Introduction
Skeletal muscle accounts for ~42% of the total body
mass in males and 36% in females (1); it is a metabolically active tissue
and is responsible for 30% of the resting metabolic rate in adults
(2). Apart from skeletal motion,
skeletal muscle plays key roles in calorigenesis, blood glucose
control, metabolic balance and the support and protection of soft
tissue (1). Exercise training has
the ability to improve pathological conditions involving metabolic
disorders and prevent various lifestyle-related chronic maladies,
partly due to its regulation of metabolic homeostasis and the
molecular responses of skeletal muscle (2). Moreover, exercise induces various
adaptive responses in the skeletal muscle, including mitochondrial
biogenesis (3), lipid metabolism
(4), glycometabolism (5) and ultrastructural changes (6).
Baar et al (7), reported that mitochondrial biogenesis
triggered by exercise is associated with the increase of the
transcriptional coactivators peroxisome proliferator-activated
receptor γ coactivator-1 (PGC-1), nuclear respiratory factor 1
(NRF-1) and NRF-2. Cantó et al (8), revealed that AMP-activated protein
kinase (AMPK) is first activated during the adaptive responses in
skeletal muscle after exercise, while sirtuin 1 (SIRT1) is
activated with deficient AMPK activity, suggesting an acetylation
regulation mechanism of the AMPK/SIRT1 axis. High-intensity
intermittent exercise training (HIIT) improves the skeletal
myopathy in patients with heart failure associated with the
increased expression of the insulin-like growth factor 1
bioregulation system (9). Exercise
training can induce the increased expression level of cytokines
secreted by skeletal muscle cells, including IL-6, IL-1 and IL-10,
which have anti-inflammatory effects (10). In addition, microRNAs
(miRNAs/miRs), a class of non-coding small RNAs regulating genes at
a post-transcriptional level, also play crucial roles in the
skeletal muscle response to exercise (1,11).
The expression level of miR-761 is reduced in the mouse skeletal
muscle response to exercise and its overexpression inhibits the P38
mitogen-activated protein kinase signaling pathway and PGC-1α,
which are associated with mitochondrial biogenesis (12). Although previous studies have been
conducted, the specific molecular mechanisms of mouse skeletal
muscle response to exercise are not fully understood (13,14).
Therefore, the present study investigated the molecular changes and
related regulatory mechanisms in skeletal muscle response to
exercise.
The microarray dataset ‘GSE109657’ of the skeletal
muscle response to HIIT used in the present study was contributed
by Miyamoto-Mikami et al (15). These authors investigated the
differentially expressed genes (DEGs) and the associated functions,
and significantly upregulated DEGs are found to be associated with
glucose metabolism and mitochondrial membranes (15). In the present study, DEGs were
identified and analyzed using weighted gene co-expression network
analysis (WGCNA), which is effective for the identification of
functional co-expressed gene modules (16). In addition, except for the
functional enrichment analysis, miRNAs and transcription factors
(TFs) were predicted in order to construct the miRNA-TF-target
regulatory network. Thus, the present results may provide a
theoretical basis for the investigation of the effect of exercise
on skeletal muscle.
Materials and methods
Microarray data
The ‘GSE109657’ gene expression dataset of human
skeletal muscle was downloaded from the Gene Expression Omnibus
(GEO) database (https://www.ncbi.nlm.nih.gov/geo/). There were 22
biopsy samples in this dataset, which were collected from the
vastus lateralis muscle of 11 young and healthy men before
(GSM2948027-GSM2948037) and after (GSM2948038-GSM2948048) a 6-week
HIIT. The platform of this dataset was GPL16686 [HuGene-2_0-st]
Affymetrix Human Gene 2.0 ST Array [transcript (gene) version].
Since the dataset was obtained from a public database, no ethical
approval was obtained in the present study.
Data preprocessing and screening of
DEGs
The Oligo in R package (v.1.34.0; http://bioconductor.org/help/search/index.html?q=oligo/)
was used to perform raw data preprocessing, including format
conversion, missing value supplement, background correction and
data standardization. The probes were annotated according to the
annotation file on the platform and were removed when the gene
symbol did not match. The differentially expressed analysis among
samples was performed utilizing the classical Bayes method in limma
package (R v.3.3.3) (17) and the
DEGs were screened with the threshold of P<0.05 and |log fold
change (FC)| >0.263.
WGCNA for DEGs
WGCNA (http://www.inside-r.org/packages/cran/WGCNA/docs/bicor)
was used to identify the modules and genes associated with HIIT
based on the expression level of DEGs, and the DEGs were clustered
into different modules according their co-expression relationships.
WGCNA was conducted according to a previous study by Langfelder and
Horvath (18), including the
definition of gene co-expression matrix Smm =
|cor(m,n)|, the definition of adjacent function
amn = power(Smnβ), the determination of
weighted coefficient β (≥0.8) and the measurement of dissimilarity
between nodes. The minimum number of genes in each module was set
as 20 and the cluster analysis height of the module was set as 0.2
in the identification of gene modules. In addition, the module
significance was calculated to identify the correlation between
modules and HIIT.
Functional enrichment analysis for the
genes in significant modules
The online database for annotation, visualization
and integrated discovery tool (v.6.8; http://david-d.ncifcrf.gov/) was used to investigate
the function of the genes in significant modules, including
biological processes in Gene Ontology (GO_BP) and the Kyoto
Encyclopedia of Genes and Genomes (KEGG) signaling pathways. The
number of enrichment genes was set as count ≥2 and P<0.05 was
selected as the threshold.
Construction of protein-protein
interaction network
The genes in significant modules were integrated and
uploaded to the STRING database (version: 10.0; http://www.string-db.org/) to retrieve the
protein-protein interactions (PPIs) with the following parameters:
Species was set as human and the PPI score was set as 0.4 (median
confidence). Cytoscape software (v.3.2.0; http://www.cytoscape.org/) was used to construct the
visualized PPI network based on the retrieved interactions from
STRING. A high node degree centrality value indicated the hub nodes
in the PPI network (19).
Construction of miRNA-TF-target
regulatory network
The over-representation analysis method of
enrichment in WebGestalt (v.2017; http://www.webgestalt.org/) was used to predict the
miRNA-target interactions and TF-target interactions for the genes
with node degree >5 in the PPI network. P<0.05 was selected
as the threshold. In addition, Cytoscape software was used to
construct the miRNA-TF-target regulatory network with the
significantly enriched miRNA-target interactions and TF-target
interactions.
Confirmatory analysis
In order to investigate the expression and function
of the DEGs, the ‘GSE41769’ gene expression dataset of human
skeletal muscle, which was contributed by Catoire et al
(20), was downloaded from the GEO
database. This dataset included 36 skeletal muscle biopsy samples,
which were collected from both the legs of nine healthy middle-aged
men before and after 1 h of one-legged exercise. The platform of
this dataset was GPL11532 [HuGene-1_1-st] Affymetrix Human Gene 1.1
ST Array [transcript (gene) version]. Data were preprocessed and
differentially expressed analysis was performed using the method
mentioned above, and the DEGs were screened within the threshold of
P<0.05. Moreover, functional enrichment analysis was also
conducted for these DEGs using the method described above.
Results
Data preprocessing and screening of
DEGs
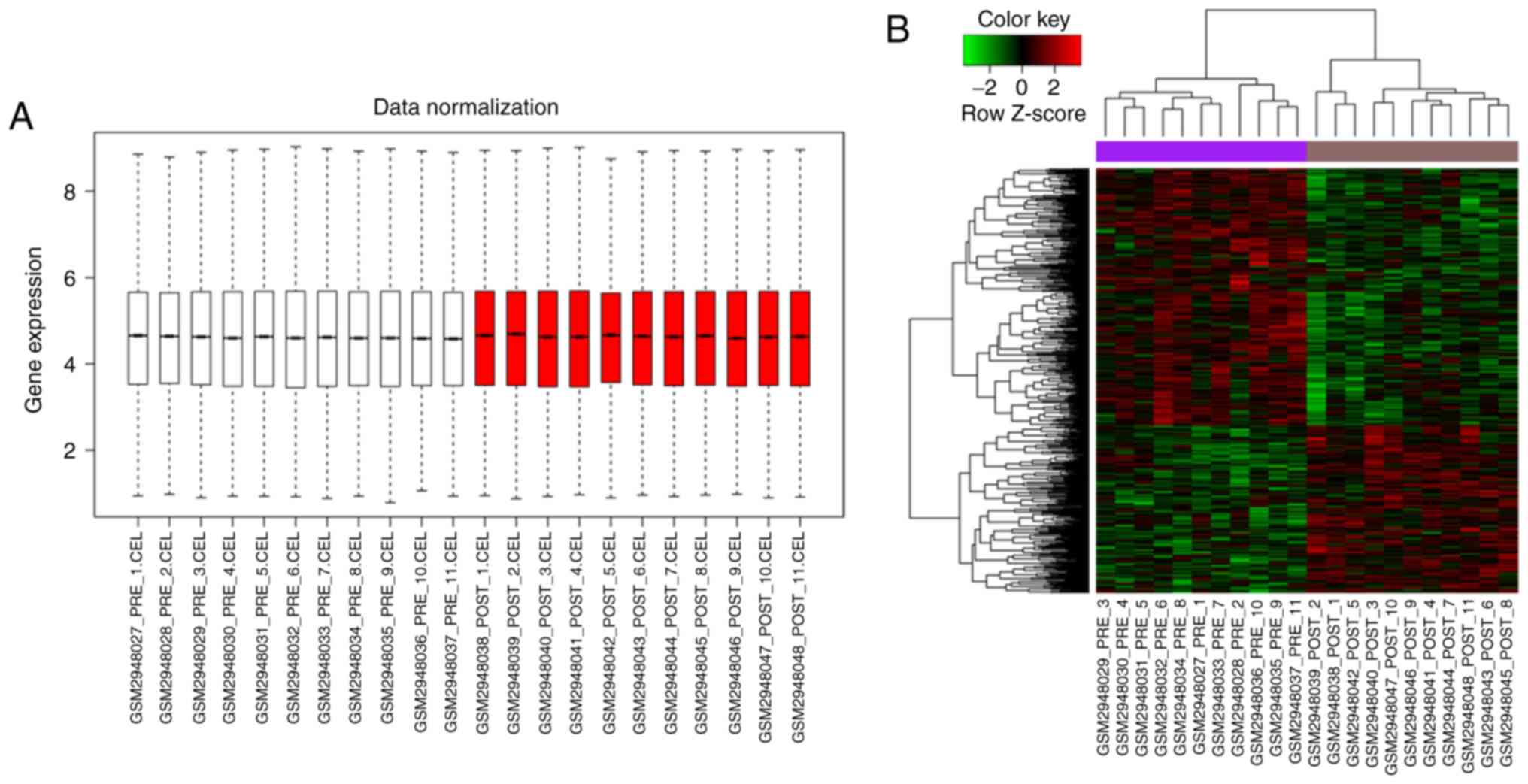
The gene expression in each sample was at the same
level after data normalization, suggesting that they could be used
in the subsequent analyses (Fig.
1A). A total of 530 DEGs were screened in the vastus lateralis
muscle after HIIT, of which 209 genes were significantly
upregulated, while 321 genes were significantly downregulated. The
heatmap of DEGs displayed in Fig.
1B indicated that DEGs could be distinguished in the muscle
biopsy samples before and after HIIT.
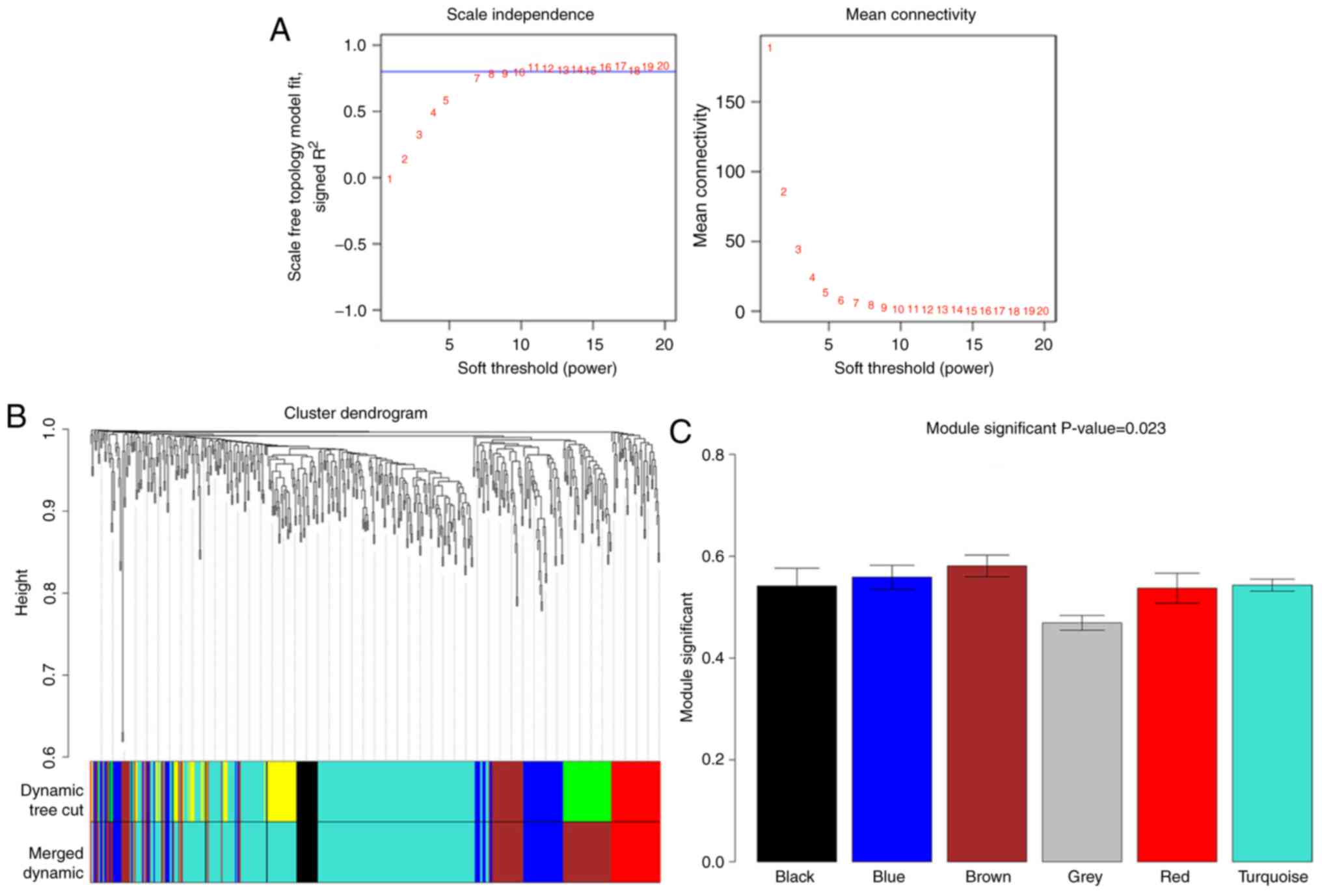
WGCNA for DEGs
The value of the power parameter in the adjacent
function was determined as eight. A total of six co-expression
modules were identified for the DEGs with absolute correlation
≥0.5, of which three modules had the absolute correlation ≥0.8;
these were the brown, blue and red modules. The DEGs in these three
modules were used in the following analysis (Table I). The DEGs in the brown module
showed the strongest correlation with HIIT and those in the grey
module were not clustered into co-expression modules with other
DEGs (Fig. 2).
 | Table I.High-intensity intermittent exercise
training correlated co-expression modules. |
Table I.
High-intensity intermittent exercise
training correlated co-expression modules.
| Module | Correlation
coefficient | P-value |
|---|
| MEbrown | 0.86 |
2.35×10−7 |
| MEblue | −0.81 | 5.94
×10−6 |
| MEred | −0.8 | 9.49
×10−6 |
| MEturquoise | −0.79 |
1.39×10−5 |
| MEblack | −0.68 | 0.0004783 |
| MEgrey | −0.63 | 0.001525 |
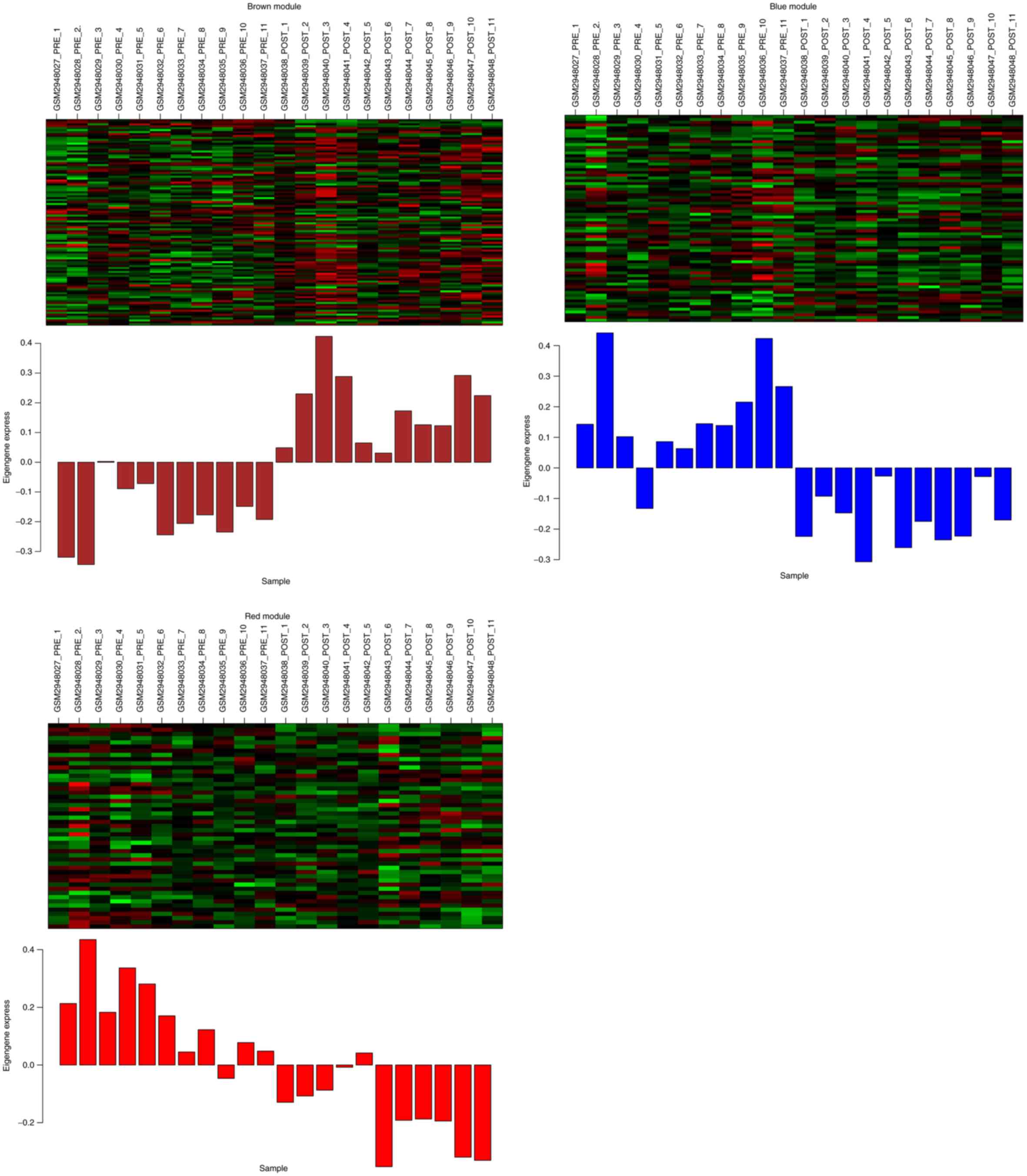
Expression levels of the genes in
significant modules
In total, three significant modules (brown, blue and
red modules) were identified after WGCNA, and a total of 106, 74
and 49 DEGs were included in the brown, blue and red module,
respectively. Fig. 3 shows the
heatmap of the DEGs in each of the three modules.
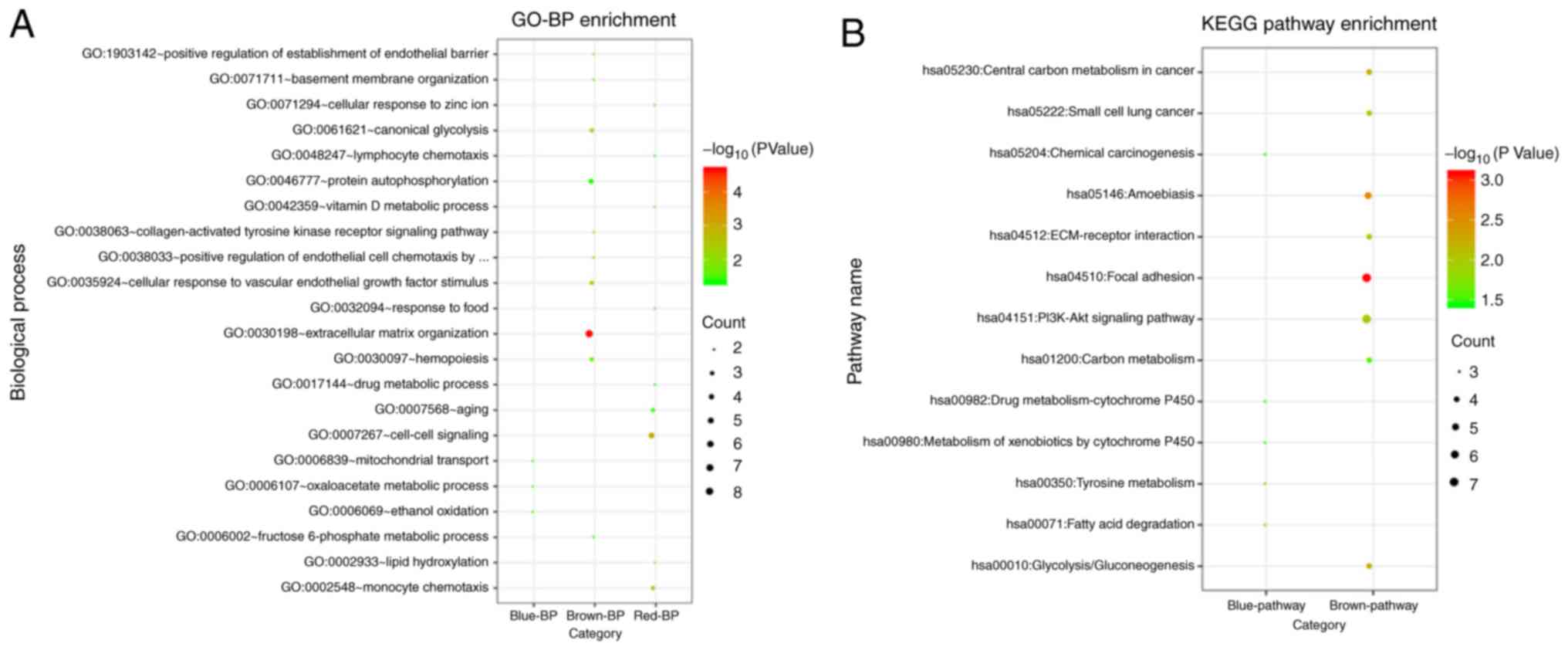
Functional enrichment analysis for the
genes in significant modules
The results of functional enrichment analysis
suggested that the DEGs in the brown module were significantly
enriched in eight KEGG signaling pathways and 14 GO_BPs, such as
‘hsa04510:Focal adhesion’ [involving collagen type IV α1 (COL4A1)
and COL4A2], ‘hsa04151:PI3K-Akt signaling pathway’ (involving
COL4A1 and COL4A2), ‘GO:0030198 extracellular matrix organization’
(involving COL4A1 and COL4A2) and ‘GO:0038063 collagen-activated
tyrosine kinase receptor signaling pathway’ (involving COL4A1 and
COL4A2). The DEGs in the blue module were significantly enriched in
five KEGG signaling pathways and three GO_BPs, such as
‘hsa00350:Tyrosine metabolism’ [involving alcohol dehydrogenase 1C,
γ polypeptide (ADH1C), alcohol dehydrogenase 1B and β polypeptide
(ADH1B)], ‘hsa00071:Fatty acid degradation’ (involving ADH1C and
ADH1B) and ‘GO:0006107 oxaloacetate metabolic process’ (involving
glutamic-oxaloacetic transaminase 1 and malate dehydrogenase 1).
The DEGs in the red module were significantly enriched in nine
GO_BPs, including ‘GO:0007267 cell-cell signaling’ [involving
fibroblast growth factor 6 (FGF6) and androgen receptor (AR)],
‘GO:0002548 monocyte chemotaxis’ (involving C-C motif chemokine
ligand 13 and C-C motif chemokine ligand 4 like 1) and others
(Fig. 4). It was found that no
KEGG pathways were significantly enriched for the DEGs in the red
module. The significantly enriched KEGG pathways and top ten GO_BPs
are presented in Fig. 4, and
detailed information of significantly enriched results is shown in
Table II.
 | Table II.Results of the significantly enriched
pathways and GO terms. |
Table II.
Results of the significantly enriched
pathways and GO terms.
| Module | Category | Term | Count | P-value | Genes | Benjamini | FDR |
|---|
| Enriched results
for the genes in brown module | KEGG_pathway | hsa04510:Focal
adhesion | 7 |
8.31×10−4 | COL4A2, LAMA4,
COL4A1, MYLK4, MYLK2, LAMB1, KDR | 0.07668634 | 0.91120178 |
|
| KEGG_pathway |
hsa05146:Amoebiasis | 5 | 0.0026281 | COL4A2, LAMA4,
COL4A1, HSPB1, LAMB1 | 0.11866249 | 2.85678542 |
|
| KEGG_pathway | hsa05230:Central
carbon metabolism in cancer | 4 | 0.0051378 | PKM, PGAM2, KIT,
PFKM | 0.15196496 | 5.51540694 |
|
| KEGG_pathway |
hsa00010:Glycolysis/Gluconeogenesis | 4 | 0.00584063 | PKM, PGAM2, FBP2,
PFKM | 0.13115115 | 6.24799315 |
|
| KEGG_pathway | hsa04151:PI3K-Akt
signaling pathway | 7 | 0.01078372 | COL4A2, LAMA4,
COL4A1, GNG11, KIT, LAMB1, KDR | 0.18793258 | 11.256198 |
|
| KEGG_pathway | hsa05222:Small cell
lung cancer | 4 | 0.01124949 | COL4A2, LAMA4,
COL4A1, LAMB1 | 0.16557454 | 11.7153268 |
|
| KEGG_pathway |
hsa04512:ECM-receptor interaction | 4 | 0.01198155 | COL4A2, LAMA4,
COL4A1, LAMB1 | 0.15236967 | 12.4325909 |
|
| KEGG_pathway | hsa01200:Carbon
metabolism | 4 | 0.023997 | PKM, PGAM2, FBP2,
PFKM | 0.25283964 | 23.4728875 |
|
| GO_BP terms |
GO:0030198~extracellular matrix
organization | 8 |
2.25×10−5 | COL4A2, LAMA4,
PXDN, COL4A1, PECAM1, NID2, LAMB1, KDR | 0.01209389 | 0.03279351 |
|
| GO_BP terms | GO:0035924~cellular
response to vascular endothelial growth factor stimulus | 3 | 0.00444559 | NRP1, HSPB1,
KDR | 0.69970374 | 6.27967557 |
|
| GO_BP terms |
GO:0061621~canonical glycolysis | 3 | 0.00566291 | PKM, PGAM2,
PFKM | 0.64020486 | 7.93401129 |
|
| GO_BP terms | GO:1903142~positive
regulation of establishment of endothelial barrier | 2 | 0.0172777 | CDH5, PROC | 0.9049046 | 22.4071852 |
|
| GO_BP terms | GO:0038033~positive
regulation of endothelial cell chemotaxis by VEGF-activated
vascular endothelial growth factor receptor signaling pathway | 2 | 0.02155092 | HSPB1, KDR | 0.90491128 | 27.1762946 |
|
| GO_BP terms |
GO:0038063~collagen-activated tyrosine
kinase receptor signaling pathway | 2 | 0.0258058 | COL4A2, COL4A1 | 0.90491796 | 31.6525377 |
|
| GO_BP terms |
GO:0030097~hemopoiesis | 3 | 0.02719289 | MKNK2, KIT,
RUNX3 | 0.88078025 | 33.0554874 |
|
| GO_BP terms | GO:0006002~fructose
6-phosphate metabolic process | 2 | 0.0342609 | FBP2, PFKM | 0.90493131 | 39.7971984 |
|
| GO_BP terms | GO:0071711~basement
membrane organization | 2 | 0.0342609 | COL4A1, NID2 | 0.90493131 | 39.7971984 |
|
| GO_BP terms | GO:0046777~protein
autophosphorylation | 4 | 0.03899119 | MKNK2, MYLK2, KIT,
KDR | 0.90803087 | 43.9498963 |
|
| GO_BP terms | GO:0048010~vascular
endothelial growth factor receptor signaling pathway | 3 | 0.03918738 | NRP1, HSPB1,
KDR | 0.88452453 | 44.1162247 |
|
| GO_BP terms | GO:0016032~viral
process | 5 | 0.0411908 | ATF7IP, CD93,
SLC25A5, CARM1, KDR | 0.8731715 | 45.7886156 |
|
| GO_BP terms |
GO:0045616~regulation of keratinocyte
differentiation | 2 | 0.04264361 | CD109, ERRFI1 | 0.85929364 | 46.9721 |
|
| GO_BP terms | GO:0030335~positive
regulation of cell migration | 4 | 0.04608079 | HAS2, KIT, LAMB1,
KDR | 0.85908958 | 49.6769433 |
|
| KEGG_pathway | hsa00350:Tyrosine
metabolism | 3 | 0.0075711 | GOT1, ADH1C,
ADH1B | 0.51050995 | 7.99894295 |
| Enriched
results | KEGG_pathway | hsa00071:Fatty acid
degradation | 3 | 0.01077982 | ACSL1, ADH1C,
ADH1B | 0.39914541 | 11.2099386 |
| for the genes
in | KEGG_pathway | hsa00982:Drug
metabolism - cytochrome P450 | 3 | 0.02686102 | ADH1C, ADH1B,
MGST1 | 0.57393167 | 25.8212278 |
| blue module | KEGG_pathway | hsa00980:Metabolism
of xenobiotics by cytochrome P450 | 3 | 0.03141257 | ADH1C, ADH1B,
MGST1 | 0.52765151 | 29.5396948 |
|
| KEGG_pathway | hsa05204:Chemical
carcinogenesis | 3 | 0.03624794 | ADH1C, ADH1B,
MGST1 | 0.50048559 | 33.3037599 |
|
| GO_BP terms |
GO:0006107~oxaloacetate metabolic
process | 2 | 0.03239246 | GOT1, MDH1 | 0.99999776 | 36.7737565 |
|
| GO_BP terms | GO:0006069~ethanol
oxidation | 2 | 0.03239246 | ADH1C, ADH1B | 0.99999776 | 36.7737565 |
|
| GO_BP terms |
GO:0006839~mitochondrial transport | 2 | 0.04032881 | SLC25A30, UCP3 | 0.99970543 | 43.6233265 |
|
| GO_BP terms |
GO:0007267~cell-cell signaling | 5 |
9.02×10−4 | FGF6, AR, CCL13,
CCL4L1, PHEX | 0.21970158 | 1.18243256 |
| Enriched
results | GO_BP terms | GO:0002548~monocyte
chemotaxis | 3 | 0.00237563 | CCL13, CCL4L1,
IL6R | 0.27894373 | 3.08747273 |
| for the genes
in | GO_BP terms | GO:0002933~lipid
hydroxylation | 2 | 0.01031897 | CYP3A4, CYP1A1 | 0.61357594 | 12.7828018 |
| red module | GO_BP terms | GO:0042359~vitamin
D metabolic process | 2 | 0.01714109 | CYP3A4, CYP1A1 | 0.69537283 | 20.3855781 |
|
| GO_BP terms | GO:0071294~cellular
response to zinc ion | 2 | 0.03232524 | ZNF658, MT1X | 0.83589656 | 35.1613714 |
|
| GO_BP terms |
GO:0007568~aging | 3 | 0.03275751 | SLC32A1, SREBF1,
CYP1A1 | 0.78271109 | 35.5422436 |
|
| GO_BP terms | GO:0032094~response
to food | 2 | 0.03566861 | SREBF1, CYP1A1 | 0.75994039 | 38.0538321 |
|
| GO_BP terms | GO:0017144~drug
metabolic process | 2 | 0.04563192 | CYP3A4, CYP1A1 | 0.79921365 | 45.9815491 |
|
| GO_BP terms |
GO:0048247~lymphocyte chemotaxis | 2 | 0.04728279 | CCL13, CCL4L1 | 0.77236779 | 47.2007199 |
Construction of PPI network
The PPI network contained 104 nodes, of which 57
belonged to the brown module, 29 belonged to the blue module and 18
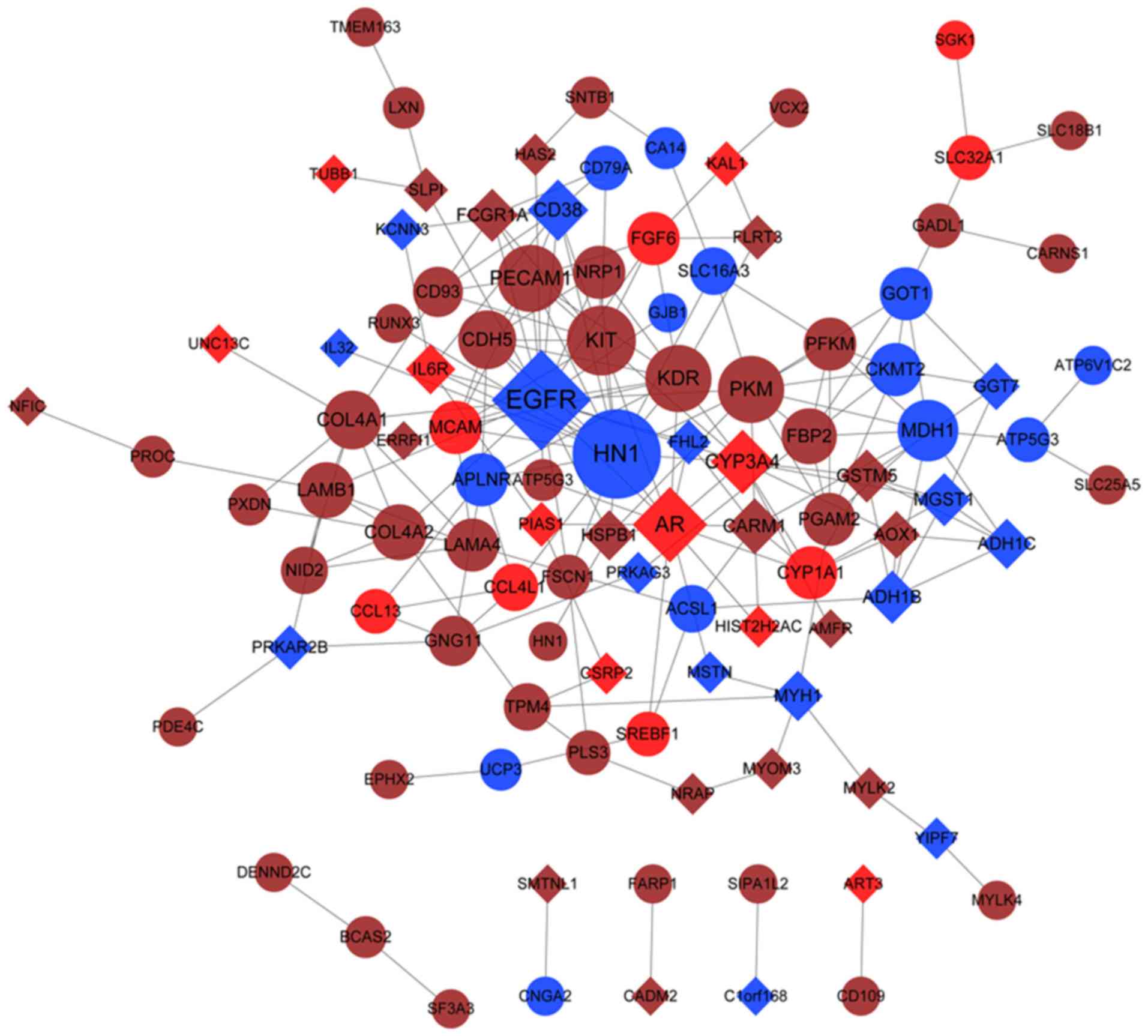
belonged to the red module, and 211 interactions (Fig. 5). The nodes in the PPI network with
a degree of >5 are presented in Table III. It was demonstrated that
epidermal growth factor receptor (EGFR, degree =23), vascular
endothelial growth factor A (VEGFA), AR, proto-oncogene receptor
tyrosine kinase (KIT), COL4A1 (degree =8) and COL4A2 (degree =7)
were the hub genes with higher degrees in the PPI network.
Furthermore, EGFR and VEGFA were the genes in the blue module, and
AR was the gene in the red module, while KIT, COL4A1 and COL4A2
were the genes in the brown module (Fig. 5).
 | Table III.Nodes in the protein-protein
interaction network with degree >5. |
Table III.
Nodes in the protein-protein
interaction network with degree >5.
| Nodes | Regulation | Module | Degree |
|---|
| EGFR | down | blue | 23 |
| VEGFA | up | blue | 19 |
| AR | down | red | 14 |
| KIT | up | brown | 12 |
| KDR | up | brown | 11 |
| PECAM1 | up | brown | 11 |
| PKM | up | brown | 11 |
| CYP3A4 | down | red | 10 |
| MDH1 | up | blue | 9 |
| CD38 | down | blue | 9 |
| COL4A1 | up | brown | 8 |
| CDH5 | up | brown | 8 |
| COL4A2 | up | brown | 7 |
| LAMB1 | up | brown | 7 |
| FBP2 | up | brown | 7 |
| CARM1 | down | brown | 7 |
| NRP1 | up | brown | 6 |
| GOT1 | up | blue | 6 |
| ADH1B | down | blue | 6 |
| LAMA4 | up | brown | 6 |
| CYP1A1 | up | red | 6 |
| PFKM | up | brown | 6 |
| APLNR | up | blue | 6 |
| FGF6 | up | red | 6 |
| PGAM2 | up | brown | 6 |
| MCAM | up | red | 6 |
| MGST1 | down | blue | 6 |
| GSTM5 | down | brown | 6 |
| CKMT2 | up | blue | 6 |
| FCGR1A | down | brown | 6 |
| ADH1C | down | blue | 5 |
| GNG11 | up | brown | 5 |
| IL6R | down | red | 5 |
| HSPB1 | down | brown | 5 |
| MYH1 | down | blue | 5 |
| CD93 | up | brown | 5 |
Construction of miRNA-TF-target
regulatory network
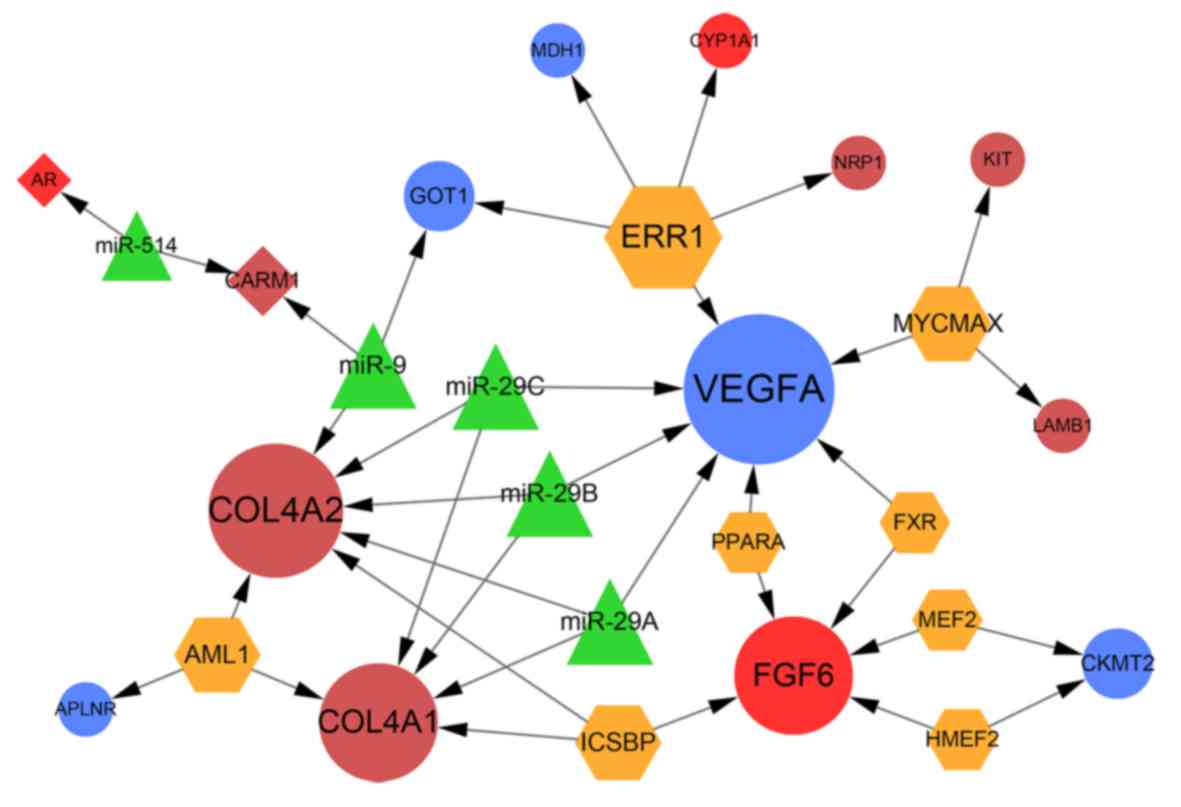
The miRNA-TF-target regulatory network included 27
nodes and 36 regulatory interactions (Fig. 6). In total, five miRNAs and eight
TFs were predicted to regulate 14 DEGs, including 12 upregulated
DEGs and two downregulated DEGs. It was found that VEGFA, COL4A1,
COL4A2 and FGF6 were the hub nodes in the regulatory network, in
which VEGFA, COL4A1 and COL4A2 were all regulated by miR-29a/b/c;
miR-29a/b/c regulated only these three DEGs in this network.
Moreover, FGF6 was regulated by five TFs, including interferon
consensus sequence binding protein (ICSBP). In addition, ICSBP also
regulated COL4A1 and COL4A2.
Confirmatory analysis
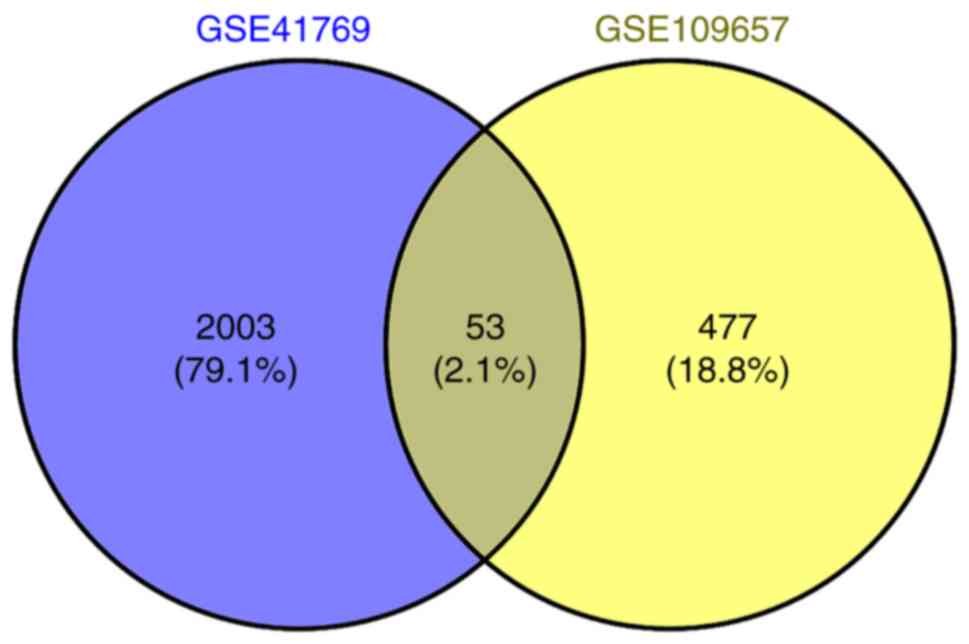
A total of 2,164 DEGs were obtained from skeletal
muscle after 1 h of one-legged exercise, including 809 upregulated
genes and 1,355 downregulated genes. There were 53 overlapping DEGs
in the two datasets, such as VEGFA and FGF6 (Fig. 7; Table SI). In the ‘GSE41769’ dataset,
collagen type VIII α 2 Chain and collagen type II α 1 chain
(COL2A1) were differentially expressed after 1 h of one-legged
exercise. However, in the ‘GSE109657’ dataset, COL4A1 and COL4A2
were differentially expressed after HIIT, suggesting that exercise
may induce expression changes of collagen-associated genes.
In addition, the DEGs were significantly enriched in
88 KEGG pathways and numerous GO_BPs, including the PI3K-Akt
signaling pathway (involving COL2A1 and VEGFA), regulation of
angiogenesis, sprouting angiogenesis, regulation of extracellular
matrix assembly, extracellular matrix organization and focal
adhesion assembly (Table SII).
Furthermore, these results were consistent with the results from
the analysis of genes in the brown module (Table II).
Discussion
In the present study, a total of 530 genes were
found to be abnormally expressed in skeletal muscle after a 6-week
HIIT, suggesting an effect of HIIT on the skeletal muscle. In
total, three significant modules (brown, blue and red modules) were
identified after WGCNA, and the genes, COL4A1 and COL4A2, in module
brown showed the strongest correlation with HIIT. There were 106,
74 and 49 DEGs in the brown, blue and red modules, respectively,
which were significantly enriched in focal adhesion, extracellular
matrix organization and the PI3K-Akt signaling pathway.
Furthermore, it was found that VEGFA, COL4A1 and COL4A2 were the
hub genes in the PPI network, and were all regulated by
miR-29a/b/c. Therefore, the present results indicated that these
genes, together with miR-29a/b/c, may have a regulatory function in
the skeletal muscle response to HIIT.
VEGFA is a protein-coding gene that plays a crucial
role in vascular endothelial cell growth and angiogenesis (21). Gustafsson et al (22), indicated that exercise can promote
the expression of VEGFA involved in the non-pathological
angiogenesis in human skeletal muscle. Moreover, Baum et al
(23) showed that exercise
training induces ultrastructural changes, such as pericyte
mobilization and basement membrane thinning in the capillaries, and
this process is associated with exercise-induced angiogenesis. The
generation of new capillaries in skeletal muscles is an adaptive
response of the skeletal muscle to exercise (24). Capillaries serve as major sites for
the transport of gas, nutrients and metabolic waste;
exercise-induced capillary angiogenesis ensures that the increased
need of active skeletal muscle for oxygen and nutrients is met
(24). The present results
suggested that VEGFA was upregulated in skeletal muscles after
HIIT, which was consistent with results from previous studies
(23,24), suggesting that skeletal muscle
angiogenesis was induced after HIIT and is associated with the
upregulation of VEGFA.
Previous studies in animal models have shown that
local VEGFA gene transfer accelerates long-term angiogenesis
(25,26). However, unregulated VEGFA
expression results in adverse changes leading to aberrant muscle
morphology (27), suggesting the
need for the regulation of VEGFA expression in long-term gene
transfer cases. Klagsbrun (28)
revealed that the extracellular matrix is a critical component in
the regulation of angiogenesis and could also provide a barrier to
angiogenesis. Furthermore, Sottile (29) reported that the extracellular
matrix controls the growth, differentiation and migration of
vascular endothelial cells in the course of angiogenesis. Moreover,
remodeling of extracellular matrix results in events that either
promote or inhibit angiogenesis (29). Focal adhesion also participates in
regulating cell migration and proliferation during angiogenesis,
and adhesion molecules may interact with the extracellular matrix
to exert an effect (30,31). The PI3K/Akt signaling pathway is
reported to regulate vascular endothelial cell elongation and
endothelial capillary stability during angiogenesis (32,33).
In the present study, COL4A1 and COL4A2 were significantly enriched
in focal adhesion, extracellular matrix organization and the
PI3K/Akt signaling pathway. COL4A1 and COL4A2 are type IV collagen
α proteins, and are major components of the basement membrane
(34). COL4A1 mutations are
reported to cause the endothelial cell defects and apoptosis in the
capillaries of skeletal muscle (35). Therefore, COL4A1 and COL4A2 may
mediate the growth and migration of vascular endothelial cells via
cell adhesion, extracellular matrix organization and the PI3K/Akt
signaling pathway, and as a result can regulate exercise-induced
skeletal muscle angiogenesis.
In the present study, miR-29a/b/c were predicted to
regulate VEGFA, COL4A1 and COL4A2 in the regulatory network. A
previous study showed that miR-29 plays an important role in
regulating skeletal muscle growth and differentiation via
decreasing Akt3 (36).
Furthermore, it was demonstrated that miR-29b mediates the
expression of collagen type I α via the PI3K/Akt signaling pathway
in human Tenon's fibroblasts (37). In addition, miR-29b targets VEGFA
via the PI3K/Akt signaling pathway to suppress angiogenesis in
endometrial carcinoma (38).
Moreover, miR-29c and miR-29a are crucial regulators in the cell
cycle progression and growth, as well as in the angiogenic
properties of human umbilical vein endothelial cells (39,40).
The present study identified the potential roles of miR-29a/b/c in
skeletal muscle and angiogenesis. Therefore, miR-29a/b/c may
regulate the exercise-induced angiogenesis in skeletal muscle by
targeting VEGFA, COL4A1 and COL4A2 via the PI3K/Akt signaling
pathway. However, further experimental studies are required to
investigate the present results in greater depth.
In conclusion, the present results suggested that
VEGFA, COL4A1 and COL4A2 were upregulated in the skeletal muscle in
response to HIIT. Furthermore, COL4A1 and COL4A2 may mediate the
growth and migration of vascular endothelial cells via cell
adhesion and extracellular matrix organization, along with the
regulation of angiogenesis. It was demonstrated that skeletal
muscle angiogenesis may be regulated by miR-29a/b/c targeting
VEGFA, COL4A1 and COL4A2 via the PI3K/Akt signaling pathway.
Therefore, the present results may facilitate continued
investigation into the effect of exercise on skeletal muscles.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LC was responsible for the conception and design of
the research, and drafting the manuscript. JB performed the data
acquisition. YL performed the data analysis and interpretation. LC
and JB participated in the design of the study and performed the
statistical analysis. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Horak M, Novak J and Bienertova-Vasku J:
Muscle-specific microRNAs in skeletal muscle development. Dev Biol.
410:1–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egan B and Zierath JR: Exercise metabolism
and the molecular regulation of skeletal muscle adaptation. Cell
Metab. 17:162–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Drake JC, Wilson RJ and Yan Z: Molecular
mechanisms for mitochondrial adaptation to exercise training in
skeletal muscle. FASEB J. 30:13–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tunstall RJ, Mehan KA, Wadley GD, Collier
GR, Bonen A, Hargreaves M and Cameron-Smith D: Exercise training
increases lipid metabolism gene expression in human skeletal
muscle. Am J Physiol Endocrinol Metab. 283:E66–E72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieman DC, Shanely RA, Zwetsloot KA,
Meaney MP and Farris GE: Ultrasonic assessment of exercise-induced
change in skeletal muscle glycogen content. BMC Sports Sci Med
Rehabil. 7:92015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoppeler H: Exercise-induced
ultrastructural changes in skeletal muscle. Int J Sports Med.
7:187–204. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baar K, Wende AR, Jones TE, Marison M,
Nolte LA, Chen M, Kelly DP and Holloszy JO: Adaptations of skeletal
muscle to exercise: Rapid increase in the transcriptional
coactivator PGC-1. FASEB J. 16:1879–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cantó C, Jiang LQ, Deshmukh AS, Mataki C,
Coste A, Lagouge M, Zierath JR and Auwerx J: Interdependence of
AMPK and SIRT1 for metabolic adaptation to fasting and exercise in
skeletal muscle. Cell Metab. 11:213–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tzanis G, Philippou A, Karatzanos E,
Dimopoulos S, Kaldara E, Nana E, Pitsolis T, Rontogianni D,
Koutsilieris M and Nanas S: Effects of high-intensity interval
exercise training on skeletal myopathy of chronic heart failure. J
Card Fail. 23:36–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peake JM, Della Gatta P, Suzuki K and
Nieman DC: Cytokine expression and secretion by skeletal muscle
cells: Regulatory mechanisms and exercise effects. Exerc Immunol
Rev. 21:8–25. 2015.PubMed/NCBI
|
|
11
|
McCarthy JJ: microRNA and skeletal muscle
function: Novel potential roles in exercise, diseases, and aging.
Front Physiol. 5:290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Zhao C, Sun X, Liu Z and Zhang J:
MicroRNA-761 regulates mitochondrial biogenesis in mouse skeletal
muscle in response to exercise. Biochem Biophys Res Commun.
467:103–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki T, Kuboyama A, Mita M, Murata S,
Shimizu M, Inoue J, Mori K and Sato R: The exercise-inducible bile
acid receptor Tgr5 improves skeletal muscle function in mice. J
Biol Chem. 293:10322–10332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henríquez-Olguín C, Renani LB,
Arab-Ceschia L, Raun SH, Bhatia A, Li Z, Knudsen JR, Holmdahl R and
Jensen TE: Adaptations to high-intensity interval training in
skeletal muscle require NADPH oxidase 2. Redox Biol. 24:1011882019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyamoto-Mikami E, Tsuji K, Horii N,
Hasegawa N, Fujie S, Homma T, Uchida M, Hamaoka T, Kanehisa H,
Tabata I, et al: Gene expression profile of muscle adaptation to
high-intensity intermittent exercise training in young men. Sci
Rep. 8:16811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song WM and Zhang B: Multiscale embedded
gene co-expression network analysis. PLOS Comput Biol.
11:e10045742015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni M, Liu X, Wu J, Zhang D, Tian J, Wang
T, Liu S, Meng Z, Wang K, Duan X, et al: Identification of
candidate biomarkers correlated with the pathogenesis and prognosis
of non-small cell lung cancer via integrated bioinformatics
analysis. Front Genet. 9:4692018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Catoire M, Mensink M, Boekschoten MV,
Hangelbroek R, Müller M, Schrauwen P and Kersten S: Pronounced
effects of acute endurance exercise on gene expression in resting
and exercising human skeletal muscle. PLoS One. 7:e510662012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng ACT, Kuraitis D, Deeke SA, Ahmadi A,
Dugan SG, Cheng BLM, Crowson MG, Burgon PG, Suuronen EJ, Chen HH,
et al: IRF2BP2 is a skeletal and cardiac muscle-enriched
ischemia-inducible activator of VEGFA expression. FASEB J.
24:4825–4834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gustafsson T, Rundqvist H, Norrbom J,
Rullman E, Jansson E and Sundberg CJ: The influence of physical
training on the angiopoietin and VEGF-A systems in human skeletal
muscle. J Appl Physiol 1985. 103:1012–1020. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baum O, Gübeli J, Frese S, Torchetti E,
Malik C, Odriozola A, Graber F, Hoppeler H and Tschanz SA:
Angiogenesis-related ultrastructural changes to capillaries in
human skeletal muscle in response to endurance exercise. J Appl
Physiol 1985. 119:1118–1126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haas TL and Nwadozi E: Regulation of
skeletal muscle capillary growth in exercise and disease. Appl
Physiol Nutr Metab. 40:1221–1232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morland C, Andersson KA, Haugen ØP, Hadzic
A, Kleppa L, Gille A, Rinholm JE, Palibrk V, Diget EH, Kennedy LH,
et al: Exercise induces cerebral VEGF and angiogenesis via the
lactate receptor HCAR1. Nat Commun. 8:155572017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Palazon A, Tyrakis PA, Macias D, Veliça P,
Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk
I, et al: An HIF-1α/VEGF-A axis in cytotoxic T cells regulates
tumor progression. Cancer Cell. 32:669–683.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karvinen H, Pasanen E, Rissanen TT,
Korpisalo P, Vähäkangas E, Jazwa A, Giacca M and Ylä-Herttuala S:
Long-term VEGF-A expression promotes aberrant angiogenesis and
fibrosis in skeletal muscle. Gene Ther. 18:1166–1172. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klagsbrun M: Regulators of angiogenesis:
Stimulators, inhibitors, and extracellular matrix. J Cell Biochem.
47:199–200. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sottile J: Regulation of angiogenesis by
extracellular matrix. BBA-Reviews on Cancer. 1654:13–22.
2004.PubMed/NCBI
|
|
30
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wary KK, Kohler EE and Chatterjee I: Focal
adhesion kinase regulation of neovascularization. Microvasc Res.
83:64–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsuji-Tamura K and Ogawa M: Inhibition of
the PI3K-Akt and mTORC1 signaling pathways promotes the elongation
of vascular endothelial cells. J Cell Sci. 129:1165–1178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee NY, Golzio C, Gatza CE, Sharma A,
Katsanis N and Blobe GC: Endoglin regulates PI3-kinase/Akt
trafficking and signaling to alter endothelial capillary stability
during angiogenesis. Mol Biol Cell. 23:2412–2423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao M, Alavi MV, Labelle-Dumais C, Gould
DB and Type IV: Type IV collagens and basement membrane diseases:
Cell biology and pathogenic mechanisms. Curr Top Membr. 76:61–116.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guiraud S, Migeon T, Ferry A, Chen Z,
Ouchelouche S, Verpont MC, Sado Y, Allamand V, Ronco P and Plaisier
E: HANAC Col4a1 mutation in mice leads to skeletal muscle
alterations due to a primary vascular defect. Am J Pathol.
187:505–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei W, He HB, Zhang WY, Zhang HX, Bai JB,
Liu HZ, Cao JH, Chang KC, Li XY and Zhao SH: miR-29 targets Akt3 to
reduce proliferation and facilitate differentiation of myoblasts in
skeletal muscle development. Cell Death Dis. 4:e6682013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li N, Cui J, Duan X, Chen H and Fan F:
Suppression of type I collagen expression by miR-29b via PI3K, Akt,
and Sp1 pathway in human Tenon's fibroblasts. Invest Ophthalmol Vis
Sci. 53:1670–1678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen HX, Xu XX, Tan BZ, Zhang Z and Zhou
XD: MicroRNA-29b inhibits angiogenesis by targeting VEGFA through
the MAPK/ERK and PI3K/Akt signaling pathways in endometrial
carcinoma. Cell Physiol Biochem. 41:933–946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu Y, Deng F, Song J, Lin J, Li X, Tang Y,
Zhou J, Tang T and Zheng L: Evaluation of miR-29c inhibits
endotheliocyte migration and angiogenesis of human endothelial
cells by suppressing the insulin like growth factor 1. Am J Transl
Res. 7:489–501. 2015.PubMed/NCBI
|
|
40
|
Yang Z, Wu L, Zhu X, Xu J, Jin R, Li G and
Wu F: MiR-29a modulates the angiogenic properties of human
endothelial cells. Biochem Biophys Res Commun. 434:143–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|