Introduction
Cerebral ischemia is associated with a number of
serious diseases, including stroke and cardiac and respiratory
arrest. Treatment usually involves blood flow recovery as soon as
possible; however, this may lead to secondary injury in the
ischemic region, referred to as ischemia/reperfusion injury (IRI)
(1–3). Following cerebral ischemia, the
recovery of the blood circulation leads to inflammation and
oxidative stress damage in areas affected by hypoxia and nutrient
deficiency. Cerebral ischemia and reperfusion also lead to impaired
mitochondrial oxidative metabolism and energy depletion in neurons,
leading to programmed cell death (4,5).
Therefore, although cerebral ischemia treatment involves the
restoration of blood flow as quickly as possible, this may induce
additional injury to the ischemic area. Appropriate drugs are
required to protect neurons from the effects of IRI, mitigate
pathological responses and control the process of neuronal death
(6). However, only few drugs to
date are clinically available for patients with cerebral ischemia
(6).
Resveratrol is a natural phytoalexin extracted from
plants that exhibits neuroprotective, anticancer and
anti-inflammatory properties (7–9).
Resveratrol has also been reported to exhibit antioxidant
properties, such as the ability to regulate nitric oxide metabolism
(10) and chemopreventive activity
(11). Moreover, resveratrol has
been demonstrated to protect against IRI in gerbils (12). The beneficial neuroprotective
effects of resveratrol may be due to its antiplatelet aggregation
and vasodilation and/or antioxidant activity (13). A previous study demonstrated that
resveratrol improves mitochondrial function and protects against
metabolic disease by activating sirtuin 1 and PPARG coactivator-1α
(14). However, whether resveratrol
serves as a mitochondrial phagocytosis regulator in brain ischemia
remains unclear.
Autophagy is a cellular housekeeping function, which
is responsible for the degradation of a number of protein
aggregates or damaged organelles and is involved in numerous
pathophysiological processes, including ischemic disease (15). There is great interest in
determining how autophagy selectively identifies and removes
damaged mitochondria. For example, it has been reported that
Parkin-dependent mitophagy is key for neuroprotection of ischemic
preconditioning (16). Furthermore,
deletion of the uncoupling protein 2 gene in endothelial cells may
lead to excessive phosphatase and tensin homolog-induced kinase 1
(PINK1)-induced mitochondrial autophagy, resulting in insufficient
mitochondrial biosynthesis and increased apoptosis of endothelial
cells (17). Resveratrol is
considered to be a key regulator of mitochondrial activity and has
been reported to attenuate mitochondrial function impairment
induced by oxidative stress via upregulating mitochondrial
antioxidant enzymes and decreasing the production of reactive
oxygen species (ROS) by organelles. Resveratrol also triggers
mitochondrial biogenesis, ameliorating the mitochondria-associated
bioenergetics status in mammalian cells (18). However, the association between the
neuroprotective effects of resveratrol during OGD/R and mitophagy
remains elusive.
The aims of the present study were to describe the
dose-dependent protective effects of resveratrol against
OGD/R-induced neuronal death and mitochondrial dysfunction,
elucidate the effect of resveratrol on mitophagic activity during
OGD/R and to investigate the involvement of the PINK1/Parkin
pathway in the neuroprotective effects of resveratrol in OGD/R. The
present results may provide novel insight into the neuroprotective
mechanisms of resveratrol and identify the potential value of
resveratrol in protecting the brain from I/R damage.
Materials and methods
Preparation of rat cortical
cultures
The present study was approved by the Ethics
Committee of the China Three Gorges University (Yichang, China).
Cultures of rat cortical neurons were prepared as previously
described (19). A total of 8 rat
pups (male; weight, 5–8 g; age, 1–2 days; China Three Gorges
University, Yichang, China) were housed in a vivarium that was
maintained at a fixed temperature (22–23°C) and moisture(70%), with
a 12-h light/dark cycle and free access to food and drinking water.
All procedures adhered to the National Institutes of Health
Guidelines for the care and use of laboratory animals (20). The rat pups were euthanized by
carbon dioxide overexposure. Once pups stopped moving (including
respiration), all pups were sterilized in 75% ethanol and
decapitated using sharp scissors, then the brains of the neonatal
rats were removed in a sterile field. The cerebral cortex and
hippocampus were isolated on ice for the preparation of primary
astrocytes and neurons, respectively. After removing the meninges
and blood vessels, the cerebral cortex and hippocampus were cut
into 1-mm3 pieces and digested with 2 mg/ml papain and
0.05 mg/ml DNase in serum-free medium at 37°C for 30 min. The
tissue suspension was gently pipetted to disperse the cells and
filtered through a 100-µM sterile filter. Neocortical cells were
plated in dishes at 37°C at a density of 4×105 cells/ml
in DMEM with 10% FBS, epidermal growth factor (10 ng/ml),
penicillin (50 U/ml) and streptomycin (50 U/ml; all Sigma-Aldrich;
Merck KGaA). The neurons were initially cultured for 2 days
(passage 0, day 2; P0D2) and subcultured for 4 days (passage 1, day
4; P1D4). Neurons at the second passage (P2D4) were used for
further study.
Cell culture and treatment
Following serum starvation, neurons were transferred
to hypoxic incubators at 37°C for 4 h containing 1% O2,
5% CO2 and 94% N2 to simulate ischemic
conditions. For reoxygenation, the hypoxic cells were cultured in
fresh medium and transferred to a normoxic incubator at 37°C for 2
h. Resveratrol in the presence or absence 10 µM mitochondrial
division inhibitor-1 (Mdivi-1) was administered immediately prior
to reoxygenation.
RNA interference (RNAi) and
transfection
Small interfering (si)RNAs against PINK1 (siPINK1;
5′GCUAGUUACAAGAGAACAA-3′), Parkin (siParkin;
5′-GAAUACAUUCCCUACCUCA-3′) and scrambled siRNA (si-Control;
5′-UUCUCCGAACGUGUCACGU-3′) were designed and synthesized by
Shanghai GenePharma Co., Ltd. A total of 1×106 cells
were transfected with 50 pg/µl siRNA, miRNA mimic, miRNA inhibitor
or corresponding NCs using Lipofectamine® RNAiMAX
transfection reagent (Thermo Fisher Scientific, Inc.) and harvested
48–72 h after transfection.
Annexin V-phycoerythrin (PE) and
7-aminoactinomycin D (7-AAD) staining
The cells were washed twice with cold PBS, then
resuspended with 1X binding buffer. Then, 100 µl cell suspension
was transferred to 5-ml culture tubes. Annexin V-PE and 7-AAD (5 µl
each; BD Biosciences) were added to each tube. The cells were
vortexed gently and incubated for 15 min at room temperature in
dark. A total of 400 µl 1X binding buffer (BD Biosciences) was
added to each tube. The reaction was performed at room temperature
and in the dark for 10 min. Flow cytometry (BD Biosciences) was
used to measure cells and data were analyzed using a FACSCanto II
flow cytometer (BD Biosciences).
Cell viability assay
Cell survival rate was determined by Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology) assay. After
being grown in 96-well microplates at a density of 10,000
cells/well, the cells were treated with hypoxia/reoxygenation (H/R)
and/or resveratrol as aforementioned. CCK-8 solution (10 µl/well)
was added to each well and incubated in 5% CO2 at 37°C
for 1 h. The absorbance was measured at 450 nm. Optical density
values were recorded as a ratio to the control.
Western blot analysis
Cell extracts were scraped into lysis buffer
containing 20 mmol/l Tris-HCl (pH 7.4), 6 mM urea and 200 mmol/l
potassium chloride with a protease inhibitor cocktail (3.6 mmol/l
leupeptin, 2.1 mmol/l pepstatin A and 50 mmol/l
phenylmethylsulfonylfluoride) followed by vigorous vortexing and
cooling on ice for 15 min, followed by 15 min centrifugation at
13,000 × g at 4°C. The protein concentration was determined using
the Bradford (Thermo Fisher Scientific, Inc.) method, according to
the manufacturer's instructions. The homogenates/lysates were
stored at −80°C. Each group (20 µg/lane, the volume/lane was kept
the same using loading buffer) was separated by 10% SDS-PAGE and
transferred to PVDF membranes and blocked in TBST (50 mM Tris-HCl,
pH 7.5, 150 mM NaCl and 0.2% Tween-20) containing 5% non-fat milk
for 1 h at 37°C. The antibodies used were as follows: Anti-LC3I/II
(1:1,000; cat. no. 3868; Cell Signaling Technology, Inc.),
anti-Caspase-3 (1:1,000; cat. no. 9662; Cell Signaling Technology,
Inc.), anti-translocase of outer mitochondrial membrane (TOMM)20
(1:1,000; cat. no. ab186734; Abcam), anti-translocase of inner
mitochondrial membrane (TIMM)23 (1:1,000; cat. no. sc-13298; Santa
Cruz Biotechnology, Inc.) and GAPDH (1:4,000; cat. no. GB11002;
Wuhan Servicebio Technology Co., Ltd.) prior to incubation
overnight at 4°C. The blots were then incubated with the secondary
antibody (1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.)
at room temperature for 1 h. The experiments were repeated ≥3 times
and bands were detected using enhanced chemiluminescence (Bio-Rad
Laboratories, Inc.). Band densities were semi-quantified using
Gel-Pro Analyzer densitometry software (version 6.3; Media
Cybernetics, Inc.).
Mitochondrial function assays
In order to investigate mitochondrial superoxide
production and membrane potential (ΔΨm), the cells were incubated
at 37°C with MitoSOX (0.5 µM mol/l; Thermo Fisher Scientific, Inc.)
or 50 nmol/l tetramethylrhodamine ethyl ester (TMRE; Thermo Fisher
Scientific, Inc.) for 15 min. Fluorescence images for TMRE and
MitoSOX were obtained using confocal laser scanning microscope
(magnification, ×200; Leica TCS SP2; Leica Microsystems GmbH). The
average fluorescent intensity for each group was analyzed with
ImageJ software (version 1.41, National Institutes of Health).
Immunofluorescence
Imaging studies for GFP-LC3 were performed as
previously described (21). Neurons
were infected with adenovirus encoding GFP-LC3 (multiplicity of
infection =20) at 37°C for 48 h. After 2 days, the samples were
fixed with 4% paraformaldehyde at 4°C for 30 min, permeated with
70% methanol/acetone at 4°C for 30 min and stained with anti-TOM20
antibody (1:200; cat. no. ab186734; Abcam) at 4°C overnight.
GFP-positive cells were selected for observation under an inverted
fluorescence microscope in order to count the number of GFP-LC3
punctas. One puncta was regarded as equal to one autophagosome. The
number of GFP dots was determined by manual counting the
fluorescent puncta. The samples were then observed under a
fluorescence confocal microscope (magnification, ×400; LSM510 META;
Carl Zeiss AG). Mander's overlap coefficient was used to quantify
the degree of colocalization using Image Pro-Plus software (version
4.5) (22).
Statistical analysis
Data are expressed as the mean ± SEM of ≥3
independent repeats. An unpaired two-tailed t-test was used for
comparisons between two groups. ANOVA or repeated ANOVA followed by
Bonferroni's post hoc test was used to perform multiple comparisons
using GraphPad Prism® software (version 6.0; GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Resveratrol inhibits OGD/R-induced
decrease in cell viability and apoptosis
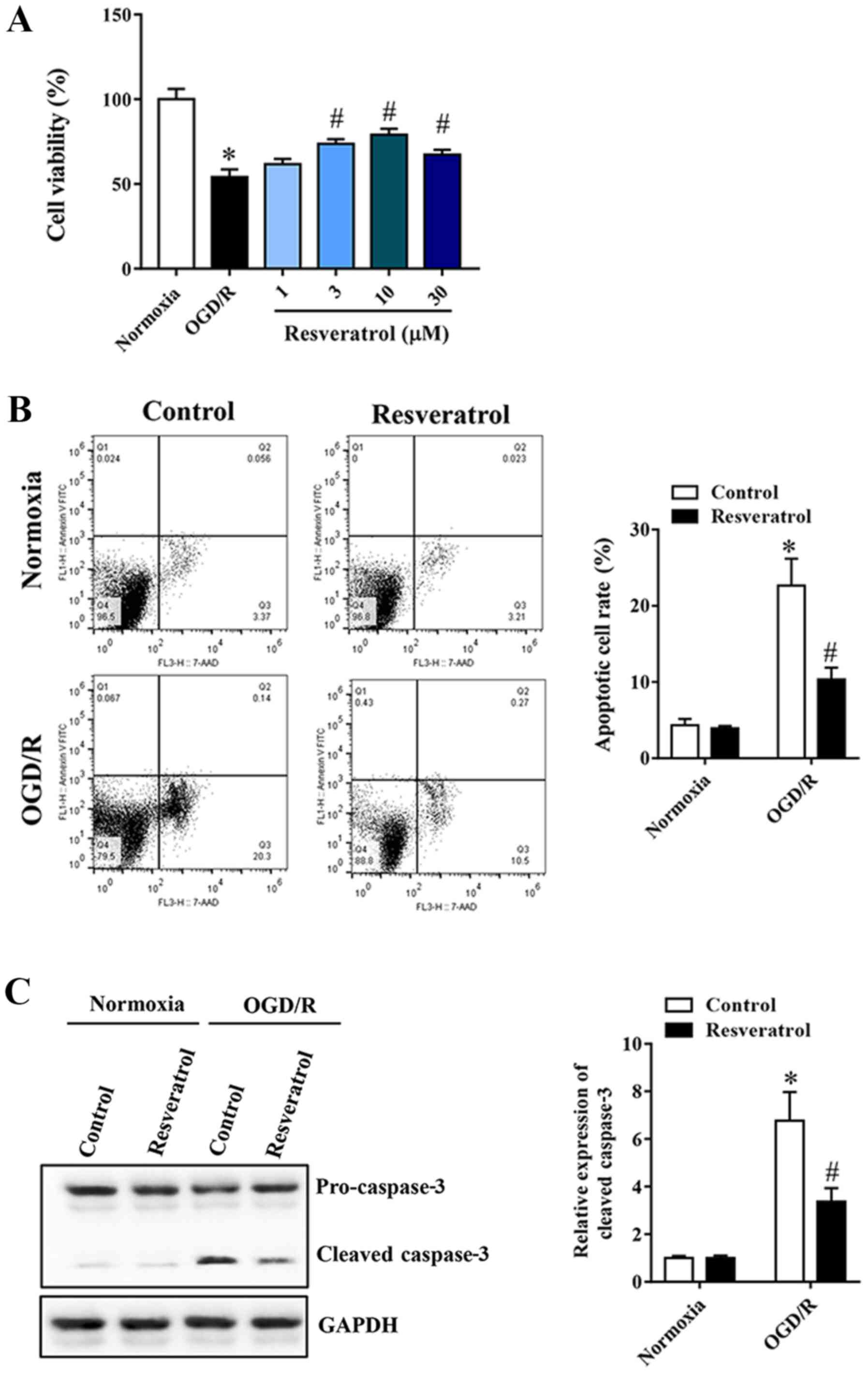
In order to study the neuroprotective effect of
resveratrol on OGD/R-injured cells, a cell viability assay was
first performed. Resveratrol at 1–30 µM administered at the
beginning of reoxygenation restored cell viability in a
concentration-dependent manner, compared with the OGD/R group
(Fig. 1A). Flow cytometry was used
to quantify OGD/R-induced neuronal apoptosis. Resveratrol inhibited
OGD/R-induced apoptosis (Fig. 1B).
Similarly, western blot analysis revealed that resveratrol
treatment significantly decreased OGD/R-induced cleavage of the
proapoptotic protein caspase-3 (Fig.
1C). Based on these results, 10 µM resveratrol was used for
subsequent experiments, as it appears to confer a greater
protection.
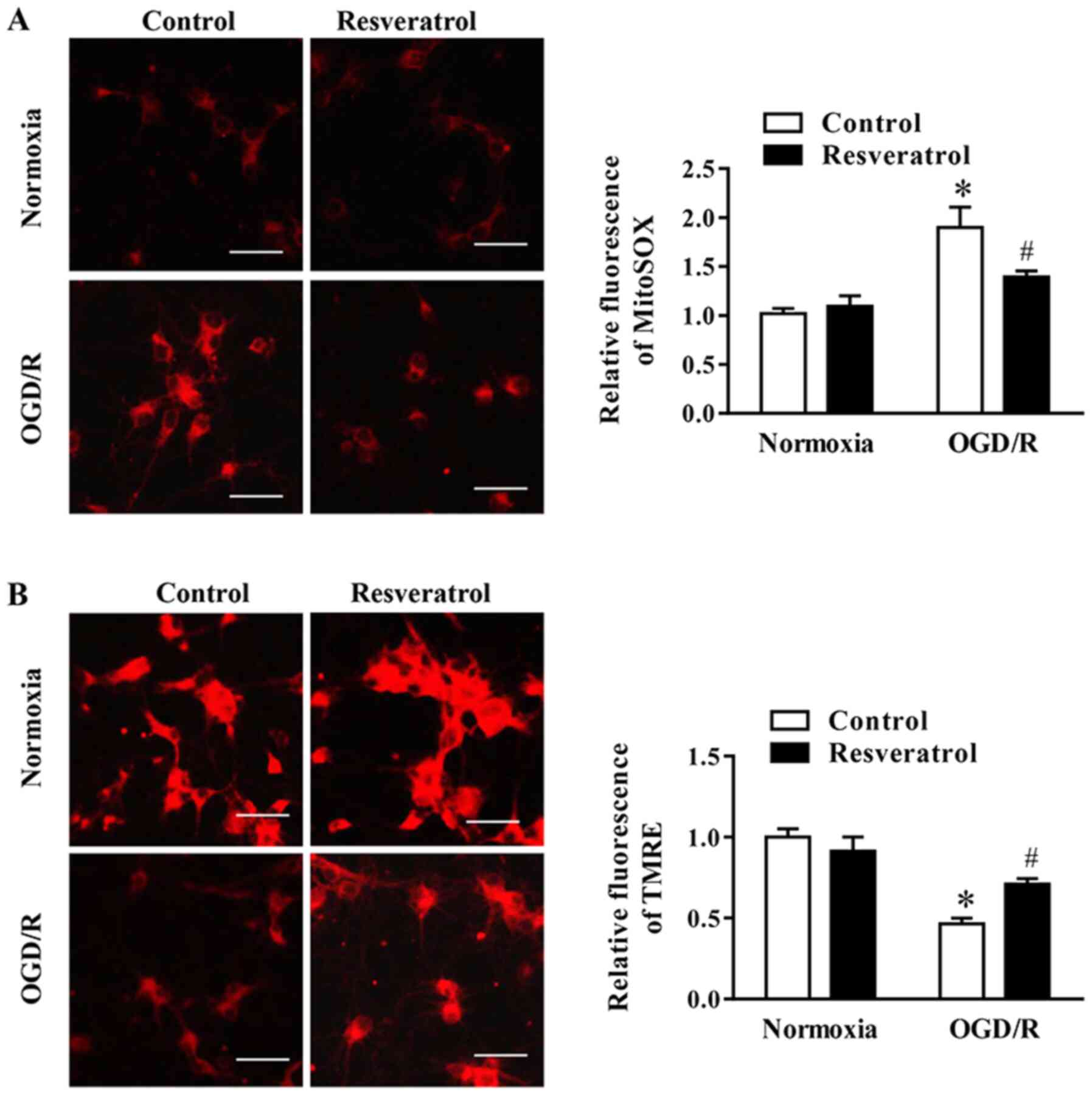
Resveratrol attenuates OGD/R-induced
oxidative stress and preserves mitochondrial function
During OGD/R, the levels of mitochondrial ROS (i.e.
superoxide) increased, whereas these changes were attenuated by
resveratrol (Fig. 2A).
Subsequently, the effect of resveratrol on ΔΨm during OGD/R, which
is a key event during cell death, was examined via assessing the
intensity of TMRE fluorescence. The data indicated that resveratrol
significantly promoted OGD/R-induced decrease in ΔΨm (Fig. 2B). In summary, these data indicated
that resveratrol prevented mitochondrial dysfunction in neuronal
cells following OGD/R.
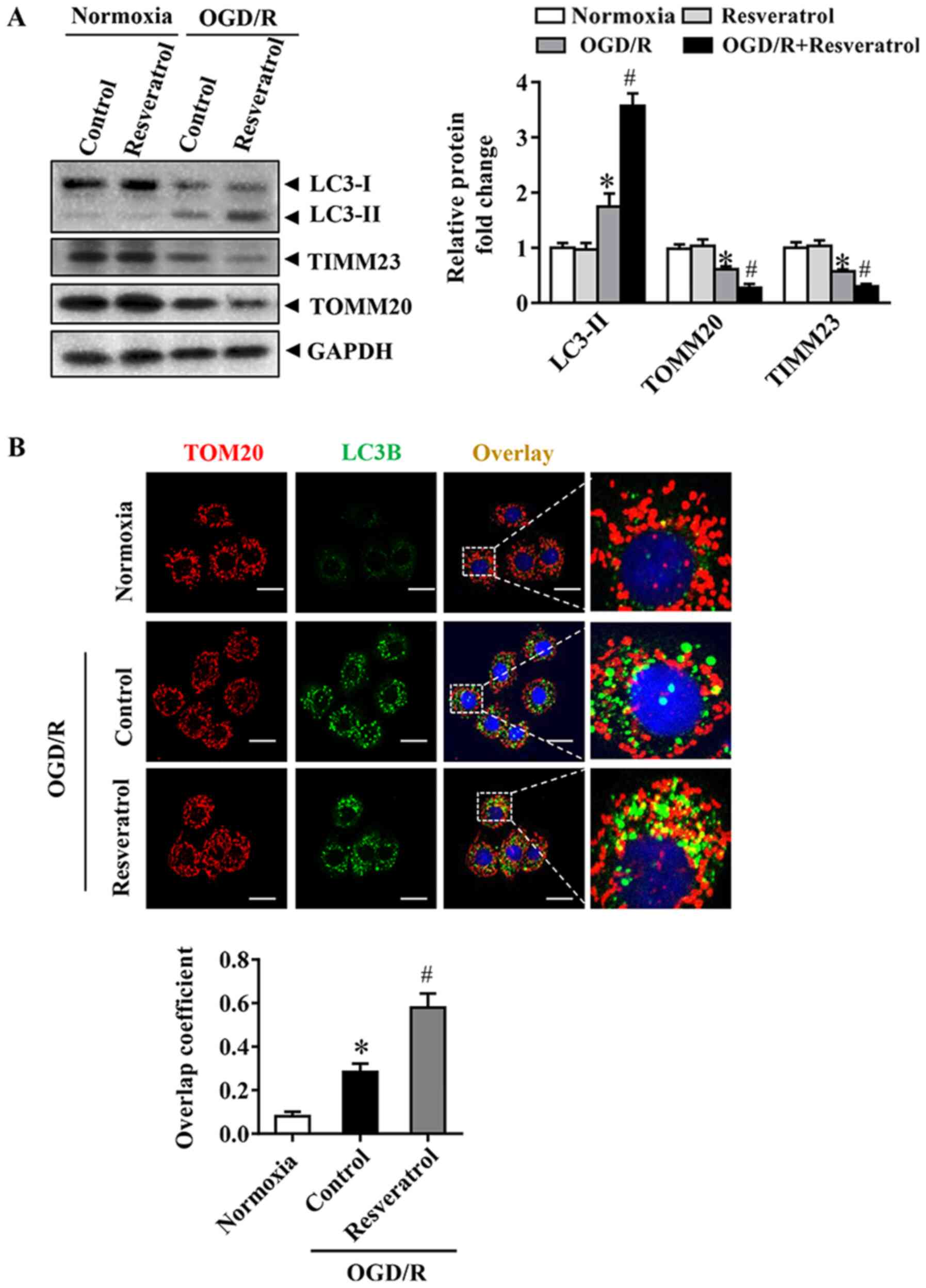
Resveratrol activates OGD/R-induced
mitophagy
The occurrence of mitophagy was detected during
OGD/R. Immunoblot analysis demonstrated that OGD/R significantly
increased the levels of the autophagy-activated biochemical marker
LC3B-II. These changes in LC3B-II during OGD/R were associated with
a significant decrease in levels of the mitochondrial inner
membrane proteins TIMM23 and TOMM20 (Fig. 3A). Activation of mitochondrial
autophagy, as well as decrease in mitochondrial mass, are
implicated in the activation of mitochondrial autophagy (16). Resveratrol activated mitophagy, as
indicated by changes in LC3B-II, TIMM23 and TOMM20 levels (Fig. 3A). In order to monitor mitochondrial
autophagy in cortical cells, mitochondria were immunostained with
anti-TOM20 antibody and autophagosomes were observed with GFP-LC3
punctate structures. Consistently, resveratrol increased the
overlap of mitochondria and autophagosomes in OGD/R-injured cells
compared with that in the control group (Fig. 3B). Collectively, these results
indicated that resveratrol enhanced OGD/R-induced mitochondrial
autophagy.
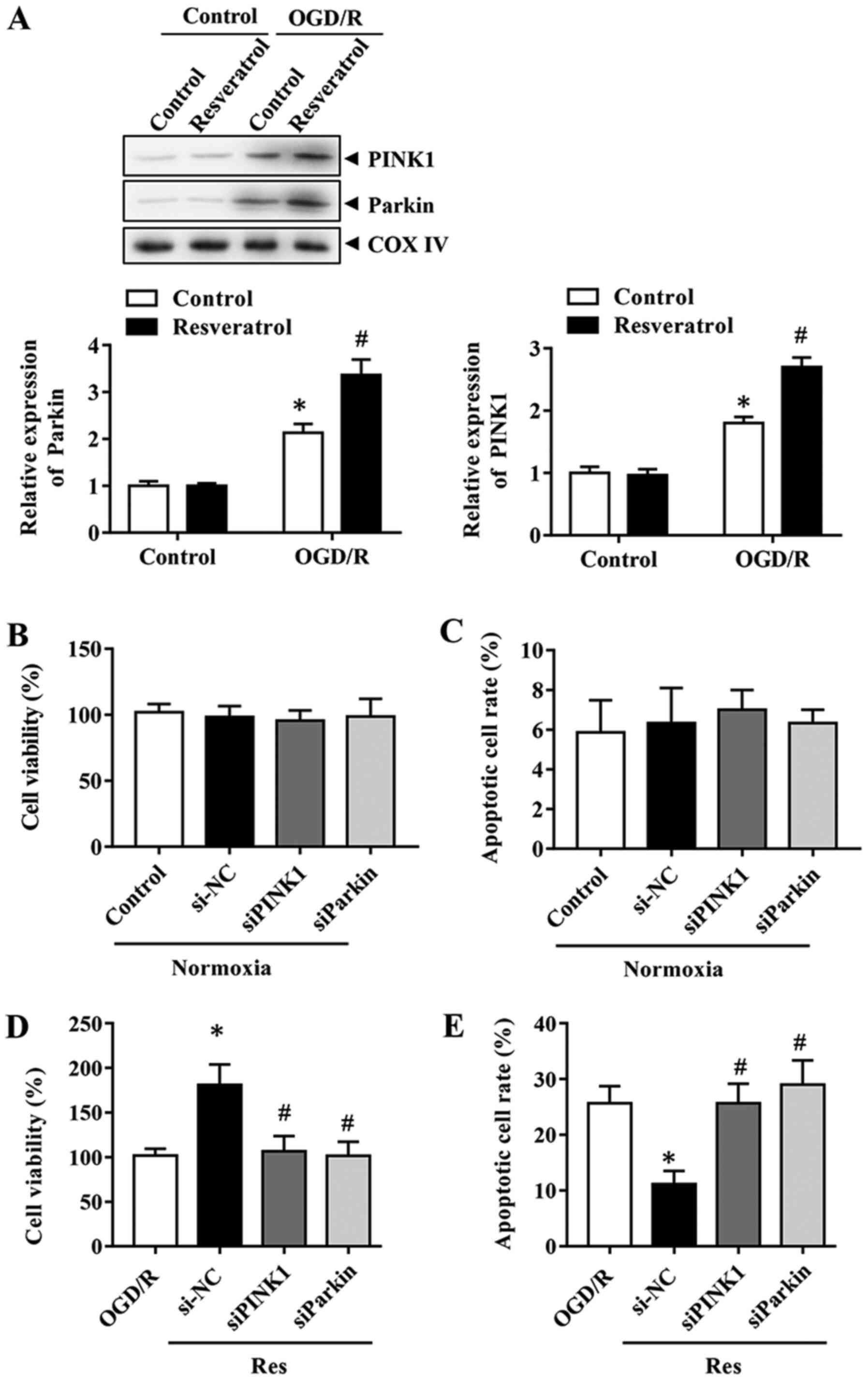
PINK1/Parkin-mediated mitophagy is
involved in the protective effects of resveratrol
In order to determine the mechanism underlying
resveratrol-induced mitophagy, the effects of the PINK1/Parkin
pathway, which serves a key role in mitophagy, were investigated
during OGD/R. The expression levels of Parkin and PINK1 were first
assessed during OGD/R in the presence or absence of resveratrol.
The results indicated that resveratrol further enhanced the
OGD/R-induced increase in PINK1 and Parkin protein expression
levels (Fig. 4A). Therefore, the
focus was placed on PINK1/Parkin to elucidate the mitophagy pathway
implicated in the neuroprotective effects of resveratrol. In order
to determine the role of PINK1/Parkin, their expression was
silenced with specific siRNAs. Cells with PINK1 or Parkin knockdown
exhibited lower mitophagy compared with control siRNA-transfected
cells (data not shown). These results revealed that PINK1 and
Parkin siRNA itself did not affect cell viability and the apoptosis
rate (Fig. 4B and C). Consistently,
the downregulation of PINK1 or Parkin reversed the the improved
cell survival and decreased apoptosis rate caused by resveratrol in
the OGD/R group (Fig. 4D and E).
Collectively, these results indicated that PINK1/Parkin-mediated
mitophagy was responsible for the neuroprotective effects of
resveratrol.
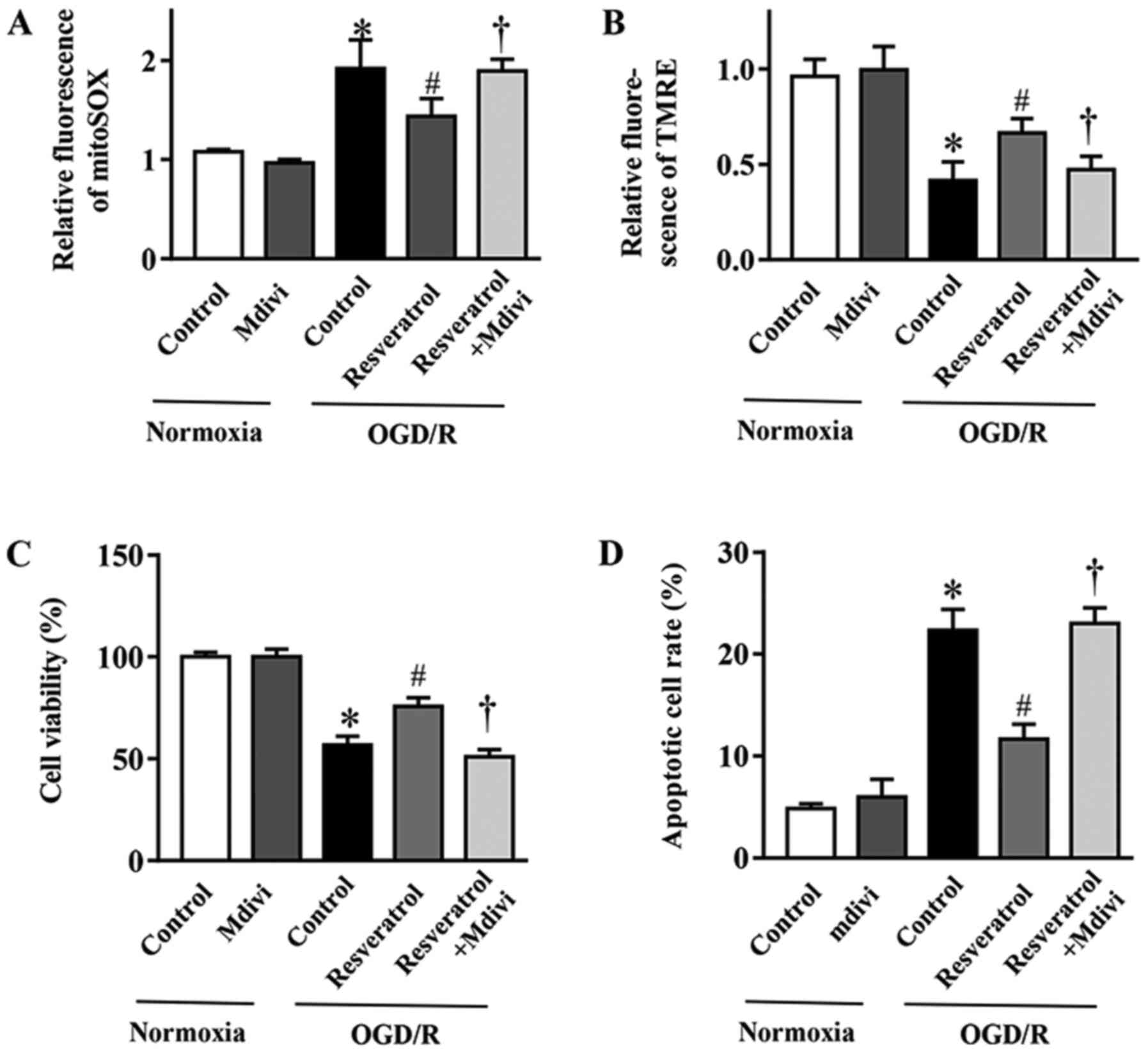
Mitophagy inhibition blocks the
protective effects of resveratrol on OGD/R-injured cells
In order to elucidate the contribution of mitophagy
to the protective effects of resveratrol against OGD/R injury, the
mitophagy inhibitor Mdivi-1 (23)
was employed. Mdivi-1 reversed resveratrol-inhibited mitochondrial
production of ROS during OGD/R (Fig.
5A). Concurrently, the resveratrol-protected ΔΨm in simulated
OGD/R cells was abolished with Mdivi-1 treatment (Fig. 5B). In addition, Mdivi-1 also
reversed the resveratrol-induced inhibition of apoptosis and
improvement in cell survival following OGD/R (Fig. 5C and D). In summary, these data
demonstrated that enhanced mitophagy served a key role in the
neuroprotective effects of resveratrol against OGD/R injury.
Collectively, the present results provided evidence supporting the
neuroprotective role of resveratrol during OGD/R, which was
mediated, at least in part, via enhancing mitophagy.
Discussion
Stroke causes brain injury in millions of
individuals worldwide each year (24). Although it is known that ischemia
causes cellular damage, the underlying mechanisms are incompletely
understood, and there is currently no approved therapy that
decreases infarct size or neurological disability (25,26).
Pharmacological interference is more clinically feasible compared
with a mechanical approach, as it is easier to implement and is
less invasive (21). However, the
availability of effective drugs for the treatment of cerebral IRI
is currently limited. To the best of our knowledge, the present
study is the first to demonstrate that resveratrol can protect
neurons against OGD/R via a mechanism that involves the modulation
of mitochondrial autophagy. The primary findings were as follows:
i) Resveratrol concentration-dependently protected neurons against
OGD/R injury; ii) resveratrol protected primary neurons from
decreased ΔΨm and improved cell survival following OGD/R; iii)
resveratrol exerted protective effects by promoting
PINK1/Parkin-mediated mitophagy. These findings demonstrated the
role and mechanisms of action of resveratrol in neuroprotection
against OGD/R injury.
Resveratrol is found in red wine and numerous edible
plants, including grapes, peanuts and plums (10). Resveratrol has been demonstrated to
have a number of biological functions, such as antiaging,
anti-inflammatory, antioxidant, anticancer and antiapoptotic
properties (27). The
resveratrol-rich Mediterranean diet significantly decreases the
risk of cardiovascular disease (28). Resveratrol pretreatment before
ischemic brain injury decreases the loss of neurons and infarct
size (29). In addition,
resveratrol administration at 6 h after ischemia or reperfusion can
also effectively decrease infarct size (30). These results provide a basis for the
clinical application of resveratrol in the prevention and treatment
of acute ischemic stroke. However, the exact underlying molecular
mechanism is unclear. The present research indicated that
resveratrol protected mitochondrial function and improved the cell
survival rate when administered at the beginning of reoxygenation,
but the underlying mechanism remains elusive.
The balance between mitochondrial autophagy and
biogenesis is a determinant of cell function disorder and disease
(31,32). The role of mitophagy in organ
ischemic disease has been controversial (33–36).
Mitochondrial autophagy is a selective form of autophagy that
removes unwanted or damaged mitochondria (37,38).
PINK1/Parkin-mediated induction of mitophagy and its protective
effects have been demonstrated in cerebral ischemia (39–41).
However, little is known regarding the role of mitophagy in
resveratrol-induced neuroprotection. The findings of the present
study may provide novel insights into the underlying mechanisms of
resveratrol and potential prevention strategies. It was observed
that resveratrol activated PINK1/Parkin-mediated mitochondrial
phagocytosis in primary cortical neurons, but further studies are
required to elucidate the underlying mechanisms.
In summary, the present study demonstrated that
resveratrol may be administered at the onset of reoxygenation and
exerts neuroprotective effects that are partly mediated by
activating the PINK1/Parkin signaling pathway in primary cortical
neurons. Further research is required to elucidate the exact
mechanism of neuroprotection of resveratrol and to determine the
potential role of resveratrol in the treatment of cerebral
ischemia.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Medical and Health Research Project of Yichang (grant no.
A17-301-13).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM and WH contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. WH, YM and SL contributed to data
collection and statistical analysis. YM contributed to data
collection, statistical analysis and manuscript preparation. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the China Three Gorges University (approval no.
20180105181532).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Puyal J, Ginet V and Clarke PG: Multiple
interacting cell death mechanisms in the mediation of
excitotoxicity and ischemic brain damage: A challenge for
neuroprotection. Prog Neurobiol. 105:24–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H, Yoshioka H, Kim GS, Jung JE, Okami
N, Sakata H, Maier CM, Narasimhan P, Goeders CE and Chan PH:
Oxidative stress in ischemic brain damage: Mechanisms of cell death
and potential molecular targets for neuroprotection. Antioxid Redox
Signal. 14:1505–1517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehta SL, Manhas N and Raghubir R:
Molecular targets in cerebral ischemia for developing novel
therapeutics. Brain Res Rev. 54:34–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabral-Costa JV and Kowaltowski AJ:
Neurological disorders and mitochondria. Mol Aspects Med.
71:1008262020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andrabi SS, Tabassum H, Parveen S and
Parvez S: Ropinirole induces neuroprotection following
reperfusion-promoted mitochondrial dysfunction after focal cerebral
ischemia in Wistar rats. Neurotoxicology. 77:94–104. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Wu S, Hou L, Zhu D, Yin S, Yang G
and Wang Y: Therapeutic effects of simultaneous delivery of nerve
growth factor mRNA and protein via exosomes on cerebral ischemia.
Mol Ther Nucleic Acids. 21:512–522. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Celotti E, Ferrarini R, Zironi R and Conte
LS: Resveratrol content of some wines obtained from dried
Valpolicella grapes: Recioto and Amarone. J Chromatogr A.
730:47–52. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pany S, Majhi A and Das J: PKC activation
by resveratrol derivatives with unsaturated aliphatic chain. PLoS
One. 7:e528882012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Das J, Pany S and Majhi A: Chemical
modifications of resveratrol for improved protein kinase C alpha
activity. Bioorg Med Chem. 19:5321–5333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carrizzo A, Puca A, Damato A, Marino M,
Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco
V and Vecchione C: Resveratrol improves vascular function in
patients with hypertension and dyslipidemia by modulating NO
metabolism. Hypertension. 62:359–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kesherwani V, Atif F, Yousuf S and Agrawal
SK: Resveratrol protects spinal cord dorsal column from hypoxic
injury by activating Nrf-2. Neuroscience. 241:80–88. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Xu J, Rottinghaus GE, Simonyi A,
Lubahn D, Sun GY and Sun AY: Resveratrol protects against global
cerebral ischemic injury in gerbils. Brain Res. 958:439–447. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao D, Zhang X, Jiang X, Peng Y, Huang W,
Cheng G and Song L: Resveratrol reduces the elevated level of MMP-9
induced by cerebral ischemia-reperfusion in mice. Life Sci.
78:2564–2570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livingston MJ, Wang J, Zhou J, Wu G,
Ganley IG, Hill JA, Yin XM and Dong Z: Clearance of damaged
mitochondria via mitophagy is important to the protective effect of
ischemic preconditioning in kidneys. Autophagy. 15:2142–2162. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haslip M, Dostanic I, Huang Y, Zhang Y,
Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC and Lee
PJ: Endothelial uncoupling protein 2 regulates mitophagy and
pulmonary hypertension during intermittent hypoxia. Arterioscler
Thromb Vasc Biol. 35:1166–1178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jardim FR, de Rossi FT, Nascimento MX, da
Silva Barros RG, Borges PA, Prescilio IC and de Oliveira MR:
Resveratrol and brain mitochondria: A review. Mol Neurobiol.
55:2085–2101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tauskela JS, Comas T, Hewitt K, Monette R,
Paris J, Hogan M and Morley P: Cross-tolerance to otherwise lethal
N-methyl-D-aspartate and oxygen-glucose deprivation in
preconditioned cortical cultures. Neuroscience. 107:571–584. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ngan E, Stoletov K, Smith HW, Common J,
Muller WJ, Lewis JD and Siegel PM: LPP is a Src substrate required
for invadopodia formation and efficient breast cancer lung
metastasis. Nat Commun. 8:150592017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Y, Gu S, Li X, Tan J, Liu S, Jiang
Y, Zhang C, Gao L and Yang HT: Berbamine postconditioning protects
the heart from ischemia/reperfusion injury through modulation of
autophagy. Cell Death Dis. 8:e25772017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zinchuk V, Zinchuk O and Okada T:
Quantitative colocalization analysis of multicolor confocal
immunofluorescence microscopy images: Pushing pixels to explore
biological phenomena. Acta Histochem Cytochem. 40:101–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Yan H, Yuan Y, Gao J, Shen Z,
Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, et al: Cerebral
ischemia-reperfusion-induced autophagy protects against neuronal
injury by mitochondrial clearance. Autophagy. 9:1321–1333. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
GBD 2016 Stroke Collaborators. Global,
regional, and national burden of stroke, 1990–2016, . A systematic
analysis for the Global Burden of Disease Study 2016. Lancet
Neurol. 18:439–458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaller B and Graf R: Cerebral ischemia
and reperfusion: The pathophysiologic concept as a basis for
clinical therapy. J Cereb Blood Flow Metab. 24:351–371. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moskowitz MA, Lo EH and Iadecola C: The
science of stroke: Mechanisms in search of treatments. Neuron.
67:181–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petrovski G, Gurusamy N and Das DK:
Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci.
1215:22–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW,
Liu HY, Zhang FB and Huang SS: Resveratrol neuroprotective effects
during focal cerebral ischemia injury via nitric oxide mechanism in
rats. J Vasc Surg. 46:346–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang SS, Tsai MC, Chih CL, Hung LM and
Tsai SK: Resveratrol reduction of infarct size in Long-Evans rats
subjected to focal cerebral ischemia. Life Sci. 69:1057–1065. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu J, Wang KZ and Chu CT: After the
banquet: Mitochondrial biogenesis, mitophagy, and cell survival.
Autophagy. 9:1663–1676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Decker RS and Wildenthal K: Lysosomal
alterations in hypoxic and reoxygenated hearts. I. Ultrastructural
and cytochemical changes. Am J Pathol. 98:425–444. 1980.PubMed/NCBI
|
|
33
|
Zhang Z and Yu J: NR4A1 promotes cerebral
ischemia reperfusion injury by repressing Mfn2-mediated mitophagy
and inactivating the MAPK-ERK-CREB signaling pathway. Neurochem
Res. 43:1963–1977. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li F, Tan J, Zhou F, Hu Z and Yang B: Heat
shock protein B8 (HSPB8) reduces oxygen-glucose
deprivation/reperfusion injury via the induction of mitophagy. Cell
Physiol Biochem. 48:1492–1504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji W, Wei S, Hao P, Xing J, Yuan Q, Wang
J, Xu F and Chen Y: Aldehyde dehydrogenase 2 has cardioprotective
effects on myocardial ischaemia/reperfusion injury via suppressing
mitophagy. Front Pharmacol. 7:1012016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andres AM, Hernandez G, Lee P, Huang C,
Ratliff EP, Sin J, Thornton CA, Damasco MV and Gottlieb RA:
Mitophagy is required for acute cardioprotection by simvastatin.
Antioxid Redox Signal. 21:1960–1973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guan R, Zou W, Dai X, Yu X, Liu H, Chen Q
and Teng W: Mitophagy, a potential therapeutic target for stroke. J
Biomed Sci. 25:872018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ashrafi G and Schwarz TL: The pathways of
mitophagy for quality control and clearance of mitochondria. Cell
Death Differ. 20:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pineda-Ramírez N, Alquisiras-Burgos I,
Ortiz-Plata A, Ruiz-Tachiquín ME, Espinoza-Rojo M and Aguilera P:
Resveratrol activates neuronal autophagy through AMPK in the
ischemic brain. Mol Neurobiol. 57:1055–1069. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Chen S, Zhang Y, Xu H and Sun H:
Electroacupuncture ameliorates neuronal injury by
Pink1/Parkin-mediated mitophagy clearance in cerebral
ischemia-reperfusion. Nitric Oxide. 91:23–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He Q, Li Z, Meng C, Wu J, Zhao Y and Zhao
J: Parkin-dependent mitophagy is required for the inhibition of
ATF4 on NLRP3 inflammasome activation in cerebral
ischemia-reperfusion injury in rats. Cells. 8:8972019. View Article : Google Scholar
|