Introduction
Allergic asthma is a chronic inflammatory disease of
the airway that is characterized by general pathological
alteration, severe eosinophilia, lymphocyte infiltration, fibrosis
deposition, and mucus overproduction (1). The pathophysiology of asthma is
related to a Th1/Th2 cell imbalance in the airways (2), and is therefore becoming a focus for
asthma treatment. Th1-associated cytokines, IFN-γ and IL-10, were
reported to reduce the asthma symptoms in patients with asthma
(3).
Nuclear factor-κB (NF-κB) plays a pivotal role in
the production of Th2 cytokines and recruitment of inflammatory
cells in the airways of murine asthma models (4,5).
Recent studies have suggested that inhibition of NF-κB can help
treat allergic asthma (6,7). NF-κB has also been shown to increase
levels of cytokines, such as IL-1β, IL-5, and IL-6, in the airway
epithelium (8).
Traditional herbal medicines are generally
recognized to be safe and exhibit various therapeutic effects
(9). Dryopteris
crassirhizoma (DC) is a semi-evergreen pteridophyte that is
widely distributed in Japan, Korea, and China (10). Several studies have demonstrated
that phloroglucinols from DC have a wide range of pharmacological
effects, such as antibacterial (11), anti-reverse transcriptase (12), and antioxidant activity (13), which are mediated by active
components, such as phloroglucinol derivatives (albaspidin,
aspidin, flavaspidic acids, and dryocrassin), triterpenes
(acylphloroglucinols), and dimethylflavanones
(desmethoxymatteucinol, matteucinol, and methoxymatteucin)
(11,14–16).
The aim of the present study was to investigate the
anti-asthmatic action of DC in vivo. In addition, to
elucidate the cellular mechanisms underlying the effects of DC on
ovalbumin (OVA)-induced allergic asthma, we performed an in
vitro study with human mast cells (HMC)-1, stimulated by A23187
and phorbol myristate acetate (A23187/PMA) treatment.
Materials and methods
Cell culture
HMC-1 cells were generously provided by Professor
Hyun-Ja Jeong (Department of Food Science and Technology and
Research Institute for Basic Science, Hoseo University, Republic of
Korea). HMC-1 cells were grown in Iscove's modified Dulbecco's
medium (IMDM), supplemented with 100 U/ml penicillin, 100 µg/ml
streptomycin, 10 µM monothioglycerol, and 10% heat-inactivated FBS
at 37°C, in 5% CO2 and 95% humidity.
Preparation of DC
DC (KFRI-SL-2021) was provided by the Division of
Nutrition and Metabolism Research, Korea Food Research Institute.
DC used in this study was purchased from the Kyeong-Dong Oriental
Pharmacy Market. DC underwent reflux extraction twice in 95%
ethanol. The ethanol extract was dried under vacuum in a rotary
evaporator. The concentrated extract was lyophilized, yielding a
dried powder that was kept at 4°C until needed and dissolved in
saline prior to use.
Ultraperformance liquid
chromatography-quadrupole-time of flight (UPLC/Q-TOF) mass
spectrometry (MS)
To identify the chemical constituents of DC, the
ethanolic extracts of DC were analyzed using UPLC/Q-TOF MS (Waters
Corp.). The extract was injected into an Acquity UPLC BEH C18
column (2.1×100 mm, 1.7 µm; Waters Corp.) at a column temperature
of 40°C. The mobile phase consisted of water with 0.1% formic acid
and acetonitrile with 0.1% formic acid, at a flow rate of 0.35
ml/min for 9 min. The capillary voltage was set at 3 or 2.5 kV, for
positive or negative mode, respectively, while the sample cone
voltage was 40 V. The desolvation flow rate was 900 l/h at 400°C
and source temperature was set at 100°C. Leucine enkephalin
[(M+H)=m/z 556.2771] was used as a reference for lock mass at a
frequency of 10 sec. The MS/MS spectra were obtained using
collision energy ramps from 20 to 45 eV. Metabolites were
identified by Unifi software using various LC/MS databases.
Animals
Pathogen-free 5-week-old male BALB/c mice, weighing
approximately 20 g, were purchased from Damool Science. Five mice
were housed per cage in a laminar air-flow cabinet, maintained at
23±2°C at a relative humidity of 55±10%, with a 12 h dark/light
cycle, throughout the study period. All animal experiments were
performed in compliance with the NIH guidelines for the care and
use of laboratory animals and were approved by the Institutional
Animal Care and Use Committees of Chonbuk National University
Laboratory Animal Center (CBNU 2016-37 and CBNU 2019-071).
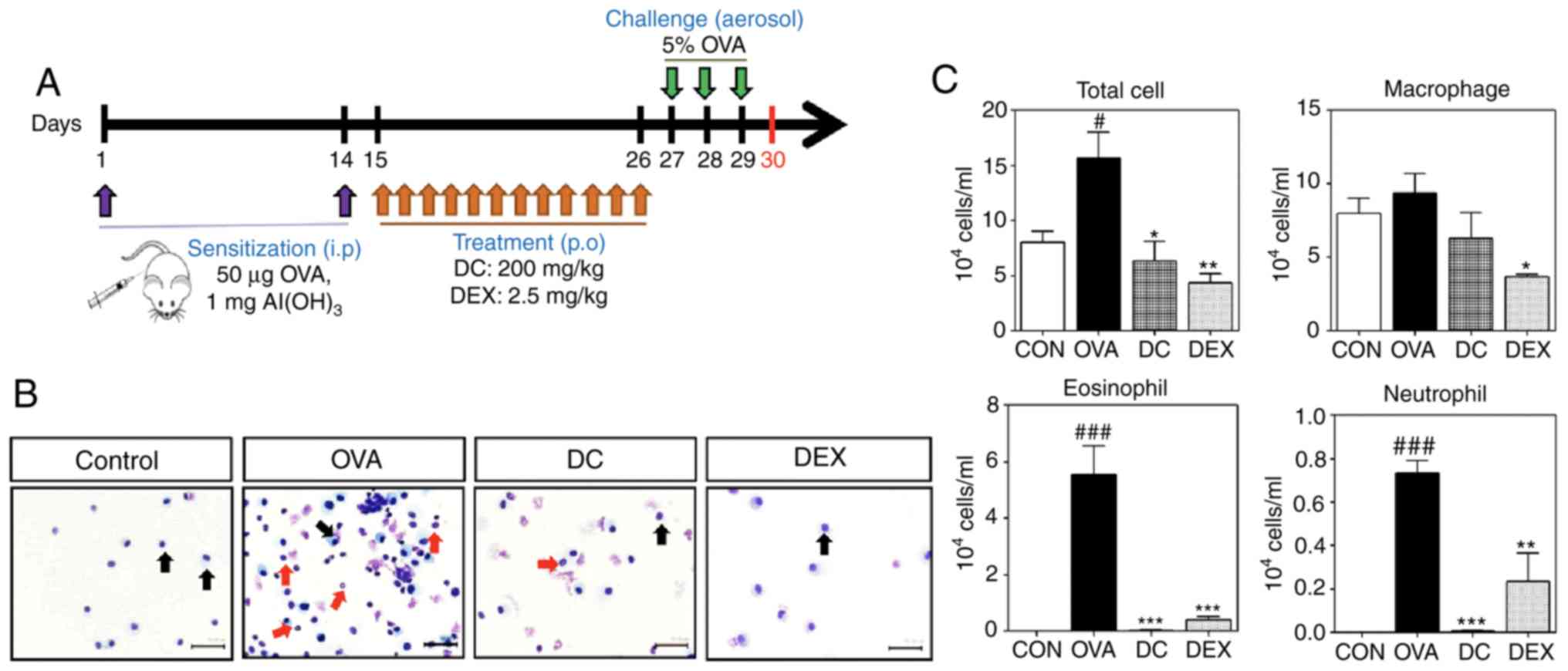
Mouse allergic asthma model and
treatment
In this study, the mice were randomly divided into
four groups (n=6 per group), namely control, OVA, DC, and Dex. The
first sensitization was performed on day 1 by intraperitoneally
injecting 50 µg of OVA (Grade V, Sigma, St. Louis, MO, USA),
emulsified in 1 mg of alum (Imject Alum; Pierce), in a total volume
of 200 µl. The control group received saline alone (NaCl 0.9%; B.
Braun Medical BV, Oss, The Netherlands). On day 14, the second
sensitization was performed by intraperitoneally injecting 50 µg of
OVA in saline. From days 15 to 26, mice of the DC group and Dex
group received DC (20 mg/ml) and Dex (2.5 mg/ml) by oral gavage.
Mice belonging to the control and OVA groups received sham saline.
On days 27, 28, and 29, the OVA, Dex, and DC groups were challenged
with inhalation of ultrasonically nebulized 5% OVA solution in
saline, for 20 min. Animals were sacrificed 24 h after day 29, to
evaluate for airway inflammation and the production of
allergen-specific cytokines (Fig.
1A).
Analysis of bronchoalveolar lavage
fluid (BALF) and lung homogenates
The airway lumina of the sacrificed animals were
washed using a tracheal cannula with 1 ml of saline twice. The BALF
so obtained, was centrifuged and supernatants were stored at −80°C
and subjected to ELISA assay using kits. Total and differential
cell numbers were counted double-blind, using a hemocytometer.
Cytospin cell preparations were made by placing the cells onto
glass slides, centrifuging at 4°C for 10 min at 1,000 × g, and
staining with Diff-Quik. Lung tissues were homogenized in saline to
a concentration of 100 mg/ml with the complete, Mini, EDTA-free
Protease Inhibitor (Roche Applied Science) and the debris-free
supernatant was used for cytokine measurement.
Histopathological examination of lung
tissue
After collecting the BALF, the lobes of the lung
were removed for histological examination, fixed in 10%
paraformaldehyde, and embedded in paraffin, using standard
methods.
Hematoxylin and eosin staining to assess the general
morphological structure of lung tissue. Periodic acid-Schiff (PAS)
staining for visualizing goblet cell hyperplasia. PAS staining was
performed on the lung tissue sections to visualize the development
of goblet cell hyperplasia. Congo red staining for visualizing
eosinophilic infiltration of the nasal mucosa. Eosinophils were
morphologically defined by the presence of granules in the
cytoplasm and a two-lobed nucleus and counted under a microscope.
Masson's trichrome staining was used to reveal the sub-epithelial
deposition of collagen in the lung tissue. Positive
trichrome-stained areas to assess the degree of sub-epithelial
fibrosis.
Measurement of cytokine levels in BALF
and lung homogenates
For assessing the Th1 response, the level of
anti-inflammatory (Th1-associated) cytokines, such as IFN-γ and
IL-10, in BALF and/or lung homogenates was assayed using ELISA kits
(R&D Systems), following the manufacturer's instructions.
For assessing the Th2 response, we evaluated the
secretion of inflammatory (Th2-associated) cytokines, such as IL-4,
IL-5, and IL-13 and proinflammatory cytokines, including IL-6, in
BALF and lung homogenates using ELISA kits (R&D Systems), as
per the manufacturer's instructions.
Measurement of serum levels of total
and OVA-specific IgE and OVA-specific IgG1 and IgG2a
Blood was collected from the orbital venous plexus
of anesthetized mice 24 h after the last challenge. The samples
were centrifuged (1,000 × g, 10 min, 4°C) to isolate the serum and
stored at −80°C until further analysis. Thereafter, the serum was
separated and levels of total IgE and OVA-specific IgE, IgG1, and
IgG2a were measured in each group using ELISA (Chondrex), following
the manufacturer's instruction.
Measurement of NF-κB, p-NF-κB, IκB,
and p-IκB in BALF and lung tissue
The expression of NF-κB p65, IκB, phosphorylation of
NF-κB p65 (p-NF-κB p65) and p-IκB in BALF and lung homogenate were
examined by using ELISA kits (eBioscience Inc.), according to the
manufacturer's instructions. The optical density was measured in
96-well plates using an ELISA reader, at 450 nm. We evaluated the
activation of NF-κB p65 and p-NF-κB p65 and the nuclear
translocalization of NF-κB in lung tissues, using ProteinSimple
capillary immunoassay (Wes) method a gel- and blot-free method
requiring less sample, antibody, and time to run than conventional
western blot assays.
Measurement of TNF-α and IL-6
production and NF-κB and p-NF-κB activation in HMC-1 cells
HMC-1 cells were pretreated with various
concentrations of DC (0.1, 1.0, and 10 mg/ml) for 30 min and
stimulated with 10 µM of A23187 and 200 nM of PMA overnight. The
samples were centrifuged (1,000 × g, 10 min, 4°C) and the
supernatants were used to evaluate the concentration of cytokines,
including IL-6 and TNF-α. The activation of NF-κB and p-NF-κB was
quantified using ELISA, according to the manufacturer's
instructions (R&D Systems).
Statistical analysis
Each experiment was repeated three times with six
mice per group. Data are expressed as mean ± SEMs. Statistical
comparisons were performed using one-way ANOVA, followed by
Fisher's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of DC on infiltration of
inflammatory cells in BALF of OVA-induced asthma mouse model
To examine the anti-inflammatory effect of DC on
allergic asthma, we used the OVA-induced allergic asthma mouse
model (Fig. 1A). The Cytospin cell
preparations showed that the number of inflammatory cells,
especially eosinophils, was markedly increased in the
OVA-challenged mice compared with the control group. However, the
DC and Dex treatment groups showed few cells-mostly macrophages
(Fig. 1B). We measured the number
of total and differential cells, including macrophages,
neutrophils, and eosinophils, in BALF. The OVA-challenged group
showed a significant increase in the number of total cells and
inflammatory cells, including eosinophils and neutrophils, compared
with the control group (Fig. 1C).
However, treatment with DC markedly reduced the number of total
cells and inflammatory cells compared with the OVA-challenged
group.
Effect of DC on pulmonary inflammation
in OVA-induced allergic asthma mouse model
To evaluate histological changes after DC treatment,
in the OVA-induced asthma model, the levels of general pathological
changes, eosinophil infiltration, mucus production, goblet cell
hyperplasia, and bronchial subepithelial fibrosis were examined in
the lung tissue. Pulmonary histopathology was found to be normal in
the control group. Mice from the OVA-challenged group exhibited a
severe infiltration of inflammatory cells around the respiratory
tract and blood vessels (Fig. 2)
compared with mice of the DC group, which showed few or no
inflammatory cells around the airway, and the difference between
the groups was statistically significant. Dex treatment mitigated
inflammation around the airway wall (bronchi). Mucus overproduction
and goblet cell hyperplasia was observed in the bronchi and
eosinophil infiltration increased in the peri-bronchial epithelium
in the OVA group compared with the control group. In contrast, the
DC group showed a significant reduction in goblet cell and
eosinophil numbers. Masson's trichrome staining revealed prominent
collagen deposition and tissue fibrosis around the bronchial
epithelia and blood vessels in the OVA group. In contrast, the DC
and Dex groups displayed reduced collagen deposition. Histological
examination of lung tissues showed similar numbers of eosinophils
as that in the BALF. Collectively, these results suggest that
cellular responses other than eosinophilic infiltration may play a
critical role in the development of allergic inflammation, in the
mouse model used in this study.
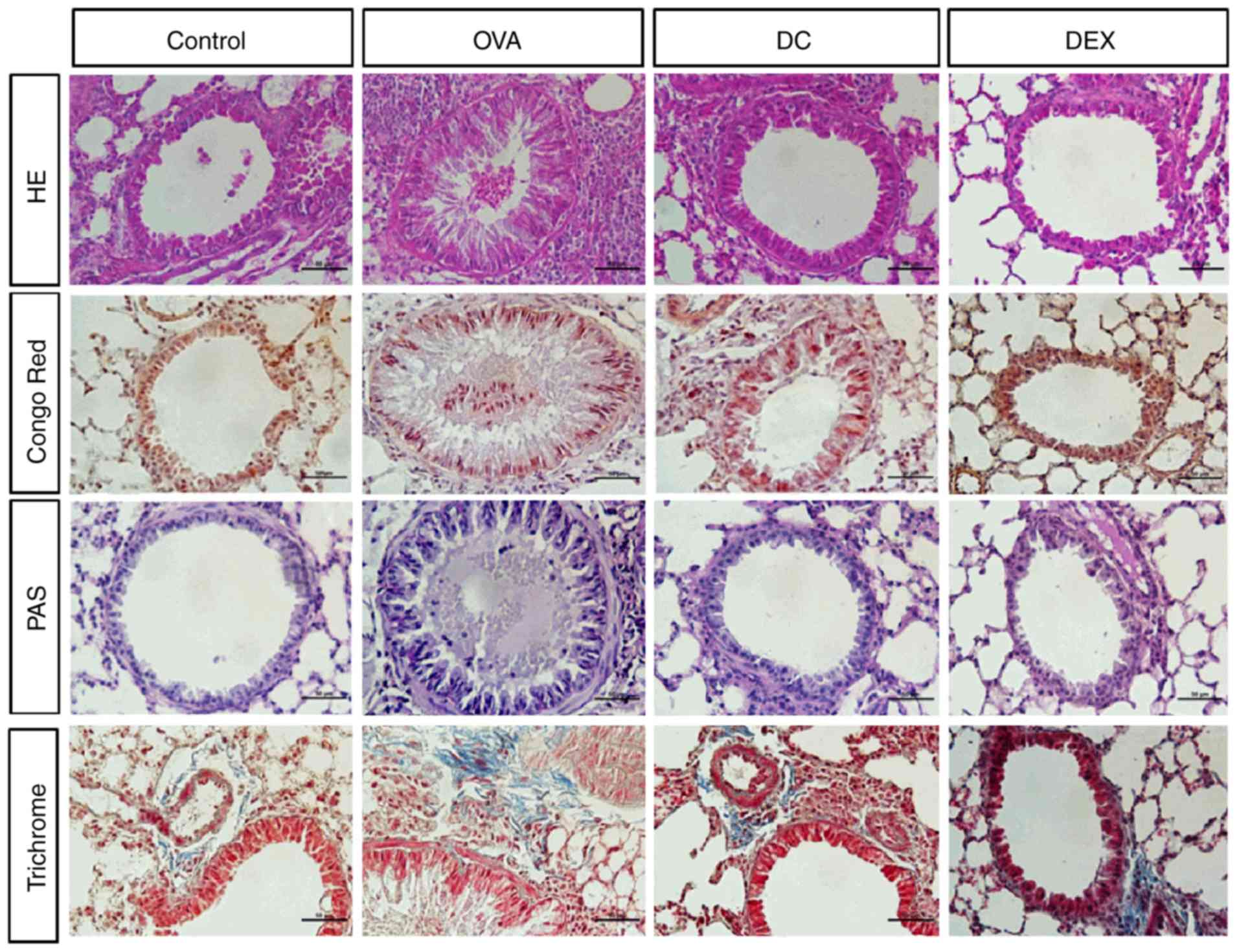 | Figure 2.Effect of DC on airway
inflammation in lung tissues. The histological changes after DC
treatment in ovalbumin-induced asthma model, hematoxylin and eosin
staining to visualize pathological changes, Congo red staining for
eosinophil infiltration, periodic acid-Schiff staining for
visualizing mucus production and goblet cell hyperplasia, and
Masson's trichrome staining for bronchial subepithelial fibrosis,
were examined in lung tissue. OVA, ovalbumin; DC, Dryopteris
crassirhizoma; DEX, dexamethasone; PAF, periodic acid-schiff; HE,
haematoxylin and eosin. |
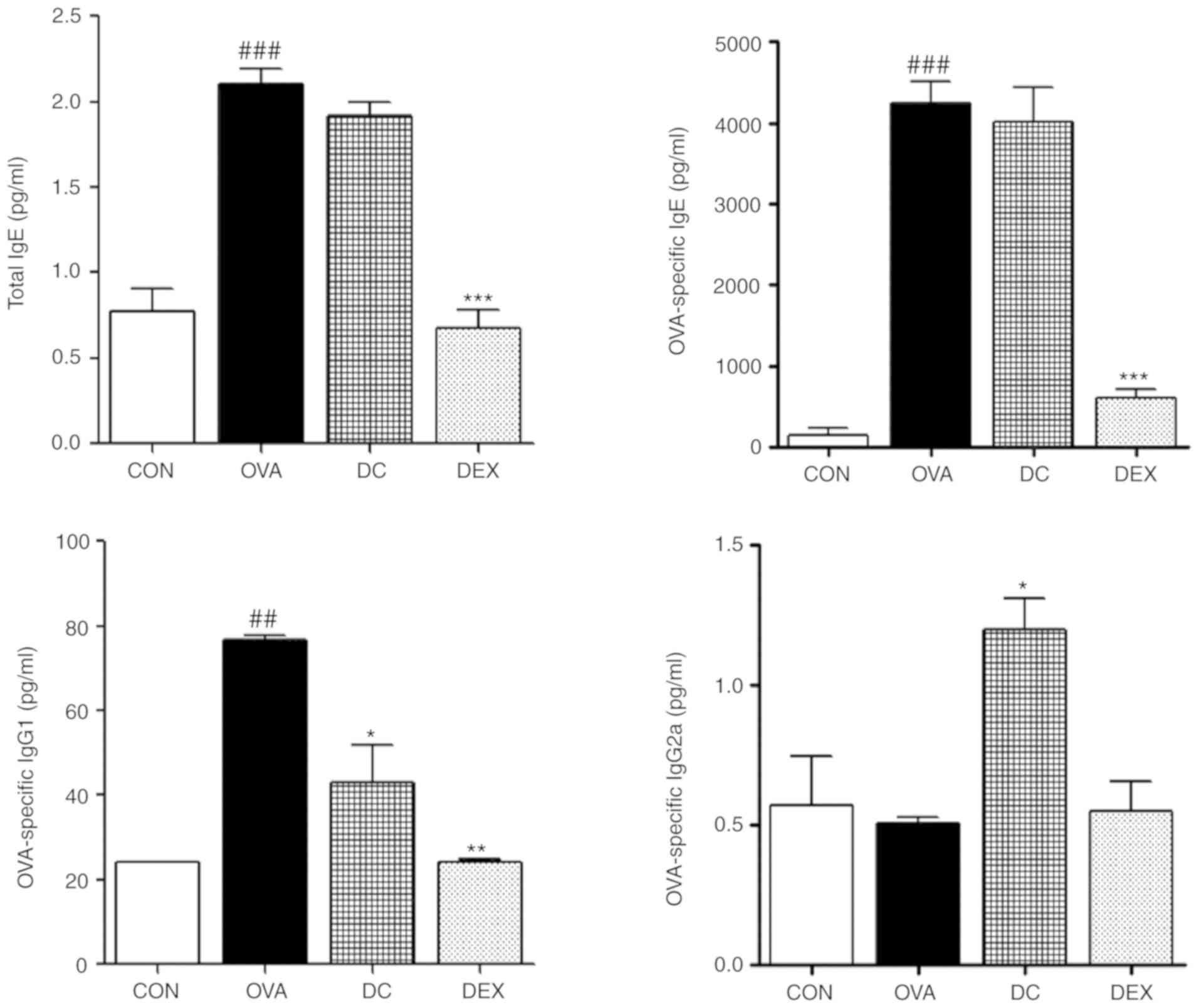
Effect of DC on the serum levels of
total and OVA-specific IgE, IgG1, and IgG2a
To investigate the therapeutic impact of DC on
OVA-specific immune responses, we determined the serum
immunoglobulin levels. As shown in Fig. 3, the levels of total and
OVA-specific IgE and IgG1 were markedly increased in the OVA group
compared with the control group. However, the DC treatment group
demonstrated substantially decreased levels of total and
OVA-specific IgE and IgG1 compared with the OVA group. Moreover,
the level of OVA-specific IgG2a was upregulated by DC treatment.
These results indicated that DC might facilitate anti-allergic
activity by suppressing the production of allergic mediators.
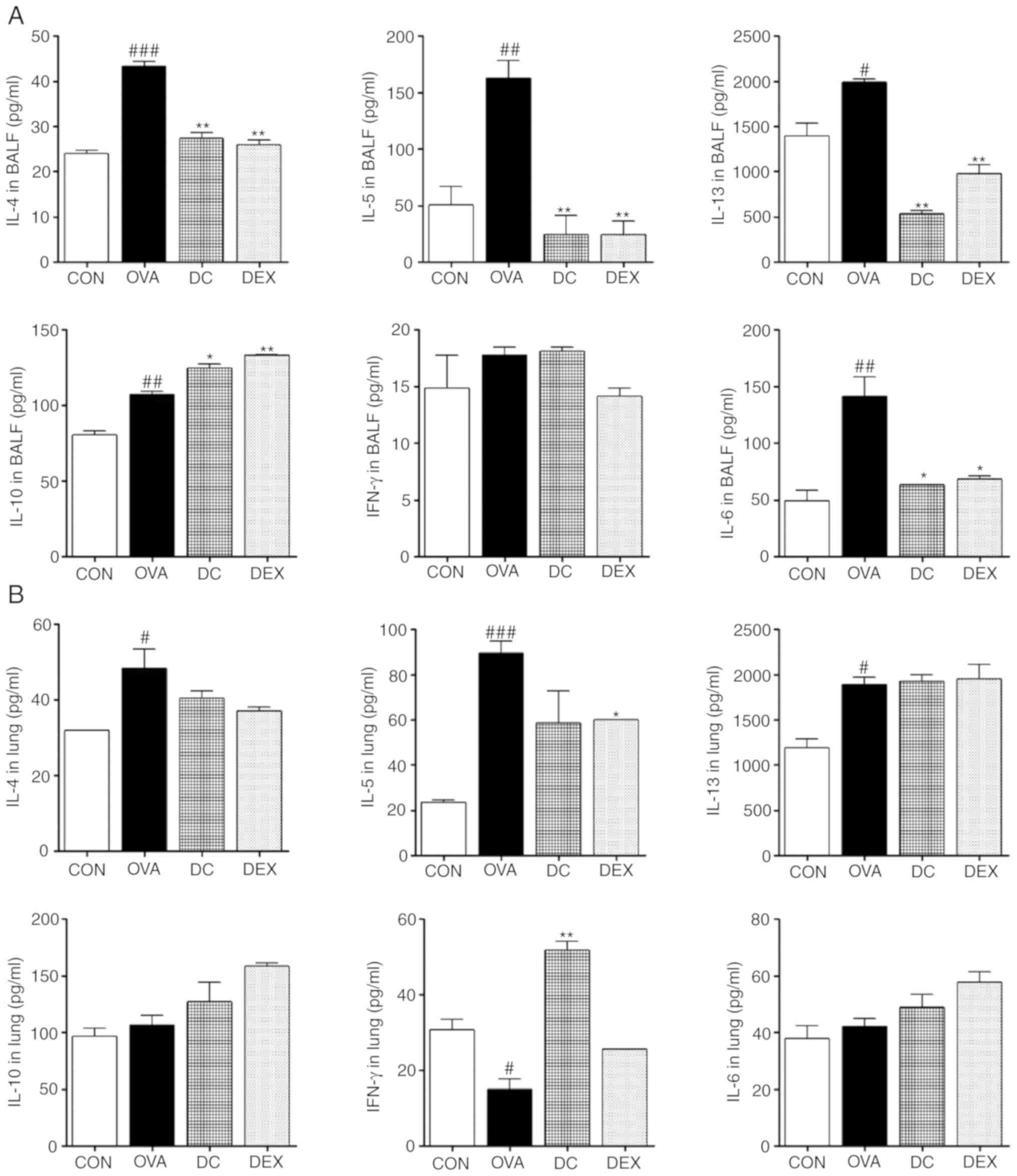
Effect of DC on the levels of Th1 and
Th2 cytokines in BALF and lung homogenates
To elucidate the anti-allergic mechanism of DC on
the Th1/Th2-mediated allergic response in the OVA-induced asthma
mice models, the levels of secreted cytokines in BALF and lung
homogenates were examined. First, we examined the secretion of the
Th2 cytokines and proinflammatory cytokines, including IL-4, IL-5,
IL-6, and IL-13, using ELISA. The OVA group showed increased levels
of both in BALF and lung homogenates compared with the control
group. However, DC treatment significantly decreased the levels of
cytokines, including IL-4, IL-5, IL-6, and IL-13. In addition, the
levels of anti-inflammatory cytokines (Th1 cytokines), such as
IFN-γ and IL-10, were assayed. The DC-treated group showed a
significant increase in the levels of IL-10 in BALF and IFN-γ in
lung homogenates, and a decrease in IL-10 in lung homogenates and
IFN-γ in BALF, as compared to the OVA group; however, no
statistically significant differences were observed (Fig. 4). These results were consistent
with the reduced infiltration of inflammatory cells in BALF and the
lung tissue of DC-treated mice, via modulation of the level of
Th1/Th2 cytokines. In this way, DC can reduce bronchial
inflammation and immune reaction in patients with asthma.
Effect of DC on the OVA-induced
activation of the NF-κB signaling pathway
To understand the inhibitory effect of DC on
allergic asthma, activation of NF-κB signaling-that regulates the
expression Th2 cytokines-was measured in OVA-challenged mice. The
levels of NF-κB p65 and p-NF-κB p65 activation in the lung tissue
were assessed via western blot analysis. As presented in Fig. 5A, the activation of NF-κB p65 and
p-NF-κB p65 was significantly upregulated in the OVA group compared
with the control group. However, this increase was effectively
blocked by DC treatment.
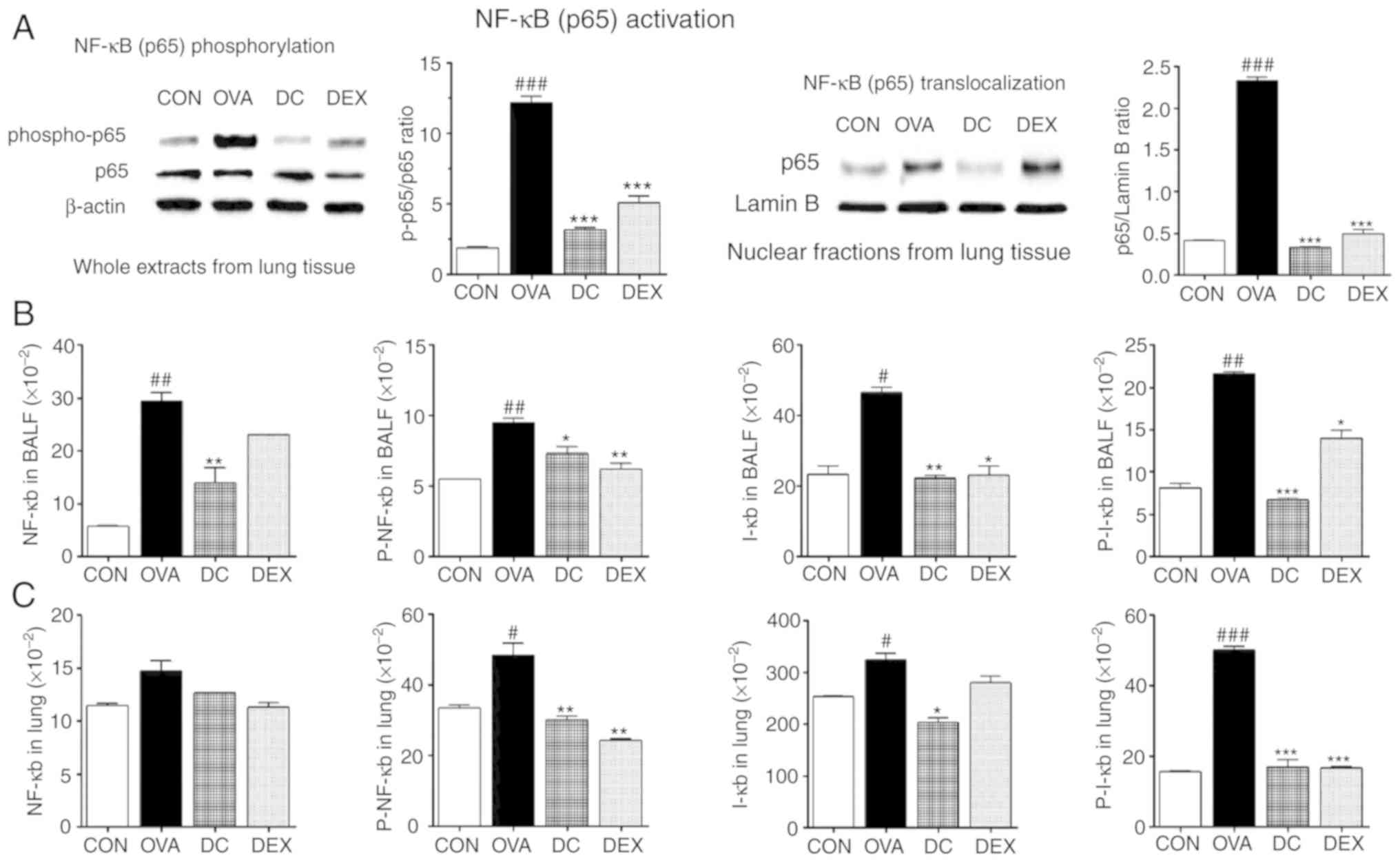 | Figure 5.Effect of DC on NF-ĸB
signaling pathway in BALF and lung homogenates. (A) Western blot
analysis of NF-ĸB p65 and p-NF-ĸB p65 from the whole cell extract
and NF-ĸB from nuclear fractions of lung tissue, (B) The levels of
NF-ĸB, p-NF-κB, IĸB, and p-IĸB in BALF and (C) lung homogenates.
###P<0.001, ##P<0.01,
#P<0.05 vs. control group. ***P<0.001, **P<0.01
and *P<0.05 vs. ovalbumin group. OVA, ovalbumin; DC, Dryopteris
crassirhizoma; DEX, dexamethasone; p, phosphorylated; BALF,
bronchoalveolar lavage fluid. |
Next, we determined whether DC suppressed nuclear
translocalization of NF-κB in the lung tissue. As shown in Fig. 5A, the OVA group showed
translocalization of p65 into the nucleus. In contrast, DC
inhibited translocalization of p65. These findings suggest a
significant anti-inflammatory role of DC.
We assessed the levels of total NF-κB and IκB, as
markers of NF-κB activation, degradation levels of p-NF-κB, and
p-IκB activation. NF-κB, p-NF-κB, IκB, and p-IκB levels were higher
in the OVA group compared with the control group, both in BALF and
lung homogenates. However, we found that DC treatment downregulated
the levels of NF-κB, p-NF-κB, IκB, and p-IκB in BALF and p-NF-κB,
IκB, and p-IκB in lung homogenates, compared with the OVA group. DC
treatment had a tendency to decrease the levels of NF-κB signaling
components in lung homogenates. In addition, the Dex group also
showed inhibitory effects on NF-κB signaling (Fig. 5B and C). These findings suggest a
potential role of the NF-κB pathway, in the suppression of
inflammatory mediators by DC, in allergic asthma.
These data combined with those presented in Fig. 5 suggest that the protective effects
of DC on allergic airway inflammation are mediated by
downregulating NF-κB activation.
Effect of DC on TNF-α and IL-6
expression in HMC-1 cells stimulated by a combination of PMA and
A23187
Mast cells play a major role in allergic
inflammation. Pro-inflammatory cytokines, such as TNF-α and IL-6,
are potent multifunctional cytokines that play an important role in
the pathogenesis of allergic disease (17). We examined whether DC could inhibit
the expression of TNF-α and IL-6 in HMC-1 cells using ELISA. As
shown in Fig. 6A, HMC-1 cells
stimulated by a combination of PMA and A23187, showed an increase
in TNF-α and IL-6 levels compared with untreated control cells.
Treatment of HMC-I cells with DC significantly attenuated the
expression of TNF-α and IL-6 levels, in a dose-dependent manner.
Thus, DC treatment showed anti-allergic effect by inhibiting the
expression of allergic and inflammatory mediators, in HMC-1 cells
stimulated by PMA and A23187.
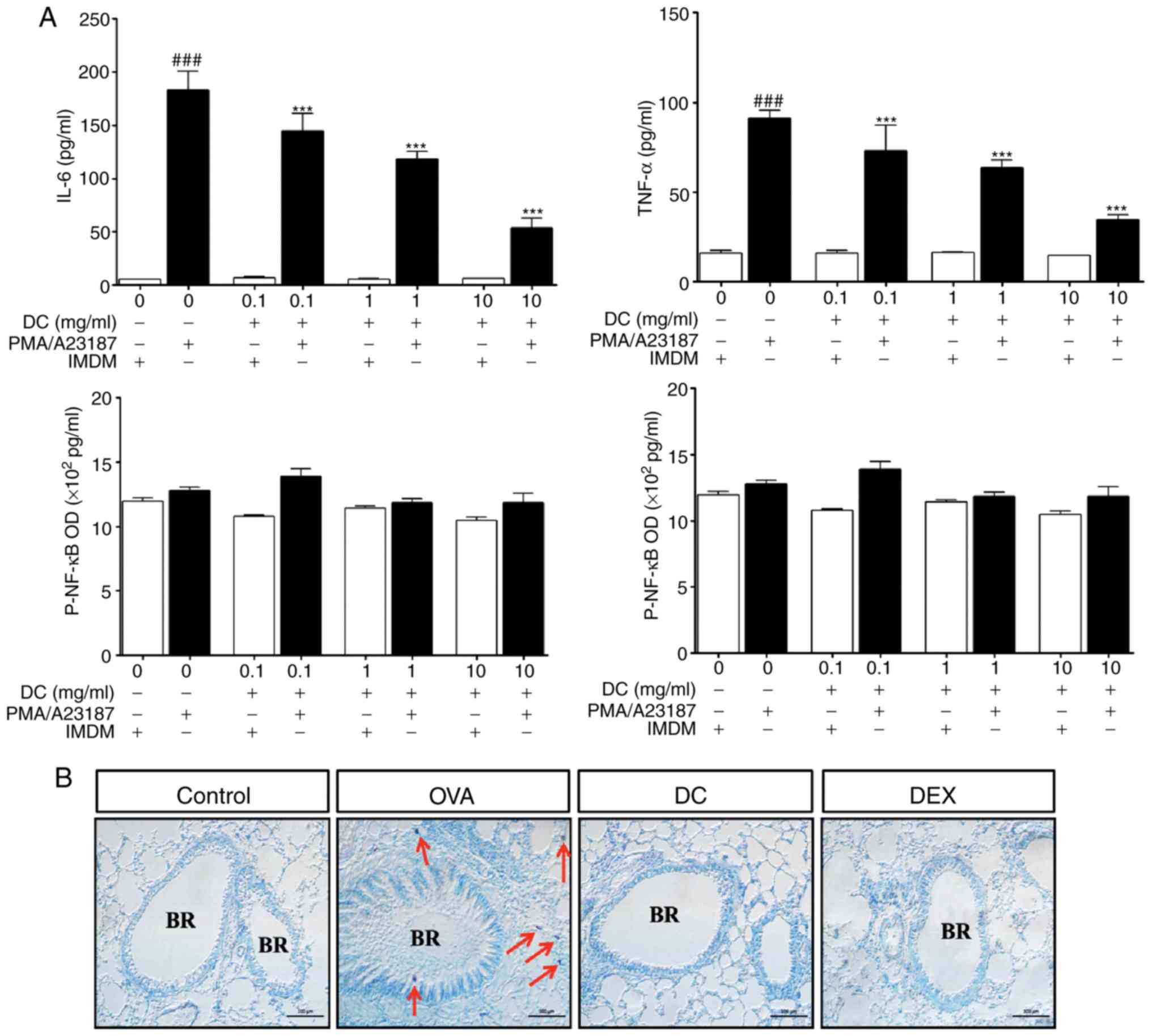 | Figure 6.Effect of DC on the expression of
TNF-α and IL-6 and NF-ĸB signaling pathway in HMC-1 cells,
stimulated by a combination of PMA and A23187. and infiltration of
mast cells in the lung tissue. HMC-1 cells were pretreated with DC
(0.1, 1.0, and 10 mg/ml) and stimulated with 10 µM of A23187 and
200 nM of PMA, overnight. The secretion levels of (A) TNF-α and
IL-6 were measured using ELISA. (B) Staining and localization of
mast cells in pulmonary sections from the ovalbumin-induced asthma
mice. Representative Giemsa staining of lung sections of different
groups of mice. Arrows point to stained mast cells.
###P<0.001, ***P<0.001 vs. ovalbumin group. BR,
bronchial; OVA, ovalbumin; DC, Dryopteris crassirhizoma; DEX,
dexamethasone; IL, interleukin. |
Effect of DC on infiltration of mast
cells in lung tissues of OVA-induced allergic asthma
Activated mast cells play a key role in allergy by
releasing numerous mediators, such as cytokines and leukotrienes,
via degranulation (18).
Therefore, we evaluated local infiltration by mast cells and
determined the inhibitory effect of DC on mast cell infiltration in
the OVA-induced allergic asthma mouse model. Giemsa staining of the
lung tissue was done for determining local infiltration by mast
cells and assessing the inhibitory effect of DC on allergic asthma.
As shown in Fig. 6B, the number of
mast cells in the lung tissue of the OVA-induced allergic asthma
mice was significantly higher than that in the control mice (red
arrows). In the DC treated mice, OVA-induced infiltration of mast
cells was markedly decreased. Thus, these findings indicate that DC
treatment can efficiently inhibit inflammatory cells, including
eosinophils, goblet cells, and mast cells in lung tissues.
Effect of DC on the activation of
NF-κB in HMC-1 cells stimulated by a combination of PMA and
A23187
To further investigate whether the NF-κB pathway
plays an important role in the DC-mediated regulation of allergic
inflammation, we analyzed the activation of NF-κB and p-NF-κB in
HMC-1 cells. In HMC-1 cells, NF-κB activation was inhibited
dose-dependently by DC treatment compared to PMA and A2318
treatment alone (Fig. 6A).
However, stimulation with DC had no effect on the expression of PMA
and A23187 induced activation of p-NF-κB (Fig. 6B). Thus, these results further
confirmed that the role of DC in mediating anti-allergic and
anti-inflammatory effects.
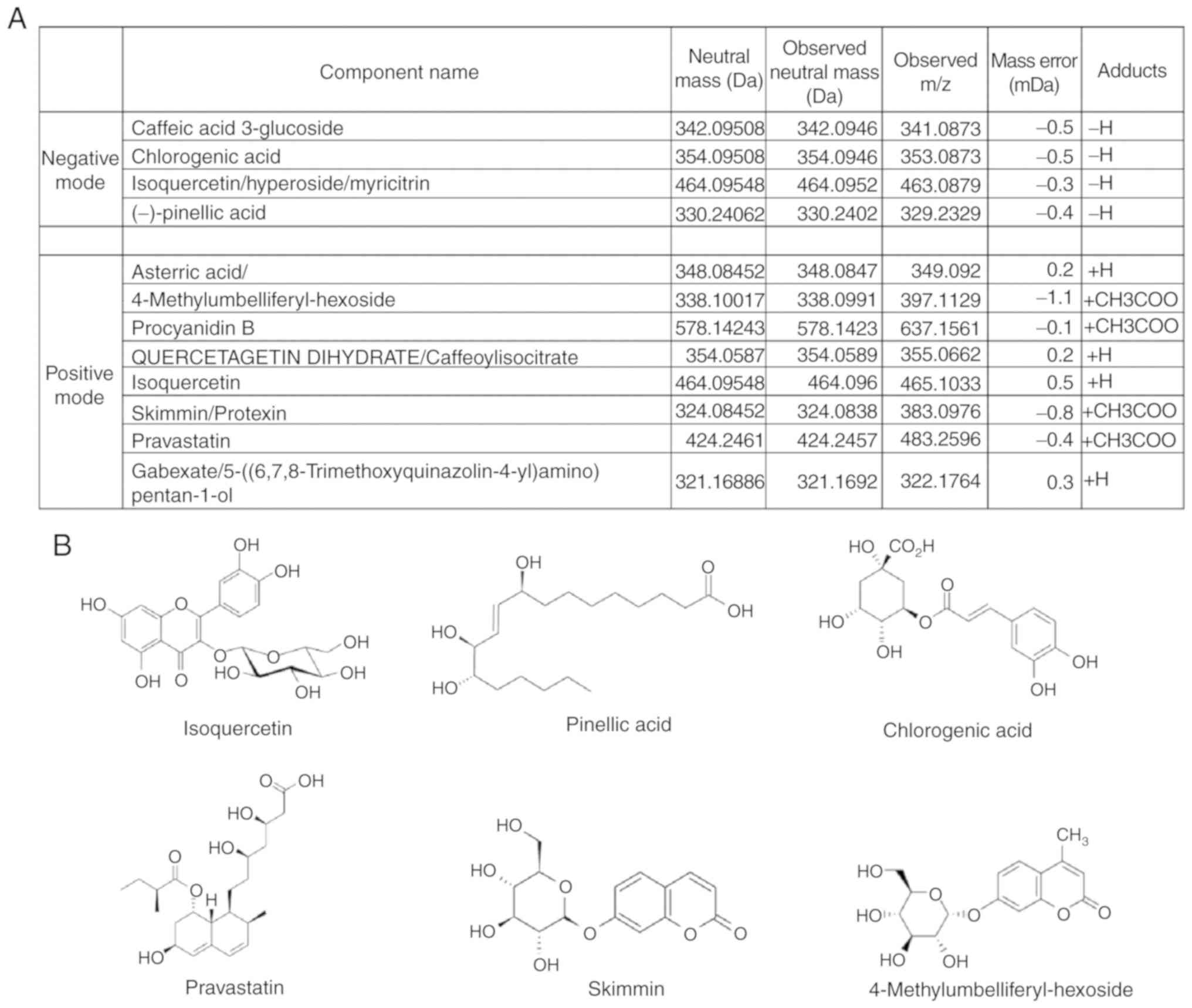
Active components of DC
To investigate the main component present in the
ethanolic extract of DC, UPLC/Q-TOF MS was used. The results showed
four negative and eight positive modes (Fig. 7), which were identified as follows:
Caffeic acid 3-glucoside, chlorogenic acid, isoquercetin, pinellic
acid, procyanidin B, quercetagetin dehydrate, asterric acid,
skimming/protexin, pravastatin, 4-methylumbelliferyl-hexoside, and
gabexate/5-[(6,7,8-trimethoxyquinazolin-4-yl)
amino)]pentan-1-ol.
Discussion
The aim of this study was to investigate the
anti-allergic and anti-inflammatory effects of DC using the
OVA-induced allergic asthma mouse model. OVA-challenged mice showed
an increased number of inflammatory cells in BALF; elevated total
IgE, anti-OVA IgG1 and IgE in the serum; increased Th2 cytokines,
including IL-4, IL-5, IL-6, and IL-13 in BALF; goblet cell
hyperplasia with excessive airway mucus and collagen deposition;
eosinophil and mast cell infiltration in lung tissues; activated
NF-κB signaling in BALF and lung homogenates; and NF-κB activation
in PMA and A23187-stimulated HMC-1 cells. However, DC treatment
increased the level of Th1 cytokines and decreased the Th2
cytokines; inhibited NF-κB signaling activation in BALF, lung
homogenates, and PMA and A23187-stimulated HMC-1 cells; inhibited
total IgE, anti-OVA IgG1, and IgE production in the serum; and
lowered the number of inflammatory cells in BALF. The
administration of DC also suppressed the infiltration of
eosinophils and mast cells, mucus overproduction, and collagen
deposition. In addition, DC treatment attenuated TNF-α and IL-6 in
PMA and A23187-stimulated HMC-1 cells. Thus, we demonstrated that
DC has anti-allergic and anti-inflammatory effects on an
OVA-induced asthma mouse model.
Medicinal herbs have been used in traditional
medicines in Asian countries, such as Korea and China, since
ancient times (19). DC is used as
a traditional herbal remedy for various diseases, especially to
treat tapeworm infestation, cold, and cancer (20). Isoquercetin was identified as a
positive mode in DC. Isoquercetin exhibits a variety of medicinal
properties, which include oxidative stress attenuation (21) and anti-inflammatory properties
(22). Statins,
hydroxymethylglutaryl-coenzyme A reductase inhibitors, currently
used as lipid-lowering agents, have pleiotropic anti-inflammatory
and immune-modulating effects. The benefits of statins in airway
inflammation have been shown in patients with allergic asthma and
smoking-related asthma (23). To
evaluate the anti-inflammatory and anti-asthmatic effects and
possible mechanism of DC, we performed an in vivo study
using an OVA-induced asthma mouse model.
Asthma is a multifactorial and complex disease in
which repetitive allergen challenge leads to permanent airway
remodeling (24). Various
medicinal herbs have been shown to downregulate the secretion of
Th2 cytokines, IL-4, IL-5, and IL-13 (25). Indeed, treatment with DC decreased
the expression of IL-4, IL-5, IL-6, and IL-13 and enhanced the
production of IL-10 and IFN-γ in BALF. A reduction in the
expression of IL4, IL-5, and IL-13 by DC, is a key indicator of
attenuation of allergic asthma symptoms, indicating inhibition of
Th2 dominant responses and regulation of Th1/Th2 immune balance by
DC.
Previous studies have shown that NF-κB plays an
important role in cytokine production (26). NF-κB signaling pathway has been
shown to play important roles in the development of inflammatory
diseases, including asthma (27,28).
To explore the protective mechanism of DC, the effects of DC on
OVA-induced NF-κB activation and in PMA and A23187-stimulated HMC-1
cells were measured. Here, we showed that the DC treated group had
significantly inhibited NF-κB activation. These findings indicate
that DC inhibited Th2 cytokine production may be related to the
suppression of NF-κB activation.
A23187 and PMA induced an increase in cytosolic
calcium concentration, leading to mast cell degranulation (29). HMC-1 have been widely used to
investigate the beneficial effects of anti-allergic drugs (30). Thus, in the present study, A23187
and PMA stimulated HMC-1 were used to investigate the anti-allergic
effects of DC in vitro. Our data showed that DC decreased
the production of TNF-α and IL-6 in HMC-1 by suppressing
degranulation. Therefore, our results suggest that DC exerts a
significant anti-allergic effect by reducing TNF-α and IL-6
production.
In summary, our data demonstrated that DC exerts a
pivotal role in OVA-induced airway inflammation, both in
vivo and in vitro, by reducing the infiltration of
inflammatory cells, particularly eosinophils, mucus overproduction,
NF-κB expression, and modulating Th1/Th2 cytokines. Serum levels of
total and OVA-specific IgE and IgG1 were significantly lower and
OVA-specific IgG2a was higher upon DC treatment compared with OVA
treatment. Furthermore, DC treatment inhibited production of
inflammatory cytokines, such as TNF-α and IL-6, and NF-κB
activation in PMA and A23187-stimulated HMC-1 cells. Collectively,
these findings suggest that DC exerts potent anti-asthmatic and
anti-inflammatory effects in the treatment of allergic asthma.
Acknowledgements
Not applicable.
Funding
This research was supported by the Korea Food
Research Institute (grant no. E0170401-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OHC designed the experiments. CHP, TTB, TVN and YF
performed the experiments. CHP and HSS analyzed the data and
performed the biological analysis. CHS and HTK collected and
analyzed data. CHP, YF and TVN sacrificed mice and performed the
ELISA assay, DS took samples and performed western blot analysis.
SYL also checked the quality of DC and performed western blot
analysis. The manuscript was written by CHP and OHC. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DC
|
Dryopteris crassirhizoma
|
|
Dex
|
dexamethasone
|
|
OVA
|
ovalbumin
|
|
Con
|
control
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
HE
|
hematoxylin and eosin
|
|
PAS
|
periodic acid-schiff
|
|
I.P
|
intraperitoneal
|
|
P.O
|
peroral
|
|
HMC
|
human mast cell
|
|
Th1
|
T helper type 1
|
|
Th2
|
T helper type 2
|
|
IFN
|
Interferon
|
|
PMA
|
Phorbol myristate acetate
|
References
|
1
|
Hamid Q and Tulic M: Immunobiology of
asthma. Annu Rev Physiol. 71:489–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spina D: Modulation of sensory nerve
function in the airways. Pulm Pharmacol Ther. 11:319–330. 1998.
View Article : Google Scholar
|
|
3
|
Barnes PJ: The cytokine network in asthma
and chronic obstructive pulmonary disease. J Clin Invest.
118:3546–3556. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi IW, Kim DK, Ko HM and Lee HK:
Administration of antisense phosphorothioate oligonucleotide to the
p65 subunit of NF-kappaB inhibits established asthmatic reaction in
mice. Int Immunopharmacol. 4:1817–1828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang NI, Yoon HY, Lee YR, Won M, Chung MJ,
Park JW, Hur GM, Lee HK and Park BH: A20 attenuates allergic airway
inflammation in mice. J Immunol. 183:1488–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desmet C, Gosset P, Pajak B, Cataldo D,
Bentires-Alj M, Lekeux P and Bureau F: Selective blockade of
NF-kappa B activity in airway immune cells inhibits the effector
phase of experimental asthma. J Immunol. 173:5766–5775. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh SW, Cha JY, Jung JE, Chang BC, Kwon HJ,
Lee BR and Kim DY: Curcumin attenuates allergic airway inflammation
and hyper-responsiveness in mice through NF-κB inhibition. J
Ethnopharmacol. 136:414–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Surh YJ, Na HK, Lee JY and Keum YS:
Molecular mechanisms underlying anti-tumor promoting activities of
heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 16
(Suppl):S38–S41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markman M: Safety issues in using
complementary and alternative medicine. J Clin Oncol. 20 (Suppl
18):S39–S41. 2002.
|
|
10
|
Wang J, Yan YT, Fu SZ, Peng B, Bao LL,
Zhang YL, Hu JH, Zeng ZP, Geng DH and Gao ZP: Anti-influenza virus
(H5N1) activity screening on the phloroglucinols from rhizomes of
Dryopteris crassirhizoma. Molecules. 22:4312017. View Article : Google Scholar
|
|
11
|
Lee HB, Kim JC and Lee SM: Antibacterial
activity of two phloroglucinols, flavaspidic acids AB and PB, from
Dryopteris crassirhizoma. Arch Pharm Res. 32:655–659. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakane H, Arisawa M, Fujita A, Koshimura S
and Ono K: Inhibition of HIV-reverse transcriptase activity by some
phloroglucinol derivatives. FEBS Lett. 286:83–85. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SM, Na MK, An RB, Min BS and Lee HK:
Antioxidant activity of two phloroglucinol derivatives from
Dryopteris crassirhizoma. Biol Pharm Bull. 26:1354–1356.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JS, Miyashiro H, Nakamura N and
Hattori M: Two new triterpenes from the Rhizome of Dryopteris
crassirhizoma, and inhibitory activities of its constituents on
human immunodeficiency virus-1 protease. Chem Pharm Bull (Tokyo).
56:711–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang X, Li W, Koike K, Wu L and Nikaido
T: Phenolic constituents from the rhizomes of Dryopteris
crassirhizoma. Chem Pharm Bull (Tokyo). 54:748–750. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang YH, Wang W, Yu SW, Ye M, He XH, Gong
NB, Lu Y, Khan IA and Guo DA: A new chiratane type triterpenoid
from the rhizomes of Drynaria fortunei. Fitoterapia. 81:988–991.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grzelewska-Rzymowska I and Pietrzkowicz M:
Role of tumor necrosis factor-alpha in allergic inflammation and
airway hyperresponsiveness. Pol Merkur Lekarski. 16:173–178.
2004.(In Polish). PubMed/NCBI
|
|
18
|
Church MK and Levi-Schaffer F: The human
mast cell. J Allergy Clin Immunol. 99:155–160. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marini-Bettolo GB: Present aspects of the
use of plants in traditional medicine. J Ethnopharmacol. 2:5–7.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang SH, Bae JH, Hong DP, Choi KD, Kim
SC, Her E, Kim SH and Kang CD: Dryopteris crassirhizoma has
anti-cancer effects through both extrinsic and intrinsic apoptotic
pathways and G0/G1 phase arrest in human prostate cancer cells. J
Ethnopharmacol. 130:248–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jayachandran M, Zhang T, Wu Z, Liu Y and
Xu B: Isoquercetin regulates SREBP-1C via AMPK pathway in skeletal
muscle to exert antihyperlipidemic and anti-inflammatory effects in
STZ induced diabetic rats. Mol Biol Rep. 47:593–602. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morikawa K, Nonaka M, Narahara M, Torii I,
Kawaguchi K, Yoshikawa T, Kumazawa Y and Morikawa S: Inhibitory
effect of quercetin on carrageenan-induced inflammation in rats.
Life Sci. 74:709–721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McKay A, Leung BP, McInnes IB, Thomson NC
and Liew FY: A novel anti-inflammatory role of simvastatin in a
murine model of allergic asthma. J Immunol. 172:2903–2908. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piao CH, Bui TT, Song CH, Shin HS, Shon DH
and Chai OH: Trigonella foenum-graecum alleviates airway
inflammation of allergic asthma in ovalbumin-induced mouse model.
Biochem Biophys Res Commun. 482:1284–1288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mueller M, Beck V and Jungbauer A: PPARα
activation by culinary herbs and spices. Planta Med. 77:497–504.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-κB pathway in asthma
and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henderson WR Jr, Chi EY, Teo JL, Nguyen C
and Kahn M: A small molecule inhibitor of redox-regulated NF-kappa
B and activator protein-1 transcription blocks allergic airway
inflammation in a mouse asthma model. J Immunol. 169:5294–5299.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baba Y, Nishida K, Fujii Y, Hirano T,
Hikida M and Kurosaki T: Essential function for the calcium sensor
STIM1 in mast cell activation and anaphylactic responses. Nat
Immunol. 9:81–88. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh HA, Kim HM and Jeong HJ: Distinct
effects of imperatorin on allergic rhinitis: Imperatorin inhibits
caspase-1 activity in vivo and in vitro. J Pharmacol Exp Ther.
339:72–81. 2011. View Article : Google Scholar : PubMed/NCBI
|