Introduction
Inflammation is a highly regulated natural
biological phenomenon in which plasma molecules and leukocytes move
to extravascular space as a secondary mechanism of endothelial
activation in response to antigen recognition or damage-involved
products (1). Psoriasis is a
genetic disease triggered by several factors with the promotion of
a proinflammatory environment, as well as dysfunctions in the
regulation of inflammation in the skin with a crucial role in the
maintenance of this pathology (2).
Typical skin lesions in psoriasis contain abundant neutrophils,
macrophages, activated plasmacytoid dendritic cells and dermal
dendritic cells which interact with naive lymphoid cells to
generate T helper (Th)1 and Th17 lymphocytes, as well as T
regulatory (Treg) cells (3–5). A
proposed etiology of psoriasis is the impaired function of Treg
cells, possibly caused by the low expression of integrin β chain-2
[cluster of differentiation (CD)18] that favors the switch of Treg
to Th17 environment, with the consequent increase in interleukin
(IL)-17 that induces hyperproliferation of keratinocytes and
angiogenesis (6). In this
environment, infiltrated and local cells of the skin, produce
several inflammatory mediators including tumor necrosis factor
(TNF)α, interferon α/β, IL-1b, IL-6, IL-8, IL-17, IL-18, IL-23,
IL-36, C-C motif chemokine ligand (CCL)2 (monocyte chemoattractant
protein-1) and CCL5 (5,7,8).
The treatments currently used for psoriasis focus
primarily on the control of keratinocyte hyperproliferation and on
the blockade of inflammatory mediators (9). Among these therapies are topic and
systemic drugs, as well as phototherapy. Topic treatment with
corticoids (including hydrocortisone, fluocinolones, betamethasone,
mometasone and triamcinolone) and D vitamin analogs (calcitriol,
calcipotriol and tacalcitol) are the most indicated drugs for
patients with mild psoriasis, but topic retinoid tazarotene,
salicylic acid, coal tar, anthralin and topic methotrexate are also
used in these patients (10).
Methotrexate, cyclosporine and acitretin have potent
immunosuppressive effects and are indicated only for patients with
severe psoriasis (11). More
recently, the use of biological pharmaceuticals has increased for
the treatment of psoriasis; adalimumab, infliximab, certolizumab
and golimumab are antibodies that block the effect of TNFα, while
etanercept is an antibody that contains a portion of the TNFα
receptor (12). Ustekinumab and
briakinumab are also antibodies used in the treatment of psoriasis
with specificity to protein-40, a common component of IL-12 and
IL-23, which are involved in the differentiation of Th1 and Th17
cells, respectively (13,14). IL-17 and IL-36 are cytokines
strongly associated with the etiology of psoriasis and recently,
anti-IL17 and anti-IL36 antibodies have been developed for the
treatment of psoriasis and other inflammatory diseases, for
example, secukinumab and ixekizumab block IL-17, whereas brodalumab
binds to the IL-17 receptor (15,16).
ANB019 is an anti-IL36 receptor antibody that is still under
clinical trial evaluation for the treatment of pustular psoriasis
(17).
Adhesion molecules are also being used as a target
to treat psoriasis (18–20). Lymphocyte function-associated
antigen 1 (LFA-1), is expressed in all T-cells, B-cells,
macrophages and neutrophils, and has been reported to be involved
in the recruitment of such cells to the site of inflammation. LFA-1
binds to intercellular adhesion molecule 1 (ICAM-1) on
antigen-presenting cells and functions as an adhesion molecule.
Efalizumab is a humanized antibody anti-CD11 that blocks the
interaction between LFA-1 and ICAM-1 expressed on endothelial
cells. Alefacept is a chimeric molecule of human immunoglobulin G
fused to LFA-3 (CD58) domain that recognizes LFA-2 (CD2) expressed
in the surface of T lymphocytes, nevertheless, it was taken off the
market due to its secondary effects (21).
The present study describes a heptapeptide obtained
from cloning a library of phage peptides that in vitro
blocked the adhesion of peripheral blood mononuclear cells (PBMCs)
to endothelial cells and in vivo inhibited the development
of murine psoriasis-like lesions. This peptide might be used in the
future as a therapeutic peptide for the treatment of psoriasis.
Materials and methods
PBMCs
Peripheral blood (6 ml) was obtained from a patient
with acute laryngitis in tubes containing EDTA. The patient signed
a written consent to participate in this study (the sample was
taken on April 17th 2019). Peripheral blood mononuclear cells
(PBMCs) were purified using Lymphoprep (Sigma-Aldrich; Merck KGaA)
and centrifuged at 50 × g for 30 min at room temperature. Cells
were maintained in DMEM medium (1 ml; cat. no. 11965-118, Gibco;
Thermo Fisher Scientific, Inc.) without serum. Cell viability was
analyzed using trypan blue and a cell suspension
(7.5×105 viable cells/ml) was prepared. The experiment
was performed according to the appropriate guidelines for human use
approved by the Institutional Committee of Bioethics of the Escuela
Nacional de Ciencias Biológicas-IPN.
Selection of phages that recognized
adhesion molecules expressed on PBMCs
PBMCs (1 ml) were washed with DMEM, diluted in 990
µl TBS (50 mM Tris-HCl; pH 7.5; 150 mM NaCl) and 10 µl Phage
Display peptide library Ph.D.-7 (New England Biolabs, Inc.) was
added. PBMCs were incubated for 1 h at 37°C under 5%
CO2, with gentle agitation every 10 min. The
PBMCs-PH.D.-7 mix was washed six times with TBST [TBS + 0.1% (v/v)
Tween-20] and centrifuged at 50 × g for 5 min at room temperature.
The phages that bound to the PBMCs were eluted with 1 ml 0.2 M
glycine-HCl (pH 2.2) and neutralized with 150 µl 1 M Tris-HCl (pH
9.1). Eluted phages were amplified by infecting Escherichia
coli ER2738 (New England Biolabs, Inc.). Briefly, the eluate
was added to 20 ml mid-log phase E. coli ER2738 culture and
incubated with vigorous shaking for 4.5 h at 37°C. Subsequently,
the solution was centrifuged for 10 min at 12,000 × g at 4°C. The
supernatant was collected and the phages were precipitated by
incubation with 20% PEG/2.5 M NaCl overnight at 4°C. The phages
were then retrieved by centrifugation at 12,000 × g for 15 min at
4°C. Finally the phages were dissolved in 200 µl TBS. The phages
were quantitated by plaque forming units (PFU) in LB agar. The
final concentration of phages was reported as plaque forming units
per milliliter (PFU/ml). This selection and amplification of phages
(biopanning) were repeated for two more rounds. After three rounds
of selection, the eluted phages, able to interact with ligands over
the surface of activated PBMCs, were dissolved in 200 µl TBST
containing 0.02% NaN3 and stored for further assays. The
total eluate was termed ‘Total phages that interact with PBMCs’
(TPhPBMCs). A non-related phage (PhNR) was obtained as a negative
control.
Isolation of single phage clones
To obtain isolated clones from TPhPBMCs, TPhPBMCs
dilutions (10−5−10−9) were prepared in TBS.
Subsequently, 10 µl of each dilution was added separately to 200 µl
E. coli ER2738 culture (mid-log growing phase), mixed with 3
ml melt Top Agar (at 45°C) and immediately spread over LB medium
plates (Sigma-Aldrich; Merck KGaA). The plates were incubated at
37°C overnight. Subsequently, 10 plaques (single colonies) were
randomly selected. E. coli ER2738 was then infected with
each single clone independently to increase the chances that every
colony forming unit contained only one peptide sequence. The
procedure was repeated twice. The isolated clones were named
Ph(1–10)PBMCs.
DNA extraction of phages, sequencing
and analysis of the peptide sequence
According to the protocol provided by New England
BioLabs, the extraction of phage DNA was performed using the E.
coli culture supernatant, which was treated with 20% PEG/2.5 M
NaCl, and centrifuged at 4,400 × g for 10 min at 4°C. The pellet
was dissolved in 100 µl iodide buffer and 250 µl ethanol and
incubated for 10–20 min at room temperature to precipitate
preferentially single-stranded phage DNA, leaving most phage
protein in solution. Finally, the pellet was retrieved after
centrifugation at 1,700 × g for 15 min at 4°C, and the phage DNA
was dissolved in 30 µl TE (10 mM Tris + 1 mM EDTA; pH 8.0)
buffer.
PCR was performed to verify the presence of the
cassette containing the sequence that coded for the inserted
peptide in the phage. The sequences of the oligonucleotides used
were as follows: Forward, 5′-GCCGTTGCTACCCTCGTTC-3′ and reverse,
5′-TTTCGGCCGAACCTCCACC-3′. The enzime used was AmpliTaq
Gold® Fast PCR Master Mix (Applied Biosystems). The
following thermocycling conditions were used for PCR: Initial
denaturation for 10 min at 95°C; 40 cycles of denaturation at 96°C
for 5 sec, primer alignment for 5 sec at 60°C and extension for 5
sec at 68°C; followed by final extension at 72°C for 1 min.
Subsequently, sequencing was performed using the Sanger method and
the primer-96pIII (5′-CCCTCATAGTTAGCGTAACG-3′). The sequences
obtained for the inserted sequences were analyzed using NCBI BLAST
software (version 2.2.25; National Center for Biotechnology
Information).
Synthesis of heptapeptides
The heptapeptide HP3 and the non-related
heptapeptide (HPNR, shuffled sequence of HP3) were manufactured by
TAG Copenhagen A/S based on the sequence obtained from Ph3PBMCs DNA
sequencing analysis.
Culture and stimulation of human
microvascular endothelial cells (HMVECs)
HMVECs (CRL-3243™, dermal microvascular endothelium
HMEC-1; American Type Culture Collection, http://www.atcc.org/products/all/CRL-3243.aspx)
were cultured in endothelial growth medium (EGM, Lonza Group Ltd.)
supplemented with MV BulletKit [Lonza Group Ltd.: Recombinant
human(rh) epidermal growth factor, GA-100, hydrocortisone, Bovine
Brain Extract (BBE) and 5% FBS], incubated at 37°C in 5%
CO2 until 100% confluence was obtained. Then, 24 h
before stimuli, HMVECs were incubated with supplemented EGM medium
without FBS. HMVECs were stimulated 4 h with 50 ng/ml rhTNFα to
induce adhesion molecule overexpression (data not shown) for
further experiments.
Adherence inhibition assays
PBMCs were obtained from one healthy donor (age, 20
years; male) who provided written informed consent on May 1st 2019.
A total of 2×106 PBMC were stained with 1 M calcein
(Thermo Fisher Scientific, Inc.) for 1 h at 37°C in 5%
CO2. Subsequently, PBMCs were washed three times with
RPMI medium and centrifuged at 50 × g for 15 min at room
temperature. PBMCs were maintained in RPMI medium. Calcein-stained
PBMCs (7.5×105 cells/ml) were incubated with different
concentrations of phages or heptapeptides in RPMI medium without
FBS for 1 h at 37°C. The concentration of TPhPBMCs and PhNR used
were 103−109 PFU/ml and 107 PUF/ml
of Ph(1–10)PBMCs. Heptapeptides were used at 0.02–3.0 pg/ml. After
2 h of incubation with phages or heptapeptides, PBMCs were washed
twice with FBS-free DMEM and centrigued at 50 × g for 5 min.
Separately, HMVECs were grown in 12-well plaques and
stimulated with rhTNFα as described above. Following stimulation,
the medium with rhTNFα was removed and HMVECs were incubated for 1
h with 7.5×105 calcein-stained PBMCs pre-treated with
phages or heptapeptides, as aformentioned. Non-adherent PBMCs were
washed six times with DMEM without FBS. The adhered cells were
observed by fluorescent microscopy or detached with Trypsin-EDTA
(Gibco; Thermo Fisher Scientific, Inc.) to be acquired and analyzed
by flow cytometry.
Analysis of cell adhesion by UV
microscopy and flow cytometry
The counting of PBMCs-calcein positive cells adhered
to HMVECs was performed in 5 different fields using fluorescent
microscopy on every assayed condition. The average adherence of
PBMCs-calcein positive cells to HMVECs stimulated with rhTNFα in
the absence of phage or peptide was considered as 100% adherence
(maximum adherence). Two-way ANOVA followed by Bonferroni's post
hoc test was used to compare the different conditions.
For the flow cytometry analysis, the co-cultures
were treated with trypsin and, after the second wash, the cells
were maintained in FACS flow fluid. The acquisition of data was
adjusted to 2.5×104 events of HMVECs (calcein-negative)
and the percentage of calcein-positive PBMCs detected in each
condition was determined. The total number of positive events was
detected and analyzed in a FACSCalibur cytometer (BD
Biosciences).
Trans-endothelial migration assay
HMVECs were cultured in the top chamber of Transwell
plates (5 µm pore size, Costar, Corning Inc.) with EBM™
supplemented medium. In the other hand, 3.75×106
calcein-PBMCs/ml was prepared. Aliquots of 200 µl calcein-PBMCs
(7.5×105) were incubated in the presence of different
concentrations of HP3 or HPNR (0.2–3 pg/ml) for 1 h. These
calcein-PBMCs pretreated with peptides were incubated in the top
chamber of Transwells with HMVECs. To induce the migration of cells
from the top chamber to the lower chamber of Transwells, 50 ng/ml
of IL-8 was added to the EBM medium contained in the lower
chambers, and after 6 h of incubation at 37°C with 5%
CO2, the cells retrieved from the top and bottom
chambers were counted in a cytometer. In the negative control
assay, no IL-8 was added (NS, not stimulated). The experiment was
repeated in five independent assays.
Treatment in imiquimod (IMQ)-induced
mice with phages and peptides
All animal experiments were performed according to
the appropriate guidelines for animal use approved by the
Institutional Committee of Bioethics of the Escuela Nacional de
Ciencias Biológicas-IPN, which follows the EU Directive 2010/63/EU
on care for animals
(ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm).
A total of 50 female Balb/c mice (age, 8 weeks; weight, 22–25 g)
were used in the present study. The animals were housed at 23°C
with 12-h light/dark cycles, 40–60% humidity and ad libitum
access to food and water. The animals were supplied by the Animal
Care Facilities of the Escuela Superior de Medicina-IPN.
The induction of psoriasis-like lesions in mice was
performed according to van der Fits et al (22). Briefly, Balb/c mice were shaved on
the dorsal region to allow free access of IMQ (Aldara™ 5% cream;
Graceway Laboratory) to the skin. IMQ (6.25 µg) was administered
topically to the dorsal regions of mice daily for six days; after
the third day of IMQ administration the psoriasis-like lesions were
visible and after the sixth day the psoriasis-like lesions were
clearly defined.
To arrest the development of psoriatic lesions, mice
were treated intravenously (i.v.) with different concentrations of
phages Ph3PBMCs or PhNR (105, 107,
109 or 1011 PFU) on days 1–3, combined with
topical treatment with IMQ; on days 4–6 mice were treated with
imiquimod. For treatment with the synthetic peptide HP3 or HPNR
(not related heptapeptide), mice were treated i.v. with different
concentrations of peptides (0.01 µg or 10 µg per mouse) dissolved
in PBS on days 1–3, combined with topical treatment with IMQ; on
days 4–6 mice were treated with IMQ. On day 7, mice were sacrificed
by intravenous injection of pentobarbital sodium (150 mg/kg) and
animal death was confirmed by lack of reflexes, heartbeat and
breathing.
Histochemistry
Skin biopsies (1 cm2) were removed from
the back of the mice and maintained in a fixative formalin solution
(10% in PBS) until use. The skin was placed into cassettes and
dehydrated using an ethanol series (70, 80, 90, 96, 100 and 100%
for 1 h each). Tissues were cleared twice in 100% xylene for 1 h
and soaked in paraffin twice at 60°C for 1 h. Subsequently, tissues
were sectioned at 5 µm using a Leica RM 2132 microtome (Leica
Microsystems GmbH). Hematoxylin and eosin (H&E) and Lillie's
trichrome staining was done to the slides, with a staining time of
5 min at room temperature. Stained sections were observed in ten
randomly selected fields of view using a transmitted light
microscope (magnification, ×40). Histological examination and
interpretation was performed blinded.
Statistical analysis
Data are presented as the mean ± SEM. Significant
statistical difference among groups were determined using one-way
ANOVA followed by Dunnett's post hoc test. Statistical analyses
were performed using GraphPad Prism software (version 7.0d;
GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
TPhPBMCs inhibit PBMC adhesion to
HMVECs
HMVECs were treated with rhTNFα for 4 h to induce
the expression of adhesion molecules (data not shown) and, in
parallel, calcein-stained PBMCs were preincubated with TPhPBMCs or
PhNR (see Materials and methods) for 2 h at different
concentrations (103−109 PUF/ml). After
several washes to remove the non-attached PBMCs, the
calcein-stained PBMCs attached to the HMVEC monolayer were observed
by fluorescent microscopy (Fig.
1A). When PBMCs were treated with TPhPBMCs the number of
attached cells to HMVECs decreased in a concentration-dependent
manner, even below basal condition (no rhTNFα stimulation; Fig. 1B). The inhibition of adhesion was
specific for the treatment with TPhPBMCs, as it did not occur with
PhNR.
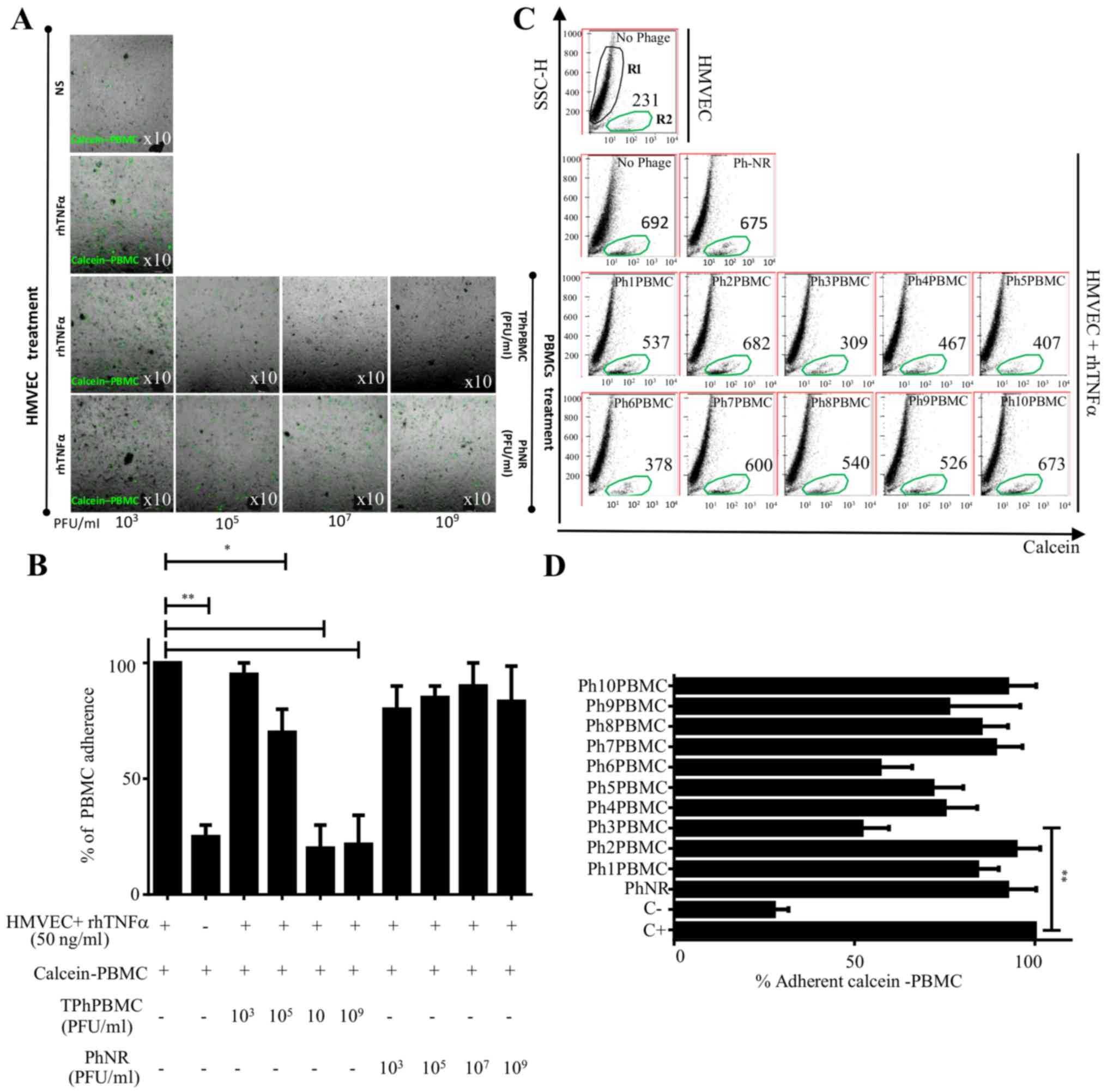 | Figure 1.Adherence assay of PBMCs to HMVECs in
the presence of TPhPBMCs. (A) HMVECs were stimulated or not with
TNFα to induce adhesion molecules. Calcein-PBMCs pretreated with
different concentrations of TPhPBMCs or PhNR phages
(103−109 PFU) were co-cultured with HMVECs
and calcein-positive PBMCs adhered to HMVECs were observed under
fluorescence microscope. (B) The same experiment as panel (A) but
cells were enzymatically detached and calcein-positive PBMCs were
counted with a cytometer. The graph shows the percentage of
calcein-positive PBMCs. (C) Calcein-PBMCs pretreated with different
clones of TPhPBMCs [Ph(1–10)PBMC] or PhNR phages were co-cultured
with HMVECs, and calcein-positive cells that adhered to HMVECs were
detached and counted by cytometry. R1, HMVECs; R2, calcein-positive
PBMCs. The number of calcein-positive cells are indicated. (D)
Graph indicates the percentage of calcein-positive PBMCs that
adhered to HMVECs from four independent assays (n=4, *P<0.05,
**P<0.01, using one-way ANOVA and Dunnett's multiple
comparison). PBMCs, peripheral blood mononuclear cells; HMVECs,
human microvascular endothelial cells; TPhPBMCs, Total phages that
interact with PBMCs TNF, tumor necrosis factor; PhNR, non-related
phage; PFU, plaque forming units per milliliter; rh, recombinant
human. |
Ph3PBMCs inhibit PBMC adhesion to
HMVECs
For further elucidation, 10 clones of phages were
randomly isolated from TPhPBMCs. Independent adhesion experiments
were performed as previously described and to corroborate the
results the proportion of calcein-positive PBMCs was also
quantified by cytometry. The percentage of adherent
calcein-positive PBMCs to HMVECs in the presence of each of the 10
isolated phage clones was determined. The maximum adherence (110%)
was considered as the number of calcein-positive PBMCs attached to
rhTNFα-stimulated HMVECs. Results showed that the clones Ph3PBMCs
and Ph6PBMCs had the highest cell adhesion inhibition (P≤0.01)
compared with C+ (Fig. 1C and D).
Analysis of the heptapeptide sequence inserted in the phages
identified that the clones Ph3PBMCs and Ph6PBMCs had the same
heptapeptide sequence (data not shown).
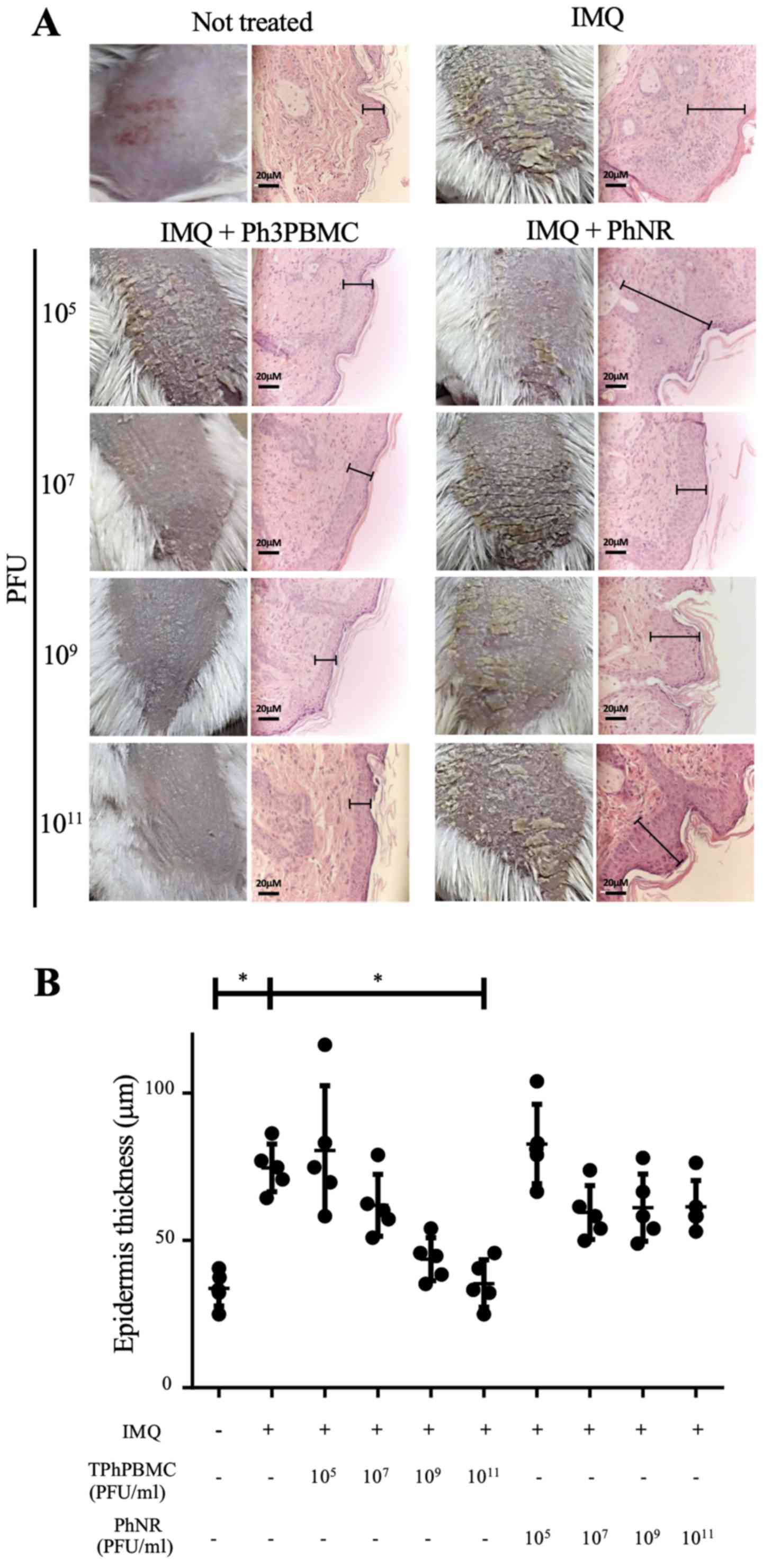
Ph3PBMCs prevent the development of
psoriasis-like lesions in a murine model
The effect of Ph3PBMCs treatment in the development
of skin lesions was evaluated using the IMQ-induced psoriasis-like
model (16). BALB/c mice were
treated with IMQ for six days to induce psoriasis and during the
first three days, they were also administered with different doses
of Ph3PBMCs or PhNR (105, 107, 109
or 1011 PFU/mouse) i.v. Fig. 2A shows squamous lesions in the back
of mice and microscopic observations of skin biopsies
(magnification, ×40). It was observed that treatment with Ph3PBMCs
prevented the development of psoriasis-like lesions in a
dose-dependent mode in comparison with mice treated with PhNR. In
mice treated with IMQ alone, the skin looked rigid and full of
flakes and in histologic analysis the thickness of epidermis
increased severely (hyperkeratosis). In mice treated with
1011 Ph3PBMCs, the skin was soft with few small flakes
and the thickness of epidermis was the same as untreated mice.
Fig. 2B shows the epidermis
thickness (µm) data of treated mice and controls and it is clear
that the administration of Ph3PBMCs decreased the thickness of the
epidermis, while PhNR didn't have such effect.
Peptide HP3 blocks adhesion of PBMCs
to rhTNFa-induced HMVECs
It was also analyzed if the synthetic peptide (HP3)
derived from the sequence of Ph3PBMCs could block the adherence of
PBMCs to rhTNFα-induced HMVECs. The adhesion assay was performed as
previously described using 0.02, 0.2 or 2 pg/ml of peptide HP3 or 2
pg/ml of an unrelated heptapeptide (HPNR). Calcein-positive PBMCs
were pretreated with the peptides and then co-cultured with
rhTNFα-induced HMVECs. After several gentle washes, the
calcein-PBMC-positive cells were quantitated by flow cytometry
(Fig. 3A) and the percentage of
adherent PBMCs was calculated for each condition (Fig. 3B). The condition of HMVECs cultured
with rhTNFα but without peptide treatment was deemed the maximum
adhesion of PBMCs to HMVECs (100%). According to the results shown
in Fig. 3A and B, the treatment
with the peptide HP3 decreased the percentage of PBMCs attached to
HMVECs as the concentration of HP3 was increased, demonstrating
that the preincubation with 2.0 pg/ml of HP3 in PBMCs reduced their
adherence to HMVECs to only 52.1% and, notably, the effect was not
observed when HPNR was used.
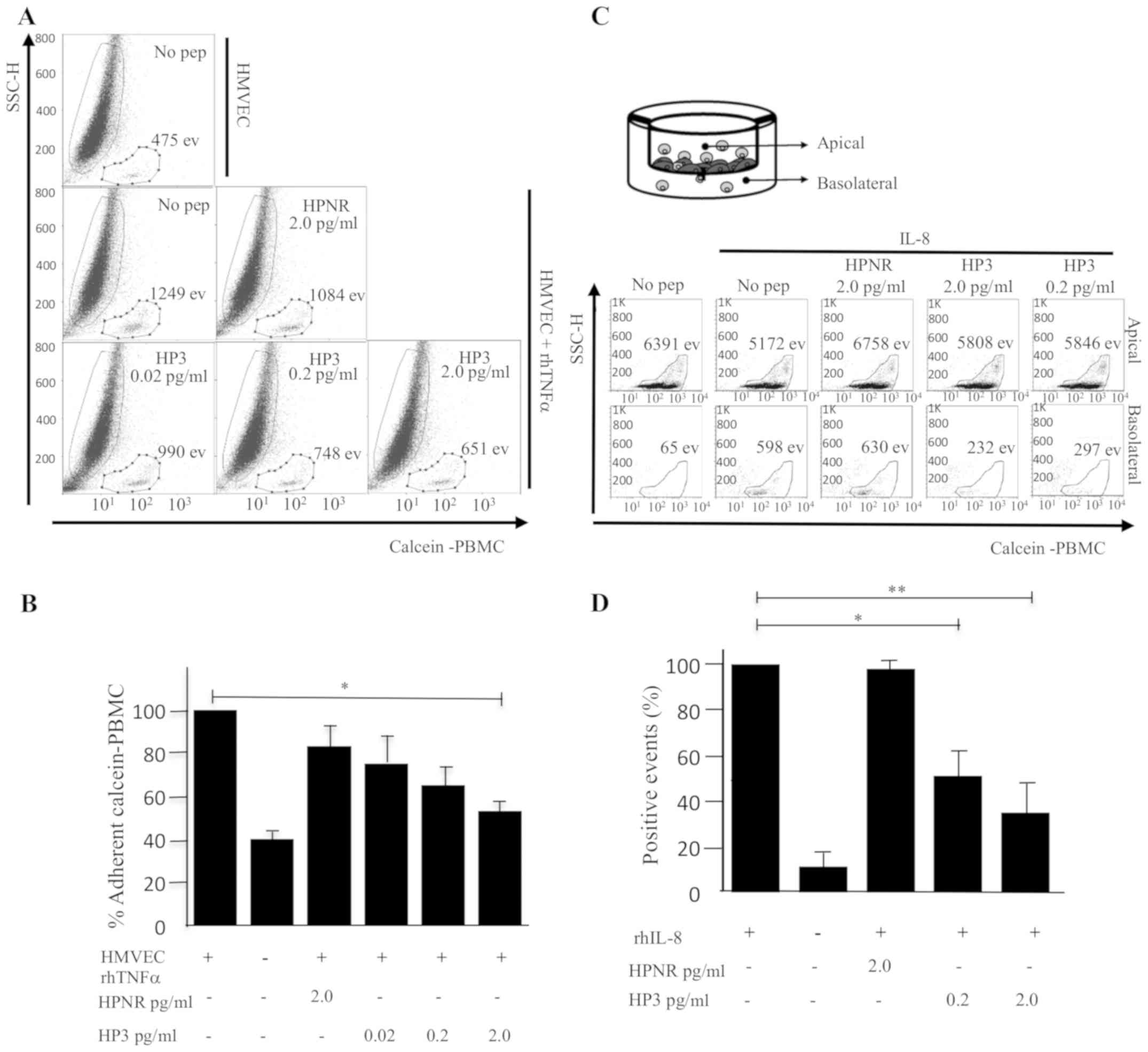 | Figure 3.Adherence assay of PBMCs to HMVECs in
the presence of peptide HP3 or HPNR. (A) Calcein-PBMCs pretreated
with different concentrations of peptide HP3 or HPNR (0.02, 0.2 2.0
pg/ml) were co-cultured with HMVECs, and calcein-positive PBMCs
adhered to HMVECs and enzymatically detached were detected by flow
cytometry. The number of calcein-positive events is indicated. (B)
Percentage of calcein-positive PBMCs adhered to HMVECs was
determined. Graph represents the average of four independent
assays. (C) Analysis of cell migration. Calcein-positive cells were
pretreated with HP3 or HPNR, and co-cultured with HMVECs attached
to the top side of Transwell plates. After 6 h of incubation the
number of calcein-positive events were quantitated from the top and
bottom sides of Transwell plates. (D) Percentage of cell migration
is represented in the graph (n=4, *P<0.05, **P<0.01, using
one-way ANOVA and Dunnett's test). PBMCs, peripheral blood
mononuclear cells; HMVECs, human microvascular endothelial cells;
HPNR, non-related heptapeptide; TNF, tumor necrosis factor; rh,
recombinant human. |
HP3 inhibits the trans-endothelial
migration of monocytes
To evaluate if HP3 could inhibit the
trans-endothelial migration of PBMCs, an in vitro assay was
performed using Transwell plates. HMVECs and calcein-positive PBMCs
were co-cultured in the top compartment of Transwell plates and
then the effect of HP3 in the migration of calcein-positive PBMCs
to the bottom compartment in the presence of the chemoattractant
IL-8 was evaluated. As shown in Fig.
3C, the bottom side of Transwell contained 13.5% of cells in
basal culture conditions and it increased to 26.1% in the presence
of IL-8. However, when calcein-PBMCs cells were pretreated with HP3
the migration decreased considerably to 11.6% with 0.2 pg/ml of HP3
and to 11.2% with 2.0 pg/ml of HP3, in contrast to the treatment
with HPNR that remained close to the control condition (28.4%).
Fig. 3D, represents the mean of
cell migration assay percentages of calcein-positive PBMCs from 4
independent experiments.
HP3 prevents the development of
psoriasis-like lesions in a murine model
Peptide HP3 (0.01 or 10 µg) or HPNR (10 µg) was
administered i.v. in the tail of mice in the first three days of
psoriasis induction protocol, as previously described. According to
the results shown in Fig. 4, the
treatment with HP3 decreased the severity of psoriasis-like lesions
even at 0.01 µg/mouse doses, but more clearly at a dose of 10
µg/mouse. In addition to the formation of squamous lesions,
angiogenesis is also increased in psoriatic lesions and when the
inner side of the skin was analyzed, a higher number and thickness
of blood vessels was observed in the IMQ-treated skins and an
apparent decrease of angiogenesis in HP3-treated skins (Fig. 4A). To confirm these observations,
sections of skin biopsies were stained with Lillie's trichrome and
a lower number of blood vessels were found in peptide HP3-treated
mice (arrowheads Fig. 4A) compared
with peptide HPNR treatment. The thickness of the skin was measured
in slides stained with hematoxylin and eosin (H&E) (Fig. 4B) and it was observed that both
concentrations of peptide HP3 decreased the thickness of the
treated skin. Van der Fits et al (22) reported that mice treated with IMQ
for psoriasis induction also develop splenomegaly, therefore it was
decided to analyze the spleen index in mice treated with the
peptide. The treatment with peptide HP3 also reduced the spleen
index, reflecting a systemic effect in the general health of
IMQ-induced mice treated with HP3 as demonstrated in Fig. 4C.
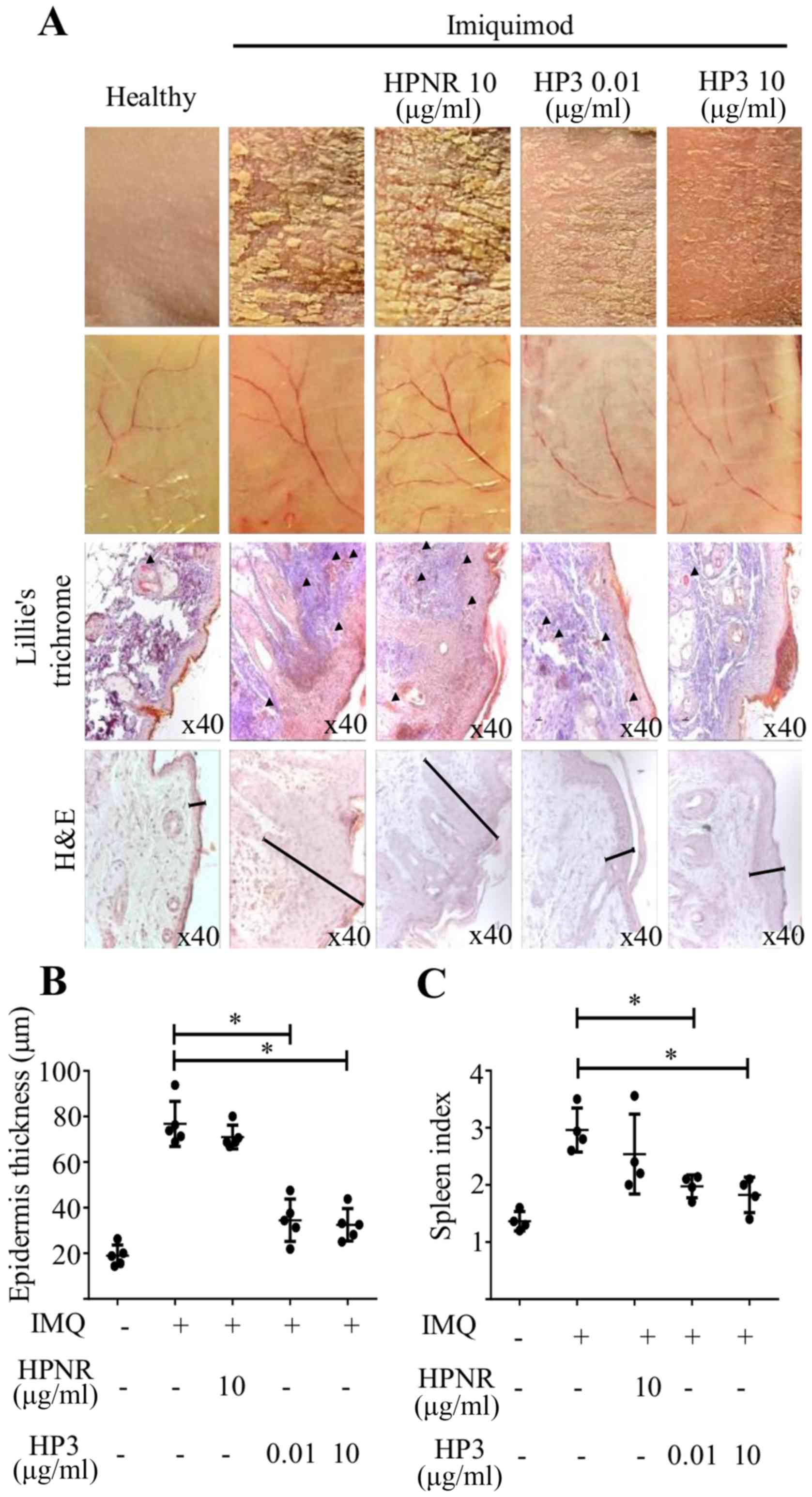 | Figure 4.Treatment with peptide HP3 protects
against psoriasis-like skin lesions development. Mice were treated
i.v. with different concentrations of peptides HP3 or HPNR (0.01
and 10 µg per mice) on days 1–3, as well as imiquimod. On days 4–6
mice were treated only with imiquimod. Some mice were also treated
only with vehicle (negative control) or only with imiquimod
(positive control). (A) Skin lesions from the back of mice are
shown, and slides of skin biopsies were stained with Lille's
trichrome solution. Arrowheads show blood vessels. The thickness of
the skins is indicated in slides stained with H&E. (B)
Epidermal skin thickness of peptide-treated mice biopsies (n=5,
*P<0.05, using one-way ANOVA, Dunnett's multiple comparison).
(C) Spleen index related to splenomegaly (n=5, *P<0.05, using
one-way ANOVA and Dunnett's multiple comparison). HPNR, non-related
heptapeptide; H&E, hematoxylin and eosin; IMQ, imiquimod. |
The results of the present study demonstrated that
peptide HP3 reduced the adhesion of PBMCs to endothelial cells,
affecting their migration through endothelial cells and frustrating
their participation in the inflammatory processes. In the murine
model of psoriasis, the treatment with HP3 in IMQ-induced mice
reduced the development of skin lesions diminishing the thickness
of the skin, the formation of squamous skin and angiogenesis, and
as a systemic effect prevents the development of splenomegaly. This
suggests a potential use of HP3 to treat psoriasis in humans.
Discussion
There are different treatments for psoriatic
patients used according to their afflictions. The treatments can be
phototherapy, systemics or biologicals. The drugs used to treat
psoriasis block the action of specific T cell population, or block
the activity of proteins such as TNFα (golimumab, infliximab,
adalimumab, etanercept and certolizumab pegol), IL-17A (secukinumab
and ixekizumab), IL-17 receptor (brodalumab) or IL-12/IL-23
(ustekinumab and tildrakizumab). These targeted cells and proteins
all play significant roles in developing psoriasis and psoriatic
arthritis (23).
The use of monoclonal antibodies as therapeutics has
provided an efficient way to inhibit proteins (ligands or
receptors) for the treatment of diverse diseases, but in a
long-term treatment its efficiency decreases as a result of
resistance, such as the generation of antibodies against the
monoclonal antibodies, or the formation of immunocomplexes. Other
common side effects for these treatments are respiratory infections
and flu-like symptoms, but also include severe side effects such as
blood and nervous system disorders, inflammation of nerves of the
eye and even certain types of cancer (24). Thus then, the generation of
different approaches to create new treatments remains crucial. The
number of peptides entering clinical development is increasing. In
2018, Lau and Dunn reported information about the existence of 484
therapeutic peptides; from these, 155 peptides were actively in
clinical development, 10% in phase I, 16% in phase II, 5% in phase
III and 5.1% in preregistration (25).
The peptide HP3 was obtained from the sequence of a
phage-peptide clone with interaction with human PBMCs. The in
vitro assays were performed with human cells, but the in
vivo assays were performed using a murine model of psoriasis.
According to the results of the present study, the peptide blocks
the adherence and migration of PBMCs in both species, suggesting
that the specific ligand/receptor that is blocked is shared between
both species. Sequence analysis of the peptide showed that it has
73% of homology with human CCR2 (data not shown) but also affinity
to a receptor of T cells. Further studies are necessary to define
the specific molecule that is recognized by the peptide.
The results of the present study demonstrated that
peptide HP3 can reduce the adhesion of human PBMCs to endothelial
cells, affecting the migration of cells and, as consequence,
interfering in their availability to participate in the development
of inflammation, as observed in the psoriasis-like murine model,
where the treatment with the peptide protects against the
generation of lesions in the skin. The same effect could occur in
human psoriasis. In such a case, this peptide could be used for the
treatment of human psoriasis.
Acknowledgements
The authors would like to thank Dr Eugenia Aguilar
Nájera from the Animal Care Facilities of the Escuela Superior de
Medicina-IPN for supplying the mice used in this work.
Funding
The present study was supported by
IPN-Multidisciplinary Projects SIP-1773 and SIP-20149. LAZC and GGG
received postgraduate scholarships from CONACyT and BEIFI–IPN. SRM,
JCCD, MECD, TAM and SMPT are COFAA-IPN, EDI–IPN and SNI-CONACyT
Fellows. FGC is Cátedra-CONACyT and SNI-CONACyT Research
Fellow.
Availability of data and materials
The datasets analyzed during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
MECD, JCCD and SRM designed the study. JSMF
performed the biopanning assay. PEBL performed the adherence assay.
LAZC, ATC and GGG performed the psoriasis experiments. IDT analyzed
the in silico results. SMPT, FGC and MECD analyzed the data
and wrote the manuscript. EAVS performed the histochemistry assays.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures, including the
purification of human mononuclear cells, treatment with psoriasis
inductors, administration of phages or peptides and death of
animals, were approved and performed according to the standards of
the Ethics Committee of the Instituto Politécnico Nacional/Escuela
Nacional de Ciencias Biológicas. The registration and approval of
the protocol of mice usage is under the file number
ENCB/CEI/036/2018, CONBIOETICA09CEI03720130520 and the approval of
the protocol of human samples usages is under the file number
CEI-ENCB-SH-002-2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hickey MJ, Reinhardt PH, Ostrovsky L,
Jones WM, Jutila MA, Payne D, Elliott J and Kubes P: Tumor necrosis
factor-alpha induces leukocyte recruitment by different mechanisms
in vivo and in vitro. J Immunol. 158:3391–3400. 1997.PubMed/NCBI
|
|
2
|
Li Q, Chandran V, Tsoi L, O'Rielly D, Nair
RP, Gladman D, Elder JT and Rahman P: Quantifying differences in
heritability among psoriatic arthritis (PsA), cutaneous psoriasis
(PsC) and psoriasis vulgaris (PsV). Sci Rep. 10:49252020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beurskens T, Chang A, van Erp PE and van
de Kerkhof PC: Epidermal proliferation and accumulation of
polymorphonuclear leukocytes in the psoriatic lesion.
Dermatologica. 178:67–72. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schlaak JF, Buslau M, Jochum W, Hermann E,
Girndt M, Gallati H, Meyer zum Büschenfelde KH and Fleischer B: T
cells involved in psoriasis vulgaris belong to the Th1 subset. J
Invest Dermatol. 102:145–149. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nestle FO, Di Meglio P, Qin JZ and
Nickoloff BJ: Skin immune sentinels in health and disease. Nat Rev
Immunol. 9:679–691. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh K, Gatzka M, Peters T, Borkner L,
Hainzl A, Wang H, Sindrilaru A and Scharffetter-Kochanek K: Reduced
CD18 levels drive regulatory T cell conversion into Th17 cells in
the CD18hypo PL/J mouse model of psoriasis. J Immunol.
190:2544–2553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He Q, Chen HX, Li W, Wu Y, Chen SJ, Yue Q,
Xiao M and Li JW: IL-36 cytokine expression and its relationship
with p38 MAPK and NF-κB pathways in psoriasis vulgaris skin
lesions. J Huazhong Univ Sci Technolog Med Sci. 33:594–599. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nedoszytko B, Sokołowska-Wojdyło M,
Ruckemann- Dziurdzińska K, Roszkiewicz J and Nowicki RJ: Chemokines
and cytokines network in the pathogenesis of the inflammatory skin
diseases: Atopic dermatitis, psoriasis and skin mastocytosis.
Postepy Dermatol Alergol. 31:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alexander H and Nestle FO: Pathogenesis
and immunotherapy in cutaneous psoriasis. what can rheumatologists
learn? Curr Opin Rheumatol. 29:71–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guenther L, Van De Kerkhof PC, Snellman E,
Kragballe K, Chu AC, Tegner E, Garcia-Diez A and Springborg J:
Efficacy and safety of a new combination of calcipotriol and
betamethasone dipropionate (once or twice daily) compared to
calcipotriol (twice daily) in the treatment of psoriasis vulgaris:
A randomized, double-blind, vehicle-controlled clinical trial. Br J
Dermatol. 147:316–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah RA, Nwannunu CE, Limmer AL, Patel RR,
Mui UN and Tyring SK: Brief update on dermatologic uses of
methotrexate. Skin Therapy Lett. 24:5–8. 2019.
|
|
12
|
Krueger G and Callis K: Potential of tumor
necrosis factor inhibitors in psoriasis and psoriatic arthritis.
Arch Dermatol. 140:218–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan C, Leonardi CL, Krueger JG, Kimball
AB, Strober BE, Gordon KB, Langley RG, de Lemos JA, Daoud Y,
Blankenship D, et al: Association between biologic therapies for
chronic plaque psoriasis and cardiovascular events: A meta-analysis
of randomized controlled trials. JAMA. 306:864–871. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tzellos T, Kyrgidis A, Trigoni A and
Zouboulis CC: Point: Major adverse cardiovascular events and
anti-IL 12/23 agents. J Am Acad Dermatol. 70:380–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thaçi D, Blauvelt A, Reich K, Tsai TF,
Vanaclocha F, Kingo K, Ziv M, Pinter A, Hugot S, You R and
Milutinovic M: Secukinumab is superior to ustekinumab in clearing
skin of subjects with moderate to severe plaque psoriasis: CLEAR, a
randomized controlled trial. J Am Acad Dermatol. 73:400–409. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordon KB, Blauvelt A, Papp KA, Langley
RG, Luger T, Ohtsuki M, Reich K, Amato D, Ball SG, Braun DK, et al:
Phase 3 trials of ixekizumab in moderate-to-severe plaque
psoriasis. N Engl J Med. 375:345–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su Z, Paulsboe S, Wetter J, Salte K,
Kannan A, Mathew S, Horowitz A, Gerstein C, Namovic M, Todorović V,
et al: IL-36 receptor antagonistic antibodies inhibit inflammatory
responses in preclinical models of psoriasiform dermatitis. Exp
Dermatol. 28:113–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi B and Constantin G: Anti-selectin
therapy for the treatment of inflammatory diseases. Inflamm Allergy
Drug Targets. 7:85–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rychly J and Nebe B: Therapeutic
strategies in autoimmune diseases by interfering with leukocyte
endothelium interaction. Curr Pharm Des. 12:3799–3806. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radi ZA, Kehrli ME Jr and Ackermann MR:
Cell adhesion molecules, leukocyte trafficking, and strategies to
reduce leukocyte infiltration. J Vet Intern Med. 15:516–529. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koo JY, Bagel J, Sweetser MT and Ticho BS:
Alefacept in combination with ultraviolet B phototherapy for the
treatment of chronic plaque psoriasis: Results from an open-label,
multicenter study. J Drugs Dermatol. 5:623–628. 2006.PubMed/NCBI
|
|
22
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strober BE, Bissonnette R, Fiorentino D,
Kimball AB, Naldi L, Shear NH, Goyal K, Fakharzadeh S, Calabro S,
Langholff W, et al: Comparative effectiveness of biologic agents
for the treatment of psoriasis in a real-world setting: Results
from a large, prospective, observational study [Psoriasis
Longitudinal Assessment and Registry (PSOLAR)]. J Am Acad Dermatol.
74:851–861.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Branisteanu DE, Voicu CM, Cretu A,
Dimitriu A, Luca MC and Salavastru CM: Adverse reactions of
biological therapy for psoriasis. Rev Med Chir Soc Med Nat Iasi.
119:38–44. 2015.PubMed/NCBI
|
|
25
|
Lau JL and Dunn MK: Therapeutic peptides:
Historical perspectives, current development trends, and future
directions. Bioorg Med Chem. 26:2700–2707. 2018. View Article : Google Scholar : PubMed/NCBI
|