Introduction
Triple-negative breast cancer (TNBC) is a
heterogeneous type of breast cancer characterized by the absent
expression of estrogen receptor (ER), progesterone receptor (PR)
and human epidermal growth factor receptor 2 (HER2) (1). In the clinic, TNBC is known to be an
aggressive subtype of breast cancer, with a higher rate of relapse
than the ER/PR- and HER2-positive breast cancer types (2). Patients with TNBC tend to have poorer
clinical prognosis compared with other breast cancer types
(3). Moreover, <30% of women
diagnosed with metastatic TNBC survive five years, and virtually
all the female metastatic TNBC cases succumb to the diseases
(4). To date, no targeted therapies
have been recommended for the treatment of TNBC, while chemotherapy
remains the primary treatment modality for TNBC. However,
traditional first-line drugs, such as paclitaxel, applied to treat
breast cancer fail to provide satisfactory therapeutic effects when
used in treating TNBC (5).
Therefore, it is of great significance to develop novel therapeutic
strategies for TNBC.
Recently, natural agents have been increasingly
investigated as an effective chemotherapy strategy for cancer, with
relatively low toxicity (6).
Sericin, a natural macromolecular protein from silkworm cocoons,
accounts for 10–35% of the total silk protein (7). Sericin is produced in the middle silk
gland, and has been widely used in the biomedical field as
anti-inflammatory drug, antioxidant, enzyme immobilizing factor,
cell culture medium supplement, dietary supplement and/or
biomaterial for drug and gene delivery (8–11). Our
previous study reported that sericin could prevent kidney injuries
in rats with diabetic nephropathy, by inhibiting the activation of
the transforming growth factor-β1/Smad3 signal pathway and reducing
glomerulosclerosis and renal interstitial fibrosis in kidney
(12). Moreover, sericin has been
revealed to decrease hippocampal neuronal apoptosis by activating
the Akt signaling pathway in diabetic rats (13). Sericin has also been shown to
improve spermatogenesis by regulating the growth
hormone/insulin-like growth factor-1 axis and to protect the
reproductive ability against diabetes-induced damages (14). Furthermore, several studies have
reported that sericin could exert antitumor effects in various
tumor types, including gastric carcinoma (15), colon carcinoma (16–18),
skin carcinoma (19,20), squamous carcinoma, breast
adenocarcinoma and tongue carcinoma (21). However, the effects of sericin in
TNBC and the underlying molecular mechanisms remain to be further
elucidated.
In the present study, the sericin-induced antitumor
activity in TNBC MDA-MB-468 cells was investigated. The effects of
sericin on the cell proliferation, cell cycle and cellular
apoptosis were analyzed. Bioinformatics analysis was performed to
predict the signaling pathway involved in TNBC pathogenesis. All
the findings may provide scientific evidence for the application of
sericin in the clinical treatment of TNBC.
Materials and methods
Cell and cell culture
Human TNBC cell lines (MDA-MB-468 and MDA-MB-453), a
non-TNBC MCF-7 cell line and a normal breast epithelial MCF-10A
cell line were purchased from Procell Life Science & Technology
Co., Ltd. MDA-MB-468 and MDA-MB-453 cells were cultured with the
Leibovitz's L-15 medium (cat. no. CM10045; Beijing Zhongke Maichen
Technology Co., Ltd.) containing 10% fetal bovine serum (cat. no.
04-001-1A; Biological Industries) and 1% penicillin-streptomycin
(cat. no. P1400-100ML; Beijing Solarbio Science & Technology
Co., Ltd.) at 37°C without CO2. MCF-7 and MCF-10A cells
were cultured with DMEM (cat. no. C11995500BT; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (cat. no.
04-001-1A; BioInd, Israel) and 1% penicillin-streptomycin (cat. no.
P1400-100ML, Solarbio, Beijing Solarbio Science & Technology
Co., Ltd.) in a 37°C, 5% CO2 incubator. The cells were
passaged every 4–5 days using 0.25% Trypsin-EDTA (v/v) (cat. no.
25200-056; Gibco; Thermo Fisher Scientific, Inc.).
MTT assay
Cell viability was assessed with the MTT assay (cat.
no. M6180; Biotopped). The cells were seeded into the 96-well
plate, at density of 1.0×104 cells/well. MDA-MB-468,
MDA-MB-453 and MCF-10A cells were treated with sericin (dissolved
with complete medium; cat. no. S5201; Sigma-Aldrich; Merck KGaA) at
indicated concentrations (0, 0.5, 1, 2, 4, 8 and 16 mg/ml) at 37°C
for 24 h. MCF-7 cells were treated with sericin (0, 2, 4 and 8
mg/ml) at 37°C for 24 h. The culture medium was then discarded.
Complete 100 µl culture medium was mixed with 10% MTT solution,
which was added into each well, to incubate the cells at 37°C for 4
h. After discarding the medium, formazan crystals were dissolved
with 100 µl DMSO. Absorbance at 570 nm was measured with a
microplate reader and the IC50 was calculated using
GraphPad prism 7.0 (GraphPad Software, Inc.).
Colony formation assay
MDA-MB-468 cells were seeded into the 6-well plates
at a density of 2.0×104 cells/well, and were incubated
at 37°C for 48 h. After adhering, the cells were treated with
sericin at indicated concentrations (0, 2, 4 and 8 mg/ml) for 24 h
at 37°C, and then cultured with L-15 complete medium at 37°C for 14
days. After fixing with 4% paraformaldehyde at 4°C for 30 min, the
cell colonies were stained with 0.1% crystal violet for 20 min at
room temperature. The number of colonies containing ≥50 cells was
counted under a light microscope (magnification, ×40), and the cell
colonies were imaged.
Flow cytometry
Cell cycle progression and apoptosis (early and late
apoptosis) were detected via flow cytometry. MDA-MB-468 cells were
treated with sericin at 0, 2, 4 and 8 mg/ml for 24 h at 37°C.
Subsequently, 1×106 cells treated with sericin were
harvested and stained with Pharmingen™ PI/RNase Staining buffer
(cat. no. 550825; BD Biosciences) or FITC Annexin V Apoptosis
Detection Kit I (cat. no. 556547; BD Biosciences) for 15–20 min at
room temperature according to the manufacturer's instructions.
After incubation for 30 min, the cells were detected using a
Coulter ELITEesp flow cytometer (Beckman Coulter, Inc.) and data
analysis was performed using FloMax 2.7 software (Sysmex
Partec).
Immunocytochemistry
Immunocytochemistry was performed with the
StreptAvidin-Biotin Complex kit (cat. no. SP-9000; OriGene
Technologies, Inc.). MDA-MB-468 cells were seeded into the 24-well
plates at a density of 5×104 cells/ml, which were
treated with sericin at 0, 2, 4 and 8 mg/ml for 24 h at 37°C. The
cells were fixed with 4% paraformaldehyde at 4°C for 30 min and
then permeabilized with 0.1% Triton X-100 in PBS at 4°C for 10 min.
Quenching of endogenous peroxidase was achieved using 3%
H2O2 at 37°C for 10 min. After blocking with
10% goat serum (cat. no. SL038; Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 30 min, the cells
were incubated with rabbit anti-human anti-P21 (1:300: Cat. no.
ab109520; Abcam) and rabbit anti-human anti-Ki67 (1:100; cat. no.
ZA-0502; OriGene Technologies, Inc.) primary antibodies at 4°C
overnight. The goat anti-rabbit secondary antibody (1:10,000; cat.
no. SP-9000; OriGene Technologies, Inc.) was added to the cells at
37°C for 30 min. Chromogenic development was performed using the
DAB kit (cat. no. ZLI-9017; OriGene Technologies, Inc.). Then, the
cells were counterstained with hematoxylin for 2 min at room
temperature, followed by observation under a light microscope (cat.
no. BX43; Olympus Corporation; magnification, ×100 or ×400).
Western blot analysis
MDA-MB-468 cells were lysed with RIPA lysis buffer
(cat. no. R0020-100ML; Beijing Solarbio Science & Technology
Co., Ltd.) and were centrifuged at 7,500 × g at 4°C for 15 min.
Protein concentration was determined using the BCA Protein Assay
kit (cat. no. PC0020-500; Beijing Solarbio Science & Technology
Co., Ltd.). Equal amounts of protein from whole-cell lysates (30
µg) were separated on 10% SDS-PAGE, which were then transferred
onto the PVDF membrane (cat. no. IPVH00010; EMD Millipore). After
blocking with skimmed milk in TBS + 0.1% Tween-20 for 3 h at room
temperature, the membrane was incubated with mouse anti-human
anti-cyclin D1 (1:4,000: Cat. no. 60186-1-1g; ProteinTech Group,
Inc.), rabbit anti-human anti-Cdk4 (1:2,000; cat. no. ab108357;
Abcam), rabbit anti-human anti-E2F transcription factor 3 (E2F3;
1:1,000; cat. no. ab50917; Abcam), rabbit anti-human anti-P21
(1:5,000; cat. no. ab109520; Abcam), rabbit anti-human anti-P27
(1:5,000; cat. no. ab32034; Abcam), rabbit anti-human anti-Bax
(1:500; cat. no. ab53154; Abcam), rabbit anti-human anti-Bcl-2
(1:500; cat. no. ab59348; Abcam), rabbit anti-human anti-cytochrome
c (Cyto-C; 1:5,000; cat. no. ab133504; Abcam), rabbit
anti-human anti-PI3K (1:500; cat. no. BS3678; Bioworld Technology,
Inc.), rabbit anti-human anti-Akt (1:10,000; cat. no. ab179463;
Abcam), rabbit anti-human anti-phosphorylated (p)-Akt (1:500; cat.
no. BS4006; Bioworld Technology, Inc.), rabbit anti-human anti-Heat
shock protein 90 (HSP90; 1:10,000; cat. no. ab13495; Abcam) and
rabbit anti-human anti-GAPDH (1:8,000; cat. no. AP0063; Bioworld
Technology, Inc.) primary antibodies at 4°C overnight. Then, the
membrane was incubated with HRP-conjugated anti-mouse (1:5,000;
cat. no. ab6789; Abcam) or anti-rabbit (1:5,000; cat. no. ab205718;
Abcam) secondary antibodies for 90 min at room temperature.
Immune-reactive proteins were visualized with the ECL luminescence
reagent (cat. no. MA0186; Dalian Meilun Biology Technology Co.,
Ltd.). The bands were detected with a Tanon 5200 imaging system
(Tanon Science and Technology Co., Ltd.), and the integrated
density was quantified using ImageJ 1.52a software (National
Institutes of Health).
Data availability
GSE112825 (22) and
GSE76124 (23), containing
information on the mRNA expression levels of normal mammary and
TNBC tissue, were obtained from the Gene Expression Omnibus (GEO)
database (https://www.ncbi.nlm.nih.gov/geo/) (24). The GSE112825 dataset contained 109
normal mammary tissue samples and the GSE76124 dataset contained
198 TNBC tissue samples, based on the GPL570 [HG-U133_Plus_2]
Affymetrix Human Genome U133 Plus 2.0 arrays platform (Affymetrix;
Thermo Fisher Scientific, Inc.). These two datasets were imported
into R software, version 3.6.1 (https://www.R-project.org/) for quality assessment. To
eliminate batch effects, ‘sva’ and ‘limma’ R packages were applied
for batch effect normalization. Differential expression analysis on
datasets was then performed using the limma package in R.
|log2(fold-change)|>2.0 and P<0.05 were set as
cutoffs for differentially expressed genes.
Gene ontology (GO) and kyoto
encyclopedia of genes and genomes (KEGG) pathway analysis
GO annotation analysis and KEGG pathway enrichment
analysis of the aforementioned differentially expressed genes were
performed using the Database for Annotation, Visualization and
Integrated Discovery (DAVID) v6.8 online tool (https://david.ncifcrf.gov/). The background was set as
Homo sapiens. P<0.05 was considered to indicate a
statistically significant difference. The GO analysis included the
following three ontologies: Biological process (BP), cellular
component (CC) and molecular function (MF). Moreover, the
reliability of the KEGG analysis was validated using the gene set
enrichment analysis (GSEA) software v3.0 (http://www.broadinstitute.org/gsea). Results for which
the normalized enrichment score (NES) absolute value was >1,
nominal P-value was <0.05 and false discovery rate q-value was
<0.25 were considered as significant. Lists of genes involved in
various pathways were acquired from the KEGG database (25), and the most critical pathway related
genes were selected to plot a diagram.
Kaplan-Meier plotter
The overall survival curves of patients with breast
cancer with different expression levels of the most critical
pathway-associated genes were constructed using the online
Kaplan-Meier Plotter tool (https://www.kmplot.com) (26). A log-rank test, the only available
method in this online analysis tool, was calculated and differences
with P<0.05 were considered as statistically significant. The
Kaplan-Meier survival curves were downloaded from the website and
the number-at-risk was indicated below the main plot.
Statistical analysis
Data are presented as the mean ± SD. Each experiment
was carried out three times. Statistical significance was assessed
with the one-way ANOVA or the independent-samples t-test and the
pairwise comparisons were performed with Bonferroni correction,
using the SPSS software (v19; IBM Corp.). GraphPad Prism software
(v7.0; GraphPad Software, Inc.) was used for illustration.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sericin inhibits proliferation of TNBC
cells
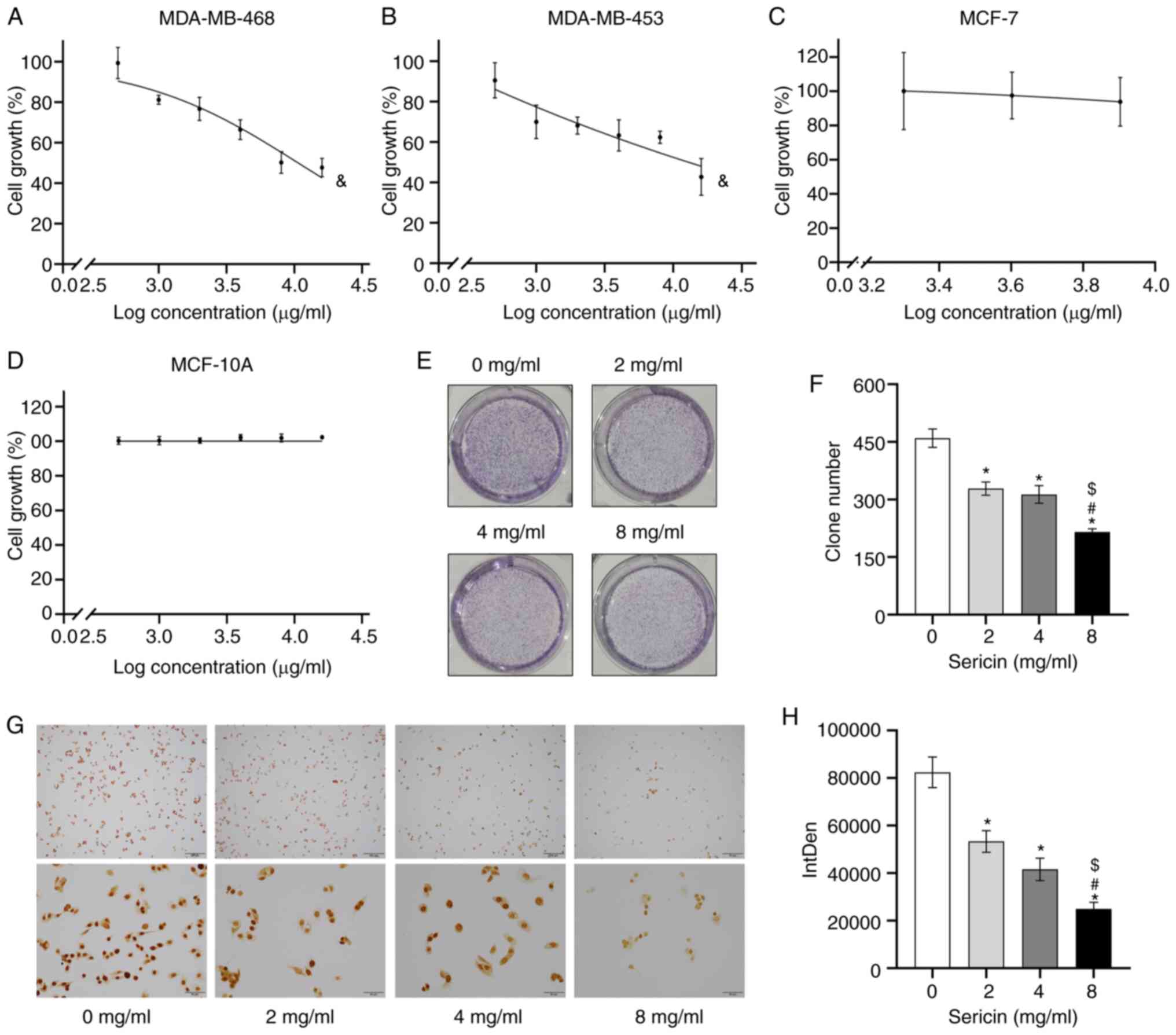
The effects of sericin on the proliferation of human
TNBC MDA-MB-468 and MDA-MB-453 cells, non-TNBC MCF-7 cells and
normal breast epithelial MCF-10A cells were investigated. The cells
were exposed to sericin at indicated concentrations for 24 h, and
cell viability was assessed with the MTT assay. Sericin treatment
inhibited the viability of both MDA-MB-468 (F=74.516; P<0.001)
and MDA-MB-453 (F=23.150; P<0.001) cells, in a dose-dependent
manner (Fig. 1A and B), suggesting
that the human TNBC cells showed sensitivity to sericin. Moreover,
the effects of sericin on MDA-MB-468 cells (IC50=10.67
mg/ml) were slightly more apparent compared with the MDA-MB-453
cells (IC50=12.06 mg/ml). Sericin had less of a growth
inhibiting effect on non-TNBC MCF-7 cells (F=0.663; P=0.587) and
normal breast epithelial MCF-10A cells (F=1.964; P=0.117) (Fig. 1C and D). According to the findings
from the MTT assay in MDA-MB-468 cells, the 1/5 IC50,
2/5 IC50 and 4/5 IC50 doses of sericin,
serving as low, middle and high concentrations, were used in the
subsequent experiments.
The results suggested that sericin significantly
suppressed the colony formation of MDA-MB-468 cells, in a
dose-dependent manner (F=81.602; P<0.001; Fig. 1E and F). The clone number for the
cells treated with sericin at concentrations of 8, 4 and 2 mg/ml
were 215.000±8.544 (P<0.001), 313.333±23.116 (P<0.001) and
328.667±17.215 (P<0.001), respectively, which were lower
compared with those of the cells treated with 0 mg/ml sericin
(459.667±24.132). Similar results were observed in the
immunocytochemistry assay. The Ki67 staining identified that
MDA-MB-468 cell proliferation was significantly inhibited by
sericin treatments in a dose-dependent manner (F=76.470;
P<0.001; Fig. 1G and H).
Therefore, the findings suggested that sericin may only inhibit the
proliferation of TNBC cells.
Sericin induces cell cycle arrest at
the G0/G1 phase
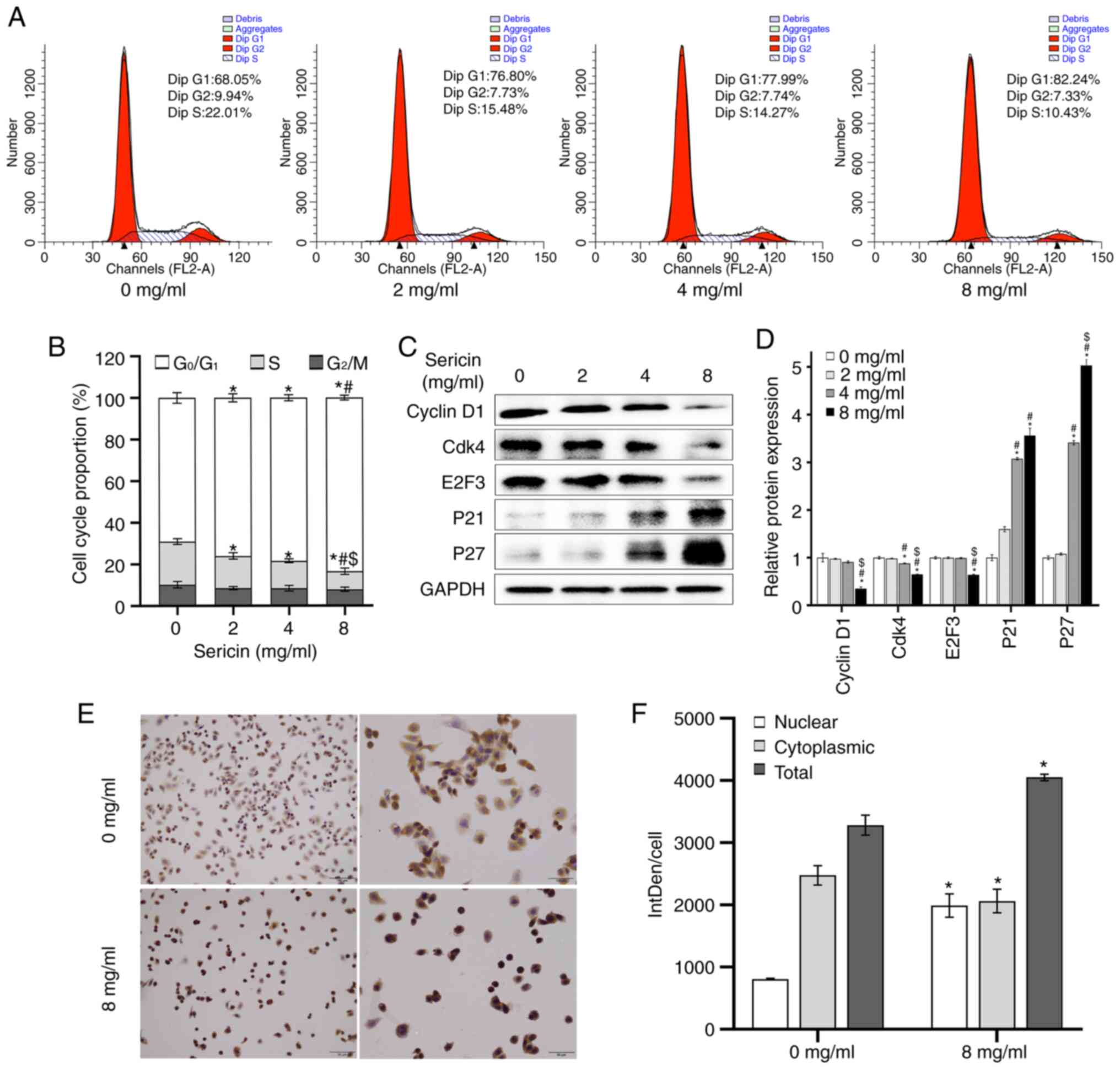
To investigate the effects of sericin on the cell
cycle of MDA-MB-468 cells, flow cytometry was performed. The
results demonstrated that the sericin treatment led to an increased
portion of cells at the G0/G1 phase
(F=32.131; P<0.001) and a reduction at the S phase (F=38.818;
P<0.001), in a dose-dependent manner (Fig. 2A and B). There was no statistical
significance among the cells treated with 0, 2, 4 and 8 mg/ml
sericin at the G2 phase (F=2.168; P=0.17). Furthermore,
to validate the effects of sericin on cell cycle progression, the
expression levels of cell cycle-regulated proteins were detected
via western blot analysis. The results suggested that sericin
treatment significantly decreased the expression levels of cyclin
D1 (F=35.866; P<0.001), Cdk4 (F=163.359; P<0.001) and E2F3
(F=257.478; P<0.001), while increased the expression levels of
P21 (F=80.107; P<0.001) and P27 (F=471.443; P<0.001)
(Fig. 2C and D).
Immunocytochemistry was also conducted to further
detect the effects of sericin on the MDA-MB-468 cells. The results
demonstrated that P21 was translocated into the nucleus (Fig. 2E), and both the nuclear (t=−10.918;
P<0.001) and total (t=−7.948; P=0.001) expressions levels of P21
were increased in the cells treated with 8 mg/ml sericin, compared
with the cells in 0 mg/ml sericin group (Fig. 2F). These results suggested that
sericin could induce cell cycle arrest at the
G0/G1 phase.
Sericin induces apoptosis in
MDA-MB-468 cells
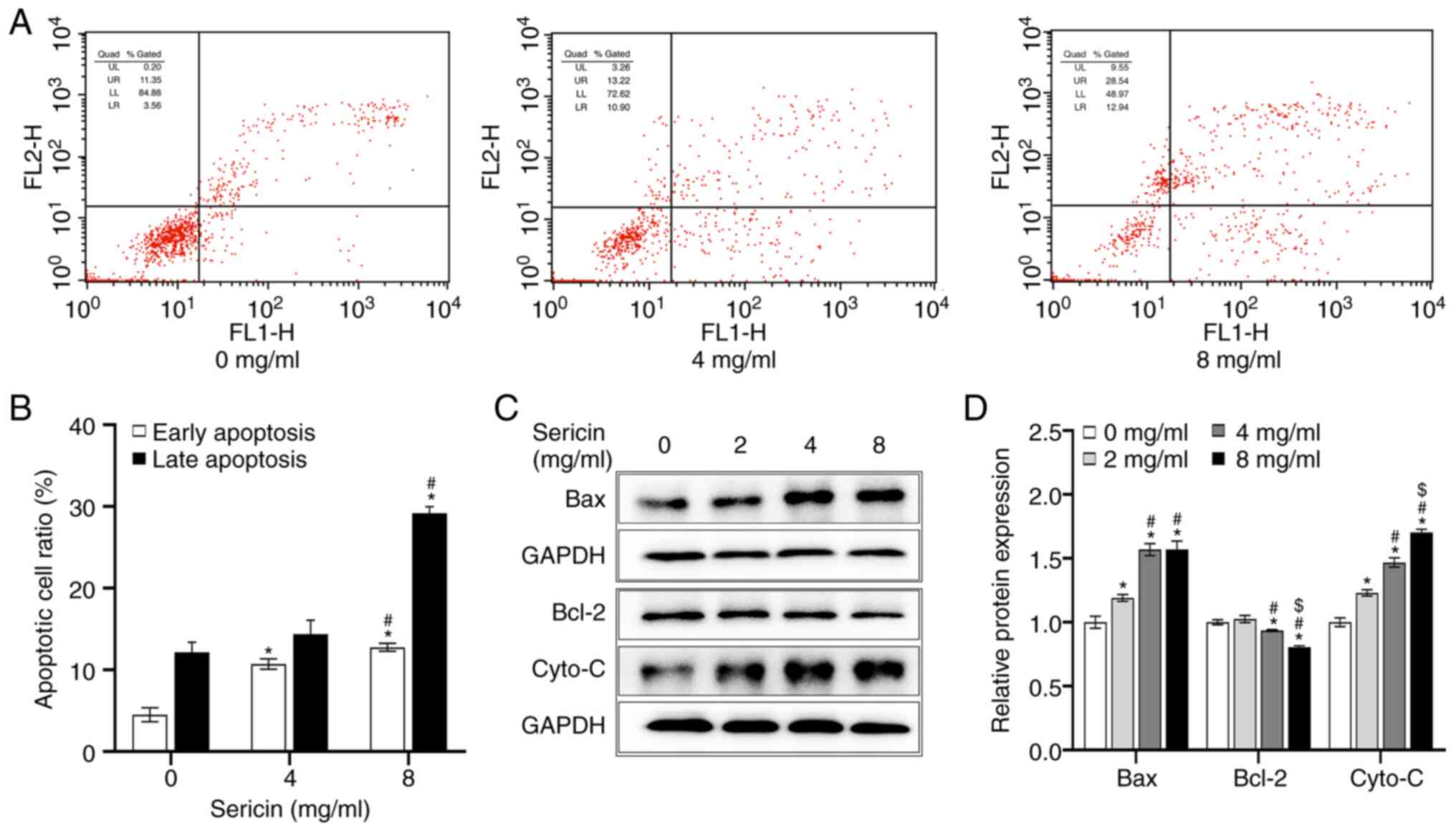
To further investigate the effects of sericin on the
MDA-MB-468 cells, cellular apoptosis was detected via flow
cytometry. The results indicated that, after incubation for 24 h,
the total apoptotic rate of the cells treated with 0 mg/ml sericin
was 16.66±1.95%, with early and late apoptotic rates of 4.51±0.84
and 12.15±1.25%, respectively. However, the total apoptotic rate of
the cells treated with 8 mg/ml sericin was increased to 41.93±1.13%
in MDA-MB-468 cells (F=233.551; P<0.001), with early and late
apoptotic rates of up to 12.76±0.49 and 29.17±0.81%, respectively
(Fig. 3A and B). In addition, the
effects of sericin on the expression levels of apoptosis-related
proteins were investigated. The protein expression levels of Bax
(F=222.130; P<0.001) and Cyto-C protein (F=55.296; P<0.001)
were upregulated, while Bcl-2 protein expression (F=92.422;
P<0.001) was significantly downregulated after treatment with
sericin, in a dose-dependent manner (Fig. 3C and D). These results suggested
that sericin could induce the cellular apoptosis in MDA-MB-468
cells.
PI3K/Akt pathway is dysregulated in
TNBC
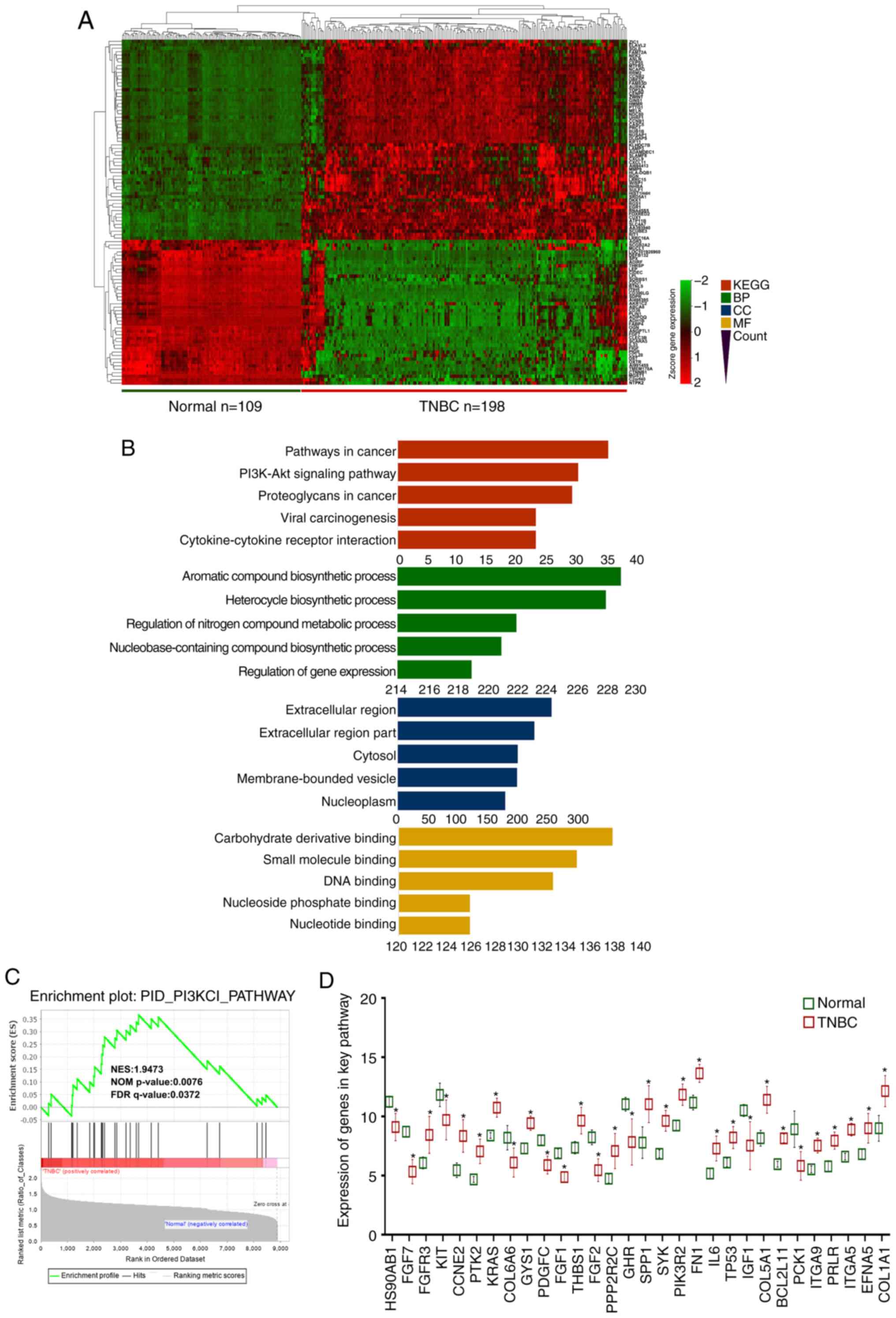
To screen the pathways associated with tumorigenesis
and progression of TNBC, the normal mammary tissue dataset
GSE112825 and TNBC tissue dataset GSE76124 were analyzed. These two
datasets consisted of 109 normal mammary tissue samples and 198
TNBC tissue samples. Based on the |log2(fold-change)|
>2 and P<0.05, a total of 1,091 differentially expressed
genes were identified, including 764 upregulated and 327
downregulated genes. As presented in Fig. 4A, the top 100 genes were selected
based on the significant changes. Moreover, the GO and KEGG pathway
analyses were applied to predict the differentially expressed genes
(Fig. 4B). The GO analysis of the
altered genes revealed that the top two enriched BPs were ‘aromatic
compound biosynthetic process’ and ‘heterocycle biosynthetic
process’. ‘Extracellular region’ and ‘carbohydrate derivative
binding’ were the top-ranked CC and MF, respectively. The KEGG
analysis indicated that the most significant pathways included
‘pathways in cancer’ and the ‘PI3K/Akt signaling pathway’ (Fig. 4B). Validation performed in GSEA
demonstrated similar results to the KEGG analysis, suggesting that
the PI3K/Akt signaling pathway was significantly altered in TNBC
(Fig. 4C). Then, the 30 genes
enriched in this pathway were extracted, the expression levels of
which in normal mammary tissues and TNBC tissues were visualized
(Fig. 4D).
It was then investigated whether the aforementioned
genes were associated with the overall survival of patients with
breast cancer, which was predicted using the Kaplan-Meier survival
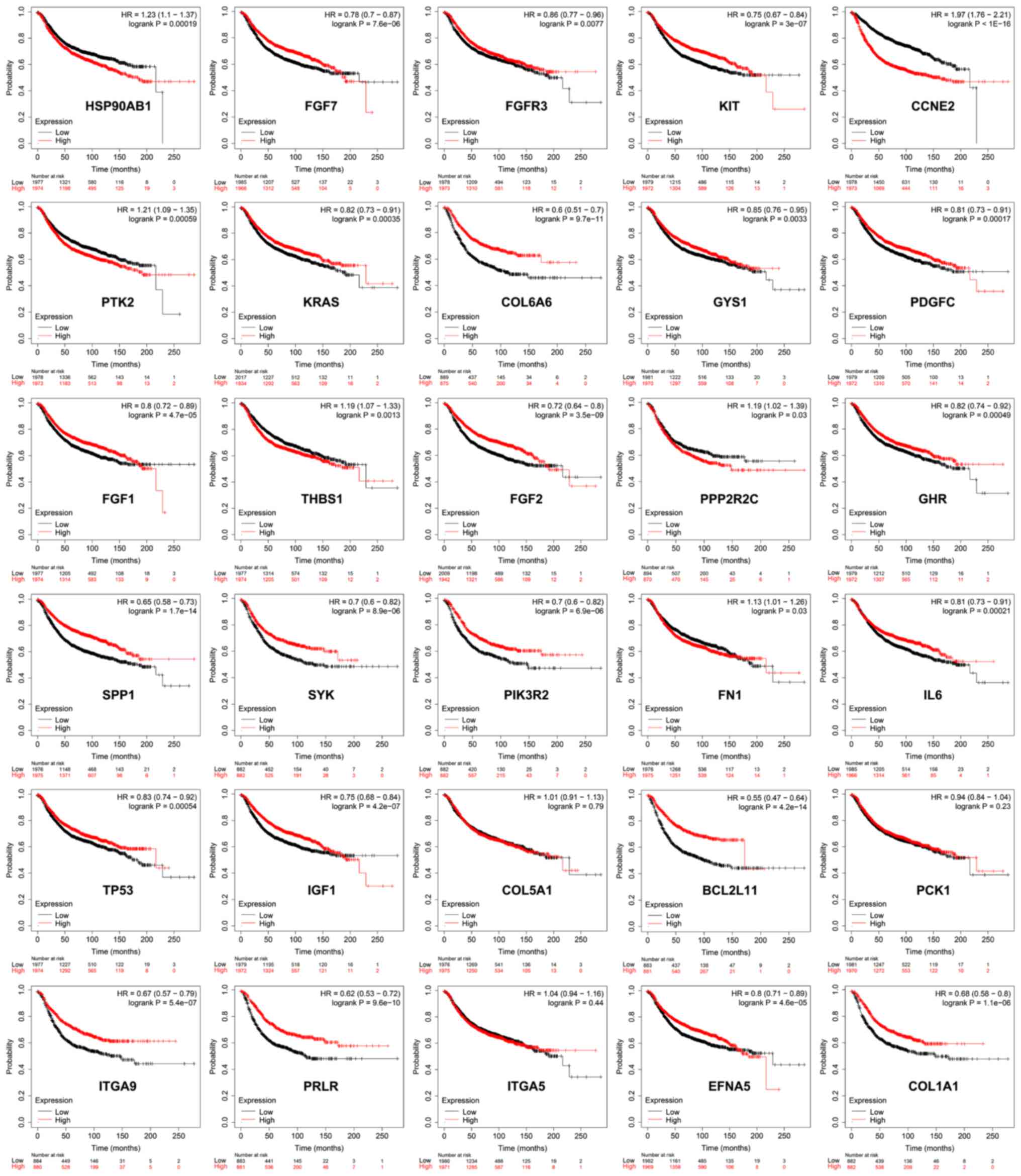
curves and log-rank test. Among the evaluated genes, 27 genes, such
as HSP90AB1, FGF7, FGFR3 and KIT, demonstrated a significant
association with the overall survival (Fig. 5). These results indicated that the
dysregulated PI3K/Akt pathway serves a major role in the
tumorigenesis and progression of TNBC.
Sericin suppresses the PI3K/Akt
signaling pathway
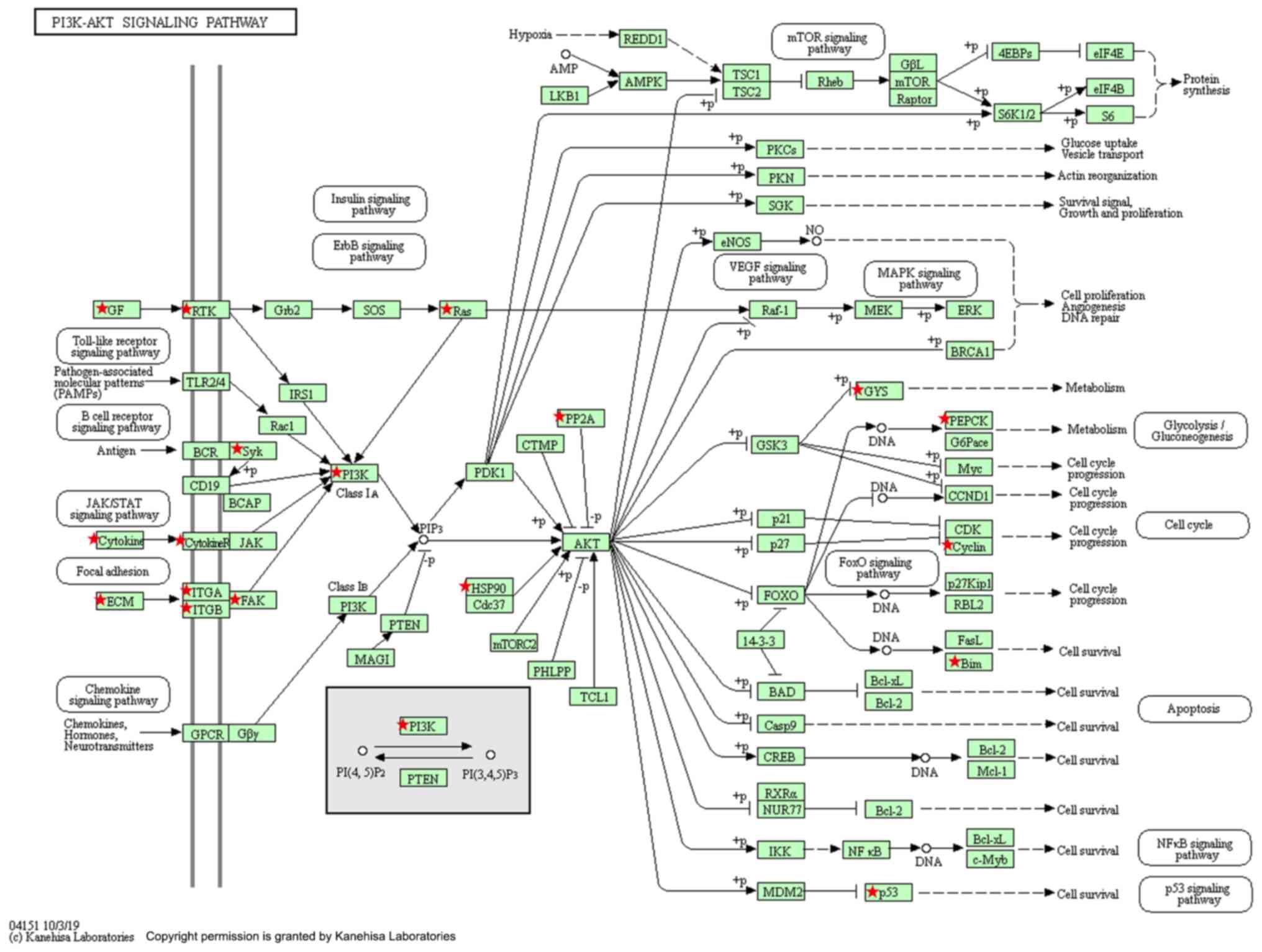
Differentially expressed genes between normal
mammary tissues and TNBC tissues involved in the PI3K/Akt signaling
pathway were screened based on the KEGG analysis (Fig. 6). To verify whether the effects of
sericin on TNBC were associated with the PI3K/Akt pathway, the
protein expression levels of PI3K, Akt and other critical genes
enriched in PI3K/Akt pathway were examined. The present results
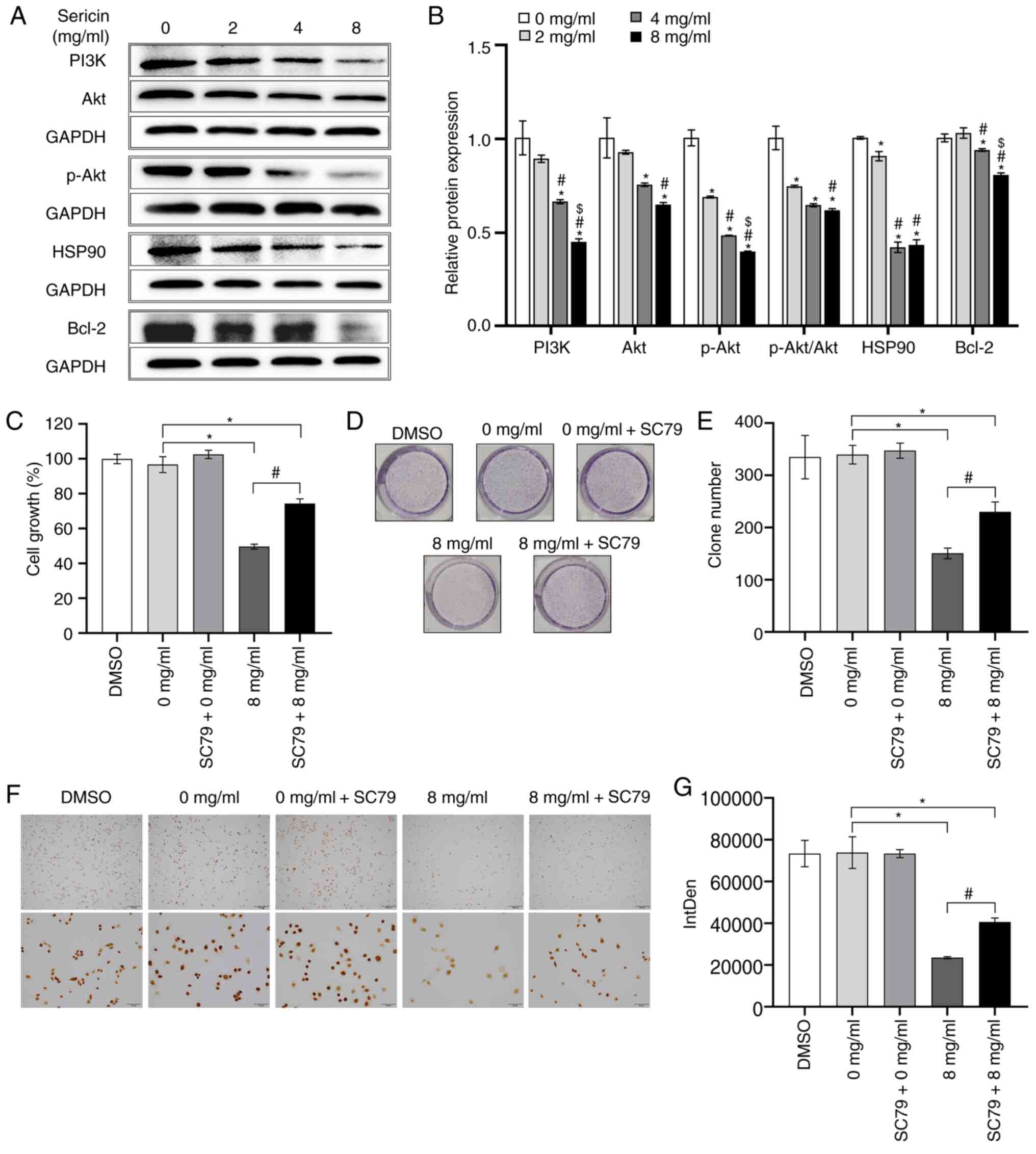
suggested that, after treatment of sericin, the protein expression
levels of PI3K (F=35.494; P<0.001), Akt (F=11.175; P=0.003),
p-Akt (F=260.046; P<0.001), HSP90 (F=398.631; P<0.001) and
Bcl-2 (F=92.422; P<0.001) were significantly decreased compared
with the cells treated with 0 mg/ml sericin (Fig. 7A and B). Additionally, the ratio of
p-Akt to total Akt was calculated and sericin treatment
significantly decreased the p-Akt/Akt ratio (F=47.365;
P<0.001).
Subsequently, the AKT agonist SC79 (10 µM; HY-18749;
MedChemExpress) was used to treat the cells for 2 h, following
sericin treatment. As demonstrated by the MTT assay, the viability
of the MDA-MB-468 cells treated with 8 mg/ml sericin in combination
with SC79 was significantly higher compared with that of the cells
treated with 8 mg/ml sericin alone (P<0.001; Fig. 7C). Moreover, the viability of these
two groups was significantly lower compared with that of the cells
treated with 0 mg/ml sericin (P<0.001). A decrease in sericin (8
mg/ml)-mediated growth inhibition was also identified in MDA-MB-468
cells after co-treatment with SC79, according to the colony
formation assay (P=0.002; Fig. 7D and
E). Similar results were observed for the expression levels of
Ki67 as examined via immunocytochemistry staining (P=0.011;
Fig. 7F and G). These findings
suggested that SC79 partially restored the sericin-induced
suppression of MDA-MB-468 cell viability. Collectively, these
results indicated that sericin modulated MDA-MB-468 cell
proliferation by suppressing the PI3K/Akt signaling pathway.
Discussion
Sericin is a major constituent of silk, produced by
silkworms and is widely used in researches of biology and medicine
(27). An increasing number of
studies have reported that sericin is a potent natural antioxidant,
which has been shown to exert anticoagulation activity,
anti-inflammatory activity, low immunogenicity and excellent
biocompatibility (11,28,29).
Despite these findings, little is known regarding the activity and
underlying mechanisms of sericin in tumor pathogenesis. The present
results demonstrated that sericin significantly suppressed TNBC
cell proliferation, induced G0/G1 cell cycle
arrest and promoted cell apoptosis by inhibiting the PI3K/Akt
signaling pathway.
Cancer types are characterized by their abilities to
sustain proliferation. Cancer cells, by controlling the
proliferating and cell cycle signals, become masters of their own
destinies (30). The present
results suggested that the TNBC cell lines MDA-MB-468 and
MDA-MB-453, but not the non-TNBC cell line MCF-7, were highly
sensitive to the exposure of sericin, which exhibited the growth
inhibiting function. It has been proposed that the proliferation of
MCF-7 cells could be suppressed by sericin (21). In total, three types of
alkali-degraded sericin were selected in a study by Kumar and
Mandal; however, only the Antheraea assamensis-derived
sericin exhibited anti-proliferative abilities in MCF-7 cells. This
may be due to fact that the properties of sericin are closely
correlated with the extraction methods and places of origin
(21). Along with the
aforementioned studies that have examined the effects of sericin on
TNBC cell viability, Guo et al (15) revealed that the sericin treatment
significantly inhibited the human gastric cancer MKN45 cell
proliferation and induced cell cycle arrest both in vitro
and in nude mice. Moreover, the present results suggested that
sericin exhibited specific antitumor properties in TNBC cell lines,
with no toxicity to the normal breast epithelial cell line MCF-10A,
which was consistent with the findings from Kumar and Mandal
(21), which indicated that sericin
could not disrupt the cellular membrane integrity, and cause little
toxicity in non-tumorigenic epithelial (MCF-10) cells and
keratinocyte (HaCaT) cells. Therefore, the role of sericin on TNBC
cells warrants further investigation.
The present results demonstrated that sericin
effectively induced MDA-MB-468 cell cycle arrest at the
G0/G1 phase. The cell cycle is regulated at
two transition points, known as the G1/S and
G2/M points (31).
Cyclin D1 can form a complex with Cdk4 to control the
G1/S biological processes (32). E2F3 is a transcriptional activator
from the E2F family, which positively regulates the transition from
the G1 to S phase (33).
Moreover, P21 and P27, as Cip/Kip family members, share a conserved
N-terminal domain that mediates the binding to Cyclins and Cdks,
thereby affecting and regulating the cell cycle (34). The present results indicated that
the expression levels of P21 and P27 were elevated after sericin
treatment. Additionally, P21, a typical cell cycle inhibitor,
serves diverse roles in tumor cells, depending on its intracellular
localization. In the nucleus, P21 acts as a potent inhibitor of
cellular proliferation, which abolishes the tumor-suppressive role,
but this factor promotes tumor growth when in the cytosol of cancer
cells (35,36). Sericin effectively led to a marked
accumulation of P21 in the nucleus. Collectively, it was suggested
that sericin could inhibit the TNBC cell proliferation and induce
the cell cycle arrest.
Evading apoptosis is one of the hallmarks of cancer
cells. Cells may achieve this function by increasing the expression
levels of antiapoptotic regulators (such as Bcl-2), and/or
downregulating the pro-apoptotic factors, such as Bax and Bak
(30). The present results
demonstrated that sericin promoted the apoptotic process in the
MDA-MB-468 cells. Sericin increased the number of early and late
apoptotic cells, as well as upregulated Bax and downregulated Bcl-2
expression levels. In line with the current findings, Kaewkorn
et al (16) reported that
sericin promoted the apoptosis of human colon cancer SW480 cells,
with increased caspase-3 activity and decreased Bcl-2 expression.
Additionally, Kumar and Mandal (21) revealed that the silk sericin can
lead to the apoptosis of tongue carcinoma SAS cells, by regulating
the expression levels of Cyto-C and Caspase-3. Mitochondrial
membrane permeability is increased, and Cyto-C is released from
mitochondria to cytoplasm, following the exposure to sericin
(21). The present results
demonstrated that the protein expression levels of Cyto-C were
significantly elevated, suggesting that the pro-apoptotic effects
of sericin could be achieved by activating the mitochondrial signal
pathway. All these findings indicate the pro-apoptotic properties
of sericin in the MDA-MB-468 cells.
In the current study, the mechanism of
sericin-induced proliferation inhibition of TNBC cells was also
investigated. TNBC is a subtype of breast cancer with an aggressive
phenotype, which leads to very poor prognosis for metastatic
diseases, and has limited treatment options (37). Several researchers have studied the
key sites, hub genes and/or specific prognostic biomarkers of TNBC,
comparing them with non-TNBC, based on bioinformatic analysis
(38,39). den Hollander et al (40) proposed that protein tyrosine
phosphatase 4A3 could serve as an independent prognostic indicator
in TNBC, compared with ER-positive breast cancer, according to the
Affymetrix microarray analysis. However, few studies have been
reported concerning the bioinformatics examination of key pathways
between TNBC tissues and normal mammary tissues.
In the present study, two gene expression profiles,
including the TNBC and normal mammary tissue samples, were obtained
from the GEO database. Differentially expressed genes were screened
out, and the pathway analysis identified that the PI3K/Akt
signaling pathway was most significantly affected in TNBC. Based on
these findings, the effects of sericin on the PI3K/Akt signaling
pathway were investigated. In addition, other related proteins in
the PI3K/Akt pathway were detected, including HSP90 and Bcl-2. It
was found that HSP90 and Bcl-2 were associated with the overall
survival of patients with breast cancer. HSP90 is a molecular
chaperone that supports the stability of the client proteins,
including the Akt and FKBP prolyl isomerase 4, which has been
considered to be responsible for multiple malignant tumors, such as
breast cancer and prostate cancer (41–43).
The present results indicated that when sericin exerted its
antitumor effects, the PI3K/Akt signaling pathway was inhibited and
HSP90 activity was suppressed. These findings suggested that the
potential antitumor role of sericin may be achieved, at least
partially, by suppressing the PI3K/Akt pathway in TNBC.
In conclusion, the present study demonstrated that
sericin, a natural water-soluble protein with excellent drug-like
characteristics, was able to suppress cell proliferation, induce
G0/G1 cell cycle arrest and promote cell
apoptosis in TNBC MDA-MB-468 cells. It was suggested that sericin
may exert its biological functions by inhibiting the PI3K/Akt
signaling pathway. However, the lack of multiple TNBC cell lines to
assess these findings was a limitation of the present study, which
remains to be examined in the future. Collectively, the present
findings may provide evidence and inspire novel ideas for the
investigation of TNBC.
Acknowledgements
Not applicable.
Funding
The research was funded by National Natural Science
Foundation of China (grant nos. 81441133 and 81703001), Natural
Science Foundation of Hebei Province (grant no. H2013406115) and
Chengde Medical University Scientific Research Major Projects
(grant no. KY2020005).
Availability of data and materials
All data generated or analyzed during this present
study are included in this article. The GSE112825 and GSE76124
datasets analyzed for this study can be found in GEO database
(https://www.ncbi.nlm.nih.gov/geo/).
Authors' contributions
LN performed most of the experiments. SY and LL
analyzed the experimental results. XZ and XL performed experiments
and acquired data. LS and MW participated in the western blot
analysis. LC helped with bioinformatics analysis. ZC and YQ
designed and directed the study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar P and Aggarwal R: An overview of
triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim A, Jang MH, Lee SJ and Bae YK:
Mutations of the epidermal growth factor receptor gene in
triple-negative breast cancer. J Breast Cancer. 20:150–159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Z, Li L, Du P, Ma L, Zhang W, Zheng
L, Lan B, Zhang B, Ma F, Xu B, et al: Transcriptional
downregulation of miR-4306 serves as a new therapeutic target for
triple negative breast cancer. Theranostics. 9:1401–1416. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abramson VG and Mayer IA: Molecular
heterogeneity of triple negative breast cancer. Curr Breast Cancer
Rep. 6:154–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abramson VG, Lehmann BD, Ballinger TJ and
Pietenpol JA: Subtyping of triple-negative breast cancer:
Implications for therapy. Cancer. 121:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pandey MK, Gupta SC, Nabavizadeh A and
Aggarwal BB: Regulation of cell signaling pathways by dietary
agents for cancer prevention and treatment. Semin Cancer Biol.
46:158–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao TT and Zhang YQ: Processing and
characterization of silk sericin from bombyx mori and its
application in biomaterials and biomedicines. Mater Sci Eng C Mater
Biol Appl. 61:940–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang YQ, Tao ML, Shen WD, Zhou YZ, Ding
Y, Ma Y and Zhou WL: Immobilization of L-asparaginase on the
microparticles of the natural silk sericin protein and its
characters. Biomaterials. 25:3751–3759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasmin C, Otoi T, Setiadi MA and Karja NW:
Maturation and fertilisation of sheep oocytes cultured in
serum-free medium containing silk protein sericin. Acta Vet Hung.
63:110–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okazaki Y, Kakehi S, Xu Y, Tsujimoto K,
Sasaki M, Ogawa H and Kato N: Consumption of sericin reduces serum
lipids, ameliorates glucose tolerance and elevates serum
adiponectin in rats fed a high-fat diet. Biosci Biotechnol Biochem.
74:1534–1538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suktham K, Koobkokkruad T, Wutikhun T and
Surassmo S: Efficiency of resveratrol-loaded sericin nanoparticles:
Promising bionanocarriers for drug delivery. Int J Pharm.
537:48–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song CJ, Fu XM, Li J and Chen ZH: Effects
of sericine on TGF-beta1/Smad3 signal pathway of diabetic
mephropathy rats kidney. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
27:102–105. 2011.(In Chinese). PubMed/NCBI
|
|
13
|
Chen Z, He Y, Song C, Dong Z, Su Z and Xue
J: Sericin can reduce hippocampal neuronal apoptosis by activating
the Akt signal transduction pathway in a rat model of diabetes
mellitus. Neural Regen Res. 7:197–201. 2012.PubMed/NCBI
|
|
14
|
Song CJ, Yang ZJ, Tang QF and Chen ZH:
Effects of sericin on the testicular growth hormone/insulin-like
growth factor-1 axis in a rat model of type 2 diabetes. Int J Clin
Exp Med. 8:10411–10419. 2015.PubMed/NCBI
|
|
15
|
Guo WH, Chen ZY, Chen H, Lin T, Zhao ML,
Liu H, Yu J, Hu YF and Li GX: Sericin regulates proliferation of
human gastric cancer MKN45 cells through autophagic pathway. Nan
Fang Yi Ke Da Xue Xue Bao. 38:148–154. 2018.(In Chinese).
PubMed/NCBI
|
|
16
|
Kaewkorn W, Limpeanchob N, Tiyaboonchai W,
Pongcharoen S and Sutheerawattananonda M: Effects of silk sericin
on the proliferation and apoptosis of colon cancer cells. Biol Res.
45:45–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki M, Kato N, Watanabe H and Yamada H:
Silk protein, sericin, suppresses colon carcinogenesis induced by
1,2-dimethylhydrazine in mice. Oncol Rep. 7:1049–1052.
2000.PubMed/NCBI
|
|
18
|
Zhaorigetu S, Sasaki M, Watanabe H and
Kato N: Supplemental silk protein, sericin, suppresses colon
tumorigenesis in 1,2-dimethylhydrazine-treated mice by reducing
oxidative stress and cell proliferation. Biosci Biotechnol Biochem.
65:2181–2186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhaorigetu S, Yanaka N, Sasaki M, Watanabe
H and Kato N: Inhibitory effects of silk protein, sericin on
UVB-induced acute damage and tumor promotion by reducing oxidative
stress in the skin of hairless mouse. J Photochem Photobiol B.
71:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhaorigetu S, Yanaka N, Sasaki M, Watanabe
H and Kato N: Silk protein, sericin, suppresses DMBA-TPA-induced
mouse skin tumorigenesis by reducing oxidative stress, inflammatory
responses and endogenous tumor promoter TNF-alpha. Oncol Rep.
10:537–543. 2003.PubMed/NCBI
|
|
21
|
Kumar JP and Mandal BB: Silk sericin
induced pro-oxidative stress leads to apoptosis in human cancer
cells. Food Chem Toxicol. 123:275–287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santucci-Pereira J, Zeleniuch-Jacquotte A,
Afanasyeva Y, Zhong H, Slifker M, Peri S, Ross EA, López de Cicco
R, Zhai Y, Nguyen T, et al: Genomic signature of parity in the
breast of premenopausal women. Breast Cancer Res. 21:462019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burstein MD, Tsimelzon A, Poage GM,
Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK,
Hilsenbeck SG, Chang JC, et al: Comprehensive genomic analysis
identifies novel subtypes and targets of triple-negative breast
cancer. Clin Cancer Res. 21:1688–1698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edgar R, Domrachev M and Lash AE: Gene
expression omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci.
28:1947–1951. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kunz RI, Brancalhão RM, Ribeiro LF and
Natali MR: Silkworm sericin: Properties and biomedical
applications. Biomed Res Int. 2016:81757012016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jena K, Pandey JP, Kumari R, Sinha AK,
Gupta VP and Singh GP: Tasar silk fiber waste sericin: New source
for anti-elastase, anti-tyrosinase and anti-oxidant compounds. Int
J Biol Macromol. 114:1102–1108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aramwit P, Kanokpanont S, De-Eknamkul W
and Srichana T: Monitoring of inflammatory mediators induced by
silk sericin. J Biosci Bioeng. 107:556–561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Purdy A, Uyetake L, Cordeiro MG and Su TT:
Regulation of mitosis in response to damaged or incompletely
replicated DNA require different levels of Grapes (Drosophila
Chk1). J Cell Sci. 118:3305–3315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
John RR, Malathi N, Ravindran C and
Anandan S: Mini review: Multifaceted role played by cyclin D1 in
tumor behavior. Indian J Dent Res. 28:187–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roninson IB: Oncogenic functions of tumour
suppressor p21(Waf1/Cip1/Sdi1): Association with cell senescence
and tumour-promoting activities of stromal fibroblasts. Cancer
Lett. 179:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki A, Tsutomi Y, Miura M and Akahane
K: Caspase 3 inactivation to suppress Fas-mediated apoptosis:
Identification of binding domain with p21 and ILP and inactivation
machinery by p21. Oncogene. 18:1239–1244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zagorac I, Fernandez-Gaitero S, Penning R,
Post H, Bueno MJ, Mouron S, Manso L, Morente MM, Alonso S, Serra V,
et al: In vivo phosphoproteomics reveals kinase activity profiles
that predict treatment outcome in triple-negative breast cancer.
Nat Commun. 9:35012018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Han Y, Huang H, Min L, Qu L and
Shou C: Integrated analysis of expression profiling data identifies
three genes in correlation with poor prognosis of triple-negative
breast cancer. Int J Oncol. 44:2025–2033. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bao C, Lu Y, Chen J, Chen D, Lou W, Ding
B, Xu L and Fan W: Exploring specific prognostic biomarkers in
triple-negative breast cancer. Cell Death Dis. 10:8072019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
den Hollander P, Rawls K, Tsimelzon A,
Shepherd J, Mazumdar A, Hill J, Fuqua SA, Chang JC, Osborne CK,
Hilsenbeck SG, et al: Phosphatase PTP4A3 promotes triple-negative
breast cancer growth and predicts poor patient survival. Cancer
Res. 76:1942–1953. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neckers L and Workman P: Hsp90 molecular
chaperone inhibitors: Are we there yet? Clin Cancer Res. 18:64–76.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Y, Peng S, Wang J, Chen H, Cong X, Chen
A, Hu M, Qin M, Wu H, Gao S, et al: Ailanthone targets p23 to
overcome MDV3100 resistance in castration-resistant prostate
cancer. Nat Commun. 7:131222016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mangé A, Coyaud E, Desmetz C, Laurent E,
Béganton B, Coopman P, Raught B and Solassol J: FKBP4 connects
mTORC2 and PI3K to activate the PDK1/Akt-dependent cell
proliferation signaling in breast cancer. Theranostics.
9:7003–7015. 2019. View Article : Google Scholar : PubMed/NCBI
|