Introduction
In 2016, statistics in China revealed that the aging
population (≥60 years) account for ~10.8% of the total population
(1), and China is predicted to
become an aging country (≥20% older) within the next 20 years
(2,3). Aging is considered a risk factor for
organ failure and a several other degenerative diseases, including
Parkinson's disease (PD), Alzheimer's disease, degenerative
osteoarthropathy and degeneration of joint disease (4–6).
Thus, a better understanding of the molecular mechanisms underlying
aging and the development of safe and effective anti-aging drugs
are required to overcome degenerative diseases associated with
aging.
Treatment with D-galactose (D-gal) has been
demonstrated to induce aging-associated changes, including
increased pathological injury and cellular senescence, as well as
the expression levels of cyclin-dependent kinase inhibitors p16,
p19 and p21 in the livers and hippocampi of mice (7). D-gal induces myocardial cell
senescence through the sirtuin 1 (SIRT1) signaling pathway in H9c2
cells (8), and icariin (ICA) can
partially restore ovarian function induced by D-gal and enhance the
fertility of mice (9).
ICA is a bioactive flavonoid component of Herba
epimedii (10), which
possesses anti-aging, antioxidant and anti-inflammatory properties
(11). For its curative effects,
Herba epimedii has been extensively used in the treatment of
several age-associated diseases, including osteoporosis,
cardiovascular diseases and sexual dysfunction (12). Previous studies have reported that
the anti-DNA damage effects of ICA can decrease the expression of
the DNA-damage marker, γ-H2AX (13), and the antioxidative effect of ICA
effectively improves β-amyloid-induced neurotoxicity and oxidative
injury in vein endothelial cells (10). Previous studies also demonstrated
that ICA extends the lifespan of human diploid fibroblasts
(14) and Caenorhabditis
elegans (15). Zhang et
al (16) reported that
long-term ICA administration significantly extended the healthy
lifespan and mean lifespan of 12-month-old C57BL/6 mice compared
with untreated mice. Furthermore, ICA has the ability to
effectively alleviate the neurotoxicity and neuroinflammation in
6-hydroxydopamine-induced PD model mice by activating nuclear
factor erythroid-2-related factor 2 (17). Collectively, these previous
findings suggest that ICA may be used as a promising drug to resist
aging and treat degenerative diseases associated with aging. Thus,
the present study aimed to investigate the potential molecular
mechanisms underlying the anti-aging ability of ICA in human lung
fibroblasts.
Materials and methods
Cell culture
IMR-90 human lung fibroblasts (American Type Culture
Collection) were cultured in minimum essential medium supplemented
with 10% fetal bovine serum and 1% penicillin-streptomycin (all
purchased from Gibco; Thermo Fisher Scientific, Inc.), at 37°C in
5% CO2.
Experimental design
To investigate the molecular mechanisms underlying
the anti-aging ability of ICA, IMR-90 cells (1×105
cells/well) were treated with different concentrations of D-gal
(50, 100 and 200 mmol/l; Beijing Solarbio Science & Technology
Co., Ltd.), in order to generate the cell aging model. Mannitol
(Man; 200 mmol/l, Beijing Solarbio Science & Technology Co.,
Ltd.) was used as the positive control. Based on the results of
senescence-associated-β-galactosidase (SA-β-Gal) staining, 200
mmol/l D-gal was used to generate the aging model.
In subsequent experimentation, different
concentrations of ICA (1, 2, 4, 8 and 16 µmol/l; Beijing Solarbio
Science & Technology Co., Ltd.) were used to pretreat IMR-90
cells at 37°C for 6 h. Cells were subsequently harvested and
incubated with D-gal (200 mmol/l) at 37°C for 72 h. The changes in
senescence level and cell viability were determined via SA-β-Gal
staining and the MTT assay.
SA-β-Gal staining assay
To evaluate senescence, SA-β-Gal staining was
performed using the Cellular Senescence Assay kit (cat. no. 9860;
Cell Signaling Technology, Inc.), according to the manufacturer's
protocol. The treated cells were seeded into 6-well plates
(5×105/ml) with complete medium and incubated in 5%
CO2 at 37°C for 48 h. Cells were subsequently washed
twice with PBS and fixed with 2% formaldehyde and 0.2%
glutaraldehyde. Following incubation for 5 min at room temperature,
the fixative solution was discarded and the cells were re-washed
twice with PBS. β-gal staining solution (35 mmol/l) was added to
each well and the plates were incubated overnight at 37°C in a dry
incubator, without CO2. When the cells became
blue/green, cells were washed with 2 ml distilled water to
terminate the reaction. Positive cells were observed in five
randomly selected fields under a light microscope (Nikon
Corporation; magnification, ×200).
MTT assay
The changes in IMR-90 cell viability were assessed
via the MTT assay. ICA-treated IMR-90 cells (3×104/ml)
were treated with D-gal or Man at 37°C for 72 h. Cells were
subsequently washed, prior to incubation with 20 µl MTT at 37°C for
4 h. Following the MTT incubation, the purple formazan crystals
were dissolved using 150 µl dimethyl sulfoxide (Beijing Solarbio
Science & Technology Co., Ltd.) and cell viability was
subsequently analyzed at a wavelength of 540 nm, using a microplate
reader (Nikon Corporation).
Cell cycle assay
Briefly, cells (1×105 cells/well) treated
with D-gal alone (200 mmol/l) or with D-gal and ICA (2, 8 and 16
µmol/l) were fixed with 70% ethanol at 4°C for 24 h. Cells were
stained with propidium iodide (50 µg/ml; Sigma-Aldrich; Merck KGaA)
for 1 h at 37°C in the dark. Cells were subsequently collected by
FACS C6 flow cytometer (BD Biosciences) and analyzed using FlowJo
6.0 software (FlowJo LLC).
Western blotting
Total protein was extracted from IMR-90 cells using
RIPA buffer (Beyotime Institute of Biotechnology), and quantified
using a bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.). Equal amounts of protein (20 µg) were loaded
onto 10% SDS-polyacrylamide gels, electrotransferred onto
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.),
which were subsequently blocked with 5% non-fat milk for 1 h at
room temperature. The membranes were incubated with primary
antibodies against: GAPDH [1:1,000 (36 kDa); cat. no. ab8245;
Abcam], p-p53 [1:500 (53 kDa); cat. no. ab1431; Abcam], p-p21
[1:1,000 (18 kDa); cat. no. ab109520; Abcam], p53 [1:500 (53 kDa);
cat. no. ab131442; Abcam], p21 [1:1,000 (18 kDa); cat. no.
ab227443; Abcam], caveolin-1 [cav-1; 1:1,000 (20 kDa); cat. no.
ab2910; Abcam], SIRT1 [1:5,000 (82 kDa); cat. no. ab32441; Abcam],
SIRT6 [1:2,000 (37 kDa); cat. no. ab62739; Abcam], P50 [1:1,000 (50
kDa); cat. no. 3035; Cell Signaling Technology, Inc.] and p65
[1:1,000 (65 kDa); cat. no. 8242; Cell Signaling Technology, Inc.],
overnight at 4°C. Following the primary incubation, membranes were
incubated with HRP-conjugated secondary antibodies [1:20,000; cat.
nos. ab205718 and ab205719 (42 and 52 kDa); Abcam]. Protein bands
were visualized using the enhanced chemiluminescence kit (GE
Healthcare Life Sciences), exposed to X-ray film and quantified
using ImageJ software (version 1.46r; National Institute of
Health).
Statistical analysis
Data are presented as the mean ± standard deviation
and all experiments were performed in triplicate. SPSS software
(version 13.0; SPSS, Inc.) was used to perform statistical
analyses. One-way analysis of variance, followed by Tukey's post
hoc test was used to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
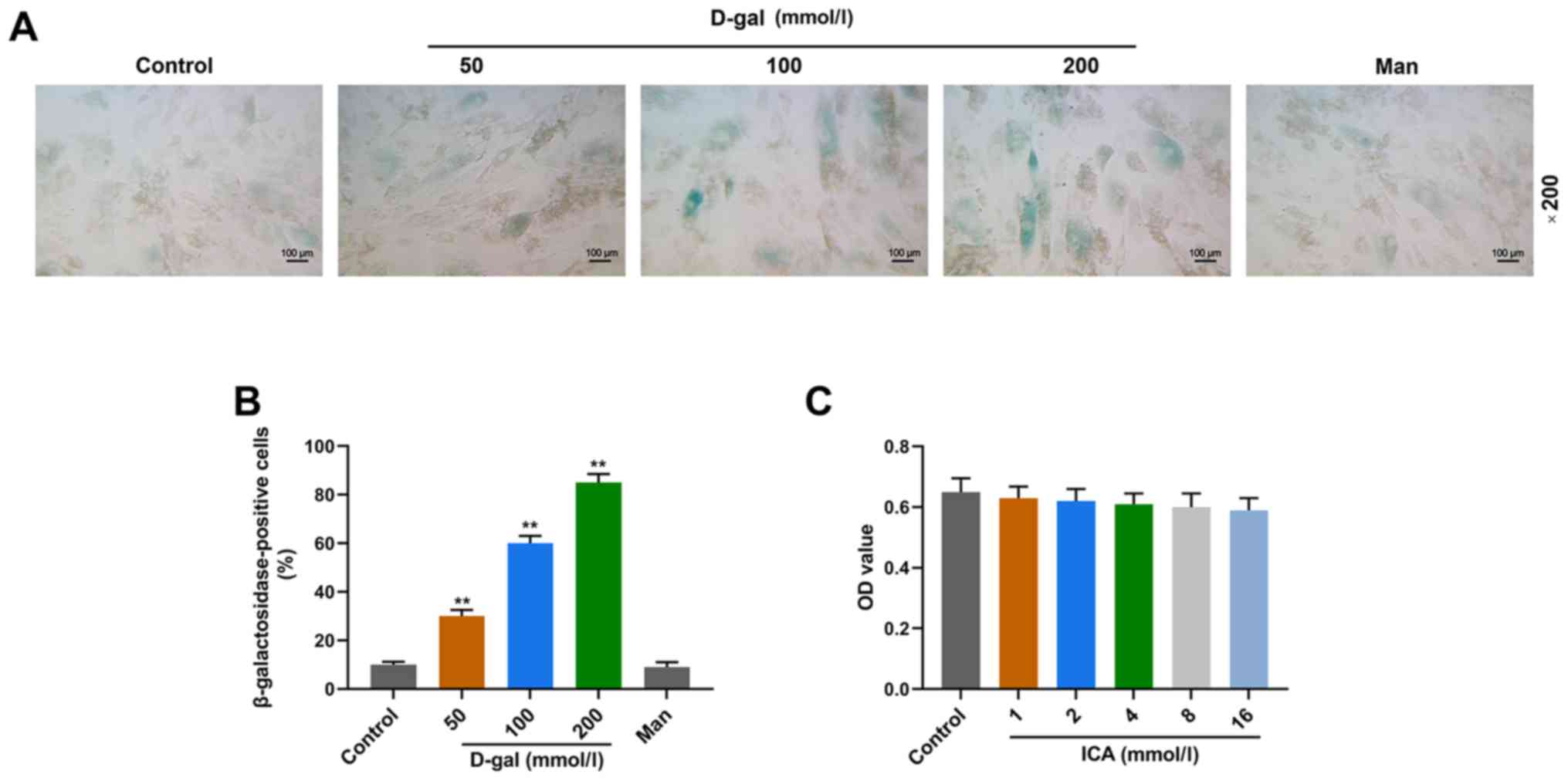
D-gal treatment promotes senescence of
IMR-90 cells
The cell aging model was induced following treatment
with D-gal and senescence of IMR-90 cells was assessed via the
SA-β-Gal staining assay (Fig. 1A and
B). Treatment with D-gal significantly increased the proportion
of SA-β-Gal positive cells in a concentration-dependent manner
compared with the control group (P<0.01; Fig. 1B). Different concentrations of ICA
were used to culture IMR-90 cells; however, no significant
differences in cell viability were observed between the
concentrations (Fig. 1C). Thus,
200 mmol/l D-gal was used to generate the cell aging model in
IMR-90 cells.
ICA pretreatment prevents
D-gal-induced aging in a concentration-dependent manner
The effects of ICA on aged IMR-90 cells induced by
D-gal were assessed via the MTT and SA-β-Gal staining assays. As
presented in Fig. 2A, D-gal
significantly decreased the viability of IMR-90 cells compared with
the control group (P<0.01), whilst pretreatment with ICA (from 4
µmol/l onwards) significantly reversed the inhibitory effect
induced by D-gal on cell viability, in a concentration-dependent
manner compared with the D-gal group (P<0.05). The results of
the SA-β-Gal staining assay demonstrated that pretreatment with ICA
significantly decreased the proportion of SA-β-Gal positive cells
in a concentration-dependent manner (Fig. 2B and C). Treatment with D-gal
significantly increased the proportion of SA-β-Gal positive cells
compared with the control group, and ICA (4, 8 and 16 µmol/l)
decreased the proportion of SA-β-Gal positive cells compared with
the control group (Fig. 2B and C).
Taken together, these results suggest that pretreatment with ICA
may prevent IMR-90 cells from D-gal-induced aging.
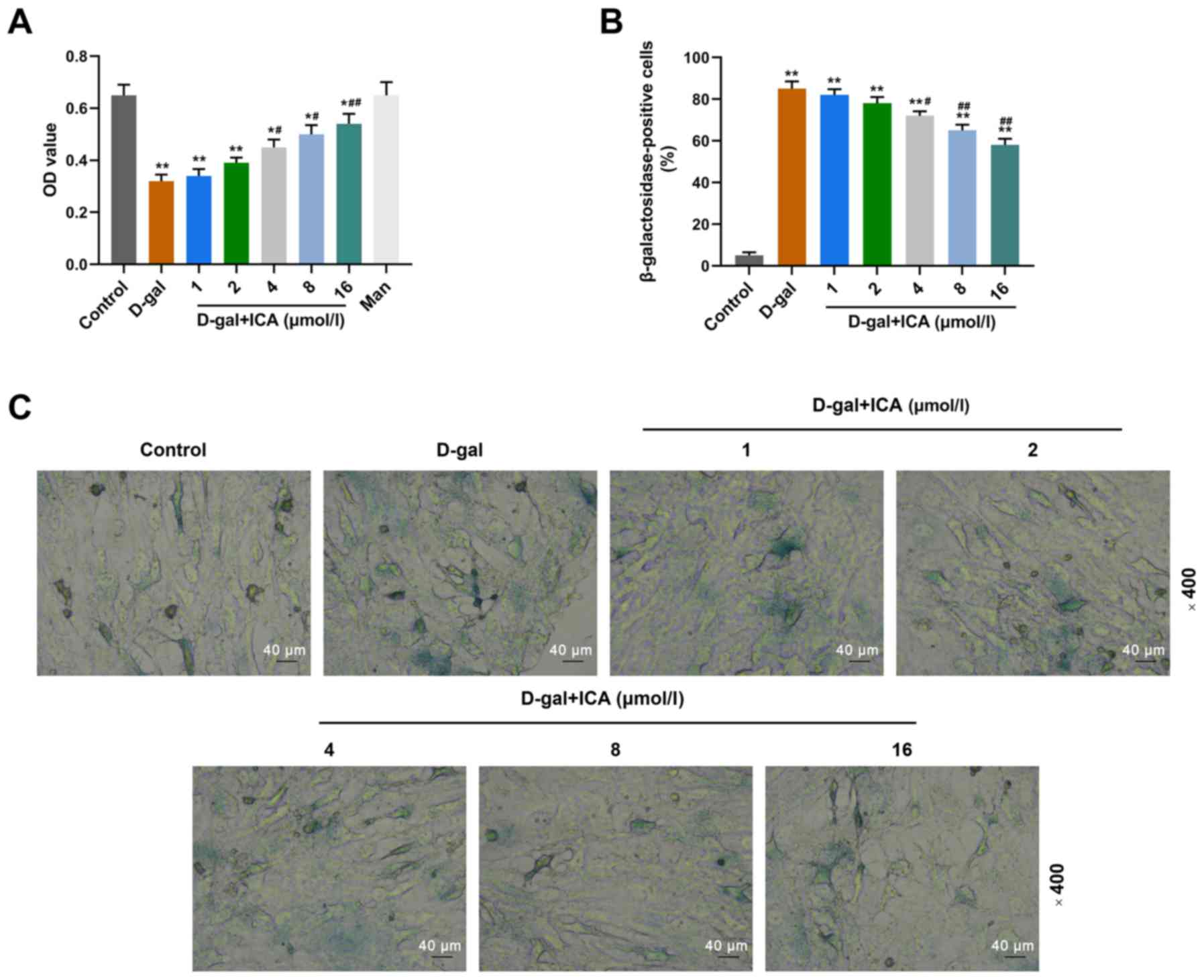 | Figure 2.Pretreatment with ICA prevents
D-gal-induced aging in a concentration-dependent manner. Cells were
pretreated with ICA (1, 2, 4, 8 and 16 µmol/l) for 6 h, followed by
incubation with D-gal (200 mmol/l) for 72 h. (A) The changes in
cell viability of IMR-90 cells were assessed via the MTT assay. (B
and C) The senescence of IMR-90 cells was evaluated via the
senescence-associated-β-galactosidase staining assay
(magnification, ×200). Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. D-gal group. ICA,
icariin; D-gal, D-galactose; OD, optical density. |
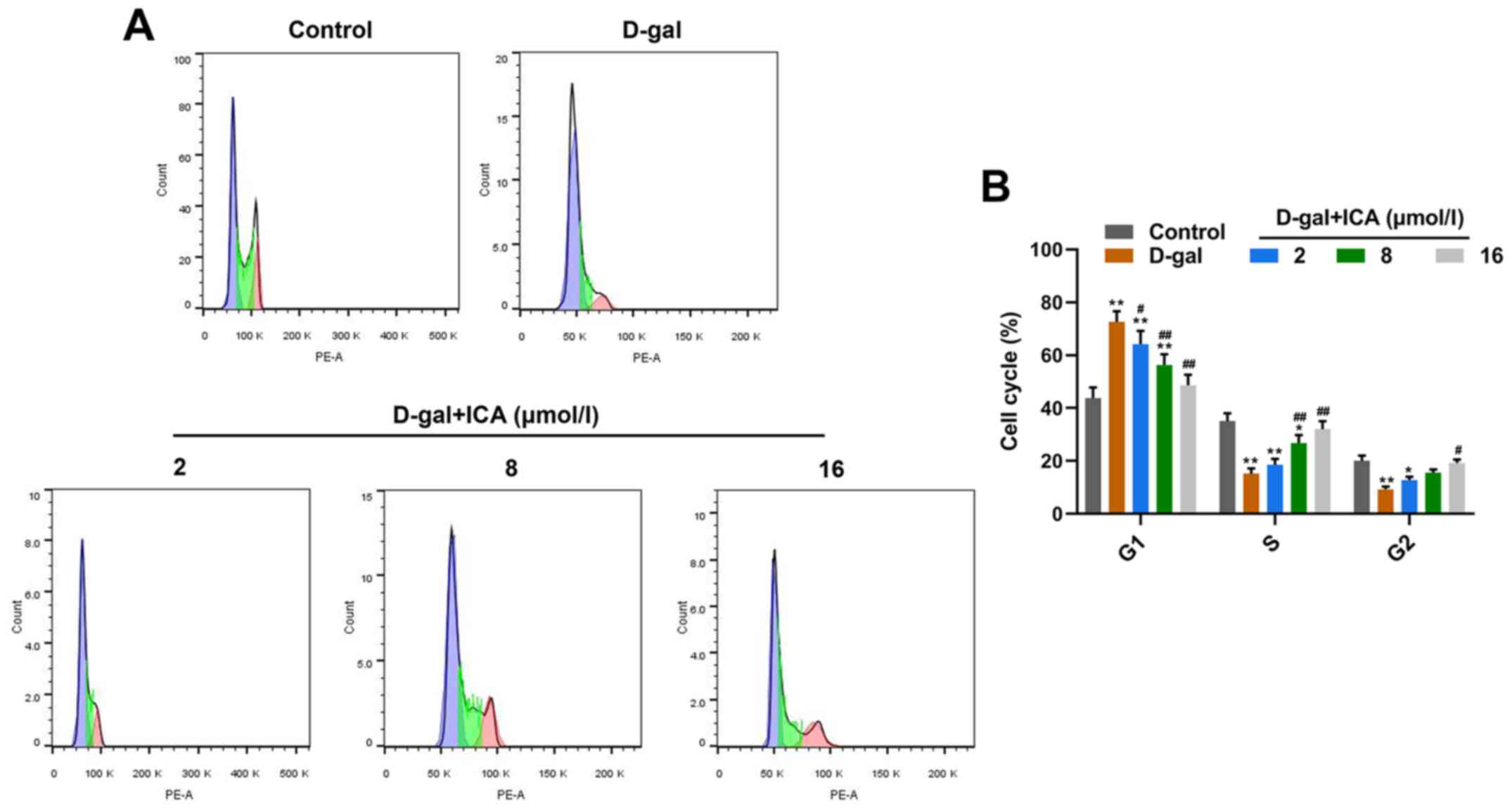
ICA pretreatment prevents
D-gal-induced cell cycle arrest in a cocentration-dependent
manner
The effects of ICA on cell cycle arrest of aged
IMR-90 cells induced by D-gal were assessed via flow cytometric
analysis. As presented in Fig. 3,
D-gal significantly accelerated cell cycle arrest of IMR-90 cells
(P<0.01), whilst pretreatment with 2, 8 and 16 µmol/l ICA
significantly reversed the suppressive effect induced by D-gal on
cell cycle arrest, in a concentration-dependent manner (P<0.05).
Collectively, these results indicate that pretreatment with ICA may
prevent IMR-90 cells from D-gal-induced cell cycle arrest.
ICA pretreatment suppresses the
activation of p53/p21 in IMR-90 cells induced by D-gal
To confirm whether regulation of the p53
transcription factor is involved in the effects of ICA pretreatment
on D-gal-induced aging, the cyclin-related protein levels of p-p53,
p-p21 and Cav-1 were measured in IMR-90 cells treated with D-gal,
or cells treated with D-gal and ICA. As presented in Fig. 4A and B, treatment with D-gal
treatment significantly promoted the activation of p53 and p21, and
increased the protein levels of Cav-1 in IMR-90 cells compared with
the control group (P<0.01). Notably, the activation of p53 and
p21, and Cav-1 protein levels significantly decreased following
pretreatment with ICA, in a concentration-dependent manner
(P<0.01).
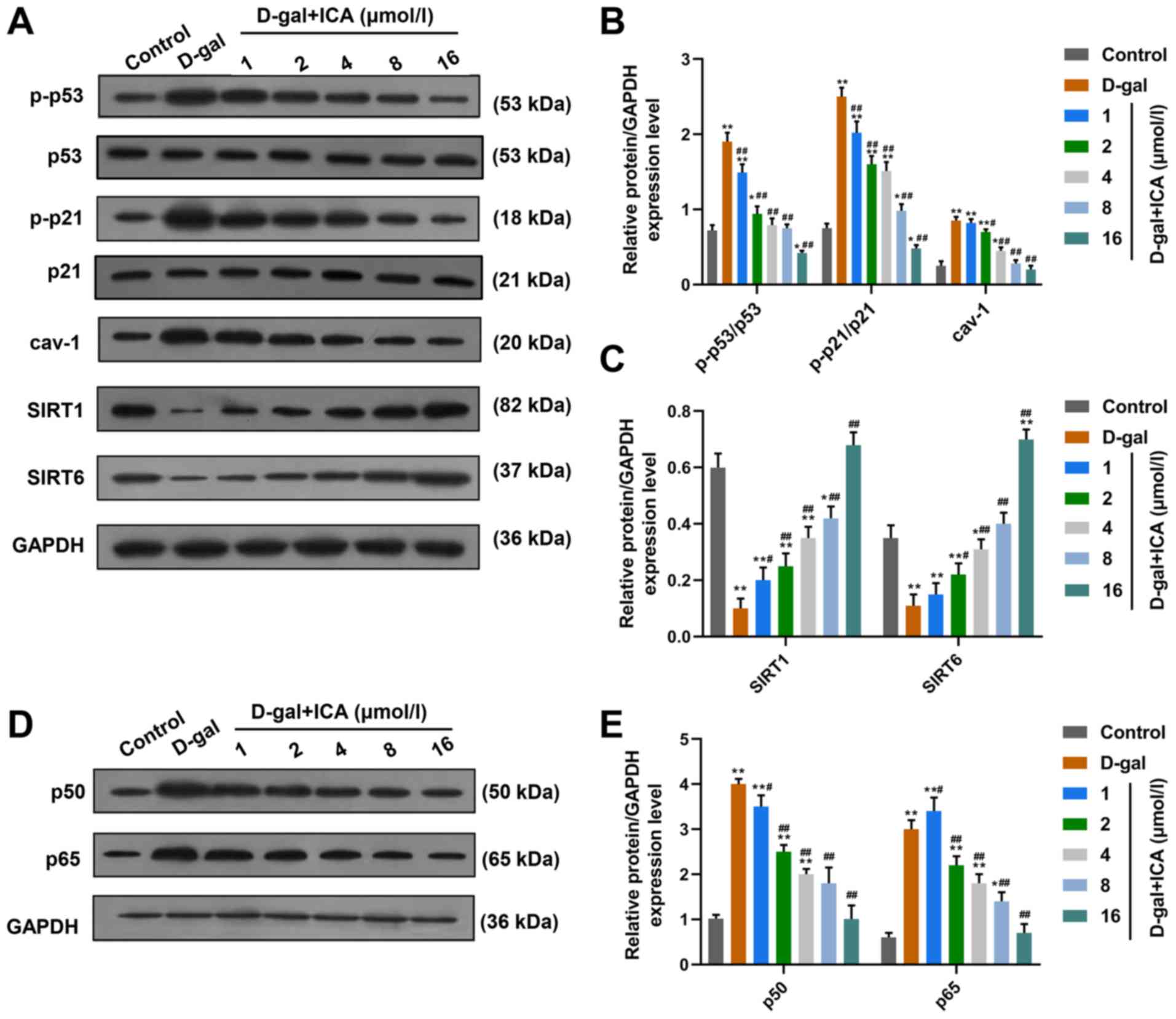 | Figure 4.Anti-aging molecular mechanisms of
ICA may be associated with the regulation of p53/p21, SIRT1/6 and
NF-κB signaling in D-gal-induced cell aging. (A) The ratio of
p-p53/p53 and p-p21/p21, and protein levels of cav-1, SIRT1 and
SIRT6 were determined via western blotting. (B) The effects of
D-gal and ICA on the ratio of of p-p53/p53 and p-p21/p21, and
protein levels of cav-1 determined via western blotting were
semi-quantified. (C) The effects of D-gal and ICA on the protein
levels of SIRT1 and SIRT6 determined via western blotting were
semi-quantified. (D and E) The protein levels of p65 and p50 in
IMR-90 cells treated with ICA and D-gal were assessed via western
blotting. GAPDH was used as the internal control. Data are
presented as the mean ± standard deviation. *P<0.05, **P<0.01
vs. control group; #P<0.05, ##P<0.01
vs. D-gal group. ICA, icariin; SIRT, sirtuin; NF-κB, nuclear factor
κB; cav-1, caveolin-1; D-gal, D-galactose. |
ICA pretreatment reverses the
decreased protein levels of SIRT1 and SIRT6 induced by D-gal in
IMR-90 cells
The protein levels of SIRT1 and SIRT6 were detected
in IMR-90 cells treated with D-gal, or cells treated with D-gal and
ICA. As presented in Fig. 4A and
C, D-gal significantly suppressed the expression levels of
SIRT1 and SIRT6 in IMR-90 cells. Notably, pretreatment with ICA
significantly reversed the suppressive effect induced by D-gal on
the levels of SIRT1 and SIRT6, in a concentration-dependent
manner.
Nuclear factor-κB (NF-κB) signaling
may be associated with the anti-aging effects of ICA on
D-gal-treated IMR-90 cells
To determine the potential pathway in the anti-aging
molecular mechanisms of ICA, the protein levels of NF-κB signaling
molecules were assessed in IMR-90 cells treated with D-gal, or
cells treated with D-gal and ICA. As presented in Fig. 4D and E, the protein levels of p50
and p65 significantly increased following treatment with D-gal
compared with the control group, suggesting that the NF-κB
signaling pathway may be involved in D-gal-induced aging in IMR-90
cells. Furthermore, pretreatment with ICA significantly decreased
the protein levels of p50 and p65 compared with the D-gal group,
indicating that NF-κB signaling may be associated with the
anti-aging ability of ICA.
Discussion
The present study aimed to investigate the molecular
mechanisms underlying the anti-aging ability of ICA. D-gal was used
to generate the cell aging model in IMR-90 cells. The results of
the present study suggested that the high concentration of D-gal
(200 mmol/l) increased the proportion of SA-β-Gal positive cells
and induced the cell aging model. However, the proportions of
SA-β-Gal positive cells and the viability of IMR-90 cells did not
change following treatment with Man (200 mmol/l), thus, no
increasing osmotic pressure of the medium was indicated.
Despite the lack of assessment of different cell
lines, the results of the present study demonstrated that
pretreatment with ICA significantly reversed the effects induced by
D-gal on viability and cell cycle arrest of IMR-90 cells.
Furthermore, the effects of D-gal on regulating proteins associated
with aging and lifespan were also significantly counteracted
following pretreatment with ICA. Western blot analysis demonstrated
that inhibiting NF-κB signaling was involved in the anti-aging
molecular mechanisms of ICA, in the D-gal-induced aging model.
P53 is a tumor suppressor gene and mutation of p53
occurs in >50% of different types of tumor (18,19).
Previous studies have demonstrated that the p53/p21 signaling
pathway is associated with aging (19,20).
For example, Lessel et al (21) reported that mutation of murine
double minute 2, which is responsible for maintaining low p53
levels or deactivating p53, leads to aberrant expression of p53,
ultimately accelerating the aging process. Furthermore, aberrant
expression of p53 plays a central role in the process of cell
senescence (22). P53 induces
upregulation of cyclin-dependent kinase inhibitor p21, which in
turn inhibits activation of the cell cycle repressor,
retinoblastoma (RB) activation to induce cell cycle arrest
(23). In addition, P53 also
promotes the expressions of downstream genes of p54, including
cyclin B1, growth arrest and DNA damage-inducible α and stratifin,
to participate in cell cycle arrest (24,25).
Jiang et al (23)
demonstrated that silencing serpine 1 can significantly decrease
the expression levels of p53 and p21, and promote phosphorylation
of RB, ultimately delaying senescence of alveolar type 2 cells.
Furthermore, cav-1 also plays a potential role in the induction of
cell senescence (26,27), as cav-1 has been reported to
interact with polymerase I and transcript release factor, and
accelerate caveolae information, subsequently promoting the
activation of the p53/p21 signaling pathway to regulate cellular
senescence (28). A previous study
also demonstrated that cav-1 is highly expressed in premature
senescence and inhibition of cav-1 by lentivirus-mediated RNA
interference has a marked effect on improving cell senescence
induced by oxidative stress (29).
The current study observed the activation of p53/p21 signaling and
significant upregulation of cav-1 in the D-gal-induced senescence
in IMR-90 cells. When cells were pretreated with ICA, activation of
the p53/p21 signaling pathway and upregulation of cav-1 were
markedly attenuated. These findings suggest that the anti-aging
ability of ICA in IMR-90 cells may be associated with the
inhibition of p53/p21 signaling.
Currently, the sirtuin family, particularly SIRT1
and SIRT6 have been extensively studied for their anti-aging
ability (30,31). SIRT1 is a class III histone
deacetylase and plays a critical role in certain biological
processes, such as individual growth, stress response, endocrine
regulation, tumorigenesis and extending lifespan (32). A previous study demonstrated that
SIRT1 knockdown in podocytes can markedly aggravate
glomerulosclerosis and albuminuria induced by aging, which, at the
same time, is accompanied by notably upregulated expression levels
of aging-associated markers in the glomeruli of aging mice
(33). Similarly, Tran et
al (34) demonstrated that
upregulating SIRT1 expression can prevent prolonged insulin-like
growth factor-1 treatment-induced cellular senescence by
attenuating the acetylation and activation of p53. Furthermore,
SIRT6-deficient rats have been reported to develop several symptoms
similar to aging-associated degenerative symptoms, such as loss of
lordokyphosis, subcutaneous fat and serious lymphopenia and
metabolic defects, at 2–3 weeks of age and ultimately the rats died
after approximately 4 weeks (35).
These study findings are consistent with the results of the present
study, which demonstrated that the protein expression levels of
SIRT1 and SIRT6 were downregulated following treatment with D-gal.
However, IMR-90 cells pretreated with ICA observably decrease the
proportion of SA-β-Gal-positive cells, which may be mediated
through the effects of ICA on preventing SIRT1 and SIRT6 from the
downregulation induced by D-gal. Recently, the effects of SIRT1 and
SIRT6 on aging were reported to be associated with the inhibition
of inflammation mediated by the NF-κB signaling pathway (36). Furthermore, studies linking aging
and the NF-κB signaling pathway suggest that the activation of
NF-κB signaling through accumulating endogenous DNA damage can lead
to aging and aging-associated degenerative changes (35,37).
Previous studies have demonstrated that the expression levels of
SIRT6 and SIRT1 can effectively suppress the activation of NF-κB
signaling by deacetylating the p65 subunit of NF-κB complex, thus
notably delaying premature and normal aging (37,38).
In the present study, treatment with D-gal treatment significantly
promoted the activation of NF-κB signaling, while treatment with 2
µmol/l ICA significantly decreased the protein levels of p65 and
p50. Taken together, these results suggested that the anti-aging
molecular mechanisms of ICA may also be associated with the
regulation of SIRT1/6 and NF-κB signaling.
In the present study, treatment with D-gal promoted
the activation of p53/p21 and NF-κB signaling through
downregulation of SIRT1/6, while ICA reversed the effects of D-gal
on IMR-90 cells. Prospective studies will aim to investigate the
translocation of NF-κB to the nucleus, binding of NF-κB to target
promoters and decreased steady-state levels of NF-κBs.
In conclusion, the present study investigated the
molecular mechanisms underlying the anti-aging ability of ICA.
D-gal was used to generate an aging model in IMR-90 cells and the
results demonstrated that D-gal notably accelerated cellular
senescence, which may be associated with the activation of p53/p21
and NF-κB signaling, and downregulation of SIRT1/6. Taken together,
the results of the present study suggest that low concentrations of
ICA may effectively prevent IMR-90 cells from aging-associated
changes induced by D-gal and markedly delay aging of IMR-90 human
lung fibroblasts, without affecting cell viability. Thus, ICA may
be implemented as a promising candidate for anti-aging.
Acknowledgements
Not applicable.
Funding
The present study was supported by the General
Project of Hangzhou Health Science and Technology (grant no.
20160533B19), the Independent Declaration Project of Hangzhou
Social Development (grant no. 20160533B18), the Zhejiang Public
Welfare Technology Application Research and Design Project (grant
no. 2015C33258) and the General Project of Hangzhou Plan (grant no.
20130633B12).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CX and XH made substantial contributions to
conception and design. YT and XF performed data acquisition, data
analysis and interpretation. YW, CW, CX and YJ performed the
experiments and drafted the initial manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
cav-1
|
caveolin-1
|
|
D-gal
|
D-galactose
|
|
ICA
|
icariin
|
|
NF-κB
|
nuclear factor κB
|
|
PD
|
Parkinson's disease
|
|
RB
|
retinoblastoma
|
|
SA-β-Gal
|
senescence-associated-β-galactosidase
|
|
SIRT1
|
sirtuin 1
|
References
|
1
|
Wang Z, Hu S, Sang S, Luo L and Yu C:
Age-period-cohort analysis of stroke mortality in China: Data from
the global burden of disease study 2013. Stroke. 48:271–275. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Sun L, Zhang W, Li H, Wang S, Mu H,
Zhou Q, Zhang Y, Tang Y, Wang Y, et al: Association of serum
glycine levels with metabolic syndrome in an elderly Chinese
population. Nutr Metab (Lond). 15:892018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu H, Guo Z, Liu J, Zhang H, Zhao W, Wu Y,
Ni J, Liu W, Tu J, Wang J, et al: Trends in stroke incidence among
elderly low-income residents of rural China: A population-based
study from 1992 to 2016. Aging (Albany NY). 10:3438–3449. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panicker N, Saminathan H, Jin H, Neal M,
Harischandra DS, Gordon R, Kanthasamy K, Lawana V, Sarkar S, Luo J,
et al: Fyn kinase regulates microglial neuroinflammatory responses
in cell culture and animal models of Parkinson's disease. J
Neurosci. 35:10058–10077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toosizadeh N, Harati H, Yen TC, Fastje C,
Mohler J, Najafi B and Dohm M: Paravertebral spinal injection for
the treatment of patients with degenerative facet osteoarthropathy:
Evidence of motor performance improvements based on objective
assessments. Clin Biomech (Bristol, Avon). 39:100–108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gondard E, Soto-Montenegro ML, Cassol A,
Lozano AM and Hamani C: Transcranial direct current stimulation
does not improve memory deficits or alter pathological hallmarks in
a rodent model of Alzheimer's disease. J Psychiatr Res. 114:93–98.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun K, Yang P, Zhao R, Bai Y and Guo Z:
Matrine attenuates d-galactose-induced aging-related behavior in
mice via inhibition of cellular senescence and oxidative stress.
Oxid Med Cell Longev. 2018:71086042018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang LF, Cao Q, Wen K, Xiao YF, Chen TT,
Guan XH, Liu Y, Zuo L, Qian YS, Deng KY and Xin HB: CD38 deficiency
alleviates d-galactose-induced myocardial cell senescence through
NAD(+)/sirt1 signaling pathway. Front Physiol. 10:11252019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JL, Liu B, Zhang C, Wang XM, Zhen D,
Huang XM, Chen W and Gao JM: Effects of icariin on ovarian function
in d-galactose-induced aging mice. Theriogenology. 125:157–167.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sze SC, Tong Y, Ng TB, Cheng CL and Cheung
HP: Herba epimedii: Anti-oxidative properties and its medical
implications. Molecules. 15:7861–7870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XA, Ho YS, Chen L and Hsiao WL: The
protective effects of icariin against the homocysteine-induced
neurotoxicity in the primary embryonic cultures of rat cortical
neurons. Molecules. 21:15572016. View Article : Google Scholar
|
|
12
|
Pei LK, Guo BL, Sun SQ and Huang WH: Study
on the identification of some species of Herba Epimedii with FTIR.
Guang Pu Xue Yu Guang Pu Fen Xi. 28:55–60. 2008.(In Chinese).
PubMed/NCBI
|
|
13
|
Shen CY, Jiang JG, Yang L, Wang DW and Zhu
W: Anti-ageing active ingredients from herbs and nutraceuticals
used in traditional Chinese medicine: Pharmacological mechanisms
and implications for drug discovery. Br J Pharmacol. 174:1395–1425.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu ZW, Shen ZY and Huang JH: Experimental
study on effect of epimedium flavonoids in protecting telomere
length of senescence cells HU. Zhongguo Zhong Xi Yi Jie He Za Zhi.
24:1094–1097. 2004.(In Chinese). PubMed/NCBI
|
|
15
|
Cai WJ, Zhang XM and Huang JH: Effect of
epimedium flavonoids in retarding aging of C. elegans.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 28:522–525. 2008.(In Chinese).
PubMed/NCBI
|
|
16
|
Zhang SQ, Cai WJ, Huang JH, Wu B, Xia SJ,
Chen XL, Zhang XM and Shen ZY: Icariin, a natural flavonol
glycoside, extends healthspan in mice. Exp Gerontol. 69:226–235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B, Wang G, He J, Yang Q, Li D, Li J
and Zhang F: Icariin attenuates neuroinflammation and exerts
dopamine neuroprotection via an Nrf2-dependent manner. J
Neuroinflammation. 16:922019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanz G, Singh M, Peuget S and Selivanova
G: Inhibition of p53 inhibitors: progress, challenges and
perspectives. J Mol Cell Biol. 11:586–599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu D and Prives C: Relevance of the
p53-MDM2 axis to aging. Cell Death Differ. 25:169–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ou HL and Schumacher B: DNA damage
responses and p53 in the aging process. Blood. 131:488–495. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lessel D, Wu D, Trujillo C, Ramezani T,
Lessel I, Alwasiyah MK, Saha B, Hisama FM, Rading K, Goebel I, et
al: Dysfunction of the MDM2/p53 axis is linked to premature aging.
J Clin Invest. 127:3598–3608. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marudamuthu AS, Shetty SK, Bhandary YP,
Karandashova S, Thompson M, Sathish V, Florova G, Hogan TB,
Pabelick CM, Prakash YS, et al: Plasminogen activator inhibitor-1
suppresses profibrotic responses in fibroblasts from fibrotic
lungs. J Biol Chem. 290:9428–9441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang C, Liu G, Luckhardt T, Antony V,
Zhou Y, Carter AB, Thannickal VJ and Liu RM: Serpine 1 induces
alveolar type II cell senescence through activating p53-p21-Rb
pathway in fibrotic lung disease. Aging Cell. 16:1114–1124. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Müllers E, Silva Cascales H, Burdova K,
Macurek L and Lindqvist A: Residual Cdk1/2 activity after DNA
damage promotes senescence. Aging Cell. 16:575–584. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De U, Son JY, Jeon Y, Ha SY, Park YJ, Yoon
S, Ha KT, Choi WS, Lee BM, Kim IS, et al: Plumbagin from a tropical
pitcher plant (Nepenthes alata Blanco) induces apoptotic cell death
via a p53-dependent pathway in MCF-7 human breast cancer cells.
Food Chem Toxicol. 123:492–500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wheaton K: Caveolar vesicles generate DNA
damage and perpetuate cellular aging. Cell Res. 21:993–994. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wheaton K, Sampsel K, Boisvert FM, Davy A,
Robbins S and Riabowol K: Loss of functional caveolae during
senescence of human fibroblasts. J Cell Physiol. 187:226–235. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai L, Deng X, Li J, Wang M, Li Q, An W, A
D and Cong YS: Regulation of cellular senescence by the essential
caveolar component PTRF/Cavin-1. Cell Res. 21:1088–1101. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding L, Zeng Q, Wu J, Li D, Wang H, Lu W,
Jiang Z and Xu G: Caveolin-1 regulates oxidative stress-induced
senescence in nucleus pulposus cells primarily via the p53/p21
signaling pathway in vitro. Mol Med Rep. 16:9521–9527. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tennen RI and Chua KF: Chromatin
regulation and genome maintenance by mammalian SIRT6. Trends
Biochem Sci. 36:39–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Oliveira RM, Pais TF and Outeiro TF:
Sirtuins: Common targets in aging and in neurodegeneration. Curr
Drug Targets. 11:1270–1280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carew JS, Giles FJ and Nawrocki ST:
Histone deacetylase inhibitors: Mechanisms of cell death and
promise in combination cancer therapy. Cancer Lett. 269:7–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chuang PY, Cai W, Li X, Fang L, Xu J,
Yacoub R, He JC and Lee K: Reduction in podocyte SIRT1 accelerates
kidney injury in aging mice. Am J Physiol Renal Physiol.
313:F621–F628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tran D, Bergholz J, Zhang H, He H, Wang Y,
Zhang Y, Li Q, Kirkland JL and Xiao ZX: Insulin-like growth
factor-1 regulates the SIRT1-p53 pathway in cellular senescence.
Aging Cell. 13:669–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Sun T, Wu J, Kalionis B, Zhang C,
Yuan D, Huang J, Cai W, Fang H and Xia S: Icariin intervenes in
cardiac inflammaging through upregulation of SIRT6 enzyme activity
and inhibition of the NF-kappa B pathway. Biomed Res Int.
2015:8959762015.PubMed/NCBI
|
|
36
|
Salminen A, Ojala J, Huuskonen J,
Kauppinen A, Suuronen T and Kaarniranta K: Interaction of
aging-associated signaling cascades: Inhibition of NF-kappaB
signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci.
65:1049–1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tilstra JS, Robinson AR, Wang J, Gregg SQ,
Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, et al:
NF-kB inhibition delays DNA damage-induced senescence and aging in
mice. J Clin Invest. 122:2601–2612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawahara TL, Michishita E, Adler AS,
Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang
HY and Chua KF: SIRT6 links histone H3 lysine 9 deacetylation to
NF-kappaB-dependent gene expression and organismal life span. Cell.
136:62–74. 2009. View Article : Google Scholar : PubMed/NCBI
|