Introduction
The Hippo signaling pathway serves an important role
in the occurrence and progression of breast cancer (1). As a terminal effector of a central
kinase cascade of the Hippo signaling pathway, dephosphorylation
and nuclear localization of yes-associated protein (YAP) regulates
the activity of many YAP downstream compounds, including connective
tissue growth factor (CTGF), cysteine-rich angiogenic inducer
(CYR61), AXL receptor tyrosine kinase (AXL) and integrin, when
combined with the DNA-binding transcription factors of TEA domain
transcription factor (TEAD) (2).
CYR61, CTGF and integrin serve an important role in cell adhesion,
migration, proliferation and angiogenesis (3). Overexpression of YAP in breast cancer
promotes the occurrence and development of breast cancer, while YAP
knockdown can reduce cell proliferation, migration and increase the
radiotherapy sensitivity of triple-negative breast cancer (TNBC)
231 cells (4,5). Activated YAP is a key regulator of
tumor cell proliferation, apoptosis, migration, invasion and
chemotherapy resistance, leading to cell malignant transformation
(6). Taken together, these studies
indicate that YAP may be a potential drug target for breast
cancer.
Verteporfin (VP) was originally designed as a
photosensitizer for macular degeneration (7). VP can produce singlet oxygen and
eliminate abnormal hyperplastic blood vessels under the activation
of a 693 nm laser (8). In recent
years, VP has lost its importance owing to the limited efficacy of
its original indications (9).
However, VP also serves a key role in the absence of light, VP can
inhibit YAP-TEAD binding without photoactivation and markedly
prevent liver hepatomegaly/tumorigenesis induced by YAP
overexpression (10). YAP is
closely associated with the occurrence and development of breast,
colon, lung and liver cancer in addition to mesothelioma, as it is
the core effector of the Hippo signaling pathway (11). Therefore, targeting YAP is may be
an attractive therapeutic strategy. VP was rapidly repositioned as
a YAP inhibitor for several types of cancer, including liver,
esophageal, lung and pancreatic cancer (12).
Non-photoactivated VP can disrupt the interaction
and transcriptional activity of YAP/TEAD and thus inhibit
YAP-mediated tumor proliferation, induce tumor cell apoptosis and
restore sensitivity to chemotherapy drugs (13). However, although VP has been
identified as a YAP/TEAD inhibitor, a detailed structural analysis
of YAP and VP interactions has yet to be presented. Therefore, the
present study analyzed the possible binding sites of VP and YAP by
molecular simulation. In addition, it evaluated the VP effect on
the migration of three breast cancer cell subtypes and on the
expression of YAP downstream cell migration-related genes.
Materials and methods
Compound preparation
VP (MedChemExpress) was dissolved in DMSO as a stock
solution at a concentration of 10 mM, stored at −20°C and protected
from light.
Breast cancer cell culture
Luminal A MCF-7, Luminal B BT-474 and TNBC BT-549
cells (14) were purchased from
Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd. The cells were
cultured with RPMI-1640 medium (HyClone; Cytiva) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.).
Cells were incubated at 37°C in a humidified atmosphere of 95% air
and 5% CO2. The cells were protected from light at all
times.
Molecular docking studies
All molecular docking studies were performed using
AutoDock 4.2.6 software package (15). The crystal structure of YAP (PDB
code 3KYS) was retrieved from the RCSB Protein Data Bank
(https://www.rcsb.org/) (16). The structure was prepared by adding
hydrogen atoms, removing waters and adding Gasteiger charge. The
three-dimensional structure of VP was retrieved from Drugbank 5.1.5
(https://www.drugbank.ca/) and was prepared by
adding hydrogen atoms and Gasteiger charge. The protonation states
of both receptor protein and VP were determined at pH 7 using
Propka 3.1 (17). Then the ligand
was docked into the binding sites of YAP with default parameters
and 20 docking poses were exported for further visual analysis. The
docking score of each pose was calculated with AutoDock 4.2. The
binding free energy was of best-scored pose calculated using the
Molecular Mechanics/Poisson-Boltzmann Surface Area (MM-PBSA) method
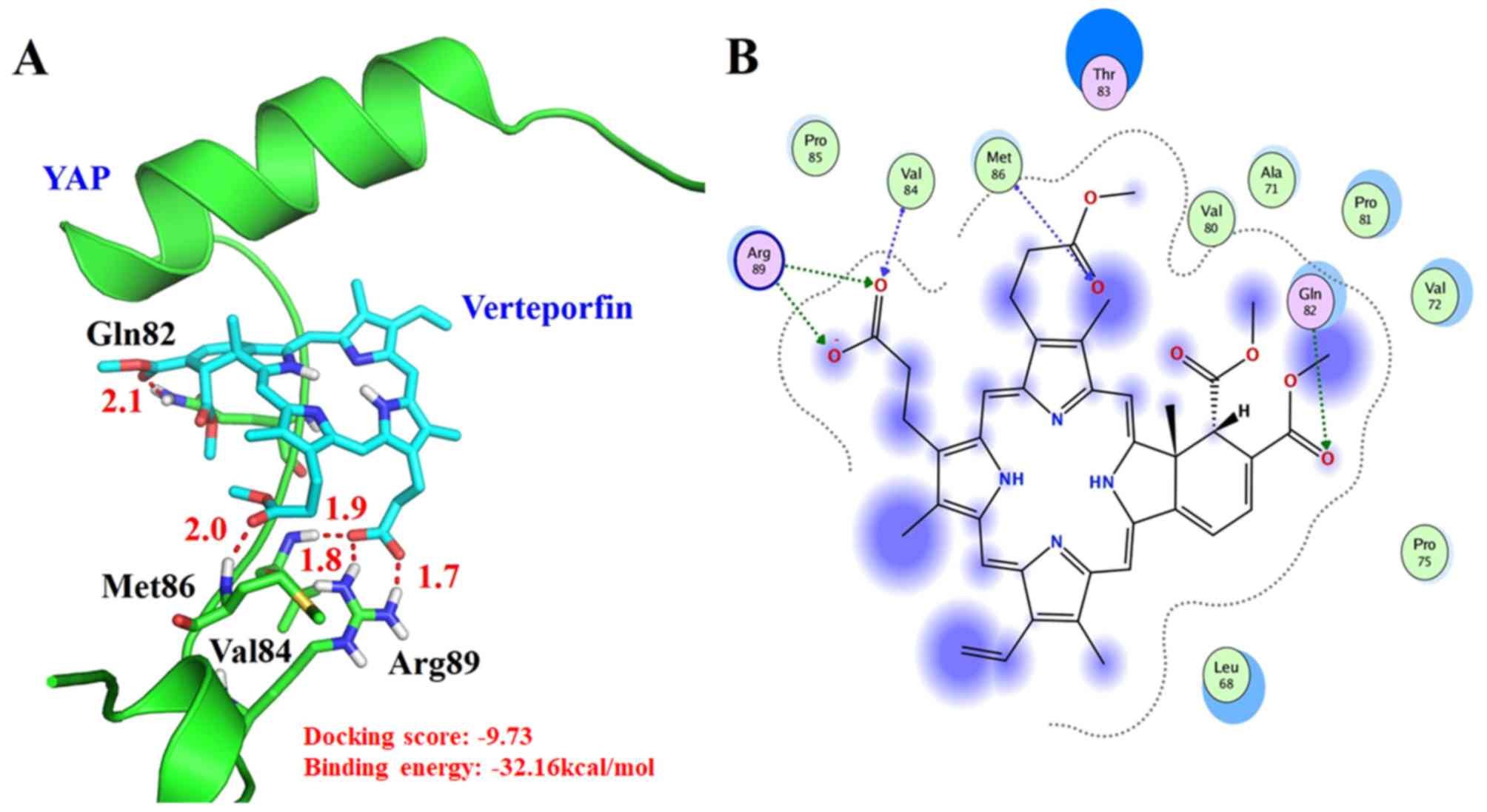
(13). Fig. 1 presents the best-scored binding
pose of VP with YAP docking result, which was constructed with
PyMOL 2.4.0 (https://pymol.org/2/).
Wound healing assay
Three parallel lines were drawn in advance on the
bottom of a 24-well plate. MCF-7, BT-474 and BT-549 cells were
placed in the plates at 1.2×105 cells per well. After
the cells reached 85–90% confluence, a 10 µl pipette tip was used
to draw a light, straight line. The floating cells in the wells
were carefully washed off with PBS buffer and images captured under
the light microscope at the 0 h time point. The blank control group
cells were cultured in fresh medium without FBS and the treatment
group was treated with 8 µM VP. After 24 h of culture, the cells
were washed with PBS buffer three times and images captured under
the microscope. Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.) was used to quantify the migration distance of cells before
and after scratches and to calculate the wound healing
distance.
Transwell assay
For these experiments, the cells were cultured at a
density of 3×105 in FBS-free medium. In the control
group, 10% FBS medium was dripped along the side wall of the lower
Transwell chamber. In the treatment group, 10% FBS medium
containing 8 µM VP was added to the lower Transwell chamber. The
3×104 cell suspensions were carefully added to the upper
Transwell chamber and then cultured at 37°C in the incubator for 24
h. The medium was then removed, and the cells were washed twice
with PBS and fixed for 20 min with 4% paraformaldehyde at room
temperature. The sample were stained with DAPI for 15 min in the
dark and images captured under a fluorescence microscope
(magnification, ×100). A total of five visual fields were randomly
selected and the results were quantified by Image Pro Plus 6.0
software (Media Cybernetics, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between VP-treated and -untreated control groups were
made by unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Molecular docking analysis
The crystal structure of YAP (PDB code 3KYS) was
used as docking receptor for VP in docking molecular experiments
and was prepared by adding hydrogen atoms, removing waters and
adding Gasteiger charge. The best-scored pose of VP was selected as
the possible bind conformation for the docking analysis (Fig. 1) and the docking score was −9.73.
VP can form a hydrogen bond with the side chain of Gln82 and the
distance was 2.1 Å (O…H-N-Gln82). In addition, VP could have a
hydrogen bond with the main chain of Val84 at the distance of 1.8 Å
(O…H-N-Val84). VP can also form a hydrogen bond with the main chain
of Met86 at the distance of 2.0 Å (O…H-N- Met86). VP can establish
two hydrogen bonds with the positively charged Arg89 side chain at
distances of 1.8 Å (O…H-N-Arg89) and 1.7 Å (O…H-N-Arg89),
respectively. In addition, VP established hydrophobic interactions
with the surrounding residues Leu68, Ala71, Val72, Pro75, Val80,
Pro81, Val84, Pro85 and Met86. These interactions tightly bound to
YAP and inhibited the interaction of YAP and TEAD. The binding free
energy was further investigate using MM-PBSA and the binding free
energy was −32.16 kcal/mol, which was favorable for the binding of
VP. This result suggested that VP was a good inhibitor for the
YAP/TEAD complex.
VP effect on the migration of
different breast cancer cell subtypes
The expression of YAP target proteins in MCF-7,
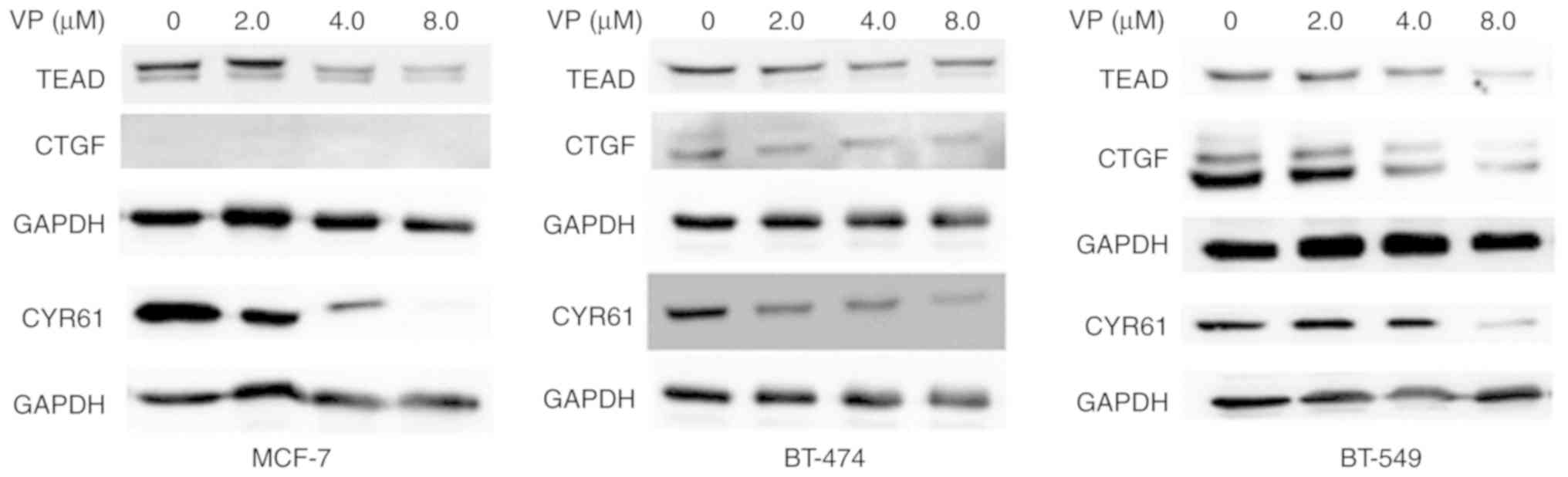
BT-474 and BT-549 cells was evaluated. Western blotting
demonstrated that 8 µM VP treatment could notably downregulate the
protein expression levels of YAP, TEAD, and CYR61. The expression
of CTGF were differential in three subtypes of breast cancer and no
CTGF expression was detected in MCF-7 cells (Fig. 2).
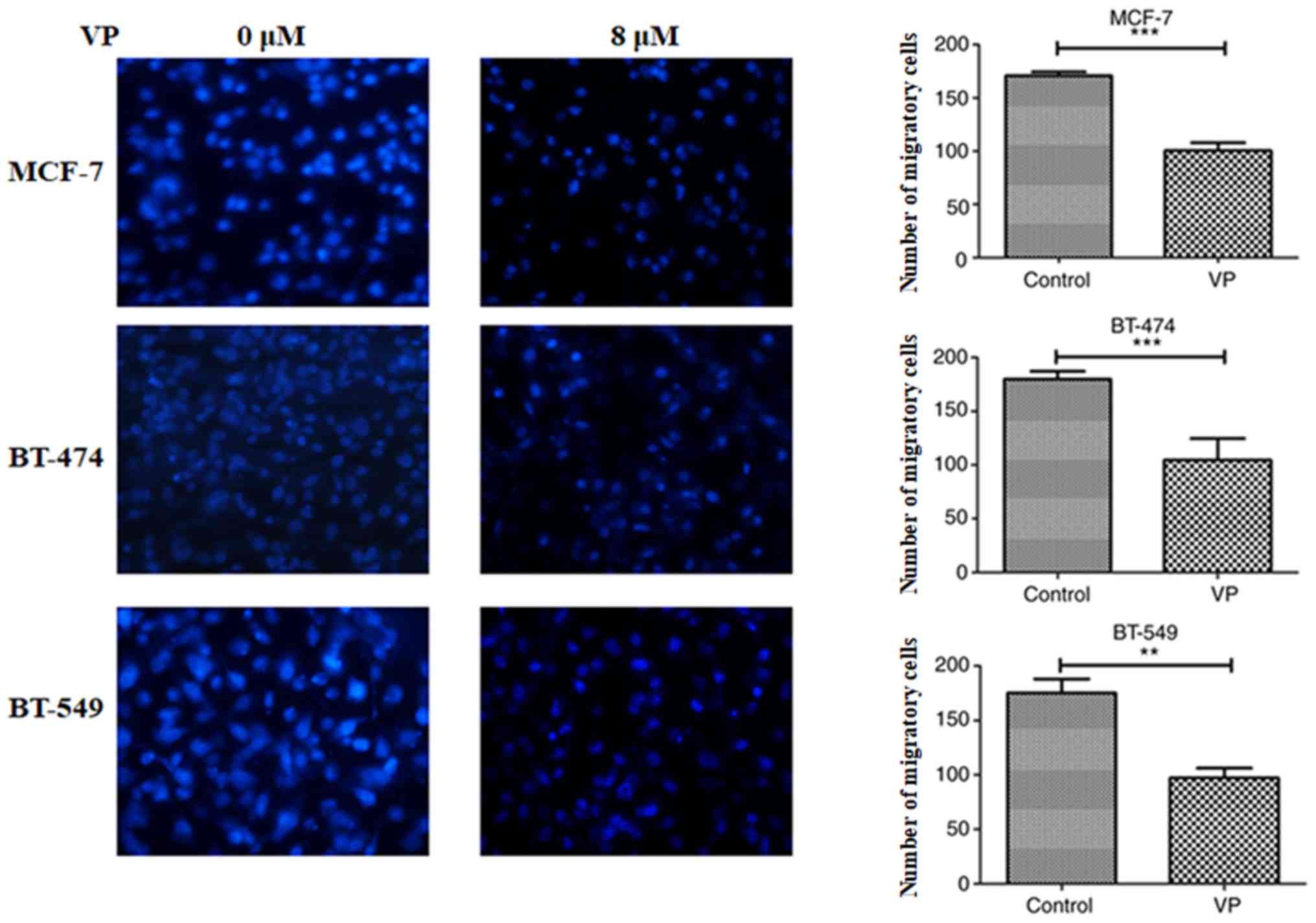
Transwell migration assays revealed that 8 µM VP
could inhibit the number of migratory cells of Luminal A MCF-7,
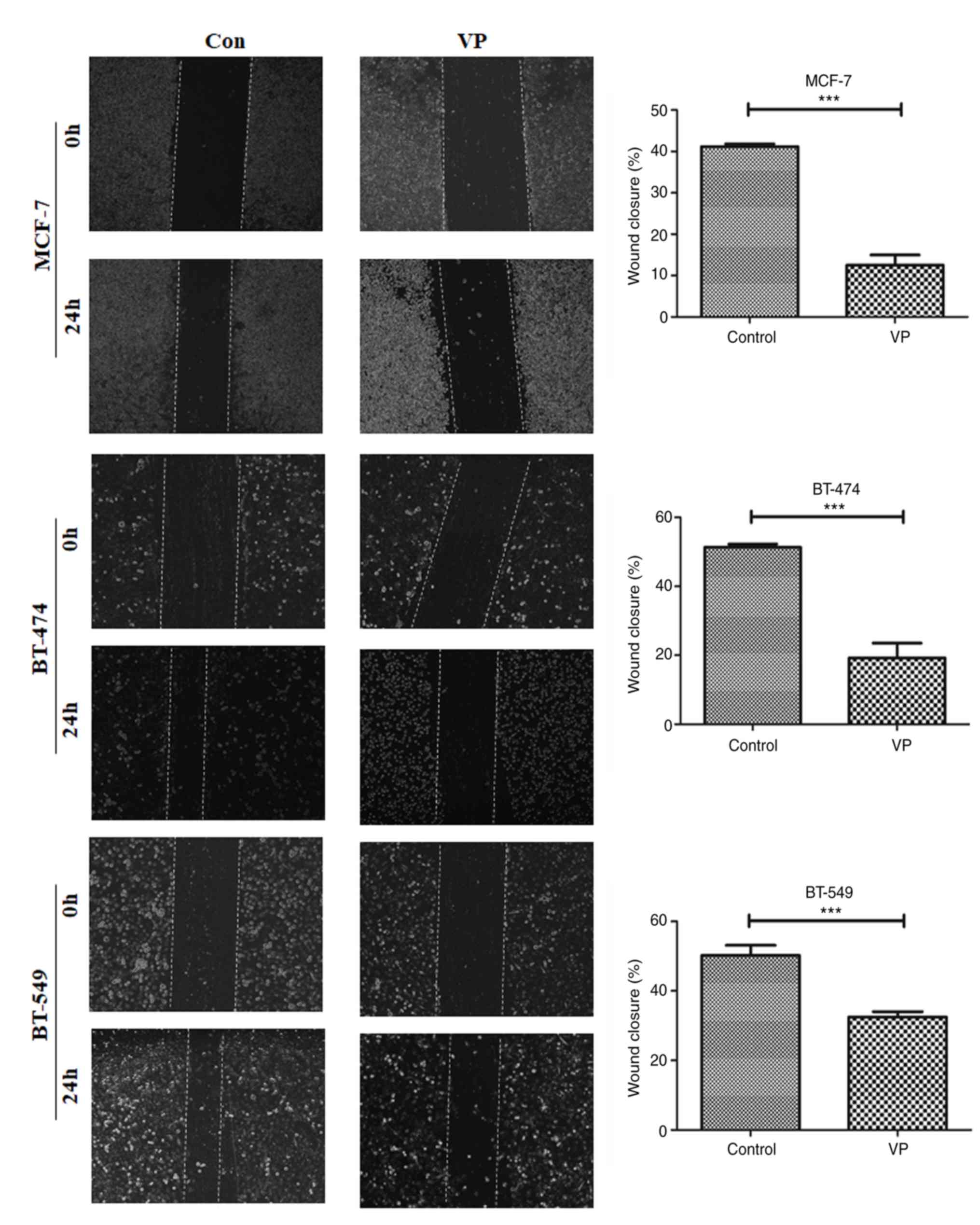
Luminal B BT-474 and TNBC BT-549 cells (P<0.01; Fig. 3). Wound healing assay results
demonstrated that 8 µM VP decreased the migratory ability of the
three different breast cancer cell subtypes (Fig. 4).
Discussion
The core components of the Hippo signaling pathway
are involved in the regulation of proliferation, migration,
invasion and chemoresistance of breast cancer cells (11). YAP is the main effector of the
Hippo signaling pathway (1). The
core kinases Mst1/2 of the Hippo signaling pathway and the
activation complex formed by the interaction of their regulatory
protein, protein salvador homolog 1, can directly phosphorylate
large tumor suppressor homolog (LATS)1 and LATS2, which interact
with Mob kinase activator 1 (Mob1). The activity of LATS1/LATS2 and
Mob1 can be inhibited by YAP phosphorylation, which is degraded by
proteasome or ubiquitination in the cytoplasm (4). By contrast, when the upstream kinase
signal of the Hippo pathway is inhibited, the unphosphorylated Yap
is transferred to the nucleus (12). Owing to the lack of a DNA-binding
domain, YAP must be combined with DNA-binding transcription factors
to serve the role of transcription coactivators (18). YAP overexpression can induce
epithelial-mesenchymal transition in the MCF-10A breast cell line
and overexpression of YAP promotes the formation and growth of
tumor in breast cancer cells in a mouse xenograft tumor model
(19). The activation of YAP in
tumor cells can ultimately promote the occurrence of breast cancer
bone metastasis (20). These data
therefore suggest that YAP is closely associated with the
occurrence and development of breast cancer. Since it was
identified as an inhibitor of the YAP/TEAD complex, several studies
have reported the therapeutic potential of VP in different types of
cancer (21–23). However, detailed structural
information on the interaction between VP and YAP remains lacking.
The N-terminal YAP region contains a TEAD-binding domain and
14-3-3-binding domain site (HXRXXS motif-containing Ser127 residue)
(24). YAP binding to 14-3-3
proteins depends on Ser127 phosphorylation, which also serves a key
role in determining the cytoplasmic localization and inactivation
of YAP (25,26). YAP-TEAD interaction is important
for the YAP-mediated transcription activation and the disruption of
this interaction is being considered as a strategy in cancer
therapy (27). Liu-Chittenden
et al found that VP selectively binds to YAP and abrogates
its interaction with TEAD (10).
In recent years, some crystal structures have been presented,
including YAP (residues 47-85)-TEAD4 (residues 210–427) complex
from Mus musculus (PDB code 3JUA), YAP (residues
50–171)-TEAD1 (residues 209–426) from Homo sapiens (PDB code 4RE1)
and YAP (residues 60–100)-TEAD4 (residues 217–434) complexes from
Homo sapiens (PDB code 3KYS) (28). Structure analysis has shown that
the 61–100 region of human YAP serves an essential role in
TEAD-binding domain, it is observed that the hydrophobic
interaction of the YAP Ω-ring formed by Met86, Arg87, Leu91, Phe95
and Phe96 residues causes the Arg89 and Ser94 side chains to form
hydrogen bonds with Glu255, Asp264 and Tyr421 residues of TEAD1
(28–30).
YAP and TEAD proteins are the best molecular target
candidates to regulate the Hippo pathway, as the formation and
activation of the YAP/TEAD complex is the last step of the
Hippo-YAP pathway. VP is a compound that directly destroys the
formation of YAP/TEAD complexes and inhibits the most essential
part of the Hippo pathway (10).
The molecular docking analysis of the present study found that VP
could form hydrogen bonds and establish hydrophobic interactions
with residues from the YAP Ω-ring that directly interacted with
TEAD1 (Met86 and Arg89). Our molecular docking results demonstrated
that VP could also interact with several surrounding residues
(Leu68, Ala71, Val72, Pro75, Val80, Pro81, Gln82, Val84 and Pro85),
particularly by hydrophobic interactions, highlighting that it
occupies the TEAD binding site and inhibits the YAP-TEAD complex
formation.
YAP is highly expressed in many solid tumors and its
carcinogenic function is mediated by its nuclear localization and
interaction with TEAD transcription factors (31,32).
By interfering with YAP/TEAD, non-photoactivated VP can inhibit the
proliferation of esophageal (33),
lung (34) and pancreatic cancer
(21), in addition to malignant
mesothelioma cells (35) and
bladder cancer cells (22),
inducing cell apoptosis. VP can significantly inhibit the
transcriptional activity of TEAD in MDA-MB-231 cells, reduce the
protein expression level of YAP target genes AXL and CTGF, inhibit
the migration of paclitaxel resistant cells and induce apoptosis
in vitro. VP reduces tumor volume and Ki67 expression in
paclitaxel-resistant mice and reverses the resistance of TNBC tumor
cells to paclitaxel therapy in vivo (23). The overexpression of YAP promotes
resistance to chemotherapy, radiotherapy and targeted treatment of
various types of cancer (13).
Non-photoactivated VP can also increase the sensitivity of HER-2
positive breast cancer cells to lapatinib (36) and increase the sensitivity of TNBC
MDA-MB-231 cells (37), urothelial
cells (38), esophageal cancer
cells (30) and colon cancer cells
(39) to chemotherapy drugs.
Therefore, non-photoactivated VP may provide a new choice for
drug-targeted YAP intervention in breast cancer.
Abnormal expression of CTGF and Cyr61 proteins is
associated with the progression of breast cancer, prostate cancer
and malignant melanoma (3,40,41).
The present study found that CTGF were expressed in Luminal B
BT-474 and TNBC BT-549 breast cancer cells and no CTGF expression
was detected in Luminal A MCF-7 cells, which was in accord with
previous data (42). CTGF specific
antibody can reduce the bone metastasis incidence in a mouse model
of breast cancer (43). The
present study found that non-photoactivated VP can inhibit the
migration of three breast cancer cell lines in vitro by
downregulating the expression of YAP/TEAD downstream target genes
CYR61, which are closely associated with cell migration and
invasion.
In conclusion, VP is an FDA-approved photosensitizer
for the treatment of age-related macular degeneration and can
inhibit the occurrence of tumor by destroying the formation of
YAP/TEAD complex in its non-photoactivated form (14). In the present study, the molecular
interface between VP and YAP was simulated and the binding site
between VP and YAP was confirmed by molecular docking experiment.
VP, a non-photoactive inhibitor of Hippo-YAP signaling, could
inhibit the migration of three molecular subtypes of breast cancer
cells by targeting YAP. The potential role of VP in cancer
progression, including cell proliferation and apoptosis, without
light activation need to be further studied and evaluated in
experimental animals and human breast cancer treatment. The results
of the present study provided a new approach for targeting the
Hippo pathway effector YAP to treat breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81473687), the
Shandong Provincial Natural Science Foundation, China (grant nos.
ZR2009CM039 and ZR2013HM038), the High Level Project Cultivation
Program of Shandong First Medical University, China (grant no.
2018GCC14) and the Academic promotion of Shandong First Medical
University, China (grant no. 2019QL017).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW acquired and analyzed the data, and drafted and
revised the manuscript. XL conceptualized the study, acquired and
analyzed the data, and drafted and revised the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fu V, Plouffe SW and Guan KL: The Hippo
pathway in organ development, homeostasis, and regeneration. Curr
Opin Cell Biol. 49:99–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holden JK and Cunningham CN: Targeting the
hippo pathway and cancer through the TEAD family of transcription
factors. Cancers (Basel). 10:812018. View Article : Google Scholar
|
|
3
|
Xie D, Nakachi K, Wang H, Elashoff R and
Koeffler HP: Elevated levels of connective tissue growth factor,
WISP-1, and CYR61 in primary breast cancers associated with more
advanced features. Cancer Res. 61:8917–8923. 2001.PubMed/NCBI
|
|
4
|
Luo XL, Yan Q, Tao DD, Hu JB, Li ZM, Li XL
and Gong JP: Effects of MST1 on cell proliferation and apoptosis of
human carcinoma cell line MCF-7. Tumor. 28:852–854. 2008.
|
|
5
|
Chen Q, Zhang N, Gray RS, Li H, Ewald AJ,
Zahnow CA and Pan D: A temporal requirement for Hippo signalling in
mammary gland differentiation, growth and tumorigenesis. Genes Dev.
28:432–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heidary Arash E, Shiban A, Song S and
Attisano L: MARK4 inhibits Hippo signaling to promote proliferation
and migration of breast cancer cells. EMBO Rep. 18:420–436. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott LJ and Goa KL: Verteporfin. Drugs
Aging. 16:139–146; discussion 147–148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richter AM, Waterfield E, Jain AK, Allison
B, Sternberg ED, Dolphin D and Levy JG: Photosensitising potency of
structural analogues of benzoporphyrin derivative (BPD) in a mouse
tumour model. Br J Cancer. 63:87–93. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ziemssen F and Heimann H: Evaluation of
verteporfin pharmakokinetics-redefining the need of
photosensitizers in ophthalmology. Expert Opin Drug Metab Toxicol.
8:1023–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Plouffe SW, Hong AW and Guan KL: Disease
implications of the Hippo/YAP pathway. Trends Mol Med. 21:212–222.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibault F, Corvaisier M, Bailly F, Huet G,
Melnyk P and Cotelle P: Non-photoinduced biological properties of
verteporfin. Curr Med Chem. 23:1171–1184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller BR III, McGee TD Jr, Swails JM,
Homeyer N, Gohlke H and Roitberg AE: MMPBSA.py: An efficient
program for end-state free energy calculations. J Chem Theory
Comput. 8:3314–3321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berman HM, Battistuz T, Bhat TN, Bluhm WF,
Bourne PE, Burkhardt K, Feng Z, Gilliland GL, Iype L, Jain S, et
al: The protein data bank. Acta Crystallogr D Biol Crystallogr.
58((Pt 6 No 1)): 899–907. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rostkowski M, Olsson MH, Søndergaard CR
and Jensen JH: Graphical analysis of pH-dependent properties of
proteins predicted using PROPKA. BMC Struct Biol. 11:62011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Wang S, Xing Z, Lin A, Liang K, Song
J, Hu Q, Yao J, Chen Z, Park PK, et al: A ROR1-HER3-lncRNA
signalling axis modulates the Hippo-YAP pathway to regulate bone
metastasis. Nat Cell Biol. 19:106–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong
J, Sun X and Li J: Verteporfin suppresses cell survival,
angiogenesis and vasculogenic mimicry of pancreatic ductal
adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci.
108:478–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong L, Lin F, Wu W, Liu Y and Huang W:
Verteporfin inhibits YAP-induced bladder cancer cell growth and
invasion via Hippo signaling pathway. Int J Med Sci. 15:645–652.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi G and Wang H, Han H, Gan J and Wang H:
Verteporfin enhances the sensitivity of LOVO/TAX cells to taxol via
YAP inhibition. Exp Ther Med. 16:2751–2755. 2018.PubMed/NCBI
|
|
24
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mesrouze Y, Meyerhofer M, Bokhovchuk F,
Fontana P, Zimmermann C, Martin T, Delaunay C, Izaac A, Kallen J,
Schmelzle T, et al: Effect of the acylation of TEAD4 on its
interaction with co-activators YAP and TAZ. Protein Sci.
26:2399–2409. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pobbati AV, Han X, Hung AW, Weiguang S,
Huda N, Chen GY, Kang C, Chia CS, Luo X, Hong W, et al: Targeting
the central pocket in human transcription factor TEAD as a
potential cancer therapeutic strategy. Structure. 23:2076–2086.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gibault F, Sturbaut M, Bailly F, Melnyk P
and Cotelle P: Targeting transcriptional enhanced associate domains
(TEADs). J Med Chem. 61:5057–5072. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Liu S, Ng EY, Li R, Poulsen A, Hill
J, Pobbati AV, Hung AW, Hong W, Keller TH and Kang C: Structural
and ligand binding analysis of the YAP-binding domain of
transcription factor TEAD4. Biochem J. 475:2043–2055. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian W, Yu J, Tomchick DR, Pan D and Luo
X: Structural and functional analysis of the YAP-binding domain of
human TEAD2. Proc Natl Acad Sci USA. 107:7293–7298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin KC, Park HW and Guan KL: Regulation of
the hippo pathway transcription factor TEAD. Trends Biochem Sci.
42:862–872. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vlug EJ, Van De Ven RA, Vermeulen JF, Bult
P, van Diest PJ and Derksen PW: Nuclear localization of the
transcriptional coactivator YAP is associated with invasive lobular
breast cancer. Cell Oncol (Dordr). 36:375–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song S, Honjo S, Jin J, Chang SS, Scott
AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et
al: The Hippo coactivator YAP1 mediates EGFR overexpression and
confers chemoresistance in esophageal cancer. Clin Cancer Res.
21:2580–2590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim J, McMillan E, Kim HS, Venkateswaran
N, Makkar G, Rodriguez-Canales J, Villalobos P, Neggers JE,
Mendiratta S, Wei S, et al: XPO1-dependent nuclear export is a
druggable vulnerability in KRAS-mutant lung cancer. Nature.
538:114–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tranchant R, Quetel L, Tallet A, Meiller
C, Renier A, de Koning L, de Reynies A, Le Pimpec-Barthes F,
Zucman-Rossi J, Jaurand MC and Jean D: Co-occurring mutations of
tumor suppressor genes, LATS2 and NF2, in malignant pleural
mesothelioma. Clin Cancer Res. 23:3191–3202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin C, Pelissier FA, Zhang H, Lakins J,
Weaver VM, Park C and LaBarge MA: Microenvironment rigidity
modulates responses to the HER2 receptor tyrosine kinase inhibitor
lapatinib via YAP and TAZ transcription factors. Mol Biol Cell.
26:3946–3953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Wang S, Wei X, Zhang S, Song Z, Chen
X and Zhang J: Role of inhibitor of yes-associated protein 1 in
triple-negative breast cancer with taxol-based chemoresistance.
Cancer Sci. 110:561–567. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ciamporcero E, Shen H, Ramakrishnan S, Yu
Ku S, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti
S, et al: YAP activation protects urothelial cell carcinoma from
treatment-induced DNA damage. Oncogene. 35:1541–1543. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li W, Cao Y, Xu J, Wang Y, Li W, Wang Q,
Hu Z, Hao Y, Hu L, Sun Y, et al: YAP transcriptionally regulates
COX-2 expression and GCCSysm-4 (G-4), a dual YAP/COX-2 inhibitor,
overcomes drug resistance in colorectal cancer. J Exp Clin Cancer
Res. 36:1442017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang F, Tuxhorn JA, Ressler SJ, McAlhany
SJ, Dang TD and Rowley DR: Stromal expression of connective tissue
growth factor promotes angiogenesis and prostate cancer
tumorigenesis. Cancer Res. 65:8887–8895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hutchenreuther J, Vincent KM, Carter DE,
Postovit LM and Leask A: CCN2 expression by tumor stroma is
required for melanoma metastasis. J Invest Dermatol. 135:2805–2813.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li MH, Sanchez T, Pappalardo A, Lynch KR,
Hla T and Ferrer F: Induction of antiproliferative connective
tissue growth factor expression in Wilms' tumor cells by
sphingosine-1-phosphate receptor 2. Mol Cancer Res. 6:1649–1656.
2008.PubMed/NCBI
|
|
43
|
Shimo T, Kubota S, Yoshioka N, Ibaragi S,
Isowa S, Eguchi T, Sasaki A and Takigawa M: Pathogenic role of
connective tissue growth factor (CTGF/CCN2) in osteolytic
metastasis of breast cancer. J Bone Miner Res. 21:1045–1059. 2006.
View Article : Google Scholar : PubMed/NCBI
|