Introduction
Ankylosing spondylitis (AS) is a common chronic
immune-mediated joint disease, which predominantly affects the
spine and pelvis (1). Between May
2005 and May 2019, the total prevalence of AS in mainland China was
0.29% (2). AS is characterized by
spinal pain, stiffness and new bone formation, which manifests
ligament atrophy and joint stiffness (3). A previous study has demonstrated that
there is no definite value in assessing the long-term prognosis and
mortality of patients with AS (4).
The number of patients with AS per 10,000 people is 23.8 in Europe,
16.7 in Asia, 31.9 in North America, 10.2 in Latin America and 7.4
in Africa (5). With the increasing
incidence of AS, the therapeutic strategies of AS are also
diversified, including the use of tumor necrosis factor blockers
(6), radiotherapy (7), ultrasound combined exercise therapy
(8) and surgical treatment
(9). Furthermore, microRNAs
(miRNAs/miRs) play a key role in regulating the immune function and
autoimmunity (10). With the
development of molecular targeting technology, research on miRNAs
is of great interest for the treatment of AS.
miRNAs play a significant role in AS pathology by
targeting the inflammation and bone remodeling genes (11). Notably, miR-204 regulates the
transformation of mesenchymal stem cells into adipose and
osteoblast cell lines (12).
miR-204 is involved in the development of several diseases. For
example, miR-204-5p plays a therapeutic role in aplastic
anemic rats via the NF-kB signaling pathway (13), which is a target for AS treatment
(14). Furthermore, the maintain
bone morphogenetic protein (BMP)/SMAD (15), Wnt/β-catenin (16) and Notch (17) signaling pathways are involved in
the process of AS. Specifically, the Notch2 signaling pathway is
required to promote cell proliferation and maintain BMP signaling
(18). There is a positive
regulatory association between the Notch and NF-κB signaling
pathways (19). However, whether
miR-204-5p is involved in the regulation of the Notch
signaling pathway, and whether it has an impact on osteogenic
differentiation of AS fibroblasts have not yet been fully
investigated.
BMP-2 is a member of the transforming growth
factor-β superfamily that is synthesized and secreted by
osteoblasts (20). BMP-2 is
considered a common osteogenic agent, which can induce
undifferentiated mesenchymal cells into cartilage and bone tissues
(21). A previous study
demonstrated that BMP-2 facilitates the osteogenic differentiation
of bone marrow-derived mesenchymal stem cells by inducing alkaline
phosphatase (ALP) activity, promoting mineralization, enhancing
adherence and mediating the expression and activation of osteogenic
markers (22).
In the current study mRNA expression was detected
using reverse transcription-quantitative PCR (RT-qPCR). The binding
site between Notch2 and miR-204-5p was predicted using
TargetScan software and assessed via the dual-luciferase reporter
assay. Moreover, ALP activity was assessed via the ALP assay, while
the mineralized nodules area was determined via the Alizarin Red S
staining assay. In addition, BMP-2 was used to induce osteogenic
differentiation of AS fibroblasts, and the regulatory role of
miR-204-5p on the osteogenic differentiation of AS
fibroblasts, and the underlying molecular mechanism involving the
Notch signaling pathway were assessed. Taken together, the results
of the present study provide a theoretical basis for the treatment
of patients with AS.
Materials and methods
Primary culture of ligament
fibroblasts
A total of 20 patients with AS (20 men; age, 25–39
years; mean age, 30.2 years) who underwent surgical intervention at
Shouguang People's Hospital between January 2016 and January 2018
were recruited in the present study. The bioptic tissues were
collected from the 20 patients with AS. All patients were in the
active stage, exhibiting inflammatory low back pain, notable
ossification of the ankle joint, positive histocompatibility
leukocyte antigen (HLA)-B27, and elevated levels of C-reactive
protein and erythrocyte sedimentation rate (ESR). All patients met
the New York Standard of the American College of Rheumatology
revised in 1984 (23).
A total of 20 patients (20 men; age, 26–43 years;
mean age, 31.5 years) who underwent hip arthroplasty for femoral
neck fracture (excluding other types of osteoarthritis) between
January 2017 and October 2017 were recruited as the control group
in the present study. The hip ligament tissues were washed with
physiological saline, immediately frozen in liquid nitrogen and
stored at −80°C until further experimentation. The present study
was approved by the Ethics Committee of Shouguang People's Hospital
(approval no. SGSRMXY-2020-09) and written informed consent was
provided by all patients prior to the study start.
The hip ligament tissues of patients with AS were
rinsed three times with PBS supplemented with 300 U/ml penicillin
and 300 µg/ml streptomycin (all Gibco; Thermo Fisher Scientific,
Inc.). The ligament tissues were subsequently cut into
1-mm3-thick sections using ophthalmic scissors, and
added into plates containing 5 ml serum-free DMEM medium and 0.2
µg/ml type I collagenase (all Invitrogen; Thermo Fisher Scientific,
Inc.). The collagen fibers were removed by filtration at 1,000
r/min, through a 0.22 µm filter (EMD Millipore). The precipitated
cells were cultured in DMEM medium supplemented with 20% serum and
1% streptomycin, at 37°C in 5% CO2 for 72 h.
Osteogenic differentiation of ligament
fibroblasts
The osteogenic differentiation of ligament
fibroblasts was induced by BMP-2 as previously described (24–26).
Cells were divided into the following groups: AS group, AS + BMP-2
group, AS + BMP-2 + miR-negative control (NC) group, AS + BMP-2 +
miR-204-5p mimics group and AS + BMP-2 + miR-204-5p
mimics + pcDNA-Notch2 group. Cells were transfected with 50 nmol/l
miR-204-5p mimics, miR-NC, pcDNA-Notch2 or pcDNA-NC
(Shanghai GenePharma Co., Ltd.), using Lipofectamine®
2000 transfection reagent (Thermo Fisher Scientific, Inc.). The
subsequent experiments were performed at 24 h post-transfection.
Subsequently, cells were cultured in DMEM/H containing 10% fetal
bovine serum, 0.05 mM vitamin C and 100 mM dexamethason (all Gibco;
Thermo Fisher Scientific, Inc.). BMP-2 (200 ng/ml; Sigma-Aldrich;
Merck KGaA) was added to all medium except the AS group. All cells
were cultured in 5% CO2 at 37°C and induced for 14
days.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the hip ligament
tissues and ligament fibroblasts using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Synthesis of cDNA
using reverse transcriptase was performed with the PrimeScript RT
Enzyme Mix I kit (Takara Bio, Inc.). The reaction mixtures were
incubated at 37°C for 60 min, 95°C for 5 min and then held at 4°C.
A total of 5 µl diluted RNA (1:20) was used to determine the
concentration and purity of total RNA. miScript SYBR Green PCR kit
(Qiagen, Inc.) was used to conduct the qPCR analysis. RT-qPCR was
performed on an ABI7500 quantitative PCR machine (Thermo Fisher
Scientific, Inc.). U6 was used as the internal control for miRNAs,
and GAPDH served as the internal control for other genes. The
primer sequences (Guangzhou Ruibo Biotechnology Co., Ltd.) are
listed in Table I. The reaction
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 10 sec, 60°C for 20 sec and 72°C for 34 sec. Relative
expression levels were calculated using the 2−ΔΔCq
method (27).
 | Table I.Primer sequences used for
quantitative PCR. |
Table I.
Primer sequences used for
quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| miR-204-5p
(F) |
TTCCCTTTGTCATCCTATGCCT |
| miR-204-5p
(R) |
TGGTGTCGTGGAGTCG |
| U6 (F) |
GCTTCGGCAGCACATATACTAAAAT |
| U6 (R) |
CGCTTCACGAATTTGCGTGTCAT |
| Notch2 (F) |
CACAGGGTTCATAGCCATCTC |
| Notch2 (R) |
GGAGGCGACCGAGAAGAT |
| RUNX2 (F) |
AGCTTCTGTCTGTGCCTTCTGG |
| RUNX2 (R) |
GGAGTAGAGAGGCAAGAGTTT |
| Osteocalcin
(F) |
CTTTGTGTCCAAGCAGGA |
| Osteocalcin
(R) |
CTGAAAGCCGATGTGGTCAE |
| GAPDH (F) |
GAAGGTGAAGGTCGGAGTC |
| GAPDH (R) |
GAAGATGGTGATGGGATTTC |
ALP staining and calcium salt
deposition staining
After 7 days of culturing, cells (1×104
cells/well) from each group were collected and fixed. ALP activity
was assessed using the ALP activity assay kit (cat. no. A059-2-2;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocol. ALP activity was measured at a wavelength
of 520 nm, using a microplate reader (Molecular Devices LLC).
After 14 days of culturing, cells from each group
were collected and stained with 2% Alizarin Red staining solution
(pH 8.3; Nanjing KeyGen Biotech Co., Ltd.) at 37°C for 10 min. The
solution was discarded, cells were washed with PBS and subsequently
observed under a phase contrast microscope (light microscope), and
the mineralized nodules area was counted at five high-power fields
(magnification, ×100).
Western blotting
Ligament fibroblasts were lysed using RIPA lysate
(Beyotime Institute of Biotechnology) at 4°C for 30 min. The
supernatants were collected via centrifugation at 7,200 × g at 4°C
for 10 min. Total protein was quantified using the bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology) and 60 µg
protein/lane was separated via 10% separating gum and 5%
concentrating gum. The separated proteins were subsequently
transferred onto polyvinylidene difluoride membranes and blocked
with 5% skim milk for 1 h at 37°C. The membranes were incubated
with primary antibodies against: Notch2 (cat. no. ab8926),
runt-related transcription factor 2 (RUNX2; cat. no. ab23981),
osteocalcin (cat. no. ab93876), GAPDH (cat. no. ab9485) and rabbit
anti-human (all 1:5,000 and from Abcam) overnight at 4°C. Following
the primary incubation, membranes were incubated with horseradish
peroxidase-labeled goat-anti-rabbit IgG secondary antibody
(1:5,000; ca. no. ab6721; Abcam) for 1 h at 25°C. The protein blots
were visualized using an enhanced chemiluminescence kit
(Invitrogen; Thermo Fisher Scientific, Inc.). Protein bands were
assessed using a luminescent image analysis software (Quantity One
1-D Analysis software; version 4.6.9; Bio-Rad Laboratories, Inc.).
GAPDH was used as the internal control.
Dual-luciferase reporter assay
TargetScan software v3.0 (http://starbase.sysu.edu.cn.) was used to predict the
targeting relationship between miR-204-5 and Notch2. A
3′-untranslated region (UTR) wild type (WT) plasmid of Notch2
(Notch2-3′-UTR-WT) was constructed according to the 3′-UTR sequence
of Notch 2. Based on this plasmid, a binding site was mutated to
construct a 3′-UTR mutant (MUT) plasmid (Notch2-3′-UTR-MUT). The
construction and sequencing of the plasmids were performed by
Sangon Biotech Co., Ltd. Subsequently, the constructed luciferase
reporter plasmids, pmirGLO-Notch2-WT/pmirGLO-Notch2-MUT (Shanghai
GenePharma Co., Ltd.) and miR-204-5p mimics/miR-NC were
co-transfected into 293T cells (American Type Culture Collection)
using Lipofectamine® 2000 transfection reagent (Thermo
Fisher Scientific, Inc.). The luciferase activity was measured
using the dual luciferase activity assay kit (Thermo Fisher
Scientific, Inc.), 48 h post-transfection, and was normalized to
Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 21.0; IBM Corp.) and data are presented as the
mean ± standard deviation. All experiments were repeated three
times. Unpaired Student's t-test was used to compare differences
between two groups. One-way analysis of variance followed by
Tukey's post hoc test was used to compare differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Downregulation of miR-204-5p in hip
capsules of patients with AS
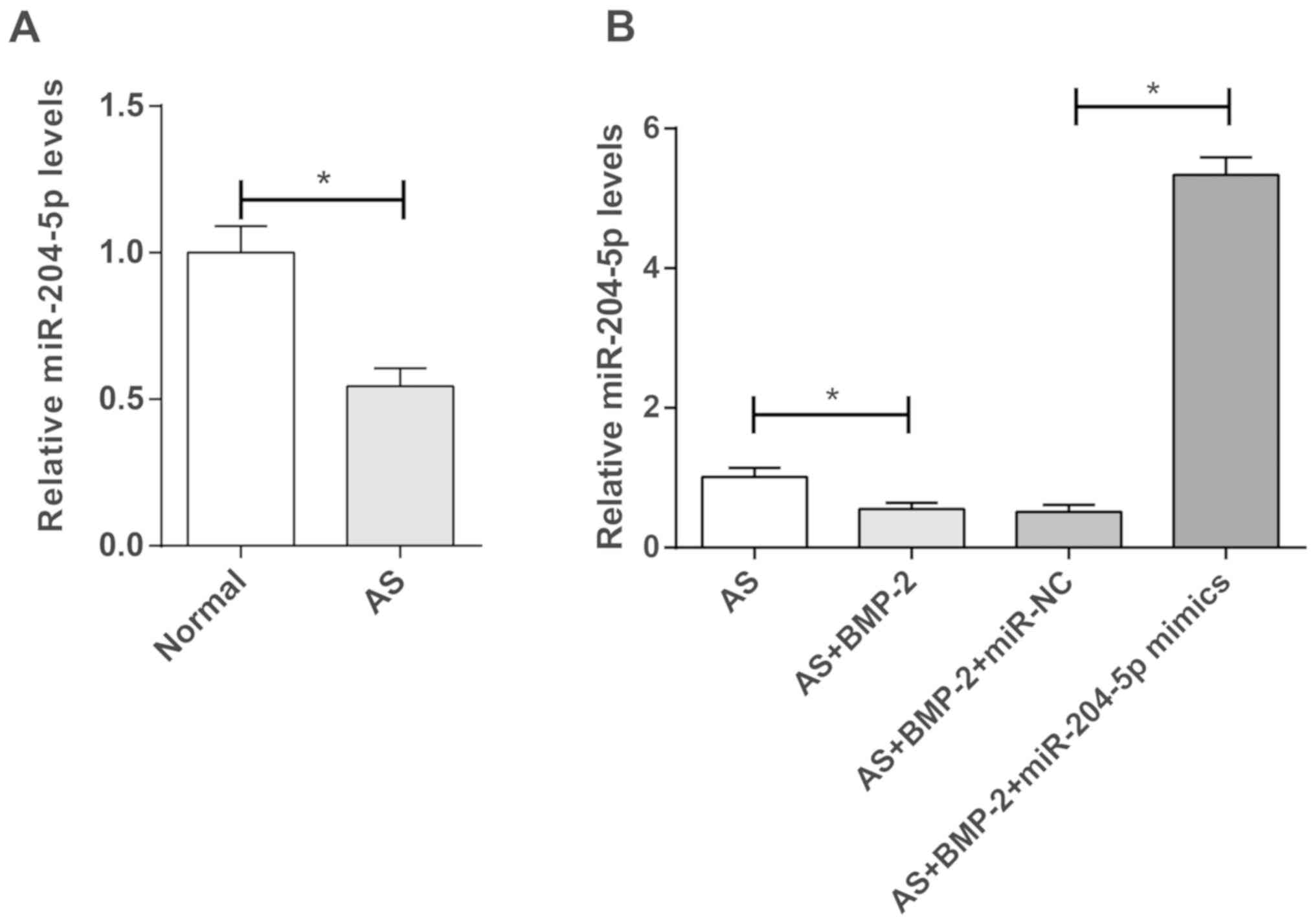
miR-204-5p expression was significantly lower
in the hip joint capsules of patients with AS than in patients with
femoral neck fracture (P<0.05; Fig.
1A). Additionally, miR-204-5p expression was
significantly decreased in BMP-2-induced AS cells compared with
untreated-cells (P<0.05; Fig.
1B). Furthermore, transfection of miR-204-5p mimics
significantly increased miR-204-5p expression in
BMP-2-induced AS cells (P<0.05; Fig. 1B).
Upregulation of Notch2 expression in
hip capsules of patients with AS
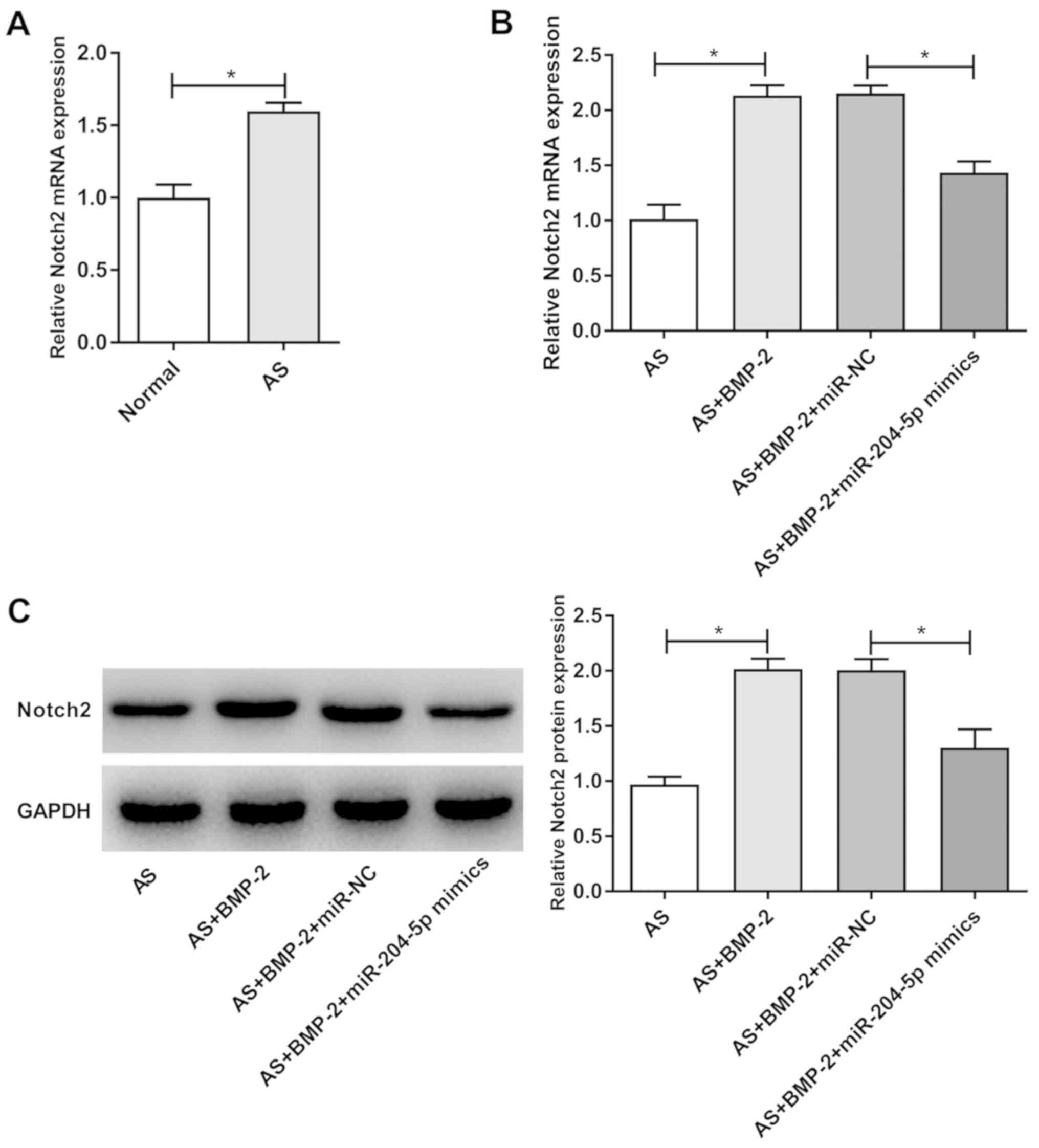
Notch2 mRNA expression was significantly higher in
the hip joint capsules of patients with AS than that in patients
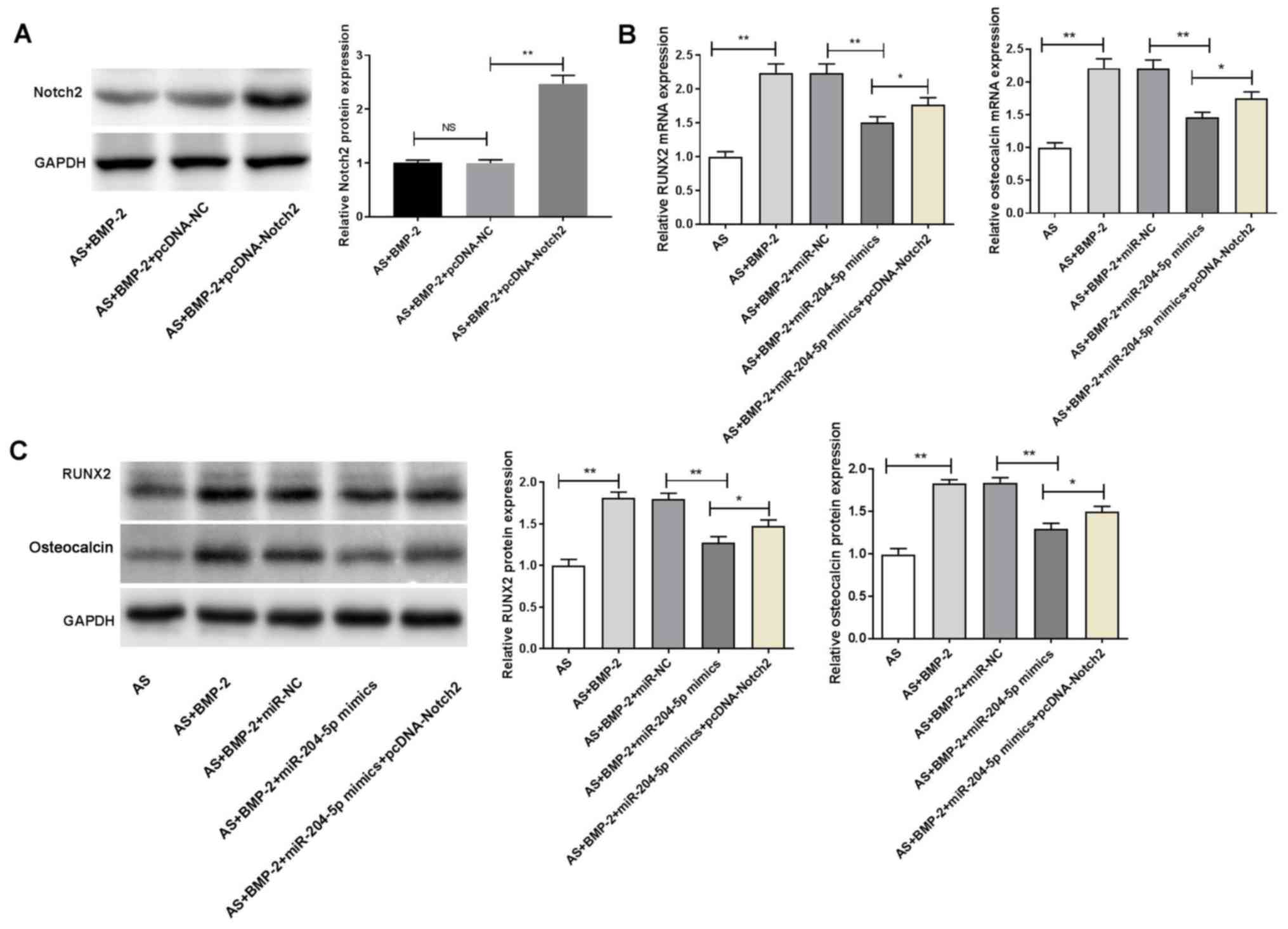
with femoral neck fracture (P<0.05; Fig. 2A). Notch2 expression was
significantly higher in the AS + BMP-2 group compared with the AS
group, at both the mRNA and protein levels (P<0.05; Fig. 2B and C). Furthermore, the mRNA and
protein levels of Notch2 were significantly decreased in the AS +
BMP-2 + miR-204-5p mimics group compared with those in the
AS + BMP-2 + miR-NC group (P<0.05; Fig. 2B and C).
Notch2 is a target gene of
miR-204-5p
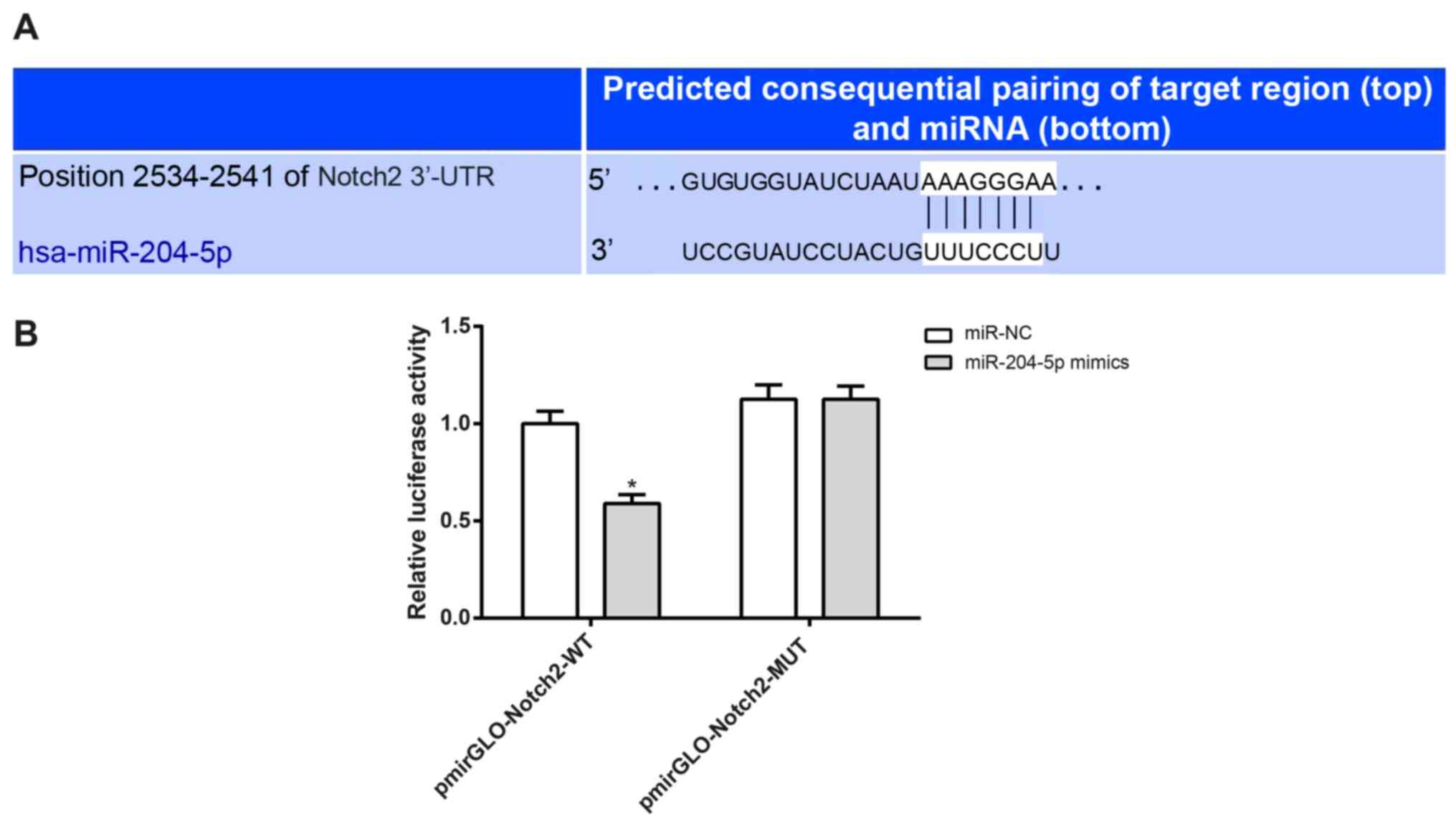
The binding site for Notch2 and miR-204-5p
was predicted using TargetScan software (Fig. 3A). The luciferase activity of cells
co-transfected with miR-204-5p mimics and pmirGLO-Notch2-WT
was significantly lower than those co-transfected with
miR-204-5p mimics and pmirGLO-Notch2-MUT (P<0.05;
Fig. 3B).
miR-204-5p inhibits osteogenic
differentiation of ligament fibroblasts by targeting Notch2
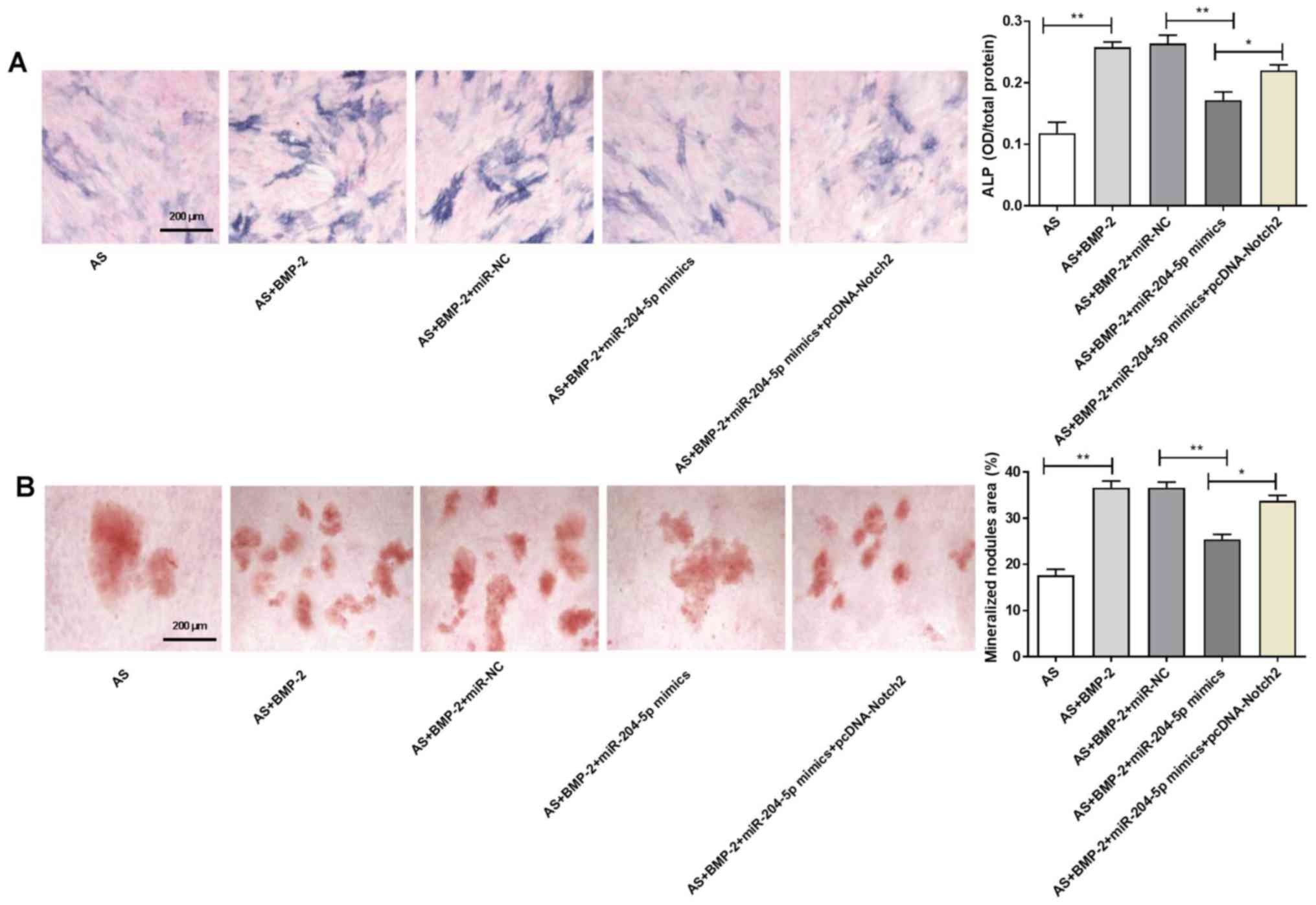
The ALP activity of the AS + BMP-2 group was higher
than that in the AS group (P<0.01; Fig. 4A). Furthermore, the ALP activity in
the AS + BMP-2 + miR-204-5p mimics group was significantly lower
than that in the AS + BMP-2 + miR-NC group (P<0.01; Fig. 4A). Notably, transfection with
pcDNA-Notch2 significantly reversed the inhibitory effect induced
by miR-204-5p mimics on the ALP activity of ligament fibroblasts
(P<0.05; Fig. 4A).
The mineralized nodules area in the AS + BMP-2 group
was significantly increased compared with the AS group (P<0.01;
Fig. 4B). Furthermore, the
mineralized nodules area in the AS + BMP-2 + miR-204-5p
mimics group was significantly decreased compared with the AS +
BMP-2 + miR-NC group (P<0.01; Fig.
4B). Notably, transfection with pcDNA-Notch2 significantly
reversed the inhibitory effect induced by miR-204-5p mimics on the
mineralized nodules area of ligament fibroblasts (P<0.05;
Fig. 4B).
miR-204-5p inhibits the expression of
RUNX2 and osteocalcin by targeting Notch2
Transfection with pcDNA-Notch2 significantly
increased Notch2 protein expression in ligament fibroblasts
(P<0.01; Fig. 5A). The
expression of RUNX2 and osteocalcin in the AS + BMP-2 group were
significantly increased compared with the AS group, at both the
mRNA and protein levels (P<0.01; Fig. 5B and C). Furthermore, the
expression of RUNX2 and osteocalcin in the AS + BMP-2 +
miR-204-5p mimics group were significantly decreased
compared with the AS + BMP-2 + miR-NC group, at both the mRNA and
protein levels (P<0.01; Fig. 5B and
C). Notably, transfection with pcDNA-Notch2 significantly
reversed the inhibitory effect induced by miR-204-5p mimics on the
expression of RUNX2 and osteocalcin in ligament fibroblasts
(P<0.05; Fig. 5B and C).
Discussion
AS is an autoimmune disease characterized by
fibroblast ossification (28).
Notably, inhibition of the ossification of AS fibroblasts is a
common treatment for patients with AS (28). The present study aimed to determine
whether miR-204-5p regulates the Notch signaling pathway,
and subsequently affects the osteogenic differentiation of AS
fibroblasts. The results demonstrated that miR-204-5p
expression decreased in the hip capsule tissues of patients with
AS, and Notch2 was identified as the target gene of
miR-204-5p. Furthermore, miR-204-5p inhibited the
osteogenic differentiation of AS fibroblasts by downregulating the
expression of Notch2, RUNX2 and osteocalcin. Heterotopic
ossification is one of the most prominent features of AS (29), and osteogenic differentiation of
fibroblasts plays a key role in the heterotopic ossification of AS
(30). miRNAs play important roles
in regulating cell-cell interactions between osteoclasts and
fibroblasts (31). For example,
miR-204-5p is involved in the adjustability of adipogenesis
and osteogenic differentiation of bone marrow stem cells (32). Zhang et al (33) reported that downregulating
miR-204-5p expression increases RUNX2 expression and
promotes osteoblast proliferation. Consistent with previous
findings, the results of the present study demonstrated the
overexpression of miR-204-5p inhibited RUNX2 expression,
thereby inhibiting osteogenic differentiation of fibroblasts. In
addition, overexpression of miR-204 has been reported to promote
adipocyte differentiation and inhibit osteogenic differentiation,
while miR-204 knockdown exerts the opposite effects (34). Taken together, these results
suggest that miR-204-5p inhibits osteogenic differentiation,
and thus can be used to treat patients with AS.
The results of the present study demonstrated that
miR-204-5p inhibited the osteogenic differentiation of
fibroblasts by targeting Notch2. Lee et al (35) and Cai et al (36) have reported that Notch2 is a target
gene of miR-204-5p. In addition, Notch family members and
their ligands are involved in the formation of articular cartilage
at different locations, and the coordination of the ossification
and extension of growth plates (37). Notably, the Notch signaling pathway
significantly enhances BMP-2-induced osteogenesis of embryonic
fibroblasts (38). BMP-2 is a
well-known bone formation stimulating factor (39). However, downregulation of miR-204
expression by BMP-2 increases RUNX2 expression and enhances
osteogenic differentiation (40).
miR-204-5p also functions in inhibiting the osteogenic
differentiation of AS fibroblasts by targeting RUNX2 (41).
RUNX2 and osteocalcin are key factors involved in
the bone-repair process (42). The
level of RUNX2 mRNA is higher in patients with AS than that in
healthy controls (43). RUNX2
controls the differentiation and formation of osteoblasts by
upregulating the transcription of the BMP-2 gene to differentiate
osteoblast precursors into osteocytes (44). Furthermore, suppressing RUNX2 can
initiate osteogenic differentiation, which participates in the
anti-osteogenic differentiation of AS fibroblasts (45). The results of the present study
indicated that miR-204-5p inhibited the osteogenic
differentiation of fibroblasts by inhibiting RUNX2 expression. Yu
et al (41) demonstrated
that miR-204-5p positively regulates RUNX2 expression to
promote osteogenic differentiation of calcific aortic valve
disease. Conversely, Wang et al (46) reported that miR-204 inhibits
RUNX2 expression and plays a negative role in regulating osteogenic
differentiation. These previous findings suggest that the
inhibition of RUNX2 expression contributes to the inhibitory effect
induced by miR-204-5p on osteogenic differentiation.
Osteocalcin is the principle non-collagen component
of the bone, which is considered a specific indicator of bone
formation (47). Osteocalcin
expression is notably higher in patients with AS than that in the
control group (48). Furthermore,
osteocalcin expression is significantly higher in patients with
ankle stiffness and hip involvement than that in healthy controls
(49). In the current study,
osteocalcin expression was decreased in AS. miR-204-5p
controls the osteogenic differentiation of fibroblasts by
inhibiting osteocalcin expression (31). Thus, when miR-204-5p is
inhibited, osteocalcin expression increases (31). Additionally, the expression of
osteocalcin is downregulated by inhibiting RUNX2 expression and
disrupting the activation of RUNX2 (50). Taken together, these results
suggest that miR-204-5p is an important target to inhibit
osteogenic differentiation through inhibiting the expression of
RUNX2 and osteocalcin.
The current study had some limitations. Firstly, a
relatively small number of studies have come from China, which
limited the ability to identify the relationships between the
miR-204-5p and AS. Moreover, the mechanism of
miR-204-5p regulation on AS was only based on the
experiments in vitro, and thus requires further
investigation in vivo. In addition, the detailed mechanisms
of action of miR-204-5p on AS are yet to be elucidated.
The present study investigated the osteogenic
differentiation of ligament fibroblasts from patients with AS. The
results demonstrated that miR-204-5p inhibited the
expression of RUNX2 and osteocalcin in AS ligament fibroblasts by
targeting Notch2, which provides a theoretical basis for the
effective treatment of AS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ: Substantial contributions to the conception and
design of the work. YZ: Substantial contributions to acquisition of
data. BL: Substantial contributions to interpretation of data. JZ
and YZ: Performed the experiments. BL: Performed the data analysis.
JZ and YZ: Drafting the manuscript and revising it critically for
important intellectual content. BL: Revised the manuscript for
critically important intellectual content. JZ, YZ and BL: Final
approval of the version to be published. JZ, YZ and BL: Agreement
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shouguang People's Hospital (Shouguang, China;
approval no. SGSRMXY-2020-09) and written informed consent was
provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robinson PC, Leo PJ, Pointon JJ, Harris J,
Cremin K, Bradbury LA, Stebbings S, Harrison AA; Australian
Osteoporosis Genetics Consortium; Wellcome Trust Case Control
Consortium, ; et al: Exome-wide study of ankylosing spondylitis
demonstrates additional shared genetic background with inflammatory
bowel disease. NPJ Genom Med. 1:160082016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao J, Huang C, Huang H, Pan JK, Zeng LF,
Luo MH, Liang GH, Yang WY and Liu J: Prevalence of ankylosing
spondylitis in a Chinese population: A systematic review and
meta-analysis. Rheumatol Int. 40:859–872. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raychaudhuri SP and Deodhar A: The
classification and diagnostic criteria of ankylosing spondylitis. J
Autoimmun. 48:128–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahsan T, Erum U, Jabeen R and Khowaja D:
Ankylosing Spondylitis: A rheumatology clinic experience. Pak J Med
Sci. 32:365–368. 2016.PubMed/NCBI
|
|
5
|
Dean LE, Jones GT, MacDonald AG, Downham
C, Sturrock RD and Macfarlane GJ: Global prevalence of ankylosing
spondylitis. Rheumatology (Oxford). 53:650–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jethwa H and Bowness P: The interleukin
(IL)-23/IL-17 axis in ankylosing spondylitis: New advances and
potentials for treatment. Clin Exp Immunol. 183:30–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darby SC, Doll R, Gill SK and Smith PG:
Long term mortality after a single treatment course with X-rays in
patients treated for ankylosing spondylitis. Br J Cancer.
55:179–190. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Şilte Karamanlioğlu D, Aktas I, Ozkan FU,
Kaysin M and Girgin N: Effectiveness of ultrasound treatment
applied with exercise therapy on patients with ankylosing
spondylitis: A double-blind, randomized, placebo-controlled trial.
Rheumatol Int. 36:653–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang T, Wang D, Cong Y, Yin C, Li S and
Chen X: Evaluating a posterior approach for surgical treatment of
thoracolumbar pseudarthrosis in Ankylosing Spondylitis. Clin Spine
Surg. 30:E13–E18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Wang L, Zhang X, Yang X, Li X, Xia
Q, Chen M, Han R, Liu R, Xu S and Pan F: Overexpression of miR-31
in Peripheral Blood Mononuclear Cells (PBMC) from patients with
Ankylosing Spondylitis. Med Sci Monit. 23:5488–5494. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perez-Sanchez C, Font-Ugalde P, Ruiz-Limon
P, Lopez-Pedrera C, Castro-Villegas MC, Abalos-Aguilera MC,
Barbarroja N, Arias-de la Rosa I, Lopez-Montilla MD,
Escudero-Contreras A, et al: Circulating microRNAs as potential
biomarkers of disease activity and structural damage in Ankylosing
Spondylitis patients. Hum Mol Genet. 27:875–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He H, Chen K, Wang F, Zhao L, Wan X, Wang
L and Mo Z: miR-204-5p promotes the adipogenic differentiation of
human adipose-derived mesenchymal stem cells by modulating DVL3
expression and suppressing Wnt/β-catenin signaling. Int J Mol Med.
35:1587–1595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Niu ZY, Guo YJ, Wang LH, Lin FR
and Zhang JY: IL-11 promotes the treatment efficacy of
hematopoietic stem cell transplant therapy in aplastic anemia model
mice through a NF-κB/microRNA-204/thrombopoietin regulatory axis.
Exp Mol Med. 49:e4102017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roozbehkia M, Mahmoudi M, Aletaha S,
Rezaei N, Fattahi MJ, Jafarnezhad-Ansariha F, Barati A and
Mirshafiey A: The potent suppressive effect of β-d-mannuronic acid
(M2000) on molecular expression of the TLR/NF-kB Signaling Pathway
in ankylosing spondylitis patients. Int Immunopharmacol.
52:191–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, Cai J, Zhang J and Li C: Mechanism
of triptolide in treating ankylosing spondylitis through the
anti-ossification effect of the BMP/Smad signaling pathway. Mol Med
Rep. 17:2731–2737. 2018.PubMed/NCBI
|
|
16
|
Zou Y, Yang X, Yuan S, Zhang P, Ye Y and
Li Y: Downregulation of dickkopf-1 enhances the proliferation and
osteogenic potential of fibroblasts isolated from ankylosing
spondylitis patients via the Wnt/β-catenin signaling pathway in
vitro. Connect Tissue Res. 57:200–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu W, Liang CG, Li YF, Ji YH, Qiu WJ and
Tang XZ: Involvement of Notch1/Hes signaling pathway in ankylosing
spondylitis. Int J Clin Exp Pathol. 8:2737–2745. 2015.PubMed/NCBI
|
|
18
|
Zhou Y, Tanzie C, Yan Z, Chen S, Duncan M,
Gaudenz K, Li H, Seidel C, Lewis B, Moran A, et al: Notch2
regulates BMP signaling and epithelial morphogenesis in the ciliary
body of the mouse eye. Proc Natl Acad Sci USA. 110:8966–8971. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruan ZB, Fu XL, Li W, Ye J, Wang RZ and
Zhu L: Effect of notch1,2,3 genes silicing on NF-κB signaling
pathway of macrophages in patients with atherosclerosis. Biomed
Pharmacother. 84:666–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith DM, Cooper GM, Mooney MP, Marra KG
and Losee JE: Bone morphogenetic protein 2 therapy for craniofacial
surgery. J Craniofac Surg. 19:1244–1259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khosla S, Westendorf JJ and Oursler MJ:
Building bone to reverse osteoporosis and repair fractures. J Clin
Invest. 118:421–428. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Li J, Li C and Yu Y: Role of bone
morphogenetic protein-2 in osteogenic differentiation of
mesenchymal stem cells. Mol Med Rep. 12:4230–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Linden SM, Valkenburg HA, de Jongh
BM and Cats A: The risk of developing ankylosing spondylitis in
HLA-B27 positive individuals. A Comparison of Relatives of
Spondylitis patients with the general population. Arthritis Rheum.
27:241–249. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hupkes M, Sotoca AM, Hendriks JM, van
Zoelen EJ and Dechering KJ: MicroRNA miR-378 promotes BMP2-induced
osteogenic differentiation of mesenchymal progenitor cells. BMC Mol
Biol. 15:12014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanayama S, Kaito T, Kitaguchi K, Ishiguro
H, Hashimoto K, Chijimatsu R, Otsuru S, Takenaka S, Makino T, Sakai
Y, et al: ONO-1301 Enhances in vitro osteoblast differentiation and
in vivo bone formation induced by bone morphogenetic protein. Spine
(Phila Pa 1976). 43:E616–E624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung JI, Park KY, Lee Y, Park M and Kim J:
Vitamin C-linker-conjugated tripeptide AHK stimulates BMP-2-induced
osteogenic differentiation of mouse myoblast C2C12 cells.
Differentiation. 101:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qin X, Jiang T, Liu S, Tan J, Wu H, Zheng
L and Zhao J: Effect of metformin on ossification and inflammation
of fibroblasts in ankylosing spondylitis: An in vitro study. J Cell
Biochem. 119:1074–1082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou YC, Yang XW, Yuan SG, Zhang P and Li
YK: Celastrol inhibits prostaglandin E2-induced proliferation and
osteogenic differentiation of fibroblasts isolated from ankylosing
spondylitis hip tissues in vitro. Drug Des Devel Ther. 10:933–948.
2016.PubMed/NCBI
|
|
30
|
Zhou YY, Huang RY, Lin JH, Xu YY, He XH
and He YT: Bushen-Qiangdu-Zhilv decoction inhibits osteogenic
differentiation of rat fibroblasts by regulating connexin 43. Exp
Ther Med. 12:347–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu F, Cui Y, Zhou X and Han J: Osteogenic
differentiation of human ligament fibroblasts induced by
conditioned medium of osteoclast-like cells. Biosci Trends.
5:46–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shang G, Wang Y, Xu Y, Zhang S, Sun X,
Guan H, Zhao X, Wang Y, Li Y and Zhao G: Long non-coding RNA
TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of
rat bone marrow mesenchymal stem cell by targeting miR-204-5p and
miR-125a-3p. J Cell Physiol. 233:6041–6051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YY, Zhou JB, Zeng XW, Zhao FM and
Zhan XQ: Effects of puerarin on proliferation of osteoblasts and
Runx2-targeting miRNAs. Chinese Pharmacological Bulletin.
32:1457–1462. 2016.
|
|
34
|
Zhao J, Wang C, Song Y and Fang B: Arsenic
trioxide and microRNA-204 display contrary effects on regulating
adipogenic and osteogenic differentiation of mesenchymal stem cells
in aplastic anemia. Acta Biochim Biophys Sin (Shanghai).
46:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee H, Kim KR, Cho NH, Hong SR, Jeong H,
Kwon SY, Park KH, An HJ, Kim TH, Kim I, et al: MicroRNA expression
profiling and Notch1 and Notch2 expression in minimal deviation
adenocarcinoma of uterine cervix. World J Surg Oncol. 12:3342014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai B, Zheng Y, Ma S, Xing Q, Wang X, Yang
B, Yin G and Guan F: BANCR contributes to the growth and invasion
of melanoma by functioning as a competing endogenous RNA to
upregulate Notch2 expression by sponging miR-204. Int J Oncol.
51:1941–1951. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayes AJ, Dowthwaite GP, Webster SV and
Archer CW: The distribution of Notch receptors and their ligands
during articular cartilage development. J Anat. 202:495–502. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei Y, Mou D, Lian J, Luo J and Tang M:
Role of Notch signaling in BMP2-induced osteogenic differentiation
of MEFs and its mechanism. Chin J Cell Biol. 40:478–489. 2018.
|
|
39
|
Wegman F, Bijenhof A, Schuijff L, Oner FC,
Dhert WJ and Alblas J: Osteogenic differentiation as a result of
BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur Cell
Mater. 21:230–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song R, Fullerton DA, Ao L, Zhao KS and
Meng X: An epigenetic regulatory loop controls pro-osteogenic
activation by TGF-β1 or bone morphogenetic protein 2 in human
aortic valve interstitial cells. J Biol Chem. 292:8657–8666. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu C, Li L, Xie F, Guo S, Liu F, Dong N
and Wang Y: LncRNA TUG1 sponges miR-204-5p to promote osteoblast
differentiation through upregulating Runx2 in aortic valve
calcification. Cardiovasc Res. 114:168–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Yao X, Yan G, Xu Y, Yan J, Zou W
and Wang G: Mediator MED23 cooperates with RUNX2 to drive
osteoblast differentiation and bone development. Nat Commun.
7:111492016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang J, Song G, Yin Z, Fu Z and Ye Z:
MiR-29a and messenger RNA expression of bone turnover markers in
canonical Wnt pathway in patients with ankylosing spondylitis. Clin
Lab. 63:955–960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang LQ, Dong CJ and Zhu S:
Osteogenesis-related factor Runx2 expression in necrotic femoral
head tissue: Study protocol for a non-randomized,
parallel-controlled trial. Chinese Journal of Tissue Engineering
Research. 2016.
|
|
45
|
Zhou YY, Liu HX, Jiang N, Feng XH, Feng
XY, Zhang HQ, Wu ZK, Liang HY, Jiang Q and Chen P: Elemene, the
essential oil of Curcuma wenyujin, inhibits osteogenic
differentiation in ankylosing spondylitis. Joint Bone Spine.
82:100–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Chen S, Deng C, Li F, Wang Y, Hu
X, Shi F and Dong N: MicroRNA-204 targets Runx2 to Attenuate
BMP-2-induced osteoblast differentiation of human aortic valve
interstitial cells. J Cardiovasc Pharmacol. 66:63–71. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Franck H and Keck E: Serum osteocalcin and
vitamin D metabolites in patients with ankylosing spondylitis. Ann
Rheum Dis. 52:343–346. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kwon SR, Lim MJ, Suh CH, Park SG, Hong YS,
Yoon BY, Kim HA, Choi HJ and Park W: Dickkopf-1 level is lower in
patients with ankylosing spondylitis than in healthy people and is
not influenced by anti-tumor necrosis factor therapy. Rheumatol
Int. 32:2523–2527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Solmaz D, Bulbul H, Uslu S, Kozaci LD,
Karaca N and Akar S: AB0157 serum level of the vascular endothelial
growth factor is elevated in Ankylosing Spondylitis and Osteocalcin
may be related with osteoproliferation. BMJ. 74 (Suppl
2):AB01572015.
|
|
50
|
Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS,
Park SW, Kim SY and Shin CS: Activation of peroxisome
proliferator-activated receptor-gamma inhibits the Runx2-mediated
transcription of osteocalcin in osteoblasts. J Biol Chem.
278:23270–23277. 2003. View Article : Google Scholar : PubMed/NCBI
|