Introduction
Cardiovascular disease (CVD) is a primary cause of
death worldwide (1), resulting in
a public health burden for society and patients. Age is considered
as a major contributor to CVD, the incidence of which increases
significantly with age (2,3). Vascular endothelial cells are a
single layer of squamous cells covering the surface of the vascular
intima, which forms the biological barrier of the vascular wall
(4). Dysfunction of vascular
endothelial cells is closely associated with senescence, increasing
the risk of CVD in the elderly population. Senescent endothelial
cells impair the function of vessels, which involves oxidative
stress and a proinflammatory phenotype (5). Inflammation and oxidative stress are
the primary factors of cell senescence. Inflammatory cytokines
secreted by senescent cells further trigger inflammation and
senescence in the surrounding tissue (6). Previous studies have demonstrated
that proinflammatory cytokines are increased, and superoxide
dismutase (SOD) and glutathione peroxidase (GSH-Px) activities are
decreased in the process of cell senescence (6–8).
Therapeutic strategies for inflammatory disorders and normalizing
oxidative stress have been demonstrated to be effective in various
types of CVD (9).
Monotropein (Mtp) is an iridoid glycoside isolated
from the roots of Morinda officinalis (10). A previous study demonstrated that
Mtp protected osteoblasts from H2O2-induced
oxidative stress via regulating autophagy (11). Mtp induced the differentiation of
bone marrow-derived endothelial progenitor cells and prevented cell
apoptosis by decreasing the release of reactive oxygen species
(ROS) (12). NF-κB is a classical
transcription factor that is activated in response to extracellular
stimulus, and serves a crucial role in oxidative stress and
inflammatory responses (13,14).
He et al (15) demonstrated
that Mtp significantly inhibited lipopolysaccharide (LPS)-induced
secretion of inflammatory cytokines by suppressing activation of
the NF-κB signaling pathway. However, to the best of our knowledge,
there is limited research available regarding the role of Mtp in
the pathogenesis of CVD. H2O2-stimulated
human umbilical vein endothelial cells (HUVECs) are a
well-established senescent cell model (16). The aim of the present study was to
simulate an oxidative environment with
H2O2-stimulated vascular endothelial cells,
and to determine the effects and mechanisms underlying Mtp in
H2O2-stimulated endothelial cells.
Materials and methods
Cell culture
HUVECs (Sigma-Aldrich; Merck KGaA) were maintained
in endothelial growth medium (Sigma-Aldrich; Merck KGaA) at 37°C
with 5% CO2. HUVECs were pretreated with Mtp (0.1, 1,
10, 100 or 1,000 µM; dissolved in deionized water; purity >98%;
Chengdu Herbpurify Co., Ltd.) for 24 h at 37°C.
H2O2 has been widely used to induce an
oxidative environment in vascular endothelial cell models in
vitro (16). To induce cell
senescence, HUVECs were treated with 100 µM
H2O2 for 12 h at 37°C. HUVECs were incubated
with 100 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich;
Merck KGaA) for 12 h at 37°C to activate NF-κB, as previously
described (17).
MTT assay
HUVECs were seeded (5×103 cells/well)
into a 96-well plate. Following treatment with
H2O2 and Mtp, 20 µl MTT reagent (5 mg/ml;
Beijing Solarbio Science & Technology Co., Ltd.) was added to
each well for 4 h at 37°C. Subsequently, 150 µl DMSO was used to
dissolve the purple formazan. Absorbance was measured at a
wavelength of 570 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
Senescence-associated β-galactosidase
(β-Gal) activity
HUVECs were collected into a centrifuge tube
containing the extracting reagent of the β-Gal assay kit (Beijing
Solarbio Science & Technology Co., Ltd.). HUVECs
(5×106) were centrifuged at 15,000 × g for 10 min at
4°C. The corresponding reagents in the kit were added into the tube
and incubated for 30 min at 37°C. The absorbance value was
immediately determined at a wavelength of 400 nm according to
manufacturer's protocol.
ELISA
Cell culture medium was centrifuged at 1,000 × g at
4°C for 10 min. The levels of secreted IL-6, TNF-α and monocyte
chemoattractant protein-1 (MCP-1) in cell culture medium were
measured using human IL-6 (cat. no. EH004-48), TNF-α (cat. no.
EH009-48) and MCP-1 (cat. no. EH019-48) ELISA kits (Shanghai ExCell
Biology, Inc.) according to the manufacturer's protocol. Optical
density values were recorded at a wavelength of 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.). Each group was
assessed in triplicate.
Western blotting
HUVECs were seeded (5×106) into a 100-mm
petri dish. Total protein was extracted using RIPA buffer
containing phosphatase inhibitors (Beijing Solarbio Science &
Technology Co., Ltd.). Proteins (30 µg) were separated via 10%
SDS-PAGE and transferred onto PVDF membranes (EMD Millipore). After
blocking with 5% skimmed milk for 2 h at room temperature, the
membranes were incubated at 4°C overnight with primary antibodies
targeted against: Phosphorylated (p)-NF-κB p65 (phosphor S276; cat.
no. ab194726; 1:1,000; Abcam), NF-κB p65 (cat. no. ab16502;
1:1,000; Abcam), high mobility group AT-hook 1 (Hmga1; cat. no.
ab129153; 1:20,000; Abcam), vascular cell adhesion molecule-1
(VCAM-1; cat. no. bs-0920R; 1:500; BIOSS), intercellular cell
adhesion molecule-1 (ICAM-1; cat. no. bs-4618R; 1:500; BIOSS),
Bcl-2 (cat. no. bs-4563R; 1:500; BIOSS), Bax (cat. no. bs-0127R;
1:500; BIOSS), GAPDH (cat. no. bsm-33033M; 1:500; BIOSS), cleaved
caspase-3 (cat. no. 9661; 1:1,000; Cell Signaling Technology,
Inc.), caspase-3 (cat. no. 9662; 1:1,000; Cell Signaling
Technology, Inc.), γ-H2A.X variant histone (H2AX; cat. no. 2577;
1:1,000; Cell Signaling Technology, Inc.), H2AX (cat. no. 2595;
1:1,000; Cell Signaling Technology, Inc.), p-activator protein-1
(AP-1; phospho Ser63; cat. no. ABP50261; 1:1,000; Abbkine
Scientific Co., Ltd.) and AP-1 (cat. no. ABP50668; 1:1,000; Abbkine
Scientific Co., Ltd.). Subsequently, the membranes were incubated
with HRP-linked anti-mouse IgG (cat. no. 7076; 1:3,000; Cell
Signaling Technology, Inc.) or HRP-linked anti-rabbit IgG (cat. no.
7074; 1:3,000; Cell Signaling Technology, Inc.) secondary
antibodies. Protein bands were visualized using Pierce™ ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). Protein
expression levels were semi-quantified using Image Lab software
(version 4.0; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cultured cells using
the TRIzol® Purification kit (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using M-MLV Reverse Transcriptase (Promega Corporation) in the
presence of oligo(dT) primers and dNTP. The following temperature
protocol was used for reverse transcription: Denaturation at 70°C
for 5 min; annealing at 25°C for 10 min; and extension at 42°C for
50 min. Subsequently, qPCR was performed using the Power SYBR Green
PCR Master mix (Thermo Fisher Scientific, Inc.) and a 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions used for qPCR were as follows: 95°C for 10
min; followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec.
The following primers were used for qPCR: VCAM-1 forward,
5′-CAGGCTGTGAGTCCCCATT-3′ and reverse, 5′-TTGACTGTGATCGGCTTCC-3′;
ICAM-1 forward, 5′-ACCATCTACAGCTTTCCGGC-3′; and reverse,
5′-TTTCTGGCCACGTCCAGTTT-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
GAPDH was used as an internal control for quantification using the
2−∆∆Cq method (18).
Measurement of malondialdehyde
(MDA)
The MDA assay kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to evaluate the content of MDA.
HUVECs (4×106) were collected and centrifuged at 8,000 ×
g for 10 min at 4°C after adding the extracting reagent of the MDA
assay kit. Subsequently, the MDA detection reagent was added and
fully mixed at 100°C for 60 min, cooled and then centrifuged at
10,000 × g for 10 min at room temperature. The supernatant (200 µl)
was plated into a 96-well plate and the absorbance value was
determined at wavelengths of 450, 532 and 600 nm according to the
manufacturer's protocol.
Measurement of SOD
A SOD assay kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to evaluate SOD activity. HUVECs
(5×106) were collected and centrifuged at 8,000 × g for
10 min at 4°C after adding the extracting reagent of the SOD assay
kit. Subsequently, corresponding reagents were added into the
sample and fully mixed at 37°C for 30 min. The absorbance value was
measured at a wavelength of 560 nm according to the manufacturer's
protocol.
Measurement of GSH-Px
The GSH-Px assay kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to evaluate GSH-Px activity. HUVECs
were collected and centrifuged at 8,000 × g for 10 min at 4°C after
adding the extracting reagent of the GSH-Px assay kit.
Subsequently, the corresponding reagents were added into the sample
and fully mixed. The absorbance value was immediately determined at
a wavelength of 412 nm according to the manufacturer's
protocol.
TUNEL apoptosis assay kit
Adherent cell slides were prepared to locate
apoptotic cells using the TUNEL apoptosis assay kit (Nanjing KeyGen
Biotech Co., Ltd.). Biotin-labeled dUTP could connect to the 3′-OH
terminal of apoptotic cells via TdT Enzyme and combine specifically
with streptavidin-HRP. Briefly, treated cells were fixed with fresh
4% paraformaldehyde for 15 min at room temperature, gently rinsed
with PBS and incubated with 0.1% Triton X-100 for 2 min at 4°C. The
TdT enzyme was added and incubated for 60 min at 37°C in the dark,
and then with streptavidin-HRP solution for 30 min in the dark at
37°C. Finally, diaminobenzidine solution was used to assess the
color-reaction for 10 min at room temperature. Apoptotic cells were
visualized in six randomly selected fields of view using a light
microscope (magnification, ×200).
Statistical analysis
Data are presented as the mean ± SD. Each experiment
was performed in triplicate. Statistical analyses were performed
using GraphPad Prism software (version 6.0; GraphPad Software,
Inc.). Comparisons between two groups were analyzed using the
unpaired Student's t-test. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Mtp regulates HUVEC viability and
senescence
Initially, varying concentrations of Mtp were
prepared for the pretreatment of HUVECs for 24 h. The results
indicated that, compared with the control group, 0.1–100.0 µM Mtp
did not significantly affect HUVEC viability, but 1,000 µM Mtp
significantly reduced cell viability (Fig. 1A). For subsequent experiments,
0.1–100.0 µM Mtp were used for pretreating HUVECs, which were
subsequently incubated with 100 µM H2O2 for
12 h. The results suggested that H2O2
significantly inhibited cell viability in the absence of Mtp
pretreatment compared with the control group. By contrast, 10 and
100 µM Mtp significantly increased cell viability in
H2O2-stimulated HUVECs compared with the
H2O2 group, and 100 µM Mtp exhibited an
improved efficacy compared with 10 µM Mtp (Fig. 1B). Subsequently, HUVECs were
pretreated with 100 µM Mtp or vehicle, and then stimulated with
H2O2 for 12 h. Compared with the control
group, H2O2 significantly enhanced β-Gal
activity, but Mtp pretreatment significantly decreased
H2O2-induced β-Gal activity (Fig. 1C). Additionally, by measuring the
expression levels of the senescence marker Hmga1 and the DNA damage
marker γ-H2AX, the results also indicated that
H2O2 increased HUVEC senescence compared with
the control group, whereas Mtp pretreatment significantly inhibited
H2O2-induced senescence (Fig. 1D). The results suggested that Mtp
reversed H2O2-mediated downregulation of cell
viability and induction of senescence.
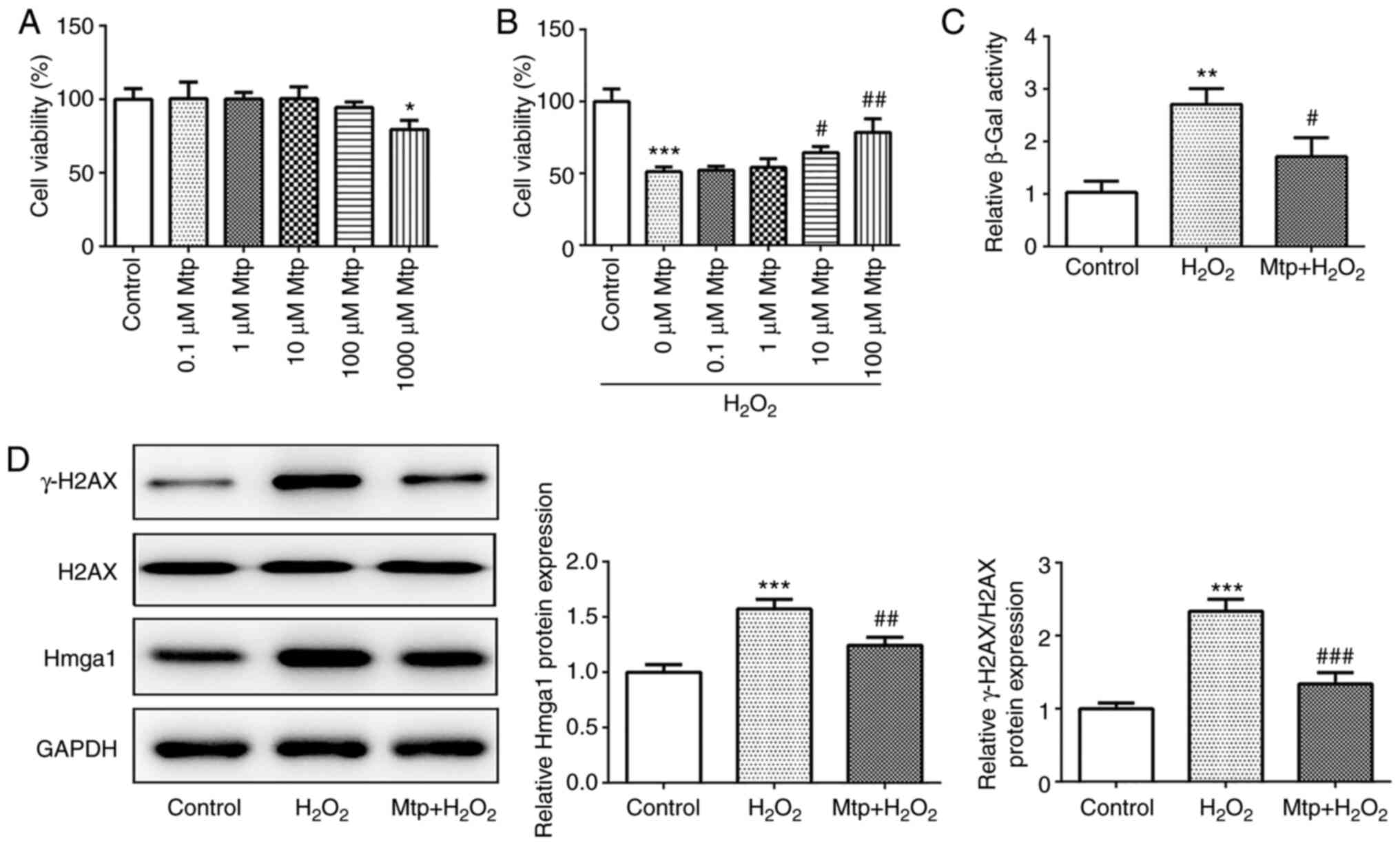 | Figure 1.Mtp regulates HUVEC viability and
senescence. (A) Following treatment with Mtp for 24 h, HUVEC
viability was assessed by performing an MTT assay. (B) Following
pretreatment with Mtp for 24 h and incubation with
H2O2 for 12 h, HUVEC viability was assessed
by performing an MTT assay. (C) Following pretreatment with 100 µM
Mtp for 24 h and incubation with 100 µM H2O2
for 12 h, β-Gal activity was measured using a β-Gal assay kit. (D)
The protein expression levels of Hmga1, γ-H2AX and H2AX were
measured via western blotting. *P<0.05, **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2. Mtp, monotropein; HUVEC, human
umbilical vein endothelial cell; β-Gal, β-galactosidase; Hmga1,
high mobility group AT-hook 1; H2AX, H2A.X variant histone. |
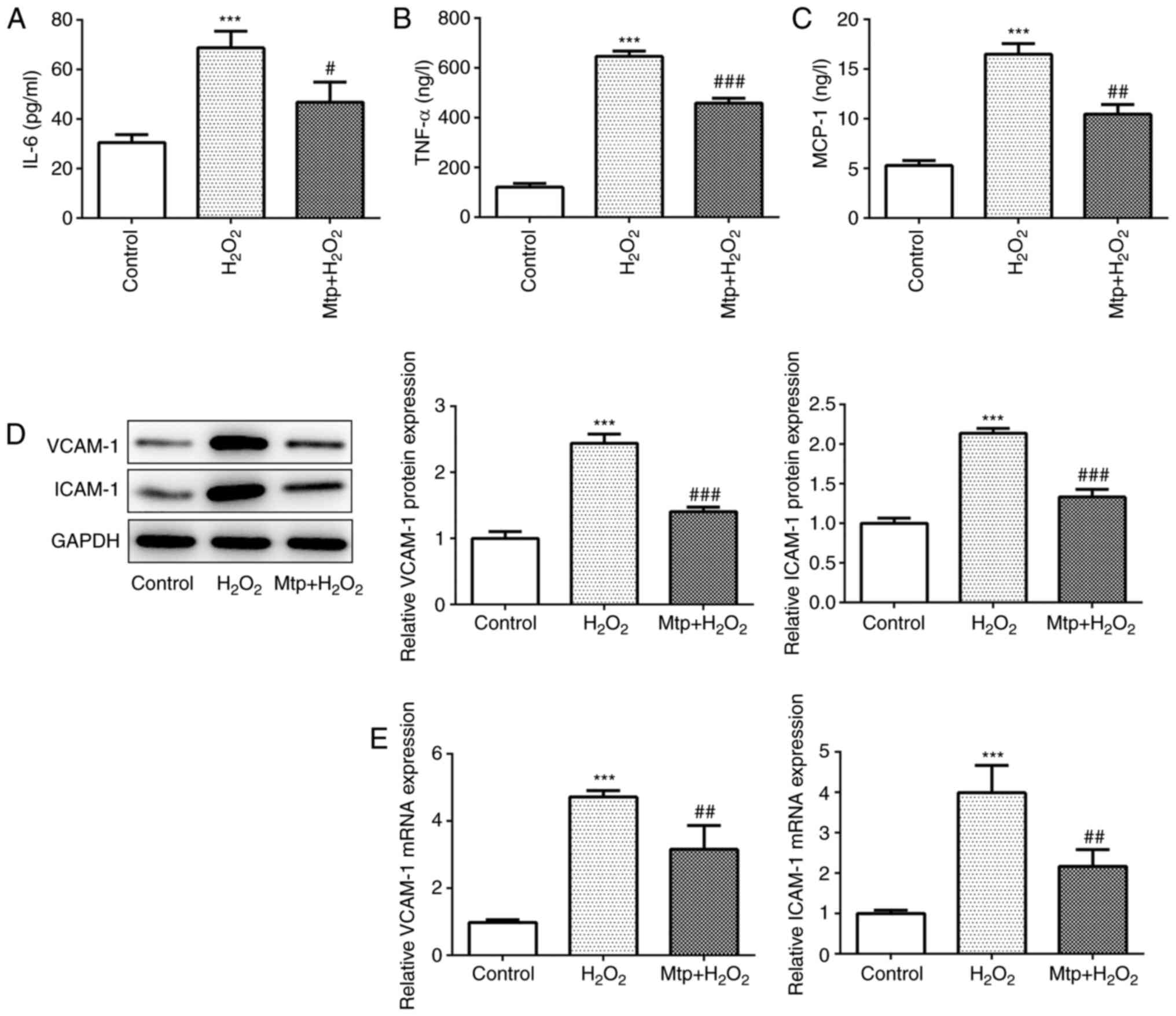
Mtp alleviates the inflammatory
response of HUVECs
To investigate the effect of Mtp on the inflammatory
response in H2O2-stimulated HUVECs, cell
culture medium was collected to estimate the release of
proinflammatory cytokines, such as IL-6, TNF-α and MCP-1. The
results indicated that H2O2 significantly
upregulated the release of proinflammatory cytokines compared with
the control group, and Mtp pretreatment significantly reduced
H2O2-induced proinflammatory cytokine
release, suggesting a potent anti-inflammatory effect of Mtp
(Fig. 2A-C). TNF-α can cause
vascular endothelial cell dysfunction, resulting in the production
of a variety of cytokines, such as ICAM-1 and VCAM-1, and
triggering vascular inflammation (19,20).
The results indicated that ICAM-1 and VCAM-1 expression levels were
significantly increased in the H2O2 group
compared with the control group, whereas Mtp pretreatment
significantly reversed H2O2-induced protein
expression (Fig. 2D). Similarly,
the mRNA levels of ICAM-1 and VCAM-1 were upregulated in the
H2O2 group compared with the control group
(Fig. 2E). Collectively, the
results indicated that Mtp protected HUVECs against
H2O2-induced inflammation.
HUVEC oxidative stress and apoptosis
are suppressed by Mtp
It has previously been reported that Mtp is capable
of inhibiting H2O2-induced ROS generation in
osteoblasts (21). In the present
study, MDA content was estimated to evaluate membrane lipid
peroxidation. The results indicated that H2O2
significantly increased MDA content compared with the control
group, whereas pretreatment with Mtp significantly decreased MDA
levels compared with the H2O2 group (Fig. 3A). In addition, significantly
decreased SOD and GSH-Px activities were observed in the
H2O2 group compared with the control group,
but Mtp pretreatment inhibited H2O2-mediated
downregulation of SOD and GSH-Px activities (Fig. 3B and C), which indicated that Mtp
protected HUVECs against H2O2-induced
oxidative injury. Subsequently, whether there was an association
between Mtp and cell apoptosis was investigated. The TUNEL assay
indicated that apoptotic cells (brown-stained) were observed in the
H2O2 group and Mtp pretreatment significantly
decreased H2O2-induced cell apoptosis
(Fig. 3D). Furthermore,
alterations to the protein expression levels of Bcl-2, Bax and
cleaved-caspase 3 indicated that Mtp pretreatment significantly
relieved H2O2-induced cell apoptosis
(Fig. 3E). Collectively, the
results indicated that Mtp ameliorated
H2O2-induced oxidative stress and
apoptosis.
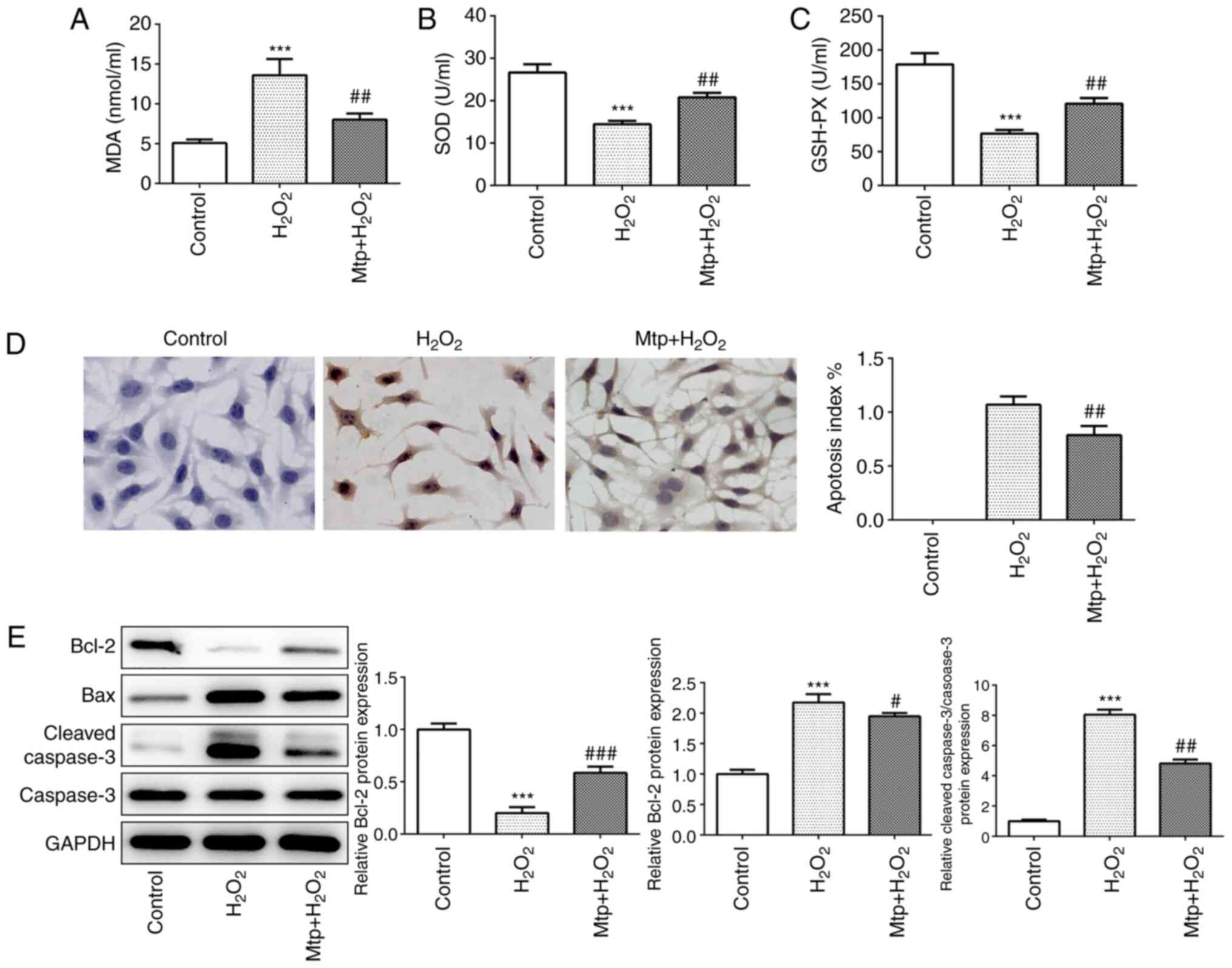 | Figure 3.HUVEC oxidative stress and apoptosis
are suppressed by Mtp. (A) MDA levels, and (B) SOD and (C) GSH-Px
activities were determined using corresponding assay kits. (D) The
TUNEL assay was performed to identify apoptotic cells
(magnification, ×200). Blue-stained cells represent normal HUVECs
and brown-stained cells indicate apoptotic cells. (E) The protein
expression levels of Bcl-2, Bax, cleaved-caspase 3 and caspase 3
were measured via western blotting. ***P<0.001 vs. control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. H2O2. HUVEC,
human umbilical vein endothelial cell; Mtp, monotropein; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase. |
Mtp anti-inflammatory effects are
reversed by PMA
NF-κB is a key transcription factor associated with
oxidative stress and the inflammatory response (13,14).
AP-1 has been demonstrated to interact with NF-κB, and NF-κB/AP-1
signaling cascades serve an important role in inflammation
(22,23). The present study suggested that the
phosphorylation of NF-κB and AP-1 was significantly increased
following H2O2 treatment compared with the
control group. By contrast, pretreatment with Mtp significantly
decreased the phosphorylation of NF-κB and AP-1 compared with the
H2O2 group (Fig.
4A). PMA (100 ng/ml) was prepared and incubated with HUVECs for
12 h to activate NF-κB as previously described (17). The phosphorylation levels of NF-κB
and AP-1 were significantly increased by PMA incubation compared
with the control group (Fig. 4B).
Furthermore, the results indicated that the anti-inflammatory
effects of Mtp were significantly reversed by PMA (Fig. 4C-E), which was further indicated by
significantly elevated protein expression levels of ICAM-1 and
VCAM-1 in the PMA + Mtp + H2O2 group compared
with the Mtp + H2O2 group (Fig. 4F). In summary, the results
suggested that Mtp exerted an anti-inflammatory effect potentially
via regulating the activation of NF-κB/AP-1 signaling cascades.
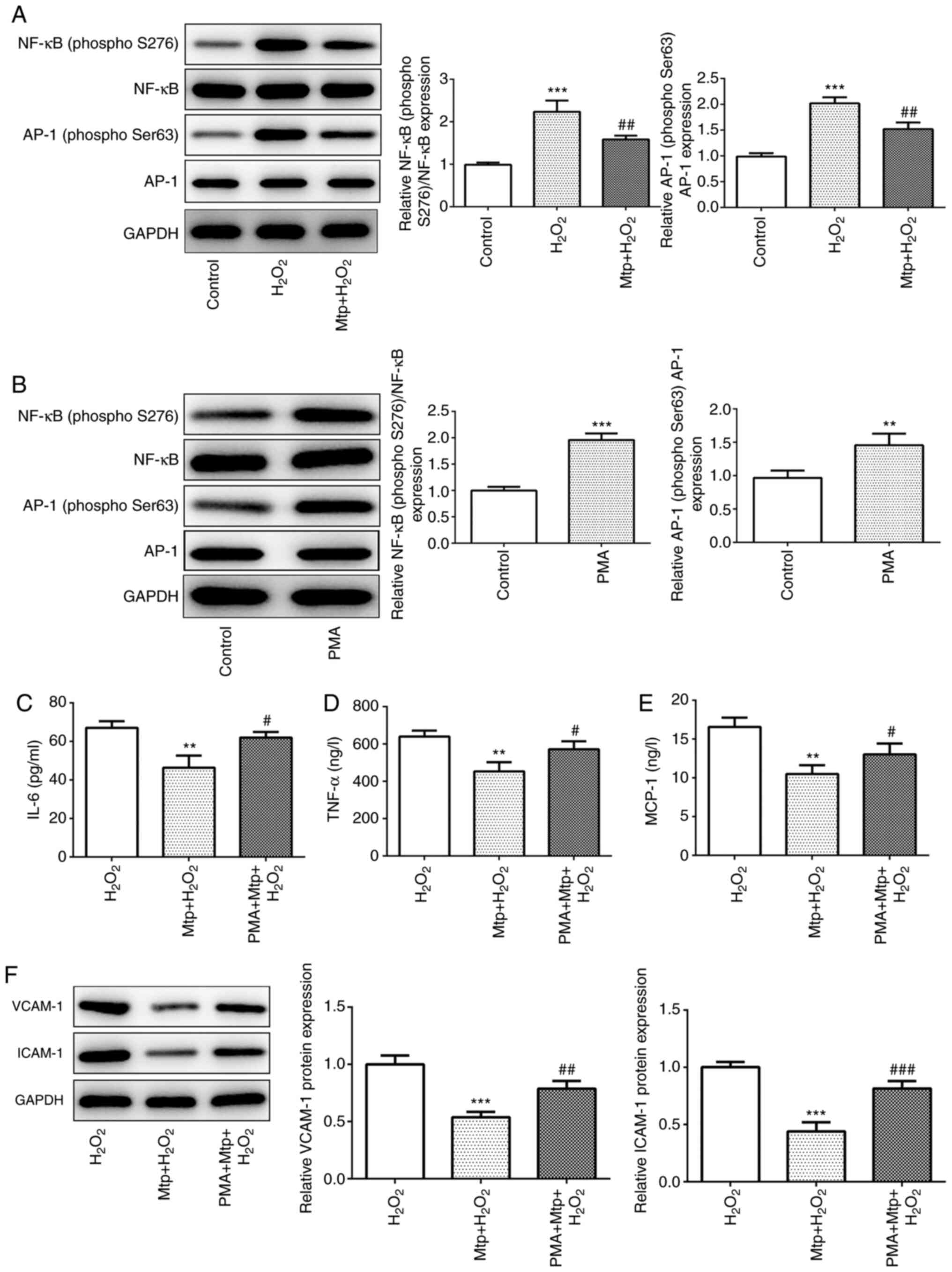 | Figure 4.Mtp anti-inflammatory effects are
reversed by PMA. (A) NF-κB and AP-1 protein expression levels were
measured via western blotting. (B) HUVECs were treated with PMA for
12 h, and NF-κB and AP-1 protein expression levels were determined
via western blotting. The levels of secreted proinflammatory
cytokines (C) IL-6, (D) TNF-α and (E) MCP-1 were determined by
performing ELISAs. (F) The protein expression levels of VCAM-1 and
ICAM-1 were measured via western blotting. **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2. Mtp, monotropein; PMA, phorbol
12-myristate 13-acetate; AP-1, activator protein-1; HUVEC, human
umbilical vein endothelial cell; MCP-1, monocyte chemoattractant
protein-1; VCAM-1, vascular cell adhesion molecule-1; ICAM-1,
intercellular cell adhesion molecule-1. |
NF-κB/AP-1 signaling may be associated
with the inhibitory effects of Mtp on oxidative stress and
apoptosis
To further evaluate the molecular mechanism
underlying HUVEC oxidative stress and apoptosis, cells were
incubated with Mtp and PMA. The results suggested that Mtp
pretreatment-mediated decreases in MDA content were counteracted by
PMA (Fig. 5A). PMA also
significantly decreased Mtp-mediated upregulation of SOD and GSH-Px
in H2O2-stimulated HUVECs (Fig. 5B and C). In addition, Mtp
pretreatment decreased H2O2-induced cell
apoptosis, which was weakened by PMA treatment (Fig. 5D). Accordingly, Mtp-mediated
downregulation of the expression levels of proapoptotic proteins
Bax and cleaved-caspase 3 was reversed by PMA, whereas the protein
expression levels of Bcl-2 displayed the opposite effect (Fig. 5E). The results suggested that the
inhibitory effects of Mtp on HUVEC oxidative stress and apoptosis
were partially counteracted by PMA.
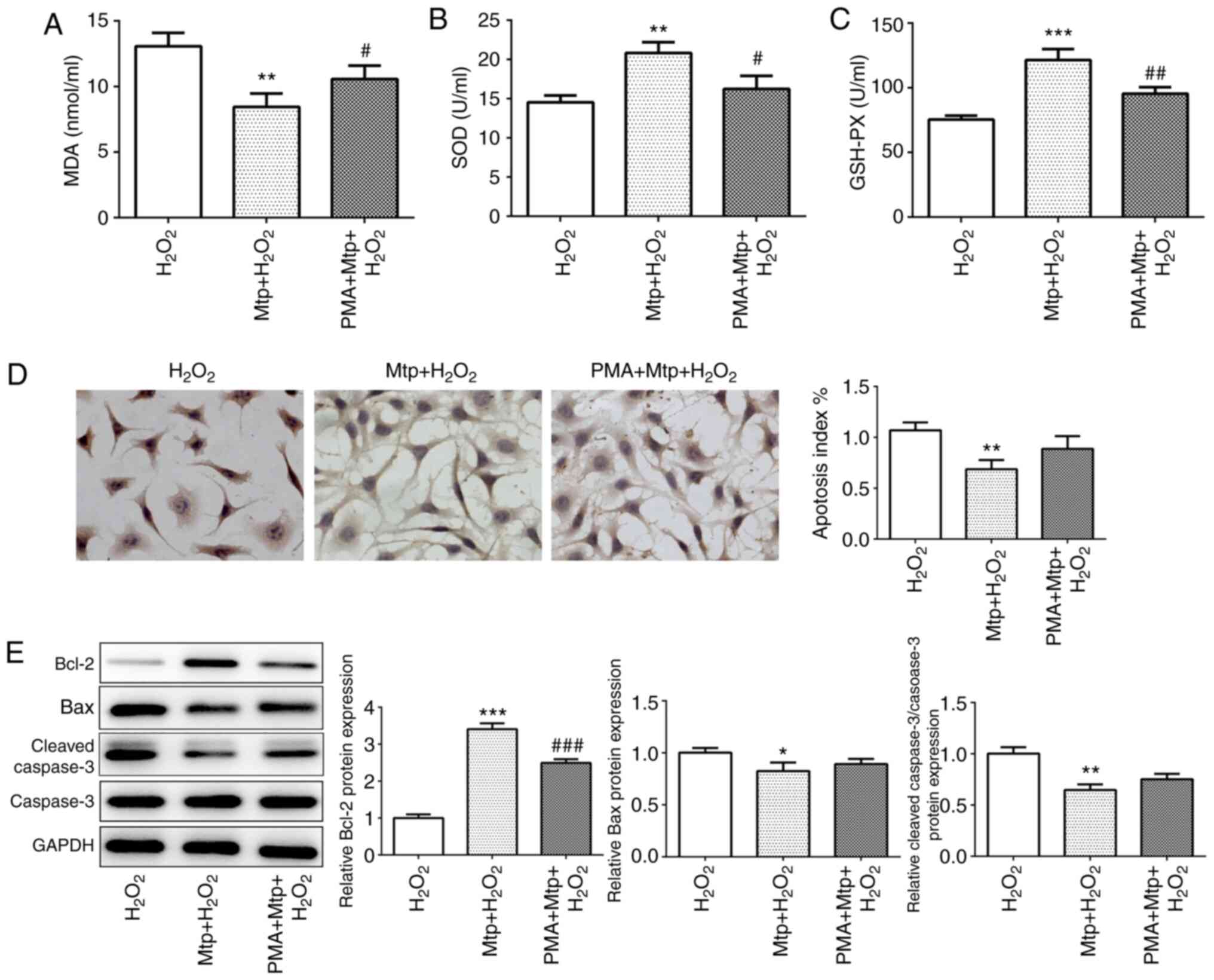 | Figure 5.NF-κB/AP-1 signaling may be
associated with the inhibitory effects of Mtp on HUVEC oxidative
stress and apoptosis. (A) MDA levels, and (B) SOD and (C) GSH-Px
activities were determined using corresponding assay kits. (D) The
TUNEL assay was performed to identify apoptotic cells
(magnification, ×200). Blue-stained cells represent normal HUVECs
and brown-stained cells indicate apoptotic cells. (E) The protein
expression levels of Bcl-2, Bax, cleaved-caspase 3 and caspase 3
were measured via western blotting. *P<0.05, **P<0.01 and
***P<0.001 vs. H2O2;
#P<0.05, ##P<0.01 and
###P<0.001 vs. H2O2 + Mtp.
AP-1, activator protein-1; Mtp, monotropein; HUVEC, human umbilical
vein endothelial cell; MDA, malondialdehyde; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase. |
Discussion
Previous studies have demonstrated that Mtp exerts
antiapoptotic and anti-inflammatory effects in osteoarthritis
chondrocytes (21,24,25).
Nevertheless, the potential functions of Mtp in the progression of
CVD are not completely understood. CVD is closely associated with
senescent vascular endothelial cells, which secrete proinflammatory
mediators and further exacerbate the progression of CVD (26). In the present study, HUVECs were
cultured in vitro and stimulated with
H2O2 to mimic a senescent cell model.
An appropriate concentration of Mtp was selected to
treat HUVECs and was assessed using an MTT assay. Subsequently, by
measuring the proinflammatory mediators secreted by HUVECs exposed
to different stimuli, the results indicated that Mtp pretreatment
ameliorated the inflammatory response triggered by
H2O2. In addition, the markers of oxidative
stress and apoptosis were also decreased in
H2O2-stimulated HUVECs in the presence of
Mtp; however, a potential limitation of the present study may be
the lack of ROS determination.
A previous study indicated that Mtp decreased the
DNA binding activity of NF-κB in LPS-induced RAW 264.7 macrophages,
and inhibited the phosphorylation and degradation of inhibitory
κB-α, thereby inhibiting the translocation of NF-κB (27). Furthermore, Mtp inhibits the
phosphorylation of NF-κB in MC3T3-E1 murine embryonic osteoblastic
precursor cells (15). AP-1 is
capable of interacting with NF-κB, which triggers inflammatory
cytokines, including TNF-α and IL-1β, via regulating their
corresponding mediator genes (28). In the present study, the
phosphorylation of NF-κB was increased in
H2O2-stimulated HUVECs compared with the
control group. Pretreatment with Mtp decreased the phosphorylation
of NF-κB and AP-1 in H2O2-stimulated HUVECs.
Moreover, the results indicated that elevating the activation of
NF-κB by PMA counteracted the ameliorative effects of Mtp on
H2O2-stimulated HUVECs, suggesting that Mtp
exerted its protective role by modulating the NF-κB/AP-1 signaling
pathway. When cells are stimulated with PMA, the phosphorylation of
p38MAPK is increased (29,30). The signaling pathway activates a
variety of transcription factors, including NF-κB (p50/p65) and
AP-1 (c-Fos/c-Jun), that coordinate the induction of numerous genes
encoding inflammatory mediators (31), such as IL-6, TNF-α and MCP-1
(32). To date, studies on Mtp
have primarily focused on osteoarthritis. He et al (15) demonstrated that Mtp attenuates
inflammatory impairment on osteoblasts via inactivation of the
NF-κB signaling pathway. Moreover, Mtp suppresses IL-1β-induced
apoptosis and catabolic responses on osteoarthritis chondrocytes
(24), and in Mtp-treated
osteoblasts, oxidative stress was alleviated via Akt/mTOR-mediated
autophagy (11). However, the role
of Mtp on endothelial cells in CVD has not been previously
reported.
In summary, the present study indicated that Mtp
protected HUVECs against H2O2-induced
inflammation, oxidative stress and apoptosis, potentially via
mediating the NF-κB/AP-1 signaling pathway. Therefore, Mtp may
serve as a candidate therapeutic for protecting HUVECs in patients
with CVD via monitoring NF-κB/AP-1 signaling cascades or inhibiting
NF-κB activation. The present study suggested the protective effect
of Mtp on H2O2-induced vascular endothelial
cells, indicating a potential therapeutic effect for patients with
CVD via targeting endothelial functions. Nevertheless, the effects
of Mtp on vascular endothelial cells were only investigated at the
cellular level in the present study. Therefore, how Mtp activates
NF-κB and whether Mtp affects other signaling pathways in
endothelial cells requires further investigation. Moreover, animal
experiments and clinical trials are required to further validate
the curative effect of Mtp.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation for the Youth (grant no. 81200075).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJ and XRX made substantial contribution to data
acquisition. WML and KX contributed to data analysis. LFW and XCY
designed the present study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xue B, Head J and McMunn A: The
associations between retirement and cardiovascular disease risk
factors in China: A 20-year prospective study. Am J Epidemiol.
185:688–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finegold JA, Asaria P and Francis DP:
Mortality from ischaemic heart disease by country, region, and age:
Statistics from world health organisation and United Nations. Int J
Cardiol. 168:934–945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paneni F, Diaz Cañestro C, Libby P,
Luscher TF and Camici GG: The aging cardiovascular system:
Understanding it at the cellular and clinical levels. J Am Coll
Cardiol. 69:1952–1967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montezano AC, Neves KB, Lopes RA and Rios
F: Isolation and culture of endothelial cells from large vessels.
Methods Mol Biol. 1527:345–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierce GL, Lesniewski LA, Lawson BR, Beske
SD and Seals DR: Nuclear factor-(kappa)B activation contributes to
vascular endothelial dysfunction via oxidative stress in
overweight/obese middle-aged and older humans. Circulation.
119:1284–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serino A and Salazar G: Protective role of
polyphenols against vascular inflammation, aging and cardiovascular
disease. Nutrients. 11:532018. View Article : Google Scholar
|
|
7
|
Salazar G, Huang J, Feresin RG, Zhao Y and
Griendling KK: Zinc regulates Nox1 expression through a NF-κB and
mitochondrial ROS dependent mechanism to induce senescence of
vascular smooth muscle cells. Free Radic Biol Med. 108:225–235.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian Y, Zhang J, Zhou X, Yi R, Mu J, Long
X, Pan Y, Zhao X and Liu W: Lactobacillus plantarum CQPC11
isolated from sichuan pickled cabbages antagonizes
d-galactose-induced oxidation and aging in mice. Molecules.
23:30262018. View Article : Google Scholar
|
|
9
|
Steven S, Frenis K, Oelze M, Kalinovic S,
Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J,
Kröller-Schön S, Münzel T and Daiber A: Vascular inflammation and
oxidative stress: Major triggers for cardiovascular disease. Oxid
Med Cell Longev. 2019:70921512019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Zhang Q, Yang H, Liu W, Zhang N,
Qin L and Xin H: Monotropein isolated from the roots of Morinda
officinalis increases osteoblastic bone formation and prevents
bone loss in ovariectomized mice. Fitoterapia. 110:166–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Liu XY, Jiang YP, Zhang JB, Zhang
QY, Wang NN and Xin HL: Monotropein attenuates oxidative stress via
Akt/mTOR-mediated autophagy in osteoblast cells. Biomed
Pharmacother. 121:1095662020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Mao C, Lou Y, Xu J, Wang Q, Zhang
Z, Tang Q, Zhang X, Xu H and Feng Y: Monotropein promotes
angiogenesis and inhibits oxidative stress-induced autophagy in
endothelial progenitor cells to accelerate wound healing. J Cell
Mol Med. 22:1583–1600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen X, Luo L, Yang M, Lin Y, Li J and
Yang L: Exendin4 inhibits lipotoxicity-induced oxidative stress in
betacells by inhibiting the activation of TLR4/NF κB signaling
pathway. Int J Mol Med. 45:1237–1249. 2020.PubMed/NCBI
|
|
14
|
Wang F, Zhou H, Deng L, Wang L, Chen J and
Zhou X: Serine deficiency exacerbates inflammation and oxidative
stress via microbiota-gut-brain axis in D-galactose-induced aging
mice. Mediators Inflamm. 2020:58214282020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He YQ, Yang H, Shen Y, Zhang JH, Zhang ZG,
Liu LL, Song HT, Lin B, Hsu HY, Qin LP, et al: Monotropein
attenuates ovariectomy and LPS-induced bone loss in mice and
decreases inflammatory impairment on osteoblast through blocking
activation of NF-κB pathway. Chem Biol Interact. 291:128–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huo J, Xu Z, Hosoe K, Kubo H, Miyahara H,
Dai J, Mori M, Sawashita J and Higuchi K: Coenzyme Q10 prevents
senescence and dysfunction caused by oxidative stress in vascular
endothelial cells. Oxid Med Cell Longev. 2018:31817592018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salman I, Jung L, Adeeb S, Eun-Mi A, You L
and Young L: Decursinol angelate inhibits LPS-induced macrophage
polarization through modulation of the NFκB and MAPK signaling
pathways. Molecules. 23:18802018. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong LT, Ju Z, Liu L, Wang L, Zhou X, Xiao
T and Zhou S: Rice-derived peptide AAGALPS inhibits TNF-α-induced
inflammation and oxidative stress in vascular endothelial cells.
Food Sci Nutr. 8:659–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CC, Pan CS, Wang CY, Liu SW, Hsiao LD
and Yang CM: Tumor necrosis factor-alpha induces VCAM-1-mediated
inflammation via c-Src-dependent transactivation of EGF receptors
in human cardiac fibroblasts. J Biomed Sci. 22:532015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu FB, Wang JY, Zhang YL, Hu YG, Yue ZS,
Zeng LR, Zheng WJ, Hou Q, Yan SG and Quan RF: Mechanisms underlying
the antiapoptotic and anti-inflammatory effects of monotropein in
hydrogen peroxide-treated osteoblasts. Mol Med Rep. 14:5377–5384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung YY, Shanmugam MK, Chinnathambi A,
Alharbi SA, Shair OHM, Um JY, Sethi G and Ahn KS: Fangchinoline, a
bisbenzylisoquinoline alkaloid can modulate cytokine-impelled
apoptosis via the dual regulation of NF-κB and AP-1 pathways.
Molecules. 24:31272019. View Article : Google Scholar
|
|
23
|
Lee JH, Kim C, Lee SG, Yang WM, Um JY,
Sethi G and Ahn KS: Ophiopogonin D modulates multiple oncogenic
signaling pathways, leading to suppression of proliferation and
chemosensitization of human lung cancer cells. Phytomedicine.
40:165–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Wu L, Li L and Chen S: Monotropein
exerts protective effects against IL-1β-induced apoptosis and
catabolic responses on osteoarthritis chondrocytes. Int
Immunopharmacol. 23:575–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karna KK, Choi BR, You JH, Shin YS, Cui
WS, Lee SW, Kim JH, Kim CY, Kim HK and Park JK: The ameliorative
effect of monotropein, astragalin, and spiraeoside on oxidative
stress, endoplasmic reticulum stress, and mitochondrial signaling
pathway in varicocelized rats. BMC Complement Altern Med.
19:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donato AJ, Morgan RG, Walker AE and
Lesniewski LA: Cellular and molecular biology of aging endothelial
cells. J Mol Cell Cardiol. 89:122–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin JS, Yun KJ, Chung KS, Seo KH, Park
HJ, Cho YW, Baek NI, Jang D and Lee KT: Monotropein isolated from
the roots of Morinda officinalis ameliorates proinflammatory
mediators in RAW 264.7 macrophages and dextran sulfate sodium
(DSS)-induced colitis via NF-κB inactivation. Food Chem Toxicol.
53:263–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han Y, Li X, Zhang X, Gao Y, Qi R, Cai R
and Qi Y: Isodeoxyelephantopin, a sesquiterpene lactone from
Elephantopus scaber Linn., inhibits pro-inflammatory mediators'
production through both NF-κB and AP-1 pathways in LPS-activated
macrophages. Int Immunopharmacol. 84:1065282020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang T, Chen T, Chen P, Zhang B, Hong J
and Chen L: MPTP-induced dopamine depletion in basolateral amygdala
via decrease of D2R activation suppresses GABAA
receptors expression and LTD induction leading to anxiety-like
behaviors. Front Mol Neurosci. 10:2472017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alvarez SE, Milstien S and Spiegel S:
Autocrine and paracrine roles of sphingosine-1-phosphate. Trends
Endocrinol Metab. 18:300–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gay NJ and Gangloff M: Structure and
function of toll receptors and their ligands. Annu Rev Biochem.
76:141–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sperlich J, Kerr R and Teusch N: The
marine natural product pseudopterosin blocks cytokine release of
triple-negative breast cancer and monocytic leukemia cells by
inhibiting NF-κB signaling. Mar Drugs. 15:2622017. View Article : Google Scholar
|