Introduction
Breast cancer (BC), one of the most common
malignancies in women worldwide, remains a major challenge despite
improved treatments (1). Based on
previous reports, it is estimated that 250,000 new cases of BC
occurred in women in 2017 and ~40,000 women will die from breast
cancer worldwide each year (2,3). The
treatment of BC primarily involves surgical resection,
chemotherapy, radiotherapy, targeted therapy and hormone therapy.
Nevertheless, patients with advanced BC have highly metastatic and
invasive tumors that are difficult to treat with targeted and
hormone therapy (4). Therefore,
novel treatments could reduce the mortality rate of patients with
BC (1). A previous study noted
that immunotherapy has become a novel and potentially effective
treatment option for solid malignancies, including BC (5). Cellular immune responses and tumor
immune escape are two key elements of cancer immunoediting
(6). Recruitment of specific
immunosuppressive leukocyte populations, including regulatory T
(Treg) cells, or inducing the tumor cells to produce
immunosuppressive cytokines, including transforming growth factor
(TGF)-β, which enhances Treg cell proliferation, contribute to
immunosuppression in the tumor microenvironment of patients with BC
who did not respond to the immunotherapy treatment (5). These findings suggest that effective
tumor immunity can be stimulated in tumor tissues by local or
systemic immunosuppression.
Treg cells are an immunosuppressive subset of
CD4+ T cells that constitutively express CD25 on their
cell surface. The lineage-defining transcription factor, forkhead
box P3 (Foxp3), is the most reliable marker of Treg cells and is
responsible for the functional maintenance and differentiation of
these regulatory cells (7).
Therefore, Treg cells can be defined as
CD4+CD25+Foxp3+ cells.
CD4+CD25+Foxp3+ Treg cells play
critical roles in the aggressiveness of disease and cancer by
modulating immune responses (8). A
number of studies have reported that alterations in the number and
function of Treg cells in peripheral blood, as well as alterations
to the particular tumor microenvironment, are closely related to
the development and progression of various disease settings and
different types of cancer (9,10).
Evidence that tumor infiltrating lymphocytes and their subsets are
parallel to BC progression suggests that these potential
interactions with immune cells in the tumor microenvironment are
important (11). An increase in
the number of Treg cells in a breast tumor biopsy is associated
with an invasive phenotype and poor overall and relapse-free
survival (12). However, the
mechanism by which CD4+CD25+Foxp3+
Treg cells are regulated during the control of autoimmunity and
maintenance of immunological tolerance in patients with BC or
healthy individuals has not been fully clarified.
In some cases, selective ablation of Treg cells in
tumors has been achieved, leading to a strong antitumor response
and reduced autoimmune toxicity, suggesting that directly targeting
the function of Treg cells in tumors is an effective therapeutic
strategy (13). Multiple molecules
are involved in Treg cell-mediated immunosuppressive mechanisms,
including interleukin (IL)-2 and TGF-β (14). IL-2 is a key cytokine involved in
the control of the survival, homeostasis and function of
CD4+CD25+Foxp3+ Treg cells
(15). In addition, TGF-β1
activates STAT5, which binds to the promoter of Foxp3 to promote
the differentiation of Treg cells via IL-2, suggesting the
involvement of TGF-β1 in the development of Treg cells and the
expression of the transcription factor Foxp3 (16). Although
CD4+CD25+Foxp3+ Treg cells are
activated by IL-2 and TGF-β1 in vitro and in vivo
(17,18), the potential of peripheral Treg
cells from healthy individuals and patients with BC as a source of
therapeutic cell products generated in vitro has not been
clarified.
There is evidence that certain malignant phenotypes
of cells release chemokines, including CCL2, CCL5 and CCL22, to
attract and activate Treg cells (19,20).
CCL11 was reported to limit angiogenesis and cause necrosis in a
murine model of osteosarcoma by inducing and attracting eosinophils
and reducing the tumor formation ability (21). Increased tissue and serum levels of
CCL11 mediate the inhibition of dendritic cell maturation and
differentiation in the tumor microenvironment, skewing the
microenvironment toward a T helper 2 (Th2) immune response and
inhibiting CD8+ T cell-mediated tumor cell lysis
(22). Additionally, the loss of
CCL11 reduced tumor outgrowth (22). Therefore, the present study
hypothesized that CCL11 may promote tumor growth by increasing
angiogenesis or inhibiting dendritic cell maturation and the role
of CCL11 in regulating tumor growth may vary in different types of
cancer. The biological effect of CCL11 is modulated by CC chemokine
receptor 3 (CCR3), which is downregulated in Th2 and Treg cells of
patients with septic shock (23).
However, the potential of CCL11/CCR3 to regulate Treg cells during
tumor immunity remains unknown. In the present study, the serum and
expression levels of CCL11 within tumors of patients with BC were
investigated. Furthermore, the present study evaluated whether
CCL11 could regulate non-tumor or BC
CD4+CD25+Foxp3+ Treg cells and
also aimed to identify the underlying molecular mechanisms.
Materials and methods
Patients and specimens
A cohort of 100 women with BC, aged between 18 and
79 years (median, 58 years), who had received mastectomies at the
Huangpu Branch, Shanghai Ninth People's Hospital between January
2015 and June 2018 was included in the present study. The healthy
group consisted of 50 healthy women, aged between 16 and 83 years
(median, 55 years), who had undergone a mammary gland examination
prior to sample collection at the Huangpu Branch, Shanghai Ninth
People's Hospital between May 2015 and March 2018. Fresh peripheral
blood and serum samples (10 ml) were collected from patients with
BC and healthy individuals for ELISA and flow cytometry analysis,
respectively. Datasets of BC, including 1,041 tumors and 112
adjacent normal tissues, were downloaded from The Cancer Genome
Atlas (TCGA) data portal (http://tcga-data.nci.nih.gov). The present study was
approved by the Ethics Committee of the Huangpu Branch, Shanghai
Ninth People's Hospital. Written informed consent was obtained from
all participants.
Cell treatment
The peripheral blood mononuclear cells (PBMCs) were
isolated from peripheral blood samples collected from each
participant in tubes with K2 ethylenediaminetetraacetic acid (EDTA)
as anticoagulant as previously described (24). CD4+ T cells were
isolated from the PBMCs of patients with BC using the MagCellect
Human CD4+ T Cell Isolation kit (cat. no. MAGH102;
R&D Systems, Inc.), according to the manufacturer's protocol.
The 1×105 CD4+ T cells were treated with
different concentrations of anti-CCL11 neutralizing antibodies
(0.1, 0.2, 0.5, 1 and 2 µg/ml; cat. no. ab9955; Abcam) or isotype
control antibodies (1:1,000 dilution; cat. no. ab171870; Abcam) for
48 h at 4°C. CD4+ T cells were isolated from the PBMCs
of healthy individuals and 1×105 CD4+ T cells
were treated with different concentrations of recombinant human
CCL11 protein (5, 10, 20, 50 and 100 ng/ml; cat. no. ab243753;
Abcam; rhCCL11) alone or 50 ng/ml rhCCL11 in the presence or
absence of 1 µM STAT5 inhibitor CAS (cat. no. 285986-31-4; EMD
Millipore) or vehicle (DMSO) was used as a negative control for 48
h at 4°C.
In vivo xenograft model
Male BALB/c nude mice (age, 6–8 weeks; weight, 18–20
g; n=24) purchased from Vital River Laboratory were housed in a
specific pathogen-free environment, with a relative humidity of
60–70%, at a controlled temperature of 25–27°C with 12-h light/dark
cycles and were fed a standard diet and water ad libitum.
The mice were intravenously injected with 5×106 4T1
breast cancer cells (ATCC) or phosphate buffer saline (PBS;
Meilunbio) as control and then intraperitoneally injected with 100
µg anti-CCL11 neutralizing antibodies every 48 h (n=6 per group)
(25). Mice were observed over 21
days for tumor formation. At 21 days post-injection, the PBMCs were
isolated from peripheral blood samples collected from mice as
previously described (26) and
CD4+ T cells were isolated from the PBMCs of the mice
using the MagCellect Mouse CD4+ T Cell Isolation kit
(cat. no. MAGM205; R&D Systems, Inc.), according to the
manufacturer's protocol. Mice were monitored until they had reached
criteria for predetermined loss of wellness endpoint. These
endpoints were defined as tumor burden where any tumor had a
diameter of 20 mm, impaired mobility, tumor ulceration, and/or
respiratory distress (27). Mice
were anesthetized with 1% pentobarbital (45 mg/kg) via
intraperitoneal injection and then sacrificed by cervical
dislocation. Tissues were fixed in neutral 10% formalin for 10 min
at 25°C, dehydrated and embedded in paraffin wax for sectioning
(slice thickness of 4 µm) by conventional methods. Sections were
stained by routine hematoxylin-eosin processing (hematoxylin for 5
min followed by counterstaining with the eosin for 2 min at 25°C),
observed under a BX53 light microscope (magnification, ×100) and
photographed with a DP72 camera (Olympus Corporation). The present
study was approved by the Ethics Committee for the Care and Use of
Laboratory Animals at the Huangpu Branch, Shanghai Ninth People's
Hospital.
Evaluation of
CD4+CD25+Foxp3+ T cells
CD4+ T cells isolated from PBMCs were
stained with either isotype control antibody (cat. no. 553452 for
anti-Foxp3 and anti-CD25 antibody; cat. no. 555909 for anti-CD4
antibody, all from BD Biosciences; Becton, Dickinson and Company)
or anti-human CD4, anti-human CD25 and anti-Human Foxp3 antibodies,
according to the protocol of the Anti-Human Foxp3 Staining kit
(cat. no. 560133; BD Biosciences; Becton, Dickinson and Company).
The proportion of CD4+CD25+Foxp3+
Treg cells was analyzed using a flow cytometer (BD Accuri C6,
software version 1.0.264.21; BD Biosciences; Becton, Dickinson and
Company).
Western blotting
Total protein was extracted from CD4+ T
cells using RIPA lysis buffer (Applygen Technologies Inc.). The
protein concentration was measured using a bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.), and
absorbance was measured using a microplate reader (SM600 Labsystem;
Shanghai Utrao Medical Instrument Co., Ltd.). Equal amounts of
protein (25 µg) were separated by 12% SDS-PAGE and transferred to
nitrocellulose membranes. The membranes were incubated overnight at
4°C with antibodies targeted against: CCL11 (cat. no. ab133604;
1:2,000; Abcam), CCR3 (cat. no. ab32512; 1:500; Abcam), Foxp3 (cat.
no. ab20034; 1:500; Abcam), STAT5 (cat. no. ab230670; 1:500;
Abcam), phosphorylated (p)-STAT5 antibody (cat. no. 32364; 1:1,000;
Abcam) and GAPDH (cat. no. ab9485; 1:2,000; Abcam). Following the
primary incubation, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. nos. A0208 and
A0216; 1:1,000; Beyotime Institute of Biotechnology) for 1 h at
37°C. Protein bands were visualized using an ECL detection kit (GE
Healthcare), according to the manufacturer's protocol. Signals were
quantified by densitometry (Quantity One 4.6.3 software; Bio-Rad
Laboratories, Inc.).
ELISA
CCL11 content of the serum of patients with BC and
healthy individuals was measured by ELISA, according to the
manufacturer's protocol. The Human Eotaxin ELISA kit (cat. no.
ab185985; Abcam) was used to measure CCL11 levels, according to the
manufacturer's protocol. The IL-2 and TGF-β1 content of the media
of CD4+ T cells from the PBMCs of patients with BC or
healthy individuals was measured using the Human IL-2 Quantikine
ELISA kit (cat. no. S2050; R&D Systems) and the Human TGF-β1
Quantikine ELISA kit (cat. no. SB100B; R&D Systems),
respectively. The CCL11, IL-2 and TGF-β1 content of the serum of
tumor-bearing mice was measured using the Mouse CCL11/Eotaxin
Quantikine ELISA kit (cat. no. MME00; R&D Systems), the Mouse
IL-2 Quantikine ELISA kit (cat. no. SM2000; R&D Systems) and
the Mouse/Rat/Porcine/Canine TGF-β1 Quantikine ELISA kit (cat. no.
SMB100B; R&D Systems), respectively.
Statistical analysis
Data are presented as the mean ± SD of triplicate
experiments. All statistical analyses were performed using SPSS
software (version 19.0; IBM Corp.). Data were analyzed using a
unpaired two-tailed Student's t-test or ANOVA followed by Tukey's
post hoc test. Correlation of CCL11 expression with
clinicopathological features of BC in TCGA database and hospital
cohort was identified by χ2 test. Pearson's correlation
analysis was used to evaluate the relationship between CCL11 level
and proportion of CD4+CD25+Foxp3+
Treg cells in BC patients. P<0.05 was considered to indicate a
statistically significant difference.
Results
Upregulation of CCL11 correlates with
the proportion of CD4+CD25+Foxp3+
Treg cells in tumor-associated CD4+ T cells
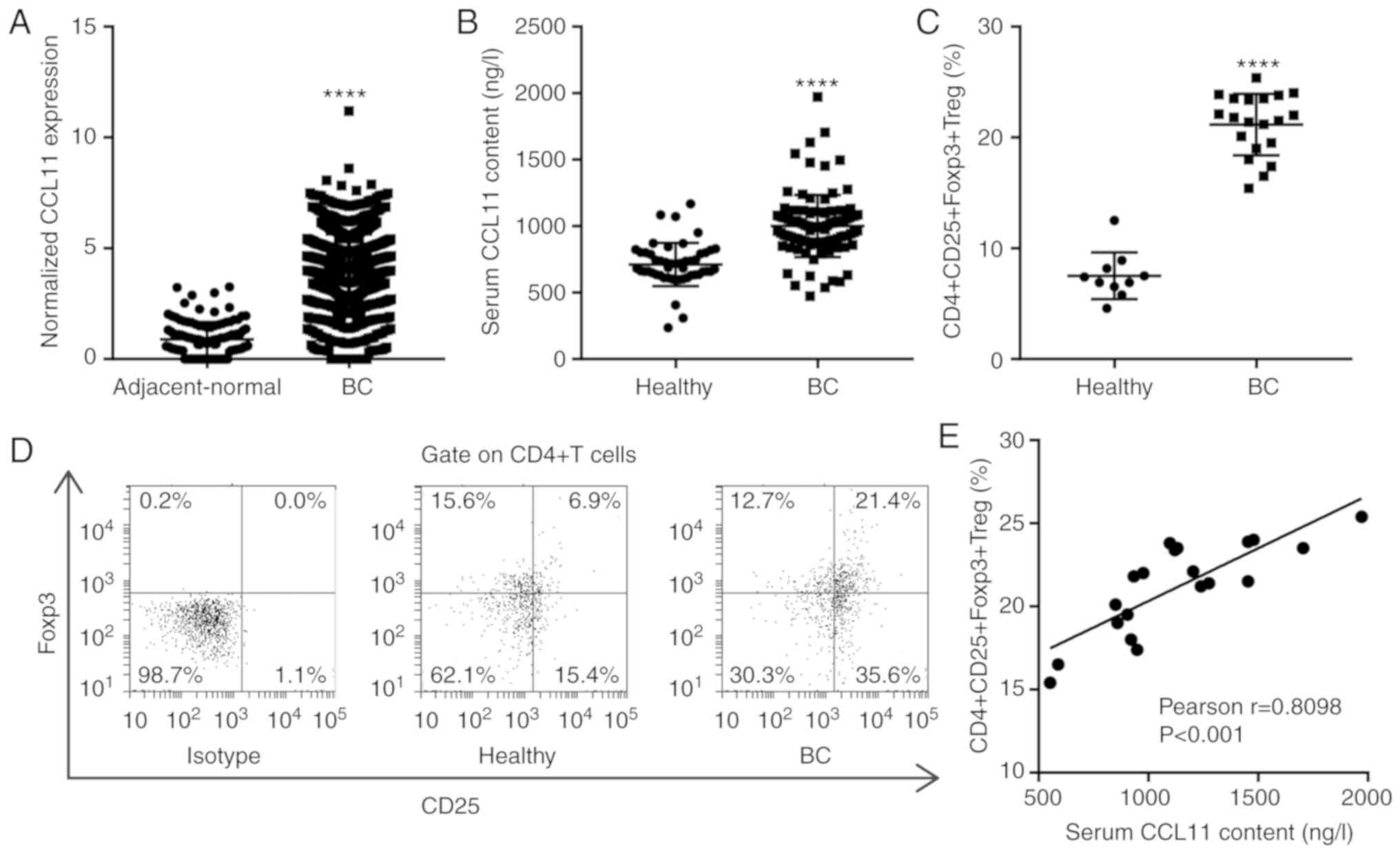
The TCGA BC database revealed that CCL11 was
significantly upregulated in BC tissues compared with the
adjacent-normal breast tissues (Fig.
1A). The association between CCL11 and the clinicopathological
features of BC in the TCGA database suggested that CCL11 expression
was associated with tumor stage, histologic grade and estrogen
receptor (ER) status, but not with the other clinicopathological
features (Table I). To further
define CCL11 expression patterns in BC, the serum CCL11 levels in
healthy individuals and patients with BC were analyzed. The serum
CCL11 levels were significantly increased in patients with BC
(n=100) compared with healthy individuals (n=50; Fig. 1B). The association of serum CCL11
levels with the clinicopathological features of BC in the hospital
cohort suggested that serum CCL11 levels were associated with tumor
diameter, tumor stage and ER status but not with the other
clinicopathological features (Table
II). Treg cells are a T cell subset with regulatory functions
that can inhibit tumor immunity. Foxp3 is the most specific marker
of CD4+CD25+ Treg cells and is critical for
the regulation of Treg cells and the maintenance of immune
tolerance in patients with cancer (7). To further study the regulation of
Treg cells in BC, the proportion of
CD4+CD25+Foxp3+ Treg cells among
the CD4+ T cells was measured by flow cytometry. The
percentage of CD4+CD25+Foxp3+ Treg
cells was significantly increased in patients with BC (n=20)
compared with healthy individuals (n=10; Fig. 1C and D). Furthermore, the serum
CCL11 levels were positively correlated with the proportion of
CD4+CD25+Foxp3+ Treg cells in
patients with BC, by Pearson's correlation analysis (Fig. 1E). The results suggested that the
increase in CCL11 in patients with BC contributed to an increase in
the proportion of peripheral
CD4+CD25+Foxp3+ Treg cells.
 | Table I.Correlation of C-C motif chemokine
ligand 11 expression with clinicopathological features of breast
cancer in The Cancer Genome Atlas database. |
Table I.
Correlation of C-C motif chemokine
ligand 11 expression with clinicopathological features of breast
cancer in The Cancer Genome Atlas database.
|
| Serum CCL11
levels |
|
|---|
|
|
|
|
|---|
| Clinicopathological
feature | High (n=521) | Low (n=520) | P-value |
|---|
| Age (years) |
|
| 0.1074 |
|
<58 | 215 | 179 |
|
|
≥58 | 207 | 216 |
|
|
Missing: 224 |
|
|
|
| Tumor stage |
|
| 0.0030 |
| I | 123 | 87 |
|
| II | 239 | 229 |
|
|
III | 30 | 49 |
|
| IV | 10 | 20 |
|
|
Missing: 254 |
|
|
|
| Histologic
grade |
|
| 0.0210 |
| I | 96 | 71 |
|
| II | 274 | 292 |
|
|
III | 111 | 109 |
|
| IV | 3 | 12 |
|
|
Missing: 73 |
|
|
|
| Histology |
|
| 0.9002 |
|
Ductal | 176 | 196 |
|
|
Lobular | 19 | 17 |
|
|
Mix | 8 | 7 |
|
|
Other | 4 | 4 |
|
|
Missing: 610 |
|
|
|
| ER status |
|
| 0.0091 |
|
Positive | 309 | 291 |
|
|
Indeterminate | 15 | 2 |
|
|
Negative | 99 | 80 |
|
|
Missing: 245 |
|
|
|
| PR status |
|
| 0.5448 |
|
Positive | 274 | 247 |
|
|
Indeterminate | 1 | 3 |
|
|
Negative | 133 | 122 |
|
|
Missing: 261 |
|
|
|
| HER2 status |
|
| 0.3407 |
|
Positive | 65 | 49 |
|
|
Negative | 329 | 322 |
|
|
Equivocal | 4 | 6 |
|
|
Missing: 266 |
|
|
|
 | Table II.Correlation of serum C-C motif
chemokine ligand 11 levels with clinicopathological features of
breast cancer in the hospital cohort. |
Table II.
Correlation of serum C-C motif
chemokine ligand 11 levels with clinicopathological features of
breast cancer in the hospital cohort.
|
| CCL11
expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
feature | High (n=50) | Low (n=50) | P-value |
|---|
| Age (years) |
|
| 0.6853 |
|
<58 | 30 | 28 |
|
|
≥58 | 20 | 22 |
|
| Tumor diameter
(cm) |
|
| 0.0394 |
|
<2 | 26 | 36 |
|
| ≥2 | 24 | 14 |
|
| Tumor stage |
|
| 0.0302 |
| I | 9 | 20 |
|
| II | 30 | 27 |
|
|
III | 8 | 2 |
|
| IV | 3 | 1 |
|
| Histologic
grade |
|
| 0.5860 |
| I | 7 | 11 |
|
| II | 25 | 26 |
|
|
III | 14 | 11 |
|
| IV | 4 | 2 |
|
| Histology |
|
| 0.6701 |
|
Ductal | 40 | 43 |
|
|
Lobular | 8 | 5 |
|
|
Other | 2 | 2 |
|
| ER status |
|
| 0.0211 |
|
Positive | 38 | 27 |
|
|
Negative | 12 | 23 |
|
| PR status |
|
| 0.8399 |
|
Positive | 29 | 28 |
|
|
Negative | 21 | 22 |
|
| HER2 status |
|
| 0.8595 |
|
Positive | 25 | 20 |
|
|
Negative | 25 | 30 |
|
CCL11 blockade decreases the
proportion of CD4+CD25+Foxp3+ Treg
cells and the level of STAT5 activation in tumor-associated
CD4+ T cells
To assess the role of CCL11 in regulating the
proportion of Treg cells, CD4+ T cells were isolated
from patients with BC and treated with anti-CCL11 neutralizing
antibody. The anti-CCL11 neutralizing antibody significantly
reduced the proportion of
CD4+CD25+Foxp3+ Treg cells in a
dose-dependent manner, compared with the control group (Fig. 2A). Moreover, the expression of
CCL11, CCR3 and Foxp3 in CD4+ T cells was also measured.
The anti-CCL11 neutralizing antibody decreased the expression of
CCL11, CCR3 and Foxp3 in a dose-dependent manner, compared with the
control group (Fig. 2B).
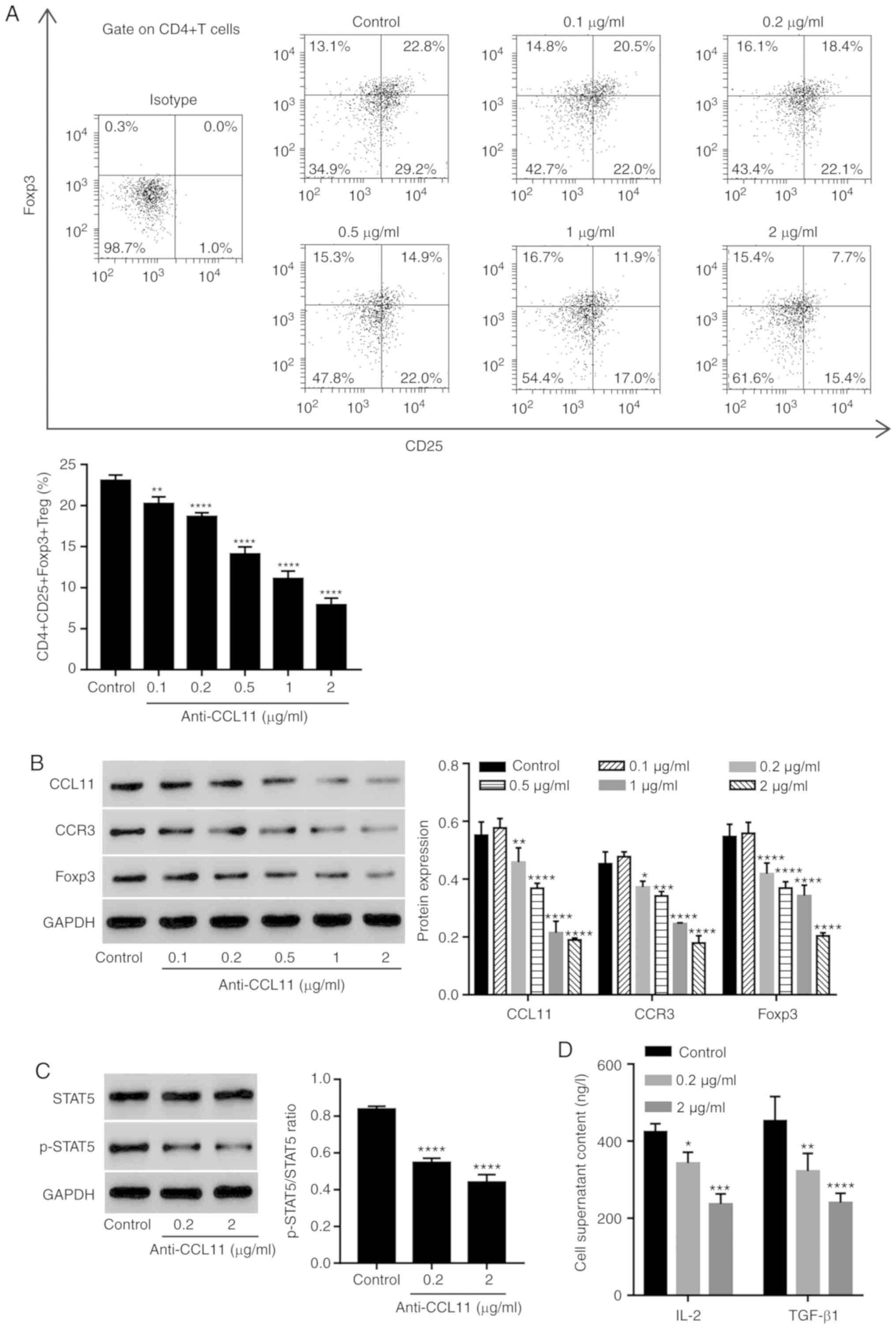 | Figure 2.CCL11 blockade reduces the proportion
of CD4+CD25+Foxp3+ Treg cells and
the level of STAT5 activation in tumor-associated CD4+ T
cells. CD4+ T cells collected from the peripheral blood
mononuclear cells of patients with BC were incubated with
anti-CCL11 neutralizing antibodies (0.1, 0.2, 0.5, 1 and 2 µg/ml;
n=3 per group). (A) Proportion of
CD4+CD25+Foxp3+ Treg cells,
measured by flow cytometry. (B) CCL11, CCR3 and Foxp3 expression,
measured by western blotting. CD4+ T cells were
incubated with anti-CCL11 neutralizing antibody (0.2 and 2 µg/ml),
and the expression levels of (C) p-STAT5 and STAT5, as well as (D)
the serum content of IL-2 and TGF-β1, were measured by western
blotting and ELISA, respectively (n=3 per group). *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001 vs. the control
group. CCL11, C-C motif chemokine ligand 11; Foxp3, forkhead box
P3; Treg, T regulatory; BC, breast cancer; CCR3, C-C motif
chemokine receptor 3; p, phosphorylated; IL, interleukin; TGF,
transforming growth factor. |
Since STAT5 plays a role in the maintenance of
normal immune function and homeostasis (28), especially in the function and
development of Treg cells, and induces Foxp3 transcription by
binding to multiple regulatory regions in the Foxp3 gene (29), the effect of CCL11 on the STAT5
signaling pathway was assessed. The anti-CCL11 neutralizing
antibody significantly inhibited the level of phosphorylated-STAT5,
but not STAT5 compared with the control group, suggesting the
inactivation of STAT5 signaling (Fig.
2C). To further investigate the regulation of CCL11 in the
STAT5 signaling pathway, the expression of IL-2 and TGF-β1, which
are important for IL-2-driven STAT5 phosphorylation in Treg cells,
was also measured (16,30). The anti-CCL11 neutralizing antibody
significantly inhibited the level of IL-2 and TGF-β1 protein
expression in the media of CD4+ T cells compared with
the control group (Fig. 2D). The
data suggested that CCL11 increased the proportion of BC-associated
CD4+CD25+Foxp3+ Treg cells and
therefore may have a role during BC immunity.
CCL11 blockade reduces the proportion
of CD4+CD25+Foxp3+ Treg cells and
the serum levels of IL-2 and TGF-β1 in tumor-bearing mice
The aforementioned in vitro studies indicated
that CCL11 increases the proportion of
CD4+CD25+Foxp3+ Treg cells and the
release of IL-2 and TGF-β1 by CD4+ T cells. Thus,
whether CCL11 affected the proportion of
CD4+CD25+Foxp3+ Treg cells and the
release of IL-2 and TGF-β1 in vivo was investigated. Mice
were simultaneously treated with 4T1 breast cancer cells and the
anti-CCL11 neutralizing antibody or its isotype antibody, as
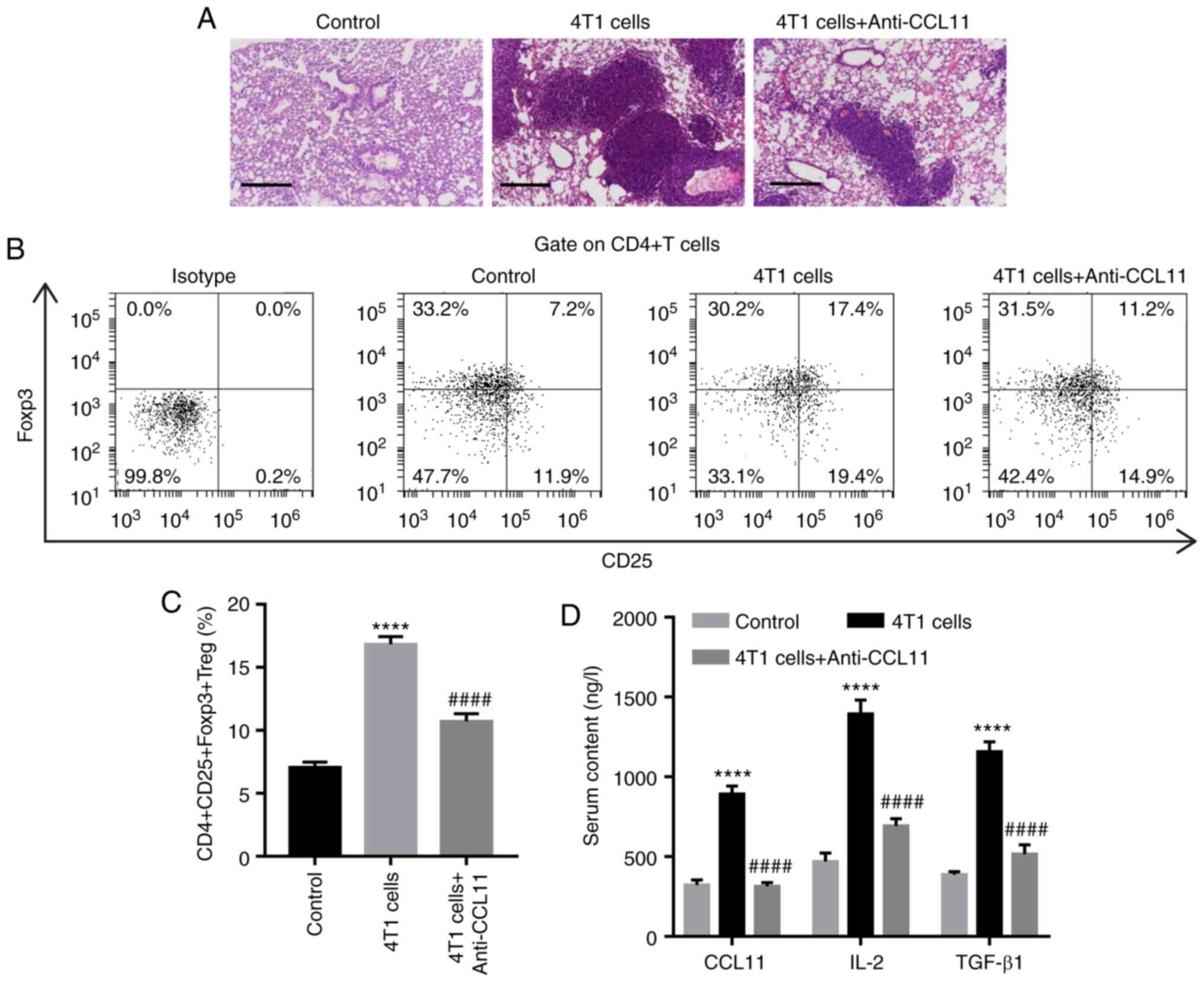
indicated. At 28 days post-treatment, lung tissues displayed
increased microscopic lesions, the proportion of
CD4+CD25+Foxp3+ Treg cells and
serum levels of IL-2 and TGF-β1 compared with the control group
(Fig. 3). However, CCL11 blockade
decreased microscopic lesions, the proportion of
CD4+CD25+Foxp3+ Treg cells and the
serum levels of IL-2 and TGF-β1 in mice, compared with mice treated
with 4T1 cells alone (Fig. 3).
Taken together, these results suggested that CCL11 also increased
the proportion of BC-associated
CD4+CD25+Foxp3+ Treg cells in
vivo.
CCL11 upregulation increases the
proportion of CD4+CD25+Foxp3+ Treg
cells in non-tumor-associated CD4+ T cells via the STAT5
signaling pathway
To assess whether the effects of CCL11 on the
proportion of CD4+CD25+Foxp3+ Treg
cells were also present in non-tumor-associated CD4+ T
cells, CD4+ T cells isolated from healthy individuals
were treated with or without rhCCL11, at the indicated doses.
RhCCL11 treatment significantly increased the proportion of
CD4+CD25+Foxp3+ Treg cells and the
protein expression levels of CCL11, CCR3 and Foxp3 in
CD4+ T cells in a dose-dependent manner, compared with
the control group (Fig. 4A and
B).
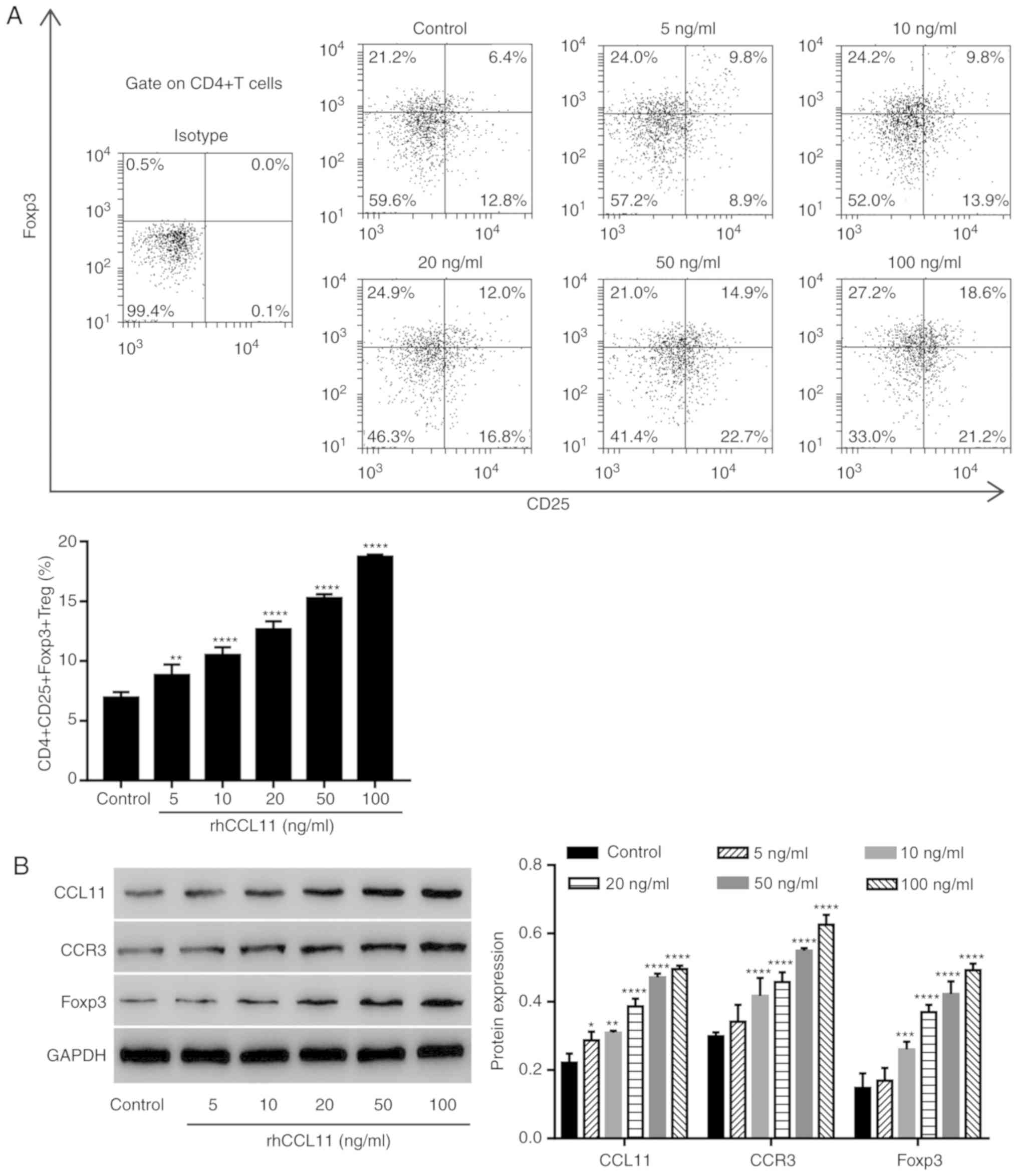 | Figure 4.CCL11 upregulation increases the
proportion of CD4+CD25+Foxp3+ Treg
cells in non-tumor associated CD4+ T cells.
CD4+ T cells collected from peripheral blood mononuclear
cells of healthy individuals were treated with or without rhCCL11
(5, 10, 20, 50 and 100 ng/ml; n=3 per group). (A) Proportion of
CD4+CD25+Foxp3+ Treg cells,
measured by flow cytometry. (B) CCL11, CCR3 and Foxp3 protein
expression levels, measured by western blotting. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001 vs. the control
group. CCL11, C-C motif chemokine ligand 11; Foxp3, forkhead box
P3; Treg, T regulatory; rhCCL11, recombinant human CCL11; CCR3, C-C
motif chemokine receptor 3. |
To further assess whether the role of CCL11 in
regulating the proportion of
CD4+CD25+Foxp3+ Treg cells was
dependent on the STAT5 signaling pathway, CD4+ T cells
were treated with rhCCL11 and the STAT5 inhibitor CAS. A vehicle
was used as a negative control. RhCCL11 treatment significantly
increased the proportion of
CD4+CD25+Foxp3+ Treg cells and the
levels of Foxp3 expression, STAT5 activation and release of IL-2
and TGF-β1 by CD4+ T cells, compared with the control
group (Fig. 5). However, the STAT5
inhibitor CAS significantly limited the RhCCL11-induced effects
(Fig. 5). These data suggested
that CCL11 increased the proportion of
CD4+CD25+Foxp3+ Treg cells via the
STAT5 signaling pathway.
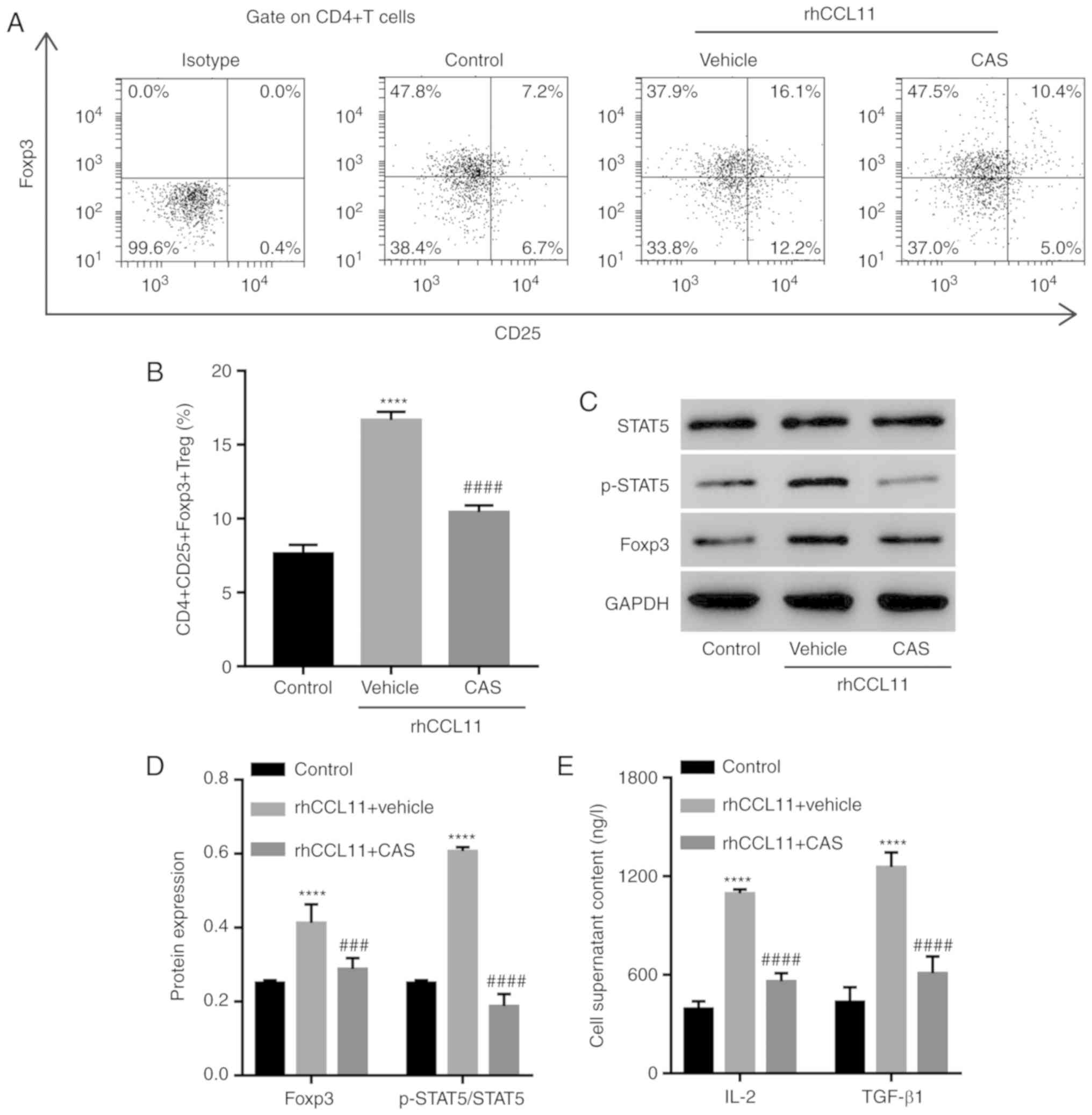 | Figure 5.Involvement of the STAT5 signaling
pathway in the function of CCL11. CD4+ T cells collected
from peripheral blood mononuclear cells of healthy individuals were
treated with rhCCL11 (50 ng/ml) in the presence or absence of the
STAT5 inhibitor CAS (1 µM) or vehicle (n=3 per group). (A)
Representative plots and (B) quantification of the proportion of
CD4+CD25+Foxp3+ Treg cells,
measured by flow cytometry. (C) Western blots and (D)
quantification of p-STAT5, STAT5 and Foxp3 protein expression
levels, measured by western blotting. (E) IL-2 and TGF-β1 levels in
the cell media, measured by ELISA. ****P<0.0001 vs. the control
group; ###P<0.001 and ####P<0.0001 vs.
the rhCCL11 + vehicle group. CCL11, C-C motif chemokine ligand 11;
rhCCL11, recombinant human CCL11; Foxp3, forkhead box P3; Treg, T
regulatory; p, phosphorylated; IL, interleukin; TGF, transforming
growth factor. |
Discussion
CD4+CD25+Foxp3+
Treg cells in patients with solid tumors have been reported to be
attracted to and activated by chemokines (19,20,31).
Increased infiltration of
CD4+CD25+Foxp3+ Treg cells was
observed in BC tissues and was associated with high histologic
grade, negative estrogen receptor and progesterone receptor status,
and human epidermal growth factor receptor type 2 overexpression,
as well as decreased overall and progression-free survival
(32). However, patients with
triple-negative breast cancer and a high number of intratumoural
CD4+CD25+Foxp3+ Treg cells
displayed a higher tumor grade, lymph node status and improved
prognosis (33). These data
indicate that the presence of tumor-infiltrating lymphocytes is not
enough to reliably predict their effects. Therefore, the present
study focused on the peripheral proportion of
CD4+CD25+Foxp3+ Treg cells and the
regulatory mechanism of these Treg cells in patients with BC and
healthy individuals. Patients with BC displayed altered serum CCL11
levels and proportions of
CD4+CD25+Foxp3+ Treg cells in
PBMCs compared with healthy individuals. A positive correlation
between CCL11 expression and the proportion of
CD4+CD25+Foxp3+ Treg cells was
identified, which is required for the pathogenesis and development
of BC (31). The present study
found that CD4+CD25+Foxp3+ Treg
cells were induced by CCL11 and higher CCL11 expression and
proportions of CD4+CD25+Foxp3+
Treg cells were associated with an increased probability of BC
occurring. Similarly, CCL11 levels are increased in the serum and
tissues of patients with melanoma and are involved in tumorigenesis
by regulating the function of CD8+ T cells and Th2 cells
(22). However, in gastric cancer,
the frequency of CD4+CD25+Foxp3+
Treg cells was significantly increased in tumor-infiltrating
lymphocytes but not in tumor-associated peripheral blood
lymphocytes compared with healthy individuals (7).
CCL11 signaling is involved in the regulation of the
tumor microenvironment and cancer progression. The CCL11/CCR3
signaling pathway potently stimulates cell proliferation, invasion
and migration in ovarian carcinoma (34), prostate cancer (35) and lymphoma (36), and regulates necrosis and
angiogenesis via the induction and attraction of eosinophils in
murine osteosarcoma (21). These
data suggest that the CCL11/CCR3 signaling pathway broadly
contributes to pathological and physiological processes of cancer.
In the present study, blockade of CCL11 by administration of an
anti-CCL11 neutralizing antibody, in tumor-associated
CD4+ T cells, resulted in decreased expression levels of
CCR3 and Foxp3 and the proportion of
CD4+CD25+Foxp3+ Treg cells in a
dose-dependent manner. Furthermore, a decreased proportion of
CD4+CD25+Foxp3+ Treg cells was
also observed in tumor-bearing mice in the presence of the
anti-CCL11 neutralizing antibody. Foxp3 is crucial for
immunosuppression by Treg cells. Foxp3 undergoes methylation or
ubiquitination results in aberrant perinuclear accumulation and
disrupted regulatory function in Treg cells (37,38).
Moreover, upregulation of CCL11 in non-tumor-associated
CD4+ T cells by administration of rhCCL11 resulted in
increased expression levels of CCR3 and Foxp3 and the proportion of
CD4+CD25+Foxp3+ Treg cells in a
dose-dependent manner. These data suggested a role for the
CCL11/CCR3 signaling pathway in the regulation of
CD4+CD25+Foxp3+ Treg cells among
tumor and non-tumor-associated CD4+ T cells. Similarly,
hepatocellular carcinoma cells recruit
CD4+CD25+Foxp3+ Treg cells to
promote angiogenesis under hypoxic conditions and regulate Foxp3
expression by CCL28 upregulation (39).
The present study also further suggested that STAT5
activation is involved in the control of Foxp3, TGF-β1 and IL-2
expression levels and the increased proportion of
CD4+CD25+Foxp3+ Treg cells in
healthy individuals in the presence of rhCCL11. Previous studies
have reported that TGF-β1 and IL-2 regulate Foxp3 expression in
human CD4+CD25+ Treg cells via the STAT5
signaling pathway, and are involved in the generation, function and
stabilization of peripheral Treg cells (40–42).
Moreover, TGF-β1 activates the transcription factor STAT5 via IL-2
(16). The present study suggested
that CCL11 may regulate the proportion of
CD4+CD25+Foxp3+ Treg cells via a
positive feedback loop involving the STAT5 signaling pathway, IL-2
and TGF-β1. However, CCL11 inhibits granulocyte-macrophage colony
stimulating factor-mediated STAT5 activation in a range of
hematopoietic cells (43).
Therefore, further investigation is required to clarify the
alternative mechanism by which the TGF-β1/IL-2/STAT5 signaling
pathway regulates the CCL11-mediated effects on the proportion of
CD4+CD25+Foxp3+ Treg cells in
non-tumor or BC-associated CD4+ T cells.
To the best of our knowledge, the present study is
the first to report that CCL11 increased the proportion of
CD4+CD25+Foxp3+ Treg cells and the
production of IL-2 and TGF-β1 via the STAT5 signaling pathway. The
results of the present study may aid in the development of new
therapeutics for BC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
RW and KH conceived the study, collected and
analyzed the data, performed the experiments and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Huangpu Branch, Shanghai Ninth People's Hospital. All
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mansoori B, Mohammadi A, Ghasabi M,
Shirjang S, Dehghan R, Montazeri V, Holmskov U, Kazemi T, Duijf P,
Gjerstorff M and Baradaran B: miR-142-3p as tumor suppressor miRNA
in the regulation of tumorigenicity, invasion and migration of
human breast cancer by targeting Bach-1 expression. J Cell Physiol.
234:9816–9825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giovannelli P, Di Donato M, Galasso G, Di
Zazzo E, Bilancio A and Migliaccio A: The androgen receptor in
breast cancer. Front Endocrinol (Lausanne). 9:4922018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Domschke C, Schneeweiss A, Stefanovic S,
Wallwiener M, Heil J, Rom J, Sohn C, Beckhove P and Schuetz F:
Cellular immune responses and immune escape mechanisms in breast
cancer: Determinants of immunotherapy. Breast Care (Basel).
11:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizukami Y, Kono K, Kawaguchi Y, Akaike H,
Kamimura K, Sugai H and Fujii H: CCL17 and CCL22 chemokines within
tumor microenvironment are related to accumulation of
Foxp3+ regulatory T cells in gastric cancer. Int J
Cancer. 122:2286–2293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Workman CJ and Vignali DA:
Targeting regulatory T cells in tumors. FEBS J. 283:2731–2748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Liu X, Wang W, Wang S, Zhang J,
Jiang S, Wang Y, Li L, Li J, Zhang Y and Huang H: Low-dose IL-2
expands CD4+ regulatory T cells with a suppressive
function in vitro via the STAT5-dependent pathway in patients with
chronic kidney diseases. Ren Fail. 40:280–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roychoudhuri R, Eil RL and Restifo NP: The
interplay of effector and regulatory T cells in cancer. Curr Opin
Immunol. 33:101–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: Crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9:2122007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plitas G, Konopacki C, Wu K, Bos PD,
Morrow M, Putintseva EV, Chudakov DM and Rudensky AY: Regulatory T
cells exhibit distinct features in human breast cancer. Immunity.
45:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugiyama D, Nishikawa H, Maeda Y, Nishioka
M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y,
et al: Anti-CCR4 mAb selectively depletes effector-type
FoxP3+CD4+ regulatory T cells, evoking
antitumor immune responses in humans. Proc Natl Acad Sci USA.
110:17945–17950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thornton AM, Donovan EE, Piccirillo CA and
Shevach EM: Cutting edge: IL-2 is critically required for the in
vitro activation of CD4+CD25+ T cell
suppressor function. J Immunol. 172:6519–6523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang TT, Song SJ, Xue HB, Shi DF, Liu CM
and Liu H: Regulatory T cells in the pathogenesis of type 2
diabetes mellitus retinopathy by miR-155. Eur Rev Med Pharmacol
Sci. 19:2010–2015. 2015.PubMed/NCBI
|
|
17
|
Hippen KL, Merkel SC, Schirm DK, Sieben
CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL,
Riley JL, et al: Massive ex vivo expansion of human natural
regulatory T cells (T(regs)) with minimal loss of in vivo
functional activity. Sci Transl Med. 3:83ra412011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu L, Zhou X, Wang J, Zheng SG and Horwitz
DA: Characterization of protective human CD4CD25 FOXP3 regulatory T
cells generated with IL-2, TGF-β and retinoic acid. PLoS One.
5:e151502010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Wang SH, Chen SC, Chen CY and Lin
TM: Zoledronic acid blocks the interaction between breast cancer
cells and regulatory T-cells. BMC Cancer. 19:1762019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higuchi T, Matsuo K, Hashida Y, Kitahata
K, Ujihara T, Taniguchi A, Yoshie O, Nakayama T and Daibata M:
Epstein-Barr virus-positive pyothorax-associated lymphoma expresses
CCL17 and CCL22 chemokines that attract CCR4-expressing regulatory
T cells. Cancer Lett. 453:184–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing Y, Tian Y, Kurosawa T, Matsui S,
Touma M, Yanai T, Wu Q and Sugimoto K: CCL11-induced eosinophils
inhibit the formation of blood vessels and cause tumor necrosis.
Genes Cells. 21:624–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Hawkins OE, Barham W, Gilchuk P,
Boothby M, Ayers GD, Joyce S, Karin M, Yull FE and Richmond A:
Myeloid IKKβ promotes antitumor immunity by modulating CCL11 and
the innate immune response. Cancer Res. 74:7274–7284. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venet F, Lepape A, Debard AL, Bienvenu J,
Bohe J and Monneret G: The Th2 response as monitored by CRTH2 or
CCR3 expression is severely decreased during septic shock. Clin
Immunol. 113:278–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez Rivas E, Ximenez C,
Nieves-Ramirez ME, Moran Silva P, Partida-Rodríguez O, Hernandez
EH, Rojas Velázquez L, Serrano Vázquez A and Magaña Nuñez U:
Entamoeba histolytica calreticulin induces the expression of
cytokines in peripheral blood mononuclear cells isolated from
patients with amebic liver abscess. Front Cell Infect Microbiol.
8:3582018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pinho V, Oliveira SH, Souza DG,
Vasconcelos D, Alessandri AL, Lukacs NW and Teixeira MM: The role
of CCL22 (MDC) for the recruitment of eosinophils during allergic
pleurisy in mice. J Leukocyte Biol. 73:356–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Liu M, Ding N, Li Y, Shao J, Zhu
M, Xie Z and Sun K: Vaccine based on antibody-dependent
cell-mediated cytotoxicity epitope on the H1N1 influenza virus
increases mortality in vaccinated mice. Biochem Biophys Res Commun.
503:1874–1879. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clark-Knowles KV, Dewar-Darch D, Jardine
KE and McBurney MW: SIRT1 catalytic activity has little effect on
tumor formation and metastases in a mouse model of breast cancer.
PLoS One. 8:e821062013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rani A and Murphy JJ: STAT5 in cancer and
immunity. J Interferon Cytokine Res. 36:226–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldstein JD, Burlion A, Zaragoza B,
Sendeyo K, Polansky JK, Huehn J, Piaggio E, Salomon BL and Marodon
G: Inhibition of the JAK/STAT signaling pathway in regulatory T
cells reveals a very dynamic regulation of Foxp3 expression. PLoS
One. 11:e01536822016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janssen E, Kumari S, Tohme M, Ullas S,
Barrera V, Tas JM, Castillo-Rama M, Bronson RT, Usmani SM, Irvine
DJ, et al: DOCK8 enforces immunological tolerance by promoting IL-2
signaling and immune synapse formation in Tregs. JCI insight.
2(pii): 942982017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramos RN, Chin LS, Dos Santos AP,
Bergami-Santos PC, Laginha F and Barbuto JA: Monocyte-derived
dendritic cells from breast cancer patients are biased to induce
CD4+CD25+Foxp3+ regulatory T
cells. J Leukocyte Biol. 92:673–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu F, Lang R, Zhao J, Zhang X, Pringle
GA, Fan Y, Yin D, Gu F, Yao Z and Fu L: CD8+ cytotoxic T
cell and FOXP3+ regulatory T cell infiltration in
relation to breast cancer survival and molecular subtypes. Breast
Cancer Res Treat. 130:645–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeong J, Thike AA, Lim JC, Lee B, Li H,
Wong SC, Hue SS, Tan PH and Iqbal J: Higher densities of
Foxp3+ regulatory T cells are associated with better
prognosis in triple-negative breast cancer. Breast Cancer Res
Treat. 163:21–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levina V, Nolen BM, Marrangoni AM, Cheng
P, Marks JR, Szczepanski MJ, Szajnik ME, Gorelik E and Lokshin AE:
Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res.
15:2647–2656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu F, Liu P, Li J and Zhang Y: Eotaxin-1
promotes prostate cancer cell invasion via activation of the
CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol Rep.
31:2049–2054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyagaki T, Sugaya M, Murakami T, Asano Y,
Tada Y, Kadono T, Okochi H, Tamaki K and Sato S: CCL11-CCR3
interactions promote survival of anaplastic large cell lymphoma
cells via ERK1/2 activation. Cancer Res. 71:2056–2065. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ni X, Kou W, Gu J, Wei P, Wu X, Peng H,
Tao J, Yan W, Yang X, Lebid A, et al: TRAF6 directs FOXP3
localization and facilitates regulatory T-cell function through
K63-linked ubiquitination. EMBO J. 38(pii): e997662019.PubMed/NCBI
|
|
38
|
Kagoya Y, Saijo H, Matsunaga Y, Guo T,
Saso K, Anczurowski M, Wang CH, Sugata K, Murata K, Butler MO, et
al: Arginine methylation of FOXP3 is crucial for the suppressive
function of regulatory T cells. J Autoimmun. 97:10–21. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ren L, Yu Y, Wang L, Zhu Z, Lu R and Yao
Z: Hypoxia-induced CCL28 promotes recruitment of regulatory T cells
and tumor growth in liver cancer. Oncotarget. 7:75763–75773. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mahmud SA, Manlove LS and Farrar MA:
Interleukin-2 and STAT5 in regulatory T cell development and
function. JAKSTAT. 2:e231542013.PubMed/NCBI
|
|
41
|
Horwitz DA, Zheng SG, Wang J and Gray JD:
Critical role of IL-2 and TGF-beta in generation, function and
stabilization of Foxp3+CD4+ Treg. Eur J
Immunol. 38:912–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zorn E, Nelson EA, Mohseni M, Porcheray F,
Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ,
et al: IL-2 regulates FOXP3 expression in human
CD4+CD25+ regulatory T cells through a
STAT-dependent mechanism and induces the expansion of these cells
in vivo. Blood. 108:1571–1579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stevenson NJ, Addley MR, Ryan EJ, Boyd CR,
Carroll HP, Paunovic V, Bursill CA, Miller HC, Channon KM, McClurg
AE, et al: CCL11 blocks IL-4 and GM-CSF signaling in hematopoietic
cells and hinders dendritic cell differentiation via suppressor of
cytokine signaling expression. J Leukocyte Biol. 85:289–297. 2009.
View Article : Google Scholar : PubMed/NCBI
|