Introduction
Obesity is characterized by excessive triglyceride
(TG) stored in white adipocytes. There are two major types of
adipose tissue with distinct functions in mammals, white adipose
tissue (WAT) and brown adipose tissue (BAT). Of these, BAT is less
abundant and specializes in non-shivering thermogenesis via
mitochondrial lipid oxidation. However, WAT is primarily
responsible for lipid storage, mostly of TG and participates in
lipid metabolism (1). WAT in the
body is broadly classified into subcutaneous and visceral fat
depots, and is recognized as a versatile endocrine organ that
produces a variety of adipokines, which can influence nutrient
metabolism and immune responses. Moreover, visceral WAT secretes
higher levels of inflammatory cytokines compared with subcutaneous
WAT and is more likely to cause metabolic disorders (2). Therefore, WAT is the main adipose
tissue involved in obesity and associated metabolic
alterations.
Enzymatic hydrolysis of TG in adipocytes is a highly
regulated process, which releases glycerol and free fatty acids
(FFAs) in response to stimulation of certain hormones, cytokines or
drugs, accompanied by activation of related signaling pathways.
Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase
(HSL) are two rate-limiting enzymes that control the hydrolysis of
TG (3,4). Furthermore, released FFAs from
adipocytes lipolysis can be used for thermogenesis in adipose
tissue via β-oxidation and mitochondrial uncoupling, or is
transported to peripheral tissues to provide energy (5). FFAs also function as modulators of
glucose and insulin action (6),
and insulin secretion (7). The
elevation of plasma FFA levels is the result of increased fat
mobilization and is thought to be associated with insulin
resistance and type 2 diabetes (8). Moreover, sustained high FFAs in
pancreatic β cells may impair insulin secretion (9). Thus, the regulation of adipocytes
lipolysis is vital to energy homeostasis, body fat controlling and
metabolic health.
Berberine (BBR), a natural isoquinoline alkaloid
derived from the Chinese herb Coptis chinensis, has been
shown to have anti-obesity, antidiabetic, antihyperlipidemia and
anti-inflammatory effects (10).
Previous studies have mainly focused on the effect of BBR on
lipogenesis and have primarily involved the use of mice and 3T3-L1
adipocytes (11–13). A limited number of studies have
investigated the role of BBR in adipocyte lipolysis. It has been
reported that BBR attenuates catecholamines-stimulated lipolysis in
3T3-L1 adipocytes via reducing the inhibition of phosphodiesterase
3B and 4, which are two enzymes involved in decreasing
intracellular cAMP production (14). Furthermore, Jiang et al
(15) revealed that BBR stimulated
basal lipolysis in mature 3T3-L1 adipocytes by upregulating ATGL
and inactivating HSL via the AMP-activated protein kinase (AMPK)
pathway. Therefore, it was hypothesized that the different effects
of BBR on adipocytes lipolysis may be attributed to different types
or states of the cells; however, the precise mechanism by which BBR
regulates lipolysis in adipocytes is not fully understood.
AMPK is a heterotrimeric serine/threonine protein
kinase, composed of a catalytic α subunit and regulatory β and γ
subunits. Moreover, two isoforms of the catalytic subunit have been
identified as α1 and α2 (16).
Threonine phosphorylation of AMPKα is required for activation of
AMPK and its downstream signaling cascades. Furthermore, AMPK is
thought to act as a crucial cellular energy sensor that maintains
energy homeostasis by regulating glucose and lipid metabolism
(17). However, the role of AMPK
in lipolysis in adipose tissue has been controversial. It was
revealed that AMPK activation inhibits basal and
isoproterenol-stimulated lipolysis in homocysteine-treated primary
murine adipocytes and 3T3-L1 adipocytes (18). However, another previous study
reported an opposite effect, in which activation of AMPK by BBR
treatment promotes basal lipolysis in differentiated 3T3-L1
adipocytes (15). Additionally,
Kim et al (19)
demonstrated that adipose-tissue specific knockout of AMPKα1/α2 in
mice results in a decrease in basal lipolysis and enhances protein
kinase A (PKA)-stimulated lipolysis in adipose tissue, suggesting
that AMPK activation increases basal lipolysis and inhibits
PKA-stimulated lipolysis. Thus, in BBR-treated adipocytes, whether
AMPK is activated requires further investigation, as well as the
regulatory role of AMPK activation in adipocyte lipolysis and the
underlying molecular mechanism.
Pigs have abundant body fat and have many similar
anatomical, genetical, metabolic and physiological characteristics
to humans. Furthermore, pigs are more closely evolutionarily
related to humans compared with rats and mice, which are two
commonly used animal models. Thus, the pig is considered as an
ideal medical model for researching human obesity and related
metabolic diseases (20). In
addition, pigs are an important source of meat products for humans
worldwide and reduction of porcine fat deposition helps improve
pork quality and production efficiency. Therefore, the aims of the
present study were to investigate the effect of BBR on lipolysis in
porcine adipocytes and identify the potential molecular
mechanisms.
Materials and methods
Reagents
DMEM/Nutrient Mixture F12 (DMEM/F12) and
penicillin-streptomycin were obtained from Gibco (Thermo Fisher
Scientific, Inc.). HEPES, BSA, FBS, glycerol assay kit (cat. no.
MAK117), cAMP Enzyme Immunoassay kit (cat. no. CA 200), recombinant
human tumor necrosis factor α (TNFα), isoprenaline (ISO), DMSO,
H89, compound C and polybrene were obtained from Sigma-Aldrich
(Merck KGaA). Lentiviral vector pRNAT-U6.2/Lenti was obtained from
GenScript Biotech Corporation. ViraPower Packaging mix, 293FT cell
line, Lipofectamine 2000, type I collagenase and TRIzol®
reagent were purchased from Invitrogen (Thermo Fisher Scientific,
Inc.). PrimeScript reverse transcription (RT) Master mix was
obtained from Takara Biotechnology Co., Ltd. SYBR Premix Ex Taq II
kit was obtained from Takara Bio, Inc.
Phosphatase inhibitor cocktail (cat. no. 5870),
protease inhibitor cocktail (cat. no. 5871), rabbit polyclonal
antibodies to AMPKα1 (cat. no. 2795), phosphorylated (p)-AMPKα
(cat. no. 2531) and rabbit anti-β-actin monoclonal antibody (cat.
no. 4970) were obtained from Cell Signaling Technology, Inc. Rabbit
anti-HSL polyclonal antibody (cat. no. abs131895) was obtained from
Absin Biotechnology, Co. Ltd. Rabbit polyclonal antibody to p-HSL
(cat. no. bs-3222R) was obtained from Bioss Biotechnology Co., Ltd.
Rabbit polyclonal antibodies to Perilipin A (cat. no. ab126639),
ATGL (cat. no. ab99532), p-ATGL (cat. no. ab135093), p-IRS1 (cat.
no. ab1194), carnitine palmitoyl-transferase-1 (CPT-1; cat. no.
ab83862), uncoupling protein 2 (UCP2; cat. no. ab97931) and goat
anti-peroxisome proliferator-activated receptor γ coactivator-1α
(PGC-1α) polyclonal antibody (cat. no. ab106814) were obtained from
Abcam. Rabbit anti-IRS-1 monoclonal antibody (cat. no. MA5-15068)
was obtained from Invitrogen (Thermo Fisher Scientific, Inc.).
Monoclonal secondary antibodies of mouse anti-rabbit
IgG-horseradish peroxidase (HRP)-conjugate (cat. no. sc-2357) and
mouse anti-goat IgG-HRP (cat. no. sc-2354) were obtained from Santa
Cruz Biotechnology, Inc. Enhanced chemiluminescence (ECL) reagent
and bicinchoninic acid (BCA) protein assay kit were purchased from
Pierce (Thermo Fisher Scientific, Inc.). Cell Counting Kit-8
(CCK-8) was obtained from Vazyme Biotech Co., Ltd. TG assay kit
(cat. no. E1013) was obtained from Applygen Technologies, Inc. The
specific primer sequences for reverse transcription-quantitative
PCR were synthesized in Aoke Biotechnology Corporation. BBR (with
purity of >98%) was obtained from the National Institute for the
Control of Pharmaceutical and Biological Products.
Experimental animals
Healthy male or female crossbred piglets (Duroc ×
Landrace × Large-White; age, 3 days) were provided by the Jinfeng
Livestock Farm of Linfen. The animals were housed in a
temperature-controlled room (23±2°C) with 40–50% air relative
humidity and a 12-h light/12-h dark cycle under specific-pathogen
free conditions, and allowed access to food and water ad libitum. A
total of 76 piglets were used in the present study (38 male and 38
female; weight, 1.5–2.0 kg). In each experiment, two piglets (1
female and 1 male) were euthanized by CO2 asphyxiation
to reduce suffering. All experimental procedures involving animals
were strictly carried out according to The Guide for The Care and
Use of Laboratory Animals and were approved by The Institutional
Ethics Committee of Shanxi Normal University.
Porcine preadipocytes primary culture,
differentiation and drug treatment
The WAT of newborn piglets mainly resides in
subcutaneous fat depots (21).
According to our previous established method (22), adipose tissue was isolated from
neck and back of the piglets, and was rinsed with DMEM/F12 basal
culture medium (pH 7.4; 1:1 mixture of DMEM and F12; 100 mmol/l
HEPES; 50 U/ml penicillin-streptomycin) under sterile conditions.
Tissue was then minced and digested with 1 mg/ml type I collagenase
in DMEM/F12 basal culture medium containing 20 mg/ml BSA, in a 37°C
shaking water bath for 1 h. This was then filtered through a 200-µm
nylon mesh and centrifuged at 395 × g for 8 min at room
temperature. Collected pellets from the stromal-vascular fraction
were used as preadipocytes and were resuspended in DMEM/F12
complete culture medium consisting of DMEM/F12 basal culture medium
and 10% FBS.
The preadipocytes were seeded in 35 mm diameter
culture dishes at a density of 5×104
cells/cm2 and maintained at 37°C in a 5% CO2
humidified atmosphere. DMEM/F12 complete medium can be used for
proliferation and differentiation of primary porcine preadipocytes,
without having to add a specific differentiation cocktail (23). Medium was changed every 2 days. The
day of seeding was set as day 0. Morphology of lipids accumulation
in adipocytes was observed with an Olympus IX53 inverted light
microscope (magnification, ×200; Olympus Corporation). During
differentiation of preadipocytes, the small lipid droplets in cells
converged into larger lipid droplets, indicating that the cultured
adipocytes were unilocular white adipocytes, rather than
multilocular brown adipocytes.
In contrast to fresh isolated mature adipocytes,
preadipocytes have the ability to divide and proliferate. After
proliferation and differentiation, additional adipocytes can be
obtained for subsequent experimental studies. Therefore, in the
present study, preadipocytes were isolated and cultured instead of
using mature adipocytes.
To examine the effect of BBR on lipolysis,
differentiated porcine adipocytes were incubated with various
concentrations (0-40 µM) of BBR (National Institute for the Control
of Pharmaceutical and Biological Products) at 37°C for 24–72 h. To
assess whether BBR activated the AMPK signaling pathway,
differentiated porcine adipocytes on day 5 were pre-treated with or
without various concentrations of AMPK inhibitor compound C (10 or
20 µM) at 37°C for 24 h, then incubated with or without 30 µM BBR
in the continued absence or presence of the inhibitor at 37°C for a
further 48 h. Furthermore, to determine the effect of BBR-induced
lipolysis on insulin signaling, a positive control trial was
performed, in which differentiated porcine adipocytes on day 6 were
incubated with 10 ng/ml TNFα (Sigma-Aldrich; Merck KGaA) at 37°C
for 30 min. BBR was dissolved in DMSO and the final concentration
of DMSO in culture medium was <0.1% (v/v). The control group was
treated with 2 µl DMSO.
Lipolysis assay
Differentiated porcine adipocytes were treated with
(+) or without (−) BBR (10–40 µM), H89 (20 µM), ISO (10 µM) or
compound C (10–20 µM) at 37°C for 24–72 h, 26–72 h, 2 h and 72 h,
respectively. Then, the glycerol content of the incubation medium
was used as an index for lipolysis and was measured by a
colorimetric method using glycerol assay kits according to the
manufacturer's protocol. Glycerol concentration is presented as
glycerol (µmol/ml).
Measurement of TG levels
Cells in 35-mm diameter culture dishes
(1×106 cells/dish) were washed twice with ice-cold PBS
and lysed using the lysis buffer provided in the TG assay kit, at
room temperature for 10 min. Lysates were divided into two parts
and the concentrations of TG and total protein in each plate were
measured with TG and BCA assay kits according to the manufacturer's
protocols, respectively. TG content was expressed as mmol of TG/g
of protein. Each group of cells was analyzed in triplicate and the
experiments were repeated three times.
Cell viability assay
Cell viability was determined using CCK-8 assay
according to the manufacturer's protocol. Porcine preadipocytes
were seeded in 96-well culture plates at a density of
1.0×104 cells/well in 100 µl volume. Cells were cultured
in DMEM/F12 complete culture medium supplemented with 10% FBS and
50 U/ml penicillin-streptomycin for 72 h, and then treated with
different concentrations of BBR (0, 10, 20, 30 and 40 µM) for 48 h.
Then, 10 µl CCK-8 solution was added into each well and incubated
for a further 4 h at 37°C. Absorbance was quantified at 450 nm
using a Benchmark Plus microplate reader (Bio-Rad Laboratories,
Inc.), and the relative viability of cells was presented as a
percentage of the control.
Intracellular cAMP concentration
assay
Differentiated porcine adipocytes on day 6 were
incubated at 37°C with BBR (10 and 30 µM) for 48 h or ISO (10 µM)
for 2 h. Then, intracellular cAMP content was measured using an
ELISA kit according to the manufacturer's protocol. Data are
presented as cAMP (pmol/l).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted with TRIzol®
reagent according to the manufacturer's protocol. Total RNA
integrity was detected using a 2% agarose gel and the purity and
concentration of the RNA preparation was assessed using a UV
spectrophotometer. Possible genomic DNA contamination was
eliminated by incubating total RNA with 3 U/10 µl deoxyribonuclease
I for 30 min at 37°C. Then, 2 µg total RNA was RT to synthesize
first-strand cDNA using the PrimeScript RT-reagent kit using the
following thermocycling conditions: 37°C for 15 min, 85°C for 5 sec
and 4°C for 5 min.
qPCR reactions were performed in triplicate on a
Bio-Rad iQ5 system (Bio-Rad Laboratories, Inc.) using a SYBR Premix
Ex Taq II system in a final volume of 25 µl (containing 12.5 µl
SYBR Premix Ex Taq II; 1 µl sense primer; 1 µl anti-sense primer; 1
µl template cDNA; 9.5 µl ddH2O). The conditions of qPCR were as
follows: Initial denaturation at 95°C for 5 min, followed by 40
cycles of melting at 95°C for 15 sec and annealing/extension at
60°C for 35 sec. A melt curve was established at 60–95°C at the end
of the amplification in order to determine specificity of the
primers. The primer sequences used for qPCR were designed by
Premier 5.0 software (Premier Biosoft International) and are listed
in Table I. Amplification
efficiency of the primers was between 90 and 100%. Furthermore, 18S
rRNA was amplified as an internal control. The 2−ΔΔCq
method was used to analyze the relative mRNA expression of each
gene of interest (24).
 | Table I.Specific primers used for
quantitative PCR. |
Table I.
Specific primers used for
quantitative PCR.
| Gene | GenBank accession
no. | Primer (5′-3′) | Product length,
bp |
|---|
| Perilipin A | NM_001038638 | Sense:
5′-GAGGATGGCAATCAACAAGG-3′ | 110 |
|
|
| Anti-sense:
5′-ACTCACAGGTGCCGCTCA-3′ |
|
| ATGL | EU373817 | Sense:
5′-ACCCTGTCCAACCTGCTGC-3′ | 153 |
|
|
| Anti-sense:
5′-GCCTGTCTGCTCCTTTATCCA-3′ |
|
| HSL | AJ000482 | Sense:
5′-TTTGAAATGCCACTGACTGC-3′ | 101 |
|
|
| Anti-sense:
5′-TAGGAGATGAGCCTGACGAG-3′ |
|
| PGC-1α | NM_213963 | Sense:
5′-CAGCGAAGATGAAAGTGA-3′ | 135 |
|
|
| Anti-sense:
5′-AATAAGGATTTGGGTGGT-3′ |
|
| TFAM | NM_001130211 | Sense:
5′-TCCTTCGTCGTAGTCCCG-3′ | 159 |
|
|
| Anti-sense:
5′-TGAACTCGCAAGCAACTC-3′ |
|
| CPT-1 | NM_001129805 | Sense:
5′-CATTTGTCCCATCTTTCG-3′ | 146 |
|
|
| Anti-sense:
5′-CTTGTCCACTTGCTACGC-3′ |
|
| UCP-2 | NM_214289 | Sense:
5′-CCCAATGTCGCTCGTAATG-3′ | 126 |
|
|
| Anti-sense:
5′-GAAGGCGGACGTGAAGTG-3′ |
|
| 18S rRNA | AY265350 | Sense:
5′-CCCACGGAATCGAGAAAGAG-3′ | 122 |
|
|
| Anti-sense:
5′-TTGACGGAAGGGCACCA-3′ |
|
Western blotting
The cellular total protein was extracted with lysis
buffer (pH 7.5) containing 50 mmol/l Tris-HCl, 0.5% Triton X-100, 2
mmol/l EDTA, 150 mmol/l NaCl, 1 mmol/l PMSF, 1% protease inhibitor
cocktail and 1% phosphatase inhibitor cocktail. Protein quality was
determined on 12% SDS-PAGE gel with 0.25% (w/v) coomassie brilliant
blue R250 staining at room temperature for 2 h followed by
decolorization for 4 h, and the protein concentration was measured
using a BCA protein assay kit.
Total proteins (50 µg) were electrophoresed using
12% SDS-PAGE under reducing conditions, followed by
electro-transfer onto PVDF membranes. After blocking with 5% BSA in
TBST (pH 7.5; 100 mmol/l Tris; 154 mmol/l NaCl; 0.1% v/v Tween-20)
for 60 min at room temperature, the membranes were reacted with
primary antibodies to Perilipin A (1:800), ATGL (1:1,000), p-ATGL
(1:1,000), HSL (1:400), p-HSL (1:500), AMPKα1 (1:1,000),
phosphorylated-AMPKα (1:1,000), PGC-1α (1:400), CPT-1 (1:1,000),
UCP2 (1:800), IRS-1 (1:1,000), p-IRS-1 (1:200) and β-actin
(internal control; 1:1,000) overnight at 4°C. Subsequently, the
membranes were washed three times with TBST (0.1% Tween-20),
followed by incubation with HRP-conjugated secondary antibodies of
mouse anti-rabbit IgG (1:1,000) or mouse anti-goat IgG (1:1,000)
for 60 min at room temperature. Membranes were then visualized
using an ECL reagent. Densitometric analysis of protein bands was
performed using ImageJ software (version 1.8.0.112; National
Institutes of Health).
Lentivirus-mediated RNA interference
(RNAi)
AMPKα1 small hairpin RNA (shRNA)design was based on
122–140 of porcine AMPKα1 mRNA (GenBank accession no. AB530142;
National Center for Biotechnology Information). A scrambled shRNA
(based on the sequence 5′-GCATCATCACTCAATCCAA-3′) was generated as
a negative control to investigate the non-specific effect of AMPKα1
shRNA on adipocytes lipolysis. To construct recombinant expression
plasmids, AMPKα1 shRNA and scrambled shRNA oligonucleotides were
cloned into pRNAT-U6.2/Lenti vectors between BamHI and
XhoI sites.
shRNA-expressing plasmids (3 µg) and ViraPower
Packaging mix (9 µg) containing pLP1, pLP2 and pLP/VSVG plasmids
were co-transfected into 293FT cells (6×106) in a 10 cm
culture dish using Lipofectamine 2000, according to the
manufacturer's protocol. The 10 ml supernatants containing
lentivirus particles in each dish were collected 48–72 h
post-transfection and passed through sterile 0.45 µm PVDF filters
to remove cellular debris. Then, titers of the recombinant
lentivirus supernatants were determined and the virus stocks were
stored at −80°C.
In 35 mm culture dish, differentiated porcine
adipocytes on day 5 were infected with 200 µl lentivirus stock
(5×106 TU/ml) containing AMPKα1 shRNA or scrambled shRNA
in the presence of 6 µg/ml polybrene. Medium was replaced with
fresh DMEM/F12 complete culture medium after 24 h. Adipocytes were
cultured for a further 48 h, then RT-qPCR and western blotting were
used to assess RNAi efficiency. The recombinant lentivirus that was
identified to have the highest inhibitory effect on AMPKα1 gene
expression was selected for AMPKα1 knockdown in subsequent
experimentations.
Statistical analysis
All experiments were conducted three times. Data are
presented as the mean ± SEM and were analyzed using SPSS 17.0
software (IBM Corp.). Individual comparisons were evaluated using
an unpaired Student's t-test. Multiple comparisons were determined
by one-way or two-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
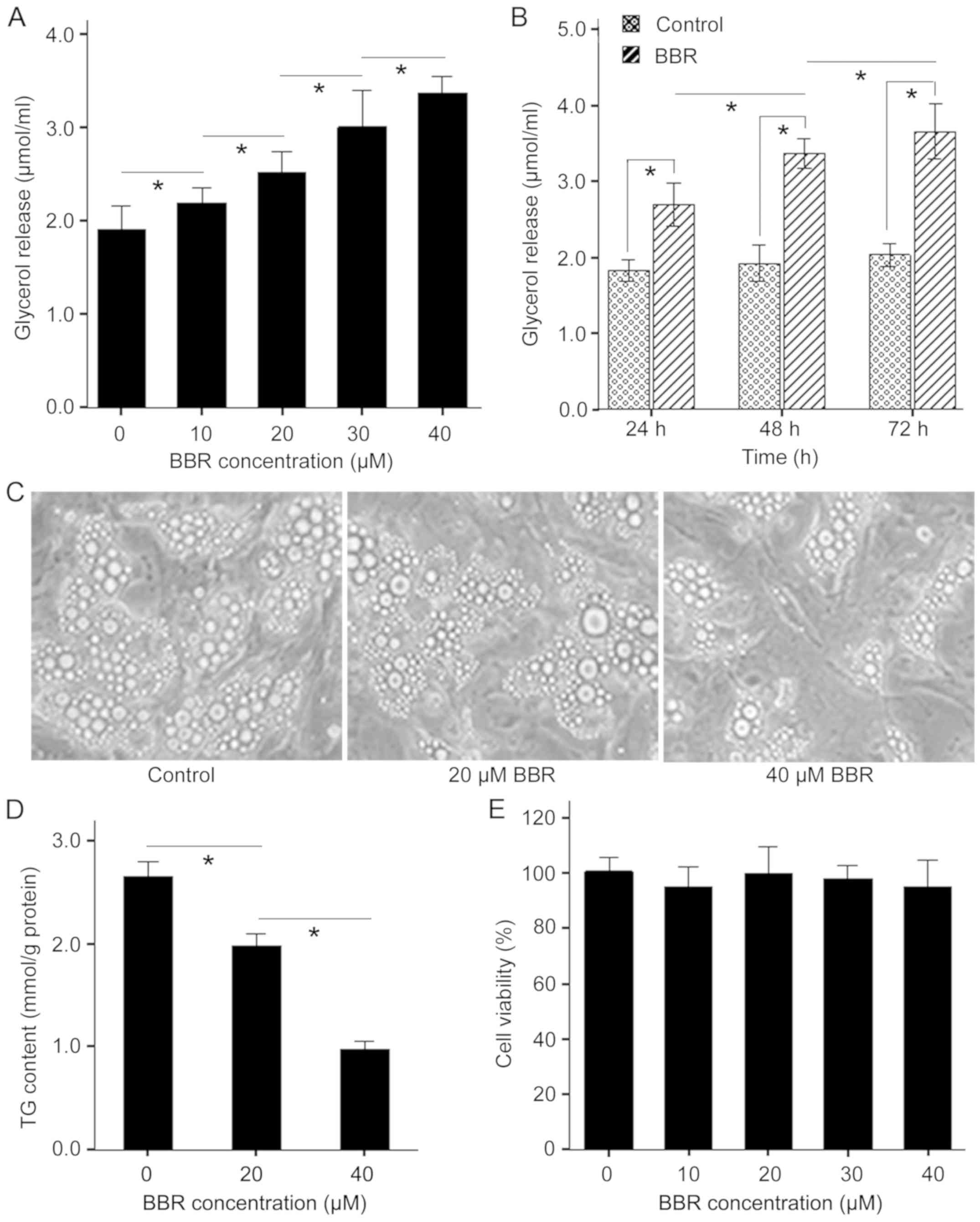
BBR induces lipolysis in
differentiated porcine adipocytes
To investigate the mechanism by which BBR regulates
lipolysis in porcine adipocytes, the present study examined the
effect of BBR on lipolysis. It was found that treatment of
adipocytes with BBR (10–40 µM) for 48 h caused a dose-dependent
increase in glycerol release (Fig.
1A). Moreover, the maximal glycerol release was seen in the 40
µM treated group and was ~47.2% higher compared with the control.
Furthermore, it was demonstrated that BBR treatment significantly
reduced lipid content in fat droplets of porcine adipocytes in a
dose-dependent manner (Fig. 1C and
D). However, BBR (10–40 µM) did not alter cell viability after
48 h treatment (Fig. 1E).
Furthermore, adipocytes released more glycerol at 72 h compared
with 48 h in response to 30 µM BBR and the minimal glycerol release
was seen in 24 h after treatment (Fig.
1B). Therefore, the present results suggested that BBR induced
lipolysis in porcine adipocytes in a dose- and time-dependent
manner, which was not related to cytotoxicity.
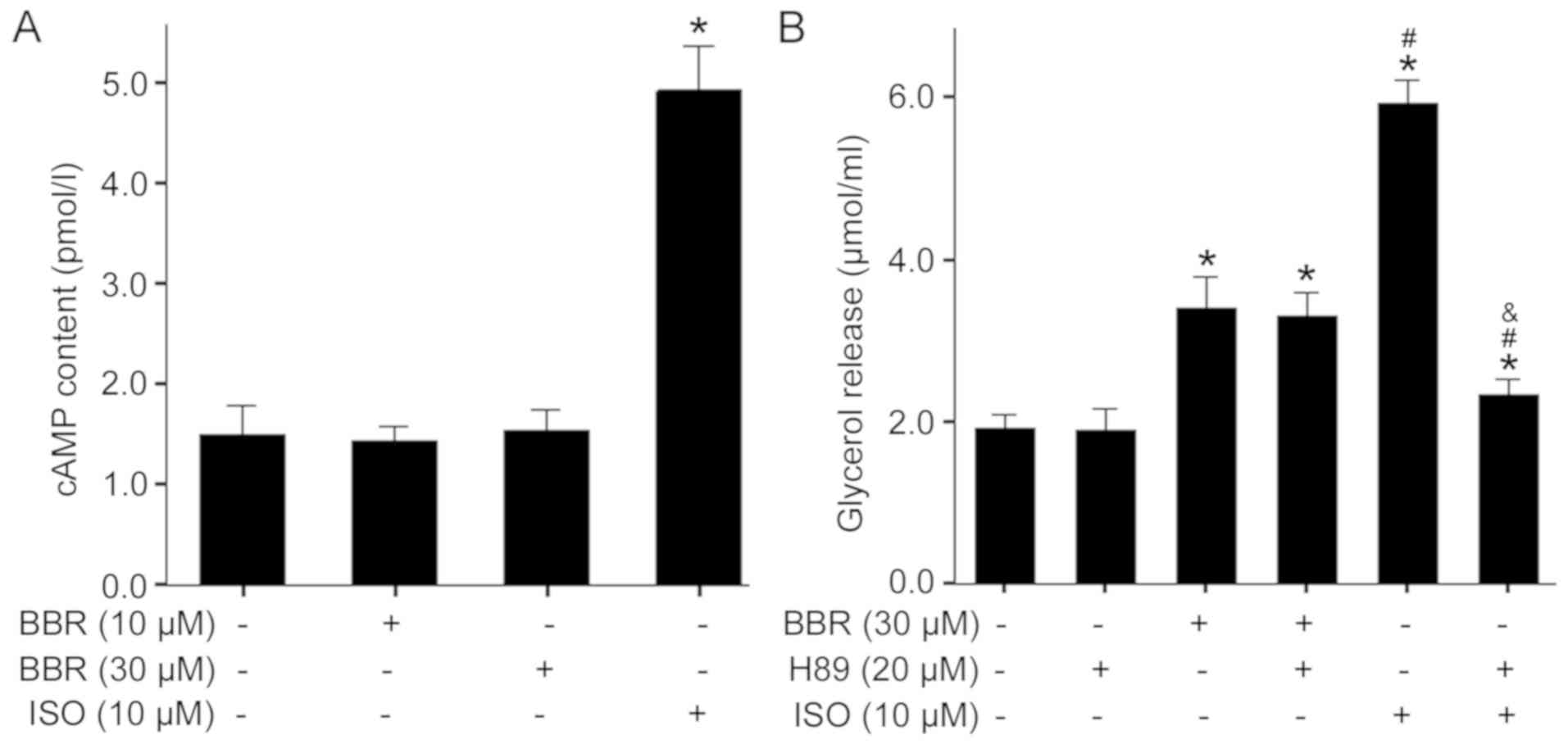
cAMP/PKA pathway is not involved in
BBR-induced lipolysis
In adipocytes, cAMP-dependent PKA activation is an
important event in lipolysis, which is stimulated by catecholamines
(25). To assess whether the
cAMP/PKA pathway mediated BBR-induced lipolysis, the present study
directly measured intracellular cAMP content after treatment with
BBR or ISO, a β-adrenergic agonist and an activator of adenylyl
cyclase. It was found that treatment of adipocytes with 10 µM ISO
caused a significant elevation in cAMP (Fig. 2A). However, both 10 and 30 µM BBR
treatments did not have effect on cAMP content in cells compared
with the control. To further examine the role of the cAMP/PKA
pathway in BBR-induced lipolysis, a specific PKA inhibitor H89 was
used to block activation of PKA. The present results identified
that stimulation of adipocytes with 10 µM ISO resulted in a
significant increase in glycerol release and this effect was
abrogated by 20 µM H89 (Fig. 2B).
In contrast, H89 pretreatment failed to suppress 30 µM BBR-induced
lipolysis. Collectively, the present results indicated that
BBR-induced lipolysis does not involve the activation of the
cAMP/PKA pathway.
Effect of BBR on expression of related
genes to lipolysis and activation of lipases
Hydrolysis of TG in adipocytes is caused by
catalysis of lipases and regulation of several protein factors. To
investigate the effect of BBR on the expression levels of lipolysis
related genes, the present study analyzed mRNA and protein
expression levels of ATGL, HSL and Perilipin A. It was demonstrated
that 30 µM BBR treatment significantly decreased Perilipin A mRNA
and protein expression levels compared with the control, whereas
the mRNA and protein expression levels of ATGL and HSL were not
affected (Fig. 3A-C).
Subsequently, the effects of BBR on the activation of ATGL and HSL
were examined. It was found that BBR treatment significantly
increased phosphorylation of ATGL, but had no effect on
phosphorylation of HSL at the PKA site (Fig. 3B and C). Thus, the present results
indicated that BBR induced lipolysis in porcine adipocytes via a
reduction of Perilipin A and elevation of ATGL phosphorylation.
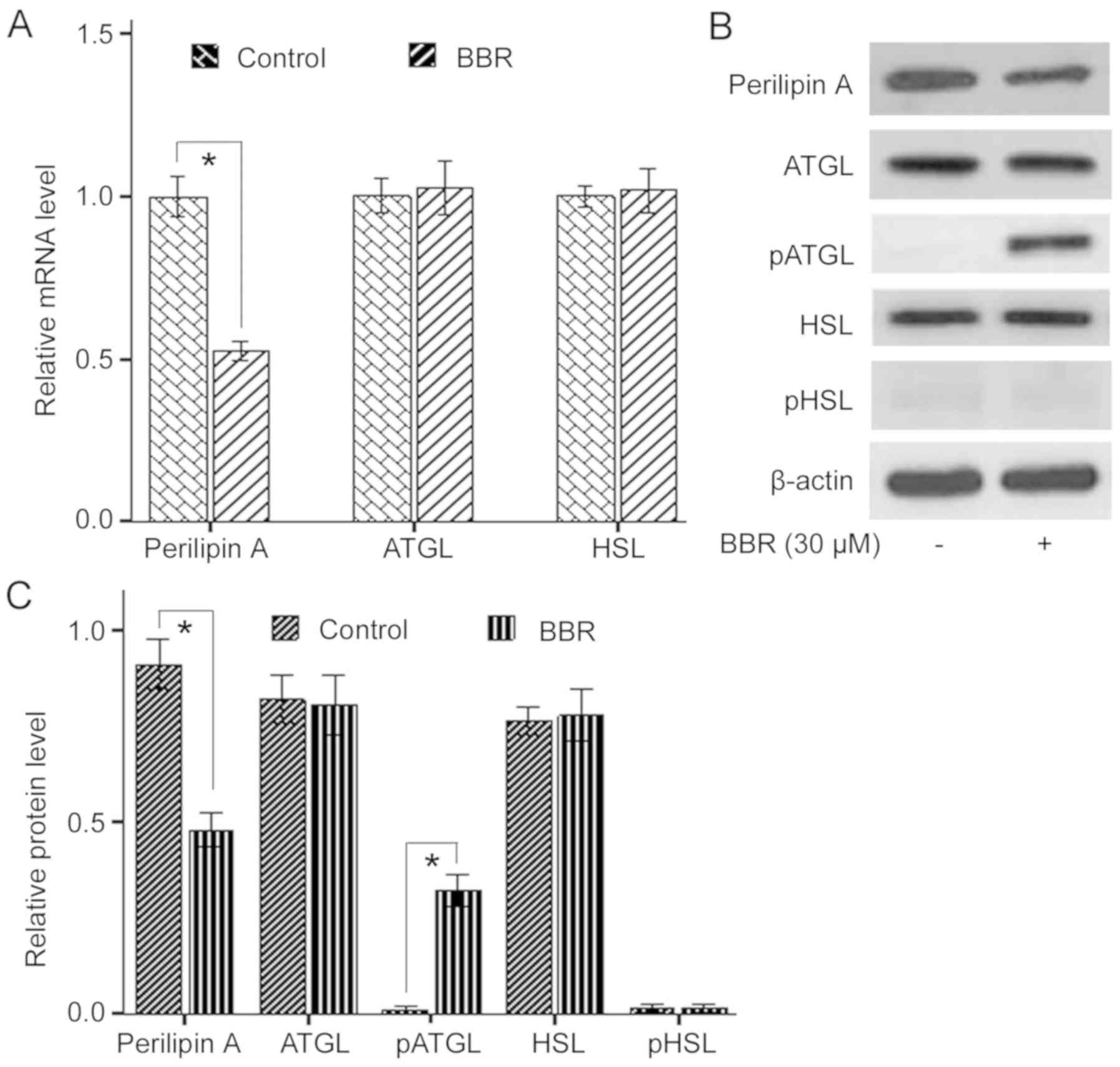 | Figure 3.Effect of BBR on expression levels of
lipolysis-related genes and phosphorylation of lipases.
Differentiated porcine adipocytes on day 6 were incubated with 30
µM BBR for 48 h. (A) Effect of BBR on mRNA expression levels of
Perilipin A, ATGL and HSL. (B) Effect of BBR on protein expression
levels of Perilipin A, ATGL, HSL, pATGL and pHSL. Representative
images were obtained from four or five independent experiments. (C)
Relative protein expression levels of Perilipin A, ATGL, HSL, pATGL
and pHSL normalized to β-actin. Data are presented as the mean ±
SEM of triplicate measurements. *P<0.05. p-, phosphorylated;
ATGL, adipose triglyceride lipase; HSL, hormone sensitive lipase;
BBR, berberine. |
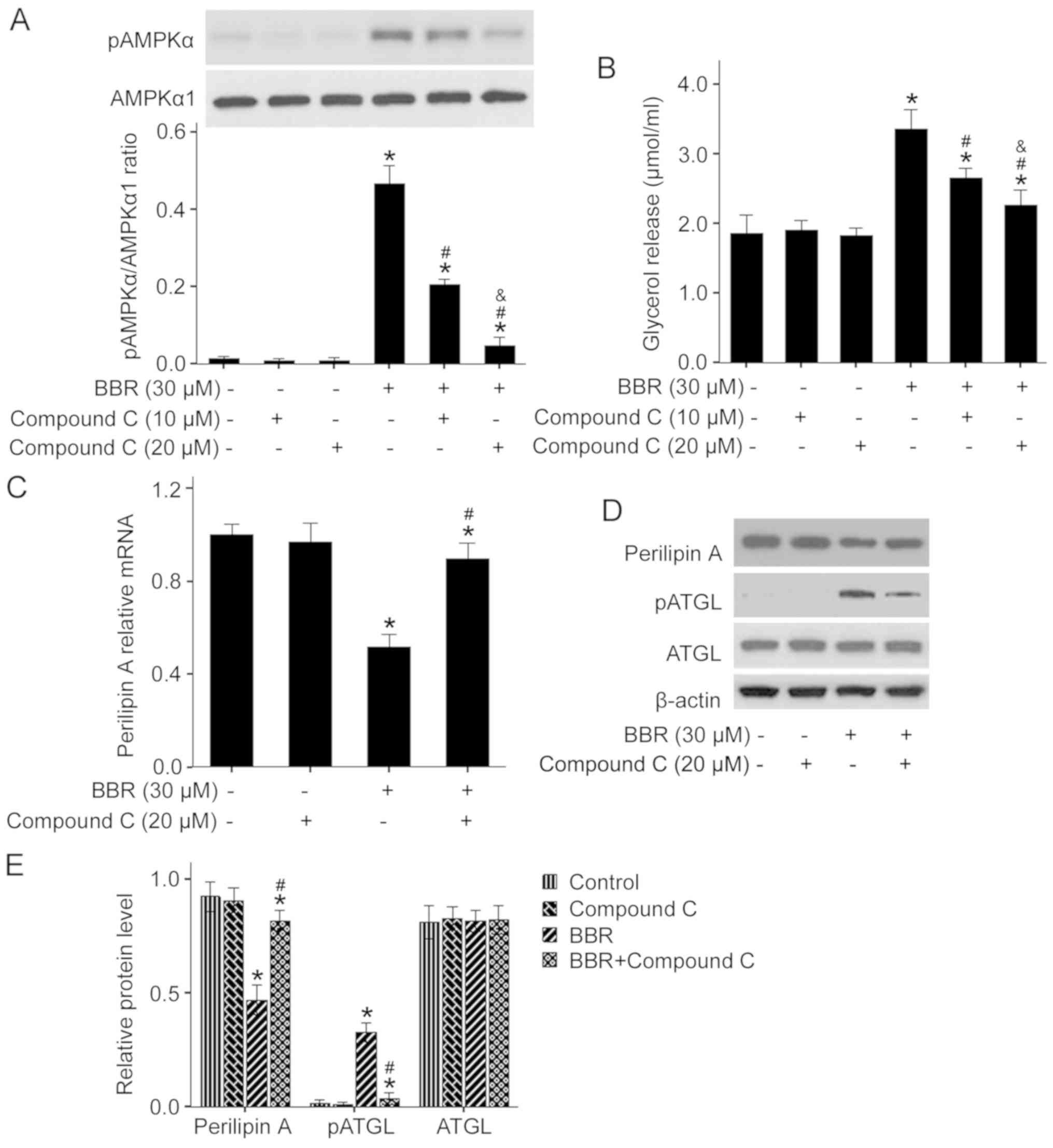
AMPK pathway mediates BBR-induced
lipolysis
AMPK is a master regulator of cellular energy
homeostasis and plays a vital role in lipid metabolism (17,26).
To determine the relationship between the AMPK pathway and
BBR-induced lipolysis, the effect of BBR on AMPK activation was
examined. The present results identified that 30 µM BBR treatment
significantly enhanced AMPKα phosphorylation (Fig. 4A). Moreover, pretreatment of
adipocytes with 10 and 20 µM compound C, a specific inhibitor of
AMPK, attenuated BBR-stimulated phosphorylation of AMPKα, thus
indicating that BBR activates AMPK in porcine adipocytes.
Furthermore, it was demonstrated that 20 µM compound C could
efficiently suppress the activation of AMPK.
To further investigate the possible role of the AMPK
pathway in BBR-induced lipolysis, the specific AMPK inhibitor
compound C was used to block activation of the AMPK signaling
pathway. It was found that pretreatment of porcine adipocytes with
a low dose of compound C (10 µM) only partly impaired BBR-induced
lipolysis, whereas a high dose of compound C (20 µM) almost
completely abolished BBR-induced lipolysis (Fig. 4B). Moreover, 20 µM compound C
significantly reversed BBR-suppressed mRNA and protein expression
of Perilipin A, as well as BBR-enhanced phosphorylation of ATGL,
however the protein expression level of ATGL was unchanged
(Fig. 4C-E). Therefore, the AMPK
pathway may mediate BBR-induced lipolysis in porcine adipocyte via
downregulation of Perilipin A and activation of ATGL.
BBR promotes expression of related
genes to FFA oxidation via the AMPK pathway
FA oxidation can decrease the end products of
lipolysis, which helps to maintain and promote adipocytes lipolysis
(27). In order to assess the
effect of BBR on FA oxidation in porcine adipocytes, the present
study used sh-AMPKα1 to block the AMPK pathway and then evaluated
expression levels of the genes related to mitochondrial FA
oxidation in response to BBR treatment. It was identified that
adipocytes transfected with AMPKα1 shRNA for 72 h had a significant
decrease in the expression of AMPKα1 compared with the control
(Fig. 5A and B). The AMPKα1 mRNA
expression level was ~86.6% lower compared with the control.
Furthermore, adipocytes transfected with AMPKα1 shRNA had a
significant reduction in protein expression level of AMPKα1, which
paralleled decreased mRNA expression. In contrast, transfection
with scrambled shRNA had no effect on the mRNA and protein
expression levels of AMPKα1. Thus, the present results suggested
that AMPKα1 was successfully knocked down by the transfection of
AMPKα1 shRNA in porcine adipocytes. Therefore, the recombinant
lentivirus containing AMPKα1 shRNA was used to knockdown AMPKα1 in
the subsequent experiments.
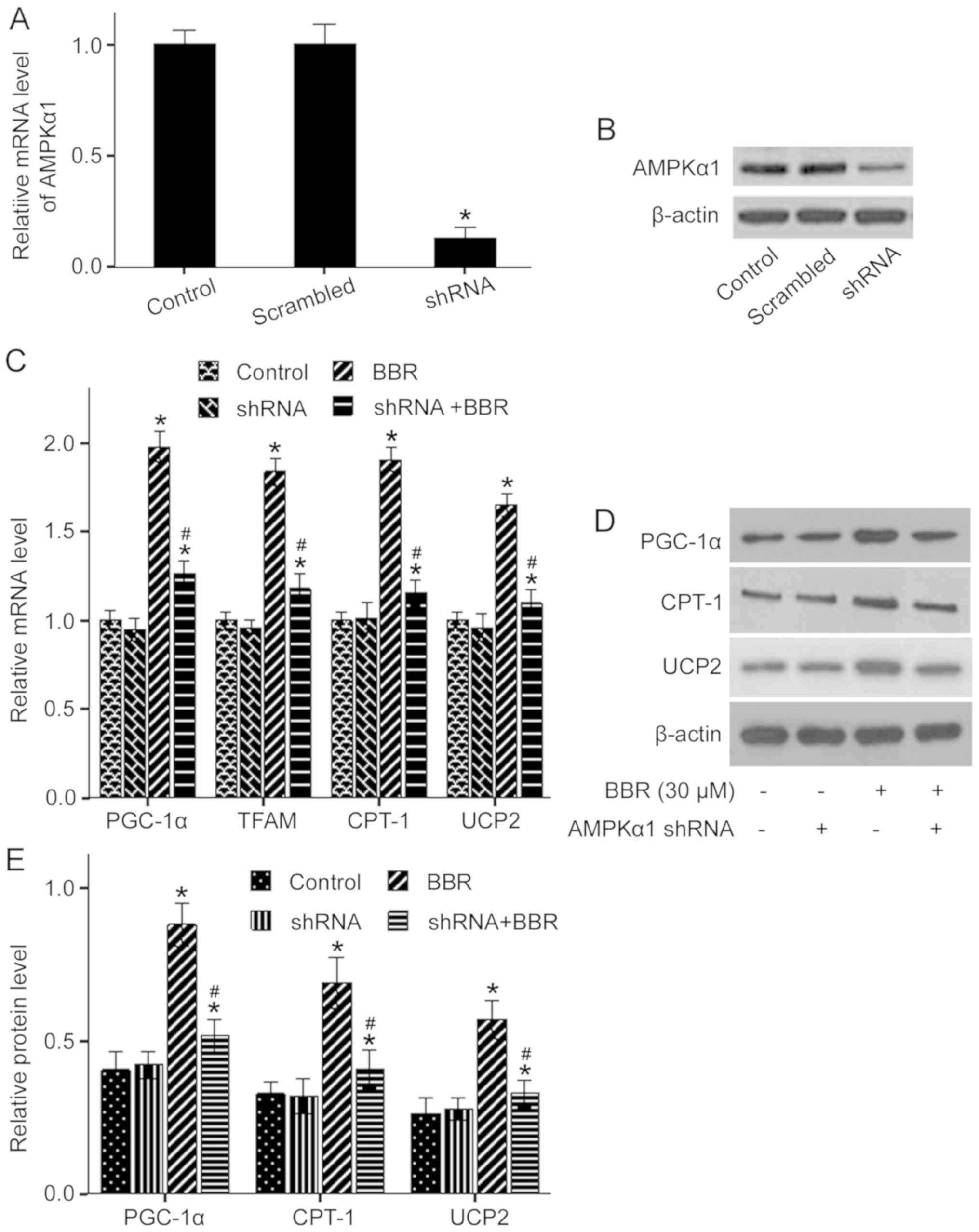 | Figure 5.Effect of AMPKα1 knockdown on
expression of related genes to free fatty acid oxidation in
BBR-treated adipocytes. AMPKα1 gene was successfully knocked down
by specific shRNA transfection. (A) Relative mRNA expression of
AMPKα1 gene. (B) Western blot analysis of AMPKα1. Differentiated
porcine adipocytes on day 5 were infected + or - AMPKα1 shRNA for
24 h and then incubated + or - 30 µM BBR for a further 48 h. (C)
Relative mRNA expression levels of PGC-1α, TFAM, CPT-1 and UCP2.
(D) Western blot analysis of PGC-1α, CPT-1 and UCP2. Representative
images were obtained from four or five independent experiments. (E)
Relative protein expression levels of PGC-1α, CPT-1 and UCP2
normalized to β-actin. Data are presented as the mean ± SEM of
triplicate measurements. *P<0.05 vs. untreated control cells.
#P<0.05 vs. BBR-treated group. shRNA, short hairpin
RNA; AMPKα, AMP-activated protein kinase α; Scrambled group,
scrambled shRNA; shRNA group, AMPKα1 shRNA; +, with; -, without;
BBR, berberine; CPT-1, carnitine palmitoyl-transferase-1; PGC-1α,
peroxisome proliferator-activated receptor γ coactivator-1α; TFAM,
mitochondrial transcription factor A; UCP2, uncoupling protein
2. |
The present study demonstrated that 30 µM BBR alone
significantly increased the mRNA expression levels of PGC-1α, TFAM,
CPT-1 and UCP2 compared with the control, which were significantly
abrogated by AMPKα1 knockdown (Fig.
5C). Moreover, alterations in protein expression levels of
PGC-1α, CPT-1 and UCP2 were similar to that of the mRNA expression
levels (Fig. 5C-E). Collectively,
the present results suggested that BBR may promote FFA oxidation in
porcine adipocytes via the upregulation of PGC-1α, TFAM, CPT-1 and
UCP2, which may be mediated by the AMPK pathway.
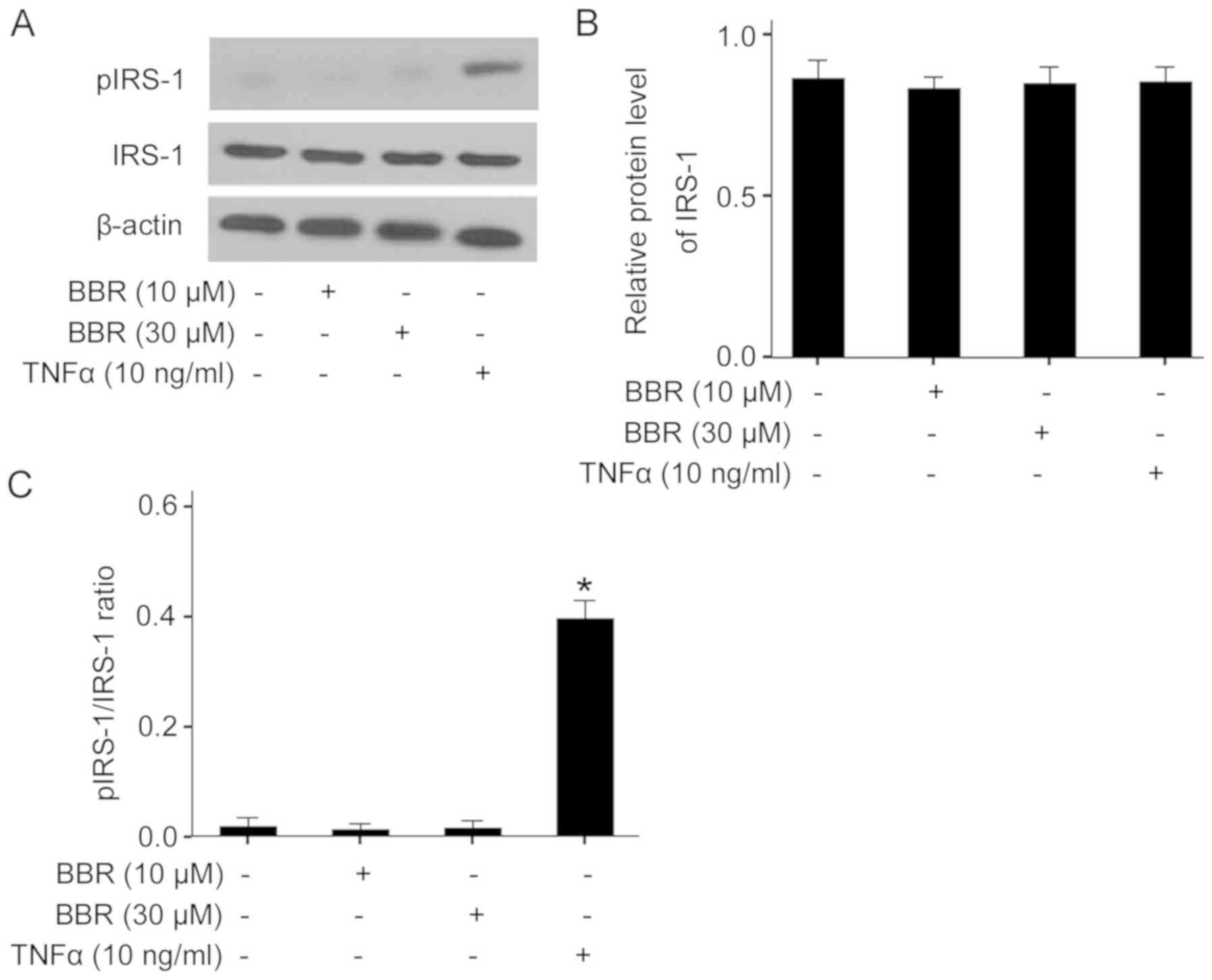
BBR-induced lipolysis does not block
insulin signaling at the level of IRS-1
FFA from increased lipolysis may block insulin
signaling via serine phosphorylation of IRS proteins, leading to
insulin resistance (28). To
determine the effect of BBR-induced lipolysis on insulin signaling,
the present study examined the protein expression level and serine
phosphorylation of IRS-1 in BBR-treated adipocytes. It was found
that both 10 and 30 µM BBR treatment did not affected the protein
expression of IRS-1, and did not increase serine phosphorylation of
IRS-1 compared with the control (Fig.
6A-C). In contrast, treatment of adipocytes with TNFα, a known
inducer of lipolysis, significantly increased IRS-1 serine
phosphorylation, although it also did not alter the protein
expression of IRS-1. Therefore, BBR-induced lipolysis may not block
insulin signaling at the level of IRS-1 in porcine adipocytes.
Discussion
BBR, a traditional Chinese herb active ingredient,
has been used to treat gastroenteritis and diarrhea (29). Previous studies have focused on its
regulatory effect on obesity and insulin resistance (30–32).
Lipolysis decreases TG content in adipocytes, which is important
for combating obesity (19,22).
However, continuous lipolysis may increase plasma FFA level and
induce systematic insulin resistance (8), which is one of the most important
factors of the pathogenesis of obesity-associated metabolic
disorders, involving hyperglycemia, hyperlipidemia, hypertension
and type 2 diabetes (33).
Moreover, enhancement of FA oxidation during adipocyte lipolysis
can contribute to a decrease in FFA accumulation and thus helps to
maintain lipolysis and improve insulin resistance (34).
The aims of the present study were to investigate
the lipolytic role of BBR in primary porcine adipocytes and to
identify the underlying signaling mechanisms. To the best of our
knowledge, the present study was the first demonstration of this
effect in porcine adipocytes. It was demonstrated that BBR induced
lipolysis by activation of the AMPK pathway, suppression of
Perilipin A, phosphorylation of ATGL and upregulation of genes
related to FA oxidation. Furthermore, this lipolytic action did not
impair insulin signaling at IRS-1 level, which may be attributed to
enhanced FA oxidation in adipocytes. Therefore, the present results
provided evidence that BBR may be used as a lipolytic inducer and
could exert beneficial regulatory effects on obesity and insulin
resistance.
Adipocytes lipolysis produces glycerol and FFA.
Furthermore, glycerol is released into culture medium as it cannot
be reused directly by the adipocytes. In contrast, only part of the
FFA is released into culture medium, because FFA can be
re-esterified into triglycerides or consumed via β-oxidation in the
adipocytes, depending on the different states of the cells
(35). Thus, the glycerol content,
rather than FFA content, in the incubation medium is used as an
ideal index for lipolysis. The present result suggested that BBR
induced lipolysis in a dose- and time-dependent manner in porcine
adipocytes, and no cell toxicity was detected, thus indicating that
BBR can effectively increase mobilization of TG from porcine
WAT.
The cAMP-dependent PKA pathway mediates
hormone-stimulated lipolysis in adipocytes. In response to
stimulation of catecholamine hormones and other agonists,
intracellular cAMP production elevates and activates PKA, which
subsequently activates HSL and promotes HSL translocation from the
cytosol to lipid droplets, thus facilitating hydrolysis of TG
(36). In the present study, it
was found that BBR did not increase cAMP content in porcine
adipocytes. Furthermore, the PKA inhibitor H89 did not block
BBR-induced lipolysis, suggesting that BBR-induced lipolysis is
independent of the cAMP/PKA pathway and that this lipolytic action
occurs in the basal state.
Terminal regulation of lipolysis in adipocytes
mainly involves Perilipin A and two rate-limiting enzymes, ATGL and
HSL. Furthermore, ATGL catalyzes the hydrolysis of TG to
diglyceride (DG) and FFA, and HSL is mainly responsible for
hydrolysis of DG (3,4). Activation of HSL is associated with
phosphorylation/dephosphorylation events, which is regulated by the
activities of PKA and AMPK. It has been shown that PKA
phosphorylates HSL at Ser563, Ser659 and Ser660, resulting in
activation of HSL. In contrast, AMPK phosphorylates HSL at Ser565,
which may reduce HSL phosphorylation at the above PKA sites,
leading to inactivation of HSL (14,37,38).
In the present study, BBR did not elevate HSL phosphorylation at
the PKA site, thus suggesting that the cAMP/PKA pathway is not
involved in BBR-induced lipolysis.
Phosphorylation of ATGL by AMPK can enhance its
lipase activity (19). Moreover,
ATGL can also be activated by comparative gene identification 58
(CGI-58) via direct interaction, which is associated with Perilipin
A (39,40). In the basal state, CGI-58 combines
with Perilipin A, an important protein coating the surface of lipid
droplets, which forms a barrier to protect the lipid droplets from
lipases, such as ATGL and HSL. When Perilipin A is phosphorylated
by PKA or is downregulated, the barrier is broken and lipid
droplets are exposed to lipases. Furthermore, CGI-58 is released by
Perilipin A to activate ATGL, which leads to increased lipolysis
(36,40,41).
In the present study, it was demonstrated that BBR decreased the
mRNA and protein expression levels of Perilipin A, and increased
ATGL phosphorylation, while the expression levels of ATGL and HSL
were unchanged. Therefore, the present results indicated that
Perilipin A downregulation and ATGL activation contribute to
BBR-induced lipolysis, which may involve AMPK activation.
AMPK is considered to be an important sensor of
cellular energy homeostasis and is activated upon energy depletion
to enhance fat mobilization and FA oxidation for ATP or heat
production (17). The present
study identified an increase in the phosphorylation of AMPKα in
response to BBR treatment. Moreover, the AMPK inhibitor compound C
suppressed AMPKα phosphorylation and blocked lipolysis in
BBR-treated adipocytes. Moreover, compound C reversed the
inhibitory effect of BBR on the mRNA and protein expression levels
of Perilipin A, as well as BBR-stimulated phosphorylation of ATGL.
Collectively, the present results provided evidence that AMPK
activation mediated BBR-induced lipolysis in porcine adipocytes via
the AMPK/Perilipin A/ATGL pathway. Furthermore, the present results
were partly in line with results from previous studies in 3T3-L1
adipocytes and mice (15,19). However, a previous study showed
that AMPK activation by homocysteine inhibited basal lipolysis in
primary murine adipocytes and 3T3-L1 adipocytes (18). This discrepancy between studies may
be due to variation in the stage of differentiation of
adipocytes.
Lentiviral vectors can produce high viral titers and
induce high gene expression in target cells. Moreover, lentiviral
vectors can be used for delivery of target genes or siRNAs to
terminally differentiated mammalian cells such as adipocytes,
myocytes and hepatocytes, using a replication incompetent
lentivirus (42). The present
results suggested that lentiviral vectors-mediated AMPKα1 shRNA
transfection suppressed the mRNA and protein expression levels of
AMPKα1 in porcine adipocytes. It was found that the inhibitory rate
was ~86% compared with the control and scrambled shRNA group, thus
suggesting that AMPKα1 was efficiently knocked down by the specific
shRNA. Therefore, the recombinant lentivirus containing AMPKα1
shRNA could be used to block the AMPK signaling pathway in the
subsequent experiments.
Mitochondrial FA oxidation is associated with the
density and activity of mitochondria in the cells, which is
regulated by a series of related genes. PGC1-α has been shown to be
a critical transcriptional regulator in mitochondrial biogenesis
and energy metabolism by controlling the expression of its
downstream target genes, such as TFAM and nuclear respiratory
factor 1 (NRF1), as well as the marker genes related to
thermogenesis CPT-1 and UCP2 (43–45).
Moreover, TFAM and NRF1 act as two key transcription factors that
activate mitochondrial DNA transcription and replication
synergistically, thus resulting in increased mitochondrial content
and enhanced mitochondrial respiratory function (46,47).
CPT-1, a rate-limiting enzyme of FA oxidation, functions as a
‘shuttle’ to deliver long-chain FA from the cytoplasm into
mitochondria for β-oxidation (48). UCP2 is thought to drive uncoupling
of oxidative phosphorylation from the mitochondrial respiratory
chain, leading to elevated energy expenditure by heat production in
adipocytes (49). In the present
study, it was demonstrated that BBR treatment increased mRNA
expression levels of PGC-1α, TFAM, CPT-1 and UCP2, which paralleled
alterations to the protein expression levels. In addition, the
present results suggested that knockdown of AMPKα1 in porcine
adipocytes significantly reversed BBR-stimulated upregulation of
the above genes. Thus, the present results indicated that BBR
promoted mitochondrial FA oxidation and thermogenesis in porcine
adipocytes by activating the AMPK/PGC-1α pathway, which may
contribute to the maintenance and enhancement of BBR-induced
lipolysis at least in part due to decreased FFA accumulation.
Moreover, the present results are consistent with previous results
in murine adipose tissue (44).
However, this effect requires further investigation using FFA
radioactive labeling, which will be carried out in future
studies.
Mild mitochondrial uncoupling can induce ATGL/HSL-
independent lipolysis, which relies on a form of autophagy in
3T3-L1 adipocytes (50). Whether
BBR-induced lipolysis involves this pathway is not fully
understood. However, a previous conflicting study revealed that
AMPK has a suppressive effect on FA oxidation and energy
utilization within adipose tissue (19). The discrepancy between studies may
be attributed to the differential physiological status of the
adipocytes.
Insulin signaling plays vital regulatory roles in
energy metabolism and health status (51). It has been shown that serine
phosphorylation of IRS proteins impairs insulin signaling, which is
a mechanism shared by several inducers of insulin resistance
including inflammatory cytokines and FFA (51,52).
Mitochondrial FA oxidation helps alleviate the inhibitory effect of
FFA on insulin signaling in adipocytes (45). In the present study, treatment with
TNFα, a known inflammatory cytokine and lipolytic inducer, enhanced
serine phosphorylation of IRS-1 in porcine adipocytes. However, BBR
treatment did not alter IRS-1 serine phosphorylation, thus
indicating that BBR and BBR-induced lipolysis does not block
insulin signaling at IRS-1 level. Therefore, the present results
suggested that enhanced FA oxidation may accompany BBR-induced
lipolysis to decrease FFA accumulation.
In conclusion, the present results indicated that
BBR induced basal lipolysis in porcine adipocytes via multiple
AMPK-dependent mechanisms. Moreover, this lipolytic process did not
block insulin signaling at the IRS-1 level. Therefore, the present
study provides novel evidence that BBR is a beneficial lipolytic
inducer, and may provide potenital molecular targets for the
reduction of porcine body fat. Furthermore, BBR may be used to
facilitate the development of prevention and treatment strategies
for human obesity and insulin resistance.
Acknowledgements
The authors would like to thank Dr Bin Wu (Arizona
Center for Reproduction, USA) and Dr Shupei Wang (University of
Montreal, Canada) for their helpful suggestions and corrections of
the English manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 30972091), the
Program for Top Young Academic Leaders of Higher Learning
Institutions of Shanxi (grant no. 201004) and the Project of 131
Leading Talent of Higher Learning Institutions of Shanxi (grant no.
2013209).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
YY and RL conceived and designed the experiments.
YY, RL, FG, JZ and FL performed the experiments. FG, JZ and FL
analyzed and interpreted the data. YY, RL and FG wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
strictly carried out according to The Guide for the Care and Use of
Laboratory Animals and were approved by The Institutional Ethics
Committee of Shanxi Normal University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMPKα
|
AMP-activated protein kinase α
|
|
ATGL
|
adipose triglyceride lipase
|
|
BBR
|
berberine
|
|
CPT-1
|
carnitine palmitoyl-transferase-1
|
|
DG
|
diglyceride
|
|
FA
|
fatty acids
|
|
FFAs
|
free fatty acids
|
|
HSL
|
hormone sensitive lipase
|
|
IRS-1
|
insulin receptor substrate-1
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor γ coactivator-1α
|
|
TFAM
|
mitochondrial transcription factor
A
|
|
TG
|
triglyceride
|
|
UCP2
|
uncoupling protein 2
|
References
|
1
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gunawardana SC: Benefits of healthy
adipose tissue in the treatment of diabetes. World J Diabetes.
5:420–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eichmann TO, Kumari M, Haas JT, Farese RV
Jr, Zimmermann R, Lass A and Zechner R: Studies on the substrate
and stereo/regioselectivity of adipose triglyceride lipase,
hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J
Biol Chem. 287:41446–41457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haemmerle G, Zimmermann R, Hayn M, Theussl
C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF and Zechner R:
Hormone-sensitive lipase deficiency in mice causes diglyceride
accumulation in adipose tissue, muscle, and testis. J Biol Chem.
277:4806–4815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SP, Yang H, Wu JW, Gauthier N, Fukao
T and Mitchell GA: Metabolism as a tool for understanding human
brain evolution: Lipid energy metabolism as an example. J Hum Evol.
77:41–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boden G: Effects of free fatty acids (FFA)
on glucose metabolism: Significance for insulin resistance and type
2 diabetes. Exp Clin Endocrinol Diabetes. 111:121–124. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hauke S, Keutler K, Phapale P, Yushchenko
DA and Schultz C: Endogenous fatty acids are essential signaling
factors of pancreatic β-cells and insulin secretion. Diabetes.
67:1986–1998. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xin Y and Wang Y, Chi J, Zhu X, Zhao H,
Zhao S and Wang Y: Elevated free fatty acid level is associated
with insulin-resistant state in nondiabetic Chinese people.
Diabetes Metab Syndr Obes. 12:139–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kashyap S, Belfort R, Gastaldelli A,
Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L,
DeFronzo R and Cusi K: A sustained increase in plasma free fatty
acids impairs insulin secretion in nondiabetic subjects genetically
predisposed to develop type 2 diabetes. Diabetes. 52:2461–2474.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin Y, Khadka DB and Cho WJ:
Pharmacological effects of berberine and its derivatives: A patent
update. Expert Opin Ther Pat. 26:229–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JS, Kim JH, Ali MY, Min BS, Kim GD
and Jung HA: Coptis chinensis alkaloids exert
anti-adipogenic activity on 3T3-L1 adipocytes by downregulating
C/EBP-α and PPAR-γ. Fitoterapia. 98:199–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhao X, Feng X, Liu X, Deng C and Hu
CH: Berberine alleviates olanzapine-induced adipogenesis via the
AMPKα-SREBP pathway in 3T3-L1 cells. Int J Mol Sci. 17:E18652016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Wang Y, Ma SR, Zuo ZY, Wu YB, Kong
WJ, Wang AP and Jiang JD: Berberine inhibits adipocyte
differentiation, proliferation and adiposity through
down-regulating galectin-3. Sci Rep. 9:134152019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou L, Wang X, Yang Y, Wu L, Li F, Zhang
R, Yuan G, Wang N, Chen M and Ning G: Berberine attenuates
cAMP-induced lipolysis via reducing the inhibition of
phosphodiesterase in 3T3-L1 adipocytes. Biochim Biophys Acta.
1812:527–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang D, Wang D, Zhuang X, Wang Z, Ni Y,
Chen S and Sun F: Berberine increases adipose triglyceride lipase
in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis.
15:2142016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carling D: The AMP-activated protein
kinase cascade-a unifying system for energy control. Trends Biochem
Sci. 29:18–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Li S, Wang F and Xin F: Structural
and biochemical insights into the allosteric activation mechanism
of AMP-activated protein kinase. Chem Biol Drug Des. 89:663–669.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Pini M, Yao T, Zhou Z, Sun C,
Fantuzzi G and Song Z: Homocysteine suppresses lipolysis in
adipocytes by activating the AMPK pathway. Am J Physiol Endocrinol
Metab. 301:E703–E712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SJ, Tang T, Abbott M, Viscarra JA,
Wang Y and Sul HS: AMPK phosphorylates desnutrin/ATGL and
hormone-sensitive lipase to regulate lipolysis and fatty acid
oxidation within adipose tissue. Mol Cell Biol. 36:1961–1976. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koopmans SJ and Schuurman T:
Considerations on pig models for appetite, metabolic syndrome and
obese type 2 diabetes: From food intake to metabolic disease. Eur J
Pharmacol. 759:231–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hausman GJ, Basu U, Wei S, Hausman DB and
Dodson MV: Preadipocyte and adipose tissue differentiation in meat
animals: Influence of species and anatomical location. Annu Rev
Anim Biosci. 2:323–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y and Yang G: Rosiglitazone regulates
IL-6-stimulated lipolysis in porcine adipocytes. Biochem Cell Biol.
88:853–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai L, Pang WJ, Yang YJ and Yang GS:
Modulation of Sirt1 by resveratrol and nicotinamide alters
proliferation and differentiation of pig preadipocytes. Mol Cell
Biochem. 307:129–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding L, Zhang F, Zhao MX, Ren XS, Chen Q,
Li YH, Kang YM and Zhu GQ: Reduced lipolysis response to adipose
afferent reflex involved in impaired activation of
adrenoceptor-cAMP-PKA-hormone sensitive lipase pathway in obesity.
Sci Rep. 6:343742016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Liu F, Lu R and Jia J: Berberine
inhibits adipogenesis in porcine adipocytes via AMP-Activated
protein kinase-dependent and -independent mechanisms. Lipids.
54:667–678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Ju D, Zhang M and Yang G:
Interleukin-6stimulates lipolysis in porcine adipocytes. Endocrine.
33:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Capurso C and Capurso A: From excess
adiposity to insulin resistance: The role of free fatty acids.
Vascul Pharmacol. 57:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Yu Z, Li Y, Fichna J and Storr M:
Effects of berberine in the gastrointestinal tract - a review of
actions and therapeutic implications. Am J Chin Med. 42:1053–1070.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu JH, Liu XZ, Pan W and Zou DJ: Berberine
protects against diet-induced obesity through regulating metabolic
endotoxemia and gut hormone levels. Mol Med Rep. 15:2765–2787.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye L, Liang S, Guo C, Yu X, Zhao J, Zhang
H and Shang W: Inhibition of M1 macrophage activation in adipose
tissue by berberine improves insulin resistance. Life Sci.
166:82–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Zhao Y, Zhang M, Pang X, Xu J,
Kang C, Li M, Zhang C, Zhang Z, Zhang Y, et al: Structural changes
of gut microbiota during berberine-mediated prevention of obesity
and insulin resistance in high-fat diet-fed rats. PLoS One.
7:e425292012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barazzoni R, Gortan Cappellari G, Ragni M
and Nisoli E: Insulin resistance in obesity: An overview of
fundamental alterations. Eat Weight Disord. 23:149–157. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ribas V, Nguyen MT, Henstridge DC, Nguyen
AK, Beaven SW, Watt MJ and Hevener AL: Impaired oxidative
metabolism and inflammation are associated with insulin resistance
in ERalpha-deficient mice. Am J Physiol Endocrinol Metab.
298:E304–E319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zambell KL, Horn WF and Keim NL:
Conjugated linoleic acid supplementation in humans: Effects on
fatty acid and glycerol kinetics. Lipids. 36:767–772. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyoshi H, Souza SC, Zhang HH, Strissel
KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC,
Obin MS, et al: Perilipin promotes hormone-sensitive
lipase-mediated adipocyte lipolysis via phosphorylation-dependent
and -independent mechanisms. J Biol Chem. 281:15837–15844. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith AJ, Thompson BR, Sanders MA and
Bernlohr DA: Interaction of the adipocyte fatty acid-binding
protein with the hormone-sensitive lipase: Regulation by fatty
acids and phosphorylation. J Biol Chem. 282:32424–32432. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jocken JW, Roepstorff C, Goossens GH, van
der Baan P, van Baak M, Saris WH, Kiens B and Blaak EE:
Hormone-sensitive lipase serine phosphorylation and glycerol
exchange across skeletal muscle in lean and obese subjects: Effect
of beta-adrenergic stimulation. Diabetes. 57:1834–1841. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lass A, Zimmermann R, Haemmerle G,
Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG,
Gorkiewicz G and Zechner R: Adipose triglyceride lipase-mediated
lipolysis of cellular fat stores is activated by CGI-58 and
defective in Chanarin-Dorfman Syndrome. Cell Metab. 3:309–319.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramanian V, Rothenberg A, Gomez C,
Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R,
Lisanti MP, et al: Perilipin A mediates the reversible binding of
CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem.
279:42062–42071. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sahu-Osen A, Montero-Moran G, Schittmayer
M, Fritz K, Dinh A, Chang YF, McMahon D, Boeszoermenyi A, Cornaciu
I, Russell D, et al: CGI-58/ABHD5 is phosphorylated on Ser239 by
protein kinase A: Control of subcellular localization. J Lipid Res.
56:109–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Durand S and Cimarelli A: The inside out
of lentiviral vectors. Viruses. 3:132–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan Y, Yang X, Zhao T, Zou Y, Li R and Xu
Y: Salicylates promote mitochondrial biogenesis by regulating the
expression of PGC-1α in murine 3T3-L1 pre-adipocytes. Biochem
Biophys Res Commun. 491:436–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan M, Audet-Walsh É, Manteghi S, Dufour
CR, Walker B, Baba M, St-Pierre J, Giguère V and Pause A: Chronic
AMPK activation via loss of FLCN induces functional beige adipose
tissue through PGC-1α/ERRα. Genes Dev. 30:1034–1046. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kleiner S, Mepani RJ, Laznik D, Ye L,
Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman
GI and Spiegelman BM: Development of insulin resistance in mice
lacking PGC-1α in adipose tissues. Proc Natl Acad Sci USA.
109:9635–9640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scarpulla RC: Transcriptional paradigms in
mammalian mitochondrial biogenesis and function. Physiol Rev.
88:611–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Z, Tao S, Li X and Yao Q: Resistin
destroys mitochondrial biogenesis by inhibiting the PGC-1α/
NRF1/TFAM signaling pathway. Biochem Biophys Res Commun. 504:13–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Calderon-Dominguez M, Sebastián D, Fucho
R, Weber M, Mir JF, García-Casarrubios E, Obregón MJ, Zorzano A,
Valverde ÁM, Serra D, et al: Carnitine palmitoyltransferase 1
increases lipolysis, UCP1 protein expression and mitochondrial
activity in brown adipocytes. PLoS One. 11:e01593992016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jia JJ, Zhang X, Ge CR and Jois M: The
polymorphisms of UCP2 and UCP3 genes associated with fat
metabolism, obesity and diabetes. Obes Rev. 10:519–526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Demine S, Tejerina S, Bihin B, Thiry M,
Reddy N, Renard P, Raes M, Jadot M and Arnould T: Mild
mitochondrial uncoupling induces HSL/ATGL-independent lipolysis
relying on a form of autophagy in 3T3-L1 adipocytes. J Cell
Physiol. 233:1247–1265. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tanti JF and Jager J: Cellular mechanisms
of insulin resistance: Role of stress-regulated serine kinases and
insulin receptor substrates (IRS) serine phosphorylation. Curr Opin
Pharmacol. 9:753–762. 2009. View Article : Google Scholar : PubMed/NCBI
|