Introduction
Aortic dissection (AD) is a major complication of
thoracic aortic disease, which is initiated by tears in the aortic
intima and media. The tears allow blood to enter into the media and
separate the medial layer along the long axis of the aorta, thus
leading to the formation of a false lumen (1,2).
Multiple factors, including poorly controlled hypertension, older
age, male gender, smoking, genetic conditions, pre-existing aortic
diseases, aortic instrumentation or surgery, and
immune/inflammatory diseases, are associated with an increased risk
of AD (3–5). Although AD is an uncommon disease with
an estimated annual incidence of 5–30 cases per million individuals
(6), the disease displays high
mortality and is ranked among the most lethal vascular diseases
worldwide (7–9). Despite advances in therapeutic
strategies, the cellular and molecular mechanisms underlying AD are
not completely understood and require further investigation.
AD is pathologically characterized by the
degeneration of the aortic media, which includes depletion of
smooth muscle cells, destruction of elastic fibers and disruption
of the extracellular matrix (ECM) network (10,11).
ECM components in the aortic wall not only provide structural
support for vascular cells, but also integrate extracellular
signals and modulate cellular responses (12). Alterations in ECM components serve
critical roles in the pathogenesis of AD (13). Increasing evidence has suggested
that an increase in the expression of proteoglycans, a major group
of nonfibrillar ECM components, is a crucial event in AD and is
closely associated with the degeneration of the aortic media
(12,14). Therefore, exploring the expression
profiles of proteoglycans and their functional effects on medial
smooth muscle cells is important for understanding the development
of AD.
In our previous study, 99 aortic tissue samples from
patients with AD were collected, and the genomic profiles were
analyzed (15). A total of
3,425,873 SNPs, 685,245 insertion-deletions and 1,177 copy number
variations were identified. By performing disease correlation
analysis, 20 candidate genes were identified. A number of
identified genes, such as myosin heavy chain 11, fibrillin 1 and
actin α2, smooth muscle, were consistent with previous studies
(16–18), whereas, to the best of our
knowledge, MAX dimerization protein MLX, DAB2 interacting protein,
E1A binding protein p300, zinc finger FYVE-type containing 9, PML
nuclear body scaffold, protein kinase C-δ and osteoglycin (OGN)
were identified as AD-associated genes for the first time in our
previous study.
OGN, which belongs to cluster III of the small
leucine-rich proteoglycans (SLRP), is an ECM component that
modulates various biological processes, including cell
proliferation, inflammation and collagen fibrillogenesis (19–21).
OGN is involved in numerous pathological conditions, including bone
disease, eye disease, neurological damage and cancer (22). Plasma OGN expression levels are
lower in patients with coronary artery disease with complex
atherosclerotic lesions compared with patients with coronary artery
disease without lesions (23).
Proteomic analysis has demonstrated decreased OGN expression levels
in calcified abdominal aortic aneurysm tissues compared with
healthy adjacent aortic tissues (24). Although emerging evidence has
revealed the effects of OGN on vascular diseases, the exact role of
OGN in AD formation requires further investigation.
The present study aimed to investigate the effect of
OGN, an ECM proteoglycan, on AD, as well as the underlying
mechanism. Therefore, OGN expression profiles in thoracic aortic
tissues from patients with AD and healthy thoracic aortic tissues
from control subjects were determined. The effect of OGN on
cellular proliferation and migration was determined in cultured rat
aortic smooth muscle cells (RASMCs). RASMCs are typically used to
study alterations in cellular and molecular biology during the
progression of vascular disease under the influence of internal and
external factors, and are a good model for studying mechanisms
in vitro (25). The present
study investigated the possible mechanisms underlying OGN in the
pathological process of AD.
Materials and methods
Cell culture
RASMCs (Procell Life Science & Technology Co.,
Ltd.) were cultured in DMEM (Procell Life Science & Technology
Co., Ltd.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin in a humidified
incubator at 37°C with 5% CO2. RASMCs at passage 3–6
were used for subsequent experiments. Following transfection, cells
were treated with recombinant human VEGF protein (10 ng/ml; R&D
Systems China Co., Ltd.) for 5, 15 or 30 min at 37°C.
Sample collection
In the present study, all volunteers were recruited
from Fuwai Hospital Chinese Academy of Medical Sciences Shenzhen,
although volunteers were from residence across China. Pathological
aortic tissues from 20 patients with AD (age, 45–62 years; 16 male
patients and 4 female patients) and healthy aortic tissues from 5
patients who underwent coronary artery bypass grafting surgery
(age, 55–74 years; 3 male patients and 2 female patients) were
collected between October 2018 and October 2019. The following
inclusion criteria were used in the present study: Pain symptoms
for <48 h; and diagnosed with acute AD after aorta computed
tomography angiography examination. The following exclusion
criteria were used in the present study: Heart failure, acute
myocardial infarction, connective tissue disease or tumor
disease.
The present study was approved by the Research
Ethics Committee of Fuwai Hospital Chinese Academy of Medical
Sciences Shenzhen (approval no. SP2019004). Written informed
consent was obtained from each patient.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from each sample using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using PrimeScript™ RT Master Mix [cat. no.
RR036A; Takara Biomedical Technology (Beijing) Co., Ltd.] according
to the manufacturer's protocol. Subsequently, qPCR was performed
using SYBR green fluorescence [Takara Biomedical Technology
(Beijing) Co., Ltd.]. The following primers were used for qPCR: OGN
forward, 5′-TCTACACTTCTCCTGTTACTGCT-3′ and reverse,
5′-GAGGTGGTGGTGTT-ATTGCCTCA-3′; and GAPDH forward,
5′-GGCAGTGATGGCATGGACTGT-3′ and reverse,
5′-CCTTCATTGACCTCAACTACA-3′. The following thermocycling conditions
were used for qPCR: 95°C for 2 min; followed by 40 cycles of 95°C
for 5 sec, 60°C for 30 sec and 72°C for 60 sec. mRNA expression
levels were quantified using the 2−∆∆Cq method (26) and normalized to the internal
reference gene GAPDH.
Western blotting
Total protein was extracted from tissues or cultured
cells using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.). Protein concentration was determined using a
BCA Protein Assay kit (Beijing Solarbio Science & Technology
Co., Ltd.). Equal amounts of protein (30 µg) were separated via 10%
SDS-PAGE and transferred to PVDF membranes. Following blocking with
5% skimmed milk in TBST (0.05% Tween-20) for 1 h at room
temperature, the membranes were incubated at 4°C overnight with the
following primary antibodies: anti-GAPDH (cat. no. 5174; 1:10,000;
Cell Signaling Technology, Inc.), anti-Tubulin (cat. no. 2148;
1:10,000; Cell Signaling Technology, Inc.), anti-OGN (cat. no.
ab211456; 1:2,000; Abcam), anti-phosphorylated (p)-AKT (cat. no.
4060; 1:2,000; Cell Signaling Technology, Inc.), anti-total AKT
(cat. no. 9272; 1:2,000; Cell Signaling Technology, Inc.),
anti-p-ERK1/2 (cat. no. 9102; 1:1,000; Cell Signaling Technology,
Inc.), anti-total ERK1/2 (cat. no. 9101; 1:1,000; Cell Signaling
Technology, Inc.), anti-p-VEGFR2 (cat. no. 2478; 1:2,000; Cell
Signaling Technology, Inc.) and anti-total VEGFR2 (cat. no. 9698;
1:2,000; Cell Signaling Technology, Inc.). After washing with TBST
for three times for 5 min each, the membranes were incubated with
an anti-rabbit IgG (cat. no. 7074; 1:10,000; Cell Signaling
Technology, Inc.) secondary antibody for 1 h at room temperature.
Following washing with TBST for three times for 5 min each, protein
bands were visualized using SuperSignal West Femto Maximum
Sensitivity Substrate (Thermo Fisher Scientific, Inc.) and an
iBright bioimaging system (Invitrogen; Thermo Fisher Scientific,
Inc.). Protein expression levels were semi-quantified using
Quantity One Analysis software (version 4.0; Bio-Rad Laboratories,
Inc.) with GAPDH or Tubulin as the loading control.
Transfection of OGN-targeting small
interfering (si)RNA
At 80% confluence, RASMCs were transfected with 50
nM OGN-specific siRNA or control siRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. The OGN-specific siRNA (si-OGN;
forward, 5′-GUGCCCACCAAGAAAGAAATT-3′ and reverse,
5′-UUUCUUUCUUGGUGGGCACTT-3′) and control siRNA [si-negative control
(NC); non-specific; forward, 5′-ACAACGAACAAGCGAACAACA-3′ and
reverse, 5′-UGUUGUUCGCUUGUUCGUUGU-3′] were synthesized by Sangon
Biotech Co., Ltd. Cells were divided into three groups: i) Blank,
cells were left untreated; ii) NC, cells were transfected with
si-NC; and iii) OGN (marked as OGN-Rat-401), cells were transfected
with si-OGN. At 48 h post-transfection, cells were used for
subsequent experiments.
Cell proliferation assay
The effect of OGN knockdown on RASMC viability was
assessed by performing a Cell Counting Kit-8 (CCK-8) assay.
Briefly, RASMCs were seeded (2.5×103 cells/well) into
96-well plates. At 48 h post-transfection, platelet-derived growth
factor-BB (PDGF-BB; final concentration, 60 µg/l; Cyagen
Biosciences, Inc.) was added to the culture medium to stimulate
cell proliferation for 24 h at 37°C. Subsequently, 10 µl CCK-8
reagent (Dojindo Molecular Technologies, Inc.) was added to each
well and incubated in the dark for 1.5 h at 37°C. The optical
density was measured at a wavelength of 450 nm using a microplate
reader (Promega Corporation).
A BrdU immunofluorescence assay was performed on
OGN-knockdown RASMCs. RASMCs were seeded (2×104
cells/well) into 6-well plates. At 48 h post-transfection, cells
were incubated with 50 µM BrdU for 12 h at 37°C in humidified
incubator with 5% CO2. Cells were fixed with 4%
paraformaldehyde at room temperature for 20 min. Subsequently,
cells were washed with PBS and desaturated with 2 M HCl at 37°C for
10 min. H3BO3 (pH 8.5) was used for
renaturation at room temperature for 10 min. Samples were washed
three times with PBS for 5 min. Cells were permeabilised using 0.5%
Triton X-100 at room temperature for 15 min. Cells were washed
three times with PBS for 5 min, blocked with non-immunized goat
serum (Beijing Dingguo Changsheng Biotechnology Co., Ltd.; 1:1,000)
at 37°C for 30 min and incubated with a BrdU primary antibody (cat.
no. ab8152; 1:100; Abcam) overnight at 4°C. Following washing three
times with PBS for 5 min, cells were incubated with the secondary
antibody (cat. no. 8890; 1:1,000; Cell Signaling Technology, Inc.)
at 37°C for 2 h. Samples were washed three times with PBS and then
were incubated with DAPI (1:100) at room temperature for 5 min.
Following two washes with PBS for 5 min, samples were washed with
distilled water. The slides were dried and sealed with 50%
glycerin. Stained sections were observed in five fields of view
using an LH-M100CB inverted fluorescence microscope (Nikon
Corporation; magnification, ×200).
Wound healing assay
For the wound healing assay, RASMCs were seeded into
6-well plates. At 95% confluence, the cell monolayer was
mechanically scraped using a sterile pipette tip to create a single
scratch. Cells were maintained in serum-free medium. At 0, 24 and
48 h, the wound was observed using a light optical microscope
(magnification, ×40). The results are presented as the distance of
wound healing, which was measured using ImageJ software (version
1.8.0.112; National Institutes of Health).
Cell invasion assay
RASMCs were seeded (5×104 cells/well)
into the upper chamber of the Matrigel-coated Transwell plate (pore
size, 8 µm). DMEM supplemented with 10% FBS was plated into the
lower chambers. Following incubation for 48 h at 37°C, invading
cells were fixed with 4% paraformaldehyde at room temperature for
20 min, and stained with 0.5% crystal violet solution at room
temperature for 10 min. Invading cells were visualized in five
randomly selected fields of view using a light microscope
(magnification, ×200).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 6; GraphPad Software, Inc.). Comparisons
among multiple groups were analyzed using one-way ANOVA followed by
Dunnett's post hoc test. Comparisons between two groups were
analyzed using an unpaired Student's t-test. Data are presented as
the mean ± SD of three independently repeated experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
OGN is downregulated in aortic tissues
from patients with AD
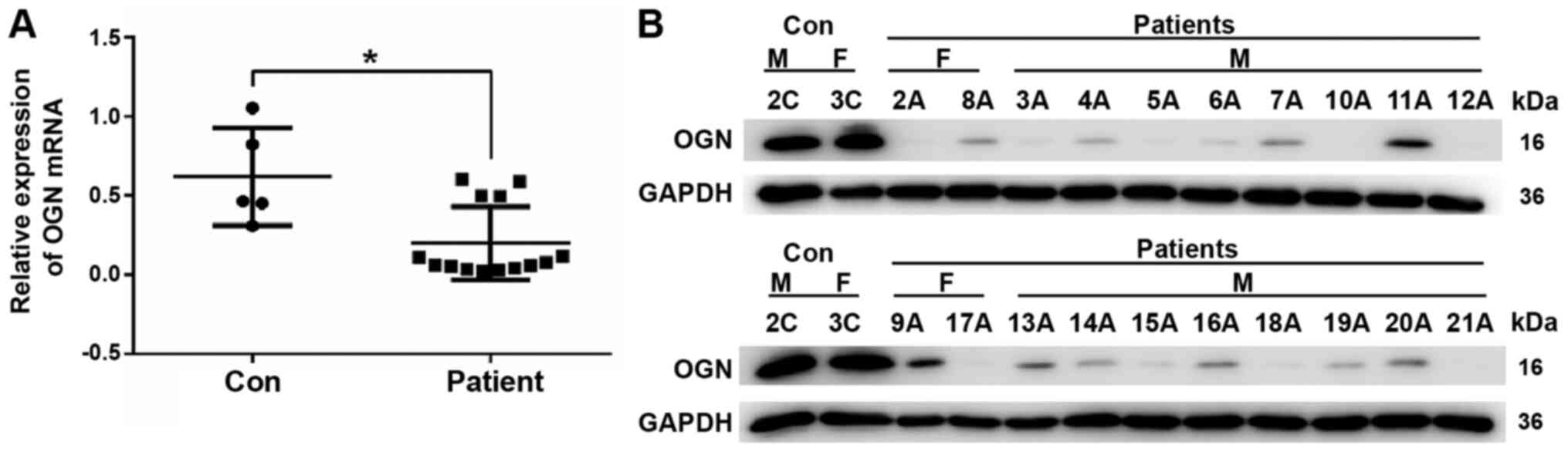
OGN mRNA expression levels were significantly lower
in the thoracic aortic tissues of 20 patients with AD compared with
the healthy thoracic aortic tissues of 5 control subjects (Fig. 1A). The western blotting results
demonstrated that the protein expression levels of OGN in the
thoracic aortic tissues from 20 patients with AD were markedly
reduced compared with the thoracic aortic tissues from 3 out of the
5 control subjects (Fig. 1B).
OGN knockdown enhances RASMC
proliferation and migration
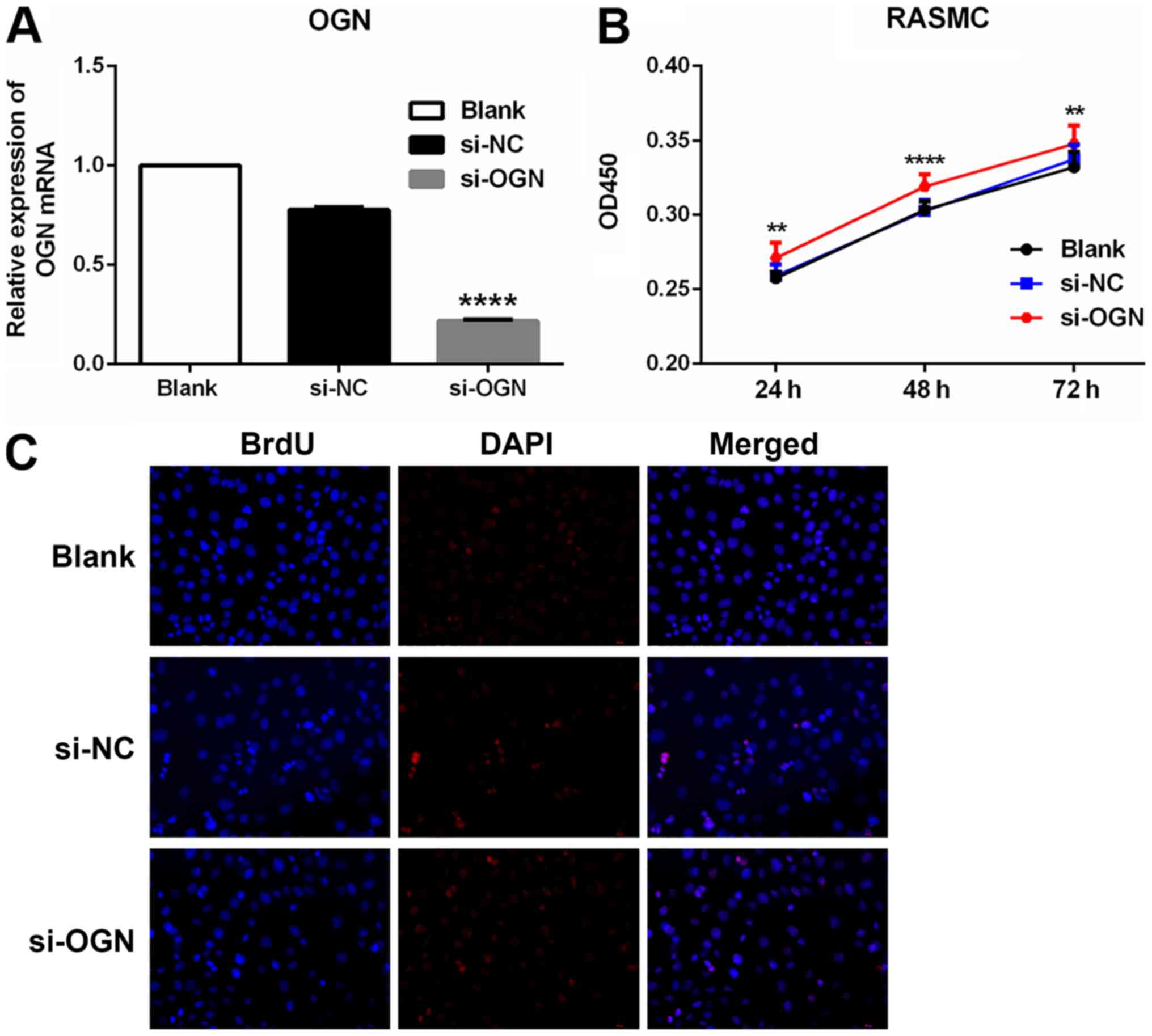
Following transfection with si-OGN, OGN mRNA
expression levels were significantly decreased compared with the
si-NC group (Fig. 2A). The CCK-8
assay was performed to investigate whether OGN knockdown affected
PDGF-BB-induced cell proliferation and survival. OGN knockdown
significantly enhanced RASMC proliferation at 24, 48 and 72 h
compared with the si-NC group (Fig.
2B). The immunofluorescence assay displayed similar results
(Fig. 2C); cell proliferation was
also notably increased in the si-OGN group compared with the si-NC
group.
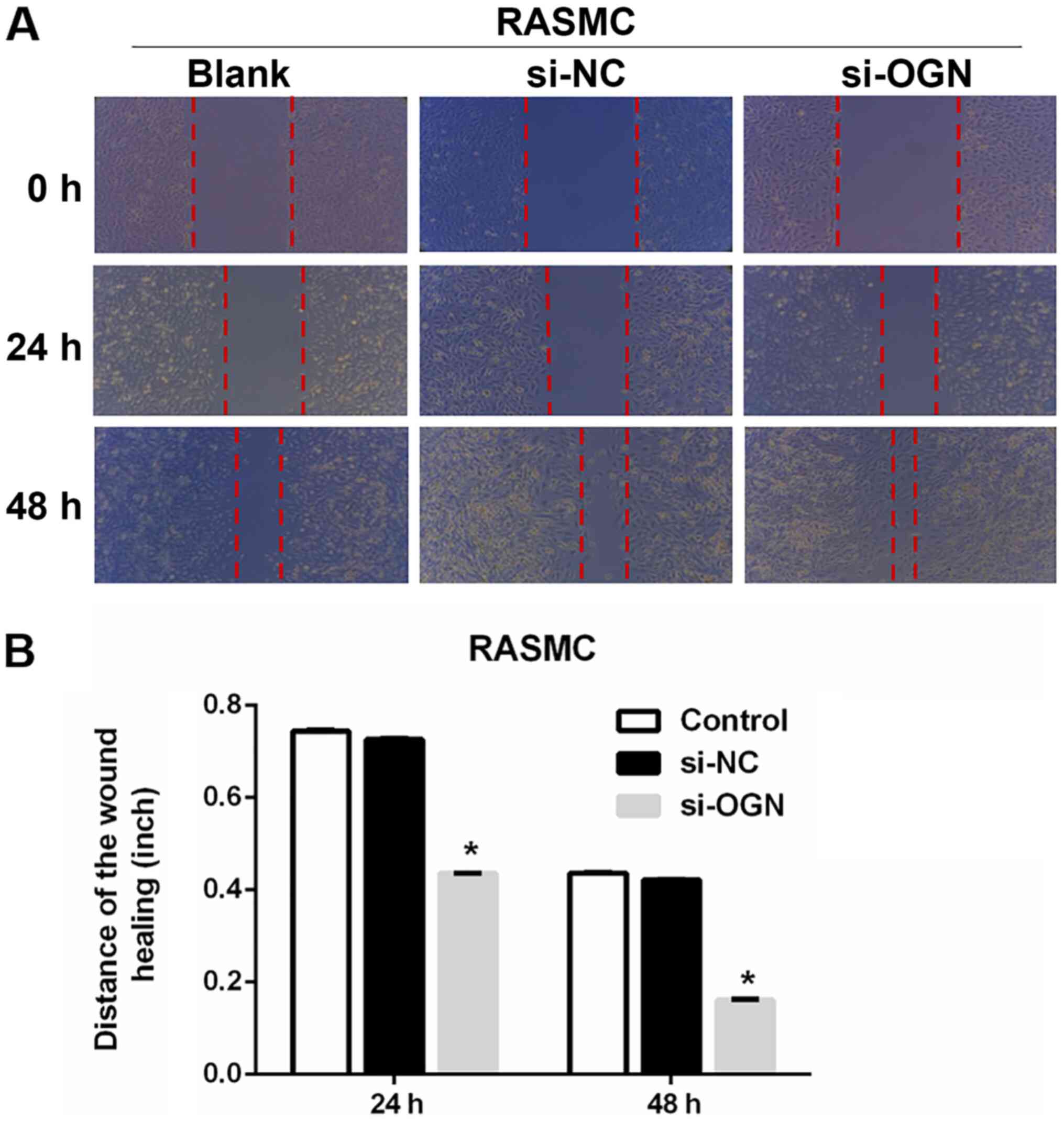
To assess the effect of OGN on RASMC migration,
wound healing and Transwell invasion assays were performed.
Compared with the si-NC group, OGN knockdown significantly
decreased the width of the wound by 41.1 and 61.9% at 24 and 48 h,
respectively (Fig. 3A and B).
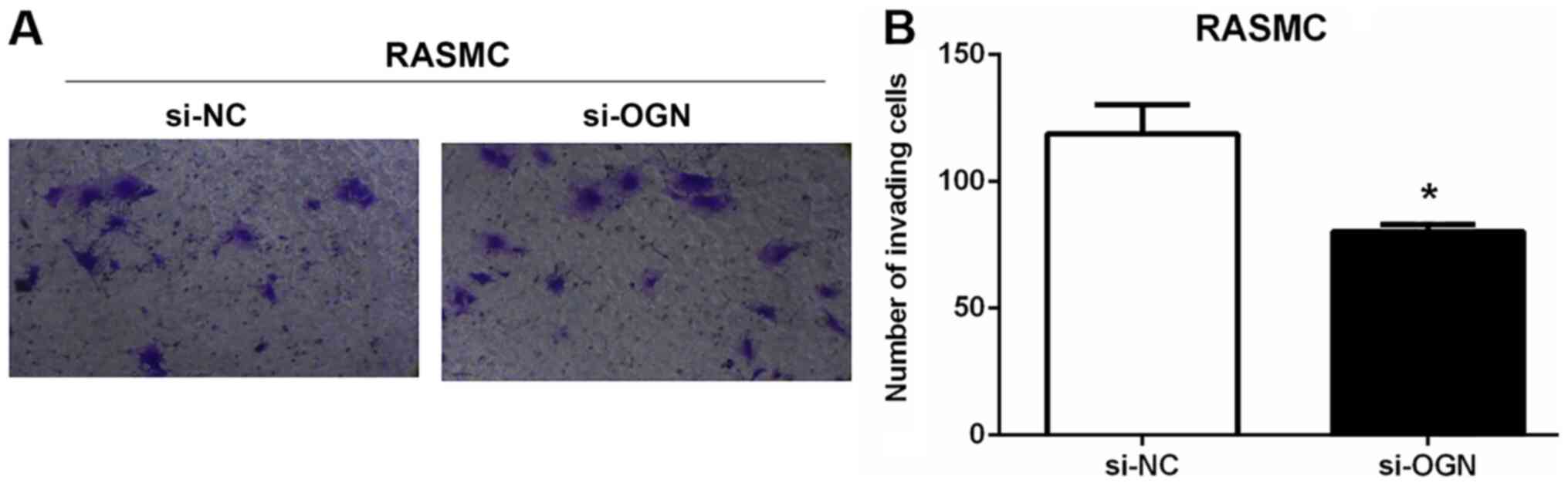
Moreover, the number of invading RASMCs was decreased by ~30% in
the si-OGN group compared with the si-NC group (Fig. 4A and B). The results indicated that
OGN knockdown was associated with increased RASMC proliferation and
migration, and decreased invasion.
OGN knockdown further increases the
phosphorylation of AKT and ERK1/2 in VEGF-treated RASMCs
In mouse models of limb ischemia, increased
expression levels of OGN are closely related to inhibition of the
VEGF/VEGFR2 signaling pathway (27,28).
In the present study, OGN-knockdown RASMCs displayed significantly
reduced VEGFR2 phosphorylation compared with si-NC-transfected
RASMCs (Fig. 5A and E).
VEGF-mediated stimulation of si-NC-transfected RASMCs significantly
increased the levels of VEGPR2, and increased the expression level
of AKT at 5 min compared with 0 min, which returned to the baseline
level at 30 min (Fig. 5A, B and C).
The protein expression levels of ERK1/2 were not significantly
altered among the different groups (Fig. 5A and D). In si-NC-transfected
RASMCs, VEGF stimulation notably increased the phosphorylation
levels of ERK1/2 and AKT in a time-dependent manner (Fig. 5A, F and G). Moreover, ERK1/2 and AKT
phosphorylation levels were significantly increased in
si-OGN-transfected RASMCs compared with si-NC-transfected RASMCs,
although a significant difference in ERK1/2 phosphorylation between
the si-NC and si-OGN groups was not observed following 30 min VEGF
stimulation (Fig. 5A, F and G). The
results suggested that OGN knockdown facilitated VEGF-induced
activation of AKT and ERK1/2.
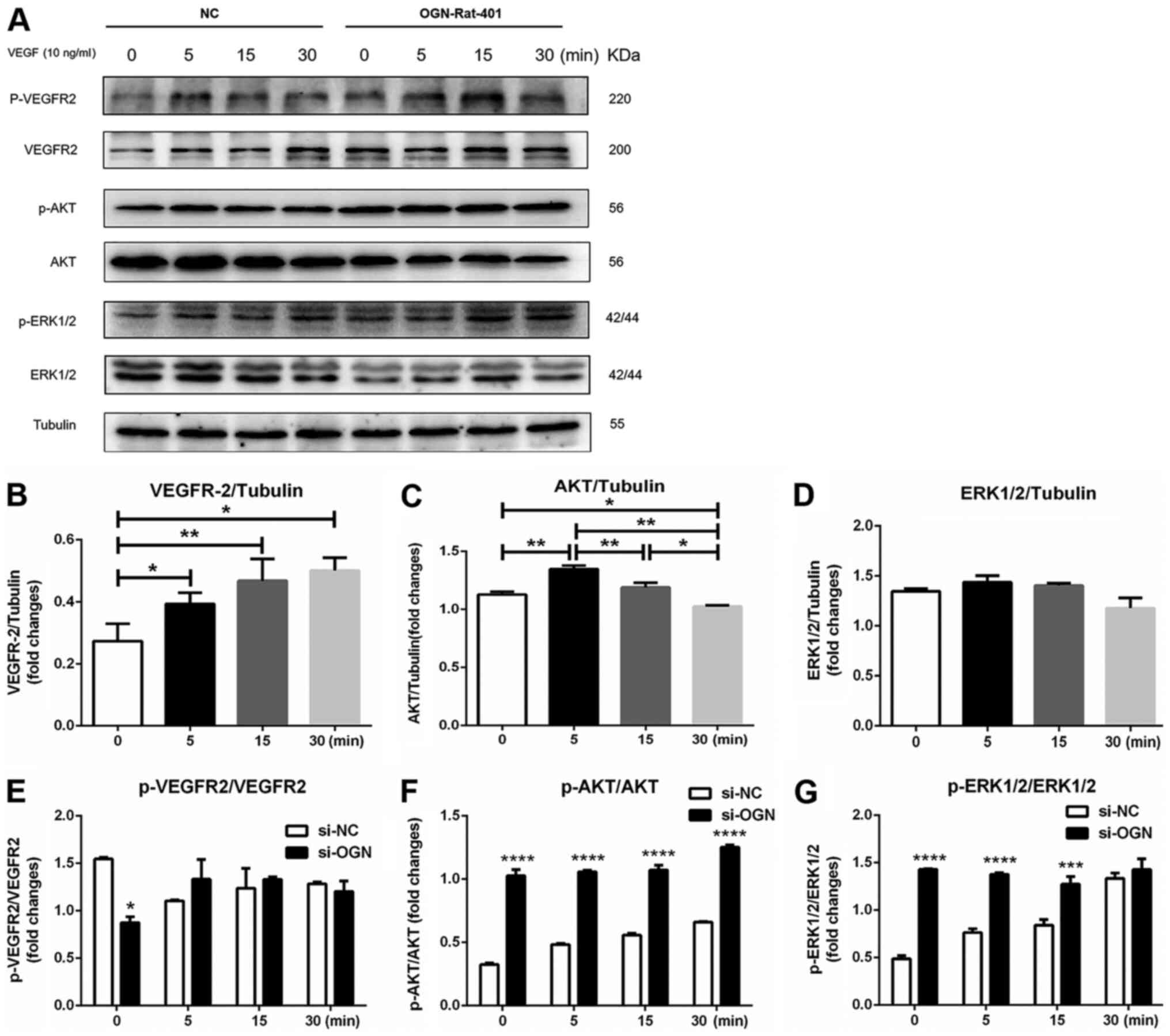 | Figure 5.OGN regulates the VEGFR/AKT signaling
pathway. Cells were transfected with si-OGN or si-NC for 48 h.
Subsequently, cells were treated with VEGF (10 ng/ml) for 5, 15 or
30 min. Protein expression levels were (A) determined via western
blotting and semi-quantified for (B) VEGFR2, (C) AKT, (D) ERK1/2,
(in si-NC-transfected cells) (E) p-VEGFR2/VEGFR2, (F) p-AKT/AKT and
(G) p-ERK1/2/ERK1/2. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. si-NC. OGN, osteoglycin; si, small interfering
RNA; NC, negative control; p, phosphorylated. |
Discussion
In the present study, the results demonstrated that
OGN expression levels were significantly downregulated in patients
with AD compared with healthy controls. Furthermore, the results
suggested that OGN expression was negatively associated with cell
proliferation and migration. Inspired by our previous work and
other previous studies, the present study indicated that OGN may
regulate the downstream signaling molecules AKT and ERK1/2 via the
VEGF/VEGFR2 signaling pathway, thereby affecting cell
proliferation, migration and angiogenesis.
Increasing evidence has demonstrated that the
altered expression of proteoglycans is associated with degeneration
of the aortic wall in AD (29).
Versican, a large chondroitin sulfate proteoglycan, is required for
RASMC proliferation and migration, and its degradation leads to
fragmentation of elastin and predisposition to AD (30,31).
Genetic depletion of biglycan, a member of the class I family of
SLRPs, results in spontaneous AD and rupture (32). Alterations in the expression levels
of OGN, which was originally identified as a modulator of bone
formation, have been implicated in atherosclerosis, myocardial
fibrosis, schaemia-induced angiogenesis and other vascular diseases
(28,33,34).
The expression levels of OGN in aortas are increased in adult rats
at 2 weeks post-balloon injury and in 2-week-old neonatal rats
(33). OGN mRNA expression levels
are downregulated in rat RASMCs stimulated with basic fibroblast
growth factor, TGFβ, PDGF and angiotensin II (33). Similarly, OGN expression levels are
decreased in the hypertrophic aortas of sinoaortic-denervated rats
(35). Consistent with the
aforementioned studies, the present study demonstrated that OGN
expression levels were significantly downregulated in the thoracic
aortic tissues of patients with AD compared with healthy controls,
suggesting that OGN might be involved in the pathological
progression of AD.
Aortic vascular smooth muscle cells, the predominant
cell type in the medial layer of the aortic wall, serve an
important role in maintaining structural integrity and regulating
vascular tone (36). RASMC
proliferation and migration occur in response to various vascular
injuries, and contribute to the development of pathological
remodeling and vascular diseases (37). Mutations in genes encoding proteins
required for SMC contraction leads to the occurrence of thoracic
aortic aneurysms and dissections (38). The switch from a contractile RASMC
phenotype to a synthetic, migratory and proliferative RASMC
phenotype is a pivotal contributor to the development of AD
(39). The present study indicated
that compared with si-NC-transfected RASMCs, OGN-knockdown RASMCs
displayed enhanced proliferation and migration, which may
facilitate the development of AD. Previous studies have indicated
that OGN is closely related to proliferation and migration in a
number of different cell types (40,41).
OGN overexpression reduces proliferation and inhibits invasion in
human colon cancer cell lines (20,42).
In the present study, compared with si-NC, OGN knockdown increased
RASMC migration but decreased invasion. Therefore, it was
hypothesized that RASMCs might respond to multiple growth factors,
inflammatory cytokines and vasoactive substances, leading to OGN
downregulation, which promotes cell proliferation and migration,
and thereby modulating AD progression. The different effects of OGN
knockdown on the invasion abilities of RASMCs and colon cancer
cells may be attributed to the distinct extracellular stimuli and
intracellular signaling pathways of the two cell types.
The VEGF/VEGFR axis is involved in numerous
physiological and pathological processes, including embryologic
development, normal growth, tissue repair and tumorigenesis
(43,44). In the process of angiogenesis, VEGF
binds to VEGFR on endothelial cells, resulting in endothelial cell
proliferation and migration (45).
OGN competitively binds to VEGFR2 in cultured human umbilical vein
endothelial cells (HUVECs) (46),
and a coimmunoprecipitation assay confirmed the direct interaction
between OGN and VEGFR2. Wu et al (28) further reported that OGN negatively
modulates the activation of VEGFR2 and its downstream signaling
pathways. In the present study, no significant alterations in the
levels of phosphorylated VEGFR2 after exposure to VEGF for 5–30 min
were observed between si-NC- and si-OGN-transfected RASMCs;
however, VEGF-induced activation of AKT and ERK1/2 was
significantly enhanced in OGN-knockdown cells compared with
si-NC-transfected cells. AKT and ERK1/2 are important downstream
signaling molecules that are required for the VEGFR2-induced
proliferation and migration of lymphatic endothelial cells and
human brain RASMCs (47,48). The results of the present study
indicated that compared with the si-NC group, OGN knockdown
significantly enhanced the phosphorylation of AKT and ERK1/2 in
RASMCs, which triggered cell proliferation and migration. The
results of the present study were consistent with the finding that
OGN knockdown in HUVECs resulted in enhanced AKT and ERK1/2
phosphorylation in response to VEGF (46).
Other VSMC-related mechanisms may exist in AD. ECM
softening serves a pivotal role in regulating the VSMC phenotype
switch and provides a potential target for treating VSMC
dysfunction and AD disease, which has been reported in
Cardiovascular Toxicology (49).
The aforementioned study focused on synthetic phenotype-related
genes and ECM softening phenotype, whereas the present study
focused on the role of OGN in VSMC proliferation and migration,
providing two possible mechanisms underlying AD. The two identified
mechanisms may display cross-talk and share certain targets and
factors, but further investigation is required. Synthetic
phenotype-related genes, including osteopontin, matrix
metalloproteinases and inflammatory cytokines, are upregulated in
VSMCs (49). On the other hand, OGN
is a component of the vascular extracellular matrix and may also
influence the vascular system (50). Therefore, it was hypothesized that
OGN may contribute to ECM softening in the regulation of the VSMC
phenotype switch.
The present study had a number of limitations.
First, the number of samples included in the present study was
limited due to the low incidence of AD. The RASMC model used in the
present study is a common tool used in AD research (25), but cell proliferation and migration
can occur in a number of other vascular diseases, such as
atherosclerosis and vascular neointima formation (51,52).
In addition, the function of OGN in AD may be related to the
process of angiogenesis. However, no angiogenesis-related genes
were analyzed in the AD tissues or in the RASMC model in the
present study. If angiogenesis is the target of OGN, further
research in vascular endothelial cells rather than vascular smooth
muscle cells is required. Therefore, future studies should
investigate the effect of OGN on angiogenesis in AD to reveal the
possible underlying mechanisms.
In conclusion, the present study suggested that OGN
knockdown facilitated the stimulatory effect of PDGF-BB on RASMC
proliferation and migration. The results indicated that OGN
regulated the VEGF/VEGFR2 axis and the downstream signaling
molecules AKT and ERK1/2, thus affecting the biological activity of
RASMCs. Therefore, OGN may serve as a novel therapeutic target for
AD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81600208, 81970210
and 81570256), the Medical Scientific Research Foundation of
Guangdong Province of China (grant no. A2018019) and the Science
and Technology Project of Shenzhen of China (grant nos.
JCYJ20180302173909492, JCYJ20180508152222104 and
KQJSCX20180329104902378).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and MW performed the literature review, wrote the
initial draft and analyzed the data. XZ and BC performed the
experiments and collected the data. MW provided ideas and
recommendations, designed the research and reviewed the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the procedures involving human participants were
performed in accordance with the ethical standards of the
institutional and the 1964 Declaration of Helsinki and its later
amendments or comparable ethical standards. The present study was
approved by the Ethics Committee of Fuwai Hospital Chinese Academy
of Medical Sciences Shenzhen (approval no. SP2019004). All patients
signed an informed consent form, which was approved by the
Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clough RE and Nienaber CA: Management of
acute aortic syndrome. Nat Rev Cardiol. 12:103–114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherrah AG, Grieve SM, Jeremy RW, Bannon
PG, Vallely MP and Puranik R: MRI in chronic aortic dissection: A
systematic review and future directions. Front Cardiovasc Med.
2:52015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Divchev D, Najjar T, Tillwich F, Aboukoura
M, Rehders T and Nienaber CA: Risk assessment of acute aortic
dissection. Dtsch Med Wochenschr. 139:1947–1951. 2014.(In German).
PubMed/NCBI
|
|
4
|
Ridge CA and Litmanovich DE: Acute aortic
syndromes: Current status. J Thorac Imaging. 30:193–201. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Segreto A, Chiusaroli A, De Salvatore S
and Bizzarri F: Biomarkers for the diagnosis of aortic dissection.
J Card Surg. 29:507–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar A and Allain RM: Aortic dissection.
Critical Care Secrets (Fifth Edition). 204–211. 2013. View Article : Google Scholar
|
|
7
|
Elsayed RS, Cohen RG, Fleischman F and
Bowdish ME: Acute type A aortic dissection. Cardiol Clin.
35:331–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gawinecka J, Schönrath F and von
Eckardstein A: Acute aortic dissection: Pathogenesis, risk factors
and diagnosis. Swiss Med Wkly. 147:w144892017.PubMed/NCBI
|
|
9
|
Evangelista A, Isselbacher EM, Bossone E,
Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S,
Braverman AC, Myrmel T, et al: Insights from the international
registry of acute aortic dissection: A 20-Year experience of
collaborative clinical research. Circulation. 137:1846–1860. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen D, Zhou XL, Li JJ and Hui RT:
Biomarkers in aortic dissection. Clin Chim Acta. 412:688–695. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang WJ, Ren WH, Liu XJ, Liu Y, Wu FJ,
Sun LZ, Lan F, Du J and Zhang HJ: Disruption of mechanical stress
in extracellular matrix is related to Stanford type A aortic
dissection through down-regulation of Yes-associated protein. Aging
(Albany NY). 8:1923–1939. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gentili C and Cancedda R: Cartilage and
bone extracellular matrix. Curr Pharm Des. 15:1334–1348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu D, Shen YH, Russell L, Coselli JS and
LeMaire SA: Molecular mechanisms of thoracic aortic dissection. J
Surg Res. 184:907–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Billaud M, Hill JC, Richards TD, Gleason
TG and Phillippi JA: Medial hypoxia and adventitial vasa vasorum
remodeling in human ascending aortic aneurysm. Front Cardiovasc
Med. 5:1242018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Zhuang X, Chen B, Wen J, Peng F,
Liu X and Wei M: 99-case study of sporadic aortic dissection by
whole exome sequencing indicated novel disease-associated genes and
variants in Chinese population. Biomed Res Int.
2020:78570432020.PubMed/NCBI
|
|
16
|
Zhu L, Vranckx R, Khau Van Kien P, Lalande
A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F,
et al: Mutations in myosin heavy chain 11 cause a syndrome
associating thoracic aortic aneurysm/aortic dissection and patent
ductus arteriosus. Nat Genet. 38:343–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marshall LM, Carlson EJ, O'Malley J,
Snyder CK, Charbonneau NL, Hayflick SJ, Coselli JS, Lemaire SA and
Sakai LY: Thoracic aortic aneurysm frequency and dissection are
associated with fibrillin-1 fragment concentrations in circulation.
Circ Res. 113:1159–1168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seike Y, Minatoya K, Sasaki H, Tanaka H,
Itonaga T, Inoue Y, Morisaki H, Morisaki T, Ishibashi-Ueda H and
Kobayashi J: Clinical outcomes of aortic repair in young adult
patients with ACTA2 mutations. Gen Thorac Cardiovasc Surg.
65:686–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa RA, Martins RST, Capilla E, Anjos L
and Power DM: Vertebrate SLRP family evolution and the
subfunctionalization of osteoglycin gene duplicates in teleost
fish. BMC Evol Biol. 18:1912018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Li YQ, Li QG, Ma YL, Peng JJ and Cai
SJ: Osteoglycin-induced VEGF inhibition enhances T lymphocytes
infiltrating in colorectal cancer. EBioMedicine. 34:35–45. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ge G, Seo NS, Liang X, Hopkins DR, Höök M
and Greenspan DS: Bone morphogenetic protein-1/tolloid-related
metalloproteinases process osteoglycin and enhance its ability to
regulate collagen fibrillogenesis. J Biol Chem. 279:41626–41633.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deckx S, Heymans S and Papageorgiou AP:
The diverse functions of osteoglycin: A deceitful dwarf, or a
master regulator of disease? FASEB J. 30:2651–2661. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seki T, Saita E, Kishimoto Y, Ibe S,
Miyazaki Y, Miura K, Ohmori R, Ikegami Y, Kondo K and Momiyama Y:
Low levels of plasma osteoglycin in patients with complex coronary
lesions. J Atheroscler Thromb. 25:1149–1155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto K, Maniwa T, Tanaka T, Satoh K,
Okunishi H and Oda T: Proteomic analysis of calcified abdominal and
thoracic aortic aneurysms. Int J Mol Med. 30:417–429. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Zhuang X, Chen B, Feng D, Li G and
Wei M: The role of miR-107 as a potential biomarker and cellular
factor for acute aortic dissection. DNA Cell Biol. 39:1895–1906.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu X, Li YQ, Li QG, Ma YL, Peng JJ and Cai
SJ: Osteoglycin (OGN) reverses epithelial to mesenchymal transition
and invasiveness in colorectal cancer via EGFR/Akt pathway. J Exp
Clin Cancer Res. 37:412018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu QH, Ma Y, Ruan CC, Yang Y, Liu XH, Ge
Q, Kong LR, Zhang JW, Yan C and Gao PJ: Loss of osteoglycin
promotes angiogenesis in limb ischaemia mouse models via modulation
of vascular endothelial growth factor and vascular endothelial
growth factor receptor 2 signalling pathway. Cardiovasc Res.
113:70–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cikach FS, Koch CD, Mead TJ, Galatioto J,
Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F,
Roselli EE and Apte SS: Massive aggrecan and versican accumulation
in thoracic aortic aneurysm and dissection. JCI Insight.
3:e971672018. View Article : Google Scholar
|
|
30
|
Evanko SP, Angello JC and Wight TN:
Formation of hyaluronan- and versican-rich pericellular matrix is
required for proliferation and migration of vascular smooth muscle
cells. Arterioscler Thromb Vasc Biol. 19:1004–1013. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wight TN: Arterial remodeling in vascular
disease: A key role for hyaluronan and versican. Front Biosci.
13:4933–4937. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heegaard AM, Corsi A, Danielsen CC,
Nielsen KL, Jorgensen HL, Riminucci M, Young MF and Bianco P:
Biglycan deficiency causes spontaneous aortic dissection and
rupture in mice. Circulation. 115:2731–2738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shanahan CM, Cary NR, Osbourn JK and
Weissberg PL: Identification of osteoglycin as a component of the
vascular matrix. Differential expression by vascular smooth muscle
cells during neointima formation and in atherosclerotic plaques.
Arterioscler Thromb Vasc Biol. 17:2437–2447. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deckx S, Heggermont W, Carai P, Rienks M,
Dresselaers T, Himmelreich U, van Leeuwen R, Lommen W, van der
Velden J, Gonzalez A, et al: Osteoglycin prevents the development
of age-related diastolic dysfunction during pressure overload by
reducing cardiac fibrosis and inflammation. Matrix Biol.
66:110–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu XS, Lei JP, Shi JB, Lian WL, Yang X,
Zheng X and Qin YW: Mimecan is involved in aortic hypertrophy
induced by sinoaortic denervation in rats. Mol Cell Biochem.
352:309–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Korneva A and Humphrey JD: Maladaptive
aortic remodeling in hypertension associates with dysfunctional
smooth muscle contractility. Am J Physiol Heart Circ Physiol.
316:H265–H278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang D, Wang Q, Yan G, Qiao Y and Tang C:
Phloretin inhibits platelet-derived growth factor-BB-induced rat
aortic smooth muscle cell proliferation, migration, and neointimal
formation after carotid injury. J Cardiovasc Pharmacol. 65:444–455.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Milewicz DM, Trybus KM, Guo DC, Sweeney
HL, Regalado E, Kamm K and Stull JT: Altered smooth muscle cell
force generation as a driver of thoracic aortic aneurysms and
dissections. Arterioscler Thromb Vasc Biol. 37:26–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Inamoto S, Kwartler CS, Lafont AL, Liang
YY, Fadulu VT, Duraisamy S, Willing M, Estrera A, Safi H, Hannibal
MC, et al: TGFBR2 mutations alter smooth muscle cell phenotype and
predispose to thoracic aortic aneurysms and dissections. Cardiovasc
Res. 88:520–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zuo C, Li X, Huang J, Chen D, Ji K, Yang
Y, Xu T, Zhu D, Yan C and Gao P: Osteoglycin attenuates cardiac
fibrosis by suppressing cardiac myofibroblast proliferation and
migration through antagonizing lysophosphatidic acid 3/matrix
metalloproteinase 2/epidermal growth factor receptor signalling.
Cardiovasc Res. 114:703–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu T, Zhang R, Dong M, Zhang Z, Li H, Zhan
C and Li X: Osteoglycin (OGN) inhibits cell proliferation and
invasiveness in breast cancer via PI3K/Akt/mTOR signaling pathway.
Onco Targets Ther. 12:10639–10650. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jia LX, Zhang WM, Zhang HJ, Li TT, Wang
YL, Qin YW, Gu H and Du J: Mechanical stretch-induced endoplasmic
reticulum stress, apoptosis and inflammation contribute to thoracic
aortic aneurysm and dissection. J Pathol. 236:373–383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marti HH and Risau W: Angiogenesis in
ischemic disease. Thromb Haemost. 82 (Suppl 1):S44–S52. 1999.
View Article : Google Scholar
|
|
44
|
Rapisarda A and Melillo G: Role of the
VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res.
114:237–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bastiaansen AJ, Ewing MM, de Boer HC, van
der Pouw Kraan TC, de Vries MR, Peters EA, Welten SM, Arens R,
Moore SM, et al: Lysine acetyltransferase PCAF is a key regulator
of arteriogenesis. Arterioscler Thromb Vasc Biol. 33:1902–1910.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dellinger MT and Brekken RA:
Phosphorylation of Akt and ERK1/2 is required for
VEGF-A/VEGFR2-induced proliferation and migration of lymphatic
endothelium. PLoS One. 6:e289472011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang GY, Yao JS, Huey M, Hashimoto T and
Young WL: Participation of PI3K and ERK1/2 pathways are required
for human brain vascular smooth muscle cell migration. Neurochem
Int. 44:441–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shao Y, Li G, Huang S, Li Z, Qiao B, Chen
D, Li Y, Liu H, Du J and Li P: Effects of extracellular matrix
softening on vascular smooth muscle cell dysfunction. Cardiovasc
Toxicol. 20:548–556. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moncayo-Arlandi J, López-García A,
Fernández MC, Durán AC and Fernández B: Osteoglycin deficiency does
not affect atherosclerosis in mice. Atherosclerosis. 237:418–425.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee KP, Sudjarwo GW, Jung SH, Lee D, Lee
DY, Lee GB, Baek S, Kim DY, Lee HM, Kim B, et al: Carvacrol
inhibits atherosclerotic neointima formation by downregulating
reactive oxygen species production in vascular smooth muscle cells.
Atherosclerosis. 240:367–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li HY, Leu YL, Wu YC and Wang SH:
Melatonin inhibits in vitro smooth muscle cell inflammation and
proliferation and atherosclerosis in apolipoprotein E-deficient
mice. J Agric Food Chem. 67:1889–1901. 2019. View Article : Google Scholar : PubMed/NCBI
|