Introduction
Renal damage is common in the end-stage of numerous
types of disease, such as diabetic nephropathy and hypertension
nephropathy (1,2). Therefore, the identification of
effective methods that can attenuate this condition is a major
focus of investigations. Angiotensin II (AngII) exerts a
vasoconstrictor effect, which may contribute to the onset and
progression of renal damage (3,4). In
addition, AngII may directly accelerate renal damage by recruiting
inflammatory cells and by sustaining cell proliferation and
fibrosis (5,6). Several studies have revealed that
AngII induces pathological responses in ischemia-reperfusion (I/R)
injury, exhibiting tubular epithelial cell necrosis, cellular
failure and increased permeability (7,8).
Moreover, accumulating evidence has suggested that autophagy serves
an important role in renal damage (9,10).
However, whether the induction of autophagy contributes to cell
survival or cell death remains unclear.
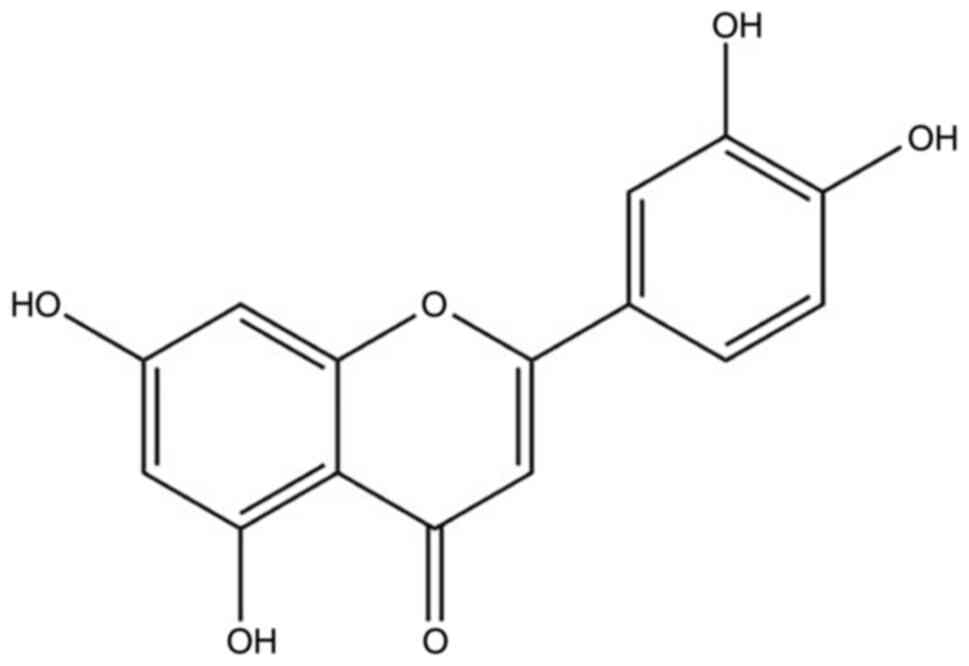
Luteolin (Lut; 3′,4′,5,7-tetrahydroxyflavone) is
widely found in vegetables, fruits and plants. For instance,
dietary products, such as carrots, cabbage, celery, olive oil,
peppermint and apples are good sources of Lut (11). The molecular formula of Lut is
C15H10O6 and its structure is
presented in Fig. 1 (12,13).
It has been reported that a variety of traditional Chinese
medicines, such as Chrysanthemum, Herba Ajugae and
honey-suckle contain excess amounts of Lut (14). Accumulating evidence has suggested
that Lut exhibits anti-inflammatory, anti-fibrotic and anti-tumor
effects (14–17). Previous studies have also reported
that Lut can protect against renal I/R injury, lipopolysaccharide
(LPS)-induced acute renal injury and cisplatin-induced
nephrotoxicity (13,14,18,19).
However, to the best of our knowledge, the role of Lut treatment on
AngII-induced renal damage in apolipoprotein E-deficient
(ApoE−/−) mice has not been previously examined.
Therefore, in the present study, the effects of Lut were
investigated in AngII-induced renal damage in ApoE−/−
mice.
Materials and methods
Animal studies
According to the previous research,
ApoE−/− mice were selected as the experimental animals
(20,21). ApoE−/− mice (n=18) were
provided from Beijing Vital River Lab Animal Technology Co., Ltd.
All the ApoE−/− mice had free access to feed on a
conventional and standard mice chow diet with clean water, and were
caged under constant conditions, including a 12-h dark/light cycle,
and 40–60% humidity at 24°C. At 8 weeks of age, male mice (23.7±0.9
g) were randomly divided into three groups as follows: i) Control
group (n=6); ii) AngII group (n=6); and iii) AngII + Lut group
(n=6). Lut (cat. no. MB2172; Dalian Meilun Biology Technology Co.,
Ltd.) was administered by gavage (100 mg/kg/d) (8,22).
ApoE−/− mice were implanted with Alzet osmotic minipumps
(Model2004; Durect Corporation), filled with either saline vehicle
or AngII solution (1,000 ng/kg/min) for a maximum period of 4 weeks
(23,24). Each group of mice was subjected to
its respective treatment for 4 weeks. The health and behaviour of
the mice were monitored every day, and after 4 weeks, all mice were
alive. The mice were anesthetized with a high dose of pentobarbital
(100 mg/kg, intraperitoneally), blood samples (0.5–0.6 ml) were
collected from the abdominal aorta, and then the mice were
euthanized via cervical dislocation. The cessation of respiration
and heartbeat indicated the death of the mice. Kidney tissues were
harvested for further analysis. Serum samples were subsequently
stored at −80°C until further use. The kidneys were frozen in
liquid nitrogen for mRNA, histopathological and immunoblotting
analyses. The current study was performed in accordance with the
Guide for the Care and Use of Laboratory Animals (National
Institutes of Health) (25) and was
approved by the Ethics Committee of Dalian Municipal Central
Hospital Affiliated of Dalian Medical University.
Biochemical measurements
Serum concentrations of creatinine (CRE) were
measured using an ELISA kit (cat. no. C011-2-1; Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocols.
Hematoxylin and eosin (H&E)
staining
The kidney tissues at room temperature were fixed in
10% buffered formalin solution and subsequently dehydrated in 75%
ethanol overnight, followed by paraffin embedding. Serial sections
(thickness, 4 µm) were subjected to hematoxylin staining for 15 min
and eosin staining for 5 min at room temperature to assess the
pathological changes. Renal injury scores were determined by two
blinded researchers according to the extent of the kidney injury,
as previously described (13,26).
The score grading was mainly based on the presence or absence of
hemorrhage, tubular cell necrosis, tubular dilatation and
cytoplasmic vacuole formation. The grading system was scored as
follows: i) 0, normal kidney; ii) 1, 0–5% injury (minimal damage);
iii) 2, 5–25% injury (mild damage); iv) 3, 25–75% injury (moderate
damage); and v) 4, 75–100% injury (severe damage). All sections
were examined using a BX40 upright light microscope (Olympus BX43;
Olympus Corporation; scale bar, 100 µm).
Masson's trichrome staining
Kidney tissues from each group were at room
temperature stored in 10% formalin, dehydrated in an ascending
series of alcohol (75, 85, 90 and 100%; 5 min each) and finally
embedded in paraffin wax. Then, 4 µm-thick paraffin sections were
sliced from these paraffin-embedded tissue blocks. The tissue
sections were subsequently deparaffinized via immersion in xylene
(3 times; 5 min each) and rehydrated using a descending series of
alcohol (100, 90, 85 and 75%; 5 min each). Biopsy samples were
stained at room temperature using Masson's trichrome stain to
investigate changes in the kidney morphology and fibrosis. Blue
staining represented collagen accumulation. All sections were
examined using a BX40 upright light microscope (Olympus BX43;
Olympus Corporation).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted with TransZol Up Plus
RNA Kit (cat. no. ER501-01; TransGen Biotech Co., Ltd.), and then
RNA concentration and purity were measured using ISOGEN (cat. no.
317-02503; Nippon Gene Co., Ltd.). cDNA was synthesized using a
first-strand cDNA synthesis kit (SuperScript VILO cDNA synthesis
kit; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. RT-qPCR was performed using fluorescent
SYBR-Green technology (Light Cycler; Roche Molecular Diagnostics).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 20 sec; followed by 45 cycles of 60°C for
30 sec and 72°C for 60 sec; and a cooling step at 4°C. The relative
expression of the target genes was normalized to that of β-actin.
The relative gene expression was determined using the
2−ΔΔCq method (27). The
primer sequences used for amplification are listed in Table I.
 | Table I.Primer oligonucleotide sequences. |
Table I.
Primer oligonucleotide sequences.
| Gene | Primer
sequences |
|---|
| IL-1β | F:
5′-TGCCACCTTTTGACAGTGAT-3′ |
|
| R:
5′-TGTGCTGCTGCGAGATTTGA-3′ |
| IL-6 | F:
5′-TACCAGTTGCCTTCTTGGGACTGA-3′ |
|
| R:
5′-TAAGCCTCCGACTTGTGAAGTGGT-3′ |
| TNF-α | F:
5′-TCTCATGCACCACCATCAAGGACT-3′ |
|
| R:
5′-ACCACTCTCCCTTTGCAGAACTCA-3′ |
| β-actin | F:
5′-CGATGCCCTGAGGGTCTTT-3′ |
|
| R:
5′-TGGATGCCACAGGATTCCAT-3′ |
Western blot analysis
Total protein was extracted from kidney tissues
using RIPA lysis buffer (cat. no. R0020; Beijing Solarbio Science
& Technology Co., Ltd.), according to the manufacturer's
protocols. Kidney tissue total protein concentrations were
determined using a BCA Protein assay reagent kit (cat. no.
DQ111-01; Beijing Transgen Biotech Co., Ltd.). Protein samples (30
µg per lane) were separated by SDS-PAGE on 10–15% gels, and were
subsequently transferred to PVDF membranes. Subsequently, membranes
were blocked in Tris-buffered saline with 0.1% Tween-20 (TBST)
containing 5% skimmed milk, and then incubated at room temperature
for 2 h. The membranes were then incubated with primary antibody
diluent (cat. no. P0023A; Beyotime Institute of Biotechnology) and
lightly shaken overnight at 4°C. Primary antibody rabbit against
collagen I (cat. no. 14695-1-AP; 1:1,000; ProteinTech Group, Inc.),
collagen III (cat. no. 22734-1-AP; 1:1,000; ProteinTech Group,
Inc.), microtubule associated protein 1 light chain 3α (LC3; cat.
no. 14600-1-AP; 1:1,000; ProteinTech Group, Inc.), p62 (cat. no.
55274-1-AP; 1:1,000; ProteinTech Group, Inc.) and β-actin (cat. no.
20536-1-AP; 1:1,000; ProteinTech Group, Inc.) was performed.
Briefly, the kidney tissues were harvested, and the protein
extracts were prepared according to established methods. The
extracts were separated via SDS-PAGE (8–15%) and transferred to a
PVDF membrane (EMD Millipore). The membranes were blocked overnight
with 5% milk blocking reagent at 4°C. The proteins were visualized
by the addition of the respective antibodies, followed by the
incubation with species-specific peroxidase-labeled secondary
antibodies (cat. no. 7074; anti-rabbit IgG; 1:2,000; Cell Signaling
Technology, Inc.) for 1 h. β-actin was used as a control of the
equal protein loading. The protein levels were expressed as
protein/β-actin ratios to minimize loading Chemiluminescence
reagent (ProteinTech Group, Inc.) was used to visualize bands. This
analysis was performed three times independently. The intensity of
the bands was semi-quantified using ImageJ software version 1.8.0
(National Institutes of Health).
Immunohistochemical analysis
Kidney tissues were fixed in 10% buffered formalin
solution for 30 min at room temperature and dehydrated in 75%
ethanol overnight, followed by paraffin embedding.
Immunohistochemical analysis was performed using the SP-9001 SPlink
Detection kits (OriGene Technologies, Inc.), according to the
manufacturers' instructions. Paraffin-embedded sections (4 µm) were
deparaffinized with xylene and subsequently rehydrated in a
descending series of ethanol washes (100, 90, 85 and 75%; 5 min
each). The sections were treated for 15 min at 98°C with 3%
H2O2 in methanol to inactivate the endogenous
peroxidase activity and were subsequently incubated at room
temperature for 1 h with primary antibodies rabbit against collagen
I (cat. no. 14695-1-AP; 1:200; ProteinTech Group, Inc.), collagen
III (cat. no. 22734-1-AP; 1:200; ProteinTech Group, Inc.), LC3
(cat. no. 14600-1-AP; 1:200; ProteinTech Group, Inc.) and p62 (cat.
no. 55274-1-AP; 1:200; ProteinTech Group, Inc.). The secondary
antibody from SPlink Detection kits (OriGene Technologies, Inc.)
was incubated with the tissue for 30 min at room temperature. All
sections were examined using a BX40 upright light microscope
(Olympus BX43; Olympus Corporation). For each staining, 3×7
sections (6 mice) per group were analyzed and the representative
images were presented. All image analyses were performed by a
blinded reviewer.
Statistical analysis
All data are presented as the mean ± SEM, and all
experiments were repeated at least three times. SPSS version 19.0
software (IBM Corp.) was used for statistical analysis. The
Kolmogorov-Smirnov test was used to detect the normality of all
data. Inter-group variation was measured using a one-way ANOVA,
followed by individual comparisons with a Tukey's tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
Lut increases CRE concentration and
improves renal function
The kidney/body weight ratio did not differ among
the three groups. The metabolic characteristics of
ApoE−/− mice from the three groups following 4 weeks of
treatment are presented in Table
II. The CRE levels in the AngII-induced ApoE−/− mice
were increased by 198% compared with those of the control group
(93.21±6.61 vs. 31.24±3.73 µmol/l; P<0.05), indicating a
reduction of renal function. The CRE levels of the AngII + Lut
group were markedly decreased by 60.5% following the treatment of
the mice with Lut compared with those in the AngII group
(36.86±4.53 vs. 93.21±6.61 µmol/l; P<0.05). No difference was
noted between the AngII + Lut and control groups.
 | Table II.Metabolic data from the three groups
after 4 weeks of treatment. |
Table II.
Metabolic data from the three groups
after 4 weeks of treatment.
| Groups | Control (n=6) | AngII (n=6) | AngII + Lut
(n=6) |
|---|
| Body weight, g | 26.12±2.60 | 25.33±1.06 | 26.43±0.81 |
| Kidney/body weight
ratio, mg/g | 6.80±1.45 | 6.23±0.54 | 6.31±0.22 |
| Creatinine,
µmol/l |
31.24±3.73a | 93.21±6.61 |
36.86±4.53a |
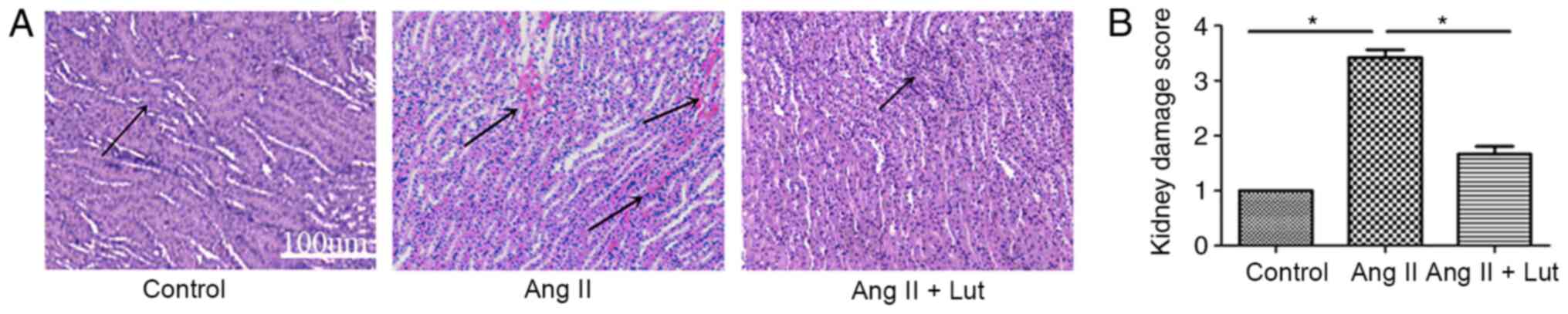
Lut improves the histopathological
characteristics of the AngII-treated kidney tissues
To evaluate the histopathological damage in the
kidney tissue, H&E staining was performed (Fig. 2A). The AngII + Lut group had
markedly reduced inflammatory cell infiltration in the kidney
tissues compared with that observed in the AngII group. These
results suggested that Lut decreased inflammatory cell infiltration
in the AngII-induced renal damage of ApoE−/− mice. Renal
injury scoring (Fig. 2B)
demonstrated that Lut treatment could significantly decrease renal
injury caused by AngII in mice, which was consistent with the
H&E results.
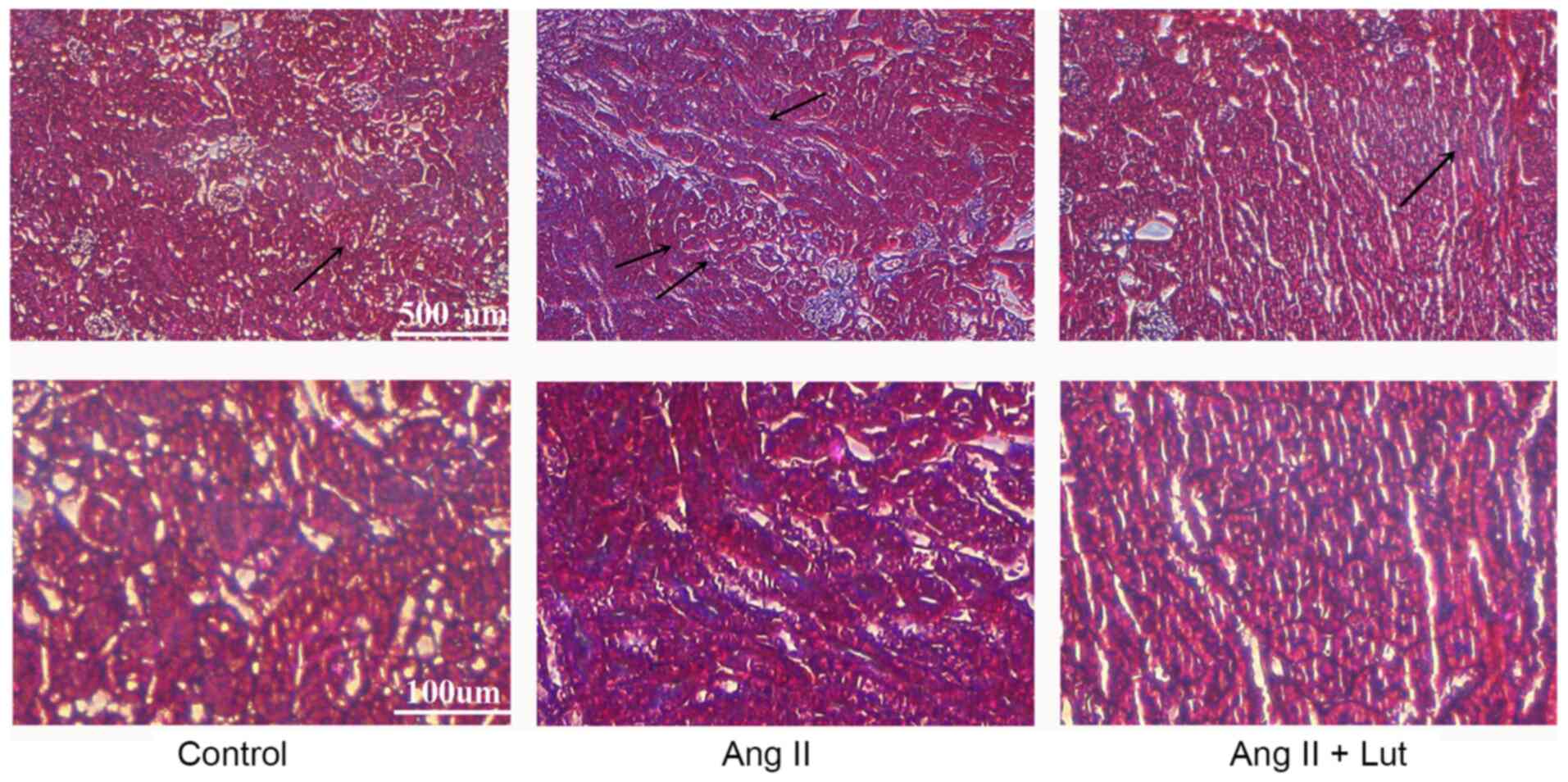
Lut inhibits collagen deposition in
AngII-treated kidney tissues
Masson's trichrome staining indicated that collagen
deposition was significantly increased in the interstitium and the
perivascular and subvascular areas, suggesting that AngII induced
renal interstitial fibrosis. Lut treatment markedly attenuated the
AngII-induced collagen deposition (Fig.
3). Taken together, these data demonstrated that Lut attenuated
renal fibrosis in AngII-induced kidney tissues.
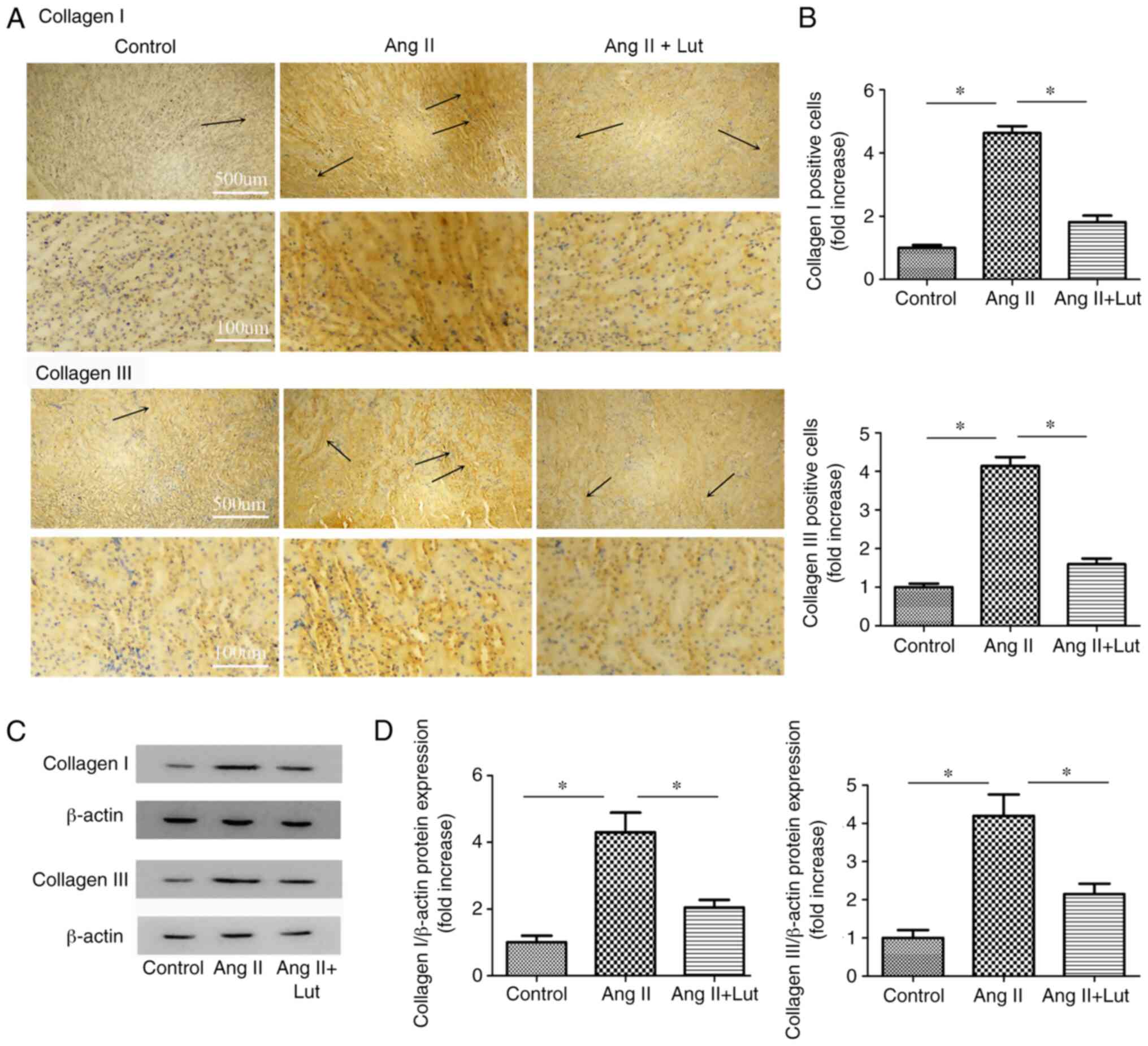
Lut inhibits collagen I and III
protein expression levels in AngII-treated kidney tissues
Collagen I and collagen III (Fig. 4A) immunostaining was performed. The
AngII + Lut group exhibited markedly decreased collagen I and III
expression levels in the kidney tissues compared with those
observed in the AngII group (Fig.
4B). The expression levels of collagen I and III proteins in
the kidney tissues were also determined via western blotting
(Fig. 4C). The results indicated
that the protein expression levels of collagen I and III in the
mice of the AngII + Lut group were significantly suppressed
compared with those in the AngII group (Fig. 4D).
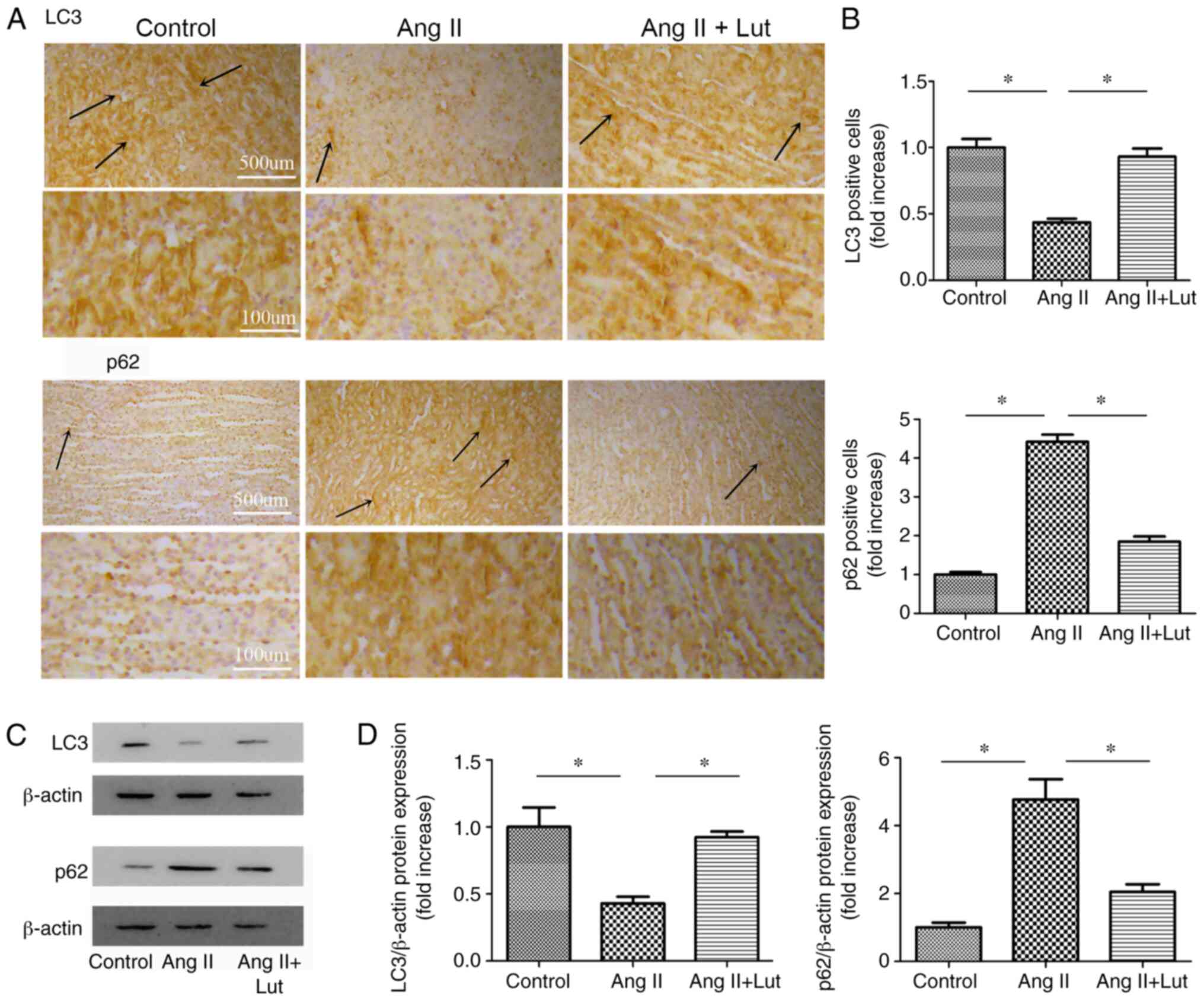
Lut attenuates autophagy in
AngII-treated kidney tissues
To evaluate the expression levels of LC3 and p62 in
kidney tissues, LC3 and p62 (Fig.
5A) immunostaining was performed. The AngII + Lut group
exhibited markedly increased LC3 and decreased p62 expression
levels in the kidney tissues compared with those observed in the
AngII group (Fig. 5B). The protein
expression levels of LC3 and p62 in the kidney tissues were also
determined via western blotting (Fig.
5C). The results demonstrated that LC3 protein expression in
the AngII + Lut group was significantly increased compared with
that in the AngII group, whereas p62 protein expression in the
AngII + Lut group was significantly decreased compared with those
in the AngII group (Fig. 5D). These
results indicated that Lut increased LC3 expression and decreased
p62 expression in ApoE−/− mice with AngII-induced renal
damage.
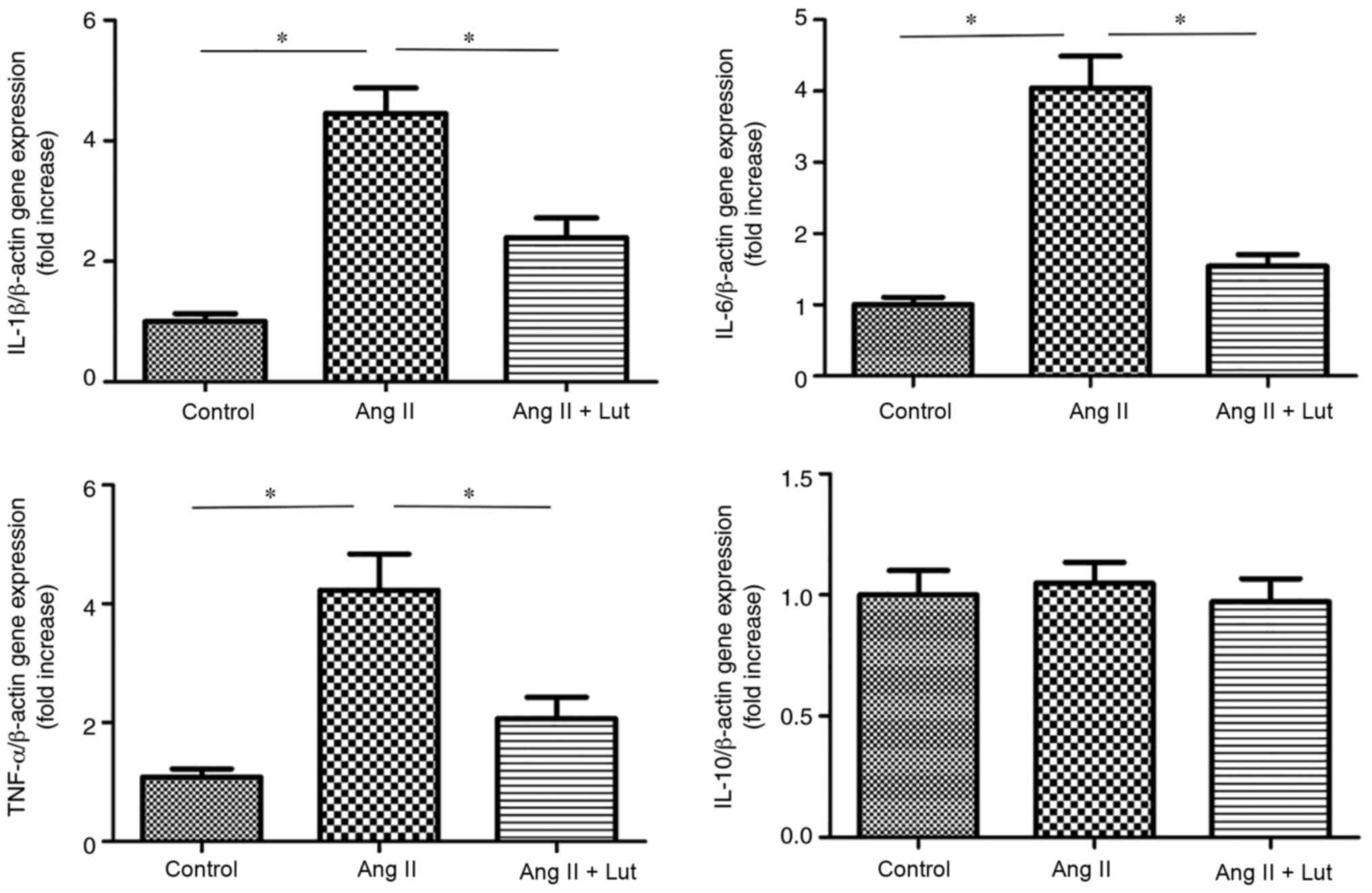
Lut inhibits pro-inflammatory cytokine
expression in AngII-treated kidney tissues
The expression levels of IL-1β, IL-6, TNF-α and
IL-10 were measured using RT-qPCR (Fig.
6). The expression levels of IL-1β, IL-6 and TNF-α were
significantly elevated in the AngII group compared with those of
the control group. However, Lut treatment decreased significantly
the expression levels of IL-1β, IL-6 and TNF-α compared with the
AngII group. The expression levels of IL-10 did not show these
changes.
Discussion
In the present study, the protective effects of Lut
were examined using a model of AngII-induced nephropathy in
ApoE−/− mice. The results suggested that Lut attenuated
AngII-induced collagen deposition and inflammation. These processes
were accompanied with the induction of autophagy.
The AngII group in ApoE−/− mice had a
marked increase in CRE levels, whereas this parameter was notably
decreased in the AngII + Lut group. No significant differences were
identified between the AngII + Lut and control groups. This
indicated that Lut attenuated impaired renal function. A number of
studies have reported similar results. For instance, Xin et
al (14) revealed that CRE
levels were increased 2.10-fold in LPS-treated mice compared with
those of normal mice. In addition, CRE levels were significantly
decreased by 38.5% following Lut treatment compared with the
LPS-treated mice (14). However,
the Lut group did not differ with regard to renal function indices
compared with the control group. Hong et al (13) also demonstrated that Lut treatment
resulted in significantly reduced serum levels of CRE in I/R
mice.
Collagen deposition was increased significantly in
the interstitium and the perivascular and subvascular areas of
AngII-induced ApoE−/− mice, as determined via Masson's
trichrome staining. Lut treatment significantly attenuated the
AngII-induced collagen deposition. This observation was further
confirmed by protein expression studies. The protective effects of
Lut were mediated by a reduction of the AngII-induced increase in
collagen I and III proteins. These results indicated that Lut
exhibited a therapeutic effect on kidney fibrosis induced by AngII.
Moreover, several studies have reported that Lut decreases the
extent of collagen deposition and fibrosis in other organs, such as
lung, liver and myocardium (12,28,29).
Moreover, Domitrović et al (28) reported that the protective effect of
Lut on CCl4-induced liver fibrosis was mediated by promoting
extracellular matrix degradation and the stimulation of hepatic
regenerative capability.
It has been shown that inflammatory cell
infiltration is a key factor in renal damage (30). The present study demonstrated that
Lut reduced the AngII-induced increase in the expression levels of
inflammatory molecules, including IL-1β, IL-6 and TNF-α. In
addition, Lut decreased inflammatory cell infiltration in
AngII-induced renal damage of ApoE−/− mice, as
determined via H&E staining. Seelinger et al (31) reported similar results, suggesting
that TNF-α, IL-1β and IL-6 were all targets of Lut. In acute
ischemic renal failure, TNF-α is an upstream molecule of the
inflammatory cascade, which can initiate the upregulation of
cytokines and chemokines (32).
IL-1β and IL-6 are downstream molecules in the inflammatory
cascade, which can directly impair kidney function (32). Hashmat et al (33) revealed that IL-6 production may
participate in the development of salt-sensitive hypertension and
end-organ damage by mediating increased infiltration into the
kidney. Furthermore, Hong et al (13) reported that Lut exhibited
anti-inflammatory effects by inhibiting pro-inflammatory cytokine
release. The results were consistent with those observed in the
present study.
The mechanism of autophagy has been investigated in
detail due to its involvement in several diseases, including
tumorigenesis, cardiomyopathy, diabetes, fatty liver, pulmonary,
neurodegenerative and kidney diseases (34–39).
In the present study, autophagy participated in AngII-induced renal
damage of ApoE−/− mice. LC3 and p62 are central
autophagy-related proteins involved in the autophagic flux
(40). Autophagy is a dynamic
cellular catabolic process accompanied by LC3-II conversion from
LC3-I and p62 degradation (41).
LC3-II is regarded as a promising autophagosome marker (42,43).
p62 is a LC3-interacting protein that transports ubiquitinated
protein aggregates to autophagosomes via its association with
LC3-II (44). When autophagy is
impaired, p62 expression is increased in cells and tissues
(9). Schläfli et al
(45) have demonstrated that high
LC3 levels are associated with lower tumor aggressiveness, whereas
p62 expression in general, is significantly associated with
aggressive tumor behavior. Guan et al (10) reported that autophagy reduces the
initiation of the apoptotic process in I/R mice with renal damage.
The authors suggested that the levels of autophagy were increased
earlier than the induction of apoptosis. This effect was noted
after 2 h of reperfusion and reached its maximum level on day 2
(10). Subsequently, autophagy
declined from day 3 when renal damage had been nearly recovered to
normal levels (10). A consistent
trend was noted in the present study. For instance, the p62 protein
levels in the AngII + Lut group were downregulated compared with
those noted in the AngII group, whereas LC3 protein levels in the
AngII + Lut group were upregulated compared with those in the AngII
group. Consistent results were obtained using immunostaining
analysis. The data suggested that Lut protected against
AngII-induced renal damage by inducing autophagy and inhibiting
apoptosis.
In conclusion, the present study demonstrated that
Lut treatment exhibited a protective effect on AngII-induced renal
injury of ApoE−/− mice. This compound protected the
kidney mainly by inhibiting collagen deposition and inflammation,
while it could also induce autophagy. The findings of the present
study may be used in the development of novel strategies for the
prevention and treatment of renal damage. But, this study does not
provide enough in-depth evidence, and thus further experiments and
clinical research are needed to prove the effectiveness and safety
of Lut.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Dalian Medical Science Research Program of China (grant no.
1712009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZNG and HYL designed this study. YSL and QY aided in
performing the experiments. XYL, LL and SL analyzed the data and
interpreted the results of the experiments. ZNG and YSL confirm the
authenticity of all the raw data. SL prepared the figures. QY
drafted the manuscript. XYL and LL aided in the revisions of the
manuscript. HYL provided the research funds. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the ethics
committee of Dalian Municipal Central Hospital Affiliated to Dalian
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Umanath K and Lewis JB: Update on diabetic
nephropathy: Core curriculum 2018. Am J Kidney Dis. 71:884–895.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skov J, Christiansen JS and Poulsen PL:
Hypertension and diabetic nephropathy. Endocr Dev. 31:97–107. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma N, Malek V, Mulay SR and Gaikwad
AB: Angiotensin II type 2 receptor and angiotensin-converting
enzyme 2 mediate ischemic renal injury in diabetic and non-diabetic
rats. Life Sci. 235:1167962019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urushihara M and Kagami S: Role of the
intrarenal renin-angiotensin system in the progression of renal
disease. Pediatr Nephrol. 32:1471–1479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaliappan G, Nagarajan P, Moorthy R, Kalai
Gana Selvi S, Avinash Raj T and Mahesh Kumar J: Ang II induce
kidney damage by recruiting inflammatory cells and up regulates
PPAR gamma and Renin 1 gene: Effect of β carotene on chronic renal
damage. J Thromb Thrombolysis. 36:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly TN, Raj D, Rahman M, Kretzler M,
Kallem RR, Ricardo AC, Rosas SE, Tao K, Xie D, Hamm LL, et al CRIC
Study Investigators, : The role of renin-angiotensin-aldosterone
system genes in the progression of chronic kidney disease: Findings
from the Chronic Renal Insufficiency Cohort (CRIC) study. Nephrol
Dial Transplant. 30:1711–1718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuksal N, Chalker J and Mailloux RJ:
Progress in understanding the molecular oxygen paradox - function
of mitochondrial reactive oxygen species in cell signaling. Biol
Chem. 398:1209–1227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Shi B, Li Y and Zhang H: Protective
Effect of Luteolin Against renal ischemia/reperfusion injury via
modulation of pro-inflammatory cytokines, oxidative stress and
apoptosis for possible benefit in kidney transplant. Med Sci Monit.
23:5720–5727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanida I: Autophagy basics. Microbiol
Immunol. 55:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan X, Qian Y, Shen Y, Zhang L, Du Y, Dai
H, Qian J and Yan Y: Autophagy protects renal tubular cells against
ischemia/reperfusion injury in a time-dependent manner. Cell
Physiol Biochem. 36:285–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imran M, Rauf A, Abu-Izneid T, Nadeem M,
Shariati MA, Khan IA, Imran A, Erdogan Orhan I, Rizwan M, Atif M,
et al: Corrigendum to ‘Luteolin, a flavonoid, as an anticancer
agent: A review’ [Biomed. Pharmacother. 112 (2019) 108612]. Biomed
Pharmacother. 116:1090842019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, Shang P and Li D: Luteolin: A
flavonoid that has multiple cardio-protective effects and its
molecular mechanisms. Front Pharmacol. 8:6922017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong X, Zhao X, Wang G, Zhang Z, Pei H and
Liu Z: Luteolin treatment protects against renal
ischemia-reperfusion injury in rats. Mediators Inflamm.
2017:97838932017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xin SB, Yan H, Ma J, Sun Q and Shen L:
Protective effects of luteolin on lipopolysaccharide-induced acute
renal injury in mice. Med Sci Monit. 22:5173–5180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung J and Lee J: Anti-inflammatory
activity of Butein and Luteolin through suppression of NFκB
activation and induction of heme oxygenase-1. J Med Food.
18:557–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi JS, Islam MN, Ali MY, Kim YM, Park
HJ, Sohn HS and Jung HA: The effects of C-glycosylation of luteolin
on its antioxidant, anti-Alzheimer's disease, anti-diabetic, and
anti-inflammatory activities. Arch Pharm Res. 37:1354–1363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seelinger G, Merfort I, Wölfle U and
Schempp CM: Anti-carcinogenic effects of the flavonoid luteolin.
Molecules. 13:2628–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Domitrović R, Cvijanović O, Pugel EP,
Zagorac GB, Mahmutefendić H and Škoda M: Luteolin ameliorates
cisplatin-induced nephrotoxicity in mice through inhibition of
platinum accumulation, inflammation and apoptosis in the kidney.
Toxicology. 310:115–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang KP, Park SK, Kim DH, Sung MJ, Jung
YJ, Lee AS, Lee JE, Ramkumar KM, Lee S, Park MH, et al: Luteolin
ameliorates cisplatin-induced acute kidney injury in mice by
regulation of p53-dependent renal tubular apoptosis. Nephrol Dial
Transplant. 26:814–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Honjo T, Chyu KY, Dimayuga PC, Lio WM,
Yano J, Trinidad P, Zhao X, Zhou J, Cercek B and Shah PK:
Immunization with an ApoB-100 related peptide vaccine attenuates
angiotensin-II induced hypertension and renal fibrosis in mice.
PLoS One. 10:e01317312015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cha J, Ivanov V, Ivanova S, Kalinovsky T,
Rath M and Niedzwiecki A: Evolution of angiotensin II-mediated
atherosclerosis in ApoE KO mice. Mol Med Rep. 3:565–570.
2010.PubMed/NCBI
|
|
22
|
Xu Y, Zhang J, Liu J, Sai Li, Cheng Li,
Wang W, Ma R and Liu Y: Luteolin attenuate the D-galactose-induced
renal damage by attenuation of oxidative stress and inflammation.
Nat Prod Res. 29:1078–1082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Wang W, Yu H, Zhang Y, Dai Y,
Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, et al: Interleukin
6 underlies angiotensin II-induced hypertension and chronic renal
damage. Hypertension. 59:136–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong J, Guo D, Chen CB, Wang W, Schuster
M, Loibner H, Penninger JM, Scholey JW, Kassiri Z and Oudit GY:
Prevention of angiotensin II-mediated renal oxidative stress,
inflammation, and fibrosis by angiotensin-converting enzyme 2.
Hypertension. 57:314–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. 8th edition. National Academies Press; Washington, DC:
2011
|
|
26
|
Shingu C, Koga H, Hagiwara S, Matsumoto S,
Goto K, Yokoi I and Noguchi T: Hydrogen-rich saline solution
attenuates renal ischemia-reperfusion injury. J Anesth. 24:569–574.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Domitrović R, Jakovac H, Tomac J and Sain
I: Liver fibrosis in mice induced by carbon tetrachloride and its
reversion by luteolin. Toxicol Appl Pharmacol. 241:311–321. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CY, Peng WH, Wu LC, Wu CC and Hsu SL:
Luteolin ameliorates experimental lung fibrosis both in vivo and in
vitro: Implications for therapy of lung fibrosis. J Agric Food
Chem. 58:11653–11661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Shetty S, Zhang P, Gao R, Hu Y,
Wang S, Li Z and Fu J: Aspirin-triggered resolvin D1 down-regulates
inflammatory responses and protects againstendotoxin-induced acute
kidney injury. Toxicol Appl Pharmacol. 277:118–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okusa MD: The inflammatory cascade in
acute ischemic renal failure. Nephron. 90:133–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashmat S, Rudemiller N, Lund H,
Abais-Battad JM, Van Why S and Mattson DL: Interleukin-6 inhibition
attenuates hypertension and associated renal damage in Dahl
salt-sensitive rats. Am J Physiol Renal Physiol. 311:F555–F561.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Corrochano S, Renna M, Tomas-Zapico C,
Brown SD, Lucas JJ, Rubinsztein DC and Acevedo-Arozena A:
α-synuclein levels affect autophagosome numbers in vivo and
modulate Huntington disease pathology. Autophagy. 8:431–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eskelinen EL and Saftig P: Autophagy: A
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Virgin HW and Levine B: Autophagy genes in
immunity. Nat Immunol. 10:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luciani A, Villella VR, Esposito S,
Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L,
Giardino I, Pettoello-Mantovani M, et al: Defective CFTR induces
aggresome formation and lung inflammation in cystic fibrosis
through ROS-mediated autophagy inhibition. Nat Cell Biol.
12:863–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He L, Livingston MJ and Dong Z: Autophagy
in acute kidney injury and repair. Nephron Clin Pract. 127:56–60.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schmitz KJ, Ademi C, Bertram S, Schmid KW
and Baba HA: Prognostic relevance of autophagy-related markers LC3,
p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients
with respect to KRAS mutational status. World J Surg Oncol.
14:1892016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee YK, Jun YW, Choi HE, Huh YH, Kaang BK,
Jang DJ and Lee JA: Development of LC3/GABARAP sensors containing a
LIR and a hydrophobic domain to monitor autophagy. EMBO J.
36:1100–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schläfli AM, Berezowska S, Adams O, Langer
R and Tschan MP: Reliable LC3 and p62 autophagy marker detection in
formalin fixed paraffin embedded human tissue by
immunohistochemistry. Eur J Histochem. 59:24812015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin Z, Li Y, Pitti R, Lawrence D, Pham VC,
Lill JR and Ashkenazi A: Cullin3-based polyubiquitination and
p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis
signaling. Cell. 137:721–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schläfli AM, Adams O, Galván JA, Gugger M,
Savic S, Bubendorf L, Schmid RA, Becker KF, Tschan MP, Langer R, et
al: Prognostic value of the autophagy markers LC3 and p62/SQSTM1 in
early-stage non-small cell lung cancer. Oncotarget. 7:39544–39555.
2016. View Article : Google Scholar : PubMed/NCBI
|