Introduction
Hypertrophic scar (HS) is a well-known complication
of skin injury, which is commonly observed after wound healing of
human skin caused by burns, lacerations and surgery (1,2). A
previous study reported that >90% of burn injuries and >40%
of surgical damages lead to HS, which has become a rising health
problem worldwide (3). Currently,
there are several therapies used to treat HS, mainly including
surgery, silicone gel and laser, but no treatment has proven to be
optimal, primarily due to the limited understanding of the precise
underlying mechanisms of HS (4,5).
Therefore, it is of high importance and urgency to identify novel
and viable therapies to treat HS.
Previous studies have revealed that scar formation
is attributed to the abnormal proliferation and apoptotic
resistance of fibroblasts, as well as excessive deposition of
extracellular matrix proteins, including collagen (6–8). It
has been revealed that sphingosine kinases (Sphks) catalyze the
formation of sphingosine 1-phosphate, which could regulate fibrotic
events in various organs, including the lungs, liver, skin and
kidneys (9,10). Moreover, Sphks are ubiquitously
expressed and Sphk2 is one of the most common subtypes (11). A previous study reported that
silencing of Sphk2 could increase the proliferation of renal mouse
mesangial cells and fibroblasts (12). Emerging evidence also supports the
hypothesis that Sphk2 deficiency could inhibit collagen expression
and attenuate unilateral ureteral obstruction-induced mouse kidney
fibrosis by enhancing the expression of Smad7 (13). In addition, the Sphk2 inhibitor,
ABC294640, was reported to suppress proliferation and promote
apoptosis in a human skin squamous cell carcinoma cell line
(14). Furthermore, it has been
revealed that inhibition of Sphk2 can alleviate psoriasis-like skin
disease (15). However, the effect
of Sphk2 in HS formation remains unknown.
Therefore, the present study investigated the effect
of Sphk2 in scar formation and its underlying regulatory mechanisms
in human HS fibroblasts (HSF).
Materials and methods
Tissue samples
A total of 20 paired HS tissues (HS group) and
adjacent healthy skin tissues (healthy group) were collected from
20 patients who underwent plastic surgery from February 2017 to
March 2018 at The Second Affiliated Hospital, University of South
China (Table I). The size of the
tissue was 1×1×1 cm. The ages of all the patients ranged from 19–50
years. The specimens were selected according to the following
criteria: i) Skin or HS tissue specimens were identified by
clinicians, which was in accordance with a previous study (16); ii) patients with pituitary
diseases, adrenal diseases, infectious diseases, skin diseases,
local infections and ulcers were excluded from the study; and iii)
patients with scars who had undergone previous treatments were
excluded from the study (17). All
tissues were immediately immerged in liquid nitrogen (−196°C) after
surgery until further use. The present study was approved by the
Ethics Committee of The Second Affiliated Hospital, University of
South China. Written informed consent was provided from each
patient or their legal guardians.
 | Table I.Profile of each sample from each
volunteer. |
Table I.
Profile of each sample from each
volunteer.
| Sample | Sex | Age, years | Localization | Time after trauma
or burn, months |
|---|
| 1 | Female | 19 | Shoulder | 7 |
| 2 | Female | 21 | Chest | 10 |
| 3 | Male | 28 | Chest | 11 |
| 4 | Female | 39 | Back | 10 |
| 5 | Male | 50 | Shoulder | 8 |
| 6 | Male | 33 | Back | 9 |
| 7 | Male | 25 | Ear | 12 |
| 8 | Female | 31 | Trunk | 10 |
| 9 | Female | 44 | Forehead | 6 |
| 10 | Female | 38 | Chest | 11 |
| 11 | Male | 26 | Lower leg | 8 |
| 12 | Male | 40 | Back | 12 |
| 13 | Male | 41 | Shoulder | 7 |
| 14 | Female | 27 | Forehead | 8 |
| 15 | Female | 29 | Shoulder | 7 |
| 16 | Male | 37 | Nose | 7 |
| 17 | Male | 49 | Back | 12 |
| 18 | Female | 20 | Lower leg | 9 |
| 19 | Female | 32 | Shoulder | 9 |
| 20 | Male | 23 | Chest | 11 |
Cell culture
Human HSF were purchased from Shanghai Guandao
Biological Engineering Co., Ltd. (cat. no. c0618) and human
embryonic skin fibroblasts, CCC-ESF-1 (control), were purchased
from the Shanghai Zibo Biological Technology Co., Ltd. (cat no.
YB-aTcc-3084). Cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% ascorbic acid (Sigma-Aldrich; Merck
KGaA). All cells were incubated at 37°C in a humidified incubator
with 5% CO2.
Cell transfection
Prior to cell transfection, cells were seeded into
6-well plates (1×106 cells/well) and incubated at 37°C
for 24 h to reach 80% confluency. Subsequently, 100 nmol/l small
interfering (si)RNA-Sphk2 (5′-CAGGATTGCGCTCGCTTTCAT-3′), the
negative control (NC; Sphk2-NC; 5′-UUCUCCGAACGUGUCACGUTT-3′),
siRNA-Smad7 (5′-GGCTGGAGGTCATCTTCAA-3′), Smad7-NC
(5′-AATTGTCCGAACGTGTCACGT-3′), pcDNA3.1-Smad7 or empty pcDNA3.1
vector (pcDNA-NC) were transfected into HSF, which were all
synthesized by Shanghai GenePharma Co., Ltd.. To perform the cell
transfection experiments, Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used following the
manufacturer's instructions. Following 48 h of transfection at
37°C, the cells were collected for subsequent experimentation.
Immunohistochemical staining
The tissues were fixed with 10% formalin at room
temperature for 24 h and then embedded in paraffin at 62°C for 45
min. Paraffin-embedded specimens were cut into 4-µm thick sections,
deparaffinized and rehydrated with a graded ethanol and xylene
series at room temperature. Following blocking with 10% normal goat
serum (Wuhan Servicebio Technology Co., Ltd.) for 10 min at 37°C,
slides were incubated overnight (12 h) at 4°C with the following
primary antibodies: Anti-Sphk2 (ProteinTech Group, Inc.; cat. no.
17096-1-AP; 1:100) and anti-Smad7 antibody (Santa Cruz
Biotechnology, Inc.; cat. no. sc-101152; 1:1,000). Slides were then
incubated with horseradish peroxidase (HRP)-secondary antibodies
(Abcam; cat. nos. ab6721 or ab6728; 1:1,000) for 2 h at room
temperature, stained with diaminobenzidine (Beyotime Institute of
Biotechnology) at room temperature for 5 min, and counterstained
with 0.2% hematoxylin at room temperature for 1 min. A light
microscope (magnification, ×200; Carl Zeiss AG) was used to analyze
the degree of staining for each image. Brown cellular staining was
considered to indicate positive protein expression (18).
Immunofluorescence assay
Cells were washed with PBS, fixed with 4%
paraformaldehyde for 30 min at room temperature, permeabilized with
0.1% Triton X-100 and blocked with 5% BSA (Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature. Cells were then incubated with
an anti-collagen I primary antibody (Abcam; cat. no. ab34710;
1:1,000) overnight at 4°C. After washing with PBS, cells were
immersed in fluorescein isothiocyanate-conjugated goat anti-rabbit
secondary antibody (Boster Biological Technology; cat. no. BA1105;
1:10,000) at 37°C for 1 h. Nuclei were stained with DAPI (Roche
Diagnostics) in the dark at room temperature for 5 min, and a
fluorescence microscope (magnification, ×200; Nikon Corporation)
was used to obtain fluorescence images.
Cell proliferation assay
A Cell Counting Kit-8 assay (CCK-8; Shanghai Yi
Sheng Biotechnology Co., Ltd.) was used to analyze the ability of
cell proliferation, according to the manufacturer's instructions.
At 48 h after transfection, cells were seeded in 96-well plates
(1×104 cells/well). At 24, 48 and 72 h, 10 µl CCK-8
solution was added to each well. Following incubation at 37°C for 1
h, the optical density was measured at 450 nm on a microplate
reader.
Cell apoptosis assay
Following transfection for 48 h, HSF were subjected
to an apoptosis assay. HSF (8×105) were stained with
Annexin V-PE/7AAD for 15 min at room temperature using a cell
apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's instructions. Then, cell apoptosis
was analyzed using a BD FACSVerse™ flow cytometer (BD Biosciences).
Subsequently, the data were analyzed using FlowJo software (version
10; FlowJo LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). First-strand cDNA synthesis was conducted using a
Sensiscript RT kit (Takara Biotechnology Co., Ltd.) according to
the manufacturer's protocol at 37°C for 15 min and 85°C for 5 sec.
qPCR was performed using iTaq™ Universal SYBR® Green
Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 7 min; and 40 cycles of 95°C for 15 sec and 60°C for 30 sec;
and a final extension at 72°C for 30 sec. The following primers
were used: Sphk2 forward, 5′-CCAGTGTTGGAGAGCTGAAGGT-3′ and reverse,
5′-GTCCATTCATCTGCTGGTCCTC-3′; Smad7 forward,
5′-GCTATTCCAGAAGATGCTGTTC-3′ and reverse,
5′-GTTGCTGAGCTGTTCTGATTTG-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′, and reverse
5′-GGCTGTTGTCATACTTCTCATGG-3′. The 2−ΔΔCq method was
used for data analysis with normalization to GAPDH (19).
Western blot analysis
Proteins in tissues and cells were extracted using
protein lysis buffer (RIPA; Beyotime Institute of Biotechnology)
and the concentration was determined using a bicinchoninic acid
assay protein assay kit (Beyotime Institute of Biotechnology).
Equal amounts of protein (40 µg per lane) were loaded on 10%
SDS-PAGE and transferred onto a PVDF membrane (EMD Millipore). The
membranes were subsequently blocked with 5% skimmed milk for 1 h at
room temperature and incubated with primary antibodies overnight at
4°C. After washing three times with TBS-0.2% Tween-20, membranes
were probed with a goat anti-rabbit HRP-conjugated secondary
antibody (Cell Signaling Technology, Inc.; cat. no. 7074S; 1:3,000)
or horse anti-mouse HRP-conjugated secondary antibody (Cell
Signaling Technology, Inc.; cat. no. 7076S; 1:3,000) at room
temperature for 1 h. Proteins were visualized with an enhanced
chemiluminescence detection system (Applygen Technologies, Inc.)
and subsequently quantified using ImageJ software (version 1.52r,
National Institutes of Health). The protein expression of the bands
was normalized against the gray value of GAPDH. Anti-Smad7 antibody
(cat. no. sc-101152; 1:1,000) was purchased from Santa Cruz
Biotechnology, Inc. Anti-collagen I antibody (cat. no. ab34710;
1:1,000) was purchased from Abcam. Anti-Sphk2 (cat. no. 32346S;
1:1,000), anti-cyclin D1 (cat. no. 55506T; 1:1,000), anti-p21 (cat.
no. 2947T; 1:1,000), anti-Bax (cat. no. 5023T; 1:1,000), anti-Bcl-2
(cat. no. 4223T; 1:1,000), anti-cleaved caspase-3 (cat. no. 9661T;
1:1,000), anti-caspase-3 (cat. no. 14220T; 1:1,000),
anti-transforming growth factor (TGF)-β1 (cat. no. 3709S; 1:1,000),
anti-phosphorylated (p)-Smad2 (cat. no. 18338T; 1:1,000),
anti-p-Smad3 (cat. no. 9520T; 1:1,000), anti-Smad2 (cat. no. 5339T;
1:1,000), anti-Smad3 (cat. no. 9523T; 1:1,000) and anti-GAPDH (cat.
no. 5174T; 1:1,000) antibodies were obtained from Cell Signaling
Technology, Inc.
Statistical analysis
All results were obtained from ≥3 independent
experiments and all data were analyzed using SPSS 20.0 software
(SPSS, Inc.). Data are presented as the mean ± SD. Quantitative
data between two groups was analyzed using an unpaired Student's
t-test, and the comparisons among multiple groups were conducted
using a one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Sphk2 is upregulated and Smad7 is
downregulated in HS tissues and HSF
To investigate the effect of Sphk2 and Smad7 in scar
formation, HS tissues and adjacent healthy skin tissues were
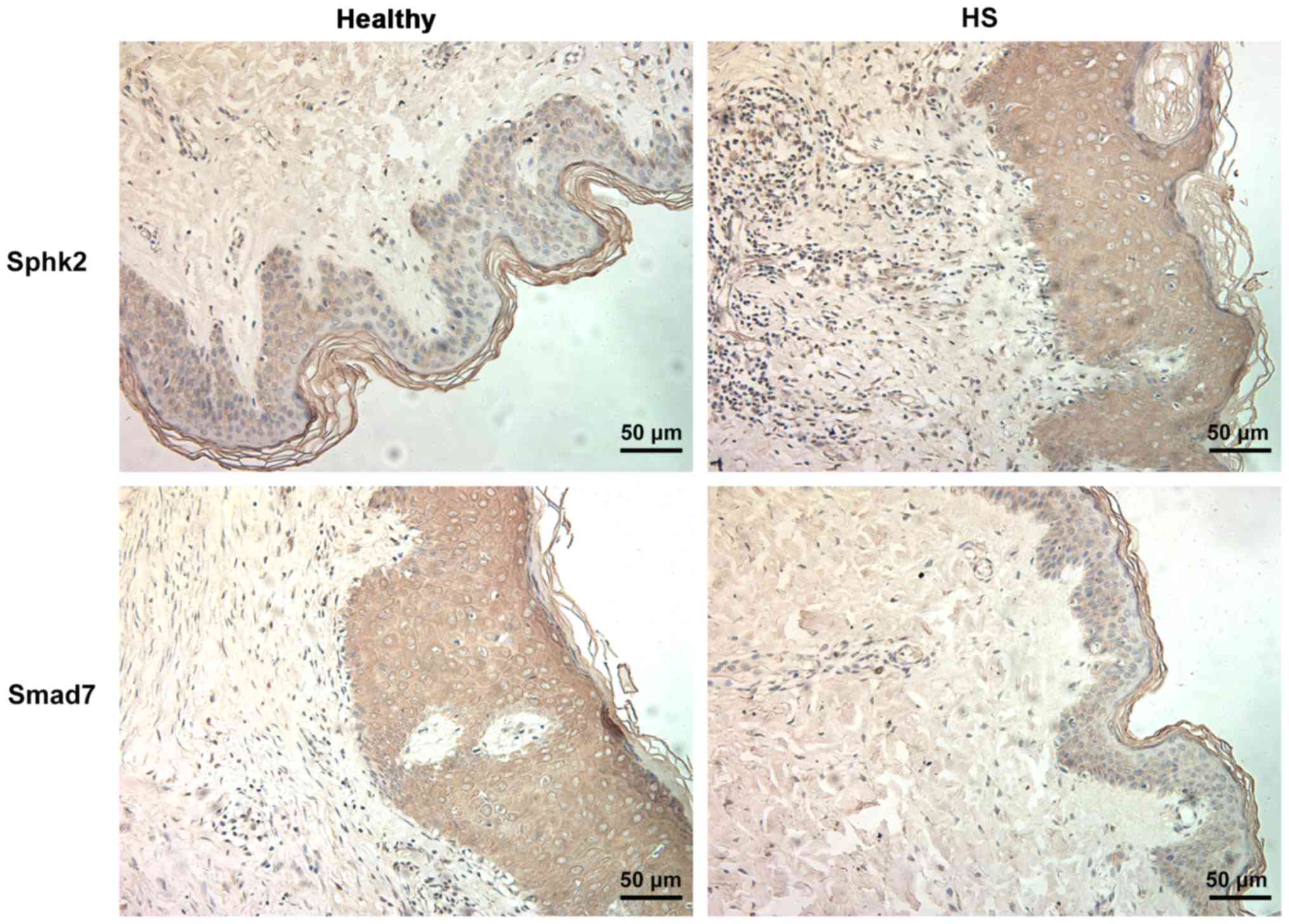
obtained to assess the expression levels Sphk2 and Smad7. It was
determined that the expression of Sphk2 was increased in the HS
group compared with the healthy group (Fig. 1). In addition, Smad7 expression was
downregulated in HS tissues.
To further assess the possible mechanisms of Sphk2
in scar formation, HSF and CCC-ESF-1 cells were used in the
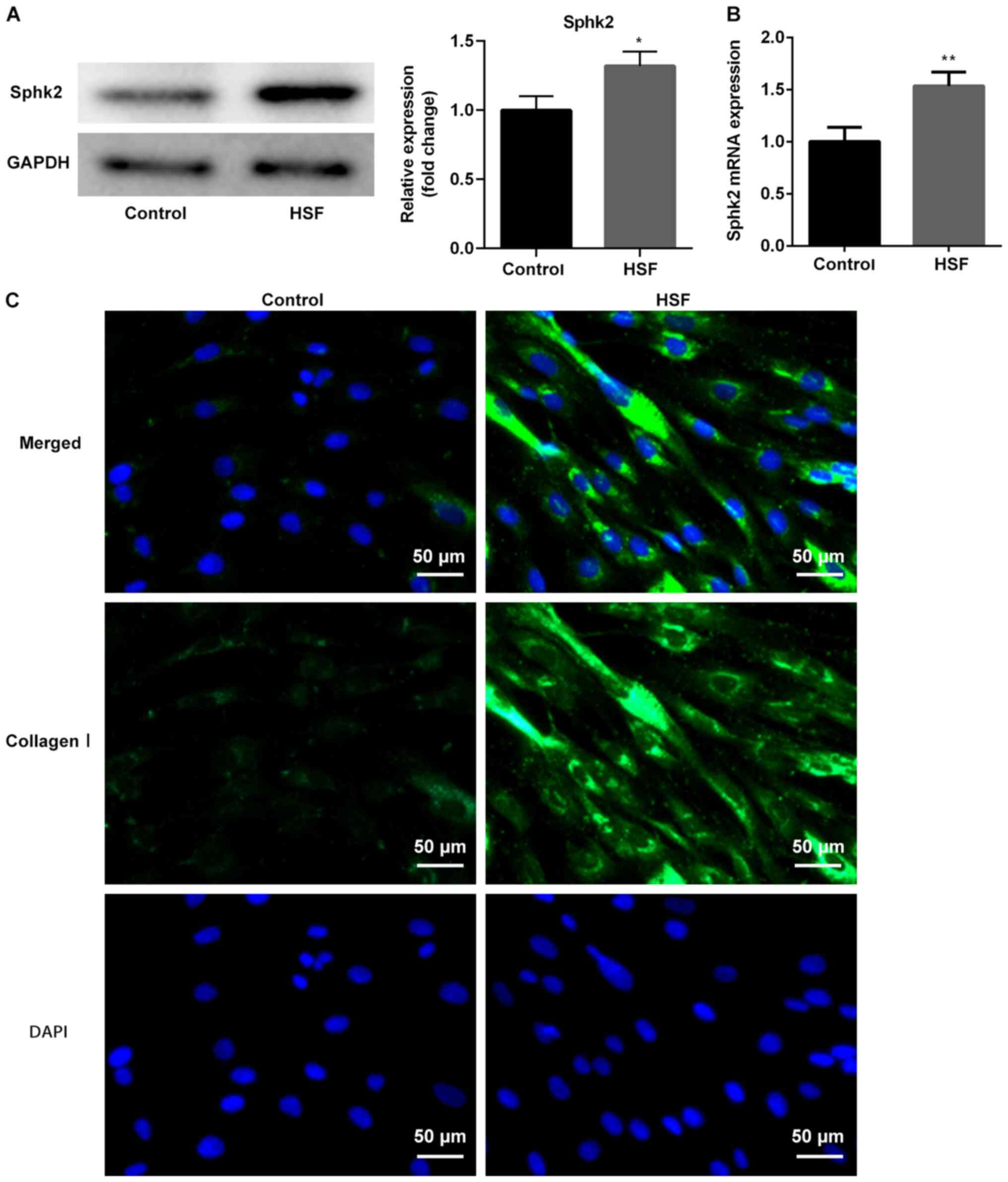
subsequent experiments. The results indicated that the protein and
mRNA expression levels of Sphk2, detected by western blot analysis
and RT-qPCR, respectively, were significantly increased in the HSF
group (Fig. 2A and B). In
addition, the immunofluorescence assay results revealed that the
protein expression of collagen I was increased in HSF compared with
the control group. Therefore, the results demonstrated that Sphk2
was upregulated, whereas Smad7 was downregulated, in HS tissues and
HSF, thus suggesting a potential regulatory effect between Sphk2
and Smad7.
Sphk2 silencing or Smad7
overexpression is successfully established in HSF
Sphk2-siRNA or Smad7-pcDNA were transfected into
HSF, and the transfection efficiency was determined using western
blot analysis and RT-qPCR. It was demonstrated that Sphk2-siRNA
transfection significantly downregulated the expression of Sphk2
(Fig. 3A and B). Moreover, the
expression of Smad7 was significantly upregulated in HSF
transfected with Smad7-pcDNA compared with the pcDNA-NC group
(Fig. 3C and D). Thus, the results
indicated that Sphk2 silencing or Smad7 overexpression were
successfully established.
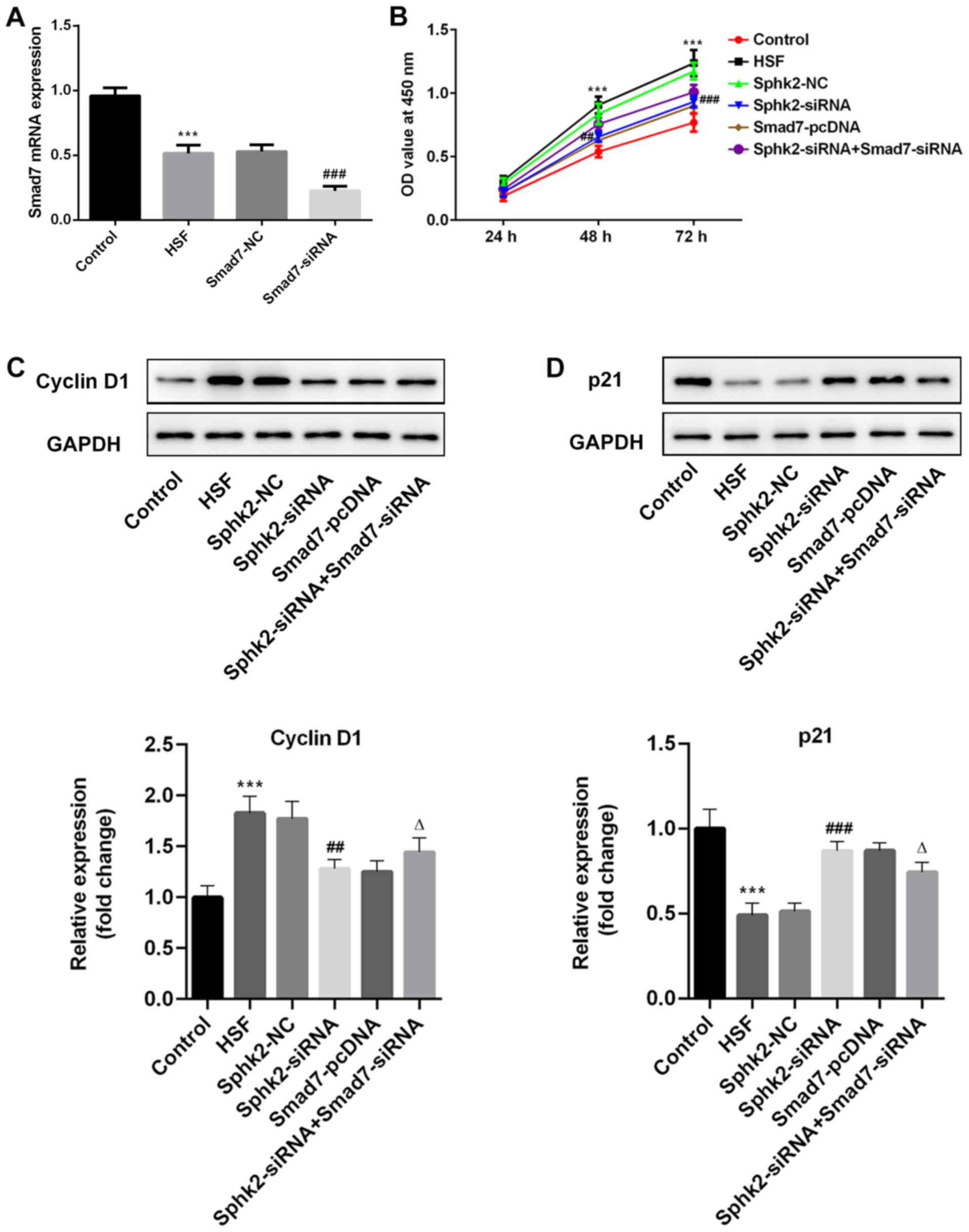
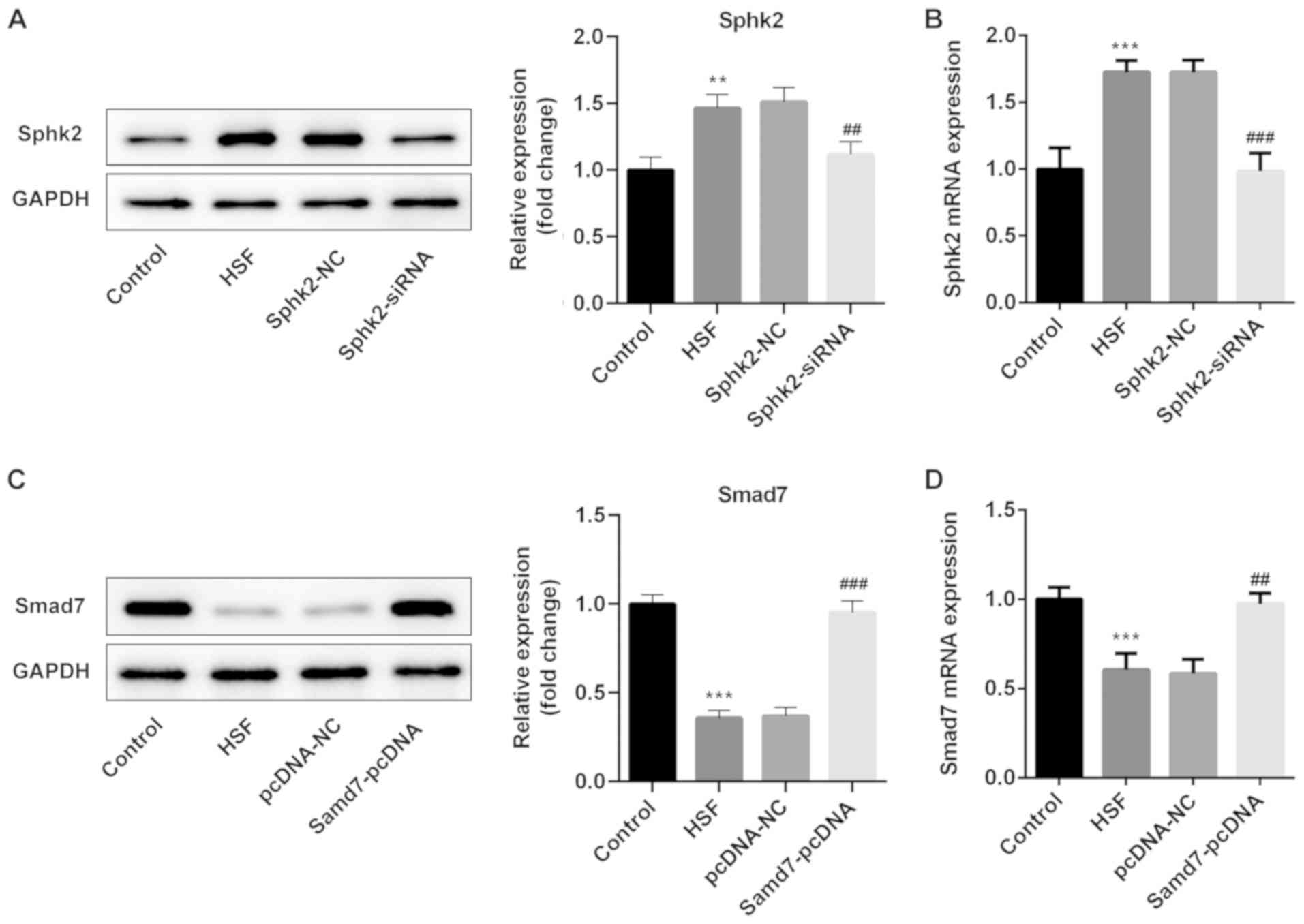 | Figure 3.Sphk2-siRNA or Smad7-pcDNA3.1
transfection into HSF. (A) Western blot analysis and (B) RT-qPCR
were used to evaluate the protein and mRNA expression levels of
Sphk2 after transfection with or without Sphk2, respectively.
**P<0.01, ***P<0.001 vs. the control; ##P<0.01,
###P<0.001 vs. Sphk2-NC. Protein and mRNA expression
levels of Smad7 were detected by (C) western blot analysis and (D)
RT-qPCR, respectively. ***P<0.001 vs. the control;
##P<0.01, ###P<0.001 vs. Smad7-NC. All
experiments were repeated three times independently. Data are
presented as the mean ± SD. Sphk2, sphingosine kinase 2; NC,
negative control; siRNA, small interfering RNA; RT-qPCR, reverse
transcription-quantitative PCR; HSF, hypertrophic scar
fibroblasts. |
Sphk2 silencing inhibits proliferation
of HSF via upregulation of Smad7 expression
The expression of Smad7 was examined by RT-qPCR
after transfection with Smad7-siRNA, and it was revealed that the
expression of Smad7 was significantly decreased in the Smad7-siRNA
group compared with the Smad7-NC group (Fig. 4A). To examine the effect of Sphk2
silencing on proliferation in HSF, a CCK-8 kit was used to
determine cell proliferation ability. It was determined that Sphk2
silencing inhibited the proliferation of HSF compared with the
Sphk2-NC group, and that Smad7 overexpression also inhibited cell
proliferation; however, this suppression of proliferation was
reversed following transfection with Sphk2-siRNA and Smad7-siRNA
(Fig. 4B). Moreover, the
expression levels of the proliferation-associated proteins were
evaluated by western blot analysis. It was demonstrated that Sphk2
silencing decreased the expression of cyclin D1, accompanied by a
significant increase in p21 expression compared with the Sphk2-NC
group (Fig. 4C and D). The
overexpression of Smad7 demonstrated the same results as Sphk2
silencing on the expression levels of the proliferation-associated
proteins. However, cells transfected with both Sphk2-siRNA and
Smad7-siRNA had increased expression of cyclin D1 and decreased
expression of p21 compared with cells transfected with Smad7-siRNA
alone. Therefore, these results indicated that Sphk2 silencing
inhibited the proliferation of HSF by upregulating Smad7.
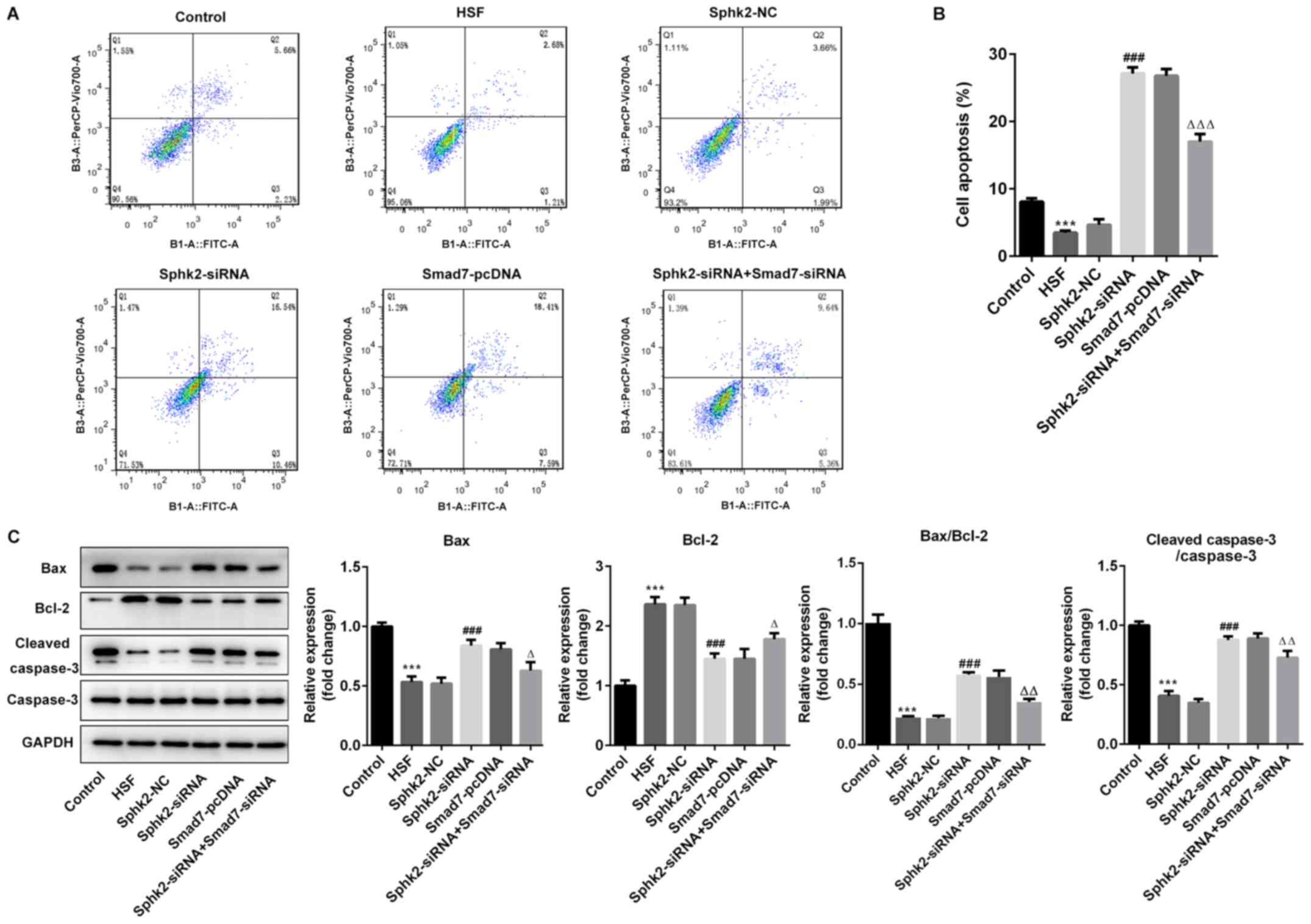
Sphk2 silencing promotes apoptosis of
HSF via upregulation of Smad7 expression
To evaluate the effect of Sphk2 silencing on
apoptosis of HSF, flow cytometry was performed, and it was
demonstrated that the number of apoptotic cells was significantly
decreased in the HSF group compared with the control group
(Fig. 5A and B). After
transfection with Sphk2-siRNA or Smad7-pcDNA, the ratio of cell
apoptosis was enhanced compared with the untreated cells. However,
transfection with both Sphk2-siRNA and Smad7-siRNA reversed the
increase in the number of apoptotic cells.
The expression levels of apoptosis-associated
proteins were subsequently investigated. It was revealed that
Sphk2-siRNA significantly upregulated the expression levels of
pro-apoptotic proteins, Bax and cleaved caspase-3, whereas the
expression of the anti-apoptotic protein Bcl-2 was significantly
downregulated compared with the Sphk2-NC group (Fig. 5C). Similar results were obtained
with Smad7-pcDNA, with the expression levels of the aforementioned
apoptosis-related proteins demonstrating the same trend. By
contrast, the transfection with both Sphk2-siRNA and Smad7-siRNA
partially reversed the effects of Sphk2-siRNA alone on the
expression levels of apoptotic proteins. Collectively, the results
indicated that Sphk2 silencing promoted apoptosis of HSF via
upregulation of Smad7.
Sphk2 silencing inactivates
TGF-β1/Smad signaling and collagen I expression in HSF via
upregulation of Smad7 expression
To examine the regulatory mechanisms of Sphk2 on
scar formation, western blot analysis was performed to assess the
expression levels of TGF-β1/Smad signaling proteins and collagen I.
The results revealed that Sphk2 silencing or Smad7 overexpression
alone decreased the expression levels of TGF-β1, p-Smad2, p-Smad3
and collagen I, while cells transfected with both Sphk2- and
Smad7-siRNA had increased expression levels of the aforementioned
proteins compared with cells transfected with Sphk2-siRNA and
Smad7-pcDNA alone (Fig. 6A and B).
Therefore, the results indicated that Sphk2 silencing inhibited
TGF-β1/Smad signaling and collagen I expression in HSF via
upregulation of Smad7.
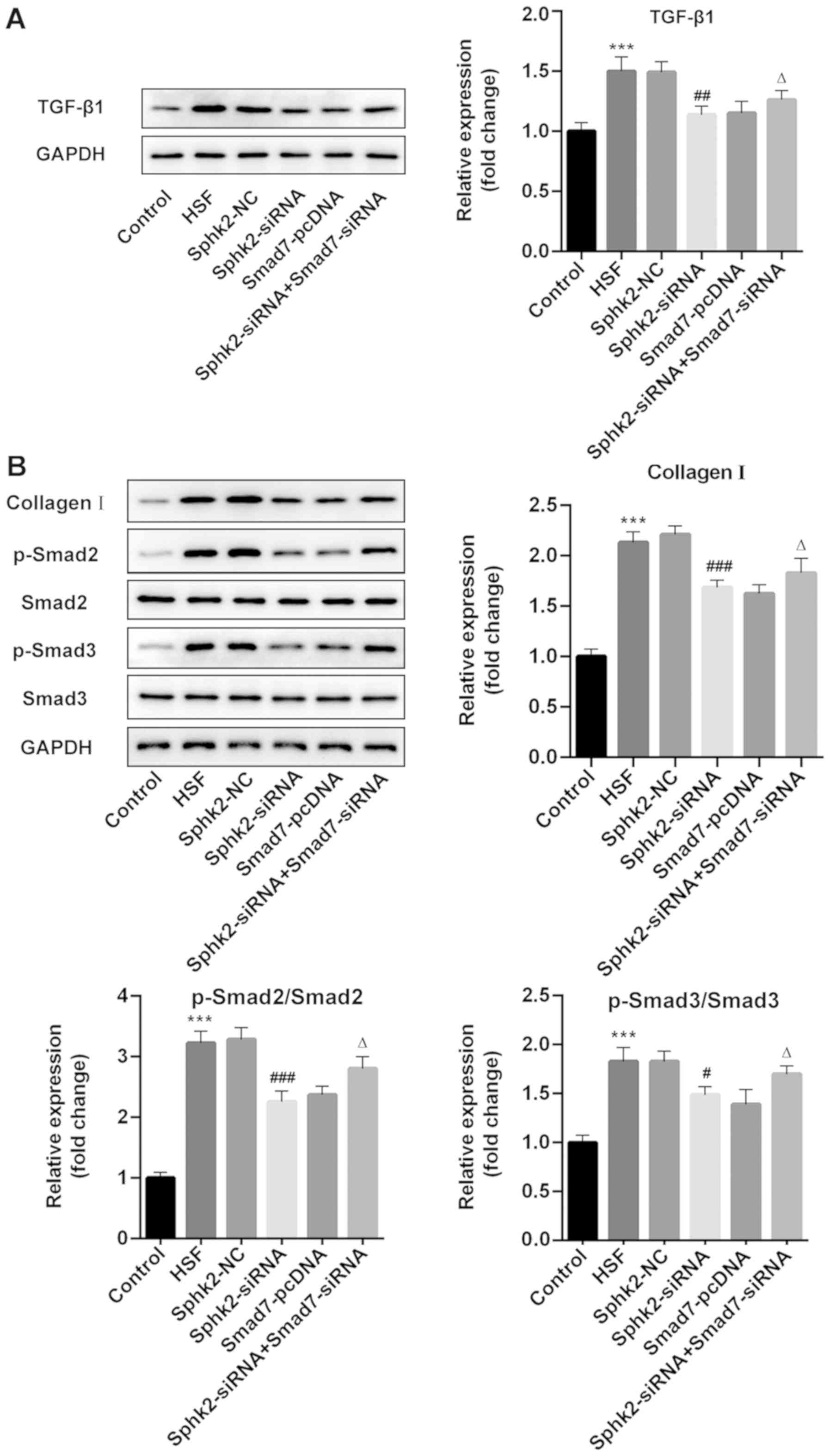 | Figure 6.Effect of Sphk2-siRNA, Smad7-pcDNA
and Sphk2-siRNA + Smad7-siRNA on the expression levels of
TGF-β1/Smad signaling key proteins and collagen I in HSF. Protein
expression levels of (A) TGF-β1 and (B) collagen I, p-Smad2 and
p-Smad3 were determined using western blot analysis. ***P<0.001
vs. the control; #P<0.05, ##P<0.01,
###P<0.001 vs. Sphk2-NC; ∆P<0.05 vs.
Sphk2-siRNA. All experiments were repeated three times
independently. Data are presented as the mean ± SD. Sphk2,
sphingosine kinase 2; siRNA, small interfering RNA; TGF-β1,
transforming growth factor-β1; NC, negative control; HSF,
hypertrophic scar fibroblasts; p-, phosphorylated. |
Discussion
HS is a fibrotic disease characterized by the
over-proliferation and activation of fibroblasts, which is often
considered as a benign skin tumor (20). The role of Sphk2 in skin diseases
has been reported in several previous studies (14,15);
however, to the best of our knowledge, the expression and function
of Sphk2 in HS has yet to be elucidated. In the present study, it
was initially found that Sphk2 was upregulated, but Smad7 was
downregulated in HS tissues compared with healthy skin tissues.
Furthermore, Sphk2 silencing or Smad7 overexpression inhibited
proliferation, promoted apoptosis and inactivated Smad signaling
and collagen expression in HSF, which were eliminated by the
silencing of both Sphk2 and Smad7. Therefore, it was speculated
that inhibition of Sphk2 could alleviate scar formation by
upregulating Smad7 expression.
Previous studies have revealed that the abnormal and
excessive proliferation of fibroblasts is one of the dominant
factors in the occurrence and development of HS (21). Moreover, it has been reported that
inhibition of HSF proliferation could suppress the development of
HS (22,23). In addition, Sphk2 silencing can
reduce proliferation of various types of tumor cells, such as
non-small cell lung cancer (NSCLC), papillary thyroid carcinoma,
colorectal cancer and skin squamous cell carcinoma (14,24–26).
It has also been revealed that the activation of Sphk2 may
contribute to bile duct ligation-induced liver fibrosis and
cholangiocyte proliferation (27).
Therefore, the present study investigated whether Sphk2 silencing
could affect the proliferation of HSF. The present results
indicated that silencing of Sphk2 and the overexpression of Smad7,
reduced the proliferative ability of HSF, decreased cyclin D1
expression and increased p21 expression. Furthermore, it was
revealed that transfecting Sphk2-siRNA and Smad7 siRNA into HSF
abrogated the reduction in cell proliferation. Collectively, these
results indicated that Sphk2 silencing can inhibit proliferation of
HSF via upregulation of Smad7 expression.
Previous studies have reported that apoptotic
resistance of fibroblasts contributes to the development and
progression of scar formation, and the induction of fibroblast
apoptosis reduces HS formation (28,29).
Furthermore, silencing of Sphk2 was revealed to suppress cell
proliferation and promote cell apoptosis in NSCLC and skin squamous
cell carcinoma (14,30). It has also been revealed that Sphk2
silencing suppresses the proliferation and induces the apoptosis of
osteoarthritis chondrocytes (31).
In the present study, HSF apoptosis was observed following
transfection with Sphk2-siRNA or Smad7-pcDNA. Moreover, the
expression levels of the pro-apoptosis proteins, Bax and cleaved
caspase-3, were significantly upregulated, coupled with a
downregulation in the expression of the anti-apoptosis protein
Bcl-2 in the Sphk2-siRNA group or the Smad7-pcDNA group, which was
eliminated by silencing of both Sphk2 and Smad7. Thus, it was
demonstrated that Sphk2 silencing promoted apoptosis of HSF via
upregulation of Smad7 expression.
It is speculated that an increase in collagen
synthesis may be one of the main features of HS formation (32). Collagen I is the main structural
element of the extracellular matrix, which plays as a vital role in
the development and progression of HS (33). A previous study also reported that
the expression of collagen I in HS tissues and HSF was notably
higher compared with that in adjacent healthy skin tissues and
normal cells, and the present results were in line with this
previous study (34). Moreover,
emerging evidence supports the hypothesis that Sphk2 could regulate
the expression of collagen I in human corneal fibrosis (35). In addition, Sphk2 deficiency was
revealed to decrease collagen accumulation in kidney tissues in a
kidney fibrosis mouse model (13).
The TGF-β1/Smad signaling pathway plays a significant role during
HS formation (36,37). A previous study has revealed that
activation of the TGF-β1/Smad signaling pathway promoted HSF
proliferation and collagen synthesis (38). Furthermore, Smad7, an inhibitor of
Smads, is an essential negative regulator in the TGF-β1/Smad
signaling pathway (39), and it
has been revealed that overexpression of Smad7 inhibits the
fibrosis of hepatic stellate cells by regulating the TGF-β1/Smad
signaling pathway (40). Moreover,
Smad 7 acts as a negative feedback regulator, which can antagonize
the activities of the Smad2 and Smad3 (41). In the present study, it was
revealed that Sphk2 silencing or Smad7 overexpression decreased the
expression levels of TGF-β1, p-Smad2, p-Smad3 and collagen I, which
were reversed following transfection with both Sphk2- and
Smad7-siRNA. Overall, the present results indicated that Sphk2
silencing inactivated TGF-β1/Smad signaling and collagen I
expression in HSF by upregulating Smad7 expression.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that Sphk2 silencing may
suppress HS formation via the inhibition of HSF proliferation,
promotion of apoptosis, and inactivation of TGF-β1/Smad signaling
and collagen I expression in HSF by upregulating Smad7 expression.
Thus, Sphk2 may be a novel target for the treatment of HS. However,
the fact that the specific relationship between Sphk2 and Smad7 was
not determined is a limitation of the present research and
therefore, a comprehensive analysis resolving these issues is
required in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and BJ wrote the manuscript, interpreted the data
and performed experiments. XX and RZ collected the data, searched
the literature and designed the study. RZ revised the manuscript.
All authors read and approval the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital, University of South
China. Written informed consent was obtained from each patient or
their legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miletta NR, Donelan MB and Hivnor CM:
Management of trauma and burn scars: The dermatologist's role in
expanding patient access to care. Cutis. 100:18–20. 2017.PubMed/NCBI
|
|
2
|
van der Veer WM, Bloemen MC, Ulrich MM,
Molema G, van Zuijlen PP, Middelkoop E and Niessen FB: Potential
cellular and molecular causes of hypertrophic scar formation.
Burns. 35:15–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sideek MA, Teia A, Kopecki Z, Cowin AJ and
Gibson MA: Co-localization of LTBP-2 with FGF-2 in fibrotic human
keloid and hypertrophic scar. J Mol Histol. 47:35–45. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuccaro J, Ziolkowski N and Fish J: A
systematic review of the effectiveness of laser therapy for
hypertrophic burn scars. Clin Plast Surg. 44:767–779. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Willows BM, Ilyas M and Sharma A: Laser in
the management of burn scars. Burns. 43:1379–1389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gras C, Ratuszny D, Hadamitzky C, Zhang H,
Blasczyk R and Figueiredo C: miR-145 contributes to hypertrophic
scarring of the skin by inducing myofibroblast activity. Mol Med.
21:296–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang XQ, Song F and Liu YK: Hypertrophic
scar regression is linked to the occurrence of endothelial
dysfunction. PLoS One. 12:e01766812017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu B, Guo Z and Gao W: miR-181b-5p
promotes proliferation and inhibits apoptosis of hypertrophic scar
fibroblasts through regulating the MEK/ERK/p21 pathway. Exp Ther
Med. 17:1537–1544. 2019.PubMed/NCBI
|
|
9
|
Pyne NJ, Dubois G and Pyne S: Role of
sphingosine 1-phosphate and lysophosphatidic acid in fibrosis.
Biochim Biophys Acta. 1831:228–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwalm S, Pfeilschifter J and Huwiler A:
Sphingosine-1- phosphate: A Janus-faced mediator of fibrotic
diseases. Biochim Biophys Acta. 1831:239–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ravichandran S, Finlin BS, Kern PA and
Özcan S: Sphk2(−/-) mice are protected from obesity and insulin
resistance. Biochim Biophys Acta Mol Basis Dis. 1865:570–576. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwalm S, Timcheva TM, Filipenko I, Ebadi
M, Hofmann LP, Zangemeister-Wittke U, Pfeilschifter J and Huwiler
A: Sphingosine kinase 2 deficiency increases proliferation and
migration of renal mouse mesangial cells and fibroblasts. Biol
Chem. 396:813–825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwalm S, Beyer S, Frey H, Haceni R,
Grammatikos G, Thomas D, Geisslinger G, Schaefer L, Huwiler A and
Pfeilschifter J: Sphingosine kinase-2 deficiency ameliorates kidney
fibrosis by up-regulating Smad7 in a mouse model of unilateral
ureteral obstruction. Am J Pathol. 187:2413–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Chen J and Yu H: Targeting
sphingosine kinase 2 by ABC294640 inhibits human skin squamous cell
carcinoma cell growth. Biochem Biophys Res Commun. 497:535–542.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shin SH, Cho KA, Hahn S, Lee Y, Kim YH,
Woo SY, Ryu KH, Park WJ and Park JW: Inhibiting sphingosine kinase
2 derived-sphingosine-1-phosphate ameliorates psoriasis-like skin
disease via blocking Th17 differentiation of naive CD4 T
lymphocytes in mice. Acta Derm Venereol. 99:594–601. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi J, Li J, Guan H, Cai W, Bai X, Fang X,
Hu X, Wang Y, Wang H, Zheng Z, et al: Anti-fibrotic actions of
interleukin-10 against hypertrophic scarring by activation of
PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts.
PLoS One. 9:e982282014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo J, Chen Z, Zhong X, Lan W, Kuang Y and
Huang D: FBP1 is highly expressed in human hypertrophic scars and
increases fibroblast proliferation, apoptosis, and collagen
expression. Connect Tissue Res. 59:120–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Xu Y, Yang G, Zhang Q, Huang X, Yu
L and Dong X: Mast cell chymase promotes hypertrophic scar
fibroblast proliferation and collagen synthesis by activating
TGF-β1/Smads signaling pathway. Exp Ther Med. 14:4438–4442.
2017.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao Y, Xu D, Song H, Shu F, Wei P, Yang
X, Zhong C, Wang X, Müller WE, Zheng Y, et al: Cuprous oxide
nanoparticles reduces hypertrophic scarring by inducing fibroblast
apoptosis. Int J Nanomedicine. 14:5989–6000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Zhao Y, Du H, Suo Y, Chen H, Li H,
Liang X, Li Q and Huang X: Downregulation of CFTR is involved in
the formation of hypertrophic scars. Biomed Res Int.
2020:95262892020.PubMed/NCBI
|
|
22
|
Song Y, Guo B, Ma S, Chang P and Tao K:
Naringin suppresses the growth and motility of hypertrophic scar
fibroblasts by inhibiting the kinase activity of Akt. Biomed
Pharmacother. 105:1291–1298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou X, Xie Y, Xiao H, Deng X, Wang Y,
Jiang L, Liu C and Zhou R: MicroRNA-519d inhibits proliferation and
induces apoptosis of human hypertrophic scar fibroblasts through
targeting Sirtuin 7. Biomed Pharmacother. 100:184–190. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu W, Yang Z, Fan Y and Zheng Q:
MicroRNA-613 inhibits cell growth, migration and invasion of
papillary thyroid carcinoma by regulating SphK2. Oncotarget.
7:39907–39915. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Liu X, Zuo Z, Hao C and Ma Y:
Sphingosine kinase 2 promotes colorectal cancer cell proliferation
and invasion by enhancing MYC expression. Tumor Biol. 37:8455–8460.
2016. View Article : Google Scholar
|
|
26
|
Leili H, Nasser S, Nadereh R, Siavoush D
and Pouran K: Sphingosine kinase-2 inhibitor ABC294640 enhances
doxorubicin-induced apoptosis of NSCLC cells via altering survivin
expression. Drug Res (Stuttg). 68:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao Y, Liu R, Li X, Gurley EC, Hylemon
PB, Lu Y, Zhou H and Cai W: Long noncoding RNA H19 contributes to
cholangiocyte proliferation and cholestatic liver fibrosis in
biliary atresia. Hepatology. 70:1658–1673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang XC, Wang T, Zhang Y, Wang LL, Zhao RY
and Tan W: Tacrolimus inhibits proliferation and induces apoptosis
by decreasing survivin in scar fibroblasts after glaucoma surgery.
Eur Rev Med Pharmacol Sci. 22:2934–2940. 2018.PubMed/NCBI
|
|
29
|
Li XY, Li T, Li XJ, Wang JN and Chen Z:
TSG-6 induces apoptosis of human hypertrophic scar fibroblasts via
activation of the Fas/FasL signalling pathway. Folia Biol (Praha).
64:173–181. 2018.PubMed/NCBI
|
|
30
|
Zhang G, Zheng H, Zhang G, Cheng R, Lu C,
Guo Y and Zhao G: MicroRNA-338-3p suppresses cell proliferation and
induces apoptosis of non-small-cell lung cancer by targeting
sphingosine kinase 2. Cancer Cell Int. 17:462017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan X, Yuan J, Xie J, Pan Z, Yao X, Sun X,
Zhang P and Zhang L: Long non-protein coding RNA DANCR functions as
a competing endogenous RNA to regulate osteoarthritis progression
via miR-577/SphK2 axis. Biochem Biophys Res Commun. 500:658–664.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma L, Li LY and Zhao TL: Anti-inflammatory
effects of ginsenoside Rg3 on the hypertrophic scar formation via
the NF-κB/IκB signaling pathway in rabbit ears. Pharmazie.
75:102–106. 2020.PubMed/NCBI
|
|
33
|
Volk SW, Wang Y, Mauldin EA, Liechty KW
and Adams SL: Diminished type III collagen promotes myofibroblast
differentiation and increases scar deposition in cutaneous wound
healing. Cells Tissues Organs. 194:25–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou R, Zhang Q, Zhang Y, Fu S and Wang C:
Aberrant miR-21 and miR-200b expression and its pro-fibrotic
potential in hypertrophic scars. Exp Cell Res. 339:360–366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicholas SE, Rowsey TG, Priyadarsini S,
Mandal NA and Karamichos D: Unravelling the interplay of
sphingolipids and TGF-β signaling in the human corneal stroma. PLoS
One. 12:e01823902017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Shan S, Wang J, Cheng X, Yi B,
Zhou J and Li Q: Galangin inhibits hypertrophic scar formation via
ALK5/Smad2/3 signaling pathway. Mol Cell Biochem. 413:109–118.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao JC, Zhang BR, Shi K, Wang J, Yu QH
and Yu JA: Lower energy radial shock wave therapy improves
characteristics of hypertrophic scar in a rabbit ear model. Exp
Ther Med. 15:933–939. 2018.PubMed/NCBI
|
|
38
|
Chen H, Xu Y, Yang G, Zhang Q, Huang X, Yu
L and Dong X: Mast cell chymase promotes hypertrophic scar
fibroblast proliferation and collagen synthesis by activating
TGF-β1/Smads signaling pathway. Exp Ther Med. 14:4438–4442.
2017.PubMed/NCBI
|
|
39
|
Seki N, Toh U, Kawaguchi K, Ninomiya M,
Koketsu M, Watanabe K, Aoki M, Fujii T, Nakamura A, Akagi Y, et al:
Tricin inhibits proliferation of human hepatic stellate cells in
vitro by blocking tyrosine phosphorylation of PDGF receptor and its
signaling pathways. J Cell Biochem. 113:2346–2355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu SP, Yang Z, Li FR, Liu XD, Chen HT and
Su DN: Smad7-overexpressing rat BMSCs inhibit the fibrosis of
hepatic stellate cells by regulating the TGF-β1/Smad signaling
pathway. Exp Ther Med. 14:2568–2576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang B, Zhu B, Liang Y, Bi L, Hu Z, Chen
B, Zhang K and Zhu J: Asiaticoside suppresses collagen expression
and TGF-β/Smad signaling through inducing Smad7 and inhibiting
TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res.
303:563–572. 2011. View Article : Google Scholar : PubMed/NCBI
|