Introduction
Acute myocardial ischemia leads to an augmentation
in the production of reactive oxygen species, and the activation of
deleterious cellular signaling that causes damage of cardiomyocytes
and endothelial cells (1).
Endothelial dysfunction results in the production of
proinflammatory cytokines and the upregulation of adhesion
molecules, which leads to the infiltration of inflammatory cells to
the infarction region (2).
Furthermore, the impairment of endothelial cell has been identified
as an important factor in aggravating cardiac remodeling (3). Moreover, reduction of capillary
densities within infarcted hearts further accelerates the
remodeling process, which results in heart failure (4).
The Hippo pathway is an evolutionally conserved
pathway that plays an important role in cell proliferation,
apoptosis and angiogenesis (5,6). The
core components of the Hippo pathway include the large tumor
suppressor 2 (Lats2), Yes-associated protein (Yap) and its paralog
protein Transcriptional co-activator with PDZ-binding motif
(5). Activation of Lats2 induces
the phosphorylation of Yap, causing its cytoplasmic retention and
functional inactivation (7,8).
Previous studies have shown that inactivation of the Hippo pathway
is essential for the regulation of cardiac regeneration, function
and remodeling after myocardial infarction (MI) (9,10).
Furthermore, Lats2 is necessary to regulate the ability of Yap in
the epicardium for coronary vascular formation (11). It has been shown that inhibition of
endogenous Lats2 results in reduced infarct size and augmented Yap
activation after myocardial ischemic injury (8,12).
Moreover, loss of Lats2 in the developing heart induces an increase
in Yap activation and is sufficient to cause cardiomyocyte
proliferation (13). Additionally,
Lats2 knockdown in endothelial cells decreases cell apoptosis, and
increases migration and endothelial tube formation (6,14,15).
MicroRNAs (miRs) are small, non-coding RNAs that
function as post-transcriptional regulators to suppress target gene
expression via mRNA degradation or translational inhibition
(16). Previous studies, including
our own, have demonstrated the involvement of miRs in the
regulation of angiogenesis and cardiac remodeling in response to MI
(4,17–19).
It has been reported that miR-302-367 promotes cardiomyocyte
proliferation by repressing Lats2 expression via regulation of the
Hippo pathway (20). In addition,
a previous study showed that miR-93 is involved in angiogenesis and
metastasis by targeting Lats2 (21).
The aim of the present study was to investigate
whether miR-93 exerts a protective role in endothelial cells and
the myocardium after MI via modulation of the Hippo/Lats2/Yap
pathway. The present results suggested that upregulation of miR-93
protected endothelial cells from hypoxia/reoxygenation (H/R)
injury. Furthermore, increased expression of miR-93 promoted
cardiac angiogenesis in ischemic hearts by regulating the Hippo/Yap
pathway and suppressing Lats2 expression.
Materials and methods
Animals
Shanghai populations of Kunming mice (n=220; age,
7–8 weeks; male; weight, 28–30 g) were obtained from the Division
of Laboratory Animal Resources at Xuzhou Medical University. The
mice were maintained under controlled conditions of temperature at
23–25°C, relative humidity of 50–60% and a 12-h light/dark light
cycle with free access to food and water. The experiments were
performed in accordance with The Guide for The Care and Use of
Laboratory Animals published by The National Institutes of Health
(NIH Publication, 8th edition, 2011) (22). All aspects of the animal care and
experimental protocols were approved by Xuzhou Medical University
Committee on Animal Care.
In vitro experiments
Human umbilical vein endothelial cells (HUVECs; cat.
no. 8000; ScienCell Research Laboratories, Inc.) were cultured in
EBM-2 (Gibco; Thermo Fisher Scientific, Inc.; cat. no. 11111044)
supplemental with EGM-2 SingleQuots kit (Lonza Group Ltd.; cat. no.
cc-4147) and 20% FBS (Gibco; Thermo Fisher Scientific, Inc.; cat.
no. 10099141) at 37°C and 5% CO2. Human cardiac
microvascular endothelial cells (HCMECs; cat. no. 6000; ScienCell
Research Laboratories, Inc.) were seeded onto a
fibronectin-precoated culture dishes (2 µg/cm2;
ScienCell Research Laboratories, Inc.; cat. no. 8248) and
maintained in endothelial cell medium (ScienCell Research
Laboratories, Inc.; cat. no. 1001) at 37°C and 5% CO2.
miR-93 mimic (5′-CAAAGUGCUGUUCGUGCAGGUAG-3′) and scrambled (scr)
miR mimic (5′-ACUACUGAGUGACAGUAGA-3′) were purchased from Shanghai
GenePharma Co., Ltd. Cells were transfected with miR-93 mimic (40
nmol) or scr-miR mimic (40 nmol) using RNAifectin (Applied
Biological Materials, Inc.; cat. no. G073). After 48 h of
transfection, the cells were collected for isolation of miRNAs.
miR-93 levels were examined by reverse transcription-quantitative
PCR (RT), as described below. After transfection, the cells were
incubated with hypoxia buffer containing 118 mM NaCl, 24 mM NaHCO3,
1.0 mM NaH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, 20 mM sodium lactate,
16 mM KCl, and 10 mM 2-deoxyglucose (pH 6.2) at 37°C with 5%
CO2 and 95% N2 for 4 h, and subjected to reoxygenation
with EBM-2 (cat. no. 11111044; Gibco; Thermo Fisher Scientific,
Inc.) supplemental with EGM-2 SingleQuots kit (cat. no. cc-4147;
Lonza Group Ltd.) for 24 h at 37°C with 5% CO2. Cells
subjected to H/R without transfection served as positive controls
(H/R). Cells that were not exposed to H/R served as the normoxia
group.
Measurement of lactate dehydrogenase
(LDH) activity and cell viability
Cell injury was determined by measurement of LDH
activity in culture medium using a cytotoxicity detection kit
(Beyotime Institute of Biotechnology; cat. no. C0017). Cell
viability was examined with 10 µl Cell Counting Kit-8 assay
(concentration not commercially available) (Dojindo Laboratories,
Inc; cat. no. CK40) at 37°C for 2 h according to the manufacturer's
protocol. The optical density was measured at a wavelength of 450
nm.
Migration assay
A previously described method was used to evaluate
cell migration (4). Following
transfection, the migration of HUVECs or HCMECs was determined
using a scratch assay with 10% FBS. HUVECs or HCMECs were scratched
with 200 µl tips, and cells were imaged at 0 and 12 h after scratch
using a light microscope (magnification, ×200). The percentage of
the wound area was analyzed with ImageJ software (NIH; version
1.48; http://rsb.info.nih.gov/ij/).
Mouse model of MI
MI was induced as previously described (4). Mice (n=160, weight, 28–30 g) were
anesthetized by intraperitoneal injection of 60 mg/kg sodium
pentobarbital (Sigma-Aldrich; Merck KGaA), intubated and ventilated
using a rodent ventilator. Body temperature was regulated at 37°C
with a heating pad. Following skin incision, hearts were exposed
via a left thoracotomy in the fourth intercostal space. The left
anterior descending (LAD) coronary artery was permanently ligated
with an 8-0 silk ligature. Sham-operated mice underwent the same
surgical procedure except for ligation of the LAD coronary artery.
All animals were recovered in pre-warmed cages.
In vivo transfection of lentivirus
expressing miR-93
Mouse hearts were transfected with lentivirus
expressing miR-93 or scr-miR via the right common carotid artery,
as described previously (4). The
recombinant lentiviruses containing miR-93 (1×108
PFU/ml) and lentiviruses containing scrambled miR (1×108
PFU/ml) were obtained from Shanghai GenePharma Co., Ltd. Mice
(28-30 g) were anesthetized with an intraperitoneal injection of 60
mg/kg sodium pentobarbital. Following the skin incision, the right
common carotid artery was exposed. A micro-catheter was introduced
into the right common carotid artery and positioned into the aortic
root. 50 µl lentivirus expressing miR-93 or 50 µl scrambled miR was
injected via the micro-catheter. The micro-catheter was removed and
the right common carotid artery was tightened before the skin was
closed. After 7 days of transfection, hearts were harvested for
miRNA isolation. miR-93 expression was measured by RT-qPCR for
evaluation of transfection efficiency.
RT-qPCR
Ischemic hearts or cultured cells were harvested and
isolation of miRNAs was performed using the miR isolation kit (cat.
no. RC201; Vazyme Biotech Co., Ltd.). RT-qPCR was conducted using
LightCycler 480 RT-PCR instrument (Roche Diagnostics). According to
the manufacture's protocol, miR-93 expression was quantified using
one-step RNA TaqMan qPCR mix kit (Xinhai Gene Testing Co., Ltd.;
cat. no. A2302) and TaqMan qPCR mix kit (Xinhai Gene Testing Co.,
Ltd.; cat. no. A2301). RT conditions were as follows: 16°C for 30
min, 42°C for 30 min and 75°C for 15 min. qPCR amplification
conditions were as follows: Initial denaturation at 95°C for 15
min; 40 cycles of 95°C for 10 sec and 60°C for 60 sec. Specific
primers were obtained from Applied Biosystems (mmu-miR-93, primer
identification no. 001090; miR-93: Forward,
5′-AGGCCCAAAGTGCTGTTCGT-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6
small nucleolar RNA, primer identification no. 001973; U6: Forward,
5′-GCTTCGAGGCAGGTTACATG-3′ and reverse,
5′-GCAACACACAACATCTCCCA-3′). miR-93 levels were quantified using
the 2−ΔΔCq method and were normalized to the U6
expression level (23).
Ischemic hearts were collected for
isolation of RNA
Total RNA was isolated with total RNA extraction
reagent (Vazyme Biotech Co., Ltd.; cat. no. R401-01). According to
the manufacturer's instructions, cDNA was synthesized using RT
MasterMix (Applied Biological Materials, Inc.; cat. no. G485). The
following conditions were used for RT: 25°C for 10 min, followed by
42°C for 15 min. qPCR reactions (10 µl) consisted of 5 µl
PrimeScipt RT master mix (Takara Bio, Inc.), 0.5 µl primer (final
concentration, 10 nM), 2 µl DEPC water and 2 µl cDNA. Reactions
were run in a LightCycler 480 instrument II (Roche Diagnostics Co.,
Ltd.). qPCR with SYBR Green detection (Takara Bio, Inc.) was
performed. qPCR amplification conditions were as follows: Initial
denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for
15 sec, 56°C for 30 sec and 72°C for 30 sec. The following primers
were used for cDNA amplification: Lats2: Forward,
5′-GACGATGTTTCCAACTGTCGCTGTG-3′ and reverse,
5′-CAACCAGCATCTCAAAGAGAATCACAC-3′; matrix metalloproteinase
(MMP)-2: Forward, 5′-CAAGTTCCCCGGCGATGTC-3′ and reverse,
5′-TTCTGGTCAAGGTCACCTGTC-3′; and GAPDH: Forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-UUCUCCGAACGUGUCACGUTT-3′. Relative levels were quantified with
the 2−ΔΔCq method and were normalized to GAPDH (23).
Echocardiography
Echocardiography was induced as previously described
(4,24,25).
After 7 days of transfection with lentivirus expressing miR-93 or
scr-miR, mice were subjected to MI. Cardiac function was examined
by echocardiography prior to MI (baseline), and after MI at days 3,
14 and 21. The percentage of ejection fraction (EF) and fractional
shortening (FS) were calculated as follows: EF = (end-diastole
volume - end-systole volume)/end-diastole volume × 100; FS = (left
ventricular end-diastole diameter - left ventricular end-systole
diameter)/left ventricular end-diastole diameter × 100.
Masson's trichrome stain
Cardiac fibrosis was determined using Masson's
trichrome staining. Mice were transfected with lentivirus
expressing miR-93 or scr-miR 7 days before induction of MI.
Subsequently, 3 weeks after MI, hearts were harvested, post-fixed
with 4% paraformaldehyde for 12 h at 4°C, and sliced horizontally.
Sections (5 µm) were stained using Masson's trichrome staining kit
(cat. no. G1340; Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 1 h according to the manufacturer's
protocol (concentrations not commercially available). Stained
sections were examined using light microscope (magnification,
×12.5) and analyzed using ImageJ software (NIH; version 1.48;
http://rsb.info.nih.gov/ij/).
Immunohistochemistry staining
Immunohistochemical staining was performed as
described previously (26). Mice
were scarified after MI and perfused with 4% buffered
paraformaldehyde via the ascending aorta. Heart tissues were
post-fixed with 4% paraformaldehyde for 12 h at 4°C and embedded in
paraffin. Sections (5 µm) were blocked with 4% donkey serum (cat.
no. 136110; Jackson ImmunoResearch, Inc.) for 1 h at room
temperature and incubated with anti-CD31 (1:50; cat. no. ab28364;
Abcam), anti-intracellular adhesion molecule 1 (ICAM-1; 1:50; cat.
no. sc8439; Santa Cruz Biotechnology, Inc.) and anti-vascular cell
adhesion molecule 1 (1:50; cat. no. sc8304; VCAM-1; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. Subsequently, sections were
incubated with 50 µl IHC detection reagent at 25°C (cat. nos. 8114
and 8125, respectively; Cell Signaling Technology, Inc.) according
to the manufacturer's protocol (concentrations not commercially
available). Slides were stained with DAB Substrate kit (cat. no.
8059; Cell Signaling Technology, Inc.) and then counterstained with
100 µl hematoxylin (concentration not commercially available) (cat.
no. C0107; Beyotime Biotechnology, Inc.) at 25°C for 10 sec. Images
were captured using light microscopy (magnification, ×400).
Infiltration of neutrophils and
macrophages
At 3 days post-MI, hearts were harvested, post-fixed
with 4% paraformaldehyde for 12 h at 4°C and sliced horizontally.
According to the manufacture's protocol, infiltrated neutrophils in
heart sections (5 µm) were stained with naphtol AS-D chloroacetate
esterase kit (cat. no. 90C2; Sigma-Aldrich; Merck KGaA) followed by
hematoxylin (concentrations not commercially available) at 25°C for
10 sec. Accumulation of macrophages in the ischemic hearts was
evaluated using the macrophage specific antibody F4/80 (1:50; cat.
no. 70076; Cell Signaling Technology, Inc.), followed by
counterstaining with 100 µl hematoxylin (concentration not
commercially available) (cat. no. C0107; Beyotime Biotechnology,
Inc) at 25°C for 10 sec. A total of four independent areas of each
section were examined using light microscopy at a magnification of
×400.
Luciferase reporter assay
Lats2 was predicted as a target of miR-93 by the
targetScan (http://www.targetscan.org/), miRanda (http://www.microrna.org/), and PicTar (http://pictar.mdc-berlin.de/) database. Partial
fragment of Lats2 wild-type 3′-untranslated regions (UTR;
Lats2-3′-UTR-WT) containing the putative miR-93 binding sites or
mutant sequence (Lats2-3′-UTR-MUT) were cloned into psiCHECK-2
vectors (Promega Corporation; cat. no. C8021). HUVECs were
co-transfected with Lats2-3′-UTR-WT (1 µg) or Lats2-3′-UTR-MUT (1
µg) reporter, and miR-93 mimics (50 nM) or scr-miR mimics (50 nM)
using Lipofectamine 2000 (cat. no. 11668027; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. At 48
h of transfection, luciferase activities were examined via
comparison with Renilla luciferase activity using the Dual
Luciferase reporter assay system (Promega Corporation; cat. no.
E1910).
Western blotting
Western blotting was performed as described
previously (4). Mice were
transfected with lentivirus expressing miR-93 or scr-miR 7 days
before induction of MI. Subsequently, 3 days after MI, ischemic
hearts were harvested for isolation of cellular proteins. In
additional, HUVECs were transfected with miR-93 mimic (40 nmol) or
scr-miR mimic (40 nmol) prior to H/R injury, and the cell lysates
were prepared using RIPA lysis buffer (Beyotime Biotechnology, Inc;
cat. no. P0013B). The protein concentration was determined using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.; cat. no. 23227). A total of 100 µg cellular proteins were
separated by 12% SDS-PAGE and then transferred to PVDF membranes
(EMD Millipore). PVDF membranes were incubated with following
primary antibodies at 4°C overnight: Anti-phosphorylated (p)-Yap
(Cell Signaling Technology, Inc.; cat. no. 13008; 1:500),
anti-cleaved caspase-3 (Cell Signaling Technology, Inc.; cat. no.
9661; 1:500), anti-Lats2 (ProteinTech Group, Inc.; cat. no.
20276-1-AP; 1:100), anti-Yap (Santa Cruz Biotechnology, Inc.; cat.
no. sc101199; 1:100), anti-Bax (Cell Signaling Technology, Inc.;
cat. no. 2744; 1:500), anti-Bcl2 (Santa Cruz Biotechnology, Inc.;
cat. no. sc56015; 1:100), anti-ICAM-1 (Santa Cruz Biotechnology,
Inc.; cat. no. sc8439; 1:100), anti-VCAM-1 (Santa Cruz
Biotechnology, Inc.; cat. no. sc8304; 1:100), anti-vascular
endothelial growth factor (VEGF; Santa Cruz Biotechnology, Inc.;
cat. no. sc7269; 1:100) and anti-GAPDH (ProteinTech Group, Inc.;
cat. no. 60004-1-1g; 1:4,000). The membranes were then incubated
with peroxidase-conjugated secondary antibodies at 25°C for 2 h
(Cell Signaling Technology, Inc.; cat. no. 7074, 1:4000; cat. no.
7076; 1:4,000) and analyzed with an ECL system (Thermo Fisher
Scientific, Inc.; cat. no. 32106). The signals were quantified
using ImageJ software (NIH; version 1.48; http://rsb.info.nih.gov/ij/).
Statistical analysis
Data are presented as the mean ± SEM. Comparisons of
data between groups were performed using one ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-93 attenuates H/R-induced cell
injury and cell apoptosis in HUVECs
As endothelial cells are critical for angiogenesis
in response to MI (4), the present
study investigated the effect of H/R on miR-93 expression in
HUVECs. It was identified that H/R induced a time-dependent
reduction in the expression of miR-93 (Fig. 1A).
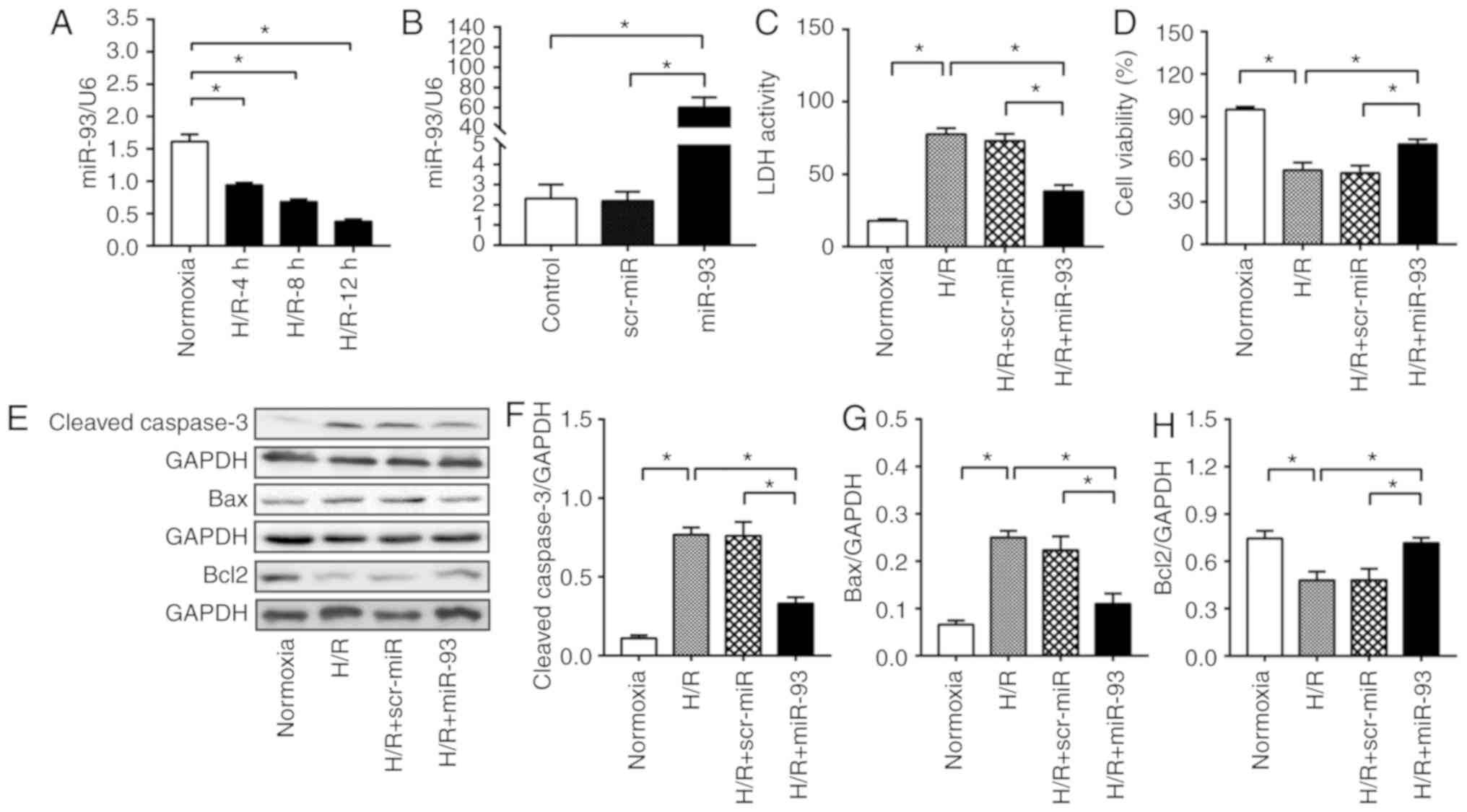 | Figure 1.miR-93 attenuates H/R-induced cell
injury and cell apoptosis in HUVECs. (A) Time course of miR-93
expression in HUVECs before and after H/R was examined using
RT-qPCR. n=4-5 replicates per group. (B) RT-qPCR analysis revealed
an increased expression of miR-93 in HUVECs transfected with miR-93
mimic. n=4–5 replicates per group. After 48 h of transfection,
HUVECs were exposed to 4 h hypoxia, followed by 24 h reoxygenation.
(C) Transfection of HUVECs with miR-93 mimic decreased LDH activity
and (D) increased cell viability following H/R injury. n=4
replicates per group. (E) Representative bands of (F) cleaved
caspase-3, (G) Bax and (H) Bcl2. Transfection of HUVECs with miR-93
mimic decreased the expression levels of cleaved caspase-3 and Bax,
and increased the expression of Bcl2 after H/R injury. n=4–5
replicates per group. Data are presented as the mean ± SEM. Each
experiment was repeated three times. *P<0.05 vs. the indicated
group. H/R, hypoxia/reoxygenation; HUVECs, human umbilical vein
endothelial cells; scr, scrambled; miR, microRNA; LDH, lactate
dehydrogenase; RT-qPCR, reverse transcription-quantitative PCR. |
To examine the effect of miR-93 on H/R-induced cell
injury in HUVECs, HUVECs were transfected with miR-93 mimic or
scrambled miR mimic for 48 h. It was found that transfection with
miR-93 mimic induced a significant increase in miR-93 expression by
25.1-fold compared with the control (Fig. 1B). Moreover, transfection with
scr-miR mimic did not increase miR-93 expression. The present
results suggested that H/R resulted in an increase in LDH activity
(Fig. 1C). However, transfection
with miR-93 mimic significantly decreased LDH activity compared
with H/R (38.3±4.3 vs. 77.5±4.3). However, transfection with
scr-miR mimic did not affect LDH activity in HUVECs. The results of
the CCK-8 assay further demonstrated that H/R significantly
decreased cell viability (by 44.9%) compared with normoxia
(Fig. 1D). However, it was found
that the H/R-induced decrease in cell viability was significantly
reversed by miR-93 mimic. Moreover, transfection with scr-miR mimic
did not affect the H/R-induced decrease in cell proliferation and
viability.
To assess the effect of miR-93 on the apoptosis of
HUVECs, the present study examined the expression levels of cleaved
caspase-3, Bax and Bcl2. Cleaved caspase-3 and Bax are important
pro-apoptotic proteins, while Bcl2 is an important anti-apoptotic
protein (27). The present results
indicated that the miR-93 mimic significantly attenuated the
expression levels of cleaved caspase-3 by 56.8% and Bax by 56.1%
compared with H/R (Fig. 1F-H).
However, H/R-decreased Bcl2 expression was significantly higher in
miR-93-transfected HUVECs (0.7±0.03) compared with H/R group
(0.5±0.06). Furthermore, scr-miR mimic did not affect the
H/R-induced increase in Bax and cleaved caspase-3 expression
levels, nor the decreased in Bcl2 expression.
miR-93 promotes HUVEC migration
Subsequently, the effect of miR-93 on HUVEC
migration was determined by scratch assay. It was demonstrated that
the control group cells migrated by 34.6% at 12 h. However, the
miR-93 mimic-transfected HUVECs migrated into the wound area by
60.0% at 12 h post-scratch (Fig.
2A). In line with this observation, scr-miR mimic-transfected
HUVECs exhibited a lower capacity to migrate compared with the
miR-93 mimic group. miR-93 suppresses Lats2 expression and
inactivates the Hippo pathway in HUVECs. Lats2, as a core kinase in
the Hippo signaling pathway, was predicted to be a potential target
of miR-93 using a miRNA database. It has been reported that the
Hippo pathway regulates cell proliferation and apoptosis, as well
as angiogenesis (5,6). Therefore, the present study examined
whether miR-93 could suppress Lats2 expression in HUVECs, thus
leading to inactivation of the Hippo/Yap pathway. To assess this
hypothesis, Lats2-3′-UTR-WT or Lats2-3′-UTR-MUT vectors containing
WT or MUT miR-93 binding sites were constructed, respectively. It
was demonstrated that co-transfection of the reporters with miR-93
mimic induced a significant reduction in luciferase activity
compared with scrambled miR (Fig.
2B). Furthermore, it was identified that the miR-93 mimic
induced a significant reduction in Lats2 protein expression by
36.7% compared with H/R group (Fig. 2C
and D). In addition, Lats2 expression was significantly lower
in miR-93-transfected HUVECs (0.7±0.1) compared with scr-miR
mimic-transfected HUVECs (1.2±0.1) after H/R injury. Subsequently,
the protein expression levels of major downstream modulators in the
Hippo pathway were examined. It was found that transfection with
miR-93 mimic significantly attenuated Yap phosphorylation, thus
increasing the expression of Yap in the cytosol of HUVECs (Fig. 2E and F). However, transfection with
scr-miR mimic did not alter the expression levels of p-Yap and Yap
after H/R injury in HUVECs.
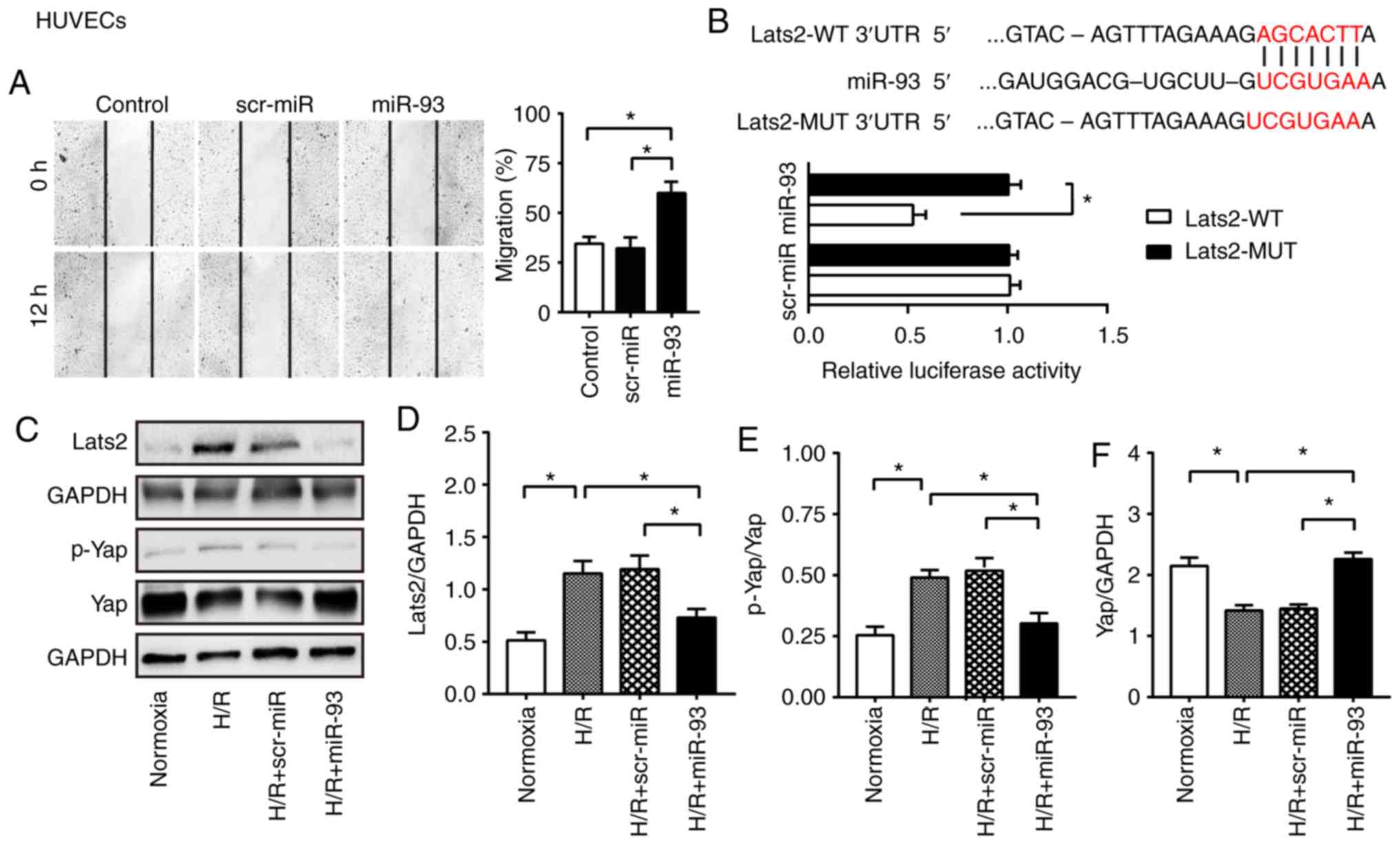 | Figure 2.miR-93 enhances endothelial cell
migration, and suppresses Lats2 expression and Yap phosphorylation
in endothelial cells. (A) Representative images of scratch-wound
assay before and 12 h after scratch formation in HUVECs
(magnification, ×200). The line indicates the width of the gap.
Transfection with miR-93 mimic promoted HUVEC migration into the
wound area. n=4 replicates per group. (B) Predicted binding sites
between miR-93 and Lats2 3′UTR. miR-93 mimics decreased luciferase
activity. n=4 replicates per group. (C) Representative bands of
Lats2, p-Yap and Yap in HUVECs. Expression of (D) Lats2 was
suppressed in miR-93 mimic-transfected HUVECs. Transfection with
miR-93 mimic (E) decreased Yap phosphorylation and (F) increased
Yap expression in the cytosol after HUVECs H/R. n=4 replicates per
group. HCMECs were transfected with miR-93 mimics or scr-miR mimics
for 48 h. (G) Reverse transcription-quantitative PCR showed the
upregulation of miR-93 in HCMECs by miR-93 mimics transfection. n=4
replicates per group. (H) Transfection of HCMECs with miR-93 mimic
increased cell viability following H/R injury. n=4 replicates per
group. (I) Representative images of scratch-wound assay at 0 and 12
h after scratch formation in HCMECs (magnification, ×100). The line
indicates the width of the gap. Transfection with miR-93 mimic
promoted HCMEC migration into the wound area. n=4 replicates per
group. (J) Representative bands of Lats2 and p-Yap in HCMECs.
Western blotting results identified the attenuation of (K) Lats2
and (L) p-Yap expression levels by miR-93 transfection after H/R in
HCMECs. Data are presented as the mean ± SEM. Each experiment was
repeated three times. *P<0.05 vs. the indicated group. Lats2,
large tumor suppressor 2; Yap, Yes-associated protein; HUVECs,
Human umbilical vein endothelial cells; HCMECs, human cardiac
microvascular endothelial cells; scr, scrambled; miR, microRNA;
H/R, hypoxia/reoxygenation; p, phosphorylated; 3′UTR, 3′
untranslated region; WT, wild-type; MUT, mutant. |
miR-93 enhances HCMEC viability and
migration
To further investigate the effect of miR-93 on the
viability and migration of endothelial cells, HCMECs were
transfected with miR-93 mimic or scr-miR mimic for 48 h. The
present results suggested that transfection of miR-93 mimic induced
a significant upregulation of miR-93 expression in HCMECs (Fig. 2G). Furthermore, transfection of
miR-93 mimics significantly increased cell viability (Fig. 2H) and the capacity to stimulate
endothelial cell migration (Fig.
2I) after H/R injury. In addition, it was found that miR-93
mimic-transfected HCMECs exhibited a downregulation in Lats2
expression (Fig. 2J and K) and Yap
phosphorylation (Fig. 2J and L)
following H/R injury, when compared with HCMECs transfected with
scr-miR mimic after H/R injury.
miR-93 results in an improvement of
cardiac function after MI
To evaluate the effect of miR-93 on MI in
vivo, the present study examined whether miR-93 expression in
the myocardium could be altered after MI. The present results
indicated that the expression of miR-93 was significantly decreased
by 53.0% on day 1, 73.1% on day 3 and 80.9% on day 7 after MI
compared with sham-operated mice (Fig.
3A), thus suggesting that increased expression of miR-93 may
serve a protective role against MI. To further investigate the role
of miR-93 in the development of MI, lentivirus vectors expressing
miR-93 mimic were used to increase miR-93 expression in the
myocardium. Hearts that were not subjected to transfection served
as the controls. RT-qPCR results identified that
miR-93-transfection significantly increased miR-93 expression by
2.2-fold in the myocardium after MI compared with scr-miR
transfection (Fig. 3B). Moreover,
miR-93 expression was also significantly elevated in the myocardium
in sham-operated mice transfected with miR-93 compared with the
scr-miR group (Fig. 3B).
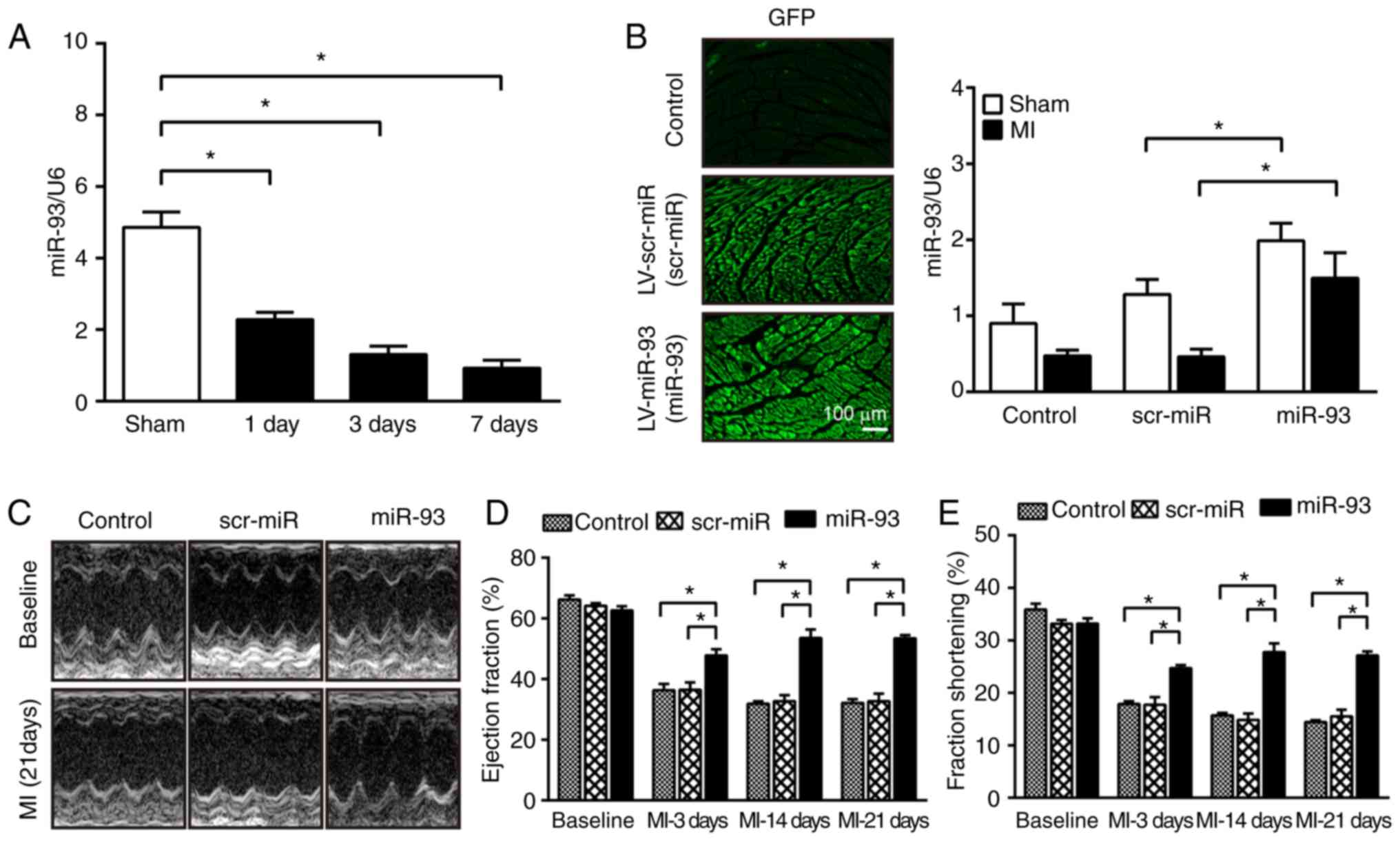 | Figure 3.Increased expression of miR-93
attenuates cardiac dysfunction after MI. (A) miRNAs were isolated
from sham-operated and infarcted hearts at 1, 3 and 7 days after
MI. RT-qPCR analysis revealed a downregulation of miR-93 expression
in the myocardium after MI. n=4-5 mice per group. (B)
Representative images of heart sections at 7 days after
transfection with lentiviral vectors (magnification, ×200). RT-qPCR
revealed the expression level of miR-93 in the myocardium after
transfection. n=4-5 mice per group. (C) Representative images of
M-mode echocardiography at baseline and 21 days after MI.
Echocardiography parameters were measured at baseline and at 3, 14
and 21 days after MI. Increased expression of miR-93 reversed
MI-induced decreases in (D) EF% and (E) FS%. n=5-7 mice per group.
Data are presented as the mean ± SEM. *P<0.05 vs. the indicated
group. scr, scrambled; miR/miRNA, microRNA; MI, myocardial
infarction; LV, lentivirus; EF, ejection fraction; FS, fraction
shortening; RT-qPCR, reverse transcription-quantitative PCR. |
The present study then evaluated the effect of
miR-93 on cardiac function after MI. It was demonstrated that MI
induced significant decreases in EF% and FS% values in the control
and scr-miR-transfected mice from at days 3–21 compared with the
respective baseline (Fig. 3D and
E). However, the values of EF% and FS% in miR-93-transfected
mice were significantly higher compared with scr-miR-transfected
mice at all the time points measured after MI. Furthermore, there
were no significant differences in the baseline of cardiac function
among the three groups.
miR-93 enhances angiogenesis and
attenuates cardiac fibrosis after MI
The present in vitro data indicated that
miR-93 promoted endothelial cell proliferation and migration.
Angiogenesis is an important factor in the restoration of cardiac
function after MI (28).
Therefore, the present study examined whether upregulation of
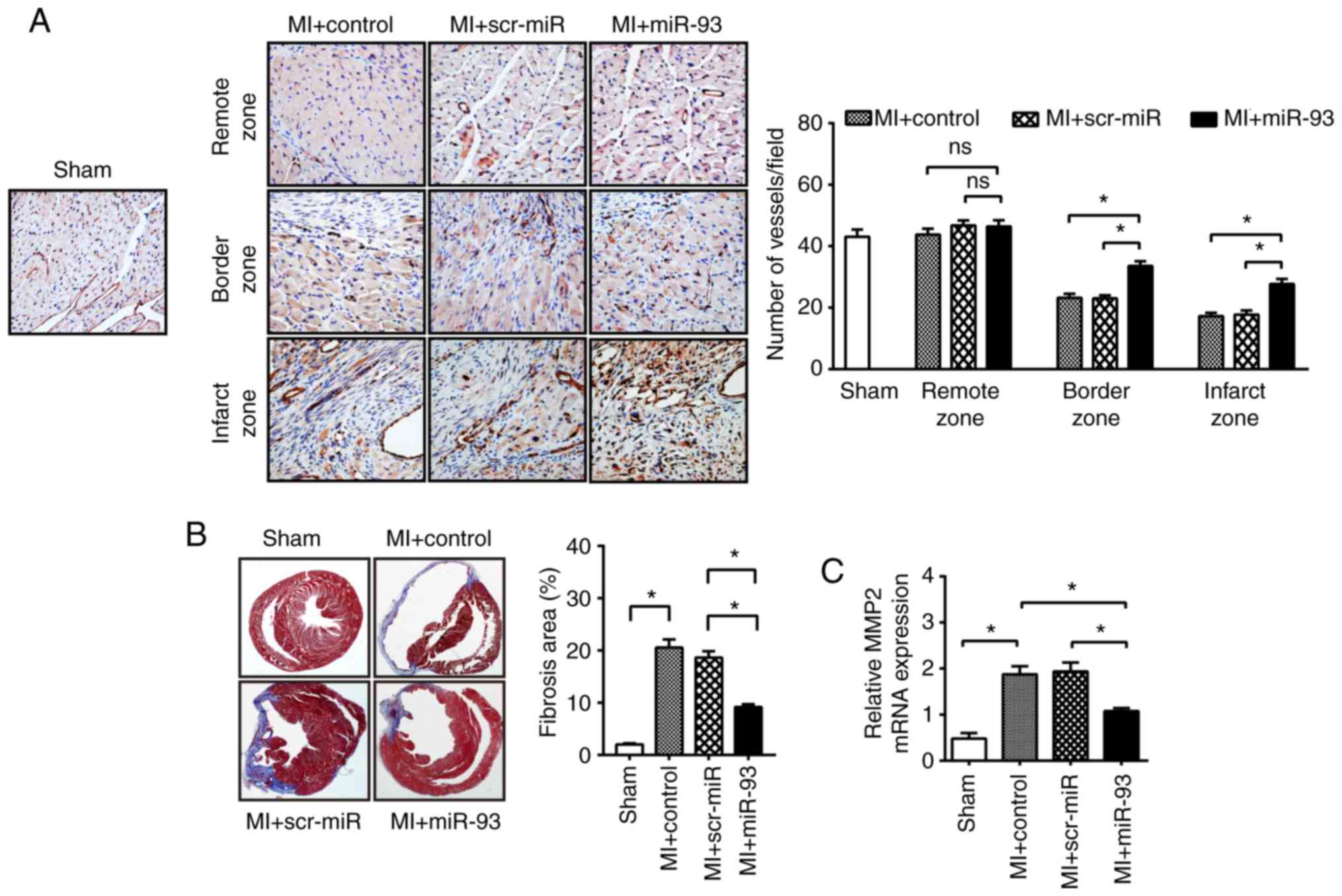
miR-93 could enhance angiogenesis in an ischemic heart. It was
identified that the microvascular density in the border area and
infarcted area at 21 days after MI was greatly decreased by 46.1
and 60.2%, respectively, compared with the sham control group
(Fig. 4A). Furthermore, miR-93
overexpression significantly promoted microvascular density in the
infarcted myocardium compared with the MI control (27.7±1.7 vs.
17.2±1.1). However, transfection with scr-miR did not inhibit the
MI-induced decrease in microvascular density.
Reduction in the microvascular density in myocardium
accelerates the cardiac remodeling process (29). Cardiac fibrosis further contributes
to cardiac dysfunction after MI (30). Thus, the present study examined
cardiac fibrosis using Masson's Trichrome staining of collagen
deposition at day 21 after MI. No difference in the deposition of
MI-induced cardiac fibrosis was observed between the
scr-miR-transfected group and the control. However, the percentage
of fibrotic area in miR-93-transfected hearts was significantly
reduced by 35.7% compared with scr-miR-transfected hearts after MI
(Fig. 4B). MMP-2 is involved in
collagen deposition after MI (31). Moreover, it was identified that
MI-induced MMP-2 mRNA expression was significantly decreased in
miR-93-transfected hearts (1.1±0.1) compared with the control
hearts (1.9±0.2; Fig. 4C).
However, transfection with scr-miR did not alter MMP-2 expression
in the myocardium after MI (Fig.
4C).
miR-93 attenuates the expression of
adhesion molecules in the myocardium after MI
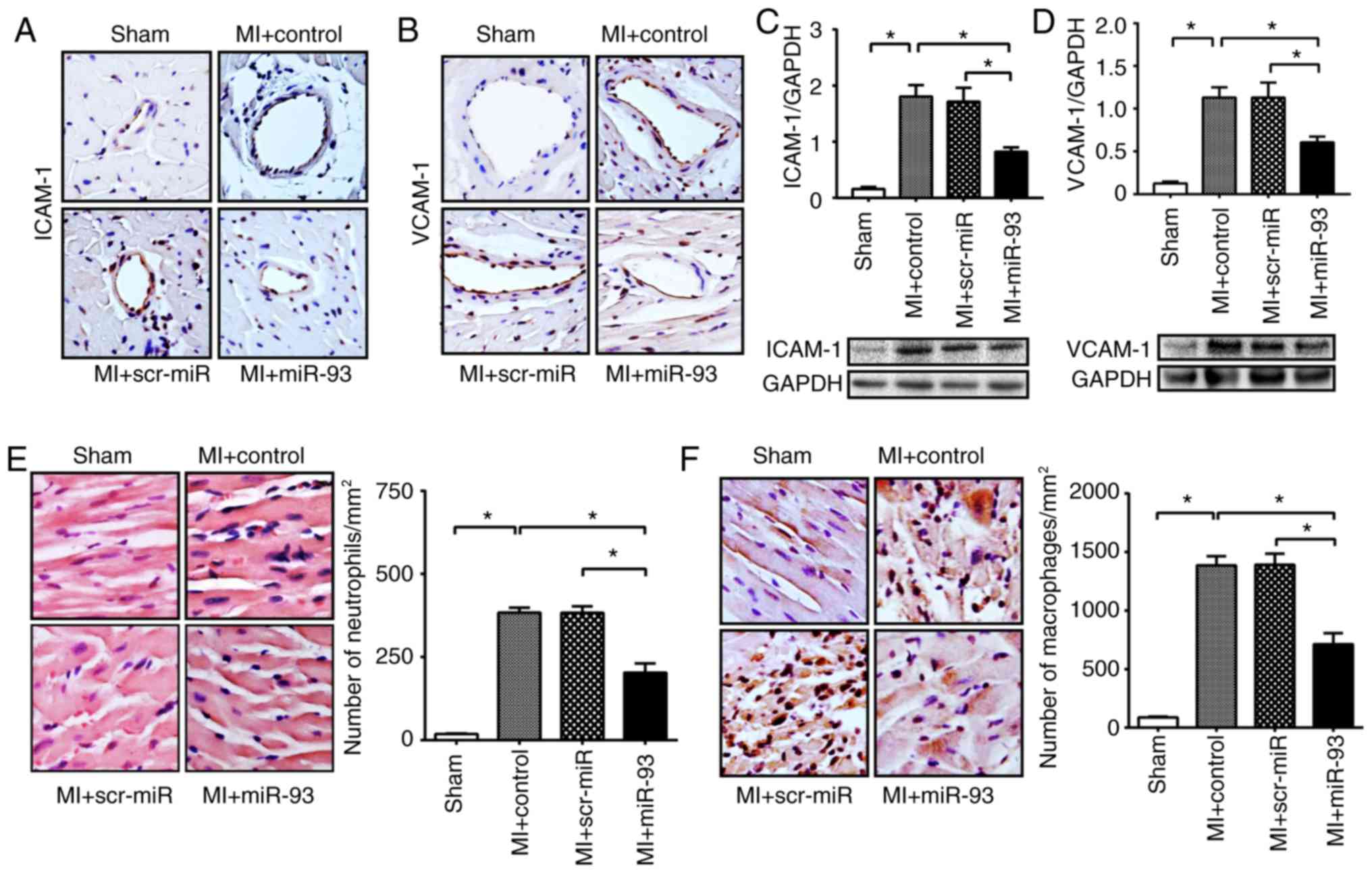
MI-induced endothelial activation is an important
determinant that causes cardiac dysfunction (32). Hence, the present study
investigated the effect of miR-93 on the expression levels of
ICAM-1 and VCAM-1, which are two important adhesion molecules that
are highly expressed in activated endothelial cells (26). It was identified that control and
scr-miR transfection groups had a higher level of positive staining
of ICAM-1 and VCAM-1 in the myocardium after MI (Fig. 5A and B). However, overexpression of
miR-93 resulted in a decreased amount of positive staining of
ICAM-1 and VCAM-1 in the ischemic myocardium. Furthermore, western
blotting results demonstrated that transfection with miR-93 mimic
significantly reduced the protein expression levels of ICAM-1 by
54.5% and VCAM-1 by 46.4%, compared with the MI control (Fig. 5C and D). However, transfection with
scr-miR did not attenuate the protein expression levels of ICAM-1
and VCAM-1 in the myocardium after MI (Fig. 5C and D).
miR-93 attenuates the infiltration of
neutrophils and macrophages into the myocardium after MI
In the early inflammatory response to MI,
circulating neutrophils and macrophages infiltrate the infarcted
area via the endothelial barrier, thus exacerbating cardiac
dysfunction (33). Therefore, the
present study examined the effect of miR-93 on the infiltration of
neutrophils and macrophages into the myocardium after MI. The
present results suggested that the number of neutrophils and
macrophages was significantly increased following MI compared with
the respective sham control (Fig. 5E
and F). Furthermore, miR-93 overexpression significantly
attenuated MI-induced infiltration of neutrophils by 40.7% and
macrophages by 47.3% compared with the respective MI control
(Fig. 5E and F). However,
transfection with scr-miR did not prevent MI-induced accumulation
of neutrophils and macrophages in the myocardium.
miR-93 suppresses Lats2 expression and
inactivates the Hippo/Yap pathway after MI
The present in vitro data indicated that
miR-93 attenuated endothelial injury by targeting Lats2. Thus, the
present study subsequently investigated whether increased
expression of miR-93 could suppress Lats2 expression in the
myocardium following MI. Abundant mRNA expression levels of Lats2
were observed in sham and MI control hearts compared with the
respective miR-93-transfected hearts (Fig. 6A). Moreover, western blot analysis
results revealed that miR-93 overexpression signficantly
downregulated the expression of Lats2 by 44.4% in the sham group
and by 45.4% in MI hearts, compared with the scr-miR transfected
group (Fig. 6B). It was found that
increased expression of miR-93 significantly attenuated Yap
phosphorylation (S127) by 53.1% after MI compared with the MI
control (Fig. 6D). However,
transfection with scr-miR did not prevent the downregulation of
p-Yap after MI. The present results indicated that overexpression
of miR-93 significantly increased total Yap expression by 65.6 and
83.6% after MI compared with the control and scr-miR-transfected
group, respectively (Fig. 6E). The
present study also examined the effect of miR-93 on VEGF expression
after MI, and it was identified that increased expression of miR-93
in the myocardium had no effect on the expression of VEGF after MI
(Fig. 6F).
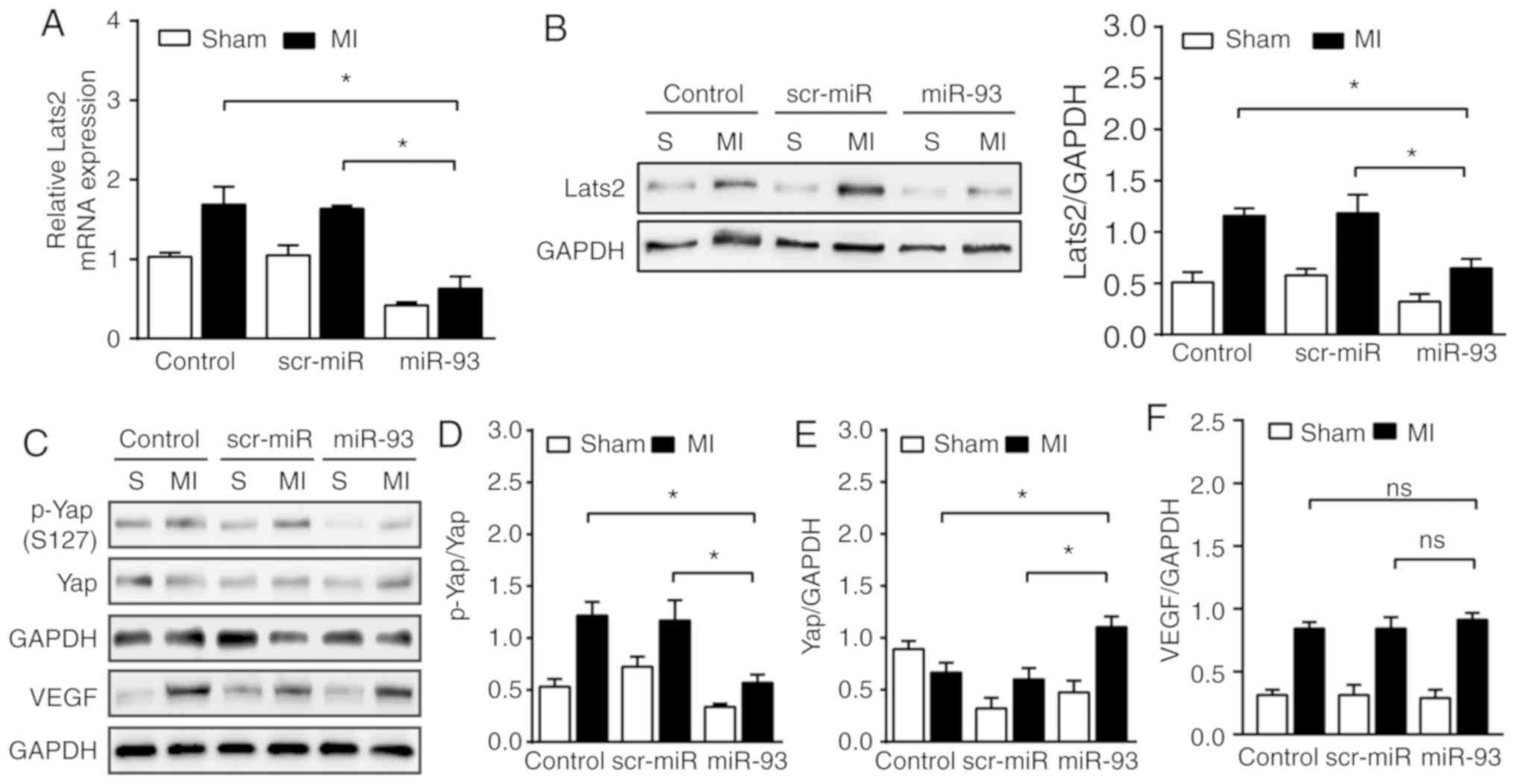 | Figure 6.Increased expression levels of miR-93
suppress Lats2 expression levels and inactivates the Hippo/Yap
pathway after MI. (A) Reverse transcription-quantitative PCR
analysis identified the downregulation of Lats2 mRNA expression by
transfection of lentivirus expressing miR-93 on day 3 of MI. n=4
mice per group. (B) Western blot analysis revealed the suppression
of Lats2 expression by lentivirus transfection expressing miR-93
after MI. n=4-5 mice per group. (C) Representative bands of p-Yap,
Yap and VEGF on day 3 of MI. Increased expression of miR-93 induced
a (D) decrease in the phosphorylation levels of Yap and an (E)
increase in total Yap expression after MI. n=4-6 mice per group.
(F) Increased expression of miR-93 in the myocardium had no effect
on the expression of VEGF after MI. n=4 mice per group. Data are
presented as the mean ± SEM. *P<0.05 vs. the indicated group.
ns, not significant. MI, myocardial infarction; scr, scrambled;
miR, microRNA; Lats2, large tumor suppressor 2; Yap, Yes-associated
protein; p, phosphorylated; VEGF, vascular endothelial growth
factor; S, sham. |
Discussion
The present results suggested that miR-93 was
involved in the pathogenesis of MI. It was identified that
transfection of HUVECs with miR-93 mimic resulted in a
cytoprotective effect, along with the enhancement of cell
proliferation and viability, and the attenuation of cell apoptosis
after H/R injury. Moreover, an increased expression of miR-93 led
to the improvement of cardiac function, promotion of angiogenesis
and reduction of cardiac remodeling, as well as attenuation of the
infiltration of neutrophils and macrophages into the myocardium
following MI. Furthermore, the suppression of Lats2 expression and
inactivation of the Hippo/Yap pathway were involved in the
underlying mechanisms of the protective role of miR-93 against MI,
both in vitro and in vivo. Collectively, the present
results indicated that there may be an association between miR-93
and the development of MI, and thus may provide a promising
therapeutic target.
Previous findings have shown that miRNAs, which are
small non-coding RNAs, play critical roles in MI (17–19).
A recent study showed that adipose-derived stromal cells-derived
miR-93-5p-containing exosomes prevents MI-induced cardiac injury by
inhibiting autophagy and the inflammatory response (34). In line with this, the present study
identified that increased expression of miR-93 reversed MI-induced
cardiac dysfunction and remodeling. However, the protective
mechanism of miR-93 after MI has not been fully elucidated. It has
been revealed that angiogenesis is a critical step for heart repair
in response to MI (4). Previous
studies, including our own, have demonstrated that the promotion of
angiogenesis by miRNAs induces functional recovery of ischemic
hearts after MI (4,17–19).
Moreover, previous studies have reported the effect of miR-93 on
cardiomyocytes after H/R injury but not on endothelial cells
(34,35). Endothelial cells are the most
abundant non-cardiomyocytes in the heart of adult mammals (32). Therefore, in the present study, the
effect of miR-93 on the endothelial cells were evaluated. Previous
findings have shown that miR-93 enhances endothelial cell viability
and inhibits endothelial cell apoptosis under normoxia (36). However, the cell function of miR-93
in endothelial cells under hypoxia remains to be investigated. The
present results indicated that transfection with miR-93 mimic
significantly increased endothelial cell viability in response to
H/R injury. Furthermore, H/R-induced endothelial apoptosis was
significantly inhibited by transfected with miR-93 mimic. Cleaved
capase-3 and Bax are two important pro-apoptotic proteins, while
Bcl2 is an anti-apoptotic protein (27). In the present study, it was found
that transfection with miR-93 decreased in Bax expression and
increased Bcl2 expression after H/R. In addition, transfection with
miR-93 mimic led to an enhancement in the migratory capacity of
endothelial cells. Therefore, the present in vitro data
suggested that miR-93 may exhibit a cytoprotective effect against
H/R injury in endothelial cells.
The Hippo pathway is an evolutionally conserved
pathway that plays an important role in the regulation of cell
proliferation, differentiation and apoptosis (5,6).
Activation of Lats1/2, a core kinase of the Hippo pathway, results
in the phosphorylation of Yap, which leads to its cytoplasmic
retention and functional inactivation (5). Fang et al (21) reported that miR-93 could suppress
the transcription of the Lats2 3′-UTR and inhibit Lats2 expression.
Consistent with Fang et al (21), the present study identified that
transfection with a miR-93 mimic suppressed Lats2 expression levels
in endothelial cells after H/R injury. Furthermore, it was observed
that the mRNA and protein expression levels of Lats2 in the
myocardium were significantly downregulated by lentivirus
expressing miR-93. Thus, the present results suggested that miR-93
may interact with the 3′-UTR of Lats2, which results in Lats2 mRNA
degradation and translational repression of Lats2. Previous studies
have shown that inactivation of the Hippo pathway decreases cardiac
remodeling and preserves cardiac function after MI (9,10).
In the heart, Lats1 and Lats2 regulate cardiomyocyte renewal
(37). Moreover, Lats2, but not
Lats1, plays an important role in mediating cardiomyocytes
apoptosis (38). Lats2 is highly
increased in the myocardium after myocardial I/R injury and MI, and
depletion of Lats2 reduces the infarct size after myocardial I/R
injury (8). However,
overexpression of Lats2 leads to a reduction of left ventricle
systolic function (38). In the
present study, it was found that increased expression of miR-93
significantly downregulated Lats2 expression in the myocardium
after MI. Moreover, suppression of Lats2 expression by miR-93
attenuated the infarct size and cardiac dysfunction after MI.
Collectively, the present results indicated that miR-93 plays a
protective role against H/R injury in endothelial cells via the
suppression of Lats2 expression.
Induction of angiogenesis plays a key role in the
infarct healing process, such as improvement of cardiac function
and attenuation of cardiac remodeling (39). The Hippo/Lats2/Yap pathway is an
important determinant of endothelial proliferation and migration
(6,14). Lats2-knockdown decreases
endothelial cell apoptosis, and increases migration and endothelial
tube formation (6). However,
overexpression of Yap induces an enhancement of the angiogenic
activity of endothelial cells (14,40).
The present results identified that transfection with miR-93 mimic
significantly decreased Lats2 expression and increased Yap
expression in HUVECs. Therefore, it was speculated miR-93 may
promote angiogenesis via the modulation of the Hippo pathway.
Moreover, the present in vivo results demonstrated that
miR-93 overexpression significantly increased the number of small
vessels in the myocardium after MI. In addition, it was found that
increased expression of miR-93 significantly attenuated cardiac
fibrosis after MI. Western blotting results demonstrated that
miR-93 overexpression decreased the phosphorylation of Yap, and
increased Yap expression in ischemic myocardium by targeting Lats2.
Therefore, it is speculated that miR-93 enhanced angiogenesis by
suppressing Lats2 expression, resulting in inactivation of the
Hippo/Yap pathway, which is positively associated with the
improvement of cardiac function and attenuation of remodeling after
MI.
VEGF is a critical pro-angiogenic factor (41). The present results suggested that
increased expression of miR-93 did not alter VEGF expression in the
myocardium after MI. Long et al (42) reported that miR-93 reduces VEGF-A
expression in cultured podocytes and glomeruli, while another
previous study showed that VEGF was not affected by upregulation of
miR-93 in both cultured HUVECs and in ischemic gastrocnemius muscle
from BALB/cJ mice (43). Thus,
these results suggest that miR-93 regulation of its target gene may
be dependent on context and cell type.
ICAM-1 and VCAM-1, two important adhesion molecules
that are highly expressed in activated endothelial cells, play an
important role in promoting the infiltration of inflammatory cells
across the endothelium and into the myocardium (44,45).
The accumulation of neutrophils and macrophages in ischemic
myocardium contributes to cardiac dysfunction following MI
(46,47). Therefore, preservation of
endothelial function is an important factor for the attenuation of
MI-induced cardiac dysfunction. The present study found that miR-93
overexpression significantly prevented MI-induced ICAM-1 and VCAM-1
expression, and significantly attenuated MI-induced infiltration of
neutrophils and macrophages into the myocardium. Moreover, Yap is
involved in the regulation of endothelial inflammation and
activation (48). Thus, it is
possible that miR-93 attenuates adhesion molecules and the
infiltration of inflammatory cells by targeting the Hippo/Lats2/Yap
pathway after MI.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that miR-93 may be an
important regulator of cardiac angiogenesis in ischemic hearts via
the suppression of Lats2 and inactivation of the Hippo/Yap pathway.
The present results help to further the understanding of the
interaction between miR-93 and endothelial cells, and its role in
MI. Thus, miR-93 may be a potential therapeutic target for MI.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Science
Foundation for Young Scientists of Jiangsu Province (grant nos.
BK20150214 and BK20170258), The National Natural Science Foundation
of China (grant nos. 81600967 and 81703493), and Jiangsu College
Students for Innovation and Entrepreneurship Training Program
(grant no. 201610313065X).
Availability and data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CM and PP carried out cell experiments, animal
surgery and molecular biology studies. YZ and TL analyzed the data.
LW performed western blotting and Masson's trichrome staining. CL
designed and supervised the study, and drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All aspects of the animal care and experimental
protocols were approved by Xuzhou Medical University Committee on
Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fiedler J and Thum T: MicroRNAs in
myocardial infarction. Arterioscler Thromb Vasc Biol. 33:201–205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernández-Reséndiz S, Muñoz-Vega M,
Contreras WE, Crespo-Avilan GE, Rodriguez-Montesinos J,
Arias-Carrión O, Pérez-Méndez O, Boisvert WA, Preissner KT and
Cabrera-Fuentes HA: Responses of Endothelial Cells Towards Ischemic
Conditioning Following Acute Myocardial Infarction. Cond Med.
1:247–258. 2018.PubMed/NCBI
|
|
3
|
Segers VFM, Brutsaert DL and De Keulenaer
GW: Cardiac Remodeling: Endothelial Cells Have More to Say Than
Just NO. Front Physiol. 9:3822018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X,
Yu H, Miao J, Kao R, Kalbfleisch J, et al: Attenuation of cardiac
dysfunction and remodeling of myocardial infarction by
microRNA-130a are mediated by suppression of PTEN and activation of
PI3K dependent signaling. J Mol Cell Cardiol. 89:(Pt A). 87–97.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The Hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating Yorkie, the Drosophila
Homolog of YAP. Cell. 122:421–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Kim YH, Kim J, Park DY, Bae H, Lee
DH, Kim KH, Hong SP, Jang SP, Kubota Y, et al: YAP/TAZ regulates
sprouting angiogenesis and vascular barrier maturation. J Clin
Invest. 127:3441–3461. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao D, Zhai P, Del Re DP, Sciarretta S,
Yabuta N, Nojima H, Lim DS, Pan D and Sadoshima J: A functional
interaction between Hippo-YAP signalling and FoxO1 mediates the
oxidative stress response. Nat Commun. 5:33152014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Q, Li L, Zhao B and Guan KL: The
hippo pathway in heart development, regeneration, and diseases.
Circ Res. 116:1431–1447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikeda S and Sadoshima J: Regulation of
Myocardial Cell Growth and Death by the Hippo Pathway. Circ J.
80:1511–1519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh A, Ramesh S, Cibi DM, Yun LS, Li J,
Li L, Manderfield LJ, Olson EN, Epstein JA and Singh MK: Hippo
Signaling Mediators Yap and Taz Are Required in the Epicardium for
Coronary Vasculature Development. Cell Rep. 15:1384–1393. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Del Re DP, Yang Y, Nakano N, Cho J, Zhai
P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, et al:
Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte
survival and growth to protect against myocardial ischemic injury.
J Biol Chem. 288:3977–3988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heallen T, Zhang M, Wang J,
Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF: Hippo
pathway inhibits Wnt signaling to restrain cardiomyocyte
proliferation and heart size. Science. 332:458–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakabe M, Fan J, Odaka Y, Liu N, Hassan A,
Duan X, Stump P, Byerly L, Donaldson M, Hao J, et al: YAP/TAZ-CDC42
signaling regulates vascular tip cell migration. Proc Natl Acad Sci
USA. 114:10918–10923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones PD, Kaiser MA, Ghaderi Najafabadi M,
Koplev S, Zhao Y, Douglas G, Kyriakou T, Andrews S, Rajmohan R,
Watkins H, et al: JCAD, a Gene at the 10p11 Coronary Artery Disease
Locus, Regulates Hippo Signaling in Endothelial Cells. Arterioscler
Thromb Vasc Biol. 38:1711–1722. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonauer A, Carmona G, Iwasaki M, Mione M,
Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et
al: MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Rooij E and Olson EN: MicroRNAs:
Powerful new regulators of heart disease and provocative
therapeutic targets. J Clin Invest. 117:2369–2376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang J, Huang W, Cai W, Wang L, Guo L,
Paul C, Yu XY and Wang Y: Inhibition of microRNA-495 Enhances
Therapeutic Angiogenesis of Human Induced Pluripotent Stem Cells.
Stem Cells. 35:337–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Liu Y, Wang T, Zhou N, Kong J,
Chen L, Snitow M, Morley M, Li D, Petrenko N, et al: A
microRNA-Hippo pathway that promotes cardiomyocyte proliferation
and cardiac regeneration in mice. Sci Transl Med. 7:279ra382015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C,
Li H, O'Malley YQ, Askeland RW, Sugg S, Liu M, et al: MiR-93
enhances angiogenesis and metastasis by targeting LATS2. Cell
Cycle. 11:4352–4365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. (8th).
National Academies Press (US). (Washington, DC). 2011.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao XM, Dart AM, Dewar E, Jennings G and
Du XJ: Serial echocardiographic assessment of left ventricular
dimensions and function after myocardial infarction in mice.
Cardiovasc Res. 45:330–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindsey ML, Kassiri Z, Virag JAI, de
Castro Brás LE and Scherrer-Crosbie M: Guidelines for measuring
cardiac physiology in mice. Am J Physiol Heart Circ Physiol.
314:H733–H752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ,
Hu J, Kalbfleisch J, Gao X, Kao R, et al: Toll-like receptor 3
plays a role in myocardial infarction and ischemia/reperfusion
injury. Biochim Biophys Acta. 1842:22–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Empel VP, Bertrand AT, Hofstra L,
Crijns HJ, Doevendans PA and De Windt LJ: Myocyte apoptosis in
heart failure. Cardiovasc Res. 67:21–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tse HF, Kwong YL, Chan JK, Lo G, Ho CL and
Lau CP: Angiogenesis in ischaemic myocardium by intramyocardial
autologous bone marrow mononuclear cell implantation. Lancet.
361:47–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oka T, Akazawa H, Naito AT and Komuro I:
Angiogenesis and cardiac hypertrophy: Maintenance of cardiac
function and causative roles in heart failure. Circ Res.
114:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Ha T, Gao X, Kelley J, Williams DL,
Browder IW, Kao RL and Li C: NF-kappaB activation is required for
the development of cardiac hypertrophy in vivo. Am J Physiol Heart
Circ Physiol. 287:H1712–H1720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy S, Khanna S, Hussain SR, Biswas S,
Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ and Sen CK: MicroRNA
expression in response to murine myocardial infarction: miR-21
regulates fibroblast metalloprotease-2 via phosphatase and tensin
homologue. Cardiovasc Res. 82:21–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT,
D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA,
et al: Revisiting Cardiac Cellular Composition. Circ Res.
118:400–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion--from mechanism to translation. Nat Med. 17:1391–1401.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Jiang M, Deng S, Lu J, Huang H,
Zhang Y, Gong P, Shen X, Ruan H, Jin M, et al: miR-93-5p-Containing
Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced
Myocardial Damage. Mol Ther Nucleic Acids. 11:103–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ke ZP, Xu P, Shi Y and Gao AM: MicroRNA-93
inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by
targeting PTEN. Oncotarget. 7:28796–28805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang L, Zhao L, Zan Y, Zhu Q, Ren J and
Zhao X: MiR-93-5p enhances growth and angiogenesis capacity of
HUVECs by down-regulating EPLIN. Oncotarget. 8:107033–107043. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heallen T, Morikawa Y, Leach J, Tao G,
Willerson JT, Johnson RL and Martin JF: Hippo signaling impedes
adult heart regeneration. Development. 140:4683–4690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsui Y, Nakano N, Shao D, Gao S, Luo W,
Hong C, Zhai P, Holle E, Yu X, Yabuta N, et al: Lats2 is a negative
regulator of myocyte size in the heart. Circ Res. 103:1309–1318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Boer RA, Pinto YM and Van Veldhuisen
DJ: The imbalance between oxygen demand and supply as a potential
mechanism in the pathophysiology of heart failure: The role of
microvascular growth and abnormalities. Microcirculation.
10:113–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi HJ, Zhang H, Park H, Choi KS, Lee HW,
Agrawal V, Kim YM and Kwon YG: Yes-associated protein regulates
endothelial cell contact-mediated expression of angiopoietin-2. Nat
Commun. 6:69432015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tammela T, Enholm B, Alitalo K and
Paavonen K: The biology of vascular endothelial growth factors.
Cardiovasc Res. 65:550–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Long J, Wang Y, Wang W, Chang BH and
Danesh FR: Identification of microRNA-93 as a novel regulator of
vascular endothelial growth factor in hyperglycemic conditions. J
Biol Chem. 285:23457–23465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hazarika S, Farber CR, Dokun AO,
Pitsillides AN, Wang T, Lye RJ and Annex BH: MicroRNA-93 controls
perfusion recovery after hindlimb ischemia by modulating expression
of multiple genes in the cell cycle pathway. Circulation.
127:1818–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Frangogiannis NG and Entman ML: Chemokines
in myocardial ischemia. Trends Cardiovasc Med. 15:163–169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Formigli L, Manneschi LI, Nediani C,
Marcelli E, Fratini G, Orlandini SZ and Perna AM: Are macrophages
involved in early myocardial reperfusion injury? Ann Thorac Surg.
71:1596–1602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kakio T, Matsumori A, Ono K, Ito H,
Matsushima K and Sasayama S: Roles and relationship of macrophages
and monocyte chemotactic and activating factor/monocyte
chemoattractant protein-1 in the ischemic and reperfused rat heart.
Lab Invest. 80:1127–1136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao
YY and Hu G, Pan D, Malik AB and Hu G: YAP Controls Endothelial
Activation and Vascular Inflammation Through TRAF6. Circ Res.
123:43–56. 2018. View Article : Google Scholar : PubMed/NCBI
|