Introduction
Maxillofacial bone defects are a problem in oral
medicine and are caused by congenital deformations, traumatic
fractures, infections or tumors (1). Bone tissue engineering is considered
as a primary option for bone defect repair (2,3).
Therefore, mesenchymal stem cells (MSCs) are in high demand for the
regenerative restoration of maxillofacial bone defects (4). The osteogenic capacity of MSCs can be
affected by a number of factors, including long noncoding RNAs
(5), microRNAs (6), circular RNAs (7), chemical drugs (8) and topographically optimized scaffolds
(9). Moreover, the osteogenic
capacity of MSCs is closely related to their tissue of origin
(4,10–13).
For example, it has been reported that the osteogenic
differentiation ability of MSCs derived from cartilage (12) and adipose tissue (12,13)
are decreased compared with bone marrow-derived MSCs (BMMSCs).
Furthermore, MSCs tend to differentiate into the tissue from which
they originate (14); therefore,
orofacial MSCs (OMSCs) are the optimal choice for maxillofacial
bone defect restoration. However, the osteogenic characteristics of
OMSCs have not been fully elucidated, particularly in comparison
with BMMSCs, which are commonly isolated from the bone marrow of
the femur (15). Considering that
the mandible and femur have different modes of bone formation
during embryonic development (16), it was hypothesized that the
osteogenic differentiation ability of OMSCs and BMMSCs may
differ.
Casein kinase-2 interaction protein-1 (CKIP-1)
negatively regulates bone formation via Smad ubiquitination
regulatory factor 1 (Smurf1) signaling (17). Our previous study also reported
that CKIP-1 silencing promoted new bone formation during mandibular
distraction osteogenesis in rats (18). Moreover, in CKIP-1−/−
mice, the level of mandible formation was significantly increased
compared with femur bone formation (unpublished data), which
further indicated that cellular responses to CKIP-1 knockout (KO)
may be significantly different.
In the present study, OMSCs and BMMSCs were isolated
from wild-type (WT) and knockout (KO; CKIP-1−/−) mice.
Furthermore, the effect of CKIP-1 KO on the proliferation and
osteogenic differentiation of OMSCs and BMMSCs were
investigated.
Materials and methods
Animals
In total, ten male C57BL/6 mice (age, 4 weeks;
weight, 15±2 g; obtained from the Animal Center of The Fourth
Military Medical University), ten male CKIP-1−/− mice
(age, 4 weeks; obtained from the Institute of Radiation Medicine,
Academy of Military Medical Sciences) and 40 male nude mice (age, 6
weeks; obtained from the Animal Center of The Fourth Military
Medical University) were included in the study. A total of 10 nude
mice were in each group: BMMSCs WT, BMMSCs KO, OMSCs WT and OMSCs
KO. Mice were housed under light-, temperature-, and
humidity-controlled conditions (12-h light/dark regimen; 22°C; at a
constant humidity of 55±5%) with free access to food and water. All
procedures in this study were approved by the Institutional Animal
Care and Use Committee of The Fourth Military Medical
University.
Genotype identification of
CKIP-1−/− mice
Sections of the tails of CKIP-1−/− mice
were collected in 1.5 ml Eppendorf tubes and 200 µl lysate prepared
by 1 M Tris (pH=8.0; Sangon Biotech Co., Ltd.), 2 M NaCl (Sangon
Biotech Co., Ltd.), 0.5 M EDTA (Sangon Biotech Co., Ltd.), 10% SDS
(Sangon Biotech Co., Ltd.) and ddH2O was added. After
incubation at 55°C for 20 min, proteinase K (Takara Bio, Inc.) was
inactivated by incubation at 95°C for 5 min. The pyrolysis products
were mixed by vortex oscillation, centrifuged at 12,000 × g for 10
min at 4°C and the supernatant was used for PCR. The following
primer pairs were used to identify the WT mice: CKIP-1WTN (+)
forward, 5′-TGGTTTCCCCTCGGACCTGTAGGAAG-3′; CKIP-1500 reverse,
5′-TTCCCCCTTTGTGAAGCCCCAACTCTTGACTC-3′. The following primer pairs
were used to identify the CKIP-1 KO mice: CKIP-1WTN (+) forward,
5′-TGGTTTCCCCTCGGACCTGTAGGAAG-3′; Rin-1A+12bp (−) reverse,
5′-CCAGACTGCCTTGGGAAAAGCGCCTCCCCTACC-3′. Golden Star T6 Super PCR
Mix kit (TsingKe Biotech Co., Ltd.) was used for PCR according to
the manufacturer's protocol. The following thermocycling conditions
were used: Initial denaturation step at 98°C for 2 min; followed by
35 cycles at 98°C for 10 sec and 58°C for 30 sec; and a final
extension step at 72°C for 10 sec. Samples (10 µl) and DL-2000 DNA
Marker (10 µl; Vazyme Biotech Co., Ltd.) were loaded in each
chamber. Agarose gel electrophoresis (Biowest) on a 2% gel was
conducted under 180 V for 30 min, and images were obtained under
ultraviolet light.
Micro-computed tomography (CT)
scanning
CKIP-1−/− and WT mice were sacrificed by
excessive sodium pentobarbital (150 mg/kg; Sigma-Aldrich; Merck
KGaA), and subsequently the femurs were isolated and fixed with 4%
paraformaldehyde (PFA; FeiYang Biotech Co., Ltd.) for 24 h at room
temperature. Micro-CT (YXLON Cheetah; YXLON International GmbH; 90
kV, 45 µA, 1,000 msec) was performed to scan the fixed femora with
a layer thickness of 8 µm. Cancellous bone at the distal end of the
femur and cortical bone in the middle part of the femur were
selected as the area of interest for three-dimensional (3D)
reconstruction. Bone volume/total volume ratio (BV/TV), trabecular
number (Tb.N), trabecular thickness (Tb.Th), bone surface/bone
volume (BS/BV), trabecular separation (Tb.Sp), cortical area
(Ct.Ar), cortical inner diameter perimeter (Ct.ld.Pm), cortical
outer diameter perimeter (Ct.Od.Pm), cortical thickness (Ct.Th),
cortical bone volume (Ct.BV), trabecular area (Tb.Ar) and
trabecular width (Tb.Wi) were selected as measurement indices, and
quantified by Inveon™ Research Workplace 2.2 (Siemens AG).
Hematoxylin and eosin (H&E)
staining
The samples were fixed with 4% PFA for 48 h at room
temperature. After decalcification, paraffin embedding and
deparaffinization, the samples were cut into 3–4 µm thick sections
(Leica Microsystems GmbH). Paraffin sections were stained with
hematoxylin (Sigma-Aldrich; Merck KGaA) for 15 min at room
temperature and washed three times with distilled water for 1 min
each. After differentiation with 1% (v/v) hydrochloric acid alcohol
and washing with distilled water three times, hematoxylin-stained
sections were stained with 0.5% eosin (Sigma-Aldrich; Merck KGaA)
for 3 min at room temperature and subsequently washed. Stained
sections were observed using a DMI6000 inverted light microscope
(Leica Microsystems GmbH) at a magnification of ×40.
Isolation and culture of BMMSCs and
OMSCs
C57BL/6 mice (age, 4 weeks) were sacrificed by an
overdose of sodium pentobarbital (150 mg/kg; Sigma-Aldrich; Merck
KGaA), and subsequently the femora and mandibles were collected.
The bones were cut into pieces and transferred to 25-cm2
culture bottles (Costar; Corning, Inc.) containing 3 ml 0.25% type
II collagenase (Sigma-Aldrich; Merck KGaA) (19–21).
After digestion on a swing bed at room temperature for 90 min,
collagenase activity was terminated using 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.). Then, bone pieces were washed with α-MEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2% FBS
and cultured at 37°C with 5% CO2. Cell culture medium
(α-MEM supplemented with 10% FBS and 1% streptomycin/penicillin)
was replaced every 2 days, and the cells were passaged at ~80%
confluency. Cells at passages 3–5 were used for further analysis.
After a fixation with 4% PFA for 24 h at room temperature and gold
sputtering (22), bone pieces
before and after digestion were observed by field emission scanning
electron microscopy (FE-SEM; S-4800; Hitachi Co., Ltd). Cells of
different generations were observed using an inverted phase
contrast microscope (Nikon Corporation) at a magnification of
×100.
Surface markers validation
Following digestion with 0.25% trypsin
(Sigma-Aldrich; Merck KGaA), MSCs (5×106 cells/ml) were
seeded into Eppendorf tubes (100 µl per tube). Non-specific
detection of the Fc component of the CD antibodies were blocked by
5% BSA (Sangon Biotech Co., Ltd.) for 30 min at room temperature.
Anti-CD44 (1:100; cat. no. 553133; BD Biosciences), anti-CD29
(1:100; cat. no. 558741; BD Biosciences), anti-CD31 (1:100; cat.
no. FAB3628P; R&D Systems, Inc.), anti-CD34 (1:100; cat. no.
128609; BioLegend, Inc.) and anti-CD90 (1:100; cat. no. 105307;
BioLegend, Inc.) antibodies were added and incubated at 37°C for 45
min. Cells were washed twice with PBS, resuspended and subsequently
analyzed by flow cytometry (FCM) using a BD FACSCanto™ II flow
cytometer (BD Biosciences) and BD FACSDiva™ 6.0 software (BD
Biosciences). Data analysis was performed using FlowJo software
(version 7.2; FlowJo LLC) with a previously described gating method
(23).
Multilineage differentiation
At 80% confluency, osteogenic and adipogenic
differentiation were performed in osteogenic medium (growth medium
containing 10 nM dexamethasone, 10 mM β-glycerophosphate and 50
µg/ml ascorbic acid; all purchased from Sigma-Aldrich; Merck KGaA),
and adipogenic medium (1 µmol/l dexamethasone, 10 mg/l insulin, 0.5
mmol/l IBMX and 100 µmol/l indometacin; all purchased from
Sigma-Aldrich; Merck KGaA), respectively. After 21 days of
osteogenic induction and 14 days of adipogenic induction at 37°C,
Alizarin Red S staining (ScienCell Research Laboratories, Inc.) and
Oil Red O staining (Amresco, LLC) were performed to investigate the
multidirectional differentiation potential of the cells, according
to the manufacturer's instructions. Images were obtained using an
inverted light microscope (Leica Microsystems GmbH) at a
magnification of ×40.
Cell morphology
Cells (2×104 cells/ml) of the BMMSC WT,
BMMSC KO, OMSC WT and OMSC KO groups were cultured on a coverslip
for 1 day at 37°C prior to observation. Cells were rinsed with PBS
and fixed with 3% glutaraldehyde overnight at room temperature.
Subsequently, the cells were dehydrated with an ascending ethanol
series (50, 70, 80, 90 and 100%) and dried at room temperature.
After gold sputtering, cell morphology was observed by FE-SEM using
the aforementioned method.
Cell proliferation
Proliferation of MSCs was assessed using an MTT
assay (Amresco, LLC). In total, 200 µl MSC suspension was seeded
(1×104 cells/well) in 96-well plates and cultured at
37°C. Subsequently, 20 µl MTT was added to each well for 4 h at
37°C. The medium was removed and 150 µl DMSO was added to each
well. The absorbance was measured at a wavelength of 490 nm using a
microplate reader (Omega Bio-Tek, Inc.).
Clone formation assay
Cells were seeded (2.5×103 cells) into a
10-cm dish and routinely cultured for ~14 days at 37°C. The clones
were rinsed with PBS and fixed with 4% PFA for 30 min at room
temperature. Subsequently, the clones were stained with 1%
toluidine blue for 30 min at room temperature and visualized using
an inverted light microscope (Leica Microsystems GmbH) at a
magnification of ×100. Clones containing ≥50 cells were counted
using Image-Pro Plus software (version 7.1; Media Cybernetics,
Inc.), and the clone formation rate was calculated using the
following equation: (The number of clone colonies/number of seeded
cells) ×100%.
Cell apoptosis
Apoptotic BMMSCs and OMSCs in the α-MEM culture
medium (Gibco; Thermo Fisher Scientific, Inc.) and adherent cells
were collected and washed with PBS. Cells were adjusted to a
density of 1×106/ml and 1 ml cell suspension was
centrifuged at 1,500 × g for 5 min at room temperature.
Subsequently, the cells were resuspended in 500 µl binding buffer
(Biomiga, Inc.). Apoptotic cells were stained with 5 µl Annexin
V-FITC and 10 µl propidium iodide for 10–15 min at room
temperature, and analyzed by FACSCanto II (BD Biosciences). Cells
from the lower left, the lower right, the upper right and the upper
left represent normal, early, late and dead cells, respectively.
The apoptotic rate was calculated as the sum of early and late
apoptosis.
Osteogenic capacity evaluation
Cells of the BMMSC WT, BMMSC KO, OMSC WT and OMSC KO
groups were seeded (2×105 cells/ml) into 6-well plates
and cultured for 1 day at 37°C. Subsequently, the α-MEM culture
medium (Gibco; Thermo Fisher Scientific, Inc.) was replaced with
osteogenic inducing fluid prepared using the aforementioned method,
which was replaced every 2 days. Following 7 and 21 days of
osteogenic induction at 37°C, cells were fixed with 4% PFA for 30
min at room temperature. Alkaline phosphatase (ALP) staining
(LeaGene Biotech Co., Ltd.) and Alizarin Red staining (ScienCell
Research Laboratories, Inc.) were performed to assess osteogenic
differentiation according to the manufacturer's protocol.
Differentiated cells were observed using an inverted light
microscope (Leica Microsystems GmbH) at a magnification of ×40.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to assess the mRNA expression
levels of CKIP-1 and osteogenesis-associated genes, including the
early-expressed genes RUNX family transcription factor 2 (Runx2)
and ALP, and the late-expressed genes colicinogenic factor 1 (COL1)
and bone γ-carboxyglutamate protein (OCN). Total RNA was extracted
from the induced cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Reverse transcription was performed using
PrimeScript RT Master Mix (Takara Bio, Inc.) in a 20-µl volume. The
following temperature protocol was used for reverse transcription:
37°C for 15 min, 85°C for 5 sec and 4°C for 10 min. qPCR was
performed using SYBR PCR Master Mix kit (Takara Bio, Inc.), 10 µM
specific primers in a 25-µl volume and an iCycleri QTX detection
system (Bio-Rad Laboratories, Inc.). The following thermocycling
conditions were used: Initial denaturation step at 95°C for 1 min;
followed by 35 cycles at 95°C for 30 sec, 58°C for 30 sec; and a
final extension step at 72°C for 30 sec. All signals were
normalized to β-actin, and the 2−ΔΔCq method was used
for quantification (24). The
primers used for RT-qPCR are presented in Table I.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5→3) |
|---|
| β-actin | F:
CTGGCACCACACCTTCTAC |
|
| R:
GGTACGACCAGAGGCATAC |
| CKIP-1 | F:
AACCGCTATGTGGTGCTGA |
|
| R:
CAGGGTGAACTTGCTGTGA |
| Runx2 | F:
GGCCAGGTTCAACGATCTG |
|
| R:
GGACCGTCCACTGTCACTT |
| ALP | F:
AACCTGACTGACCCTTCCC |
|
| R:
TTCTGGGAAGTCATGGTGC |
| COL-1 | F:
CTGACGCATGGCCAAGAAG |
|
| R:
CGTGCCATTGTGGCAGATA |
| OCN | F:
GGCGCTACCTCAACAATGG |
|
| R:
ATAGATGCGCTTGTAGGCG |
Ectopic bone formation
Hydroxyapatite (HA) and tricalcium phosphate (β-TCP)
scaffolds were provided by the National Engineering Research Center
for Biomaterials. The scaffolds (40 wt.% β-TCP and 60 wt.% HA;
porosity, 60%; pore size, 300–500 µm) were fabricated by sintering
for 3 h at 1,100°C. WT and CKIP-1−/− MSCs were seeded
(2×106 cells/ml) onto the surface of the HA/β-TCP
scaffolds and incubated for 30 min at room temperature with
shaking. Subsequently, the scaffolds were incubated at 37°C with 5%
CO2 for 24 h. The α-MEM culture medium (Gibco; Thermo
Fisher Scientific, Inc.) was replaced with osteogenic medium and
incubated for 7 days at 37°C, and the osteogenic medium was
replaced every 2 days. The nude mice were anaesthetized by
intraperitoneal injection of sodium pentobarbital (60 mg/kg;
Sigma-Aldrich; Merck KGaA) and the prepared HA/β-TCP/MSCs scaffolds
were subcutaneously transplanted into the anterior and posterior
regions of the backs of nude mice. Mice were sacrificed after 2
months by an overdose of sodium pentobarbital (150 mg/kg;
Sigma-Aldrich; Merck KGaA) and the scaffolds were isolated. Then,
H&E and Masson-trichrome staining were performed to assess
osteogenesis. H&E staining was carried out using the
aforementioned method. For Masson-trichrome staining (Beijing
Solarbio Science & Technology Co., Ltd.), the samples were
fixed with 4% PFA for 48 h at room temperature, and other steps
were similar to the aforementioned H&E staining. The staining
was performed according to the manufacturer's protocols. After
staining with hematoxylin for 10 min, differentiated with 1% (v/v)
hydrochloric acid alcohol for 10 sec and washed with distilled
water three times at room temperature, the samples were incubated
with Masson blue stain for 5 min at room temperature and then
washed. Subsequently, acid fuchsin was added for 5 min at room
temperature, and then samples were washed. After staining with 1%
phosphomolybdic acid for 3 min at room temperature, aniline blue
dye was finally added for 5 min and washed quickly with distilled
water. Stained sections with a thickness of ~3 µm were observed
using a DMI6000 inverted light microscope (Leica Microsystems GmbH)
at a magnification of ×40. Image Pro Plus software (version 7.1;
Media Cybernetics, Inc.) was used to analyze the percentage of new
bone formation.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 19.0; SPSS, Inc.). Data are presented as the mean
± SD. All experiments were repeated ≥3 times. An unpaired t-test
was used for two group-comparison of the bone mass changes in
CKIP-1 KO mice. A one-way ANOVA with Bonferroni correction (α=0.05)
was used to analyze the data for each CD marker. A two-way ANOVA
with Bonferroni correction (α=0.05) was used to analyze other data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Bone formation can be regulated by
CKIP-1
CKIP-1 KO and WT mice were used to investigate the
relationship between CKIP-1 and bone formation. Following genotype
identification (Fig. 1A),
CKIP-1−/− mice were sacrificed and femoral bones were
isolated for subsequent experiments. The 3D reconstruction of the
micro-CT results identified increased compact cancellous bone in
the femoral bones obtained from the KO group. Moreover, the BV/TV,
Tb.N and Tb.Th in the KO group were increased compared with the WT
group, and the opposite trend was observed for BS/BV and Tb.Sp.
However, 3D reconstruction of the cortical femoral bones and
quantitative analysis found similar results between the KO and WT
groups, regardless of the Ct. Ar, Ct.ld.Pm, Ct.Od.Pm, Ct.Th and
Ct.BV (Fig. 1B), which indicated
that the role of CKIP-1 during cortical bone formation was
insignificant. Moreover, H&E staining was performed to
investigate the differences between WT and KO mice. Gross
observation of the femoral bones was consistent with the 3D
reconstruction images, and the results of histomorphometry also
identified increased bone formation in the KO group, accompanied by
increased Tb.Ar, Tb.Wi and Tb.N, and decreased Tb.Sp in the KO
group for cancellous bone. However, no significant difference of
Ct.Th between the WT and KO groups was observed for cortical bone
(Fig. 1C).
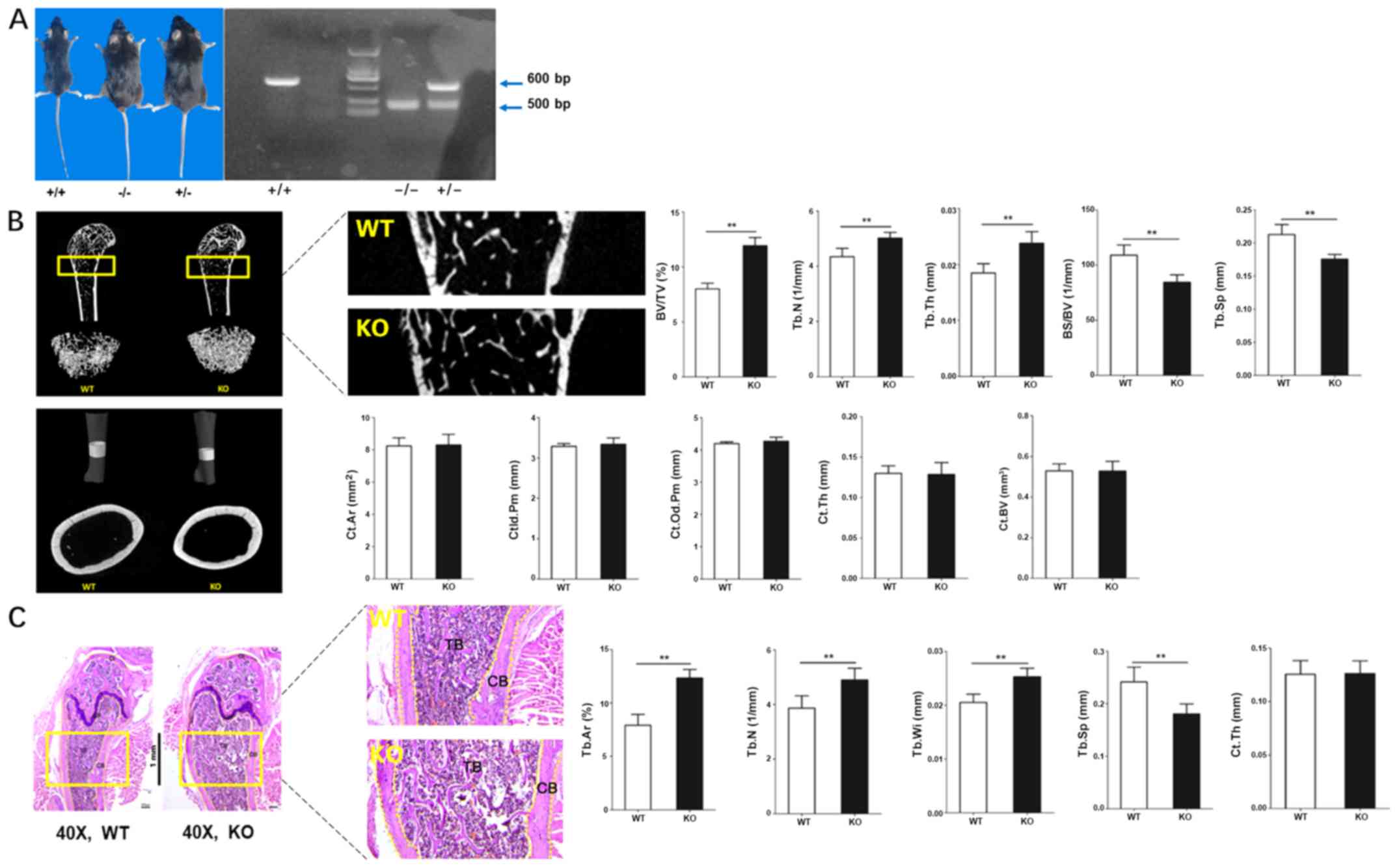 | Figure 1.Bone formation can be negatively
regulated by CKIP-1. (A) Gross observation and genotype
identification of CKIP-1 knockout mice (CKIP-1−/−; 500
bp) using PCR. (B) Three-dimensional reconstruction of the
cancellous and cortical bones of WT and CKIP-1 KO mice by
micro-computed tomography. (C) H&E staining and histological
analysis were performed to evaluate the mass of cancellous and
cortical bones of WT and KO mice. Magnification, ×40. Rectangular
boxes and the magnified images (magnification at ×200 for
micro-computed tomography and ×120 for H&E staining) display
the regions of interests for quantitative analysis. **P<0.01 vs.
WT group. CKIP-1, casein kinase-2 interaction protein-1; WT,
wild-type; KO, knockout; TB, trabecular bone; CB, cortical bone;
GP, growth plate; CI, cartilage; Tb.N, trabecular number; Tb.Sp,
trabecular separation; Tb.Ar, trabecular area; BV/TV, bone
volume/total volume ratio; Tb.Th, trabecular thickness; BS/BV, bone
surface/bone volume; Ct. Ar, cortical area; Ct.ld.Pm, cortical
inner diameter perimeter; Ct.Od.Pm, cortical outer diameter
perimeter; Ct.Th, cortical thickness; Ct.BV, cortical bone volume;
Tb.Wi, trabecular width; H&E, hematoxylin and eosin. |
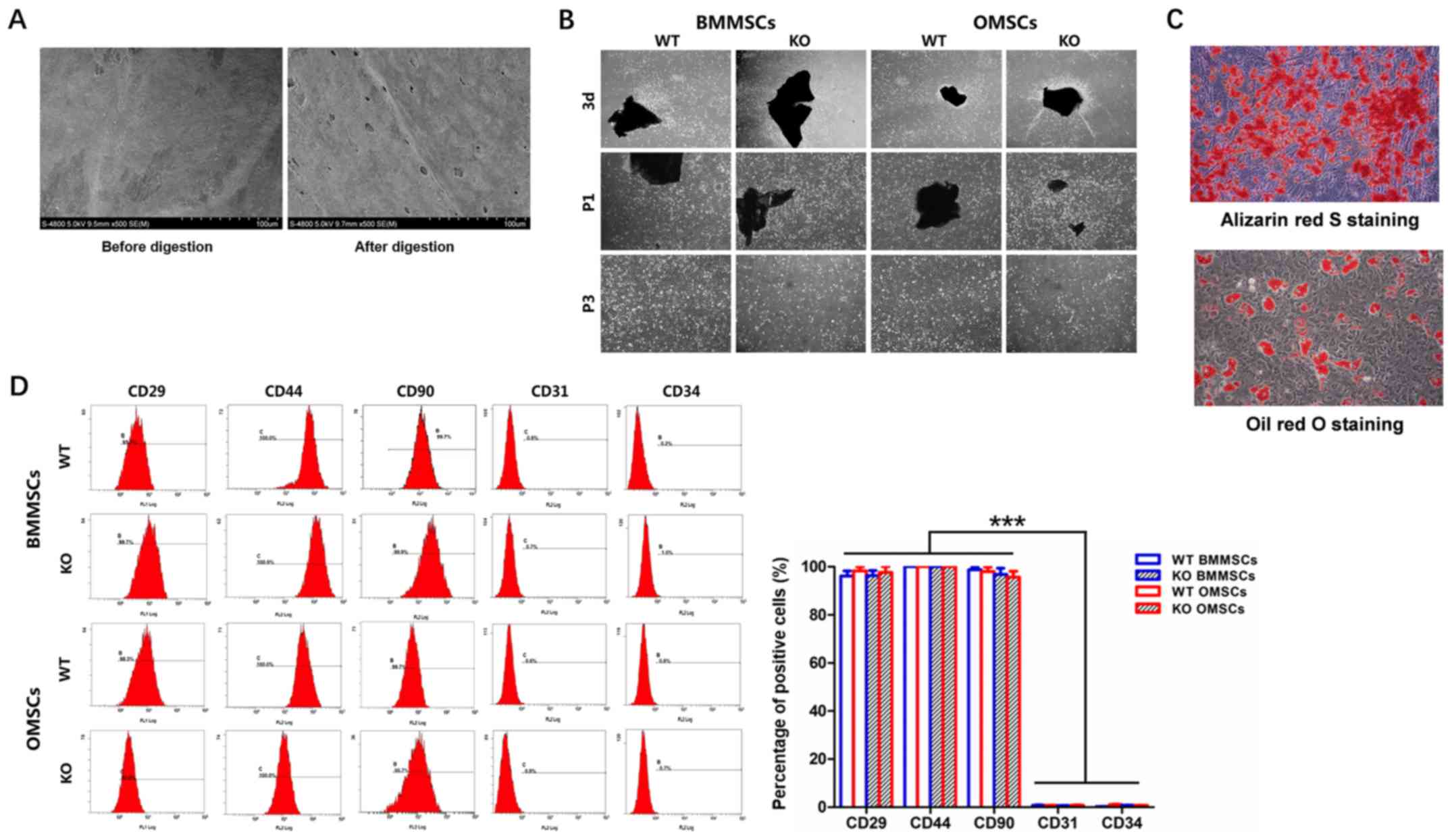
Identification of BMMSCs and
OMSCs
Microstructures on bone surfaces before and after
collagenase II digestion were observed by FE-SEM. It was
demonstrated that the digested bone had a number of micro-holes on
the surface (diameter, ~70–80 microns), which may have allowed the
stem cells to move across the bone surface (Fig. 2A). The collagenase-digested bone
pieces were inoculated for conventional culture. On day 3, stem
cells were observed on the surface of the bone pieces, and the
cells in the KO group displayed higher proliferation in generation
passage 1 (P1) and P3 (Fig. 3B).
In addition, it was determined that a small number of hematopoietic
cells were present in the P1 generation, and stem cells were
purified when passaged to the P3 generation (Fig. 2B). To identify BMMSCs and OMSCs,
in vitro differentiation and detection of surface antigens
using FCM were performed. Alizarin Red S staining on day 21 and Oil
Red O staining results on day 14 suggested that digestion-derived
MSCs had differentiated to osteogenic and adipogenic lineages,
further indicating the multidirectional differentiation ability of
MSCs (Fig. 2C). Furthermore, the
FCM results identified high expression levels of CD29, CD44 and
CD90 on the surface of BMMSCs and OMSCs, which were significantly
higher compared with the expression level of CD31 and CD34, thus
suggesting the presence of endothelial and hematopoietic cells.
Moreover, the expression levels of the aforementioned markers were
not significantly different between the four groups, which
suggested that neither CKIP-1 KO or the source of the cells altered
surface marker expression (Fig.
2D).
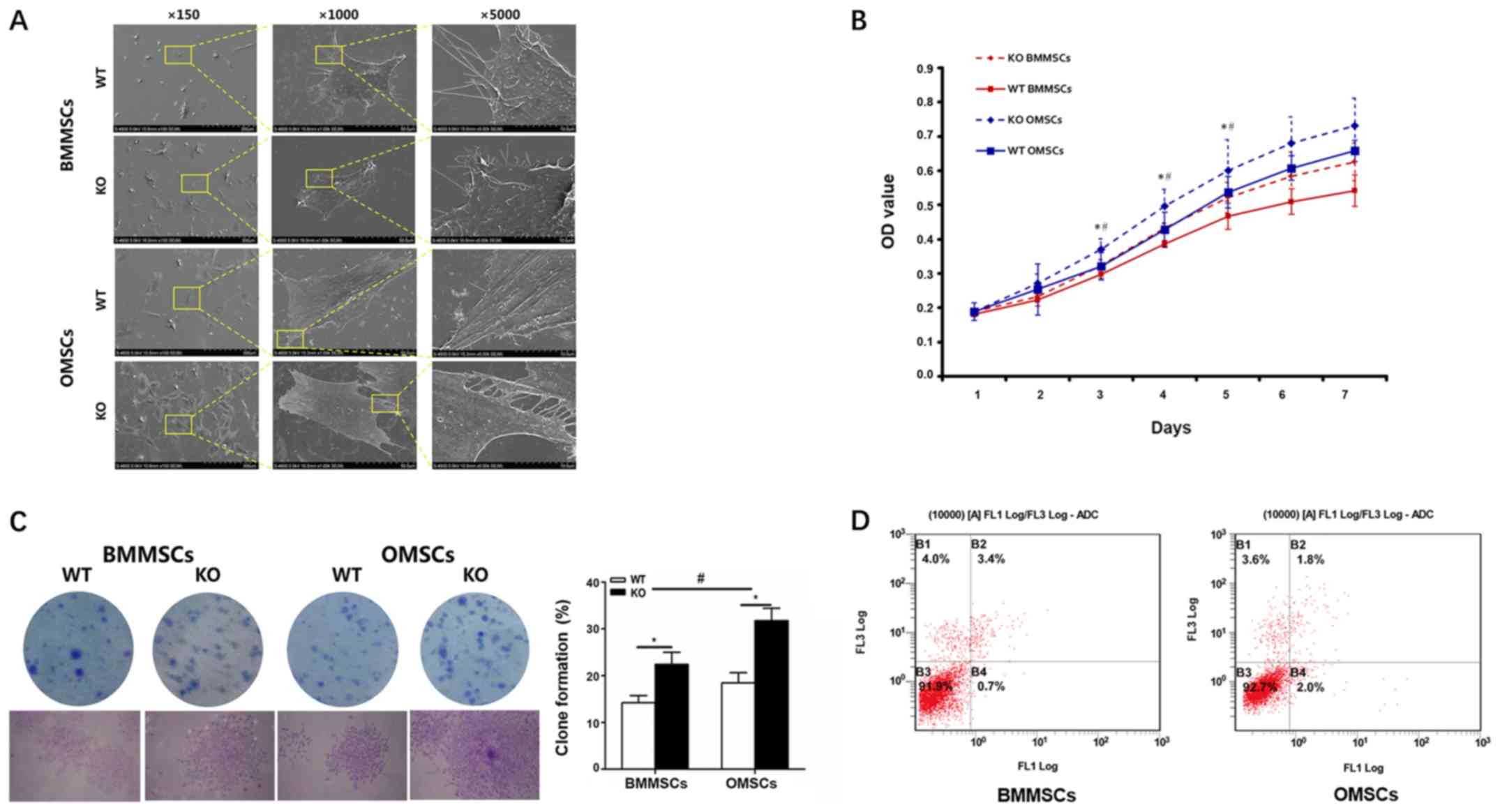
Cell morphology, proliferation and
apoptosis
After cell culture for 1 day, the morphology of
BMMSCs and OMSCs was observed by FE-SEM. The results indicated that
an increased number of MSCs were observed in the KO group at low
magnification compared with the WT group. Furthermore, at high
magnification, all cells displayed a spindle-like morphology;
however, OMSCs were relatively large and fully spread, with thicker
and higher levels of interlinked lamellipodia compared with BMMSCs
(Fig. 3A). An MTT assay was used
to assess cell proliferation in the various groups, and it was
determined that the proliferation of MSCs in the KO group was
significantly increased between days 3–5, and the proliferation
rate of OMSCs in the WT and KO groups was increased compared with
the BMMSC group (Fig. 3B). The
results also indicated that the increased rate of proliferation of
OMSCs was higher compared with BMMSCs, following CKIP-1 KO.
Moreover, a clone formation assay was also performed on MSCs
following a 2-week incubation. Consistent with the results of the
MTT assay, cloning efficiency was significantly increased in the KO
group compared with the WT group, and the increased levels of OMSCs
were more significant compared with BMMSCs (Fig. 3C). In addition, cell apoptosis was
assessed using FCM and the results indicated that there was no
significant difference in the rate of cell apoptosis between OMSCs
(4.1%) and BMMSCs (3.8%) following CKIP-1 KO (Fig. 3D).
In vitro osteogenic
differentiation
After 7 days of osteogenic induction, ALP activity
was assessed in OMSCs and BMMSCs derived from WT and KO mice. The
results indicated that osteogenic differentiation occurred in all
cells, and ALP staining of OMSCs was highest in the KO group, but
lowest in BMMSCs derived from WT mice. Moreover, quantitative
analysis of ALP staining revealed that the ALP activity of MSCs in
the KO group was increased compared with the WT group, and that ALP
activity of OMSCs following CKIP-1 KO was increased compared with
BMMSCs (Fig. 4A). Alizarin Red S
staining was performed to assess osteogenic mineralization in the
various groups following a 21-day incubation. The results indicated
that the osteogenic ability in the KO group was increased compared
with the WT group, and that the increase in osteogenesis of OMSCs
was significantly higher compared with BMMSCs (Fig. 4B). In addition, to further
investigate the aforementioned results, RT-qPCR was performed to
detect the expression levels of CKIP-1 and osteogenesis-related
genes (Runx2, ALP, COL-1 and OCN). It was demonstrated that CKIP-1
was not expressed in OMSCs and BMMSCs derived from
CKIP-1−/− mice. However, following osteogenic induction
for 7 days, the expression levels of Runx2 and ALP were
significantly increased in MSCs of the KO group compared with the
WT group, and the increase in OMSCs was higher in the KO group
compared with that of BMMSCs. Furthermore, the expression levels of
COL-1 and OCN in MSCs following a 21-day incubation exhibited a
similar trend (Fig. 4C).
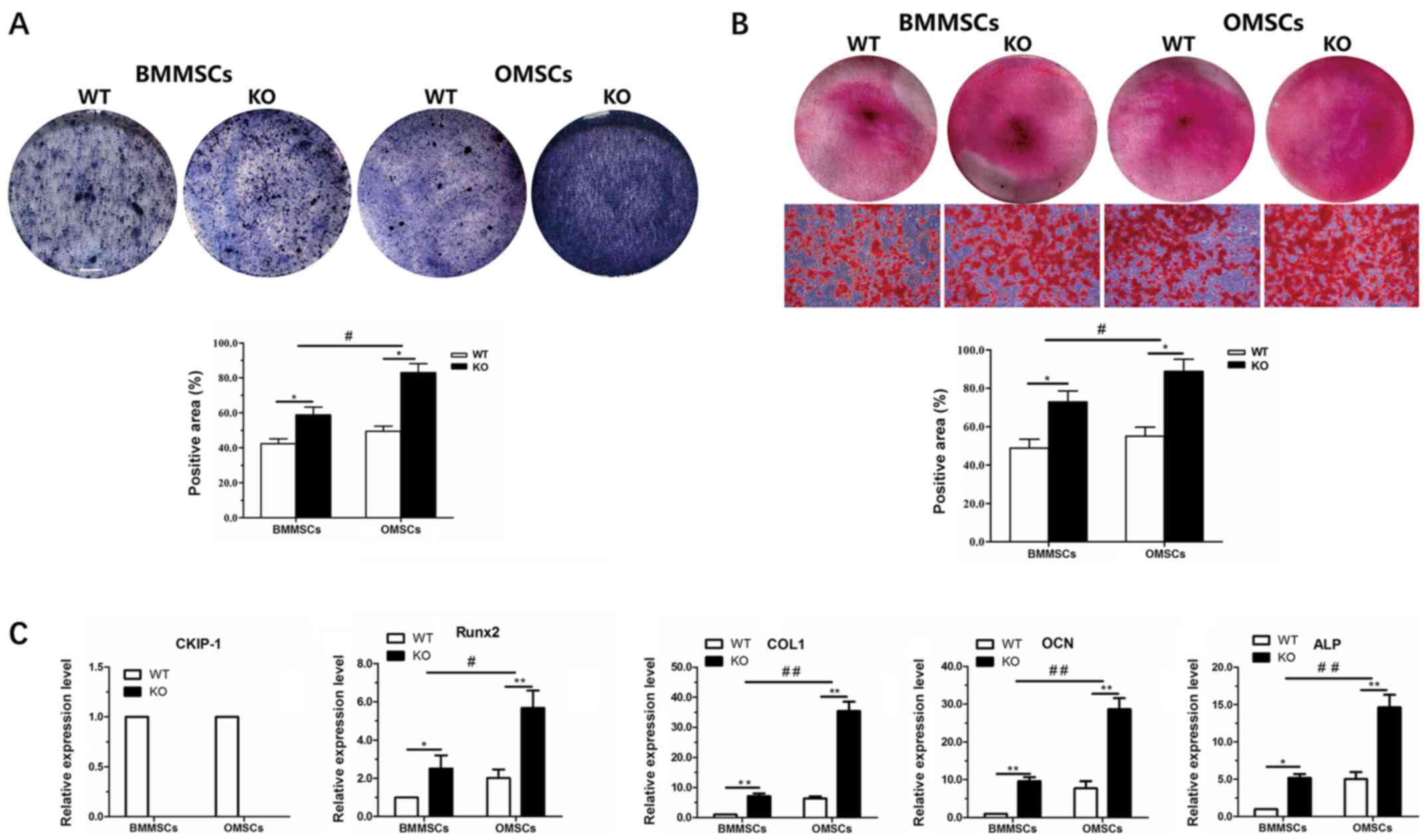 | Figure 4.In vitro osteogenic
differentiation ability of BMMSCs and OMSCs. (A) ALP staining and
quantification of BMMSCs and OMSCs in the WT and KO groups after
7-day culture (magnification, ×40). (B) Alizarin Red S staining and
quantification of BMMSCs and OMSCs in the WT and KO groups after
21-day culture (magnification, ×40). (C) Evaluation of the
expression levels of CKIP-1 and the osteogenesis-related genes
Runx2 and ALP after 7-day culture, and COL-1 and OCN after 21-day
culture. *P<0.05, **P<0.01 vs. WT group;
#P<0.05, ##P<0.01 vs. BMMSCs. BMMSCs,
bone marrow-derived MSCs; OMSCs, orofacial bone-derived MSCs; MSCs,
mesenchymal stem cells; WT, wild-type; KO, knockout; Runx2, RUNX
family transcription factor 2; ALP, alkaline phosphatase; COL-1,
colicinogenic factor 1; OCN, bone γ-carboxyglutamate protein. |
In vivo ectopic bone formation
In order to further assess the difference in
osteogenic differentiation ability between OMSCs and BMMSCs derived
from WT and KO mice, MSCs were inoculated onto HA/β-TCP scaffolds
that were subsequently subcutaneously transplanted into nude mice
at specific sites (Fig. 5A). The
gross observation, H&E staining and Masson-trichrome staining
results of the samples 2 months post-transplantation are presented
in Fig. 5B. The results revealed
that the implants in the four groups survived and formed fibrous
connective tissues. However, the amount of tissue formation
differed, with OMSCs in the KO group exhibiting the highest levels
of tissue formation and BMMSCs in the WT group having the lowest
levels. The results of H&E and Masson-trichrome staining
indicated that a large number of collagen tissues and a certain
amount of bone were formed in the four groups. Furthermore,
quantitative analysis of H&E and Masson-trichrome staining
demonstrated an increased area percentage of new bone in the KO
group compared with the WT group, and also in the OMSC group
compared with BMMSCs group. Consistent with the in vitro
results, it was determined that ectopic new bone formation was
significantly increased following CKIP-1 KO, and that OMSCs had an
improved osteogenesis ability compared with BMMSCs in the KO
groups.
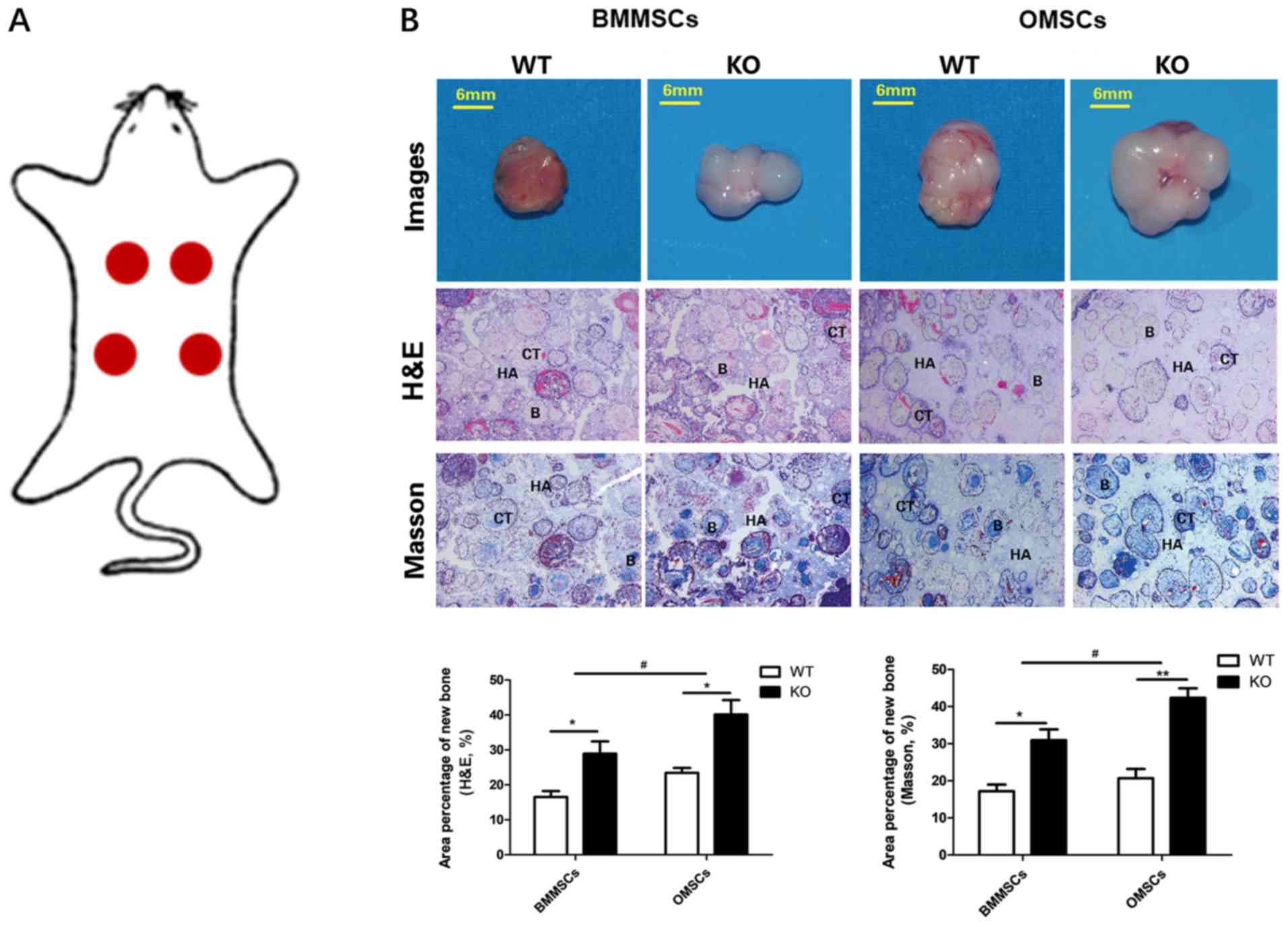 | Figure 5.Gross observation and evaluation of
ectopic bone formation. (A) Specific sites of the subcutaneous
transplantation of the MSCs + HA/β-TCP complex in nude mice. (B)
Gross observation, H&E and Masson-trichrome staining at 2
months post-subcutaneous transplantation of the complex in nude
mice. Scale bar, 6 mm; magnification, ×40. *P<0.05, **P<0.01
vs. WT group; #P<0.05 vs. BMMSCs. MSCs, mesenchymal
stem cells; HA/β-TCP, hydroxyapatite/tricalcium phosphate; WT,
wild-type; BMMSCs, bone marrow-derived MSCs; CT, collagen tissues;
B, bones; HA, HA/β-TCP; H&E, hematoxylin and eosin. |
Discussion
MSCs are a promising cell source for bone tissue
engineering with multidirectional differentiation potential
(4), and BMMSCs derived from the
bone marrow of femoral bones and the ilium are commonly used
(4). However, the mandible has a
number of disadvantages, including anatomical limitations and a
difficulty to isolate MSCs (20);
therefore, few studies have focused on OMSCs. It has been reported
that cortical bone is a novel and reliable source of MSCs, and that
the collagenase digestion method is optimal for the isolation of
MSCs (19–21). To the best of our knowledge, the
extraction of MSCs from the mandible using the collagenase
digestion method has not been previously reported; however, the
potential effects of different isolation methods on the biological
characteristics of MSCs may be avoided by using this method. In the
present study, BMMSCs and OMSCs were successfully isolated and
cultured using the collagenase digestion method.
The present results indicated that BMMSCs and OMSCs
expressed the same cell surface markers with high expression levels
of CD29, CD44 and CD90, which implied that MSCs can be successfully
isolated and cultured from the mandible using the collagenase
digestion method (19,20,25).
Furthermore, the isolated MSCs exhibited high purity, which
provided further support for the use of the collagenase digestion
method in future studies. In addition to the identification of
MSCs, the FCM results also revealed no significant difference in
the expression levels of cell surface markers between MSCs derived
from CKIP-1 KO mice and WT mice, or between BMMSCs and OMSCs. Based
on the markers that were investigated in the present study, the
expression of cell surface markers could not be used to distinguish
the two cell types, as expression was identical. However, whether
BMMSCs and OMSCs share the same cell surface markers requires
further investigation. It has been hypothesized that if cells
display different cell surface markers, it suggests that the tissue
origin has an influence on the expression of cell surface markers
by MSCs, at least for the mandible and femoral bones.
The 3D reconstruction results of micro-CT and
H&E staining indicated that the bone mass increased
significantly following CKIP-1 KO, which was consistent with our
previous study (18) and other
studies (17,26,27).
Moreover, the present results further indicated that the main site
of action of CKIP-1 is the cancellous bone, rather than the
cortical bone. In addition, compared with MSCs in the WT group, the
density of MSCs in the KO group was significantly increased, and
differences between BMMSCs and OMSCs were also observed. It was
demonstrated that the rate of apoptosis was slightly altered
following CKIP-1 KO, which was opposite to a previous report that
suggested CKIP-1 activates caspase-3 to promote apoptosis (28). Therefore, it was hypothesized that
CKIP-1 may not affect the survival and apoptosis of MSCs in the
absence of other complex stimuli. Furthermore, MSCs may have
different mechanisms for the induction and inhibition of apoptosis
compared with the cells assessed in a previous study (28). The present SEM results demonstrated
that OMSCs had larger pseudopodia and an increased extension area
compared with BMMSCs, which suggested that OMSCs were more
conducive to scaffold adhesion. It has also been previously
reported that OMSCs display an increased proliferation ability
compared with BMMSCs (29,30), which was consistent with the
results obtained in the present study.
The present results indicated that the osteogenic
differentiation ability of MSCs displayed a similar trend (29). A possible reason is that the
embryonic origin of the two cell types is different; OMSCs are
derived from neural crest cells in the neuroectoderm, while BMMSCs
are derived from mesenchymal cells in the mesoderm (16). Therefore, ectoderm-derived MSCs may
be more sensitive to CKIP-1, thus resulting in increased
alterations to the proliferation and osteogenic differentiation
ability of OMSCs following CKIP-1 KO. Furthermore, the methods of
bone formation are different between the two types of MSC;
ectodermal MSCs differentiate and develop into craniofacial bone
via a unique mechanism of intramembranous ossification, while
mesodermal MSCs form trunk and limb bones via endochondral
ossification (31,32). Moreover, CKIP-1 can negatively
regulate bone formation by suppressing Smurf1-mediated degradation
of Smad (17). Based on the
ossification mechanisms and CKIP-1, it was hypothesized that CKIP-1
may have an important role in the osteogenesis of osteoblasts
compared with osteogenesis by chondrogenic differentiation, and
that BMP signaling may be involved. In addition, CKIP-1 may be
differentially expressed and exhibit different intensities of
action in various cell types. Furthermore, it was speculated that
the lower expression of CKIP-1 in OMSCs may induce increased
proliferation and improved osteogenic differentiation ability
(17). It has also been revealed
that OMSCs have a stronger osteogenic differentiation ability
compared with BMMSCs, which may be related to homeobox A2 (Hoxa2)
gene expression, which is primarily expressed in femoral trunk
bones and not in orofacial bones (33). Moreover, Hoxa2 can inhibit the
expression of osteogenesis-related molecules that are associated
with intramembrane ossification in BMMSCs, thus resulting in a
decrease in the biological characteristics of BMMSCs (33). Therefore, a possible explanation
for the differential effects of CKIP-1 in OMSCs and BMMSCs may be
that the corresponding signaling pathways are different.
To further investigate whether the differences of
osteogenic capacity identified in vitro were also present
in vivo, an ectopic osteogenesis experiment was performed in
the present study, which involved the formation of bone tissue in
typically boneless areas (34).
Calcium phosphates are biocompatible ceramic compounds that are
involved in the formation of bones (35–37).
Moreover, the combination of HA and β-TCP can induce new bone
formation at non-osseous sites (35–37),
and therefore, HA/β-TCP was selected as the scaffold material for
the ectopic osteogenesis experiment. Consistent with the in
vitro results, H&E and Masson-trichrome staining results
further suggested that the osteogenic differentiation ability of
MSCs was enhanced following CKIP-1 KO, and that the osteogenic
differentiation ability of OMSCs was increased compared with
BMMSCs.
In conclusion, the effects of CKIP-1 on the
proliferation and osteogenesis of MSCs derived from the mandible
and femoral bones were investigated using CKIP-1−/−
mice. The present results indicated the site specificity of MSC
proliferation and osteogenesis, and revealed that OMSCs exhibited
an enhanced sensitivity to CKIP-1. Therefore, the negative
regulation of CKIP-1 in OMSCs may be a promising strategy for the
repair of maxillofacial bone defects.
Acknowledgements
The authors would like to thank Professor Zhang
Lingqiang (Institute of Radiation Medicine, Academy of Military
Medical Sciences) for the kind donation of Ckip-1−/−
C57BL/6J mice.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81670803)
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XH, YS and LK conceptualized the study. XH and WS
designed the method. Software was provided by BC. BC and LW
performed data analysis. YZ provided resources and interpreted the
data. Data acquisition was performed by YH. XH wrote the original
draft preparation, and XH and WS reviewed and edited the
manuscript. YS supervised the study. Project administration and
funding acquisition was the responsibility of LK. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures adhered to the
principles stated in the Guide for the Care and Use of Laboratory
Animals (updated 2011; National Institutes of Health), and were
approved by the Experimental Animal Usage and Welfare Ethics
Committee of the Fourth Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CKIP-1
|
casein kinase-2 interaction
protein-1
|
|
BMSCs
|
mesenchymal stem cells
|
|
OMSCs
|
orofacial bone-derived mesenchymal
stem cells
|
|
BMMSCs
|
long bone marrow-derived mesenchymal
stem cells
|
|
WT
|
wild-type
|
|
KO
|
knockout
|
|
CKIP-1−/−
|
CKIP-1 knockout
|
|
Smurf1
|
Smad ubiquitination regulatory factor
1
|
|
HA/β-TCP
|
hydroxyapatite/tricalcium
phosphate
|
References
|
1
|
Cicciù M, Cervino G, Herford AS, Famà F,
Bramanti E, Fiorillo L, Lauritano F, Sambataro S, Troiano G and
Laino L: Facial bone reconstruction using both marine or non-marine
bone substitutes: Evaluation of current outcomes in a systematic
literature review. Mar Drugs. 16:E272018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Rashidy AA, Roether JA, Harhaus L,
Kneser U and Boccaccini AR: Regenerating bone with bioactive glass
scaffolds: A review of in vivo studies in bone defect models. Acta
Biomater. 62:1–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leyendecker A Jr, Pinheiro CG, Fernandes
TL and Bueno DF: The use of human dental pulp stem cells for in
vivo bone tissue engineering: A systematic review. J Tissue Eng.
Jan 17–2018.(Epub ahead of print). doi: 10.1177/2041731417752766.
View Article : Google Scholar
|
|
4
|
Buduru SD, Gulei D, Zimta AA, Tigu AB,
Cenariu D and Berindan-Neagoe I: The potential of different origin
stem cells in modulating oral bone regeneration processes. Cells.
8:E292019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He S, Yang S, Zhang Y, Li X, Gao D, Zhong
Y, Cao L, Ma H, Liu Y, Li G, et al: LncRNA ODIR1 inhibits
osteogenic differentiation of hUC-MSCs through the
FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis. 10:9472019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carthew J, Donderwinkel I, Shrestha S,
Truong VX, Forsythe JS and Frith JE: In situ miRNA delivery from a
hydrogel promotes osteogenesis of encapsulated mesenchymal stromal
cells. Acta Biomater. 101:249–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouyang Z, Tan T, Zhang X, Wan J, Zhou Y,
Jiang G, Yang D, Guo X and Liu T: CircRNA hsa_circ_0074834 promotes
the osteogenesis-angiogenesis coupling process in bone mesenchymal
stem cells (BMSCs) by acting as a ceRNA for miR-942-5p. Cell Death
Dis. 10:9322019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J and Zhao L: Multifunction Sr, Co
and F co-doped microporous coating on titanium of antibacterial,
angiogenic and osteogenic activities. Sci Rep. 6:290692016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prasopthum A, Cooper M, Shakesheff KM and
Yang J: Three-dimensional printed scaffolds with controlled
micro-/nanoporous surface topography direct chondrogenic and
osteogenic differentiation of mesenchymal stem cells. ACS Appl
Mater Interfaces. 11:18896–18906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aghajani F, Hooshmand T, Khanmohammadi M,
Khanjani S, Edalatkhah H, Zarnani A-H and Kazemnejad S: Comparative
immunophenotypic characteristics, proliferative features, and
osteogenic differentiation of stem cells isolated from human
permanent and deciduous teeth with bone marrow. Mol Biotechnol.
58:415–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isobe Y, Koyama N, Nakao K, Osawa K, Ikeno
M, Yamanaka S, Okubo Y, Fujimura K and Bessho K: Comparison of
human mesenchymal stem cells derived from bone marrow, synovial
fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int
J Oral Maxillofac Surg. 45:124–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen
P, Ma K and Zhou C: Comparative analysis of mesenchymal stem cells
from bone marrow, cartilage, and adipose tissue. Stem Cells Dev.
17:761–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lotfy A, Salama M, Zahran F, Jones E,
Badawy A and Sobh M: Characterization of mesenchymal stem cells
derived from rat bone marrow and adipose tissue: A comparative
study. Int J Stem Cells. 7:135–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khalilifar MA, Baghaban Eslaminejad MR,
Ghasemzadeh M, Hosseini S and Baharvand H: In vitro and in vivo
comparison of different types of rabbit mesenchymal stem cells for
cartilage repair. Cell J. 21:150–160. 2019.PubMed/NCBI
|
|
15
|
Lloyd B, Tee BC, Headley C, Emam H,
Mallery S and Sun Z: Similarities and differences between porcine
mandibular and limb bone marrow mesenchymal stem cells. Arch Oral
Biol. 77:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chai Y and Maxson RE Jr: Recent advances
in craniofacial morphogenesis. Dev Dyn. 235:2353–2375. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu K, Yin X, Weng T, Xi S, Li L, Xing G,
Cheng X, Yang X, Zhang L and He F: Targeting WW domains linker of
HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat
Cell Biol. 10:994–1002. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou ZC, Che L, Kong L, Lei DL, Liu R and
Yang XJ: CKIP-1 silencing promotes new bone formation in rat
mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol
Oral Radiol. 123:e1–e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Z, Li H, Li X, Yu X, Wang H, Tang P
and Mao N: In vitro characteristics and in vivo immunosuppressive
activity of compact bone-derived murine mesenchymal progenitor
cells. Stem Cells. 24:992–1000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu H, Guo ZK, Jiang XX, Li H, Wang XY,
Yao HY, Zhang Y and Mao N: A protocol for isolation and culture of
mesenchymal stem cells from mouse compact bone. Nat Protoc.
5:550–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Short BJ, Brouard N and Simmons PJ:
Prospective isolation of mesenchymal stem cells from mouse compact
bone. Methods Mol Biol. 482:259–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang J, Xu S, Shen M, Cheng B, Li Y, Liu
X, Qin D, Bellare A and Kong L: Osteogenic activity of titanium
surfaces with hierarchical micro-/nano-structures obtained by
hydrofluoric acid treatment. Int J Nanomedicine. 12:1317–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song W, Ma Z, Zhang Y and Yang C:
Autophagy plays a dual role during intracellular siRNA delivery by
lipoplex and polyplex nanoparticles. Acta Biomater. 58:196–204.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) methods Methods. 25:402–408.
2001.PubMed/NCBI
|
|
25
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Wu K, Song W, Xu H, An R, Zhao L,
Liu B and Zhang Y: Chitosan/siCkip-1 biofunctionalized titanium
implant for improved osseointegration in the osteoporotic
condition. Sci Rep. 5:108602015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Wang Q, Wan Z, Li J, Liu L and
Zhang X: CKIP-1 knockout offsets osteoporosis induced by simulated
microgravity. Prog Biophys Mol Biol. 122:140–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Xing G, Tie Y, Tang Y, Tian C, Li
L, Sun L, Wei H, Zhu Y and He F: Role for the pleckstrin homology
domain-containing protein CKIP-1 in AP-1 regulation and apoptosis.
EMBO J. 24:766–778. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aghaloo TL, Chaichanasakul T, Bezouglaia
O, Kang B, Franco R, Dry SM, Atti E and Tetradis S: Osteogenic
potential of mandibular vs. long-bone marrow stromal cells. J Dent
Res. 89:1293–1298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaza T, Ren G, Akiyama K, Chen C, Shi Y
and Shi S: Mouse mandible contains distinctive mesenchymal stem
cells. J Dent Res. 90:317–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karaplis AC: Embryonic development of bone
and regulation of intramembranous and endochondral bone formation.
In: Principles of Bone Biology. Academic Press. (Cambridge, MA).
53–84. 2002.
|
|
32
|
Helms JA and Schneider RA: Cranial
skeletal biology. Nature. 423:326–331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leucht P, Kim JB, Amasha R, James AW,
Girod S and Helms JA: Embryonic origin and Hox status determine
progenitor cell fate during adult bone regeneration. Development.
135:2845–2854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scott MA, Levi B, Askarinam A, Nguyen A,
Rackohn T, Ting K, Soo C and James AW: Brief review of models of
ectopic bone formation. Stem Cells Dev. 21:655–667. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gallinetti S, Canal C, Ginebra MP and
Ferreira J: Development and characterization of biphasic
hydroxyapatite/β-TCP cements. J Am Ceram Soc. 97:1065–1073. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valentim RMB, Andrade SMC, Dos Santos MEM,
Santos A, Pereira V, dos Santos I, Dias C and dos Reis M: Composite
based on biphasic calcium phosphate (HA/β-TCP) and nanocellulose
from the açaí tegument. Materials (Basel). 11:E22132018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishack S, Mediero A, Wilder T, Ricci JL
and Cronstein BN: Bone regeneration in critical bone defects using
three-dimensionally printed β-tricalcium phosphate/hydroxyapatite
scaffolds is enhanced by coating scaffolds with either dipyridamole
or BMP-2. J Biomed Mater Res B Appl Biomater. 105:366–375. 2017.
View Article : Google Scholar : PubMed/NCBI
|