Introduction
Osteoporosis is the most common disorder of bone
remodeling in aging humans (1). It
is associated with high health costs, and is characterized by an
increased propensity to fragility fractures due to progressive loss
of bone mass and bone quality, coupled with decreased osteoblast
production and function (2,3). In
osteoporosis, bone homeostasis is disrupted by hormonal deficiency
and aging, leading to increased bone turnover with enhanced bone
formation and even greater rates of bone resorption, resulting in
net bone loss (4,5). Consequently, safe and effective
strategies to stimulate osteoblast formation and activity are in
great clinical demand. Anti-osteoporotic agents or food supplements
that can stimulate new bone formation and improve trabecular
microarchitecture should continue to be investigated for
postmenopausal osteoporosis, as emerging therapies hold great
promise (6–8).
MicroRNAs (miRNAs/miRs) are a diverse family of
endogenous small non-coding RNAs (ncRNAs) that are ~21 nucleotides
in length, which post-transcriptionally regulate the stability and
translational efficiency of target mRNAs (9). miRNAs have been reported to
contribute to each step of osteogenesis, starting from embryonic
bone development to adult bone tissue maintenance, by regulating
the growth, differentiation and functional activity of cells that
constitute the bone tissue (10).
In addition, substantial quantities of miRNAs are present in body
fluids such as serum and plasma as circulating ncRNAs, and have
been shown to play roles in cell communication by functioning as
hormone-like molecules to influence the behaviors of different
cells in a paracrine or endocrine manner (11,12).
Due to their high abundance and stability, circulating miRNAs have
potential utility as non-invasive, blood-based biomarkers that can
provide information on the disease and targeted therapies (13–21).
Icariin, a bioactive plant flavonoid, possesses the
ability to stimulate the osteogenic differentiation of bone
mesenchymal stem cells, improve osteoporosis in ovariectomized rats
and induce bone repair in rabbits (22–27).
In a previous study, it was demonstrated that icariin promoted
osteogenic differentiation in MC3T3-E1 cells (28). The present study screened the
differentially expressed miRNAs in icariin-treated MC3T3-E1 cells
and found that miR-27a-3p expression was negatively associated with
icariin treatment. Overexpression of miR-27a-3p inhibited
osteoblast activity in vitro, as well as decreasing the
expression levels of osterix (Osx). Furthermore, it was verified
that Osx, a primary transcription factor required for osteoblast
function, was a direct target of miR-27a-3p. Serum miR-27a-3p
expression was validated in clinical osteoporosis specimens. Hence,
the findings of the present study suggested that miRNAs could be
used as a novel diagnostic tool for osteoporosis or serve as novel
targets for therapeutic intervention.
Materials and methods
Cell culture
MC3T3-E1 preosteoblasts (obtained from the Cell
Center of the Chinese Academy of Medical Sciences) were maintained
in Minimum Essential Medium α (α-MEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (PS). 293T cells (American Type Culture
Collection) were maintained in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS and 1% PS. Cultured cells were incubated at 37°C in a
humidified chamber containing 5% CO2.
Animals
Adult female C57BL/6 mice (weight, 17–20 g; age, 6–8
weeks; n=3) were purchased from the Model Animal Research Center of
Nanjing University and maintained under standard animal housing
conditions (12-h light/dark cycle and free access to food and
water) in a temperature-controlled room (24±1°C) with relative
humidity (50±10%) at the animal center of Nanjing Medical
University. All procedures involving mice and the corresponding
experimental protocols were approved by the Animal Care and Use
Committee of Nanjing Medical University (approval no.
IACUC-1706005). Following terminal anesthesia by intraperitoneal
injection with pentobarbital sodium (100 mg/kg body weight), mice
were euthanized by cervical dislocation and the death of mouse was
verified 10 min by the following criteria: i) No breathing; ii) no
nerve reflexes; iii) no heartbeat and iv) relaxed muscles. Then,
the tissues (including the heart, liver, spleen, lung, kidney, bone
and brain) were quickly dissected and separately immersed into
liquid nitrogen.
RNA oligonucleotides and transfection
assay
For transfection, 100 nM inhibitors
(5′-GCGGAACUUAGCCACUGUGAA-3′), mimics (5′-UUCACAGUGGCUAAGUUCCGC-3′)
and non-specific control oligonucleotides
(5′-UUCUCCGAACGUGUCACGUTT-3′) of miR-27a-3p were obtained from
Guangzhou RiboBio Co., Ltd. and transfected into cultured cells
using Lipofectamine® 2000 (Invitrogen, Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions. At
48 h post-transfection, the cells were harvested and analyzed for
mRNA and protein expression.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from cultured cells, as well
as the tissues of C57BL/6 mice (including the heart, liver, spleen,
lung, kidney, bone and brain) using RNAiso Plus according to the
manufacturer's protocol (Takara Biotechnology Co., Ltd.). The
ribosomal bands were visualized on a 1% TAE agarose gel to assess
RNA integrity. cDNA was synthesized from 1 µg total RNA in RT
reactions using a PrimeScript RT reagent kit (Takara Biotechnology
Co., Ltd.), according to the manufacturer's instructions, and
RT-qPCR was performed with SYBR Green reagents (Roche Diagnostics)
on an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The expression level of mature miR-27a-3p
was determined by stem-loop RT-qPCR. U6 was used for normalization.
The following primers sequences were used: miR-27a-3p, RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCGGAACT-3′,
forward, 5′-GCGGGCGTTCACAGTGGCTA-3′ and reverse,
5′-CAGTGCAGGGTCCGAGGT-3′; U6, forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The PCR amplification
conditions were: 95°C for 5 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. The following primers sequences were
used: Runt-related transcription factor 2 (Runx2), forward,
5′-ATGATGACACTGCCACCTCTGAC-3′ and reverse,
5′-AACTGCCTGGGGTCTGAAAAAGG-3′; Osx, forward,
5′-AGCGACCACTTGAGCAAACAT-3′ and reverse,
5′-GCGGCTGATTGGCTTCTTCT-3′; alkaline phosphatase (ALP), forward,
5′-TGACCTTCTCTCCTCCATCC-3′ and reverse, 5′-CTTCCTGGGAGTCTCATCCT-3′;
osteocalcin (OC), forward, 5′-TGCTTGTGACGAGCTATCAG-3′ and reverse,
5′-GAGGACAGGGAGGATCAAGT-3′; bone sialoprotein (BSP), forward,
5′-AAGCAGCACCGTTGAGTATGG-3′ and reverse,
5′-CCTTGTAGTAGCTGTATTCATCCTC-3′; type I collagen (Col1α1), forward,
5′-GCAACAGTCGCTTCACCTACA-3′ and reverse,
5′-CAATGTCCAAGGGAGCCACAT-3′; and β-actin, forward,
5′-AGATGTGGATCAGCAAGCAG-3′ and reverse, 5′-GCGCAAGTTAGGTTTTGTCA-3′.
The relative expression levels were calculated using the
2−ΔΔCq method (29) and
normalized to β-actin. For each data point, triplicate reactions
were carried out and the experiment was repeated three times to
assess statistical significance.
RT-PCR for Osx expression
Total RNA isolation and cDNA synthesis were
conducted as described above. The amplification conditions for PCR
were: 95°C for 5 min, followed by 20–30 cycles of denaturation at
95°C for 15 sec, annealing at 56°C for 5 sec and extension at 72°C
for 30 sec. The PCR products were analyzed on 8% polyacrylamide
gels and visualized using a Tanon 4100 gel imaging analysis system
(Tanon). The primers for β-actin and Osx were same as those used
for RT-qPCR.
Western blot analysis
Cells were lysed in RIPA buffer [50 mM Tris-HCl, pH
7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate and
0.1% sodium dodecyl sulfate (SDS)] plus protease inhibitors (Roche
Diagnostics). Protein concentrations were determined using a
Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Total protein (20 µg) samples were boiled for 5 min in 1X loading
buffer, incubated on ice, separated via 10% SDS-PAGE and then
transferred to PVDF membranes (EMD Millipore) with a semidry
transfer apparatus (Bio-Rad Laboratories, Inc.). Non-specific
protein interactions were blocked by incubation with 5% fat-free
milk in TBS-Tween (TBST) buffer (50 mM Tris-HCl, 150 mM NaCl, 0.05%
Tween 20, pH 7.6) for 1 h at room temperature. The membranes were
incubated overnight with primary antibody in blocking buffer at
4°C. Unbound antibody was removed by washing three times with TBST
(10 min/wash). After rinsing, the membranes were incubated with
horseradish peroxide (HRP)-conjugated goat anti-mouse (1:5,000;
cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) or HRP-conjugated
goat anti-rabbit (1:5,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) secondary antibodies for 1 h at room
temperature, followed by washing with TBST three times (10
min/wash). The immunoreactive bands were visualized with ECL
reagent (Pierce; Thermo Fisher Scientific, Inc.). Densitometric
analysis was performed using ImageJ software (version 1.49v;
National Institutes of Health). Primary antibodies against Osx
(1:4,000; cat. no. ab94744; Abcam) and β-actin (1:1,000; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.) were used.
Plasmid construction and luciferase
reporter assay
Osx cDNA was inserted into a pRK5 vector (Addgene,
Inc.) at the EcoRI and HindIII sites to generate
wild-type pRK5-Flag-Osx plasmid. Primer sequences for cloning the
Osx CDS sequences were: Forward, 5′-CGGAATTCATGGCGTCCTCCCTGCTTGA-3′
and reverse, 5′-CCCAAGCTTTCAGATCTCCAGCAAGTTGC-3′. Fragments of the
Osx 3′-untranslated region (3′UTR) containing the miR-27a-3p
recognition sequences were inserted behind the luciferase coding
sequence at the XbaI site in the pGL3-promoter vector
(Promega Corporation). Primer sequences for cloning the Osx 3′UTR
sequences were: Wild-type (WT), forward,
5′-CTCTAGAGCTCCGACCTCCTCAACTT-3′ and reverse,
5′-CTCTAGAAAGGCATTTCAAAGGCACA-3′; mutant (MUT), forward,
5′-GCCAGAAAGCTAGTAAACTTCAAGT-3′ and reverse,
5′-ACTTGAAGTTTACTAGCTTTCTGGC-3′. The PrimeSTAR® Max DNA
Polymerase (Takara Biotechnology Co., Ltd.) was used for the PCR,
according to the manufacturer's protocol. The amplification
conditions were as follows: 98°C for 3 min; followed by 32 cycles
of 98°C for 10 sec and 60°C for 90 sec; and a final extension at
72°C for 10 min. For the luciferase assay, 1×105 293T
cells/well were co-transfected in 24-well plates with the above
reporter constructs (0.2 µg) along with phRL-null (0.01 µg;
Renilla plasmid for normalization; Promega Corporation) and
100 nM control mimic (miR-C) or miR-27a-3p mimic using
Lipofectamine® 2000. The cells were collected 48 h after
transfection and the luciferase activities were measured with a
Dual-luciferase Reporter Assay system (Promega Corporation) and a
GloMax™ Base instrument (Promega Corporation). The firefly
luciferase activity was normalized to Renilla luciferase
activity.
Bioinformatics prediction and
conservative analysis
Potential targets of miR-27a-3p were predicted from
three different algorithms: TargetScan Mouse v.6.2 (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/home.do) and RNA22
(https://cm.jefferson.edu/rna22v1.0-mus_musculus/GetInputs.jsp).
TargetScan predicts the biological targets of miRNAs by searching
for the presence of conserved 8mer, 7mer and 6mer sites that match
the seed region of each miRNA. Potential target mRNAs were selected
if they were predicted by ≥2 algorithms. The mature sequences for
mmu-miR-27a-3p and hsa-miR-27a-3p were obtained from miRBase
(http://www.mirbase.org/) and aligned by ClustalX
(http://www.clustal.org).
ALP staining
To induce osteogenic differentiation, cells were
cultured in differentiation-inducing medium containing 50 mg/l
ascorbic acid, 10 mM β-glycerophosphate and 10 nM dexamethasone for
the indicated times with regular medium changes. For ALP staining,
after induction for 5–7 days, cells were fixed with 95% ethanol and
stained with BCIP/NBT solution according to the manufacturer's
protocol (Beyotime Institute of Biotechnology) at room temperature
for 2 h. The ALP-positive cells were stained blue/purple. Stained
cells were visualized using the Canon IXUS210 camera (Canon, Inc.;
magnification, ×5). ImageJ version 1.49 software (National
Institutes of Health) was used for image analysis and
quantification.
Icariin treatment
Icariin (94.2% purity) was purchased from the
National Institutes for Food and Drug Control. Stock solutions of
Icariin (10 mM) were prepared in DMSO (≥99.7%; Sigma-Aldrich; Merck
KGaA) and stored at −20°C. Icariin treatment was performed as
previously described (28).
Briefly, MC3T3-E1 cells were cultured in differentiation-inducing
medium containing 50 mg/l ascorbic acid, 10 mM β-glycerophosphate
and 10 nM dexamethasone, and 5 µM Icariin for 48 h at 37°C in a
humidified chamber containing 5% CO2.
Clinical samples
This study conformed to the principles of the
Declaration of Helsinki and was approved by the Ethics Committee of
Jiangsu Province Hospital of Chinese Medicine. A total of 137
female participants were recruited for this study, including 85
participants with osteoporosis (50–90 years old) and 52 healthy
participants (50–90 years old) from the Jiangsu Province Hospital
of Chinese Medicine and Northern Jiangsu People's Hospital between
February 2014 and April 2019 (Table
I). Each subject underwent a clinical examination, and routine
biochemical tests were performed to exclude subjects with systemic
and metabolic bone disorders other than osteoporosis. Assessment of
the most susceptible sites of osteoporotic fractures, such as the
lumbar spine and the head of the femur, by dual X-ray
absorptiometry was used as the reference method for measuring total
bone mineral density (BMD). The osteoporosis group was defined as
BMD T-scores ≤-2.5 at the lumbar. Informed consent was obtained
from all participants prior to sample collection. Clinical samples
were obtained from the participants, including local health
volunteers and patients who were referred to the hospital, who
either presented at the bone clinic or the Department of Radiology
for a BMD scan. Serum samples were prepared by centrifugation at
2,500 × g for 30 min at room temperature. Aliquots of the
supernatants were frozen at −80°C until RNA extraction.
 | Table I.Age distribution of the patients and
controls. |
Table I.
Age distribution of the patients and
controls.
| Age, years | Normal | Osteoporosis |
|---|
| 50-59 | 9 | 5 |
| 60-69 | 31 | 32 |
| 70-79 | 8 | 32 |
| 80-90 | 4 | 16 |
Statistical analyses
Experimental data were analyzed by Student's t-test
or one-way analysis of variance with Bonferroni's correction using
GraphPad Prism 6.0 (GraphPad Software, Inc.). All pairs of columns
were compared, and the bars denote the mean ± standard deviation
from 3 independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-27a-3p expression is downregulated
during osteogenic differentiation
Our previous study demonstrated that icariin
treatment significantly elevated the gene expression of osteogenic
markers and increased ALP activity, thus promoting osteogenic
differentiation of MC3T3-E1 cells (28). The present study first examined the
levels of miR-27a-3p during icariin-induced osteogenic
differentiation. Results demonstrated that the expression of
osteogenic marker genes such as Runx2 and Osx was rapidly increased
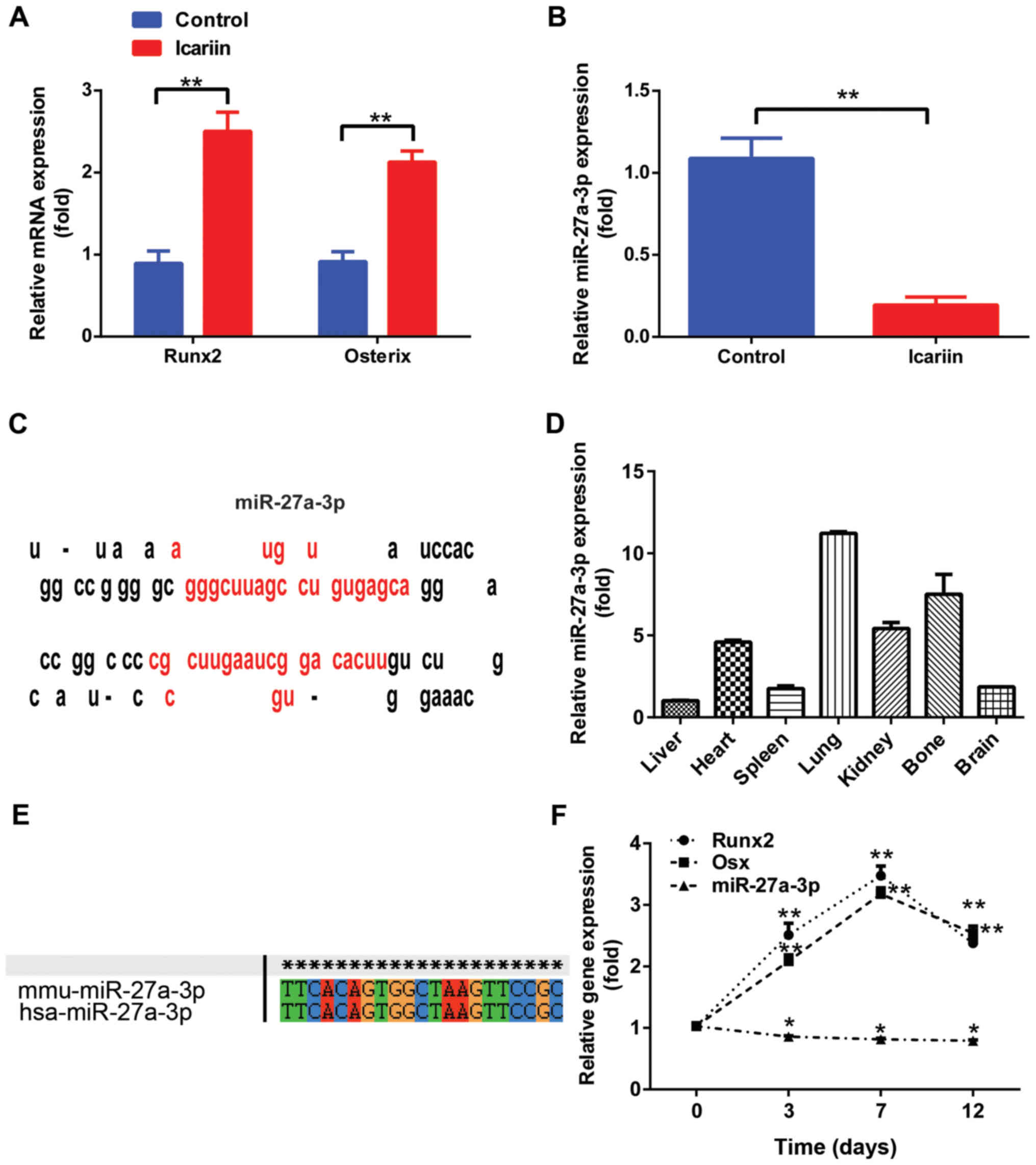
in MC3T3-E1 cells treated with icariin (5 µM) for 48 h (Fig. 1A), but that of miR-27a-3p was
significantly downregulated (Fig.
1B). Notably, miR-27a-3p, encoded by a gene located on mouse
chromosome 1, was located at a noncoding region and the precursor
sequences displayed the characteristic miRNA precursor stem-loop
secondary structure according to the miRNA repository miRBase
(Fig. 1C). To further evaluate the
function of miR-27a-3p, its tissue distribution in normal adult
mice was examined by RT-qPCR. The expression of mature miR-27a-3p
was detected in all seven types of tissues, including the heart,
liver, spleen, lung, kidney, bone and brain. In addition, results
demonstrated that miR-27a-3p was preferentially expressed in the
lung and bone tissues (Fig. 1D).
Analysis by ClustalX demonstrated that miR-27a-3p was highly
conserved in both Homo sapiens and Mus musculus
(Fig. 1E).
To determine the role of miR-27a-3p in osteoblast
lineage commitment, its expression level was detected by culturing
MC3T3-E1 preosteoblasts in osteogenic medium for different time
periods. As shown in Fig. 1F,
miR-27a-3p expression in MC3T3-E1 cells began to decrease over the
course of osteogenic differentiation (from days 3 to 12). The
expression levels of Runx2 and Osx increased gradually starting
from day 3, reaching a maximum on day 7; however, their levels
started to decline during the terminal differentiation phase.
Together, these findings suggest that miR-27a-3p may function as a
miRNA that modulates osteogenic differentiation.
Osx is a direct target of
miR-27a-3p
To establish the link between miR-27a-3p and
osteoblast function, putative protein targets involved in
osteogenic differentiation were screened. Osx, an
osteoblast-specific transcription factor, was selected as a target
for validation. Single putative miR-27a-3p recognition sites were
predicted within the Osx 3′UTR sequences by TargetScan Mouse v.6.2
and RNA22. According to the sequence analysis, Osx contained a
7-nucleotide site within its 3′UTR that matched the seed region of
miR-27a-3p (Fig. 2A). Furthermore,
tissue distribution analysis demonstrated that the Osx level was
highest in the bone (Fig. 2B).
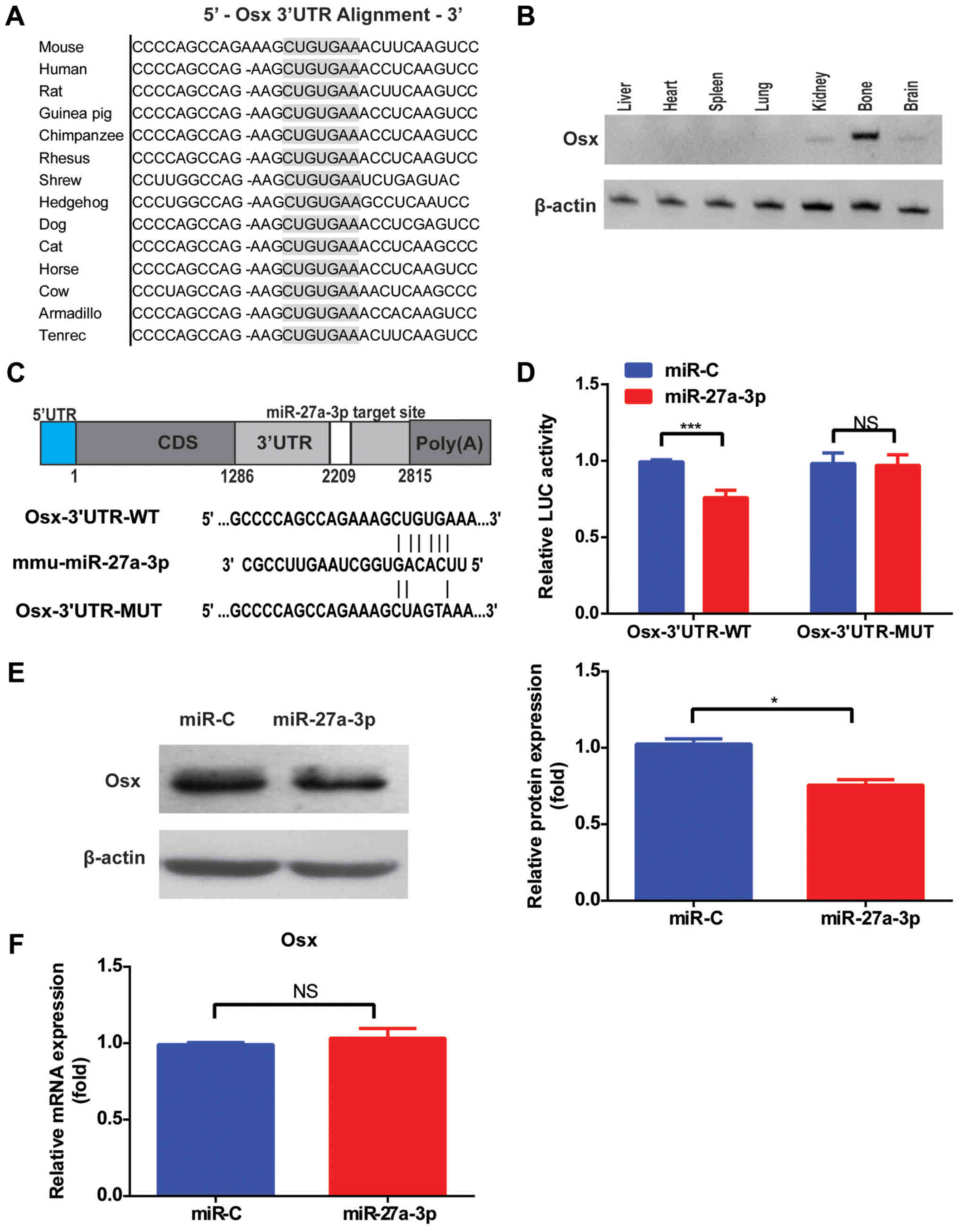 | Figure 2.Osx is a direct target of miR-27a-3p.
(A) Sequence alignment of the Osx 3′UTR by TargetScan. (B) RT-qPCR
detection of Osx expression in each tissue from mice. (C) Schematic
illustration of the design of the luciferase reporter construct
with WT Osx 3′UTR or site-directed mutant Osx 3′UTR. (D) Luciferase
activity of 293T cells co-transfected with WT or mutant Osx 3′UTR
reporter and miR-27a-3p mimic or miR-C. The relative luciferase
activity, defined as the ratio of 3′UTR reporter activity to that
of the internal control (Renilla), was determined 48 h after
transfection. (E) Western blot analysis and quantification of Osx
protein levels after transfection with miR-27a-3p mimic or miR-C in
MC3T3-E1 cells. (F) Effects of miR-27a-3p mimic or control on Osx
mRNA levels in MC3T3-E1 cells, as determined by RT-qPCR.
*P<0.05, ***P<0.001. Osx, osterix; miR, microRNA; miR-C,
mimic control; RT-qPCR, reverse transcription-quantitative PCR; WT,
wild-type; MUT, mutant; 3′UTR, 3′-untranslated region; NS, not
significant. |
To gain insight into the mechanism via which
miR-27a-3p modulates Osx expression, the 3′UTR of the Osx gene
containing one miR-27a-3p binding site was fused downstream of a
luciferase reporter (Fig. 2C).
Results demonstrated that miR-27a-3p mimic significantly inhibited
the luciferase reporter activity of the WT Osx 3′UTR compared with
miR-C (Fig. 2D). Another
luciferase reporter was constructed with an Osx 3′UTR containing
mutant sequences of the miR-27a-3p binding site (Fig. 2C). The luciferase reporter activity
of the MUT Osx 3′UTR was not suppressed by miR-27a-3p mimic
(Fig. 2D). Taken together,
introduction of mutations in these sequences abolished the ability
of miR-27a-3p to inhibit reporter activity, confirming the
selective interaction of miR-27a-3p with mRNAs and indicating that
the single recognition element identified in the 3′UTR of the Osx
mRNA is sufficient for miR-27a-3p activity. Accordingly,
transfection with miR-27a-3p mimic alone decreased the amount of
endogenous Osx protein in cultured cells (Fig. 2E), but had no significant effect on
Osx mRNA levels (Fig. 2F),
suggesting that miR-27a-3p downregulates Osx expression by
inhibiting its translation.
miR-27a-3p regulates osteoblast
activity in vitro
Due to the role of miR-27a-3p in regulating Osx
expression, the physiological effect of miR-27a-3p overexpression
on osteogenic differentiation was evaluated in vitro.
Results demonstrated that miR-27a-3p levels were substantially
upregulated after transfection with miR-27a-3p mimic (Fig. 3A). In addition, the expression of
osteoblast marker genes, including ALP, OC, BSP and Col1α1, which
are Osx downstream genes, was downregulated in MC3T3-E1 cells
transfected with miR-27a-3p mimic compared to that in cells
transfected with control mimic (Fig.
3B). Conversely, the expression of these osteoblast marker
genes was upregulated in MC3T3-E1 cells transfected with miR-27a-3p
inhibitor (Fig. 3C and D).
Consistent with the changes in osteoblast marker gene expression,
weakened ALP staining was observed in MC3T3-E1 cells transfected
with miR-27a-3p mimic compared to that in cells transfected with
control mimic (Fig. 3E).
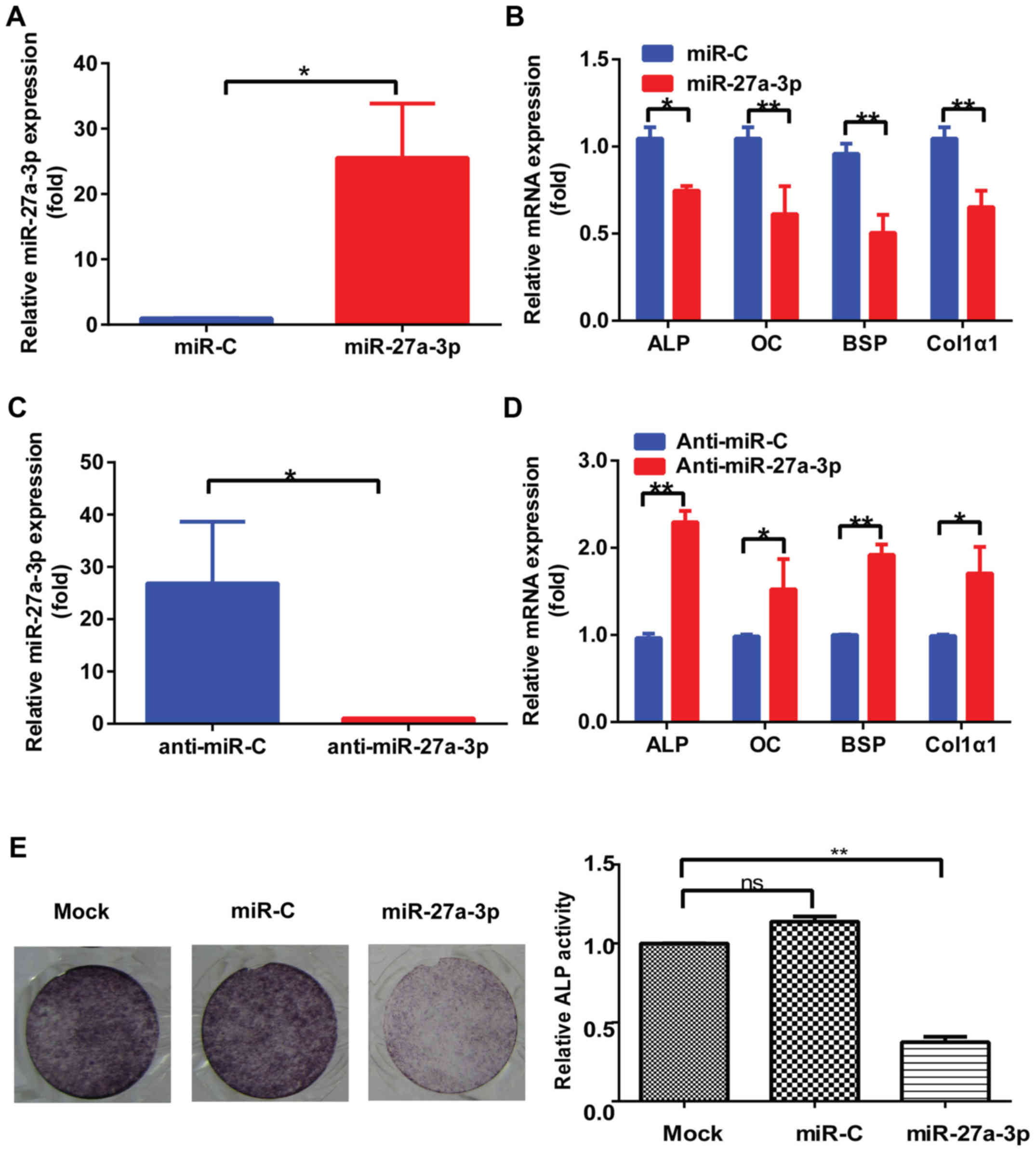 | Figure 3.miR-27a-3p regulates osteoblast
activity in vitro. (A) Expression of miR-27a-3p in MC3T3-E1
cells transfected with miR-27a-3p mimic or miR-C for 48 h, as
determined by RT-qPCR. U6 was used as the internal control. (B)
RT-qPCR detection of the levels of osteoblast marker genes in
MC3T3-E1 cells transfected with miR-27a-3p mimic or miR-C for 48 h.
β-actin was used as the internal control. (C) Expression of
miR-27a-3p in MC3T3-E1 cells transfected with miR-27a-3p inhibitor
or anti-miR-C for 48 h, as determined by RT-qPCR. U6 was used as
the internal control. (D) RT-qPCR detection of the levels of
osteoblast marker genes in MC3T3-E1 cells transfected with
miR-27a-3p inhibitor or control for 48 h. β-actin was used as the
internal control. (E) ALP staining results after induction of
osteogenic differentiation for 7 days and quantification of ALP
activity. *P<0.05, **P<0.01. miR, microRNA; miR-C, control
mimic; anti-miR-C, control inhibitor; RT-qPCR, reverse
transcription-quantitative PCR; ALP, alkaline phosphatase; OC,
osteocalcin; BSP, bone sialoprotein; Col1α1, type I collagen. |
Osx rescues the effect of miR-27a-3p
on osteogenic differentiation
Having demonstrated that miR-27a-3p suppresses
osteogenic differentiation, it was next assessed whether
overexpression of Osx could rescue the effect of miR-27a-3p on
osteogenic differentiation in vitro. As shown in Fig. 4A, Osx protein level was enhanced
after transfection with Flag-Osx expression plasmid in 293T cells.
Of note, when co-transfected with miR-27a-3p mimic and Osx
overexpression plasmid, the miR-27a-3p-induced inhibition of
osteogenic differentiation was attenuated (Fig. 4B and C). These results suggested
that the predominant effect of miR-27a-3p on osteogenic
differentiation was mediated via the downregulation of Osx.
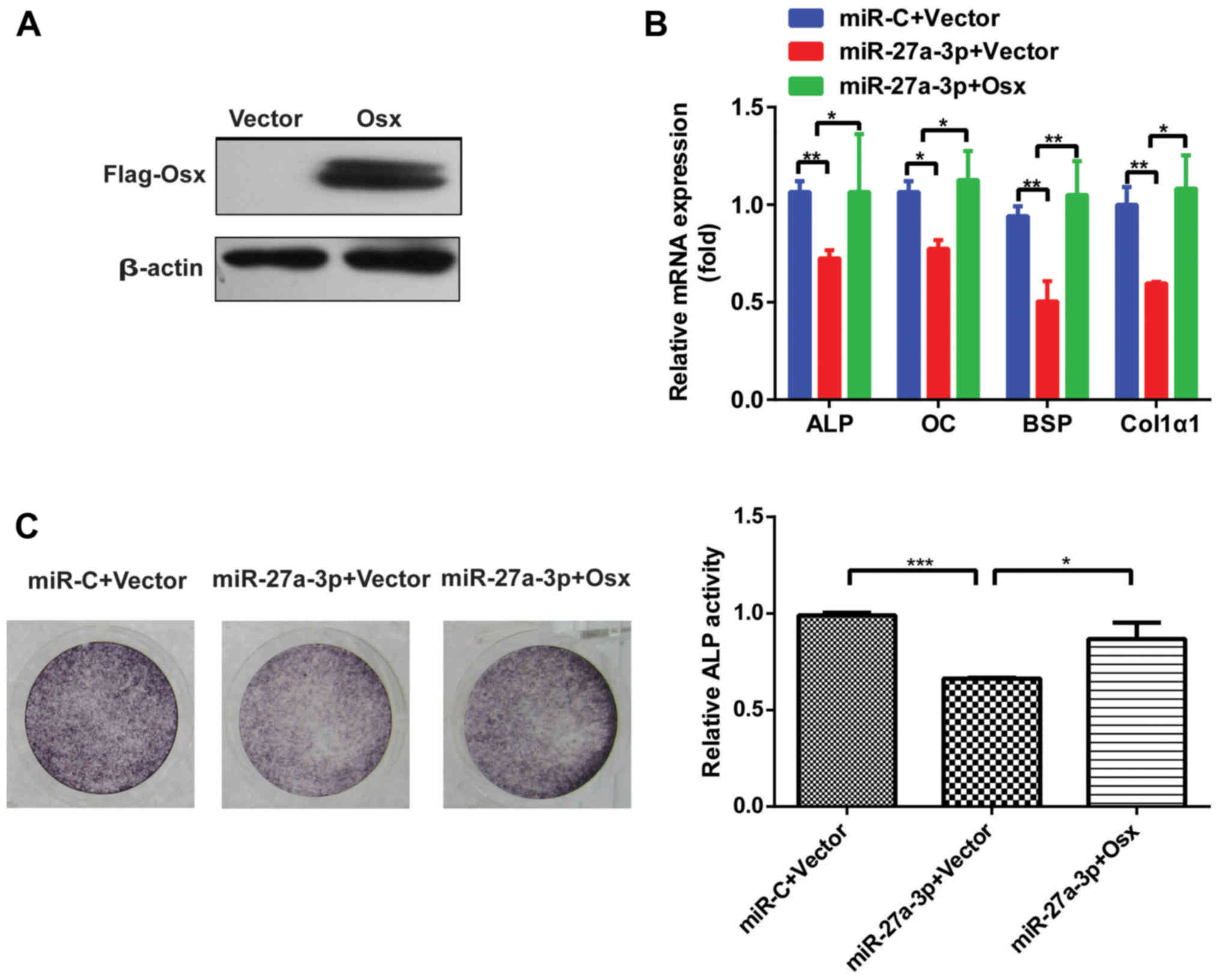 | Figure 4.Osx rescues the effect of miR-27a-3p
on osteogenic differentiation. (A) Western blot analysis of Osx
protein level after transfection with Flag-Osx expression plasmid
or vector alone in 293T cells. β-actin was used as the internal
control. (B) Expression of osteoblast marker genes, as determined
by reverse transcription-quantitative PCR following
miR-27a-3p-mediated inhibition of osteogenic differentiation in
MC3T3-E1 cells with restored expression of Osx. β-actin was used as
the internal control. (C) ALP staining analysis after
miR-27a-3p-mediated inhibition of osteogenic differentiation in
MC3T3-E1 cells with restored Osx expression and quantification of
ALP activity. *P<0.05, **P<0.01, ***P<0.001. miR,
microRNA; miR-C, control mimic; Osx, osterix; ALP, alkaline
phosphatase; OC, osteocalcin; BSP, bone sialoprotein; Col1α1, type
I collagen. |
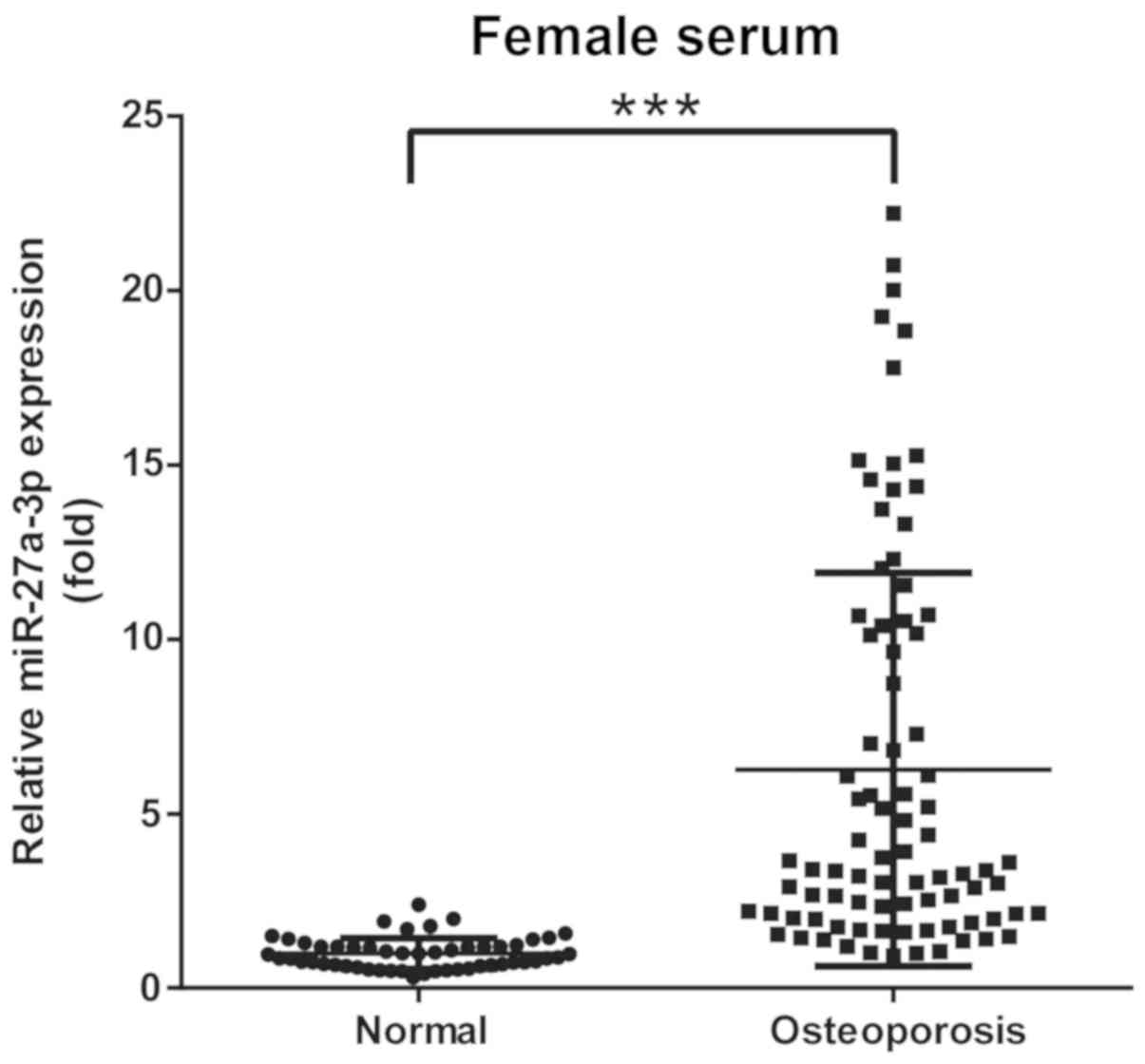
Circulating miR-27a-3p level is
associated with osteoporosis
Based on BMD, 137 participants were classified into
2 groups: Group A, 52 (female) non-osteoporotic controls; and group
B, 85 (female) patients with osteoporosis. Circulating miRNAs from
serum samples were tested using RT-qPCR. The results demonstrated
that miR-27a-3p levels were significantly elevated in patients with
osteoporosis compared with in non-osteoporotic controls (Fig. 5). These data suggested that
elevated levels of circulating miR-27a-3p in clinical samples may
be associated with the development of osteoporosis in patients.
Discussion
Osteoporosis is one of the major diseases caused by
age- or disease-related bone loss (30). A healthy mammalian skeleton is
maintained by constant bone remodeling, an active coupling process
involving bone-forming osteoblasts and bone-resorbing osteoclasts
(5,30). MC3T3-E1 cells, preosteoblasts that
mature into preosteocytes in a mineralized matrix, have been
stimulated with icariin to induce osteogenic differentiation
(28). The present study
demonstrated that miR-27a-3p expression was rapidly downregulated
during icariin-induced osteogenic differentiation in MC3T3-E1
cells.
A growing body of evidence has revealed that
miR-27a-3p acts as a key regulator of bone biology (31–36).
Compared to 293T cells, MG63 cells and osteogenic hFOBs cells have
been shown to display higher levels of miR-27a due to osteogenesis
(34). In addition, the inhibitory
effects of agomiR-27a-3p on the expression of Runx2, Osx, OC and
Col1α1 in periodontal ligament stem cells have been demonstrated
(35). Overexpression of miR-27a
has been shown to weaken ALP and alizarin red staining during
osteogenic differentiation of Satb2-induced bone marrow stromal
cells (36). Consistent with the
inhibitory role of miR-27a-3p in osteogenic differentiation
described by these studies, the present study found that the
expression of osteogenic marker genes and ALP activity were
significantly decreased in miR-27a-3p-overexpressing MC3T3-E1 cells
in vitro. These effects could be reversed by suppression of
miR-27a-3p, suggesting that miR-27a-3p is a negative regulator of
osteogenic differentiation in MC3T3-E1 cells.
To explore the molecular mechanisms via which
miR-27a-3p regulates the osteogenic differentiation of MC3T3-E1
cells, the potential targets of miR-27a-3p were predicted using
miRNA target prediction tools. Osx was identified as a potential
target of miR-27a-3p. The present study demonstrated that
miR-27a-3p directly targeted Osx by binding to its 3′UTR. Osx, a
zinc-finger-containing transcription factor, acts downstream of
Runx2, and regulates the differentiation and/or function of
osteoblasts (37). Our previous
study revealed that Osx expression in C2C12 cells is regulated by
miR-214, a suppressor of osteogenic differentiation (38). The present study reported that Osx
is a direct target of miR-27a-3p and is involved in osteogenic
differentiation. Of note, when Osx was overexpressed, osteogenic
marker gene expression and ALP activity were restored in
miR-27a-3p-overexpressing MC3T3-E1 cells. This indicated that
miR-27a-3p inhibited osteogenic differentiation via Osx. Based on
these data, it was hypothesized that miR-27a-3p was capable of
inhibiting osteogenic differentiation.
In postmenopausal osteoporosis, estrogen therapy
effectively prevents bone loss (39). Emerging evidence has demonstrated
that estrogen exhibits an inhibitory role in osteoclast formation
and enhances osteoblast function (40,41).
During estrogen-decreased osteoclast differentiation, miR-27a
remarkably enhances the inhibitory effect of estrogen (40). In addition, following treatment
with antiosteoporotic agents, serum miR-27a levels are
significantly reduced in postmenopausal women (42). The present study measured the serum
levels of miR-27a-3p in 85 samples with osteoporosis and found that
the miR-27a-3p levels were significantly higher in osteoporotic
patients compared with those in non-osteoporotic controls. These
findings implied that miR-27a-3p may serve as a promising marker
for the detection of osteoporosis.
Taken together, the results of the present study
demonstrated that miR-27a-3p suppressed osteogenic differentiation
via downregulating Osx expression. The findings revealed the
regulatory role of miR-27a-3p in osteogenic differentiation, and
suggested that therapies targeting miR-27a-3p may serve as a
promising strategy for the treatment of osteoporosis. The results
of the present study provided novel insight into the mechanism by
which miRNAs regulate bone physiology.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Natural
Science Foundation of Jiangsu Province (grant nos. BK20171057,
BK20171489 and BK20161323), the National Natural Science Foundation
of China (grant nos. 81500823, 81570804 and 81873105).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX, DL and ZZ designed the experiments. LL onducted
experiments on the clinical samples and provided material support..
YX, DL and ZZ performed most of the experiments. YX drafted the
manuscript. CM performed the bioinformatics analysis. YJ
contributed to the analysis and interpretation of the data. WZ
performed the animal experiments and modified the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all participants.
Human studies conformed to the principles of the Declaration of
Helsinki and were approved by the Ethics Committee of Jiangsu
Province Hospital of Chinese Medicine. All procedures involving
mice and the corresponding experimental protocols were approved by
the Animal Care and Use Committee of Nanjing Medical University
(approval no. IACUC-1706005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richards JB, Zheng HF and Spector TD:
Genetics of osteoporosis from genome-wide association studies:
Advances and challenges. Nat Rev Genet. 13:576–588. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Black DM and Rosen CJ: Clinical practice.
Postmenopausal osteoporosis. N Engl J Med. 374:254–262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar
G, Ajita J, Rhee Y, Kim CH and Lim SK: MiR-182 is a negative
regulator of osteoblast proliferation, differentiation, and
skeletogenesis through targeting FoxO1. J Bone Miner Res.
27:1669–1679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu B, Chang J, Liu Y, Li J, Kevork K,
Al-Hezaimi K, Graves DT, Park NH and Wang CY: Wnt4 signaling
prevents skeletal aging and inflammation by inhibiting nuclear
factor-κB. Nat Med. 20:1009–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long F: Building strong bones: Molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mulder JE, Kolatkar NS and LeBoff MS: Drug
insight: Existing and emerging therapies for osteoporosis. Nat Clin
Pract Endocrinol Metab. 2:670–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo AJ, Choi RC, Cheung AW, Chen VP, Xu
SL, Dong TT, Chen JJ and Tsim KW: Baicalin, a flavone, induces the
differentiation of cultured osteoblasts: An action via the
Wnt/beta-catenin signaling pathway. J Biol Chem. 286:27882–27893.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reid IR: Short-term and long-term effects
of osteoporosis therapies. Nat Rev Endocrinol. 11:418–428. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang TC, Yu D, Lee YS, Wentzel EA, Arking
DE, West KM, Dang CV, Thomas-Tikhonenko A and Mendell JT:
Widespread microRNA repression by Myc contributes to tumorigenesis.
Nat Genet. 40:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian JB, Stein GS, van Wijnen AJ, Stein
JL, Hassan MQ, Gaur T and Zhang Y: MicroRNA control of bone
formation and homeostasis. Nat Rev Endocrinol. 8:212–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anfossi S, Babayan A, Pantel K and Calin
GA: Clinical utility of circulating non-coding RNAs-an update. Nat
Rev Clin Oncol. 15:541–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hackl M, Heilmeier U, Weilner S and
Grillari J: Circulating microRNAs as novel biomarkers for bone
diseases-complex signatures for multifactorial diseases? Mol Cell
Endocrinol. 432:83–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng HC, Bae Y, Dawson BC, Chen Y, Bertin
T, Munivez E, Campeau PM, Tao J, Chen R and Lee BH: MicroRNA
miR-23a cluster promotes osteocyte differentiation by regulating
TGF-β signalling in osteoblasts. Nat Commun. 8:150002017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Liu J, Guo B, Liang C, Dang L, Lu C,
He X, Cheung HY, Xu L, Lu C, et al: Osteoclast-derived exosomal
miR-214-3p inhibits osteoblastic bone formation. Nat Commun.
7:108722016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeliger C, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weilner S, Schraml E, Wieser M, Messner P,
Schneider K, Wassermann K, Micutkova L, Fortschegger K, Maier AB,
Westendorp R, et al: Secreted microvesicular miR-31 inhibits
osteogenic differentiation of mesenchymal stem cells. Aging Cell.
15:744–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weilner S, Skalicky S, Salzer B, Keider V,
Wagner M, Hildner F, Gabriel C, Dovjak P, Pietschmann P,
Grillari-Voglauer R, et al: Differentially circulating miRNAs after
recent osteoporotic fractures can influence osteogenic
differentiation. Bone. 79:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mandourah AY, Ranganath L, Barraclough R,
Vinjamuri S, Hof RV, Hamill S, Czanner G, Dera AA, Wang D and
Barraclough DL: Circulating microRNAs as potential diagnostic
biomarkers for osteoporosis. Sci Rep. 8:84212018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Li K, Pang Q, Yang C, Zhang H, Wu
F, Cao H, Liu H, Wan Y, Xia W, et al: Identification of suitable
reference gene and biomarkers of serum miRNAs for osteoporosis. Sci
Rep. 6:363472016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramírez-Salazar EG, Carrillo-Patiño S,
Hidalgo-Bravo A, Rivera-Paredez B, Quiterio M, Ramírez-Palacios P,
Patiño N, Valdés-Flores M, Salmerón J and Velázquez-Cruz R: Serum
miRNAs miR-140-3p and miR-23b-3p as potential biomarkers for
osteoporosis and osteoporotic fracture in postmenopausal
Mexican-Mestizo women. Gene. 679:19–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You L, Pan L, Chen L, Gu W and Chen J:
miR-27a is essential for the shift from osteogenic differentiation
to adipogenic differentiation of mesenchymal stem cells in
postmenopausal osteoporosis. Cell Physiol Biochem. 39:253–265.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei Q, He M, Chen M, Chen Z, Yang F, Wang
H, Zhang J and He W: Icariin stimulates osteogenic differentiation
of rat bone marrow stromal stem cells by increasing TAZ expression.
Biomed Pharmacother. 91:581–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei Q, Zhang J, Hong G, Chen Z, Deng W, He
W and Chen MH: Icariin promotes osteogenic differentiation of rat
bone marrow stromal cells by activating the ERα-Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 84:931–939. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Xia L, Zhou Y, Xu Y and Jiang X:
Icariin induces osteogenic differentiation of bone mesenchymal stem
cells in a MAPK-dependent manner. Cell Prolif. 48:375–384. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Xiong Y, Zhu X, Gao H, Yin S, Wang
J, Chen G, Wang C, Xiang L, Wang P, et al: Icariin improves
osteoporosis, inhibits the expression of PPARγ, C/EBPα, FABP4 mRNA,
N1ICD and jagged1 proteins, and increases Notch2 mRNA in
ovariectomized rats. Exp Ther Med. 13:1360–1368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Wang D, Yang D, Zhen W, Zhang J
and Peng S: The effect of icariin on bone metabolism and its
potential clinical application. Osteoporos Int. 29:535–544. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Shen L, Mao Z, Wang N, Wang X,
Huang X, Hu Y, Shou D and Wen C: Icariin enhances bone repair in
rabbits with bone infection during post-infection treatment and
prevents inhibition of osteoblasts by vancomycin. Front Pharmacol.
8:7842017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Li L, Tang Y, Yang J, Jin Y and Ma
C: Icariin promotes osteogenic differentiation by suppressing Notch
signaling. Eur J Pharmacol. 865:1727942019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KM and Lim SK: Role of miRNAs in bone
and their potential as therapeutic targets. Curr Opin Pharmacol.
16:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren H, Yu X, Shen G, Zhang Z, Shang Q,
Zhao W, Huang J, Yu P, Zhan M, Lu Y, et al: MiRNA-seq analysis of
human vertebrae provides insight into the mechanism underlying
GIOP. Bone. 120:371–386. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Q, Cui Y, Luan J, Zhou X, Li H and Han
J: Exosomes from C2C12 myoblasts enhance osteogenic differentiation
of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem
Biophys Res Commun. 498:32–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Y, Shan Z, Ma J, Wang Q, Chu J, Xu P,
Qin A and Fan S: Validation of downregulated microRNAs during
osteoclast formation and osteoporosis progression. Mol Med Rep.
13:2273–2280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo D, Li Q, Lv Q, Wei Q, Cao S and Gu J:
miR-27a targets sFRP1 in hFOB cells to regulate proliferation,
apoptosis and differentiation. PLoS One. 9:e913542014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Liu C, Zhang A, Yin S, Wang T, Wang
Y, Wang M, Liu Y, Ying Q, Sun J, et al: Down-regulation of long
non-coding RNA MEG3 suppresses osteogenic differentiation of
periodontal ligament stem cells (PDLSCs) through miR-27a-3p/IGF1
axis in periodontitis. Aging (Albany NY). 11:5334–5350. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong Y, Lu J, Yu X and Yu Y: Expression of
Sp7 in Satb2-induced osteogenic differentiation of mouse bone
marrow stromal cells is regulated by microRNA-27a. Mol Cell
Biochem. 417:7–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou X, Zhang Z, Feng JQ, Dusevich VM,
Sinha K, Zhang H, Darnay BG and de Crombrugghe B: Multiple
functions of Osterix are required for bone growth and homeostasis
in postnatal mice. Proc Natl Acad Sci USA. 107:12919–12924. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B,
Li H and Ma C: MicroRNA-214 suppresses osteogenic differentiation
of C2C12 myoblast cells by targeting Osterix. Bone. 55:487–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Compston JE, McClung MR and Leslie WD:
Osteoporosis. Lancet. 393:364–376. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo L, Chen K, Yuan J, Huang P, Xu X, Li
C, Qian N, Qi J, Shao Z, Deng L, et al: Estrogen inhibits
osteoclasts formation and bone resorption via microRNA-27a
targeting PPARγ and APC. J Cell Physiol. 234:581–594. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gavali S, Gupta MK, Daswani B, Wani MR,
Sirdeshmukh R and Khatkhatay MI: Estrogen enhances human osteoblast
survival and function via promotion of autophagy. Biochim Biophys
Acta Mol Cell Res. 1866:1498–1507. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anastasilakis AD, Makras P, Pikilidou M,
Tournis S, Makris K, Bisbinas I, Tsave O, Yovos JG and Yavropoulou
MP: Changes of circulating MicroRNAs in response to treatment with
teriparatide or denosumab in postmenopausal osteoporosis. J Clin
Endocrinol Metab. 103:1206–1213. 2018. View Article : Google Scholar : PubMed/NCBI
|