Introduction
Chronic vascular inflammation is an important
pathophysiological basis of a number of cardiovascular diseases,
including atherosclerosis, polyarteritis nodosa and aneurysms
(1). Chemical, physical and other
harmful factors damage vascular endothelial cells (VECs), resulting
in functional and structural changes (2). Injured VECs release a variety of
inflammatory factors and chemokines, which induce the local
accumulation of lipids and inflammatory cells, ultimately
triggering innate and adaptive inflammatory immune responses in the
vascular intima (3). Therefore,
protecting endothelial cells (ECs) is beneficial to reducing
vascular inflammation, and preventing the occurrence and
development of cardiovascular diseases.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a
range of chemically unrelated compounds sharing certain common
therapeutic actions (4). At
present, NSAIDs that are commonly used clinically include aspirin,
paracetamol and ibuprofen, and mainly act by inhibiting
prostaglandin synthesis (5).
However, there are serious side effects associated with these
medications, such as gastrointestinal bleeding or perforation,
liver injury, and actions on the urinary and nervous systems
(6). Therefore, anti-inflammatory
components from natural compounds have attracted increasing
attention, offering a promising avenue for developing novel
anti-inflammatory drugs.
Genistein (GEN), a natural isoflavone, is
distributed widely in soybean and dentate plants, and exhibits
similarities with human estrogen in terms of its chemical structure
(7). Additionally, GEN not only
inhibits the proliferation, and promotes the differentiation and
apoptosis of various cancer cells, but it also exhibits
anti-inflammatory, antioxidant, anti-angiogenic and
lipid-regulating properties (8,9). Liu
et al (10) reported that
GEN reduced focal adhesion kinase (FAK) expression and inhibited
estradiol-induced VEC injury by downregulating the FAK pathway. Han
et al (11) suggested that
GEN protected homocysteine-induced EC inflammatory injury by
reducing the release of reactive oxygen species (ROS) and cytokines
regulating autophagy. Meanwhile, Deretic et al (12,13)
further proposed that autophagy deficiency is a predisposing factor
for inflammatory disease. Additionally, GEN protects against
oxidized low-density lipoprotein (ox-LDL)-induced senescence by
enhancing sirtuin 1 (SIRT1)/liver kinase B1/AMP kinase-mediated
autophagy flux (14), and reverses
ox-LDL- or lipopolysaccharide (LPS)-induced inflammatory responses
via miR-34a/SIRT1/FOXO3a (15) and
MyD88/NF-κB/BCL-2 signaling pathways in VECs (16). These findings suggest that GEN
regulates microRNAs (miRNAs/miRs), autophagy and apoptosis, thus
alleviating VEC injury triggered by various inducers; however, the
underlying mechanisms are yet to be clearly defined.
miRNAs are highly conserved small non-coding RNAs
that serve crucial roles in diverse physiological and pathological
processes (17,18). Increasing evidence has suggested
that changes in miRNA expression profiles may be associated with
chronic vascular inflammation, consisting of dysfunctions in ECs,
macrophages and vascular smooth muscle cells (VSMCs) (19,20).
For example, miR-126 and miR-125 directly target PI3K regulatory
subunit 2 and vascular endothelial cadherin to regulate
angiogenesis (21,22). Additionally, miR-150 targets c-Myd
and enhances the migration of ECs (23). A clinical study revealed that miR-21
expression in the serum of patients with cardiovascular disease was
significantly increased, rendering it a potential novel independent
biomarker of vascular inflammatory injury (24). Canfran-Duque et al (25) reported that miR-21 may be a
potential therapeutic target for inflammatory diseases. However,
the mechanisms via which miR-21 is associated with chronic
inflammation require further exploration.
In vivo, miR-21 knockout alters the
homeostasis of Ly-6Clo cells and reduces the formation
of early atherosclerotic lesions in apolipoprotein E knockout mice
(26), while in low-density
lipoprotein receptor knockout mice, miR-21 knockout leads to
inflammatory cell accumulation, defects in efferocytosis and
accelerated development of advanced atherosclerosis (25). Furthermore, miR-21 knockout
exacerbates angiotensin II-induced thoracic aortic aneurysm and
dissection formation in mice, which may be associated with the
TGF-β/SMAD3 signaling pathway (27). However, Li et al (28) reported that miR-21 suppresses PTEN,
and activates MMP-2 and MMP-9 to promote the proliferation and
migration of cells located within an aortic aneurysm in rats.
Collectively, these results suggest that miR-21 exerts an intricate
role in chronic vascular inflammatory response, which may be a key
mediator of GEN in inhibiting VEC damage.
Our previous studies reported that GEN and its
derivative can protect against lysophosphatidylcholine-induced VEC
injury, inhibit macrophage foaming, reduce VSMC proliferation and
migration, and inhibit the angiogenesis of HL-60 cells via the
Toll-like receptor 4 signaling pathway (29–31).
The present study was designed to investigate the effects and
molecular mechanisms of GEN on chronic vascular inflammatory
response by stimulating C57BL/6 mice and human umbilical vein (HUV)
ECs with LPS.
Materials and methods
Reagents and antibodies
GEN (purity, ≥99%) was purchased from Sigma-Aldrich
(Merck KGaA). LPS (purity, ≥99%) was purchased from Beijing
Solarbio Science & Technology Co., Ltd. miR-21 antagomir,
miR-21 mimic, miR-21 inhibitor and riboFECT CP were purchased from
Guangzhou RiboBio Co., Ltd. FBS, RPMI-1640, PBS and
penicillin/streptomycin were purchased from Biological Industries.
HiScript® II RT SuperMix for qPCR (+ gDNA wiper), miRNA
1st Strand cDNA Synthesis kit (by stem-loop) and AceQ®
qPCR SYBR Green Master Mix were obtained from Vazyme Biotech Co.,
Ltd. Antibodies against iNOS (cat. no. AF0199), NF-κB p65 (cat. no.
AF0874) and β-actin (cat. no. T0022) were purchased from Affinity
Biosciences. Goat anti-mouse HRP-conjugated IgG (cat. no. cW0102S)
and goat anti-rabbit HRP-conjugated IgG (cat. no. cW0156S) were
acquired from CoWin Biosciences. Rabbit two-step detection kits
(cat. nos. PV9001 and PV9002), goat serum and DAB staining solution
were all purchased from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.
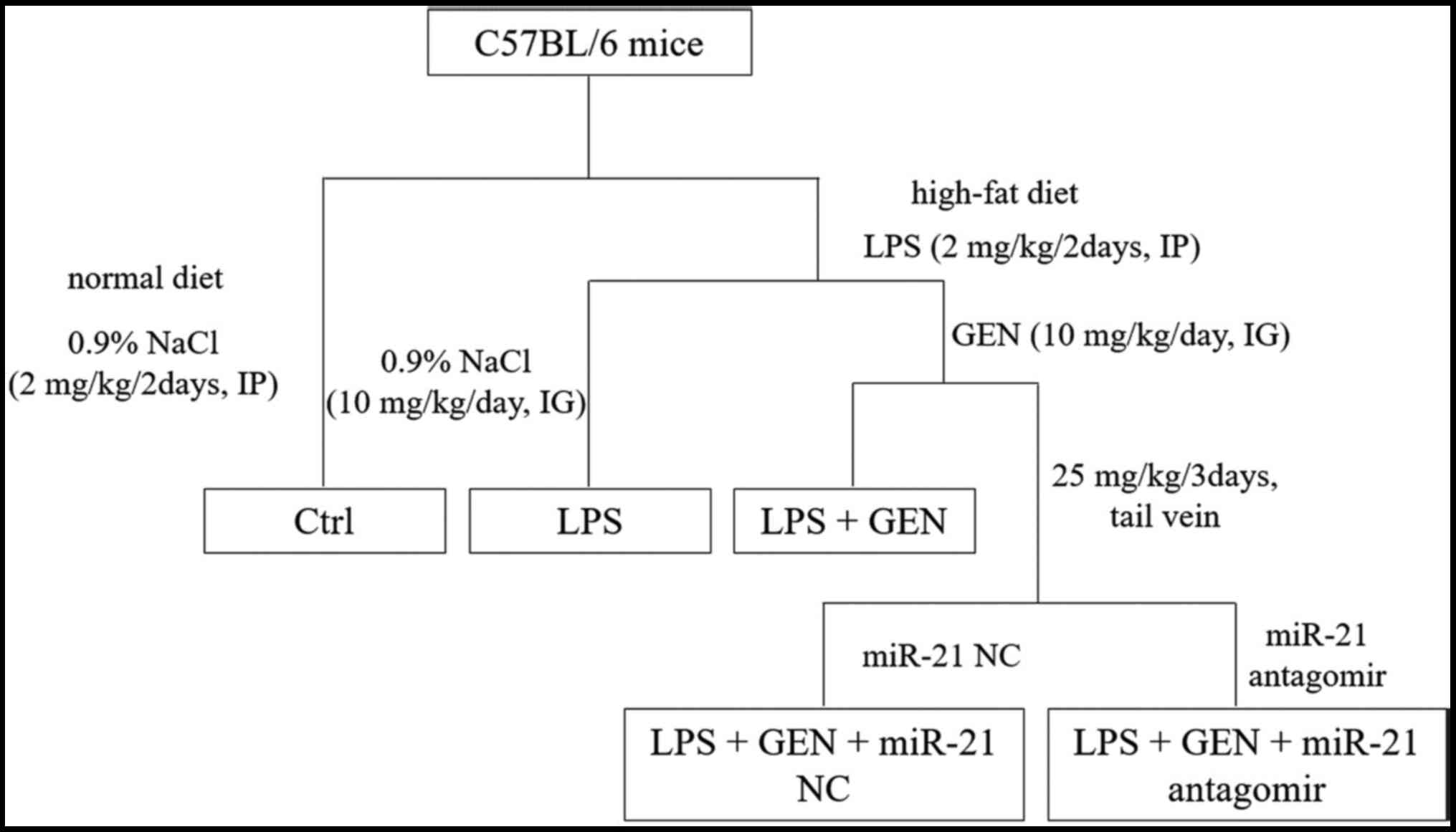
Animals and treatments
A total of 50 C57BL/6 mice (male; age, 6–8 weeks;
weight, 20±2.0 g) were purchased from Hunan SJA Laboratory Animal
Co., Ltd. (certificate no. 43004700059037). All mice were housed in
a specific pathogen-free laboratory environment under a controlled
temperature (20–24°C) and humidity (50–60%) with a 12:12-h
light/dark cycle and ad libitum access to food and water.
During the study, parameters indicating the general condition of
mice were observed daily, including fur brightness, food and water
intake, defecation and behavior. Furthermore, body weight was
measured each week. The experimental protocol was approved by the
Ethics Committee of Hunan Normal University.
A high-fat diet (10% lard, 10% egg yolk powder, 2%
cholesterol and 0.2% cholic acid) combined with LPS
(intraperitoneal injection) was used to establish a model of
chronic vascular inflammation in mice (32–34).
After 1 week of acclimatization, mice were randomly divided into 5
groups (n=10/group): Control group; LPS group; LPS + GEN group; LPS
+ GEN + miR-21 negative control (NC) group; and LPS + GEN + miR-21
Antagomir group (Fig. 1). LPS and
0.9% NaCl (2 mg/kg/2 days, IP injection) and GEN and 0.9% NaCl (10
mg/kg/day, intragastric infusion) were administered to mice from
the first week to the end of experiment. miR-21 NC, miR-21
antagomir and 0.9% NaCl (25 mg/kg/3 days) were injected into the
tail vein beginning in the week 17 until the end of week 20. After
20 weeks, mice were anesthetized with an intraperitoneal injection
of pentobarbital sodium (40 mg/kg) and then sacrificed via cervical
dislocation; their aortas were dissected and fixed in 4%
paraformaldehyde (PFA) for 2 h at room temperature for
immunohistochemistry (IHC) or frozen at −80°C for reverse
transcription-quantitative (RT-q)PCR and western blot analyses.
Cells and transfection
The HUVEC cell line HUVE-12 was supplied by China
Center for Type Culture Collection and routinely cultured in
RPMI-1640 with 10% fetal bovine serum (FBS), 1,000 U/ml penicillin
and 100 U/ml streptomycin at 5% CO2 and 37°C.
HUVE-12 cells (5×104/ml) were seeded on
6-well plates and cultured for 24 h at 37°C with 5% CO2.
Cell density at the time of transfection was 30–50%. Subsequently,
miR-21 mimic (50 nM), mimic NC (50 nM), miR-21 inhibitor (100 nM)
and inhibitor NC (100 nM) were transfected into cells using
riboFECT™ CP according to the manufacturer's protocol. At 24 h
after transfection, RT-qPCR was used to detect the transfection
efficiency. After 48 h, cells were pretreated with GEN (10 µmol/l)
for 2 h, and then incubated with LPS (1 µg/ml) for a further 24
h.
RT-qPCR
Total RNA were isolated from aorta and HUVE-12 cells
using TRIzol® (Vazyme Biotech Co., Ltd.) reagent
according to the manufacturer's protocol. Tissue (100 mg) in 1 ml
TRIzol® was placed in a grinder, and cells (10
cm2 area) were lysed with 1 ml TRIzol®. The
purity and concentration of RNA were measured using a microplate
reader with a multi-wavelength measurement system. Next, RNA was
reverse transcribed into cDNA using a HiScript Q RT SuperMix for
qPCR Kit (+ gDNA wiper) or miRNA 1st Strand cDNA Synthesis Kit (by
stem-loop) according to the manufacturer's protocol. qPCR was
conducted using AceQ qPCR SYBR Green Master Mix. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95°C for 10 min; formal denaturation at 95°C for 10 sec; 40
cycles of 30 sec at 60°C and 15 sec at 95°C; and final extension
for 60 sec at 60°C and 15 sec at 95°C. RNA expression was analyzed
using the 2−ΔΔCq method (35). The mRNA expression levels of TNF-α
and IL-6 in mice were normalized to 18S, TNF-α, IL-6 and iNOS in
HUVE-12 cells were normalized to GAPDH, and miR-21 gene expression
was normalized to U6. Primer sequences are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| 18S | F:
AGTCCCTGCCCTTTGTACACA |
|
| R:
CGATCCGAGGGCCTCACTA |
|
mmu-TNF-α | F:
CAAGGGACAAGGCTGCCCCG |
|
| R:
GCAGGGGCTCTTGACGGCAG |
|
mmu-IL-6 | F:
AACGATGATGCACTTGCAGA |
|
| R:
CTCTGAAGGACTCTGGCTTTG |
| GAPDH | F:
CAGGAGGCATTGCTGATGAT |
|
| R:
GAAGGCTGGGGCTCATTT |
|
hsa-TNF-α | F:
CGTGGAGCTGGCCGAGGAG |
|
| R:
AGGAAGGAGAAGAGGCTGAGGAAC |
|
hsa-IL-6 | F:
ATTCAATGAGGAGACTTGCCTGGTG |
|
| R:
ATCTGCACAGCTCTGGCTTGTTC |
|
hsa-iNOS | F:
GGGACCCGCACCACTACA |
|
| R:
CTGGATGTCGGACTTTGTAGATT |
| miR-21 | RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA |
|
| F:
GTGCAGGGTCCGAGGT |
|
| R:
GCCGCTAGCTTATAAGACTGATGT |
| U6 | RT:
AAAATATGGAACGCTTCACGAATTT |
|
| F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
Western blotting
Total protein was extracted from aorta and HUVE-12
cells using RIPA buffer (Beijing ComWin Biotech Co.); the protein
concentration was determined using a BCA protein assay kit
(Beyotime Institute of Biotechnology). Protein lysates (20 µg) were
separated via 10% SDS-PAGE and transferred onto PVDF membranes,
which were then blocked in 5% non-fat milk for 1 h at room
temperature. Next, membranes were incubated at 4°C overnight with
primary antibodies against NF-κB p65 (1:2,000), iNOS (1:3,000) and
β-actin (1:5,000). After being washed with PBST (0.05% Tween-20),
blots were incubated at room temperature for 2 h with goat
anti-rabbit HRP-conjugated antibody (1:10,000) or goat anti-mouse
HRP-conjugated antibody (1:10,000), followed by visualization using
ECL Western Blotting Substrate (NCM Biotech Co., Ltd) detection.
The protein levels were quantified using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc.).
IHC
Paraffin-embedded sections (4–6 µm) were incubated
in an oven at 60°C for 1 h, then immersed in xylene for 10 min,
anhydrous ethanol for 5 min, 95% alcohol for 3 min, 85% alcohol for
3 min and 75% alcohol for 3 min prior to rinsing with water for 3
min, all performed at room temperature. Later, EDTA (pH 9.0) was
used to retrieve antigens and 20% H2O2 was
used to block endogenous peroxidase activity at room temperature
for 20 min. After blocking with 5% goat serum (Beijing Solarbio
Science & Technology Co., Ltd.) for 1 h at room temperature,
sections were incubated with anti-NF-κB p65 antibody (1:200)
overnight at 4°C. Polymer Helper (cat. no. PV-9001) and Poly
Peroxidase-anti-rabbit IgG (cat. no. PV-9002) were applied for 30
min at room temperature in sequence. Finally, counterstaining was
performed using hematoxylin for 5 min at room temperature and DAB
were used to develop protein expression, respectively. Stained
samples were visualized in three fields of view using a light
microscope (Olympus Corporation; magnification, ×400) and analyzed
by Image-Pro Plus software.
Immunofluorescence (IF)
Sections were blocked with 5% goat serum at room
temperature for 1 h, then incubated with anti-NF-κB p65 antibody
(1:100) at 4°C overnight, followed by DyLight™ 594 goat anti-rabbit
IgG (1:1,000; cat. no. A23430; Abbkine Scientific Co., Ltd.) for 2
h at room temperature in the dark. Finally, DAPI was used to stain
the nuclei for 10 min at room temperature. Stained sections were
visualized in three fields of view using a fluorescence microscope
(magnification, ×400).
In vitro, HUVE-12 cells (3×105/ml)
were seeded onto coverslips. After 24 h, the cells were pretreated
with GEN (10 µM) for 2 h prior to co-incubation with LPS (1 µg/ml)
for 24 h at 37°C, then fixed with 4% PFA for 10 min at room
temperature. Subsequently, cells were permeabilized by 0.2%
Triton-100 for 5 min and blocked with 5% goat serum for 1 h at room
temperature. All subsequent steps were performed as described for
tissue sections; images were observed via fluorescence microscopy
(magnification, ×400).
Statistical analysis
Data are presented as the mean ± SD. SPSS 20.0 (IBM
Corp.) and GraphPad Prism 7.0 (GraphPad Software, Inc.) were used
for data analysis. Student's t-test was used for statistical
comparisons between two groups. One-way ANOVA was used for
comparisons among multiple groups, followed by LSD or Bonferroni
post hoc tests with equal variance and Dunnett's T3 with unequal
variance. P<0.05 (or P<0.003 with Bonferroni correction) was
considered to indicate a statistically significant difference.
Results
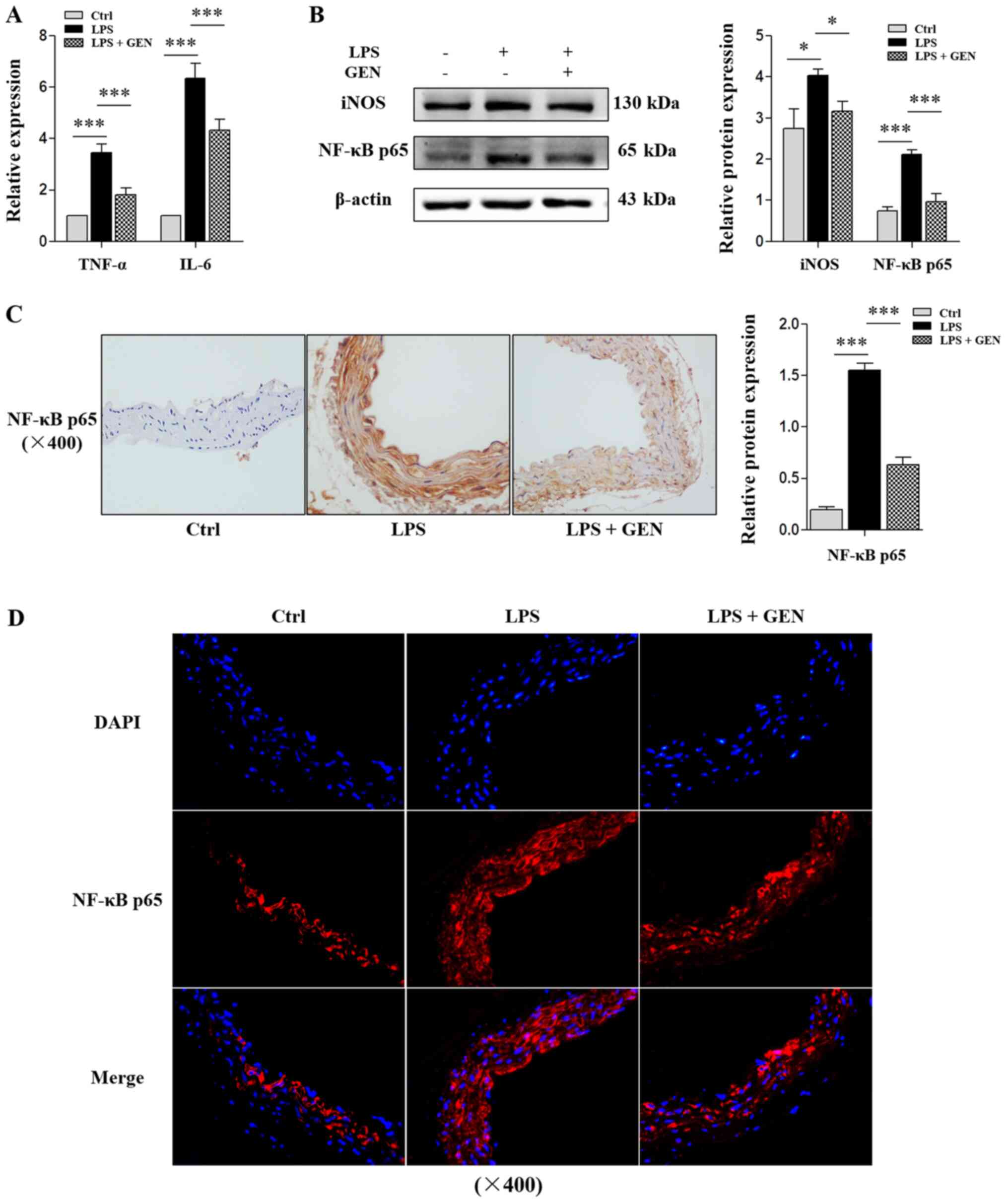
GEN attenuates the expression of
inflammation-associated factors in the aortae of LPS-treated
mice
LPS is a main component of the cell wall in
Gram-negative bacteria (36) and
has been demonstrated to be one of the pathogenic factors in
chronic vascular inflammation (37). In the present study, it was
demonstrated that intraperitoneal injection of LPS significantly
increased the mRNA expression of TNF-α and IL-6 and the protein
levels of iNOS and NF-κB p65 in the vasculature of mice (P<0.05;
Fig. 2A and B). In addition, the
relative protein expression of NF-κB p65 increased ~6-fold in aorta
from LPS-treated mice (P<0.001; Fig.
2C and D). These results indicated that a mouse model of
chronic vascular inflammatory response was successfully
established.
Furthermore, it was revealed that intragastric
administration of GEN significantly reduced the mRNA expression of
TNF-α and IL-6 in LPS-treated animals, as well as iNOS and NF-κB
p65 protein levels (P<0.05; Fig. 2A
and B). After administration of GEN, NF-κB p65 staining was
significantly reduced by ~50% in LPS-treated mice (P<0.001;
Fig. 2C and D). Collectively, these
data indicated that GEN inhibited chronic inflammation in blood
vessels.
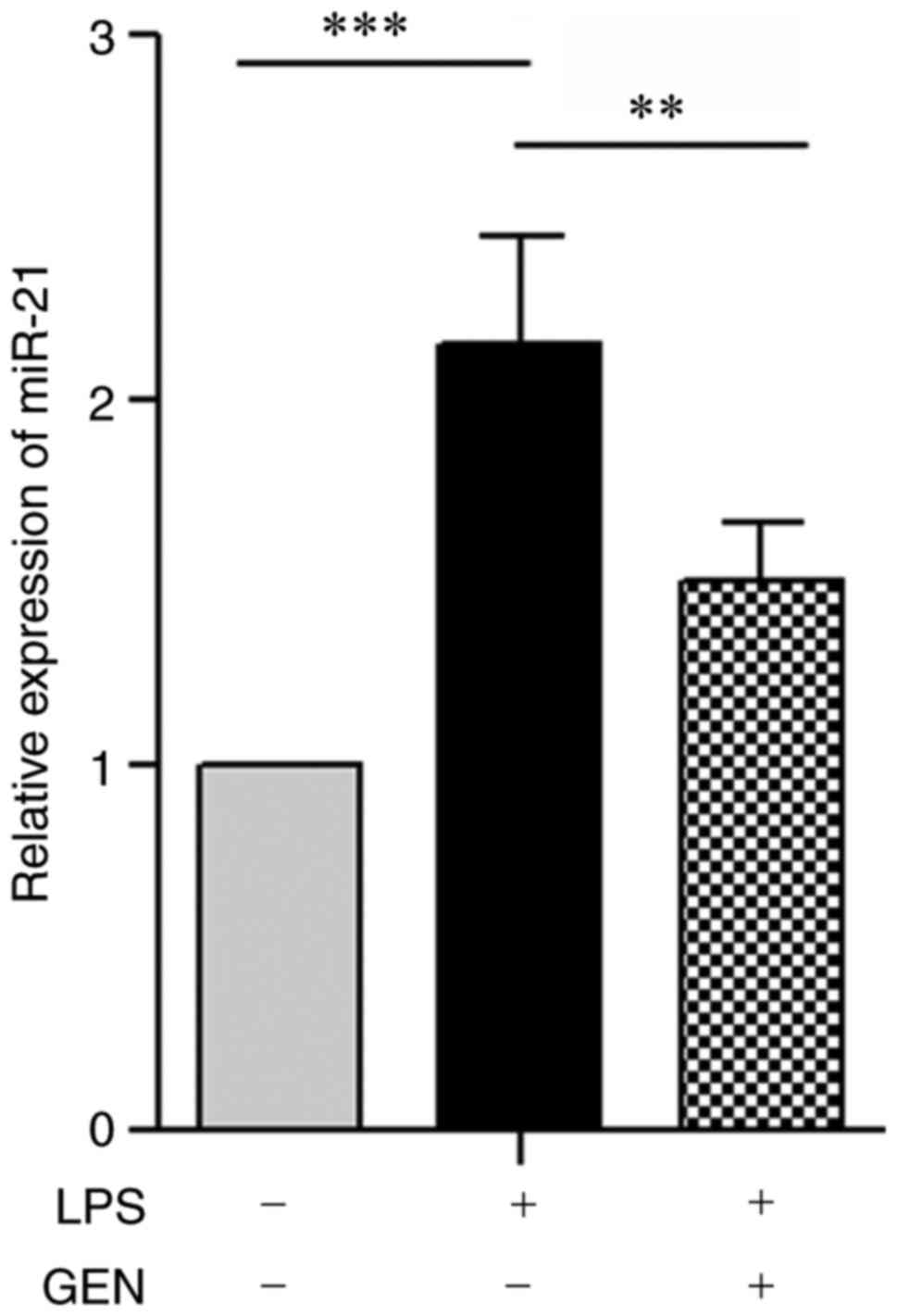
GEN suppresses miR-21 expression in
the aortae of LPS-treated mice
In atherosclerosis, aberrantly upregulated
expression miR-21 in the aorta has been independently associated
with the pathogenesis of chronic vascular inflammation (38). RT-qPCR is currently the most
convenient and popular method for detecting miRNA expression
(39) and was used to measure
miR-21 expression in aortic tissue in the present study. It was
revealed that intraperitoneal injection of LPS significantly
stimulated miR-21 expression by ~2.1-fold in C57BL/6 mice
(P<0.001; Fig. 3). Additionally,
co-treatment with GEN significantly suppressed miR-21 expression,
which was reduced by ~30% (P<0.01; Fig. 3).
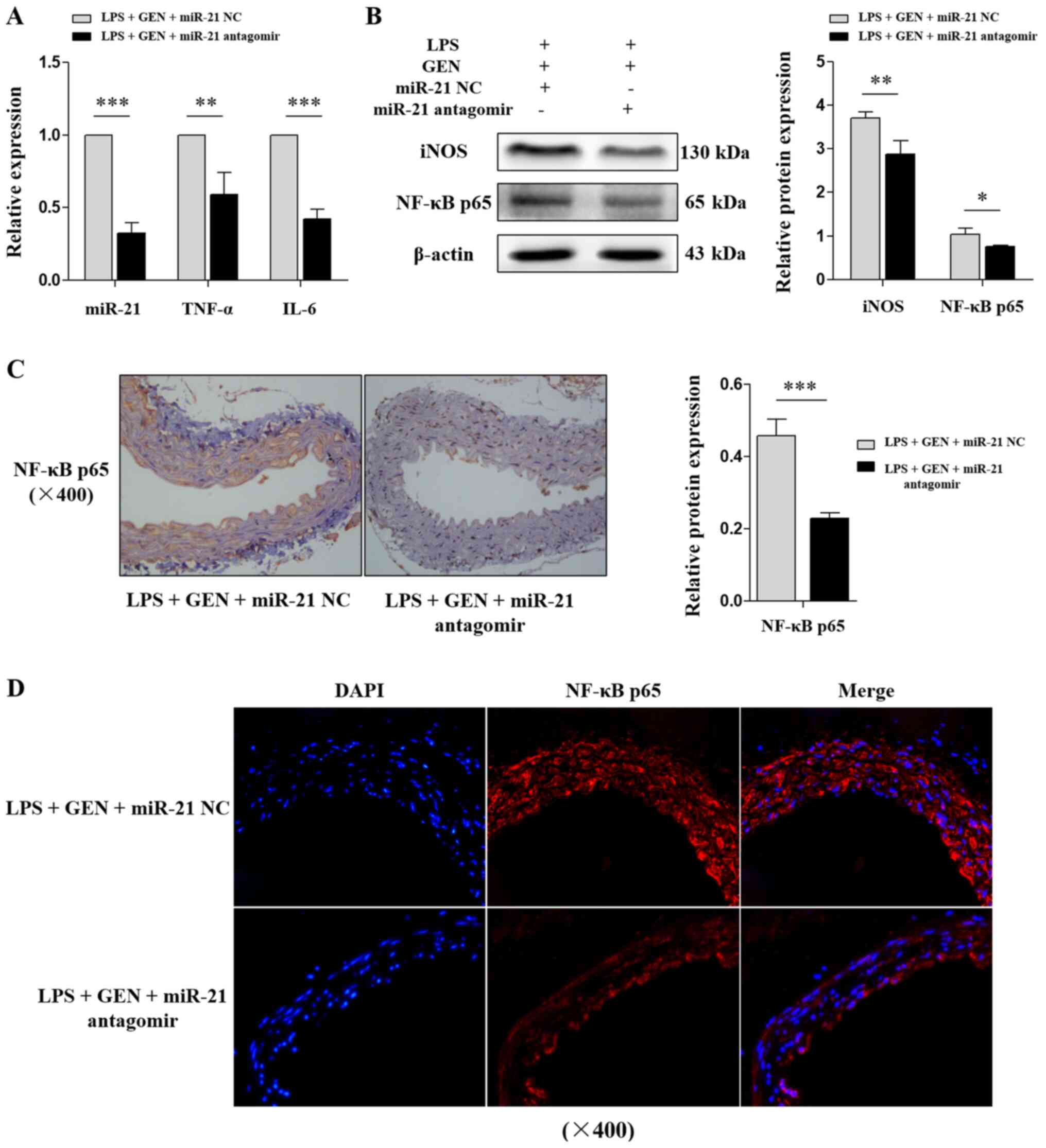
miR-21 antagomir promotes the
inhibitory effects of GEN on inflammation-associated factor
expression in the aortae of LPS-treated mice
In vivo, miRNA antagomirs pass through the
cell membrane, and inhibit the activity of their target miRNAs
(40); thus, they are frequently
used for miRNA silencing. To evaluate the effects of miR-21 on GEN,
LPS-treated mice were injected with miR-21 antagomir through the
tail vein. Compared with miR-21 NC, miR-21 antagomir significantly
decreased the levels of miR-21, TNF-α and IL-6 (P<0.01; Fig. 4A), and the protein levels of iNOS
and NF-κB p65 (P<0.05; Fig. 4B)
in mice treated with LPS + GEN. Furthermore, IHC and IF results
indicated that NF-κB p65 protein changes were consistent with the
western blot analysis (P<0.001; Fig.
4C and D). These results suggested that miR-21 served an
important role in the GEN-mediated attenuation of inflammation.
Based on these findings, it was hypothesized that GEN inhibited
chronic vascular inflammation by regulating miR-21.
miR-21 mimic attenuates GEN-mediated
inhibition of inflammation in LPS-treated VECs
ECs in arteries serve an important role in
anti-atherosclerotic processes due to unidentified pathways
(41). EC damage is a major initial
trigger for chronic vascular inflammation (42). Therefore, an inflammatory response
model was constructed in HUVE-12 cells using LPS (1 µg/ml; 24 h)
in vitro in combination with miR-21 mimic transfection.
RT-qPCR revealed that miR-21 mimic significantly upregulated miR-21
expression ~2-fold in HUVE-12 cells (P<0.001; Fig. 5A). LPS increased TNF-α, IL-6 and
iNOS mRNA expression, and iNOS and NF-κB p65 protein expression in
cells transfected with mimic NC (Fig.
5B-D), which indicated that the inflammation model was
successfully established in vitro. Furthermore, it was
demonstrated that GEN decreased TNF-α, IL-6 and iNOS mRNA
expression, and iNOS and NF-κB p65 protein levels in LPS-treated
VECs; however, miR-21 mimic significantly promoted the expression
of these factors, attenuating the inhibitory effects of GEN on
inflammation (Fig. 5B-D).
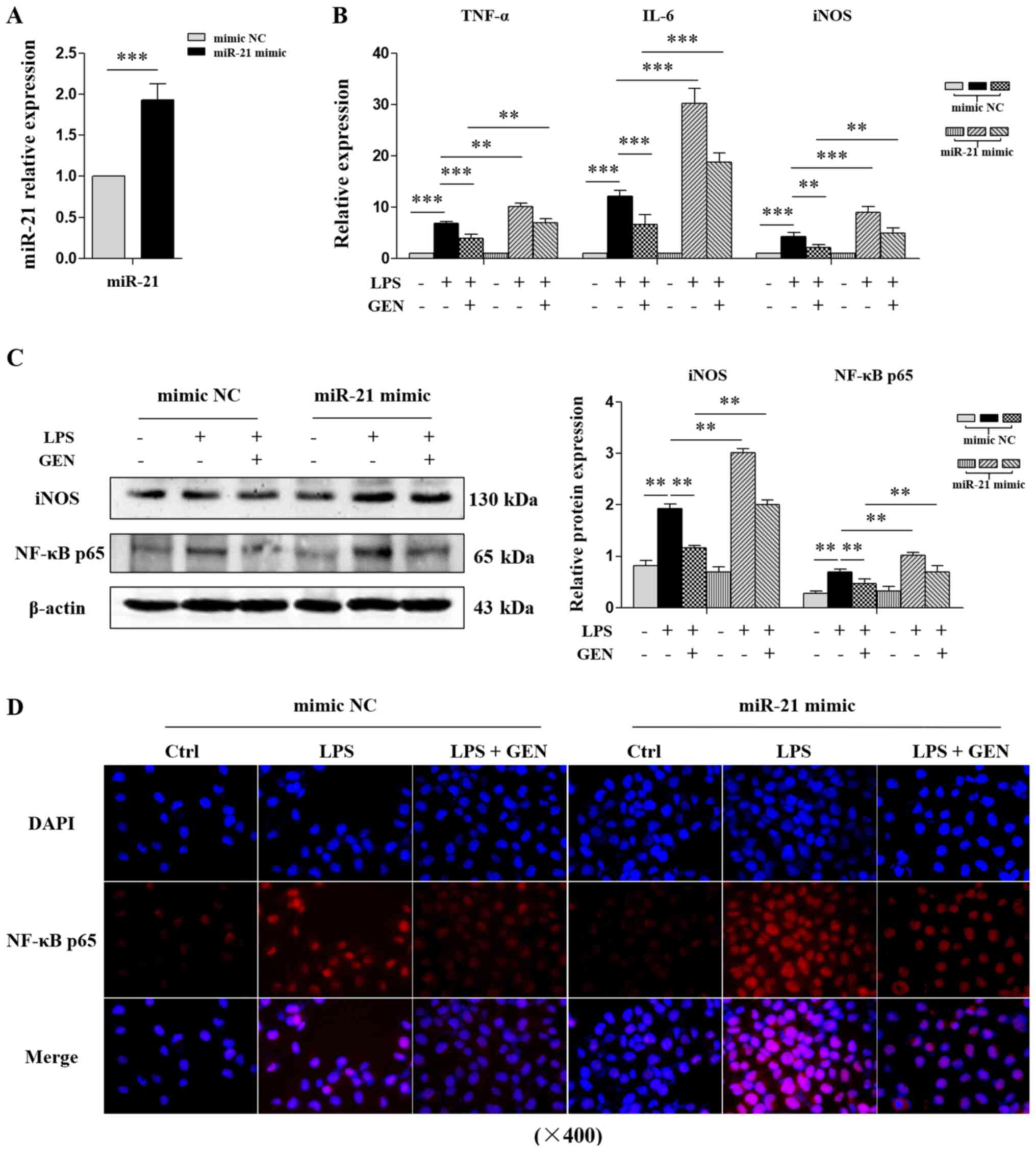 | Figure 5.Effects of miR-21 mimic on
GEN-mediated inhibition of the expression of
inflammation-associated factors in LPS-treated HUVE-12 cells. After
transfection with miR-21 mimic, HUVE-12 cells were pretreated with
GEN (10 µmol/l) for 2 h and then stimulated with LPS (1 µg/ml) for
another 24 h. (A) RT-qPCR analysis of miR-21 expression. (B)
RT-qPCR analysis of TNF-α, IL-6 and iNOS mRNA expression. (C)
Western blot analysis of the protein levels of iNOS and NF-κB p65.
(D) Immunofluorescence analysis of NF-κB p65 in the nucleus
(magnification, ×400). Data are presented as the mean ± SD of three
independent experiments. **P<0.003, ***P<0.001. GEN,
genistein; iNOS, inducible nitric oxide synthase; LPS,
lipopolysaccharide; miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; Ctrl, control. |
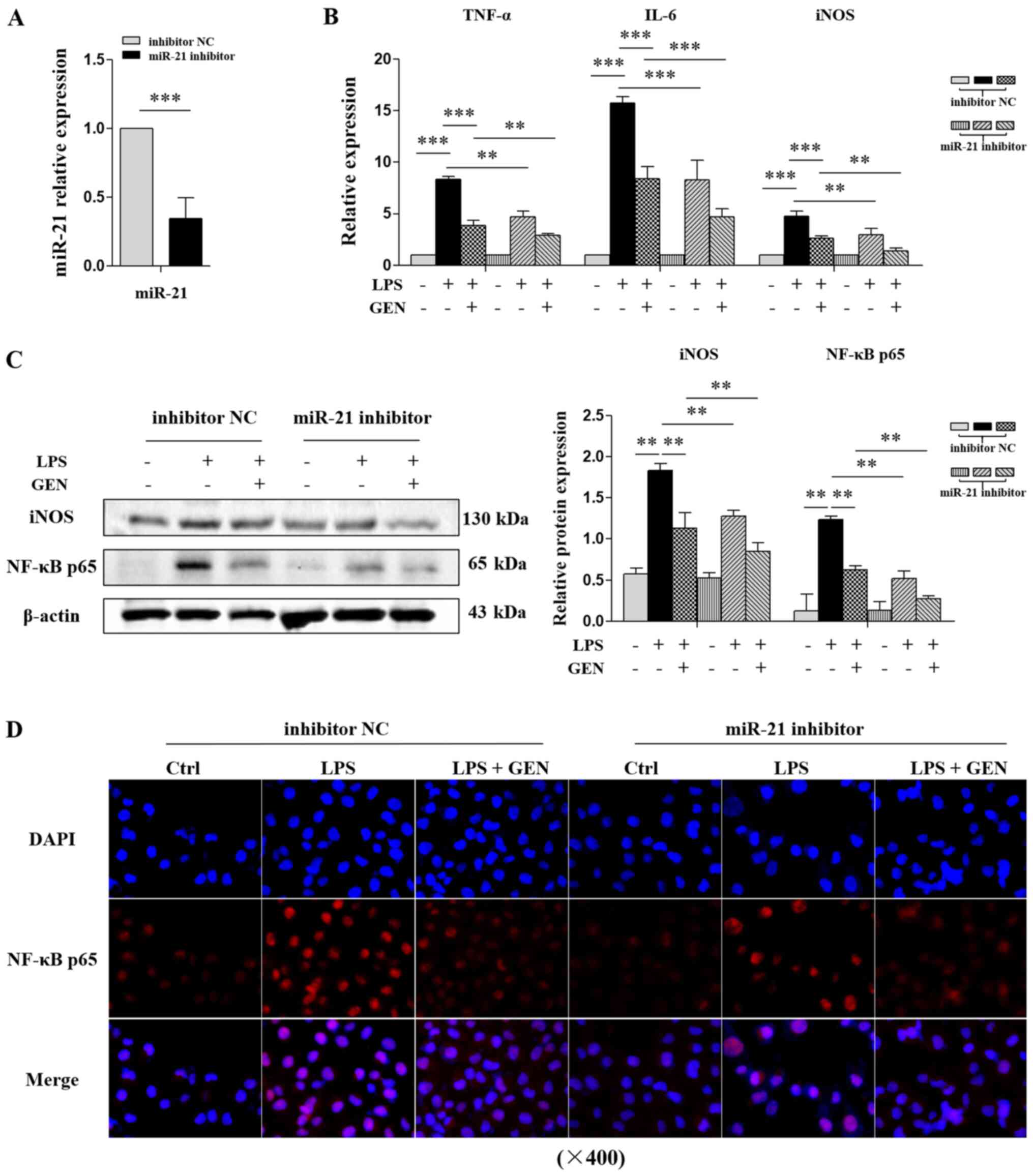
miR-21 inhibitor promotes the
GEN-mediated inhibition of inflammation in LPS-treated VECs
Additionally, miRNA inhibitors, which are chemically
modified complementary single strands to mature miRNA, were used to
silence miR-21. The results revealed that miR-21 was downregulated
by ~60% in HUVE-12 cells after transfection with miR-21 inhibitor
(P<0.001; Fig. 6A). Both GEN and
miR-21 inhibitor decreased the mRNA expression of TNF-α, IL-6 and
iNOS, and the protein levels of iNOS and NF-κB p65 in LPS-treated
VECs, and miR-21 inhibitor enhanced the effects of GEN on
inflammation-associated factor expression (Fig. 6B-D).
Discussion
Cardiovascular disease is a serious disease
affecting human health, and cases of mortality are projected to
increase from 16.7 million in 2002 to 23.3 million in 2030
worldwide (43). Chronic vascular
inflammation is an important pathological basis of cardiovascular
disease; further research into its etiology, pathogenesis and
prevention will help to delay or reverse its progression, and
reduce the morbidity and mortality of cardiovascular disease.
Inflammation is a natural and adaptive process to
noxious stimuli that the body is constantly exposed to (44,45),
and the vascular response is considered to be the central component
of inflammation. Acute inflammation is beneficial to the body, but
chronic inflammation can lead to a pathological state (46). LPS, an endotoxin found in the outer
layer of the cell wall of Gram-negative bacteria, induces
inflammatory responses by activating the NF-κB pathway, which is
important for human immune responses (47). Wu et al (48) reported that vascular inflammation
stimulated by LPS resulted in atheromatous lesions in vivo.
Therefore, chronic inflammation was induced in the aortae of mice
via a high-fat diet combined with intraperitoneal injection of LPS.
Using this method, a number of inflammatory cytokines and enzymes
were upregulated in vascular tissue, including TNF-α, IL-6, iNOS
and NF-κB p65.
GEN is the major natural isoflavone in soy and
soy-based foods (49) and can
inhibit inflammation (50), delay
aging (51) and prevent senile
dementia (52); however, the
underlying mechanisms are yet to be fully elucidated. As a potent
pro-inflammatory cytokine, TNF-α participates in early vascular
inflammation by upregulating aortic chemokines and adhesion
molecules (53). IL-6 is produced
by macrophages and T cells, and is involved in the pathogenesis of
several chronic inflammatory diseases (54). Nitric oxide (NO), an important
endogenous regulator, is of great relevance for the maintenance of
VEC homeostasis (55). iNOS is only
expressed under pathological conditions and catalyzes L-arginine to
NO, and iNOS is closely associated with vascular inflammation
(56). The NF-κB family is composed
of p65, p50, p52, RelB and c-Rel, which combine to form
transcriptionally active dimers (57). After activation, p65 is transported
from the cytoplasm to the nucleus. Where it facilitates the
transcription of inflammatory cytokines (58). The present findings indicated that
GEN downregulated the expression of TNF-α, IL-6, iNOS and p65 in
LPS-injured aortae and VECs, resulting in the inhibition of chronic
inflammation in mouse vascular, meanwhile, GEN also reduced the
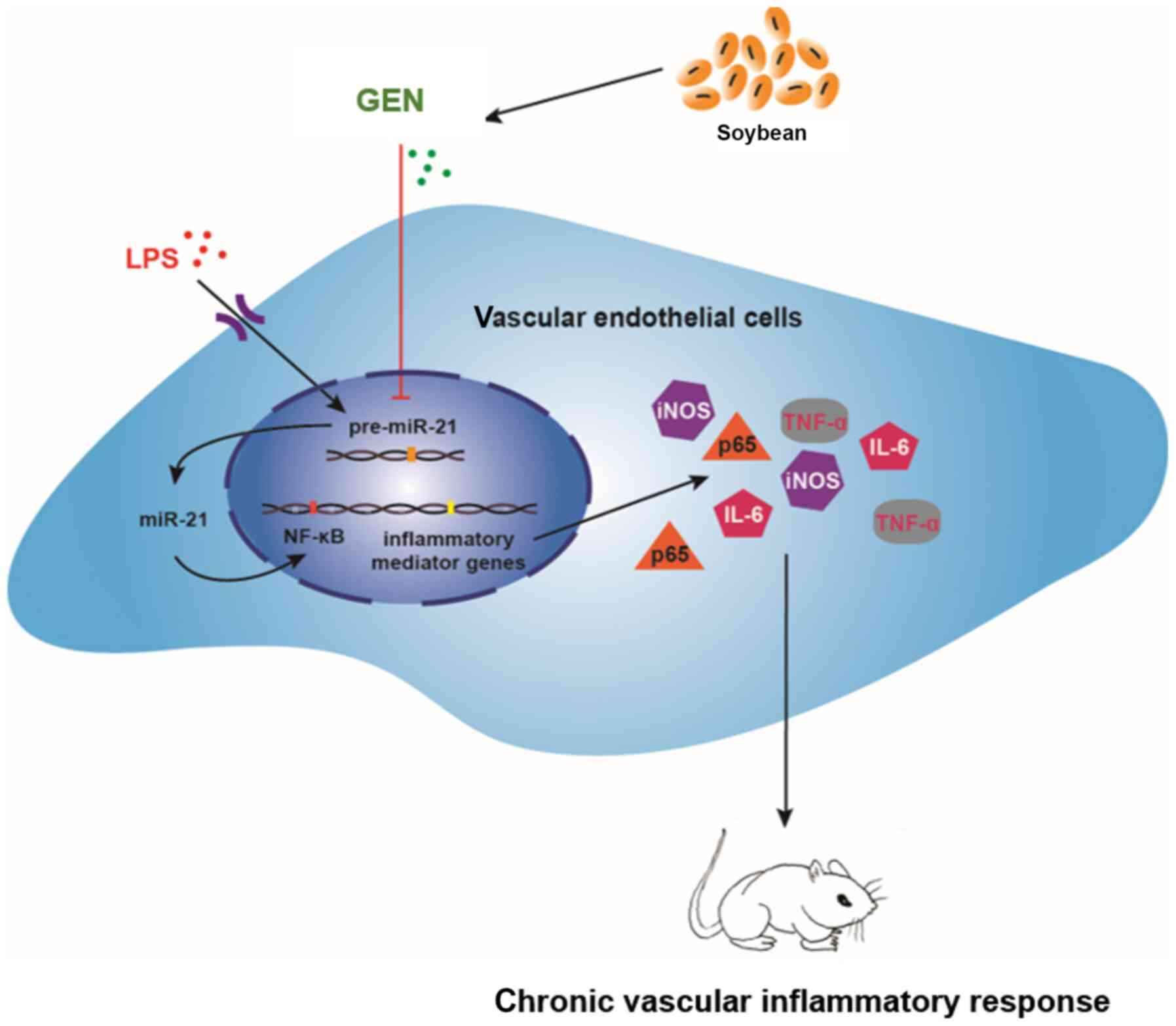
expression of miR-21 in LPS-injured aortae. Based on these
findings, it is hypothesized that GEN inhibits chronic vascular
inflammation by downregulating miR-21.
Epigenetics is one of the most popular research
fields in the post-genomic era, mainly focusing on non-coding RNA,
histone modifications, RNA methylation and DNA methylation
(59). miRNAs are non-coding small
molecule RNAs (60). It is
considered to be of major clinical importance to discover miRNAs
and their target genes in the context of inflammation (61). miR-21 is highly expressed in
functional cells related to the pathology of cardiovascular disease
and regulates inflammation (62).
Xue et al (63) found that
miR-21 deficiency inhibited the secretion of NF-κB-dependent
cytokines in macrophages, such as IL-6 and TNF-α. Therefore, the
mechanism via which GEN inhibits chronic vascular inflammation was
investigated in the present study by injecting miR-21 antagomir
into the tail veins of in mice, and transfecting miR-21 mimic or
inhibitor into HUVE-12 cells. The present findings revealed that
miR-21 antagomir enhanced GEN-mediated inhibition of chronic
inflammatory response in aortae. Furthermore, miR-21 mimic promoted
the expression of inflammation-related factors in LPS-treated VECs
and attenuated the effects of GEN, whereas miR-21 inhibitor induced
opposing effects.
Although the present study had certain limitations,
such as a lack of monitoring liver injury and inflammation, it was
still possible to obtain some conclusions. The study provided
evidence that GEN inhibited the inflammatory injury of VECs,
reduced chronic inflammatory response in the vasculature and
effectively improved inflammation, potentially by downregulating
miR-21. The mechanism may be closely associated with NF-κB p65
(Fig. 7). In subsequent
experiments, the interaction between NF-κB p65 and miR-21 will be
studied further, and the pharmacological mechanism via which GEN
inhibits chronic vascular inflammation will be elucidated.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from Natural
Science Foundation of China (grant no. 81370382) and Hunan
Provincial Natural Science Foundation of China (grant no.
2020JJ5381).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XX and LC designed the study and wrote the
manuscript. SL and LX performed the experiments and analyzed the
data. XF conceived the study and wrote the manuscript. XX and LC
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Ethics Committee of Hunan Normal University (No. 2018-172).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Libby P and Hansson GK: Inflammation and
immunity in diseases of the arterial tree: Players and layers. Circ
Res. 116:307–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacchi S, Palumbo P, Sponta A and
Coppolino MF: Clinical pharmacology of non-steroidal
anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents
Med Chem. 11:52–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LiverTox: Clinical and research
information on drug-induced liver injury (Internet). Bethesda (MD)
National Institute of Diabetes and Digestive and Kidney Diseases.
316431762012.
|
|
6
|
Harirforoosh S, Asghar W and Jamali F:
Adverse effects of nonsteroidal antiinflammatory drugs: An update
of gastrointestinal, cardiovascular and renal complications. J
Pharm Pharm Sci. 16:821–847. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sutrisno S, Aprina H, Simanungkalit HM,
Andriyani A, Barlianto W, Sujuti H, Santoso S, Dwijayasa PM,
Wahyuni ES and Mustofa E: Genistein modulates the estrogen receptor
and suppresses angiogenesis and inflammation in the murine model of
peritoneal endometriosis. J Tradit Complement Med. 8:278–281. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arakawa S, Inoue M, Kinouchi R, Morizumi
S, Yamaguchi M, Shimazu Y and Takeda M: Dietary constituent
genistein inhibits the hyperexcitability of trigeminal nociceptive
neurons associated with mechanical hyperalgesia following orofacial
inflammation. J Oral Biosci. 61:215–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaiswal N, Akhtar J, Singh SP, Badruddeen
and Ahsan F: An overview on genistein and its various formulations.
Drug Res (Stuttg). 69:305–313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu B, Xu L, Yu X, Jiao X, Yan J, Li W and
Guo M: Genistein inhibited estradiol-induced vascular endothelial
cell injury by downregulating the FAK/Focal adhesion pathway. Cell
Physiol Biochem. 49:2277–2292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han S, Wu H, Li W and Gao P: Protective
effects of genistein in homocysteine-induced endothelial cell
inflammatory injury. Mol Cell Biochem. 403:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deretic V and Levine B: Autophagy balances
inflammation in innate immunity. Autophagy. 14:243–251. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deretic V and Klionsky DJ: Autophagy and
inflammation: A special review issue. Autophagy. 14:179–180. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Yang X, Pang X, Zhao Z, Yu H and
Zhou H: Genistein protects against ox-LDL-induced senescence
through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in
HUVECs. Mol Cell Biochem. 455:127–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Zhao Z, Pang X, Yang J, Yu H,
Zhang Y, Zhou H and Zhao J: miR-34a/sirtuin-1/foxo3a is involved in
genistein protecting against ox-LDL-induced oxidative damage in
HUVECs. Toxicol Lett. 277:115–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi L, Chang M, Zhao Q, Zhou Z, Huang X,
Guo F and Huan J: Genistein-3′-sodium sulphonate protects against
lipopolysaccharide-induced lung vascular endothelial cell apoptosis
and acute lung injury via BCL-2 signalling. J Cell Mol Med.
24:1022–1035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hajibabaie F, Kouhpayeh S, Mirian M,
Rahimmanesh I, Boshtam M, Sadeghian L, Gheibi A, Khanahmad H and
Shariati L: MicroRNAs as the actors in the atherosclerosis
scenario. J Physiol Biochem. 76:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Li K and Chen X:
Inflammation-regulatory microRNAs: Valuable targets for
intracranial atherosclerosis. J Neurosci Res. 97:1242–1252. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tao L, Xu X, Fang Y, Wang A, Zhou F, Shen
Y and Li J: miR-21 targets jnk and ccr7 to modulate the
inflammatory response of grass carp following bacterial infection.
Fish Shellfish Immunol. 94:258–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shoeibi S: Diagnostic and theranostic
microRNAs in the pathogenesis of atherosclerosis. Acta Physiol
(Oxf). 228:e133532020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sessa R, Seano G, di Blasio L, Gagliardi
PA, Isella C, Medico E, Cotelli F, Bussolino F and Primo L: The
miR-126 regulates angiopoietin-1 signaling and vessel maturation by
targeting p85β. Biochim Biophys Acta. 1823:1925–1935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muramatsu F, Kidoya H, Naito H, Sakimoto S
and Takakura N: MicroRNA-125b inhibits tube formation of blood
vessels through translational suppression of VE-cadherin. Oncogene.
32:414–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Liu D, Chen X, Li J, Li L, Bian
Z, Sun F, Lu J, Yin Y, Cai X, et al: Secreted monocytic miR-150
enhances targeted endothelial cell migration. Mol Cell. 39:133–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pordzik J, Pisarz K, De Rosa S, Jones AD,
Eyileten C, Indolfi C, Malek L and Postula M: The potential role of
platelet-related microRNAs in the development of cardiovascular
events in high-risk populations, including diabetic patients: A
review. Front Endocrinol (Lausanne). 9:742018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Canfran-Duque A, Rotllan N, Zhang X,
Fernandez-Fuertes M, Ramirez-Hidalgo C, Araldi E, Daimiel L, Busto
R, Fernandez-Hernando C and Suárez Y: Macrophage deficiency of
miR-21 promotes apoptosis, plaque necrosis, and vascular
inflammation during atherogenesis. EMBO Mol Med. 9:1244–1262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chipont A, Esposito B, Challier I,
Montabord M, Tedgui A, Mallat Z, Loyer X and Potteaux S:
MicroRNA-21 deficiency alters the survival of Ly-6Clo
monocytes in ApoE−/− mice and reduces early-stage
atherosclerosis-brief report. Arterioscler Thromb Vasc Biol.
39:170–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Yue Z, Wu J, Chen J, Wwang S, Wu
J, Ren L, Zhang A, Deng P, Wang K, et al: MicroRNA-21 knockout
exacerbates angiotensin II-induced thoracic aortic aneurysm and
dissection in mice with abnormal transforming growth factor-β-SMAD3
signaling. Arterioscler Thromb Vasc Biol. 38:1086–1101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li K, Cui MZ, Zhang KW, Wang GQ and Zhai
ST: Effect of miR-21 on rat thoracic aortic aneurysm model by
regulating the expressions of MMP-2 and MMP-9. Eur Rev Med
Pharmacol Sci. 24:878–884. 2020.PubMed/NCBI
|
|
29
|
Cong L, Yang S, Zhang Y, Cao J and Fu X:
DFMG attenuates the activation of macrophages induced by coculture
with LPC-injured HUVE12 cells via the TLR4/MyD88/NFKB signaling
pathway. Int J Mol Med. 41:2619–2628. 2018.PubMed/NCBI
|
|
30
|
Cong L, Zhang Y, Huang H, Cao J and Fu X:
DFMG reverses proliferation and migration of vascular smooth muscle
cells induced by co-culture with injured vascular endothelial cells
via suppression of the TLR4-mediated signaling pathway. Mol Med
Rep. 17:5692–5699. 2018.PubMed/NCBI
|
|
31
|
Xiang X, Li L, Bo P, Kuang T, Liu S, Xie
X, Guo S, Fu X and Zhang Y: 7-Difluoromethyl-5,4′dimethoxygenistein
exerts antiangiogenic effects on acute promyelocytic leukemia HL-60
cells by inhibiting the TLR4/NF-κB signaling pathway. Mol Med Rep.
21:2251–2259. 2020.PubMed/NCBI
|
|
32
|
Lee WR, Kim KH, An HJ, Park YY, Kim KS,
Lee CK, Min BK and Park KK: Effects of chimeric decoy
oligodeoxynucleotide in the regulation of transcription factors
NF-κB and Sp1 in an animal model of atherosclerosis. Basic Clin
Pharmacol Toxicol. 112:236–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SJ, Park JH, Kim KH, Lee WR, Kim KS
and Park KK: Melittin inhibits atherosclerosis in LPS/high-fat
treated mice through atheroprotective actions. J Atheroscler
Thromb. 18:1117–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SJ, Park JH, Kim KH, Lee WR, Lee S,
Kwon OC, Kim KS and Park KK: Effect of NF-κB decoy
oligodeoxynucleotide on LPS/high-fat diet-induced atherosclerosis
in an animal model. Basic Clin Pharmacol Toxicol. 107:925–930.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Wang X, Zheng M and Luan Q:
Lipopolysaccharide from Porphyromonas gingivalis promotes autophagy
of human gingival fibroblasts through the PI3K/Akt/mTOR signaling
pathway. Life Sci. 211:133–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khedoe PPSJ, de Kleijn S, van
Oeveren-Rietdijk AM, Plomp JJ, de Boer HC, van Pel M, Rensen PCN,
Berbee JFP and Hiemstra PS: Acute and chronic effects of treatment
with mesenchymal stromal cells on LPS-induced pulmonary
inflammation, emphysema and atherosclerosis development. PLoS One.
12:e01837412017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sheane BJ, Smyth P, Scott K, Aziz R,
Buckley M, Lodge E, Kiely N, Kingston M, McGovern E, Healy M, et
al: An association between microRNA-21 expression and vitamin D
deficiency in coronary artery disease. Microrna. 4:57–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nejad C, Pepin G, Behlke MA and Gantier
MP: Modified polyadenylation-based RT-qPCR increases selectivity of
amplification of 3′-MicroRNA isoforms. Front Genet. 9:112018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krützfeldt J, Rajewsky N, Braich R, Rajeev
KG, Tuschl T, Manoharan M and Stoffel M: Silencing of microRNAs in
vivo with ‘antagomirs’. Nature. 438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hermkens DMA, Stam OCG, de Wit NM, Fontijn
RD, Jongejan A, Moerland PD, Mackaaij C, Waas ISE, Daemen MJAP and
de Vries HE: Profiling the unique protective properties of
intracranial arterial endothelial cells. Acta Neuropathol Commun.
7:1512019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng Y, Xu J, Hua YQ, Peng Y and Xu XL:
MDM2 contributes to oxidized low-density lipoprotein-induced
inflammation through modulation of mitochondrial damage in
endothelial cells. Atherosclerosis. 305:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling FN, et al: Heart disease and stroke
statistics-2020 update: A report from the American heart
association. Circulation. 141:e139–e596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Varela ML, Mogildea M, Moreno I and Lopes
A: Acute inflammation and metabolism. Inflammation. 41:1115–1127.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aghasafari P, George U and Pidaparti R: A
review of inflammatory mechanism in airway diseases. Inflamm Res.
68:59–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Skaper SD, Facci L, Zusso M and Giusti P:
An inflammation-centric view of neurological disease: Beyond the
neuron. Front Cell Neurosci. 12:722018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tan H, Zhao J, Zhang H, Zhai Q and Chen W:
Novel strains of Bacteroides fragilis and Bacteroides ovatus
alleviate the LPS-induced inflammation in mice. Appl Microbiol
Biotechnol. 103:2353–2365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu X, Zhang LL, Yin LB, Fu YJ, Jiang YJ,
Ding HB, Chu ZX, Shang H and Zhang ZN: Deregulated microRNA-21
expression in monocytes from HIV-infected patients contributes to
elevated IP-10 secretion in HIV infection. Front Immunol.
8:11222017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Y, An Y, Lv C, Ma W, Xi Y and Xiao R:
Dietary soybean isoflavones in Alzheimer's disease prevention. Asia
Pac J Clin Nutr. 27:946–954. 2018.PubMed/NCBI
|
|
50
|
Gholampour F, Mohammadi Z, Karimi Z and
Owji SM: Protective effect of genistein in a rat model of ischemic
acute kidney injury. Gene. 753:1447892020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Das G, Paramithiotis S, Sundaram
Sivamaruthi B, Wijaya CH, Suharta S, Sanlier N, Shin HS and Patra
JK: Traditional fermented foods with anti-aging effect: A
concentric review. Food Res Int. 134:1092692020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Petry FDS, Coelho BP, Gaelzer MM, Kreutz
F, Guma FTCR, Salbego CG and Trindade VMT: Genistein protects
against amyloid-beta-induced toxicity in SH-SY5Y cells by
regulation of Akt and Tau phosphorylation. Phytother Res.
34:796–807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ahmad Z, Ng CT, Fong LY, Bakar NA, Hussain
NH, Ang KP, Ee GC and Hakim MN: Cryptotanshinone inhibits
TNF-α-induced early atherogenic events in vitro. J Physiol Sci.
66:213–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Unver N and McAllister F: IL-6 family
cytokines: Key inflammatory mediators as biomarkers and potential
therapeutic targets. Cytokine Growth Factor Rev. 41:10–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Richards J, El-Hamamsy I, Chen S, Sarang
Z, Sarathchandra P, Yacoub MH, Chester AH and Butcher JT:
Side-specific endothelial-dependent regulation of aortic valve
calcification: Interplay of hemodynamics and nitric oxide
signaling. Am J Pathol. 182:1922–1931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cassini-Vieira P, Araujo FA, da Costa Dias
FL, Russo RC, Andrade SP, Teixeira MM and Barcelos LS: iNOS
activity modulates inflammation, angiogenesis, and tissue fibrosis
in polyether-polyurethane synthetic implants. Mediators Inflamm.
2015:1384612015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bellucci A, Bubacco L, Longhena F,
Parrella E, Faustini G, Porrini V, Bono F, Missale C and Pizzi M:
Nuclear Factor-κB Dysregulation and α-synuclein pathology: Critical
interplay in the pathogenesis of Parkinson's disease. Front Aging
Neurosci. 12:682020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Q, Lenardo MJ and Baltimore D: 30
Years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Skvortsova K, Iovino N and Bogdanovic O:
Functions and mechanisms of epigenetic inheritance in animals. Nat
Rev Mol Cell Biol. 19:774–790. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hulshoff MS, Del Monte-Nieto G, Kovacic J
and Krenning G: Non-coding RNA in endothelial-to-mesenchymal
transition. Cardiovasc Res. 115:1716–1731. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Singh RP, Massachi I, Manickavel S, Singh
S, Rao NP, Hasan S, Mc Curdy DK, Sharma S, Wong D, Hahn BH and
Rehimi H: The role of miRNA in inflammation and autoimmunity.
Autoimmun Rev. 12:1160–1165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Das A, Ganesh K, Khanna S, Sen CK and Roy
S: Engulfment of apoptotic cells by macrophages: A role of
microRNA-21 in the resolution of wound inflammation. J Immunol.
192:1120–1129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang
Z, Li Y, Yang G, Zhou D, Yang H, et al: miR-21 promotes NLRP3
inflammasome activation to mediate pyroptosis and endotoxic shock.
Cell Death Dis. 10:4612019. View Article : Google Scholar : PubMed/NCBI
|