Introduction
The mammary gland is specific to mammals, and serves
to produce milk as a source of nutrition and immune factors for
offspring (1). Periodic changes in
the structure and function of the mammary gland occur throughout
the life of female mammals; ductal development occurs primarily in
puberty and is vital for pregnancy and lactation (2,3).
Ductal elongation occurs rapidly at the onset of puberty and
terminal end buds are repositioned to the ductal termini and invade
through the fat pad to form the ductal tree (4). The ductal system is the result of the
concerted actions of growth hormones, estrogen and insulin-like
growth factor 1 (IGF-1) (5,6).
Puberty is also the developmental point where diet has the most
profound influence on ductal development (7,8). For
example, either protein- (9) or
energy-based (10,11) diets can adversely affect mammary
gland development.
Notably, environmental factors, such as heat stress
(12) or H2S (13), have been reported to exhibit
restrictive effects on the development of the mammary gland duct.
Furthermore, the phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt) signaling pathway has an important role in mammary gland
development (10) and in cell
proliferation by regulating the expression of proteins that mediate
G1-phase to S-phase transition in the cell cycle
(5). Additionally, the mammalian
target of rapamycin (mTOR) signaling pathway has been reported to
participate in the regulation of mammary gland biochemical changes
(14), milk protein synthesis in
bovine mammary epithelial cells (15), and cell proliferation of bovine
mammary epithelial cells and porcine mammary gland epithelial cells
(13,16).
In the cell cycle, cyclin D and cyclin E are
required for transition from the G1 phase to the S
phase. During the S phase, cyclin A has been reported to be
involved in the initiation and completion of DNA replication
(17). Proliferating cell nuclear
antigen (PCNA) is considered an essential component for DNA
replication (18). In addition,
p21 is regarded as a potent, tight-binding inhibitor of
cyclin-dependent kinase (19). It
had been reported that different fatty acids may regulate the
proliferation of HC11 cells and mammary gland development of
pubescent mice through modulating the protein and gene expression
levels of cyclin D1 and p21 (10,20,21).
Our previous study examined the effects of the
endogenous signaling molecule H2S on the proliferation
of mammary cells in culture. It was demonstrated that proliferation
of porcine mammary epithelial cells cultured with the
H2S donor sodium hydrosulfide (NaHS) at 10 µM was
stimulatory, whereas proliferation of cells cultured at 600 µM NaHS
was inhibitory (13).
H2S is a gaseous signaling molecule that is synthesized
in vivo and influences normal cellular physiological
processes, including cellular proliferation and differentiation
(22,23), cytoprotection (24), and protection of the cardiovascular
or nervous system (25).
H2S has been reported to be a regulator of numerous
signaling pathways, including the ERK1/2 (26), JAK/STAT (27), Nrf2 (28), mTOR (29) and PI3K/Akt (30) signaling pathways. In addition, our
previous study revealed that H2S affected cultured
mammary cell proliferation by regulating the phosphorylation of key
factors involved in mammary gland development in animals via the
PI3K/Akt-mTOR signaling pathway (13). Thus, the present study was designed
to investigate the effects of exogenous H2S, provided by
NaHS, on the mammary development of pubescent mice. Furthermore,
the contributions of the intracellular PI3K/Akt-mTOR signaling
pathway, IGF-1 and estradiol (E2) in this process were
investigated.
Materials and methods
Reagents
NaHS was obtained from Sigma-Aldrich; Merck KGaA.
RPMI-1640 medium, high glucose (HG)-DMEM and fetal bovine serum
(FBS) were purchased from Gibco; Thermo Fisher Scientific, Inc.
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. Cell-Light EdU in vitro kit was purchased
from Guangzhou Ribobio Co. Ltd. Cyclin D1 was identified using a
rabbit monoclonal antibody against cyclin D1 (cat. no. 2922S; Cell
Signaling Technology, Inc.), PCNA was identified using a mouse
monoclonal antibody against PCNA (cat. no. 2586; Cell Signaling
Technology, Inc.), p21 was targeted using a rabbit monoclonal
antibody against p21 (cat. no. 2947; Cell Signaling Technology,
Inc.), phosphorylated (p)-AktSer473 and Akt were
targeted with rabbit monoclonal antibodies against
p-AktSer473 and Akt (cat. nos. 4060 and 4691; Cell
Signaling Technology, Inc.), p-mTORSer2448 and mTOR were
targeted with rabbit monoclonal antibodies against
p-mTORSer2448 and mTOR (cat. nos. 5536 and 2983; Cell
Signaling Technology, Inc.), p-PI3KTyr508 was targeted
with a goat polyclonal antibody against p-PI3KTyr508
(cat. no. sc-12929; Santa Cruz Biotechnology, Inc.), PI3K was
targeted with a rabbit polyclonal antibody against PI3K (cat. no.
bs-0128R; BIOSS), p-JAK2Tyr1007/Tyr1008 and β-actin were
targeted with rabbit polyclonal antibodies against p-JAK2
Tyr1007/Tyr1008 and β-actin (cat. nos. bs-2485R and
bs-0061R; BIOSS), p-STAT5Tyr694 was targeted with a
rabbit monoclonal antibody against p-STAT5Tyr694 (cat.
no. ab32364; Abcam), JAK2 was targeted with a rabbit monoclonal
antibody against JAK2 (cat. no. 3230; Cell Signaling Technology,
Inc.), STAT5 was targeted with a rabbit polyclonal antibody against
STAT5 (cat. no. 9363S; Cell Signaling Technology Inc.) and IGF-1
was targeted with a rabbit monoclonal antibody against IGF-1 (cat.
no. 28530-1-AP; ProteinTech Group, Inc.). Primary antibodies Cyclin
D1, p21, p-AktSer473, Akt, p-mTORSer2448,
mTOR, PI3K, p-JAK2Tyr1007/Tyr1008, JAK2,
p-STAT5Tyr694, STAT5, IGF-1 and β-actin were conjugated
with goat anti-rabbit secondary antibody (cat. no. bs-0295G;
BIOSS), PCNA was conjugated with goat anti-mouse secondary antibody
(cat. no. bs-0296G; BIOSS) and p-PI3KTyr508 was
conjugated with donkey anti-goat secondary antibody (cat. no.
bs-0294D; BIOSS).
Cell preparation and culture
Due to the convenience of obtaining materials in the
laboratory, pig primary cell culture was chosen instead of the
previously used mice studies. Sow ovaries were obtained from Foshan
Food Co. Ltd. Meat United Processing Factory and primary granulosa
cells (GCs) were isolated as previously described (31). Briefly, follicles with a glossy
appearance that lacked a corpus luteum and appeared normal were
placed in PBS containing 1X penicillin-streptomycin (pen/strep) and
transported to the laboratory quickly for isolation. The follicular
fluid was extracted by superficial insertion of a 1-ml sterile
syringe into ovarian antral follicles, and follicular fluid was
centrifuged at 105 × g at 4°C for 6 min in a centrifuge tube
containing 5 ml HG-DMEM. The cells were cultured with HG-DMEM/10%
FBS and 1% pen/strep. The cells obtained from 10 pairs of ovaries
were seeded in a flask at 37°C for 24 h in an atmosphere containing
5% CO2. Adherent cells were cultured in fresh medium.
When cells reached 90% confluence, they were passaged using 0.25%
trypsin for subsequent experiments.
The mouse mammary epithelial cell line HC11 (cat.
no. SCSP-5037; The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences) were maintained in RPMI-1640
supplemented with 10% FBS and pen/strep at 37°C with 5%
CO2 for 24 h. The liver cancer cell line HepG2 (cat. no
HB-8065; American Type Culture Collection) was maintained in
HG-DMEM supplemented with 10% FBS and pen/strep at 37°C with 5%
CO2 for 24 h.
Cell treatments
HC11 cells in RPMI-1640 supplemented with 10% FBS
and pen/strep were cultured in the presence of NaHS at 0, 25, 50,
100, 250, 500, 750 or 1,000 µM for 4 days at 37°C. HepG2 cells
(4×105/well) and GCs (1×106/well) were
separately inoculated in 6-well plates, and cultured in HG-DMEM
supplemented with 10% FBS and pen/strep, followed by treatment with
0, 10, 100 or 600 µM NaHS at 37°C for 24 h.
Cell viability was assessed with HC11 cells in
96-well plates at a density of 8×103 cells/well in
replicates of eight. The cells were cultured in RPMI-1640 medium in
the presence or absence of NaHS for 4 days. Cell viability was
measured using CCK-8 assay, according to the manufacturer's
protocol, and the absorbance was measured at 450 nm.
In addition, cells were cultured in a similar manner
and EdU incorporation was used to assess number of EdU-positive
cells, as described previously (10). Briefly, 8×103 HC11 cells
were treated with NaHS for 4 days and exposed to EdU for 2 h at
37°C. Subsequently, the plate was processed using 4%
paraformaldehyde for 30 min at 25°C and the Cell-Light EdU in
vitro kit containing the nuclear dye Hoechst 33342 was used,
according to the manufacturer's protocol. Images of the cells were
captured using a Nikon Eclipse Ti-s fluorescent microscope (Nikon
Corporation).
Animals and samples
The animal experiments performed in the present
study were approved by the Ethics Committee of South China
Agricultural University (approval no. SYXK2014-0136). Care of all
animals and procedures at South China Agricultural University were
confirmed to ‘The Instructive Notions with Respect to Caring for
Laboratory Animals’ issued by the Ministry of Science and
Technology of the People's Republic of China, and were approved by
the Animal Subjects Committee of South China Agricultural
University (Guangzhou, China). A total of 48 C57BL/6 female mice (3
weeks old, 15±0.08 g) were purchased from Guangdong Medical
Laboratory Animal Center and acclimated for 1 week in laboratory
housing prior to the experiments. The mice were divided into four
groups: i) Control, which received intraperitoneal injections of
saline; ii) intraperitoneal injections of 3 mg/kg NaHS; iii)
intraperitoneal injections of 9 mg/kg NaHS; and iv) intraperitoneal
injections of 18 mg/kg NaHS. All intraperitoneal injections were
administered every 2 days for 4 weeks. The mice were housed at
25±1°C under a 12 h light/dark cycle and 60% humidity, with ad
libitum access to food and water. At the end of the
experiments, the animals were euthanized with CO2.
Animals were placed into chambers and a flow rate of 16% chamber
volume/min was used until the mouse was unconscious. Gas flow was
maintained for at least 1 min following apparent clinical death.
Death was verified by the absence of a heartbeat, performing
cervical dislocation and by perforating the diaphragm. Blood
samples were collected from the orbital sinus after euthanasia and
serum was separated by centrifugation at 1,500 × g for 20 min prior
to storage at −20°C. The fourth pairs of mammary glands were
excised and used as samples for subsequent experiments. The mammary
samples were weighed immediately. The right side of the sample, as
well as the livers, were stored at −80°C for later analyses. The
left side of the sample was collected and stained with whole mount
and H&E as described previously (10) and was quantified as described
previously (32). Livers were
homogenized according to previously described methods (33).
Reverse transcription-quantitative PCR
(RT-qPCR)
mRNA expression levels of cyclin D1, PCNA and cyclin
D3 were measured using RT-qPCR, according to previously described
methods (34). Briefly, total RNA
was extracted from HC11 cells and mammary gland samples using an
RNA extraction kit (Guangzhou Magen Biotechnology Co. Ltd.,
according to the manufacturer's protocol. Subsequently, cDNA was
synthesized from 2 µg total RNA using the M-MLV Reverse
Transcriptase (Promega Corporation) and random primers oligo-(dT)18
(cat. no. 3806; Takara Biotechnology Co., Ltd.), according to the
manufacturer's instructions. β-actin was used as a candidate
housekeeping gene. RT-qPCR was carried out on an Mx3005p instrument
(Stratagene; Agilent Technologies, Inc.,) using SYBR®
Green Real-time PCR Master Mix reagents (Toyobo Life Science). The
thermocycling conditions were as follows: 15 sec at 95°C for
denaturing, 15 sec at 55–62°C for annealing and 40 sec at 72°C for
extension (40 cycles). In the last cycle, the conditions were as
follows: 60 sec at 95°C for denaturing, 30 sec at 55–62°C for
annealing and 30 sec at 95°C for extension. Primer sequences with
their respective PCR fragment lengths are presented in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Amplification
length (bp) |
|---|
| Cyclin D1 |
CTGAAGGCTCGCGGAATAAAA |
GAGGTCTTTACGGATGTCAACG | 142 |
| Cyclin D3 |
CGAGCCTCCTACTTCCAGTG |
GGACAGGTAGCGATCCAGGT | 150 |
| PCNA |
TTTGAGGCACGCCTGATCC |
GGAGACGTGAGACGAGTCCAT | 135 |
| β-actin |
GGTCATCACTATTGGCAACGAG |
TGATCTCCTTCTGCATCCTGT | 131 |
Western blot analysis
Expression levels of total and phosphorylated
proteins were assessed by western blot analysis, according to
previously described methods (10). Proteins from HC11 cells and mammary
glands were extracted with RIPA lysis buffer (Shanghai BestBio Co.
Ltd.) containing 1 mM PMSF (cat. no. P0100; Beijing Solarbio
Science & Technology Co. Ltd.). Protein concentration was
determined using a BCA protein assays (cat. no. 23225; Thermo
Fisher Scientific, Inc.). Equivalent amounts of protein (20 µg)
were separated by 10% SDS PAGE and the samples were transferred
onto nitrocellulose membranes (BioRad Laboratories, Inc.).
Membranes were then subjected to immunoblotting with rabbit
anti-cyclin D1 (1:2,000), mouse anti-PCNA (1:2,000), rabbit
anti-p21 (1:2,000), rabbit anti-Akt (1:2,000), rabbit
anti-p-AktSer473 (1:2,000), rabbit anti-PI3K (1:2,000),
goat anti-p-PI3KTyr508 (1:800), rabbit anti-mTOR
(1:2,000), rabbit anti-p-mTOR Ser2448 (1:2,000), rabbit
anti-IGF-1 (1:1,000), rabbit anti-JAK2 (1:1,000), rabbit
anti-p-JAK2 (1:1,000), rabbit anti-STAT5 (1:1,000), rabbit
anti-p-STAT5 (1:1,000) and rabbit anti-β-actin (1:1,000) diluted in
0.05% TBS-Tween-20 (TBST) overnight at 4°C, followed by incubation
at room temperature for 1 h with donkey anti-goat, goat anti-rabbit
and goat anti-mouse (1:10,000) secondary antibodies diluted in
TBST, as required. Western blots were visualized using Super Signal
West Pico Chemiluminescence substrate (Thermo Fisher Scientific,
Inc.) and semi-quantified with ImageJ software (version 1.4.3.67;
National Institutes of Health).
Radioimmunoassay
The IGF-1 and E2 radioimmunoassay kits (cat. nos.
RF6 and RG6) were purchased from Jiuding Medical Biological
Engineering Co., Ltd. Mice serum and cell culture supernatant IGF-1
and E2 concentrations were measured by GC-1200 Gamma RIA counter
(Anhui Zhongke Zhongjia Scientific Instruments, Co., Ltd.)
according to the manufacturer's recommendation.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way ANOVA with a Dunnett's post hoc test were applied
for statistical analyses of the data using Sigma Plot 12.5 software
(Systat Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
In vitro effects of NaHS on cell
viability
HC11 cells were treated with different
concentrations (0, 25, 50, 100, 250, 500, 750 or 1,000 µM) of the
H2S donor NaHS for 4 days to determine the concentration
that enhanced viability. It was identified that treatment with
either 50 or 100 µM NaHS significantly promoted HC11 cell
viability, whereas treatment with 1,000 µM significantly inhibited
viability (Fig. 1A). This result
was also reflected by the increased number of EdU-positive cells
exposed to NaHS at 100 µM, whereas the proportion of these cells
was significantly decreased at 1,000 µM (Fig. 1B and C). Furthermore, cyclin D1 and
PCNA protein expression levels were significantly increased,
whereas p21 expression levels were significantly reduced in cells
treated with 100 µM NaHS compared with the untreated controls. By
contrast, when treated with 1,000 µM NaHS, the opposite effects
were observed on cyclin D1, PCNA and p21 expression levels
(Fig. 1D and E). Corroborating
these results, the mRNA expression levels of PCNA, cyclin D3 and
cyclin D1 were significantly increased by 100 µM NaHS, whereas
1,000 µM NaHS resulted in the opposite effect (Fig. 1F).
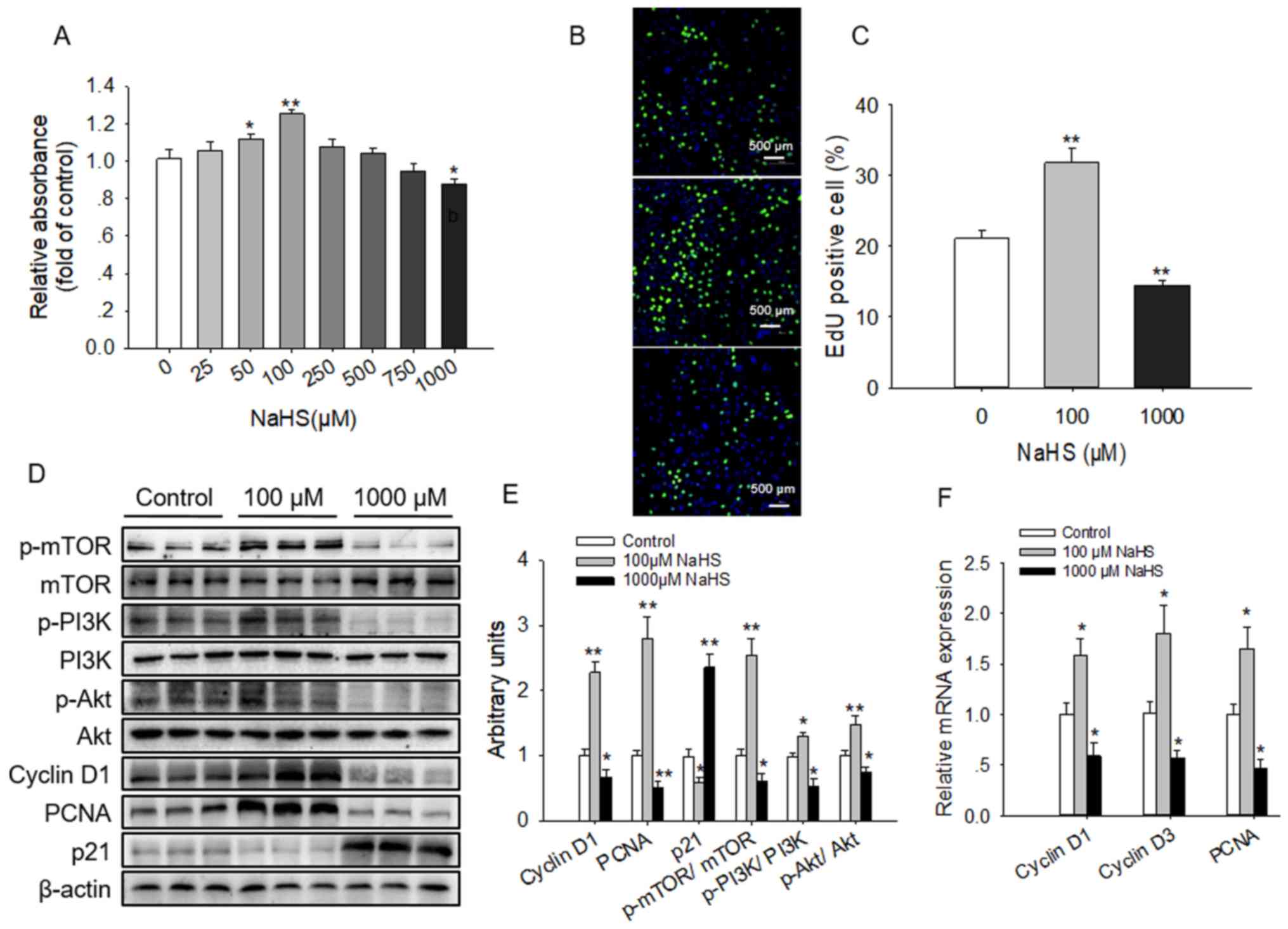 | Figure 1.Effects of NaHS on HC11 cell
viability and the PI3K/Akt-mTOR signaling pathway. HC11 cells were
cultured with the indicated concentrations of NaHS for 4 days. Cell
viability was evaluated by (A) CCK-8 assay and (B) EdU
incorporation. Blue indicated nuclei and green indicated
EdU-positive. (C) Analysis of the percentage of EdU-positive cells.
(D) Western blot analysis of HC11 cell extracts following exposure
to 0, 100 and 1,000 µM NaHS for 4 days. Blots were developed using
antibodies raised against cyclin D1, PCNA, p21, p-PI3K, p-Akt,
p-mTOR, PI3K, Akt and mTOR. β-actin was used as loading control.
(E) Intensities of the western blot bands were semi-quantified and
presented as the mean ± standard error of the mean of three
replicates. (F) Reverse transcription-quantitative PCR analysis of
the indicated genes expressed in HC11 cells exposed to NaHS. mRNA
expression levels are expressed relative to the internal control
gene β-actin. *P<0.05, **P<0.01 vs. control group. NaHS,
sodium hydrosulfide; PCNA, proliferating cell nuclear antigen; p-,
phosphorylated; PI3K, phosphatidylinositol 3-kinase; Akt, protein
kinase B; mTOR, mammalian target of rapamycin. |
The effects of NaHS treatment on the signaling
pathways associated with proliferation were also examined. It was
demonstrated that 100 µM NaHS activated in the phosphorylation of
mTOR, PI3K and Akt, whereas 1,000 µM NaHS significantly inhibited
the phosphorylation of these proteins compared with the untreated
control (Fig. 1D and E).
In vivo effects of NaHS on
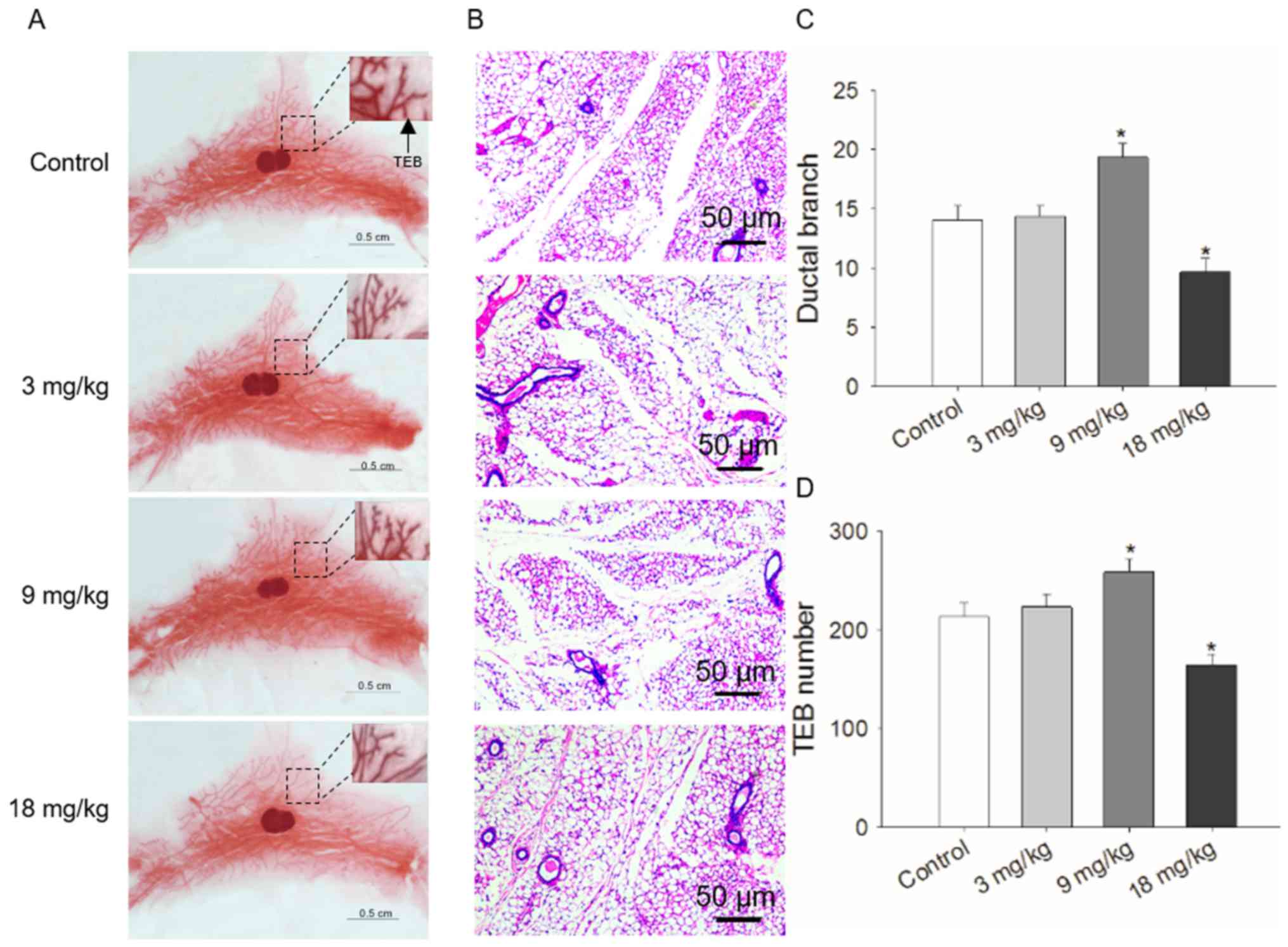
prepubescent mammary development
Prepubescent female mice were exposed to differing
treatment concentrations of NaHS via intraperitoneal injection and
ductal development was examined over 4 weeks. The number of ductal
branches observed in whole mounts of mammary tissue samples was
significantly increased in the mice that received 9 mg/kg NaHS,
whereas opposing results were seen in the mice treated with 18
mg/kg. The lowest level of NaHS used (3 mg/kg) did not result in a
significant difference (Fig. 2A and
C). Similar to the results of whole mount staining, H&E
staining of mammary tissues demonstrated that the number of
terminal end buds was also significantly increased at 9 mg/kg NaHS,
decreased at 18 mg/kg, and was not significantly affected at 3
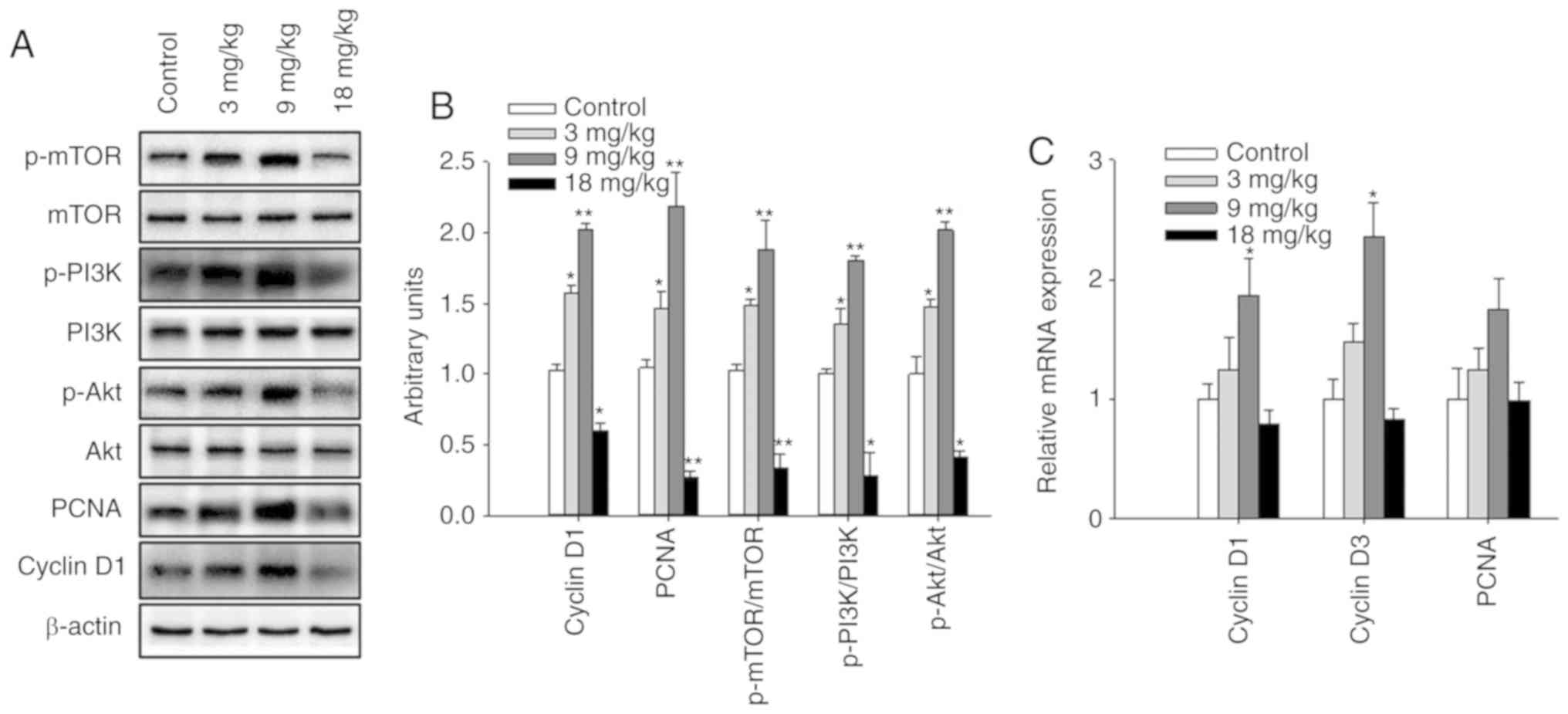
mg/kg NaHS compared with in the control group (Fig. 2B and D). Notably, when the
expression levels of mammary gland developmental proteins were
examined in the prepubescent mice, treatment with 3 and 9 mg/kg
NaHS significantly promoted PCNA and cyclin D1 expression, as well
as the phosphorylation of mTOR, PI3K and Akt compared with in the
control group. By contrast, 18 mg/kg NaHS significantly inhibited
cyclin D1 and PCNA expression, as well as the phosphorylation of
PI3K, Akt and mTOR (Fig. 3A and
B). The mRNA expression levels of PCNA and cyclin D1 were also
enhanced following treatment with 9 mg/kg NaHS (Fig. 3C).
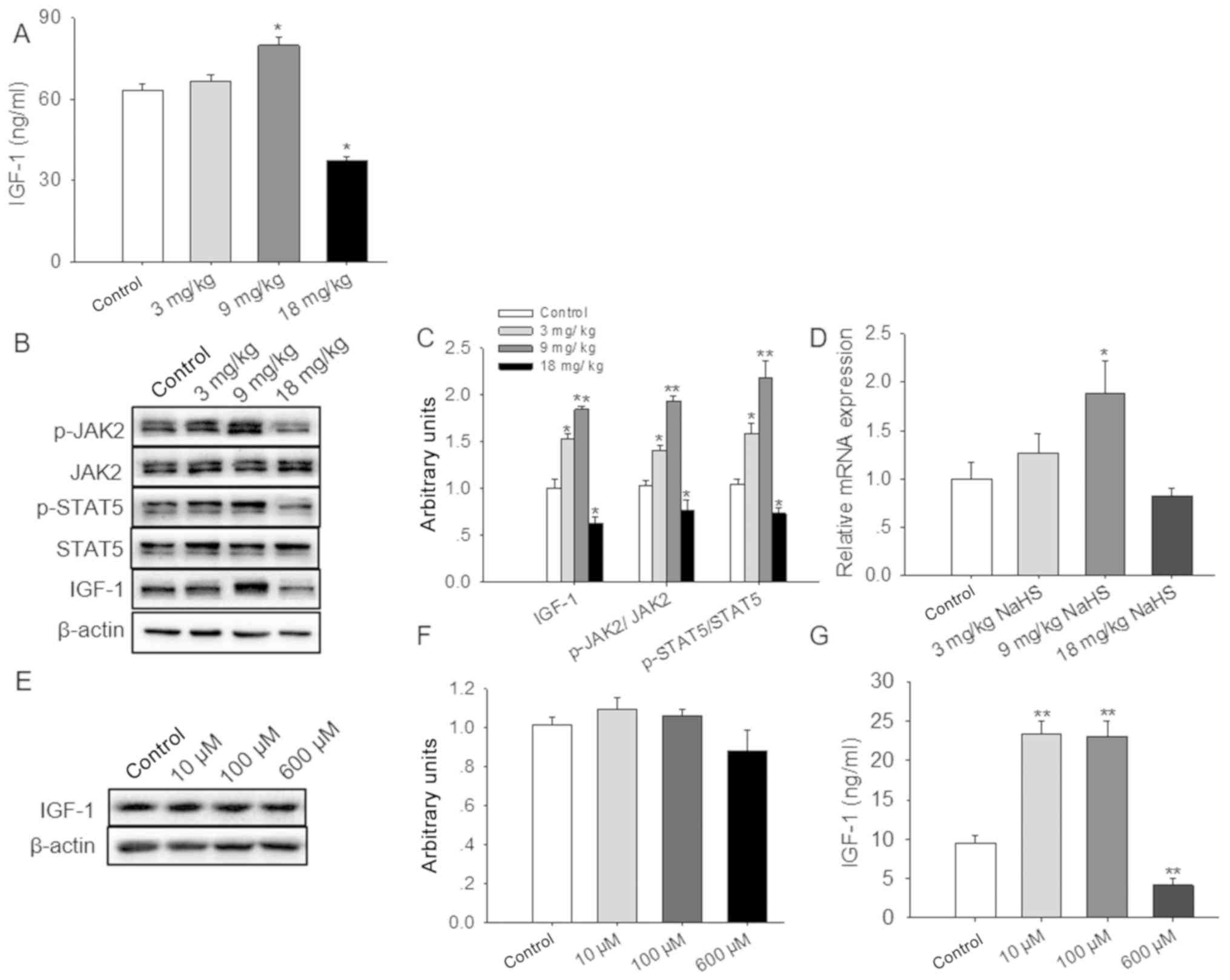
H2S affects IGF-1
production
The expression levels of IGF-1 were subsequently
detected in the serum and livers of H2S-treated animals,
which revealed that the amount of IGF-1 in the serum was
significantly increased by exposure to 9 mg/kg NaHS, whereas the
levels were significantly reduced with 18 mg/kg NaHS (Fig. 4A). In addition, IGF-1 expression,
and the phosphorylation of JAK2 and STAT5, were significantly
increased in liver tissues of the mice treated with 3 or 9 mg/kg
NaHS compared with in the control group. By contrast, a dose of 18
mg/kg NaHS significantly inhibited IGF-1 expression, and JAK2 and
STAT5 phosphorylation (Fig. 4B and
C). Furthermore, the mRNA expression levels of IGF-1 were
significantly higher in the livers of the 9 mg/kg-treated mice
compared with the untreated controls (Fig. 4D).
IGF-1 is mainly synthesized and secreted by the
liver (35); therefore, the
present study used HepG2 as a model to study whether exogenous
H2S affected the development of the mammary gland
through the synthesis and secretion of IGF-1. Consequently, the
liver-associated effects of NaHS on IGF-1 expression were examined
using the HepG2 cell line, which was treated with 0, 10, 100 or 600
µM NaHS for 24 h. No significant effects of NaHS on IGF-1 protein
expression levels in HepG2 cell extracts were observed (Fig. 4E and F). However, significantly
increased levels of IGF-1 were identified in the cell culture
medium following treatment with either 10 or 100 µM NaHS, whereas
the expression levels of IGF-1 were significantly decreased in the
600 µM NaHS-treated group (Fig.
4G).
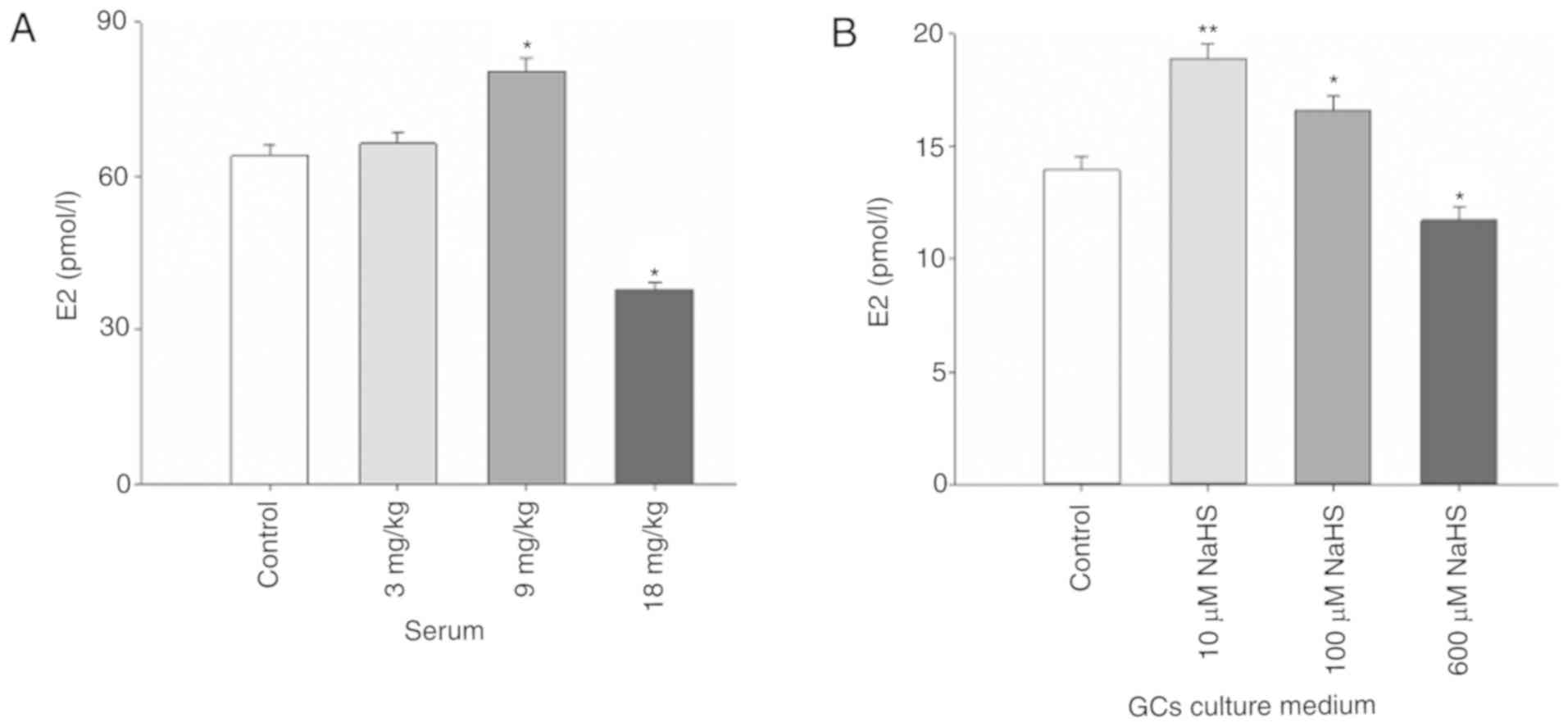
H2S affects E2
production
E2 is a key regulator of the advancement to puberty
in mammals (36). Therefore, the
present study examined whether serum E2 levels were altered in mice
treated with exogenous NaHS. NaHS administered at 9 mg/kg
significantly increased the content of E2 in the serum of mice,
whereas 18 mg/kg NaHS significantly reduced E2 levels (Fig. 5A). To examine this in more detail,
primary cultures of sow ovarian GCs exposed to NaHS for 24 h were
analyzed. Notably, E2 levels in cells treated with 10 and 100 µM
NaHS were significantly increased, whereas the expression levels of
E2 were significantly decreased by treatment with 1,000 µM NaHS
(Fig. 5B).
Discussion
The present study demonstrated that H2S
had a major effect on mammary gland development in prepubescent
female mice. Under the current additional dose condition, 9 mg/kg
of NaHS that promoted the ductal and terminal end buds branch
numbers in prepubescent mice were verified, which was also
associated with enhanced viability of cultured HC11 cells. In line
with the present results, it has previously been reported that
H2S exerts biphasic effects on the proliferation of
porcine mammary epithelial cells (PMECs) (13). The different doses of NaHS shown to
exert effects among the present and other studies may be due to the
use of different cell types and culturing times. H2S is
a gaseous messenger molecule that has been implicated in various
physiological and pathological processes in mammalian organs,
including the brain (37), liver
(38), heart (39) and other organs (40,41).
However, understanding the effect of H2S on mammary
gland hyperplasia requires further studies.
In order to determine the regulatory function of
H2S on HC11 cell proliferation and mammary ductal
growth, the expression levels of proliferative marker genes,
including cyclin D1/D3 and PCNA were detected. Accordingly, markers
of cell proliferation, including cyclin D1/D3 and PCNA, were
enhanced by the optimal levels of H2S. Cyclin D family
of proteins is critical for the G1 to S transition
(17), and PCNA is an associated
factor known to be necessary for control of DNA replication during
the S phase (42). Cyclin D1,
cyclin D3 and PCNA modulate mammary gland development and mammary
epithelial cell proliferation (10,13,20).
The present study revealed that lower doses (100 µM) of NaHS
increased cyclin D1, cyclin D3 and PCNA mRNA expression levels;
however, these were decreased when higher doses of NaHS were used
(1,000 µM). Furthermore, the number of cells in the S phase was
increased following treatment with low concentrations of NaHS, and
was decreased by high concentrations. These data suggested that
H2S may be a regulator of mammary ductal growth.
The PI3K/Akt-mTOR pathway is crucial for the
regulation of proliferation of numerous cell types, such as PMECs
(13), breast cancer cells
(43) and human osteoblast-like
cells (44). In addition,
PI3K/Akt-mTOR phosphorylation has previously been associated with
mammary gland development (10,20,45).
The results of the present study are consistent with these
observations, which confirms that PI3K/Akt-mTOR signaling may be
involved in H2S-indued modulation of HC11 cell viability
and mammary gland development.
IGF-1 and E2 are both known to be critical for
mammary ductal development (46).
Growth hormones promote cell proliferation by inducing the
expression of IGF-1 in the liver and mammary glands. Notably, IGF1
and E2 secreted by the ovary induce epithelial cell proliferation
(47). A previous study
demonstrated that IGF-1 activated the PI3K/Akt signaling pathway
via its receptor and induced epithelial cell proliferation
(48). Notably, these factors can
also be directly controlled by dietary additions of lauric acid to
increase IGF-1 and E2, which promotes mammary gland development
(10), and stearic acid to
decrease IGF-1 and E2, which inhibits terminal end buds and ductal
branches (42). These data are
consistent with the present results where intraperitoneal injection
of low concentrations of NaHS (9 mg/kg) was shown to increase serum
IGF-1 and E2 levels, and stimulate mammary gland ductal
development. By contrast, a high concentration of NaHS (18 mg/kg)
elicited the opposite effects. The expression of IGF-1 in livers of
mice exposed to NaHS also agreed with the findings for serum
expression levels. Notably, HepG2 and primary cultures of ovarian
GCs served as models for IGF-1 and E2 secretion in response to
NaHS.
In conclusion, the current results demonstrated that
exogenous H2S was able to advance mammary gland ductal
development in prepubescent female mice via PI3K/Akt-mTOR
signaling, and through secretion of IGF-1 and E2. These results may
be beneficial for the application of H2S in promoting or
suppressing mammary gland development.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 31672508 and 31972636) and
the Guangdong Special Support Program (grant no. 2014TQ01N260).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and JY performed the experiments. SW and QJ
conceptualized and designed the study. CY, QF and FZ performed the
collection and analysis of the samples. XZ, LW, PG, GS and QL
performed data analysis and interpretation. JZ drafted the
manuscript, generated and revised the figures. QL and QJ revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments performed in the present
study were performed under the permission number SYXK (Guangdong)
2014–0136. Care of all animals and procedures in South China
Agricultural University were confirmed to ‘The Instructive Notions
with Respect to Caring for Laboratory Animals’ issued by the
Ministry of Science and Technology of the People's Republic of
China and were approved by the Animal Subjects Committee of South
China Agricultural University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Akt
|
protein kinase B
|
|
CCK-8
|
Cell Counting Kit-8
|
|
FBS
|
fetal bovine serum
|
|
GCs
|
granulosa cells
|
|
mTOR
|
mammalian target of rapamycin
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
pen/strep
|
penicillin/streptomycin
|
References
|
1
|
Rezaei R, Wu Z, Hou Y, Bazer FW and Wu G:
Amino acids and mammary gland development: Nutritional implications
for milk production and neonatal growth. J Anim Sci Biotechnol.
7:202016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennighausen L and Robinson GW: Signaling
pathways in mammary gland development. Dev Cell. 1:467–475. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mcnally S and Stein T: Overview of mammary
gland development: A comparison of mouse and human. Methods Mol
Biol. 1501:1–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visvader JE and Smith GH: Murine mammary
epithelial stem cells: Discovery, function, and current status.
Cold Spring Harb Perspect Biol. 3:a0048792011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macias H and Hinck L: Mammary gland
development. Wiley Interdiscip Rev Dev Biol. 1:533–557. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brisken C and Ataca D: Endocrine hormones
and local signals during the development of the mouse mammary
gland. Wiley Interdiscip Rev Dev Biol. 4:181–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hue-Beauvais C, Laubier J, Brun N, Houtia
I, Jaffrezic F, Bevilacqua C, Provost FL and Charlier M: Puberty is
a critical window for the impact of diet on mammary gland
development in the rabbit. Dev Dyn. 248:948–960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farmer C: Review: Mammary development in
swine: Effects of hormonal status, nutrition and management.
Canadian J Anim Sci. 93:1–7. 2013. View Article : Google Scholar
|
|
9
|
Silva AL, Detmann E, Dijkstra J, Pedroso
AM, Silva LHP, Machado AF, Sousa FC, Santos GBD and Marcondes MI:
Effects of rumen-undegradable protein on intake, performance, and
mammary gland development in prepubertal and pubertal dairy
heifers. J Dairy Sci. 101:5991–6001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng Y, Zhang J, Zhang F, Ai W, Zhu X, Shu
G, Wang L, Gao P, Xi Q, Zhang Y, et al: Lauric acid stimulates
mammary gland development of pubertal mice through activation of
GPR84 and PI3K/Akt signaling pathway. J Agric Food Chem. 65:95–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farmer C, Palin MF and Martel-Kennes Y:
Impact of diet deprivation and subsequent over-allowance during
prepuberty. Part 2. Effects on mammary gland development and
lactation performance of sows. J Anim Sci. 90:872–880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapila N, Sharma A, Kishore A, Sodhi M,
Tripathi PK, Mohanty AK and Mukesh M: Impact of heat stress on
cellular and transcriptional adaptation of mammary epithelial cells
in riverine buffalo (Bubalus Bubalis). PLoS One. 11:e01572372016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Ye J, Yuan C, Fu Q, Zhang F, Zhu
X, Wang L, Gao P, Shu G, Jiang Q and Wang S: Exogenous
H2S exerts biphasic effects on porcine mammary
epithelial cells proliferation through PI3K/Akt-mTOR signaling
pathway. J Cell Physiol. 233:7071–7081. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sciascia Q, Sales F, van der Linden D,
Wards N, Oliver M, Blair H and McCoard S: Nutritional plane of
twin-bearing ewes alters fetal mammary gland biochemical
composition and mTOR/MAPK pathway signaling. J Anim Sci.
93:699–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao HN, Hu H, Zheng N and Wang JQ: Leucine
and histidine independently regulate milk protein synthesis in
bovine mammary epithelial cells via mTOR signaling pathway. J
Zhejiang Univ Sci B. 16:560–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Liu L, Qu B, Li X, Gao X and Zhang
M: Twinfilin 1 enhances milk bio-synthesis and proliferation of
bovine mammary epithelial cells via the mTOR signaling pathway.
Biochem Biophys Res Commun. 492:289–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park SY, Jeong MS, Han CW, Yu HS and Jang
SB: Structural and functional insight into proliferating cell
nuclear antigen. J Microbiol Biotechnol. 26:637–647. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng Y, Yuan C, Zhang J, Zhang F, Fu Q,
Zhu X, Shu G, Wang L, Gao P, Xi Q, et al: Stearic acid suppresses
mammary gland development by inhibiting PI3K/Akt signaling pathway
through GPR120 in pubertal mice. Biochem Biophys Res Commun.
491:192–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng Y, Zhang J, Yuan C, Zhang F, Fu Q, Su
H, Zhu X, Wang L, Gao P, Shu G, et al: Oleic acid stimulates HC11
mammary epithelial cells proliferation and mammary gland
development in peripubertal mice through activation of
CD36-Ca2+ and PI3K/Akt signaling pathway. Oncotarget.
9:12982–12994. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Bian H, Li X, Wu H, Bi Q, Yan Y
and Wang Y: Hydrogen sulfide promotes cell proliferation of oral
cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep.
35:2825–2832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu D, Wang Z, Zhan J, Zhang Q, Wang J,
Zhang Q, Xian X, Luan Q and Hao A: Hydrogen sulfide promotes
proliferation and neuronal differentiation of neural stem cells and
protects hypoxia-induced decrease in hippocampal neurogenesis.
Pharmacol Biochem Behav. 116:55–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
King AL, Polhemus DJ, Bhushan S, Otsuka H,
Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, et
al: Hydrogen sulfide cytoprotective signaling is endothelial nitric
oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA.
111:3182–3187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olas B: Hydrogen sulfide in signaling
pathways. Clin Chim Acta. 439:212–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong XB, Yang CT, Zheng DD, Mo LQ, Wang
XY, Lan AP, Hu F, Chen PX, Feng JQ, Zhang MF and Liao XX:
Inhibition of ROS-activated ERK1/2 pathway contributes to the
protection of H2S against chemical hypoxia-induced injury in H9c2
cells. Mol Cell Biochem. 362:149–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu M, Li Y, Liang B, Li Z, Jiang Z, Chu C
and Yang J: Hydrogen sulfide attenuates myocardial fibrosis in
diabetic rats through the JAK/STAT signaling pathway. Int J Mol
Med. 41:1867–1876. 2018.PubMed/NCBI
|
|
28
|
Yang B, Bai Y, Yin C, Qian H, Xing G, Wang
S, Li F, Bian J, Aschner M and Lu R: Activation of autophagic flux
and the Nrf2/ARE signaling pathway by hydrogen sulfide protects
against acrylonitrile-induced neurotoxicity in primary rat
astrocytes. Arch Toxicol. 92:2093–2108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong
S, Wu J, Zhao Y, Xu C, Zhang W and Lu F: Exogenous H2S protects
against diabetic cardiomyopathy by activating autophagy via the
AMPK/mTOR pathway. Cell Physiol Biochem. 43:1168–1187. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu M, Li Z, Liang B, Li L, Liu S, Tan W,
Long J, Tang F, Chu C and Yang J: Hydrogen sulfide ameliorates rat
myocardial fibrosis induced by thyroxine through PI3K/AKT signaling
pathway. Endocr J. 65:769–781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao P, Zhang AL and Zhong YY: Separation,
culture and identification of sow ovarian granulose cells.
Guangdong Agric Sci. 131–135. 2014.(In Chinese).
|
|
32
|
Ball SM: The development of the terminal
end bud in the prepubertal-pubertal mouse mammary gland. Anat Rec.
250:459–464. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Xu J, Wang T, Wan X, Zhang F,
Wang L, Zhu X, Gao P, Shu G, Jiang Q and Wang S: The dipeptide
pro-gly promotes IGF-1 expression and secretion in HepG2 and female
mice via PepT1-JAK2/STAT5 pathway. Front Endocrinol (Lausanne).
9:4242018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye J, Ai W, Zhang F, Zhu X, Shu G, Wang L,
Gao P, Xi Q, Zhang YL, Jiang Q and Wang S: Enhanced proliferation
of porcine bone marrow mesenchymal stem cells induced by
extracellular calcium is associated with the activation of the
calcium-sensing receptor and ERK signaling pathway. Stem Cells Int.
2016:657–671. 2016. View Article : Google Scholar
|
|
35
|
Luo XY, Jiang XK, Li J, Bai Y, Li Z, Wei
P, Sun S, Liang Y, Han S, Li X and Zhang BY: Insulin-like growth
factor-1 attenuates oxidative stress-induced hepatocyte premature
senescence in liver fibrogenesis via regulating nuclear
p53-progerin interaction. Cell Death Dis. 10:4512019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roy JR, Chakraborty S and Chakraborty TR:
Estrogen-like endocrine disrupting chemicals affecting puberty in
humans-a review. Med Sci Monit. 15:RA137–RA145. 2009.PubMed/NCBI
|
|
37
|
Hu X, Luan L, Guan W, Zhang S, Li B, Ji W
and Fan H: Hydrogen sulfide attenuates isoflurane-induced
neuroapoptosis and cognitive impairment in the developing rat
brain. BMC Anesthesiol. 17:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mani S, Cao W, Wu L and Wang R: Hydrogen
sulfide and the liver. Nitric Oxide. 41:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo Q, Feng X, Xue H, Teng X, Jin S, Duan
X, Xiao L and Wu Y: Maternal renovascular hypertensive rats
treatment with hydrogen sulfide increased the methylation of AT1b
gene in offspring. Am J Hypertens. 30:1220–1227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pichette J, Fynn-Sackey N and Gagnon J:
Hydrogen sulfide and sulfate prebiotic stimulates the secretion of
GLP-1 and improves glycemia in male mice. Endocrinology.
158:3416–3425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Askari H, Seifi B, Kadkhodaee M, Sanadgol
N, Elshiekh M, Ranjbaran M and Ahghari P: Protective effects of
hydrogen sulfide on chronic kidney disease by reducing oxidative
stress, inflammation and apoptosis. EXCLI J. 17:14–23.
2018.PubMed/NCBI
|
|
42
|
Wang SC: PCNA: A silent housekeeper or a
potential therapeutic target? Trends Pharmacol Sci. 35:178–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou R, Chen H, Chen J, Chen X, Wen Y and
Xu L: Extract from astragalus membranaceus inhibit breast cancer
cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC
Complement Altern Med. 18:832018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou H, Jiao G, Dong M, Chi H, Wang H, Wu
W, Liu H, Ren S, Kong M, Li C, et al: Orthosilicic acid accelerates
bone formation in human osteoblast-like cells through the
PI3K-Akt-mTOR pathway. Biol Trace Elem Res. 190:327–335. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rosen JM: On hormone action in the mammary
gland. Cold Spring Harb Perspect Biol. 4:a0130862012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mallepell S, Krust A, Chambon P and
Brisken C: Paracrine signaling through the epithelial estrogen
receptor α is required for proliferation and morphogenesis in the
mammary gland. Proc Natl Acad Sci USA. 103:2196–2201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou Y, Li S, Li J, Wang D and Li Q:
Effect of microRNA-135a on cell proliferation, migration, invasion,
apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt
signaling pathway in non-small cell lung cancer. Cell Physiol
Biochem. 42:1431–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|