Introduction
Both acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) are severe clinical conditions accompanied
by interstitial edema and infiltration of inflammatory cells, which
result in progressive acute respiratory failure (1–3). A
previous systematic review reported that the mortality rate of
pediatric ARDS (PARDS) was 24%, with ~one-quarter of patients
developing new morbidities after PARDS due to residual organ
dysfunction and complications related to treatments (4,5).
Radiation-induced lung injury commonly occurs in patients receiving
radiotherapy for thoracic cancer (6). Inhaled corticosteroids and other
anti-inflammatory drugs are effective in patients with inflammatory
lung disorders. However, their long-term use is associated with
severe side effects (7). While
ARDS mortality has moderately declined with improved ventilator
management and fluid management, it has remained as high as 20 and
40% in clinical studies, respectively (2).
The therapeutic potential of mesenchymal stem cells
(MSCs) for ARDS has been evaluated in previous clinical trials
(8,10). It was demonstrated that a single
intravenous infusion of allogeneic bone marrow (BM)-MSCs was well
tolerated in nine patients with moderate to severe ARDS in a
previous clinical trial (NCT01775774) (8). However, the clinical use of BM-MSCs
was hindered by low cell numbers on harvest (9). Another previous clinical study
(NCT01902082) reported that intravenous administration of
allogeneic adipose-derived MSCs was safe and feasible but
inefficient in the treatment of ARDS (10). Compared with BM-MSCs,
placenta-derived MSCs (pMSCs) are more easily obtained in large
numbers (11,12). As pMSCs have similar properties and
functions as BM-MSCs, these cells have become a promising
alternative source of MSCs in research and clinical applications
(12). A previous study
demonstrated that treatment with pMSCs was protective against the
development of bronchiolitis obliterans in a murine model (13). Evidence has shown that the progress
of pulmonary inflammation is closely related to the phenotype and
function of macrophages (14,15).
pMSCs can regulate macrophage differentiation from the
pro-inflammatory type to the anti-inflammatory type (16). However, to the best of the authors'
knowledge, the therapeutic potential of pMSC delivery in ALI
treatment has not been studied to date.
C-X-C motif chemokine 12 (CXCL12) belongs to the
family of CXC chemokines. Diallyl trisulfide, a garlic-derived
organosulfur compound, markedly reduced the expression of CXCL12 in
lipopolysaccharide (LPS)-stimulated BV2 microglial cells,
demonstrating its anti-inflammatory effects against LPS stimulation
(17). The administration of
CXCL12-neutralizing antibodies delayed disease onset or prevented
disease progression in cancer, inflammatory bowel diseases and ALI
(18). Thus, CXCL12 may act as an
inflammatory cytokine in LPS-induced ALI.
ALI and ARDS often develop as a complication of
severe sepsis, particularly after infection with Gram-negative
bacteria (19). LPS-induced
endotoxemia triggers the secretion of pro-inflammatory cytokines
and is extensively used to establish an ALI animal model (20). In the present study, pMSCs were
used to treat LPS-induced RAW264.7 macrophages and the effects of
intratracheal delivery of pMSCs on LPS-induced ALI in
Sprague-Dawley rats were investigated.
Materials and methods
Isolation and culture of pMSCs
pMSCs were isolated from chorionic villi in placenta
of Sprague-Dawley rats, which were anesthetized by intraperitoneal
anesthesia with 2% pentobarbital sodium (30 mg/kg). Rats were
sacrificed using a supraphysiological dose of pentobarbital sodium
(100 mg/kg). Briefly, placental tissue was dissected, then
thoroughly washed with ice-cold sterile PBS (Gibco; Thermo Fisher
Scientific, Inc.) containing antibiotics. After removing the
amniotic membrane, the underlying chorionic villi were minced into
a paste-like consistency. Then, 10 g tissue samples were
enzymatically digested with trypsin and neutral protease for 60 min
at 37°C. Digestion was terminated with the addition of DMEM
containing 10% FBS (both from Gibco; Thermo Fisher Scientific,
Inc.). The suspension was filtered using a 200 mesh sieve,
collected and centrifuged at 1,000 × g for 10 min at 4°C. The
supernatant was discarded, and the pellet was resuspended in DMEM
containing 10% FBS and plated in T25 Flasks (Corning, Inc.) in a 5%
CO2 air incubator at 37°C (CB 170; BINDER GmbH). pMSCs
were identified by flow cytometry (CytoFLEX; Beckman Coulter, Inc.)
and the detection of the following cell surface markers: CD11b/c
(OX42, PE; cat. no. 12-0110-80; eBioscience; Thermo Fisher
Scientific, Inc.); CD44 (OX-49, FITC; cat. no. MA5-17522; Thermo
Fisher Scientific, Inc.); CD45 (OX1, APC; cat. no. 47-0461-80;
eBioscience; Thermo Fisher Scientific, Inc.); CD90 (HIS51, FITC;
cat. no. 03013-50; BioGems; PeproTech, Inc.); CD29 (HMb1-1, FITC;
cat. no. 11-0291-80; eBioscience; Thermo Fisher Scientific, Inc.);
CD31 (TLD-3A12, FITC; cat. no. MA5-16952; Thermo Fisher Scientific,
Inc.). The unstained control was used as a negative control in this
study. After incubation in the dark at 4°C for 30 min, CytExpert
software (version 2.0; Beckman Coulter, Inc.) was used for
analysis.
Immunofluorescence
The cell density was adjusted to 3×106/ml
and the cells were laid in the glass bottom dishes specially
designed for laser confocal microscopy. Cells were fixed with 4%
paraformaldehyde for 20 min at 4°C and permeabilized with 0.1%
Triton X-100 for 10 min, blocked with 3% BSA, and sealed for 60 min
at room temperature. After incubation overnight with anti-CD44
antibody (1:200; cat. no. ab189524; Abcam), the cells were rinsed
thoroughly and treated with anti-rabbit antibodies (1:200; cat. no.
BA1032; Boster Biological Technology), respectively. Nuclei were
stained using 0.3 µM DAPI (cat. no. C1002; Beyotime Institute of
Biotechnology).
Osteogenic and adipogenic
differentiation of pMSCs
The third generation of pMSCs were seeded at
1×105 cells/cm2 on a 24-well plate (Merck
KGaA). When the density of cells reached 80%, the complete medium
was removed and cells were induced with an osteogenic induction
medium (DMEM with 10% FBS; 0.1 µM dexamethasone; 10 mM
β-glycerophosphate sodium; 50 µM ascorbic acid). Cells were
cultured for 3 weeks and the medium was changed every 72 h. After 3
weeks, differentiated pMSCs were stained with aqueous 0.5% (v/v)
Alizarin Red S (Beijing Solarbio Science & Technology Co, Ltd.)
for 30 min at room temperature. For adipogenic induction, the cells
were plated in DMEM supplemented with 10% FBS, 200 µM indomethacin,
1 µM dexamethasone, 0.5 mm isobutyl methylxanthine and 0.5 µg/ml
insulin. After 10 days, the differentiation was verified using Oil
Red O staining for 10 min at room temperature (Sigma-Aldrich; Merck
KGaA).
Cell culture and stimulation. RAW264.7 cells
(cat. no. CL-0190; Procell Life Science & Technology Co., Ltd.)
were cultured in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. The cell cultures were
maintained in a 5% CO2 air incubator at 37°C. RAW264.7
cells were then seeded and cultured in six-well plates at a density
of 5×105 cells/well, followed by treatment with LPS at
different concentrations (1, 2 or 5 µg/ml) for different time
periods (0, 0.5, 1, 2, 4 or 24 h), and tumor necrosis factor-α
(TNF-α) levels were detected by ELISA (cat. no. E-EL-M0049c;
Elabscience Biotechnology, lnc.) to determine if the LPS-induced
RAW264.7 macrophage inflammatory model was established. To detect
the therapeutic effects of pMSCs in the RAW264.7 macrophage
inflammation model in vitro, RAW264.7 cells and pMSCs were
co-cultured at 37°C for 48 h in two chambers using Millicell
(Corning Inc.) to prevent contact between the cells.
TNF-α and interleukin (IL)-10
ELISA
Cell-free supernatants were collected, and IL-10 and
TNF-α levels were measured using IL-10 (cat. no. SEA056Ra, Wuhan
USCN Business Co., Ltd.) and TNF-α (cat. no. E-EL-R0019c;
Elabscience Biotechnology, Inc.) ELISA kits according to the
manufacturers' instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from RAW264.7 cells using
TRIpure (cat. no. RN0101; Aidlab Biotechnologies Co., Ltd.) and
reverse transcribed into cDNA using the HiScript Reverse
Transcriptase (RNase H) (cat. no. R101-01/02; Vazyme Biotech Co.,
Ltd.) at the following conditions: 25°C 5 min; 50°C 15 min; 85°C 5
min. PCR amplification was independently performed with a SYBR
Green PCR kit (cat. no. Q111-02; Vazyme Biotech Co., Ltd.) using
the Applied Biosystems 7500 real-time PCR system (Applied
Biosystems QuantStudio 6; Applied Biosystems; Thermo Fisher
Scientific, Inc.) in triplicate. The thermocycling conditions of
the PCR were as follows: 5 min at 95°C, 15 sec at 95°C and 1 min at
60°C for 40 cycles, then 5 min at 72°C. The reaction specificity
was controlled by post-amplification melting curve analysis and gel
electrophoresis. The expression fold changes were analyzed using
the 2−ΔΔCq relative quantification method (21). Primer sequences were as follows:
IL-10-forward, 5′-GCTGGACAACATACTGCTAACCG-3′; IL-10-reverse,
5′-CACAGGGGAGAAATCGATGACAG-3′; TNF-α-forward,
5′-CGTCAGCCGATTTGCTATCT-3′; TNF-α-reverse,
5′-CGGACTCCGCAAAGTCTAAG-3′; CXCL12-forward,
5′-TCAACACTCCAAACTGTGCCCTTCA-3′; CXCL12-reverse,
5′-GCCTTTCTCTTCTTCTGTCGCTTCT-3′; β-actin-forward,
5′-CACGATGGAGGGGCCGGACTCATC-3′ and β-actin-reverse,
5′-TAAAGACCTCTATGCCAACACAGT−3′.
Western blotting
Cells were lysed in RIPA lysis buffer (Beyotime
Institute of Biotechnology) on ice. Protein concentrations were
determined using the BCA kit (Thermo Fisher Scientific, Inc.). The
sample proteins (40 µg/lane) were separated via SDS-PAGE on 15%
gels and transferred to a PVDF membrane (EMD Millipore). The
membranes were then blocked with TBS containing 5% non-fat dried
milk and 0.1% Tween-20 at 4°C for 2 h. After blocking, the
membranes were incubated with a primary anti-CXCL12 antibody
(1:1,000; Abcam; cat. no. ab9797) overnight at 4°C with gentle
shaking and subsequently incubated with HRP-conjugated anti-rabbit
IgG secondary antibody (1:50,000; Wuhan Boster Biological
Technology, Ltd.; cat. no. BA1054) for 2 h at room temperature. The
bands were detected using enhanced chemiluminescence western
blotting detection reagents (Thermo Fisher Scientific, Inc.). GAPDH
(1:1,000; cat. no. AB-P-R 001; Goodhere Biological Technology) was
used as a loading control, and the band density was measured using
ImageJ (version 1.52a; National Institutes of Health).
Rat model of ALI
All animal procedures were approved by The Ethics
Committee of Clinical Research, Renmin Hospital of Wuhan University
(reference no. WDRY2018-K048). All animal experiments were
performed in compliance with the Guidelines for Proper Conduct of
Animal Experiments established by the Science Council. A total of
15 adult male Sprague-Dawley rats (aged 6–8 weeks; weight, 280–350
g) were provided by Hunan SJA Laboratory Animal Co., Ltd. The rats
were housed in a specific pathogen-free environment at 18–26°C in
the dark, with 40–70% humidity, and free access to food and water.
These rats were randomly divided into three groups: A normal
control (NC) group injected with 0.5 ml PBS; an LPS-induced ALI
group intravenously receiving 7.5 mg/kg LPS dissolved in 0.5 ml
sterile saline solution (22); and
in the LPS + pMSCs group, rats were anesthetized using 2%
pentobarbital (30 mg/kg), and 1×105 pMSCs intratracheal
instillation was performed after administration of LPS for 1 h
(pMSCs group). After 3 days, the lung tissue and the
bronchoalveolar lavage fluid (BALF) samples were collected
(23,24). There were five rats in each
group.
Analysis of BALF
BALF was collected and centrifuged at 600 × g for 5
min at 25°C, for quantification of total protein content in the
BALF supernatant standard BALF collection was performed, as
described previously (25). The
protein concentration was measured using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology) following the manufacturer's
instructions. BALF cytokine levels were examined using IL-10 (cat.
no. SEA056Ra, Wuhan USCN Business Co., Ltd.) and TNF-α (cat. no.
E-EL-R0019c, Elabscience Biotechnology, Inc.) ELISA kits.
Cell counts and Giemsa staining
The number of white blood cells in BALF was counted
using a Bowman plate. The alveolar lavage fluid was coated with
glass slides and stained with Giemsa (reagent 1 solution for 1 min
and reagent 2 for 6–8 mins at room temperature), then the stained
cells were observed with a light microscope at magnification, ×100
and ×200.
Histopathological observation of
lungs
The lower lobe of the right lung was collected from
the rats, fixed in 10% formaldehyde solution for 48 h at room
temperature, embedded in paraffin, dehydrated, cut into 5-µm
sections and observed under an optical microscope (magnification,
×100 and ×200) after hematoxylin-eosin (HE) staining for 5 min at
room temperature.
Immunohistochemical analysis
Paraffin-embedded tissues were sectioned at 5 µm,
mounted onto glass slides and dewaxed, then rehydrated in a
descending alcohol gradient (100, 95, 90, 80, 70 and 50%). Antigen
retrieval was performed by microwaving at high power in 10 mM
sodium citrate buffer, pH 6.0 for 20 min. To block the non-specific
binding of antibodies, each slide was blocked with 10% normal goat
serum (cat. no. AR1009; Boster Biological Technology) for 30 min at
room temperature. Immunostaining was performed by incubation with
an anti-CXCL12 antibody (1:200, cat. no. ab9797; Abcam) at 4°C
overnight. Slides were then washed in PBS and incubated with
secondary antibody (anti-rabbit detection system; 1:200; cat. no.
GB23303; Wuhan Servicebio Technology Co., Ltd.) for 30 min at 37°C.
Tissues were stained with 3, 3-diaminobenzidine for 5 min at room
temperature and counterstained with hematoxylin for 1 min at room
temperature. Photomicrographs were taken using an Olympus BX53
microscope (Olympus Corporation), at magnification, ×100 and ×400.
Image Pro Plus version 6.0 (Media Cybernetics, Inc.) was used to
determine staining intensity.
Statistical analysis
All data were analyzed using GraphPad Prism version
5.0 software (GraphPad Software, Inc.) and presented as the mean ±
SD of three independent experiments. One-way ANOVA followed by
Newman-Keuls Multiple Comparison post hoc test were used when
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and identification of
pMSCs
pMSCs from rat embryos were isolated and cultured,
which were passed to the P2 generation and adherent in a
serum-containing medium and showed a spindle-like shape (Fig. 1A). Phenotypic analysis was carried
out by flow cytometry. CD44, CD90 and CD29 were 97.87, 97.58 and
96.02%, respectively, while CD31, CD45, CD11b/c were negative
(Fig. 1B). This was consistent
with the expected pMSC phenotype
CD44+CD90+CD29+CD31−CD45−CD11b/c−.
Subsequently, immunofluorescence staining was used to evaluate
specific expression of the CD44 marker in pMSCs (Fig. 1C). The primary antibody was
replaced with PBS in the control group, while the CD44 antibody of
anti-mouse was replaced in the experimental group. Although the
staining result was similar in non-specific nuclear staining of
both groups, specific CD44 staining in the experimental group was
much stronger than that in the control group. Thus, cells isolated
from rat embryos were indeed pMSCs that expressed CD44. The
potential pluripotent ability of pMSCs was confirmed by osteogenic
and adipogenic differentiation in vitro (Fig. 1D), this indicated that pMSCs have
multilineage differentiation ability in the cultured condition.
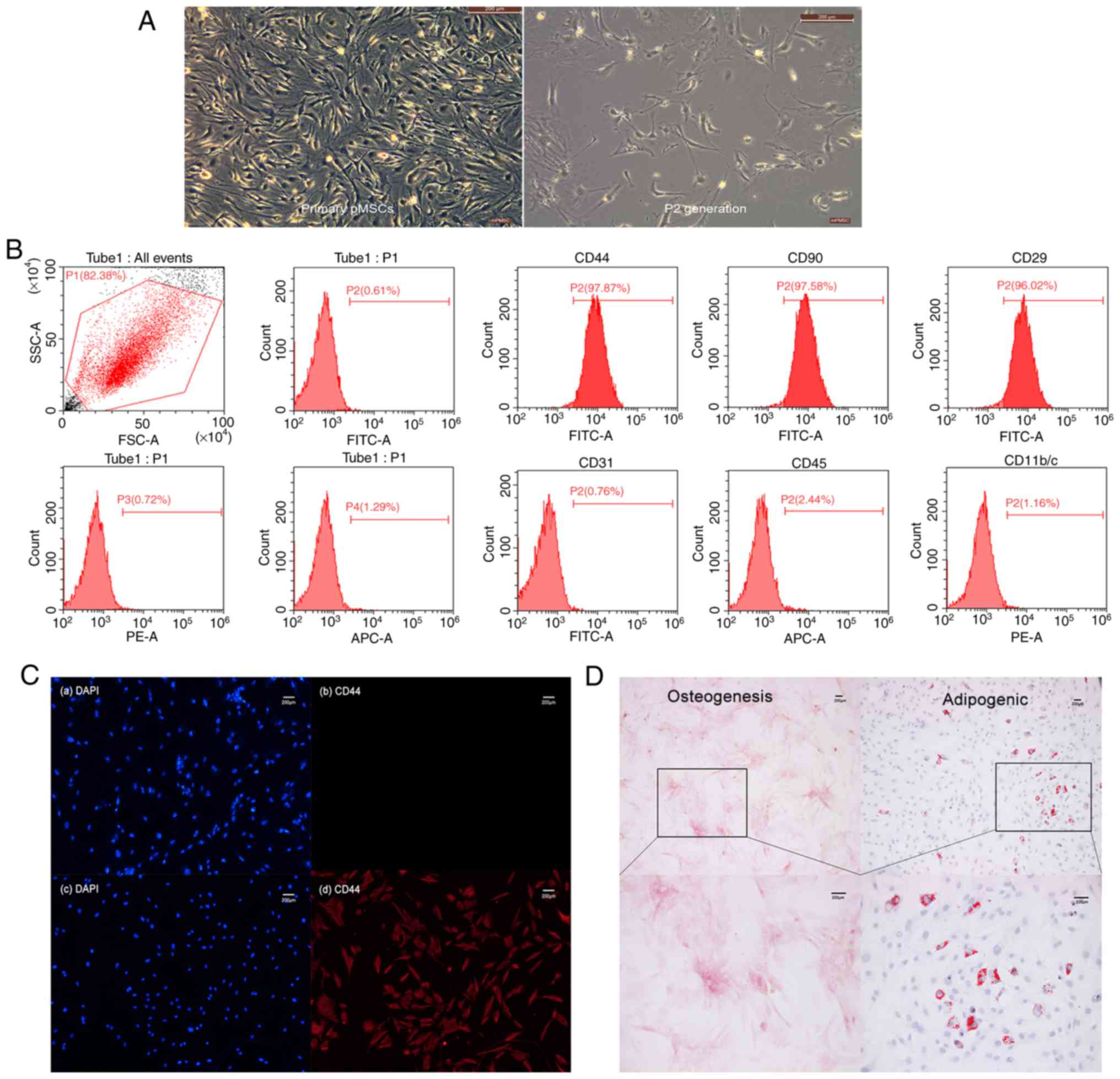 | Figure 1.Isolation and identification of rat
pMSCs. (A) Primary culture and P2 generation cell morphology
(magnification, ×100). (B) The three on the left panels are
unstained negative controls of flow cytometry analysis, while the
six on the right are the quantitative analysis of CD44, CD90, CD29,
CD31, CD45 and CD11b/c. (C) Immunofluorescence staining of pMSCs.
(a and b) Control group. (c and d) Experimental group. Non-specific
nuclear staining is shown in blue on the left-hand side, and
specific CD44 staining is in red on the right-hand side. (D)
Multi-lineage differentiation capacity of pMSCs. Osteogenesis
differentiation was demonstrated by Alizarin Red S staining
(magnification, ×100 and ×200), while adipogenic differentiation
was demonstrated by Oil Red O staining (magnification, ×100 and
×200). pMSC, placenta-derived mesenchymal stem cell; PE,
phycoerythrin; APC, allophycocyanin. |
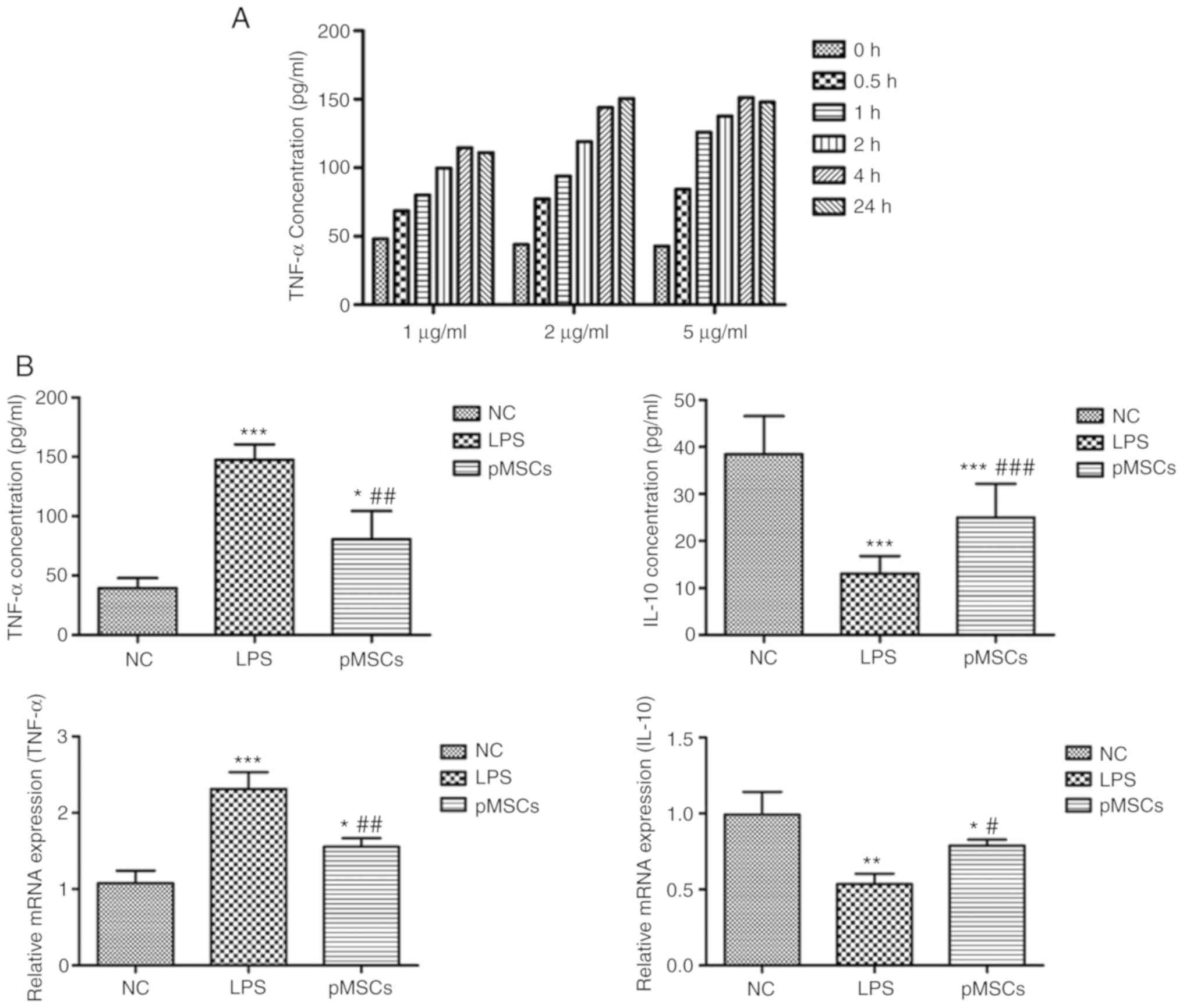
Therapeutic effects of pMSCs in
RAW264.7 macrophage inflammation model in vitro
Before establishing the inflammatory model, the
optimally induced concentration of LPS was evaluated using ELISA
and to establish an LPS-induced RAW264.7 macrophage inflammation
model. When the concentration of the pro-inflammatory cytokine
TNF-α reached the maximum, the optimum condition was achieved. As
presented in Fig. 2A, the optimal
concentration was 5 µg/ml with co-culturing for 4 h. The
inflammation model of RAW264.7 macrophages by this optimal
condition for 4 h was then induced. A total of three groups were
formed: RAW264.7 cells as the control (NC group), RAW264.7 cells
treated with LPS (LPS group), and RAW264.7 cells treated with LPS +
pMSCs (pMSCs group). The expressions of cytokines TNF-α and IL-10
were detected. As presented in Fig.
2B, pMSCs reduced the expression of TNF-α and increased IL-10
in the cell inflammatory model. These results provide evidence for
the direct involvement of the therapeutic effect of pMSCs in
LPS-induced injury.
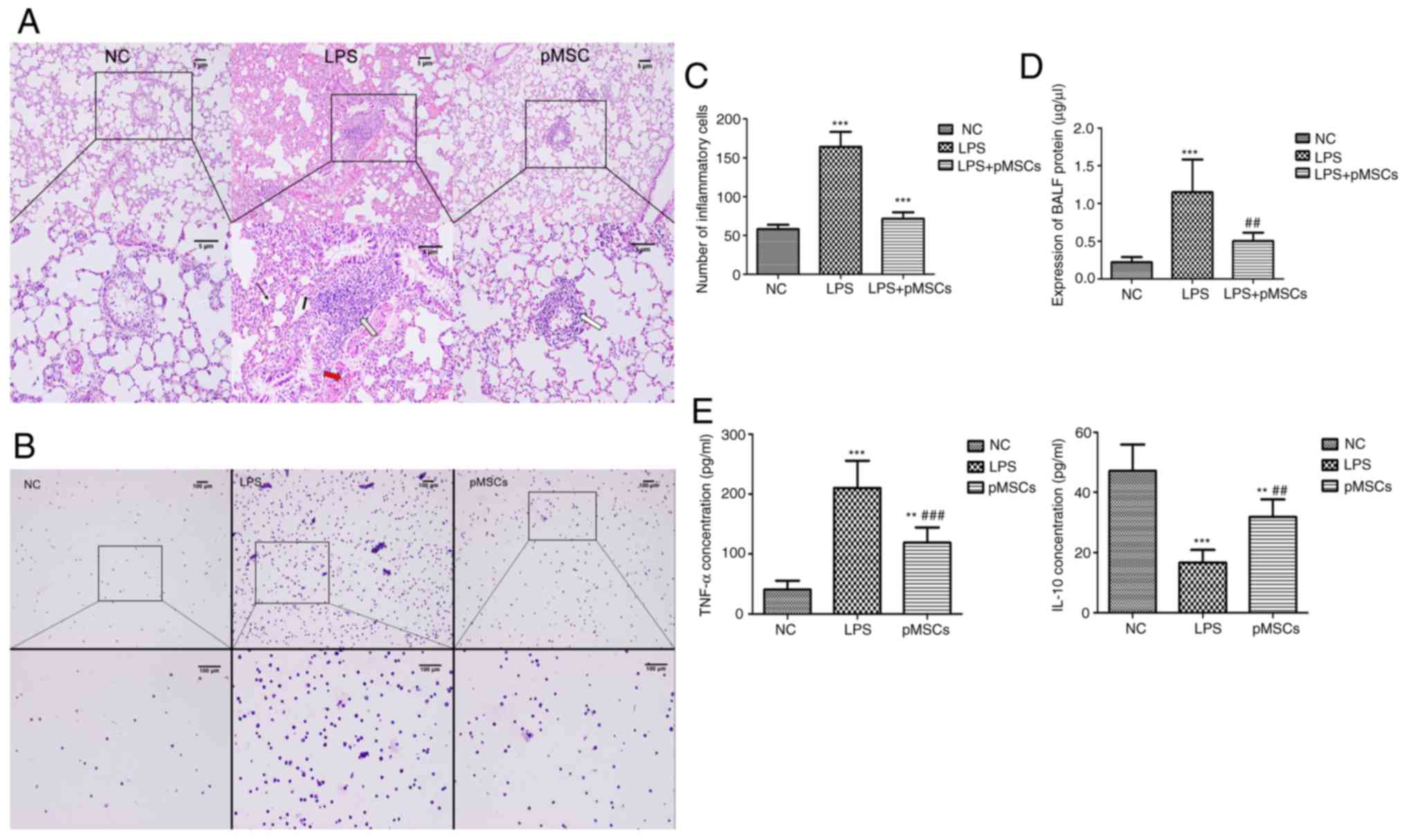
Influence of pMSCs on lung
histopathology
An LPS-induced ALI animal model was established to
determine whether pMSCs had a protective effect against LPS-induced
ALI in vivo. The histopathology of rat lung tissue from each
group was examined using HE staining (Fig. 3A). The HE staining of lung sections
before administration of LPS showed no obvious lesions in the NC
group. However, after LPS was administered, typical histological
features of ALI were observed in the LPS group, including diffuse
alveolar injury, massive pleomorphic leukocytes in the stroma,
obvious hyperemia and hemorrhage in alveolar, remarkable
interstitial edema, and thickened alveolar septum. Although the
treatment of pMSCs improved the lung histopathology by reducing
neutrophil infiltration and alleviating interstitial edema, there
was still challenge compared with that of the NC group.
Effect of pMSCs on BALF inflammatory
cells and proteins
As the hallmark of ALI, indicators of vascular
leakage in BALF were assessed, typically the total leukocyte cell
number and total protein concentration, which are common indicators
to evaluate the severity of alveolar-capillary membrane injury. The
pMSCs injected into the LPS-induced ALI rats caused a decrease in
the balance of inflammatory cells and the total cell count was
significantly reduced compared with the LPS group (Fig. 3B and C). The protein in BALF was
markedly increased in rats that received LPS compared with
untreated rats (P<0.05 vs. NC group). Moreover, a significant
decrease in the protein of BALF was observed in the pMSCs group
(P<0.05 vs. LPS group; Fig.
3D).
Reduced TNF-α and increased IL-10 by
pMSCs
TNF-α and IL-10 expression levels in BALF of the
three groups were determined using ELISA. The average of the data
was acquired after multiple measurements. The administration of
pMSCs led to decreased TNF-α and increased IL-10 (Fig. 3E) during LPS-induced ALI, which
suggested that pMSCs are an effective therapy for LPS-induced
ALI.
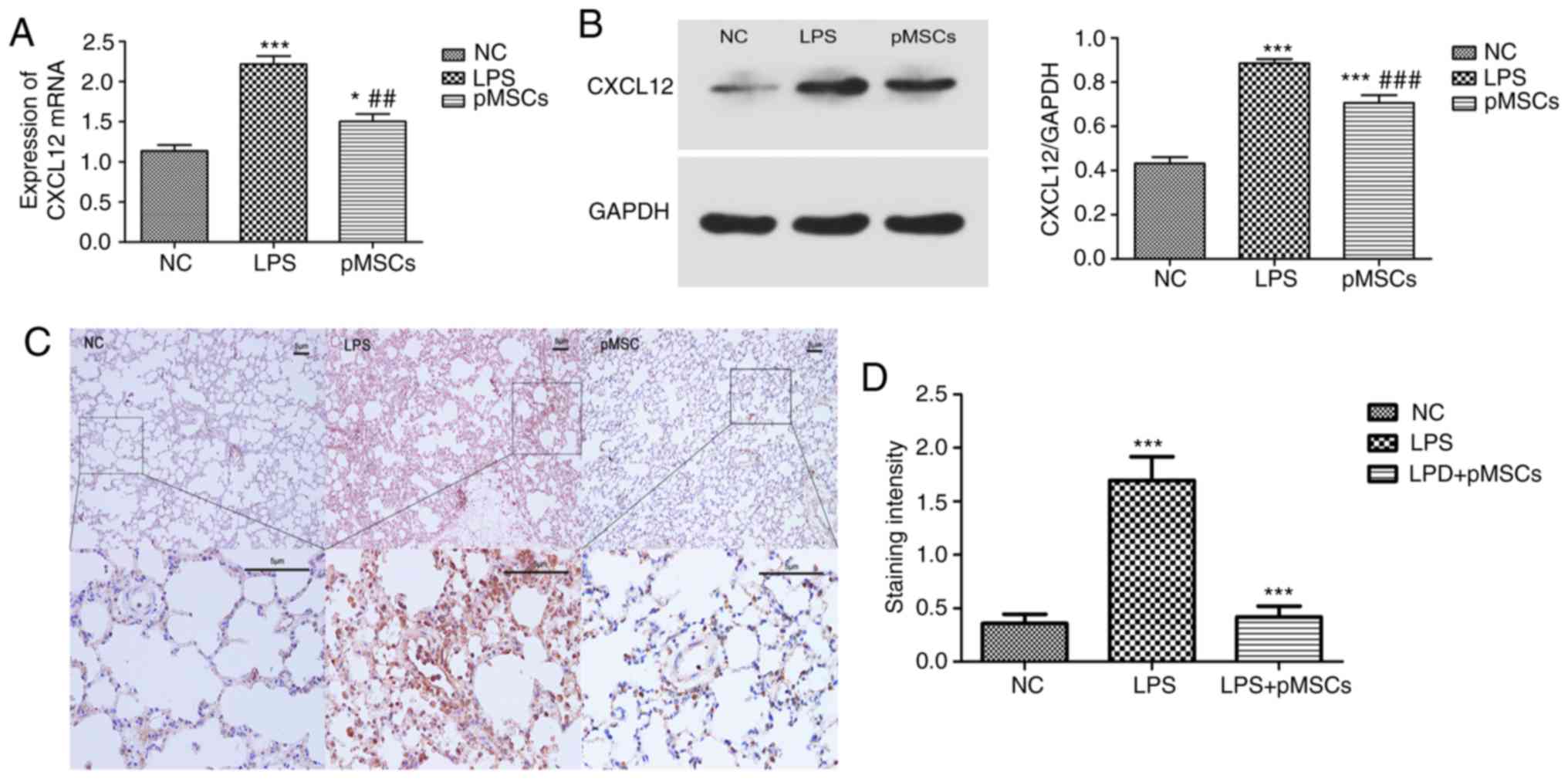
pMSC downregulates CXCL12 in
LPS-induced ALI
In our previous studies it was demonstrated that the
activation of the CXCL12/CXCR4 axis played an important role in the
metastasis and drug resistance in lung cancer (26–28).
Moreover, CXCL12 could serve as pro-inflammatory chemokines as was
reported in some previous studies (17,18).
To further investigate the mechanism underlying the effect of pMSCs
on LPS-induced ALI, mRNA levels and protein expression of CXCL12 in
LPS-induced RAW264.7 cells and LPS-induced ALI were measured. As
presented in Fig. 4A and B, the
LPS group showed a significantly higher expression of CXCL12
compared with the NC group (P<0.001). However, treatment with
pMSCs reversed this effect, suggesting that pMSCs reduced the
expression of CXCL12 in the RAW264.7 macrophage inflammatory model
(P<0.05). To demonstrate the effects of pMSCs on
histopathological changes in lung tissues in LPS-induced ALI rats,
histological analysis was carried out using immunohistochemistry.
The LPS group displayed very high expression of CXCL12, while the
pMSCs group showed a marked reduction of CXCL12 (Fig. 4C and D). These data indicated that
pMSC administration reduced the LPS-induced expression of CXCL12 in
RAW264.7 macrophage and in lung tissue of ALI rats. Therefore,
pMSCs significantly regulated levels of pro-inflammatory cytokines,
such as TNF-α (P<0.05) and anti-inflammatory IL-10 (P<0.05),
as well as accumulation of inflammatory cells and protein
concentration in BALF. This suggested that pMSCs were effective in
attenuating LPS-induced ALI.
Discussion
Despite the increased understanding of the
pathogenesis of ALI and ARDS, the underlying mechanism remains to
be investigated. Currently, there is evidence suggesting that
macrophages are key factors in the pathogenesis of ALI/ARDS
(29). Macrophages can be divided
into pro-inflammatory M1-type and anti-inflammatory M2-type
macrophages. While the M1-type can secrete TNF-α cytokines to
promote the progress of inflammation, the M2-type can secrete IL-10
to inhibit the progress of inflammation (30). A previous study suggested that
regulating the function of macrophages might be a promising
therapeutic strategy against ALI/ARDS (14). The present study demonstrated that
pMSCs could inhibit the inflammatory response by decreasing the
secretion of TNF-α cytokines and increasing the IL-10 secretion in
an LPS-induced RAW264.7 macrophage inflammatory model.
In the present study, pMSCs could inhibit the
inflammatory response of LPS-induced RAW264.7 macrophages. Thus,
the focus of the present study was to evaluate whether pMSCs had an
effect on ALI in rats and how this therapeutic effect could be
exerted. An ALI rat model was established using intravenous
instillation of LPS. Pretreatment with pMSCs significantly reduced
LPS-induced lung pathological changes, levels of pro-inflammatory
cytokines and infiltration of pleomorphic leukocytes and total
protein in BALF. Previous studies demonstrated that MSCs could be
transplanted into rabbits or humans with beneficial effects and
without immunological rejection (31,32).
In the present study, pMSC administration did not cause any adverse
effect in rats and led to decreased TNF-α and increased IL-10
levels during LPS-induced ALI. A previous study demonstrated that
IL-10 overexpression in umbilical cord MSCs enhanced their effects
in E. coli pneumosepsis and increased macrophage function,
which illustrated their therapeutic potential for infection-induced
ARDS (33). Recently, it has been
reported that erythropoietin produces protective effects against
ALI in rats by increasing the levels of anti-inflammatory cytokine
IL-10 (34). The present study
demonstrated that pMSCs increased the IL-10 secretion of
LPS-induced RAW264.7 macrophages. In addition, pMSC treatment
increased IL-10 levels, which led to beneficial effects on
LPS-induced ALI and enhanced the macrophage function. pMSCs reduced
the LPS-induced expression of CXCL12 in RAW264.7 macrophages and in
lung tissue of ALI rats. Therefore, CXCL12 may act as an
inflammatory cytokine in LPS-induced ALI and pMSCs could have a
therapeutic effect on ALI through inhibition of CXCL12
expression.
In the present study, TNF-α expression was used to
characterize the LPS-induced RAW264.7 macrophage inflammatory
model. BALF inflammatory cells and protein, lung histopathology and
pro-inflammatory cytokine TNF-α were used to confirm establishment
of the LPS-induced ALI rat model. Although TNF-α is the most
representative and commonly used inflammatory cytokine (35–37),
other inflammatory cytokines, such as IL-1β (35), nuclear factor-κB (36) and IL-6 (37) should also be assessed to further
characterize LPS-induced ALI. Several previous studies suggested
that macrophages are a key component in the initiation and
maintenance of the inflammatory response in ALI (14,38,39).
Thus, modulation of macrophage function might provide new
therapeutic modalities for ALI. The LPS-induced RAW264.7 macrophage
inflammatory model is a commonly used inflammatory model (40,41).
The results of the present study demonstrated that pMSCs could
inhibit the inflammatory response of LPS-induced RAW264.7
macrophage inflammatory model in vitro. In accordance with
other previous studies (42–44),
the NC, LPS and LPS + pMSC groups were used both in vitro
and in vivo. The effects of pMSC administration without LPS
stimulation have not been evaluated in the present study.
In summary, the present study demonstrated that
pMSCs reduced inflammation and protected against lung injury in the
LPS-induced ALI rat model. However, the mechanisms and long-term
effects need to be studied further.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Foundation of China (grant no. 81801954).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX and JH conceived the study and designed the
experiments. WY and HS performed the experiments and drafted the
manuscript. JX, WJ and GK made substantial contributions to data
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by The Ethics
Committee of Clinical Research, Renmin Hospital of Wuhan University
(reference no. WDRY2018-K048). All animal experiments were
performed in compliance with the Guidelines for Proper Conduct of
Animal Experiments established by the Science Council.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
ARDS Definition Task Force, . Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin Definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
2
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butt Y, Kurdowska A and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong JJ, Jit M, Sultana R, Mok YH, Yeo JG,
Koh JWJC, Loh TF and Lee JH: Mortality in pediatric acute
respiratory distress syndrome: A systematic review and
meta-analysis. J Intensive Care Med. 34:563–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keim G, Watson RS, Thomas NJ and Yehya N:
New morbidity and discharge disposition of pediatric acute
respiratory distress syndrome survivors. Crit Care Med.
46:1731–1738. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graves PR, Siddiqui F, Anscher MS and
Movsas B: Radiation pulmonary toxicity: From mechanisms to
management. Semin Radiat Oncol. 20:201–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miravitlles M, Cosio BG, Arnedillo A,
Calle M, Alcazar-Navarrete B, Gonzalez C, Esteban C, Trigueros JA,
Rodriguez Gonzalez-Moro JM, Quintano Jimenez JA and Baloira A: A
proposal for the withdrawal of inhaled corticosteroids in the
clinical practice of chronic obstructive pulmonary disease. Respir
Res. 18:1982017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson JG, Liu KD, Zhuo H, Caballero L,
McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al:
Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1
clinical trial. Lancet Respir Med. 3:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: A better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge
M, Deng K, Zhang L, Zou B, Cheng B and Xu J: Treatment of acute
respiratory distress syndrome with allogeneic adipose-derived
mesenchymal stem cells: A randomized, placebo-controlled pilot
study. Respir Res. 15:392014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vellasamy S, Sandrasaigaran P, Vidyadaran
S, George E and Ramasamy R: Isolation and characterisation of
mesenchymal stem cells derived from human placenta tissue. World J
Stem Cells. 4:53–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Gillen JR, Harris DA, Kron IL,
Murphy MP and Lau CL: Treatment with placenta-derived mesenchymal
stem cells mitigates development of bronchiolitis obliterans in a
murine model. J Thorac Cardiovasc Surg. 147:1668–1677.e1665. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Xiu H, Zhang S and Zhang G: The
role of macrophages in the pathogenesis of ALI/ARDS. Mediators
Inflamm. 2018:12649132018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abumaree MH, Al Jumah MA, Kalionis B,
Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW and Knawy
BA: Human placental mesenchymal stem cells (pMSCs) play a role as
immune suppressive cells by shifting macrophage differentiation
from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell
Rev Rep. 9:620–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HH, Jeong JW, Hong SH, Park C, Kim BW
and Choi YH: Diallyl trisulfide suppresses the production of
lipopolysaccharide-induced inflammatory mediators in BV2 microglia
by decreasing the NF-kappaB pathway activity associated with
toll-like receptor 4 and CXCL12/CXCR4 pathway blockade. J Cancer
Prev. 23:134–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janssens R, Struyf S and Proost P:
Pathological roles of the homeostatic chemokine CXCL12. Cytokine
Growth Factor Rev. 44:51–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buttenschoen K, Kornmann M, Berger D,
Leder G, Beger HG and Vasilescu C: Endotoxemia and endotoxin
tolerance in patients with ARDS. Langenbecks Arch Surg.
393:473–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Zhou CL, Zhou QS and Zou HD:
Galantamine protects against lipopolysaccharide-induced acute lung
injury in rats. Braz J Med Biol Res. 49:e50082016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao YF, Luo YM, Xiong W, Ding W, Li YR,
Zhao W, Zeng HZ, Gao HC and Wu XL: Mesenchymal stem cell-based FGF2
gene therapy for acute lung injury induced by lipopolysaccharide in
mice. Eur Rev Med Pharmacol Sci. 19:857–865. 2015.PubMed/NCBI
|
|
24
|
Li JW and Wu X: Mesenchymal stem cells
ameliorate LPS-induced acute lung injury through KGF promoting
alveolar fluid clearance of alveolar type II cells. Eur Rev Med
Pharmacol Sci. 19:2368–2378. 2015.PubMed/NCBI
|
|
25
|
D'Alessio FR, Tsushima K, Aggarwal NR,
West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM,
McDyer JF and King LS: CD4+CD25+Foxp3+ Tregs resolve experimental
lung injury in mice and are present in humans with acute lung
injury. J Clin Invest. 119:2898–2913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie S, Tu Z, Xiong J, Kang G, Zhao L, Hu
W, Tan H, Tembo KM, Ding Q, Deng X, et al: CXCR4 promotes
cisplatin-resistance of non-small cell lung cancer in a
CYP1B1-dependent manner. Oncol Rep. 37:921–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo
KM, Huang J, Hu W, Huang X, Pan S, et al: CXCR4 is involved in
CD133-induced EMT in non-small cell lung cancer. Int J Oncol.
50:505–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie S, Zeng W, Fan G, Huang J, Kang G,
Geng Q, Cheng B, Wang W and Dong P: Effect of CXCL12/CXCR4 on
increasing the metastatic potential of non-small cell lung cancer
in vitro is inhibited through the downregulation of CXCR4 chemokine
receptor expression. Oncol Lett. 7:941–947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnston LK, Rims CR, Gill SE, McGuire JK
and Manicone AM: Pulmonary macrophage subpopulations in the
induction and resolution of acute lung injury. Am J Respir Cell Mol
Biol. 47:417–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng G, Ge M, Qiu G, Shu Q and Xu J:
Mesenchymal stromal cells affect disease outcomes via macrophage
polarization. Stem Cells Int. 2015:9894732015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mokhber Dezfouli MR, Jabbari Fakhr M,
Sadeghian Chaleshtori S, Dehghan MM, Vajhi A and Mokhtari R:
Intrapulmonary autologous transplant of bone marrow-derived
mesenchymal stromal cells improves lipopolysaccharide-induced acute
respiratory distress syndrome in rabbit. Crit Care. 22:3532018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horwitz EM, Gordon PL, Koo WK, Marx JC,
Neel MD, McNall RY, Muul L and Hofmann T: Isolated allogeneic bone
marrow-derived mesenchymal cells engraft and stimulate growth in
children with osteogenesis imperfecta: Implications for cell
therapy of bone. Proc Natl Acad Sci USA. 99:8932–8937. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jerkic M, Masterson C, Ormesher L, Gagnon
S, Goyal S, Rabani R, Otulakowski G, Zhang H, Kavanagh BP and
Laffey JG: Overexpression of IL-10 enhances the efficacy of human
umbilical-cord-derived mesenchymal stromal cells in E. coli
Pneumosepsis. J Clin Med. 8:E8472019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X and Dong S: Protective effects of
erythropoietin towards acute lung injuries in rats with sepsis and
its related mechanisms. Ann Clin Lab Sci. 49:257–264.
2019.PubMed/NCBI
|
|
35
|
Badamjav R, Sonom D, Wu Y, Zhang Y, Kou J,
Yu B and Li F: The protective effects of Thalictrum minus L. on
lipopolysaccharide-induced acute lung injury. J Ethnopharmacol.
248:1123552020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Chang G, Huang J, Wang Y, Ma N, Roy
AC and Shen X: Sodium butyrate inhibits the inflammation of
lipopolysaccharide-induced acute lung injury in mice by regulating
the toll-like receptor 4/nuclear factor κB signaling pathway. J
Agric Food Chem. 67:1674–1682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Y, Xu D, Liu J and Gu L: Protective
effect of sophocarpine on lipopolysaccharide-induced acute lung
injury in mice. Int Immunopharmacol. 70:180–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aggarwal NR, King LS and D'Alessio FR:
Diverse macrophage populations mediate acute lung inflammation and
resolution. Am J Physiol Lung Cell Mol Physiol. 306:L709–L725.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lomas-Neira J, Chung CS, Perl M, Gregory
S, Bi W and Ayala A: Role of alveolar macrophage and migrating
neutrophils in hemorrhage-induced priming for ALI subsequent to
septic challenge. Am J Physiol Lung Cell Mol Physiol. 290:L51–L58.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang H, Lu Z, Huo C, Chen Y, Cao H, Xie P,
Zhou H, Liu D, Liu J and Yu L: Liang-Ge-San, a classic traditional
Chinese medicine formula, attenuates lipopolysaccharide-induced
acute lung injury through up-regulating miR-21. Front Pharmacol.
10:13322019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang J, Diao P, Shu X, Li L and Xiong L:
Quercetin and quercitrin attenuates the inflammatory response and
oxidative stress in LPS-induced RAW264.7 cells: In vitro assessment
and a theoretical model. Biomed Res Int. 2019:70398022019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo XY, Meng XJ, Cao DC, Wang W, Zhou K,
Li L, Guo M and Wang P: Transplantation of bone marrow mesenchymal
stromal cells attenuates liver fibrosis in mice by regulating
macrophage subtypes. Stem Cell Res Ther. 10:162019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng Y, Xu Q, Yang Y, Shi W, Meng W, Zhang
H, He X, Sun M, Chen Y, Zhao J, et al: The therapeutic effects of
bone marrow-derived mesenchymal stromal cells in the acute lung
injury induced by sulfur mustard. Stem Cell Res Ther. 10:902019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu
J, Chen J, Yang Y and Xie J: MSC-secreted TGF-β regulates
lipopolysaccharide-stimulated macrophage M2-like polarization via
the Akt/FoxO1 pathway. Stem Cell Res Ther. 10:3452019. View Article : Google Scholar : PubMed/NCBI
|