxanthin inhibited the
TGF-β1-induced expression of α-SMA, type 1 collagen and IL-6, and
inhibited the phosphorylation of p38, PI3K/AKT and Smad2/Smad3 in
human pulmonary fibroblasts (80).
Additionally, small interfering RNAs (siRNAs) against plasminogen
activator inhibitor-1 (PAI-1) reduced the expression of type I and
III collagen and increased the expression of caspase-3 in
vivo, as well as inhibiting p-ERK1/2 and PI3K/AKT expression,
and induced the proliferation of fibroblasts derived from rat
models of BLM-induced fibrosis (81). It has also been shown that microRNA
(miR)-155 reduces the expression of collagens and inhibits the
formation of hypertrophic scar fibroblasts by targeting
hypoxia-inducible factor-1α via the PI3K/AKT signaling pathway
(82). Additionally, activation of
the adenosine subtype 2B (A2B) receptor mediated the inhibition of
ET-1-induced fibroblast proliferation via the cAMP/Epac/PI3K/AKT
signaling pathway (83).
miR-542-5p mimic reduced the proliferation of mouse fibroblasts,
and inhibited silica-induced pulmonary fibrosis by directly binding
to integrin-α6, both effects at least partially via the
FAK/PI3K/AKT signaling pathway (84). However, whether PI3K is involved in
the effect of baicalin on pulmonary fibrosis, and its specific
BLM-induced mechanism, has not yet been reported.
CaMKII is known to be activated by intracellular
Ca2+ (35). He et
al (85) reported that LPS
induced a direct fibrogenic effect on lung fibroblasts by
upregulating collagen expression and cell proliferation via the
PI3K/AKT signaling pathway. However, the relationship between
baicalin and the PI3K/AKT or CaMKII signaling pathways in pulmonary
fibrosis has not been elucidated. The results of the present study
suggested that BLM increased the phosphorylation of CaMKII and AKT,
which was reversed by baicalin treatment. Moreover, baicalin
significantly decreased BLM-induced [Ca2+]i
in lung fibroblasts. The results suggested that the CaMKII and AKT
signaling pathways were involved in the inhibition of the cell
cycle and proliferation of lung fibroblasts in rats treated with
baicalin. Consistently, it has been reported that in certain cell
types, Ca2+/calmodulin-dependent protein kinases act
upstream of AMPK (86,87). Moreover, puerarin-mediated eNOS
phosphorylation is dependent on AMPK signaling via CaMKII (38). An increase in ERK1/2 expression
promotes CaMKII-induced matrix metalloproteinase-9 expression,
resulting in the proliferation and migration of cardiac fibroblasts
obtained from a rat model of pulmonary arterial hypertension
(88). Furthermore, neokyotorphin
induced the proliferation of fibroblasts, which required
Ca2+ influx and the activation of PKA, CaMKII and
MAPK/ERK (89).
Although the present study suggested that the
protective effect of baicalin on pulmonary fibrosis may be via
inhibiting the PI3K/AKT and CaMKII signaling pathways, the receptor
and downstream target genes of these inhibitory signaling pathways,
the mechanism in baicalin-treated cells requires further
investigation. Thus, further studies will help to reveal how
baicalin influences the CaMKII/PI3K/AKT complex.
In conclusion, the present study suggested that
baicalin inhibited histopathological damage and lung fibroblast
proliferation in BLM-induced pulmonary fibrosis, and that this
effect was at least in part, mediated via the CaMKII and PI3K/AKT
signaling pathways. The present results provide novel insights into
the protective effects of baicalin in pulmonary fibrosis and the
underlying mechanisms, which may become the theoretical basis for
the use of baicalin in the management of IPF.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL designed the experiments. HZ performed the
experiments. CL, LL and YR performed the animal experiments. JL, YG
and KM performed the cell experiments and western blotting. HZ, DC
and AL analyzed the data. HZ and YR wrote the manuscript. HZ and ZL
are accountable for all aspects of the work. All authors
contributed to the interpretation of the data and critical revision
of the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Medical
Ethics Committee of the Harbin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gross TJ and Hunninghake GW: Idiopathic
pulmonary fibrosis. N Engl J Med. 345:517–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Selman M, Thannickal VJ, Pardo A, Zisman
DA, Martinez FJ and Lynch JP III: Idiopathic pulmonary fibrosis:
Pathogenesis and therapeutic approaches. Drugs. 64:405–430. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thannickal VJ, Toews GB, White ES, Lynch
JP III and Martinez FJ: Mechanisms of pulmonary fibrosis. Annu Rev
Med. 55:395–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raghu G, Freudenberger TD, Yang S, Curtis
JR, Spada C, Hayes J, Sillery JK, Pope CE II and Pellegrini CA:
High prevalence of abnormal acid gastro-oesophageal reflux in
idiopathic pulmonary fibrosis. Eur Respir J. 27:136–142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ask K, Bonniaud P, Maass K, Eickelberg O,
Margetts PJ, Warburton D, Groffen J, Gauldie J and Kolb M:
Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1
but not TGF-beta3. Int J Biochem Cell Biol. 40:484–495. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gharaee-Kermani M, Gyetko MR, Hu B and
Phan SH: New insights into the pathogenesis and treatment of
idiopathic pulmonary fibrosis: A potential role for stem cells in
the lung parenchyma and implications for therapy. Pharm Res.
24:819–841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilkes DS, Chew T, Flaherty KR, Frye S,
Gibson KF, Kaminski N, Klemsz MJ, Lange W, Noth I and Rothhaar K:
Oral immunotherapy with type V collagen in idiopathic pulmonary
fibrosis. Eur Respir J. 45:1393–1402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinnula VL, Fattman CL, Tan RJ and Oury
TD: Oxidative stress in pulmonary fibrosis: A possible role for
redox modulatory therapy. Am J Respir Crit Care Med. 172:417–422.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rydell-Törmänen K, Andréasson K,
Hesselstrand R, Risteli J, Heinegård D, Saxne T and
Westergren-Thorsson G: Extracellular matrix alterations and acute
inflammation; developing in parallel during early induction of
pulmonary fibrosis. Lab Invest. 92:917–925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia L, Sun P, Gao H, Shen J, Gao Y, Meng
C, Fu S, Yao H and Zhang G: Mangiferin attenuates bleomycin-induced
pulmonary fibrosis in mice through inhibiting TLR4/p65 and
TGF-β1/Smad2/3 pathway. J Pharm Pharmacol. 71:1017–1028. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasaneen NA, Cao J, Pulkoski-Gross A,
Zucker S and Foda HD: Extracellular matrix metalloproteinase
inducer (EMMPRIN) promotes lung fibroblast proliferation, survival
and differentiation to myofibroblasts. Respir Res. 17:172016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sergew A and Brown KK: Advances in the
treatment of idiopathic pulmonary fibrosis. Expert Opin Emerg
Drugs. 20:537–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Lü W, Ge H, Tang H, Li R and Zhang
C: Protective effect of the traditional chinese patent medicine
Qing-Xuan Granule against bleomycin-induced pulmonary fibrosis in
mice. Chem Biodivers. 16:e19004672019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CC and Shieh DE: The anti-inflammatory
activity of Scutellaria rivularis extracts and its active
components, baicalin, baicalein and wogonin. Am J Chin Med.
24:31–36. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Z, Huang K and Xu H: Protective
effects of flavonoids in the roots of Scutellaria
baicalensis georgi against hydrogen peroxide-induced oxidative
stress in HS-SY5Y cells. Pharmacol Res. 43:173–178. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Tsang SY, Yao X and Chen ZY:
Biological properties of baicalein in cardiovascular system. Curr
Drug Targets Cardiovasc Haematol Disord. 5:177–184. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Zhi F, Lun W, Deng Q and Zhang W:
Baicalin inhibits PDGF-BB-induced hepatic stellate cell
proliferation, apoptosis, invasion, migration and activation via
the miR-3595/ACSL4 axis. Int J Mol Med. 41:1992–2002.
2018.PubMed/NCBI
|
|
19
|
Huang S, Chen P, Shui X, He Y, Wang H,
Zheng J, Zhang L, Li J, Xue Y, Chen C and Lei W: Baicalin
attenuates transforming growth factor-β1-induced human pulmonary
artery smooth muscle cell proliferation and phenotypic switch by
inhibiting hypoxia inducible factor-1α and aryl hydrocarbon
receptor expression. J Pharm Pharmacol. 66:1469–1477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng O, Li Z, Han Y, Jiang Q, Yan Y and
Cheng K: Baicalin improved the spatial learning ability of global
ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain
Res. 1470:111–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orzechowska B, Chaber R, Wiśniewska A,
Pajtasz-Piasecka E, Jatczak B, Siemieniec I, Gulanowski B, Chybicka
A and Błach-Olszewska Z: Baicalin from the extract of
Scutellaria baicalensis affects the innate immunity and
apoptosis in leukocytes of children with acute lymphocytic
leukemia. Int Immunopharmacol. 23:558–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Cao Y, Yu J, Liu R, Bai B, Qi H,
Zhang Q, Guo W, Zhu H and Qu L: Baicalin alleviates
ischemia-induced memory impairment by inhibiting the
phosphorylation of CaMKII in hippocampus. Brain Res. 1642:95–103.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei XL, Fang RT, Yang YH, Bi XY, Ren GX,
Luo AL, Zhao M and Zang WJ: Protective effects of extracts from
Pomegranate peels and seeds on liver fibrosis induced by carbon
tetrachloride in rats. BMC Complement Altern Med. 15:3892015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng L, Zhang C, Li L, Hu C, Hu M,
Sidikejiang N, Wang X, Lin M and Rong R: Baicalin ameliorates renal
fibrosis via inhibition of transforming growth factor β1 production
and downstream signal transduction. Mol Med Rep. 15:1702–1712.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Dai W, Li C, Liu F, Chen Y, Weng D
and Chen J: Baicalin alleviates silica-induced lung inflammation
and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J Nat
Prod. 78:3049–3057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang X, He Y, Chen Y, Wu P, Gui D, Cai H,
Chen A, Chen M, Dai C, Yao D and Wang L: Baicalin attenuates
bleomycin-induced pulmonary fibrosis via adenosine A2a receptor
related TGF-β1-induced ERK1/2 signaling pathway. BMC Pulm Med.
16:1322016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu XL, Wang LK, Yang DD, Qu M, Yang YJ,
Guo F, Han L and Xue J: Effects of Glut1 gene silencing on
proliferation, differentiation, and apoptosis of colorectal cancer
cells by targeting the TGF-β/PI3K-AKT-mTOR signaling pathway. J
Cell Biochem. 119:2356–2367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XQ, Sun P and Paller AS: Inhibition
of integrin-linked kinase/protein kinase B/Akt signaling: Mechanism
for ganglioside-induced apoptosis. J Biol Chem. 276:44504–44511.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Song YH, Mohler J and Delafontaine
P: ANG II induces apoptosis of human vascular smooth muscle via
extrinsic pathway involving inhibition of Akt phosphorylation and
increased FasL expression. Am J Physiol Heart Circ Physiol.
290:H2116–H2123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gui X, Chen H, Cai H, Sun L and Gu L:
Leptin promotes pulmonary fibrosis development by inhibiting
autophagy via PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun.
498:660–666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsoyi K, Chu SG, Patino-Jaramillo NG,
Wilder J, Villalba J, Doyle-Eisele M, McDonald J, Liu X, El-Chemaly
S, Perrella MA and Rosas IO: Syndecan-2 attenuates
radiation-induced pulmonary fibrosis and inhibits fibroblast
activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J
Respir Cell Mol Biol. 58:208–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsu HS, Liu CC, Lin JH, Hsu TW, Hsu JW, Su
K and Hung SC: Involvement of ER stress, PI3K/AKT activation, and
lung fibroblast proliferation in bleomycin-induced pulmonary
fibrosis. Sci Rep. 7:142722017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pitt GS: Calmodulin and CaMKII as
molecular switches for cardiac ion channels. Cardiovasc Res.
73:641–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mukherjee S, Sheng W, Sun R and Janssen
LJ: Ca2+/calmodulin-dependent protein kinase IIβ and IIδ
mediate TGFβ-induced transduction of fibronectin and collagen in
human pulmonary fibroblasts. Am J Physiol Lung Cell Mol Physiol.
312:L510–L519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Williams CL, Phelps SH and Porter RA:
Expression of Ca2+/calmodulin-dependent protein kinase
types II and IV, and reduced DNA synthesis due to the
Ca2+/calmodulin-dependent protein kinase inhibitor KN-62
(1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenyl
piperazine) in small cell lung carcinoma. Biochem Pharmacol.
51:707–715. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang YP, Kim HG, Hien TT, Jeong MH, Jeong
TC and Jeong HG: Puerarin activates endothelial nitric oxide
synthase through estrogen receptor-dependent PI3-kinase and
calcium-dependent AMP-activated protein kinase. Toxicol Appl
Pharmacol. 257:48–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mukherjee S, Sheng W, Michkov A, Sriarm K,
Sun R, Dvorkin-Gheva A, Insel PA and Janssen LJ: Prostaglandin E2
inhibits profibrotic function of human pulmonary fibroblasts by
disrupting Ca2+ signaling. Am J Physiol Lung Cell Mol
Physiol. 316:L810–L821. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Monaco S, Illario M, Rusciano MR,
Gragnaniello G, Di Spigna G, Leggiero E, Pastore L, Fenzi G, Rossi
G and Vitale M: Insulin stimulates fibroblast proliferation through
calcium-calmodulin-dependent kinase II. Cell Cycle. 8:2024–2030.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi EJ, Jin GY, Bok SM, Han YM, Lee YS,
Jung MJ and Kwon KS: Serial micro-CT assessment of the therapeutic
effects of rosiglitazone in a bleomycin-induced lung fibrosis mouse
model. Korean J Radiol. 15:448–455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang
MX, Li W, Li Y, Zhang WY and Li X: The angiotensin-converting
enzyme 2/angiotensin (1–7)/Mas axis protects against lung
fibroblast migration and lung fibrosis by inhibiting the
NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid Redox
Signal. 22:241–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Q, Fan K, Wang P, Yu J, Liu R, Qi H,
Sun H and Cao Y: Carvacrol induces the apoptosis of pulmonary
artery smooth muscle cells under hypoxia. Eur J Pharmacol.
770:134–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu R, Zhang Q, Luo Q, Qiao H, Wang P, Yu
J, Cao Y, Lu B and Qu L: Norepinephrine stimulation of
alpha1D-adrenoceptor promotes proliferation of pulmonary artery
smooth muscle cells via ERK-1/2 signaling. Int J Biochem Cell Biol.
88:100–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang P, Luo Q, Qiao H, Ding H, Cao Y, Yu
J, Liu R, Zhang Q, Zhu H and Qu L: The neuroprotective effects of
carvacrol on ethanol-induced hippocampal neurons impairment via the
antioxidative and antiapoptotic pathways. Oxid Med Cell Longev.
2017:40794252017.PubMed/NCBI
|
|
46
|
Li L, Cai L, Zheng L, Hu Y, Yuan W, Guo Z
and Li W: Gefitinib inhibits bleomycin-induced pulmonary fibrosis
via alleviating the oxidative damage in mice. Oxid Med Cell Longev.
2018:82496932018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen M, Guerrero AD, Huang L, Shabier Z,
Pan M, Tan TH and Wang J: Caspase-9-induced mitochondrial
disruption through cleavage of anti-apoptotic BCL-2 family members.
J Biol Chem. 282:33888–33895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Noble PW, Barkauskas CE and Jiang D:
Pulmonary fibrosis: Patterns and perpetrators. J Clin Invest.
122:2756–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moeller A, Ask K, Warburton D, Gauldie J
and Kolb M: The bleomycin animal model: A useful tool to
investigate treatment options for idiopathic pulmonary fibrosis?
Int J Biochem Cell Biol. 40:362–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gao C, Zhou Y, Li H, Cong X, Jiang Z, Wang
X, Cao R and Tian W: Antitumor effects of baicalin on ovarian
cancer cells through induction of cell apoptosis and inhibition of
cell migration in vitro. Mol Med Rep. 16:8729–8734. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li X, Zou K, Gou J, Du Q, Li D, He X and
Li Z: Effect of baicalin-copper on the induction of apoptosis in
human hepatoblastoma cancer HepG2 cells. Med Oncol. 32:722015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ren X, Zhang Z, Tian J, Wang H, Song G,
Guo Q, Tian J, Han Y, Liao Q, Liu G, et al: The downregulation of
c-Myc and its target gene hTERT is associated with the
antiproliferative effects of baicalin on HL-60 cells. Oncol Lett.
14:6833–6840. 2017.PubMed/NCBI
|
|
54
|
Mouratis MA and Aidinis V: Modeling
pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 17:355–361.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen C, Wang YY, Wang YX, Cheng MQ, Yin
JB, Zhang X and Hong ZP: Gentiopicroside ameliorates
bleomycin-induced pulmonary fibrosis in mice via inhibiting
inflammatory and fibrotic process. Biochem Biophys Res Commun.
495:2396–2403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ji YD, Luo ZL, Chen CX, Li B, Gong J, Wang
YX, Chen L, Yao SL and Shang Y: BML-111 suppresses TGF-β1-induced
lung fibroblast activation in vitro and decreases experimental
pulmonary fibrosis in vivo. Int J Mol Med. 42:3083–3092.
2018.PubMed/NCBI
|
|
57
|
Huang C, Wu X, Wang S, Wang W, Guo F, Chen
Y, Pan B, Zhang M and Fan X: Combination of Salvia miltiorrhiza and
ligustrazine attenuates bleomycin-induced pulmonary fibrosis in
rats via modulating TNF-α and TGF-β. Chin Med. 13:362018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jin M, Wang L, Wu Y, Zang BX and Tan L:
Protective effect of hydroxysafflor yellow A on bleomycin-induced
pulmonary inflammation and fibrosis in rats. Chin J Integr Med.
24:32–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Akter T, Silver RM and Bogatkevich GS:
Recent advances in understanding the pathogenesis of
scleroderma-interstitial lung disease. Curr Rheumatol Rep.
16:4112014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schoenfeld SR and Castelino FV:
Interstitial lung disease in scleroderma. Rheum Dis Clin North Am.
41:237–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lu Q, Guo Z, Xie W, Jin W, Zhu D, Chen S
and Ren T: The lncRNA H19 mediates pulmonary fibrosis by regulating
the miR-196a/COL1A1 axis. Inflammation. 41:896–903. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Honda H, Fujimoto M, Serada S, Urushima H,
Mishima T, Lee H, Ohkawara T, Kohno N, Hattori N, Yokoyama A and
Naka T: Leucine-rich α-2 glycoprotein promotes lung fibrosis by
modulating TGF-β signaling in fibroblasts. Physiol Rep.
5:e135562017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Todd NW, Luzina IG and Atamas SP:
Molecular and cellular mechanisms of pulmonary fibrosis.
Fibrogenesis Tissue Repair. 5:112012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Inghilleri S, Morbini P, Oggionni T, Barni
S and Fenoglio C: In situ assessment of oxidant and nitrogenic
stress in bleomycin pulmonary fibrosis. Histochem Cell Biol.
125:661–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kinnula VL and Myllärniemi M:
Oxidant-antioxidant imbalance as a potential contributor to the
progression of human pulmonary fibrosis. Antioxid Redox Signal.
10:727–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fubini B and Hubbard A: Reactive oxygen
species (ROS) and reactive nitrogen species (RNS) generation by
silica in inflammation and fibrosis. Free Radic Biol Med.
34:1507–1516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gao F, Kinnula VL, Myllärniemi M and Oury
TD: Extracellular superoxide dismutase in pulmonary fibrosis.
Antioxid Redox Signal. 10:343–354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lin CH, Shih CH, Lin YC, Yang YL and Chen
BC: MEKK1, JNK, and SMAD3 mediate CXCL12-stimulated connective
tissue growth factor expression in human lung fibroblasts. J Biomed
Sci. 25:192018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Arezzini B, Vecchio D, Signorini C,
Stringa B and Gardi C: F2-isoprostanes can mediate
bleomycin-induced lung fibrosis. Free Radic Biol Med. 115:1–9.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tian R, Zhu Y, Yao J, Meng X, Wang J, Xie
H and Wang R: NLRP3 participates in the regulation of EMT in
bleomycin-induced pulmonary fibrosis. Exp Cell Res. 357:328–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen X, Nishida H and Konishi T: Baicalin
promoted the repair of DNA single strand breakage caused by H2O2 in
cultured NIH3T3 fibroblasts. Biol Pharm Bull. 26:282–284. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jiang H, Lv P, Li J, Wang H, Zhou T, Liu Y
and Lin W: Baicalin inhibits colistin sulfate-induced apoptosis of
PC12 cells. Neural Regen Res. 8:2597–2604. 2013.PubMed/NCBI
|
|
73
|
Chen WC, Kuo TH, Tzeng YS and Tsai YC:
Baicalin induces apoptosis in SW620 human colorectal carcinoma
cells in vitro and suppresses tumor growth in vivo.
Molecules. 17:3844–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Q, Cao Y, Luo Q, Wang P, Shi P, Song
C, E M, Ren J, Fu B and Sun H: The transient receptor potential
vanilloid-3 regulates hypoxia-mediated pulmonary artery smooth
muscle cells proliferation via PI3K/AKT signaling pathway. Cell
Prolif. 51:e124362018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ding L, Huang Y, Dai M, Zhao X, Du Q, Dong
F, Wang L, Huo R, Zhang W, Xu X and Tong D: Transmissible
gastroenteritis virus infection induces cell cycle arrest at S and
G2/M phases via p53-dependent pathway. Virus Res. 178:241–251.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhong C, Han Y, Ma J, Zhang X, Sun M, Wang
Y, Chen J, Mi W, Xu X and Qiu J: Viral-mediated expression of c-Myc
and cyclin A2 induces cochlear progenitor cell proliferation.
Neurosci Lett. 591:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Joshaghani HR, Jafari SM, Aghaei M,
Panjehpour M and Abedi H: A3 adenosine receptor agonist induce G1
cell cycle arrest via Cyclin D and cyclin-dependent kinase 4
pathways in OVCAR-3 and Caov-4 cell lines. J Cancer Res Ther.
13:107–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ventura C, Núñez M, Gaido V, Pontillo C,
Miret N, Randi A and Cocca C: Hexachlorobenzene alters cell cycle
by regulating p27-cyclin E-CDK2 and c-Src-p27 protein complexes.
Toxicol Lett. 270:72–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lu Y, Azad N, Wang L, Iyer AK, Castranova
V, Jiang BH and Rojanasakul Y: Phosphatidylinositol-3-kinase/akt
regulates bleomycin-induced fibroblast proliferation and collagen
production. Am J Respir Cell Mol Biol. 42:432–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ma SY, Park WS, Lee DS, Choi G, Yim MJ,
Lee JM, Jung WK, Park SG, Seo SK, Park SJ, et al: Fucoxanthin
inhibits profibrotic protein expression in vitro and attenuates
bleomycin-induced lung fibrosis in vivo. Eur J Pharmacol.
811:199–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang YP, Li WB, Wang WL, Liu J, Song SX,
Bai LL, Hu YY, Yuan YD and Zhang M: siRNA against plasminogen
activator inhibitor-1 ameliorates bleomycin-induced lung fibrosis
in rats. Acta Pharmacol Sin. 33:897–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu X, Li J, Yang X, Bai X, Shi J, Gao J,
Li Y, Han S, Zhang Y, Han F, et al: miR-155 inhibits the formation
of hypertrophic scar fibroblasts by targeting HIF-1α via PI3K/AKT
pathway. J Mol Histol. 49:377–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Phosri S, Arieyawong A, Bunrukchai K,
Parichatikanond W, Nishimura A, Nishida M and Mangmool S:
Stimulation of adenosine A2B receptor inhibits
endothelin-1-induced cardiac fibroblast proliferation and α-smooth
muscle actin synthesis through the cAMP/Epac/PI3K/Akt-signaling
pathway. Front Pharmacol. 8:4282017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yuan J, Li P, Pan H, Li Y, Xu Q, Xu T, Ji
X, Liu Y, Yao W, Han L and Ni C: miR-542-5p attenuates fibroblast
activation by targeting integrin α6 in silica-induced pulmonary
fibrosis. Int J Mol Sci. 19:E37172018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
He Z, Deng Y, Li W, Chen Y, Xing S, Zhao
X, Ding J, Gao Y and Wang X: Overexpression of PTEN suppresses
lipopolysaccharide-induced lung fibroblast proliferation,
differentiation and collagen secretion through inhibition of the
PI3-K-Akt-GSK3beta pathway. Cell Biosci. 4:22014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hurley RL, Anderson KA, Franzone JM, Kemp
BE, Means AR and Witters LA: The Ca2+/calmodulin-dependent protein
kinase kinases are AMP-activated protein kinase kinases. J Biol
Chem. 280:29060–29066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Woods A, Dickerson K, Heath R, Hong SP,
Momcilovic M, Johnstone SR, Carlson M and Carling D:
Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream
of AMP-activated protein kinase in mammalian cells. Cell Metab.
2:21–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Imoto K, Okada M and Yamawaki H:
Characterization of fibroblasts from hypertrophied right ventricle
of pulmonary hypertensive rats. Pflugers Arch. 470:1405–1417. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sazonova OV, Blishchenko EY, Tolmazova AG,
Khachin DP, Leontiev KV, Karelin AA and Ivanov VT: Stimulation of
fibroblast proliferation by neokyotorphin requires Ca influx and
activation of PKA, CaMK II and MAPK/ERK. FEBS J. 274:474–484. 2007.
View Article : Google Scholar : PubMed/NCBI
|