Introduction
Diabetes is one of the major diseases threatening
human health worldwide. According to official reports, more than
400 million people worldwide suffer from diabetes (1). Diabetic cardiomyopathy (DCM) is one of
the complications of diabetes; moreover, it is one of the main
causes of diabetes-associated morbidity and mortality. Furthermore,
DMC accounts for more than 50% of all diabetes-associated deaths
each year (2). According to
epidemiological studies, people with diabetes are 2 to 5 times more
likely to develop heart failure than their healthy peers.
Therefore, it is urgent to explore new prevention and treatment
strategies for DCM (3–5). Complex biological responses, including
inflammation, oxidative stress, the renin-angiotensin system,
mitochondrial dysfunction, intracellular Ca2+ transport
disorders, myocardial fibrosis, and cardiomyocyte apoptosis, are
associated with DCM, which makes it extremely difficult to
investigate the pathophysiological mechanism of DCM (6,7).
A growing body of evidence has confirmed that
oxidative stress, inflammation, and cell death play major roles in
DCM (6,8,9). Both
hyperglycaemia and inflammation can lead to excessive reactive
oxygen species (ROS) production in cells, which can cause lipid
peroxidation reactions and reduce the antioxidant capacity of
cells, ultimately leading to cardiomyocyte apoptosis and even the
occurrence of cardiac dysfunction (10). In addition, ROS and ROS-induced DNA
damage can promote inflammatory responses and fibrotic processes
(11).
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is an important redox sensor and one of the key regulators of the
expression of various antioxidants in cells (12,13).
In response to oxidative stress, Nrf2 is activated and binds to
antioxidant response elements (AREs) to activate the transcription
and translation of antioxidant genes and proteins, respectively
(14,15). Zhao et al (16) reported that the inhibition of Nrf2
expression affected the expression level of antioxidant proteins,
thus exacerbating DOX-induced oxidative stress damage in
cardiomyocytes. Silent information regulator 2 homologue 1 (SIRT1)
is an NAD+-dependent deacetylase that can regulate a
variety of biological processes, including cell metabolism, redox
homeostasis, apoptosis, inflammation, and senescence, through
deacetylation (17,18). Existing research has confirmed that
SIRT1 suppresses cardiomyocyte apoptosis in DCM (18). Moreover, in a study of glomerular
mesangial cells, SIRT1 was revealed to activate the Nrf2/ARE
pathway (19). Nuclear factor-κB
(NF-κB) is a key transcription factor in the regulation of
inflammatory processes, and SIRT1 can also modulate NF-κB-dependent
inflammatory responses (20).
Therefore, it is critical to explore whether the SIRT1/Nrf2/NF-κB
signalling pathway plays a regulatory role in oxidative stress and
inflammation in diabetic cardiomyopathy.
Hyperglycaemia activates the renin-angiotensin (Ang)
system (RAS) and induces extracellular matrix accumulation, leading
to cardiac remodelling and dysfunction (21). Briefly, increased levels of Ang II
have been reported to regulate the production of ROS and lead to
cardiac hypertrophy, left ventricular dysfunction, and myocardial
insulin resistance (22,23). Allisartan isoproxil is a new
nonpeptide angiotensin II receptor blocker (ARB) precursor drug
that can produce a carboxylic acid derivative (EXP3174) during
absorption in vivo (24,25).
Compared with losartan, allisartan isoproxil can be completely
hydrolysed by esterase into the active metabolite EXP3174 after
being absorbed into the small intestine and stomach (26). Losartan is already widely used to
treat hypertension clinically (27), and the effect of losartan has been
investigated in ventricular hypertrophy, heart failure, kidney
diseases and DCM in rats (28–32).
However, it remains to be seen whether allisartan isoproxil can be
used for the treatment of DCM.
In the present study, it was examined whether
allisartan isoproxil protected against cardiac injuries in DCM
rats. The present study investigated the antioxidant stress and
anti-inflammatory effects of allisartan isoproxil in myocardium by
constructing DCM rats models and treating them with allisartan
isoproxil. The experiments focused on the regulatory effect of
allisartan isoproxil on SIRT1/Nrf2 and SIRT1/NF-κB signaling
pathways. The results indicated that allisartan isoproxil could
significantly attenuate cardiac injuries caused by diabetes by
inhibiting inflammation and oxidative stress.
Materials and methods
Animal model of diabetes
Wild-type SD rats were obtained from Shanghai
Sippe-Bk Lab Animal Co., Ltd. All animal study protocols were
approved by the Institutional Ethics Committee of The First
Affiliated Hospital with Nanjing Medical University (approval no.
IACUC-1803019) and were performed according to the guidelines of
the US Department of Health (NIH Publication no. 85-23, revised
1996) for the use and care of laboratory animals.
A rat diabetes model was established by streptozocin
(STZ) and a high-fat diet (HFD) (33). The HFD (10% lard oil, 10% sucrose,
2.0% cholesterol, 0.5% cholate, 5% yolk powder and 72.5% ordinary
feed) and control diets were obtained from Beijing Boaigang
Biological Technology Co., Ltd. A total of 32 adult Sprague-Dawley
rats weighing 280–300 g were used for the animal experiments. The
animals were housed with a 12-h light/dark cycle and a temperature
of 22±2°C. Rats in the control group were fed a control diet. The
other 3 groups were fed an HFD to induce diabetes. After 4 weeks of
feeding, all the animals were fasted for 12 h, and then the rats in
the diabetes group were intraperitoneally injected with STZ (35
mg/kg body weight), while the rats in the control group were
injected with the same volume of citrate buffer (34–36).
The blood glucose levels were measured 72 h after STZ injection.
When the blood glucose levels were higher than 16.7 mmol/l, the
rats were identified as diabetic. Then, the rats were continued on
an HFD for 8 weeks to induce DCM. To explore the effects of
allisartan isoproxil, after 8 weeks of continuous HFD feeding, the
3 groups of diabetic rats were treated with PBS (DCM+PBS group),
allisartan isoproxil (10.8 mg/kg/day, DCM+allisartan isoproxil
group) (24,37), and no treatment other than an HFD
(DCM group). In the present study, the allisartan isoproxil was
used as palliative treatment. The rats were fed an HFD throughout
the 16-week experimental period. The whole experiment lasted for 16
weeks. During the experimental period, the rats were monitored
every day, and 4 of them exhibited lethargy after STZ injection and
succumbed to natural causes within a week. At the end of the
experiment period, 28 rats were anesthetized with isoflurane
(2.0–2.5% isoflurane in oxygen). When the rats appeared to have
faint breathing, myasthenia, lack of independent reaction,
cyanosis, or were comatose, it was considered that rats were close
to death and were euthanized by cervical dislocation.
Echocardiography
A Vevo 2100 imaging system (VisualSonics, Inc.) was
used to measure heart function in all animals by 2D
echocardiography as previously described (7). Left ventricular ejection fraction
(EF), short axis fractional shortening (FS), the E™/A™ ratio, the
left ventricular end-systolic dimension (LVD) and left ventricular
posterior wall thickness (LVPW) were measured from the parasternal
long-axis view.
Histological analysis
Heart tissues were fixed in 10% neutral buffered
formalin for 72 h at room temperature. Histopathological analysis
was performed on paraffin-embedded sections of the heart (5 µm)
stained with hematoxylin and eosin staining. Briefly, after
dewaxing and hydration of paraffin sections, hematoxylin staining
was performed for 3 min at room temperature, the sections rinsed
with distilled water, and then stained with eosin for 2 min at room
temperature and rinsed with distilled water again. Finally, the
sections were dried and sealed. Myocardial fibrosis was determined
using Masson's trichrome staining (Sigma-Aldrich; Merck KGaA).
Briefly, hematoxylin staining for 5 min, ponceau trichrome staining
for 10 min and aniline blue staining for 2 min at room temperature.
Finally, the sections were dried and sealed. Multiple images were
acquired from each stained heart section under a light microscope.
The fibrotic areas of paraffin-embedded heart sections were
evaluated using ImageJ 6.0 software (National Institutes of
Health).
Enzyme-linked immunosorbent assay
Serum cTnT and BNP levels were measured by ELISA
kits (cat. nos. OKEH04575 and OKEH00475; Aviva Systems Biology)
according to the manufacturer's instructions. Briefly, serum
samples were added to an ELISA plate at a standard volume of 100
µl/well. The plates were incubated at 37°C for 2 h. Then, the
plates were washed, the conjugation solution was added to the wells
and incubated at 37°C for another 1 h. Finally, the results were
recorded using a microplate reader (Elx800; BioTek Instruments,
Inc.) at a wavelength of 450 nm.
Immunohistochemistry
Heart tissues were fixed in 10% neutral buffered
formalin for 72 h at room temperature. The expression of SIRT1 and
Nrf2 in cardiac tissue was examined by immunohistochemistry.
Paraffin-embedded sections (5 µm) were deparaffinized (heated in a
drying oven at 60°C for 2 h and soaked in pure xylene for 15 min
three times) and hydrated (immersion in 100, 90, 85, and 75%
alcohol, 10 min each). Then, the activity of endogenous peroxidase
was quenched with 3% H2O2 for 20 min. After
endogenous peroxidase quenching and antigen retrieval, to avoid
nonspecific antibody binding, the sections were placed in a culture
dish containing 10% goat serum (cat. no. ab7481; Abcam) and
incubated for 30 min at room temperature. Then, the sections were
incubated with SIRT1 (1:400, cat. no. ab189494) or Nrf2 (1:400,
cat. no. ab92946; both from Abcam) antibodies at 4°C for 12 h.
Following primary antibody incubation, the sections were washed
with PBS three times. The sections were then incubated in a diluted
solution of horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:50, cat. no. ab205718; Abcam) for 60 min.
3,3-diaminobenzidine was used as the detection reagent for 5 min.
Finally, the sections were counterstained with haematoxylin (1 min
at room temperature) and bluing reagent, dehydrated and mounted.
Multiple images were acquired from each stained heart section under
a light microscope. The heart sections were evaluated using ImageJ
6.0 software (National Institutes of Health).
TUNEL assay
Rat heart tissue was surgically removed, frozen in
optima cutting temperature compound and sectioned at 8 µm with a
cryostat. Apoptosis in rat heart tissue was measured using the
ApopTag plus peroxidase in situ apoptosis detection kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
guidelines. Briefly, after washing with PBS, heart frozen sections
were incubated with 1 µg/ml Proteinase K and 10 mM Tris solution
for 10 min at room temperature. The sections were washed in PBS for
5 min twice and then incubated with the TUNEL reaction mixture at
room temperature for 45 min. Finally, frozen sections were washed
with PBS 5 min twice and then the nuclei labeled with DAPI for 20
min at room temperature and mounted in 50% glycerine diluted in
water. For each rat, three sections were used for the TUNEL assay.
A total of 15 randomly chosen microscopic fields were analyzed
under a fluorescence microscope. The number of TUNEL-positive
myocardial nuclei and total myocardial nuclei in each group were
calculated. Images were captured with a fluorescent microscope. The
rate of apoptosis was determined by dividing the number of
TUNEL-positive myocardial nuclei by the total number of myocardial
nuclei.
Measurement of malondialdehyde (MDA)
production and superoxide dismutase (SOD) activity
Experimental kits were used to determine the level
of MDA (cat. no. YBA003-2) and SOD (cat. no. YBA001-3) activity in
myocardial tissue according to the manufacturer's instructions
(Shanghai Yubo Biological Technology Co., Ltd.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from heart tissue with
TRIzol reagent (CWBiotech). To reverse transcribe RNA into cDNA for
RT-qPCR, TransStart Tip Green qPCR SuperMix from TransGen Biotech
was used according to the manufacturer's instructions. Quantitative
PCR was performed using SYBR green (Thermo Fisher Scientific,
Inc.). Thermocycling conditions were 2 min at 50°C, followed by
initial denaturation at 95°C for 2 min and 45 cycles at 95°C for 15
sec (denaturation) and 60 sec at 56°C (annealing and extension).
The relative expression level was calculated using the
2−ΔΔCq method (38). The
primers are listed in Table
SI.
Western blotting
Total protein was extracted from the samples using
radioimmunoprecipitation assay (RIPA) lysis buffer (100 µl). The
lysates were centrifuged at 12,000 × g for 20 min at 4°C and the
supernatants collected. The protein concentration was determined by
the BCA assay. Protein samples (20 µg) were loaded and separated
using 8 or 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis at 90 V for 1.5 h and subsequently transferred to
polyvinylidene fluoride membranes. The membrane was blocked in 5%
bovine serum albumin (cat. no. A3858, Sigma-Aldrich) for 2 h at
room temperature. The primary antibodies used were SIRT1 (1:1,000;
cat. no. cat. no. ab189494), Nrf2 (1:1,000; cat. no. ab92946), heme
oxygenase-1 (HO-1; 1:1,000; cat. no. ab13243), NF-κB p65 (1:1,000;
cat. no. ab16502), TNF-α (1:1,000; cat. no. ab66579), IL-1β
(1:1,000; cat. no. ab9722), Bax (1:1,000; cat. no. ab32503), Bcl-2
(1:1,000; cat. no. ab196495), caspase-3 (1:1,000; cat. no. ab4051),
cleaved caspase-3 (1:1,000; cat. no. ab49822), and GAPDH (1:2,000;
cat. no. ab9485; all from Abcam). Following an overnight incubation
at 4°C the membranes were washed with TBST three times, and then
horseradish peroxidase (HRP)-conjugated rabbit IgG secondary
antibody (1:5,000; cat no. sc-2004; Santa Cruz Biotechnology, Inc.)
were added for each corresponding primary antibody for 1.5 h at
room temperature. All protein bands were visualized by enhanced
chemiluminescence (ECL) system using the ECL chemiluminescent
substrate reagent kit (cat. no. C510043 Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The grayscale value
of protein bands was calculated using ImageJ 6.0 software.
Statistical analysis
The data are expressed as the mean ± SD of at least
three independent experiments for each in vitro experimental
group and at least six independent experiments for each animal
group. All experimental data were analysed using SPSS (version
20.0; IBM Corp.) and GraphPad Prism 7.0 (GraphPad Software, Inc.)
unless otherwise stated. The data was evaluated with unpaired
Student's t-tests for comparisons between two groups. A value of
P<0.05 was considered to indicate a statistically significant
result.
Results
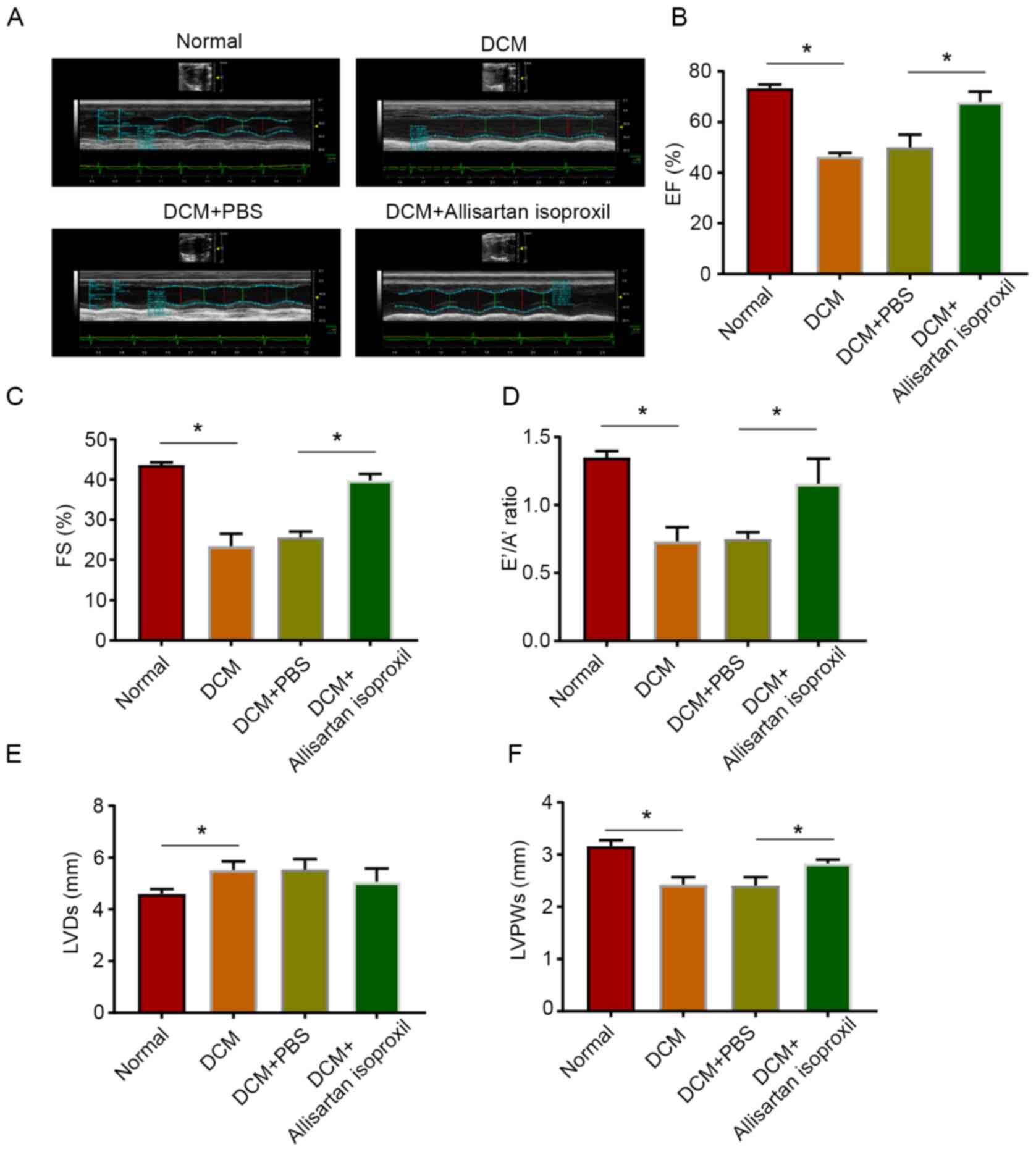
Protective effects of allisartan
isoproxil on the hearts of DCM rats
To investigate the effect of allisartan isoproxil on
cardiac function in diabetic rats, cardiac function was measured by
echocardiography in diabetic rats treated with and without
allisartan isoproxil (Fig. 1A). As
revealed in Fig. 1, the diabetic
rats exhibited abnormal EFs and FS dysfunction (Fig. 1B and C). Moreover, cardiac diastolic
dysfunction with a reduced E™/A™ ratio was also detected in
diabetic rats (Fig. 1D). In
addition, both the LVD and LVPW exhibited abnormal manifestations
(Fig. 1E and F). However, cardiac
systolic function and cardiac diastolic function were significantly
improved by allisartan isoproxil (Fig.
1A-F).
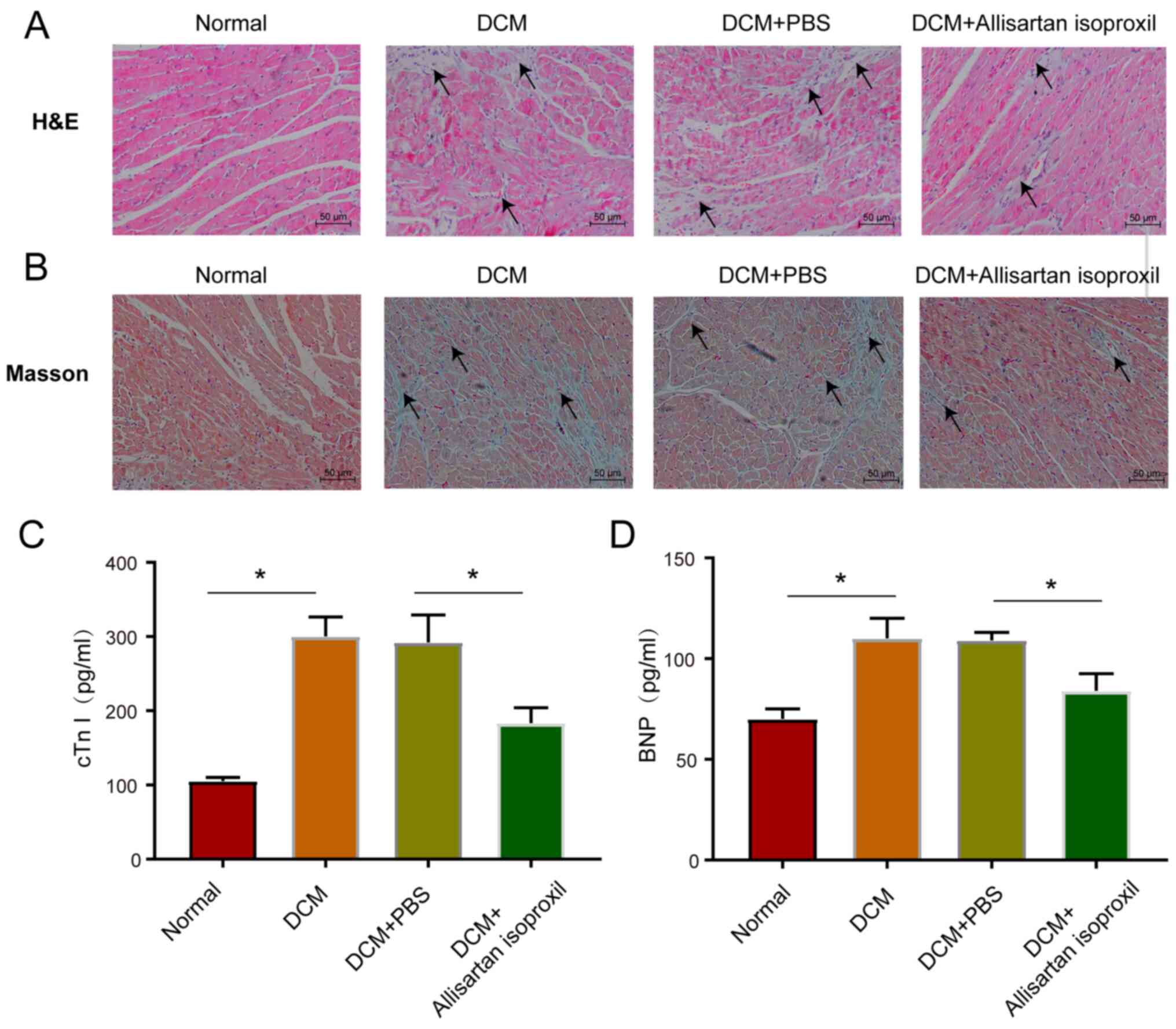
Histological and cardiac marker
analysis of DCM rats
Next, the myocardial damage caused by hyperglycaemia
and the protective effects of allisartan isoproxil were explored.
The haematoxylin-stained histopathological sections of normal
control rats exhibited normal myocardial tissue morphology and
regular nuclear arrangement. However, STZ-induced DCM rats
exhibited tissue oedema, inflammatory cell infiltration and
disorganized myocardial nuclei (arrowhead), while allisartan
isoproxil treatment significantly alleviated these pathological
effects (Fig. 2A). Moreover,
allisartan isoproxil significantly attenuated the accumulation of
collagen (arrowhead) in the myocardial tissue of DCM rats, as
revealed by Masson staining (Fig.
2B). Then, cardiac troponin I (cTnI) and B-type natriuretic
peptide (BNP), biomarkers of myocardial injury and heart failure,
respectively, were measured by ELISA. Allisartan isoproxil
effectively inhibited the expression levels of both cTnT and BNP
(Fig. 2C and D).
Allisartan isoproxil can inhibit
diabetes-induced myocardial oxidative stress in rats
It has been confirmed that SIRT1 and Nrf2 could
reduce myocardial cell apoptosis and modulate the antioxidant
response system in DCM (18). Nrf2
has been reported to modulate the antioxidant response system of
cardiomyocytes under high glucose conditions (39). When Nrf2 is activated, it can enter
the nucleus and regulate the expression level of the antioxidative
stress response gene HO-1 to protect cell viability (13,39).
Moreover, a study on glomerular mesangial cells revealed that SIRT1
could activate the Nrf2/ARE pathway (19). It was hypothesised that SIRT1 could
regulate DCM by regulating the expression of Nrf2. Therefore,
western blotting and RT-qPCR were used to assess the protein and
mRNA expression levels, respectively, of SIRT1 and Nrf2. As
revealed in Fig. 3A and C, the
protein and mRNA expression levels of SIRT1 and Nrf2 in the
myocardial tissue of diabetic rats were significantly
downregulated, while allisartan isoproxil treatment partially
restored the expression levels of SIRT1 and Nrf2 (Fig. 3A). Immunohistochemical staining also
revealed that allisartan isoproxil could reverse the downregulation
of both SIRT1 and Nrf2 protein levels in the heart tissue sections
of diabetic rats (Fig. 3B).
Moreover, as was anticipated, the expression trend of HO-1, a
downstream gene of Nrf2, was consistent with that of Nrf2 (Fig. 3C). The expression level of MDA
(Fig. 3D) and the activity of SOD)
(Fig. 3E were also assessed. These
results revealed that allisartan isoproxil could reduce the
oxidative stress response of DCM rats.
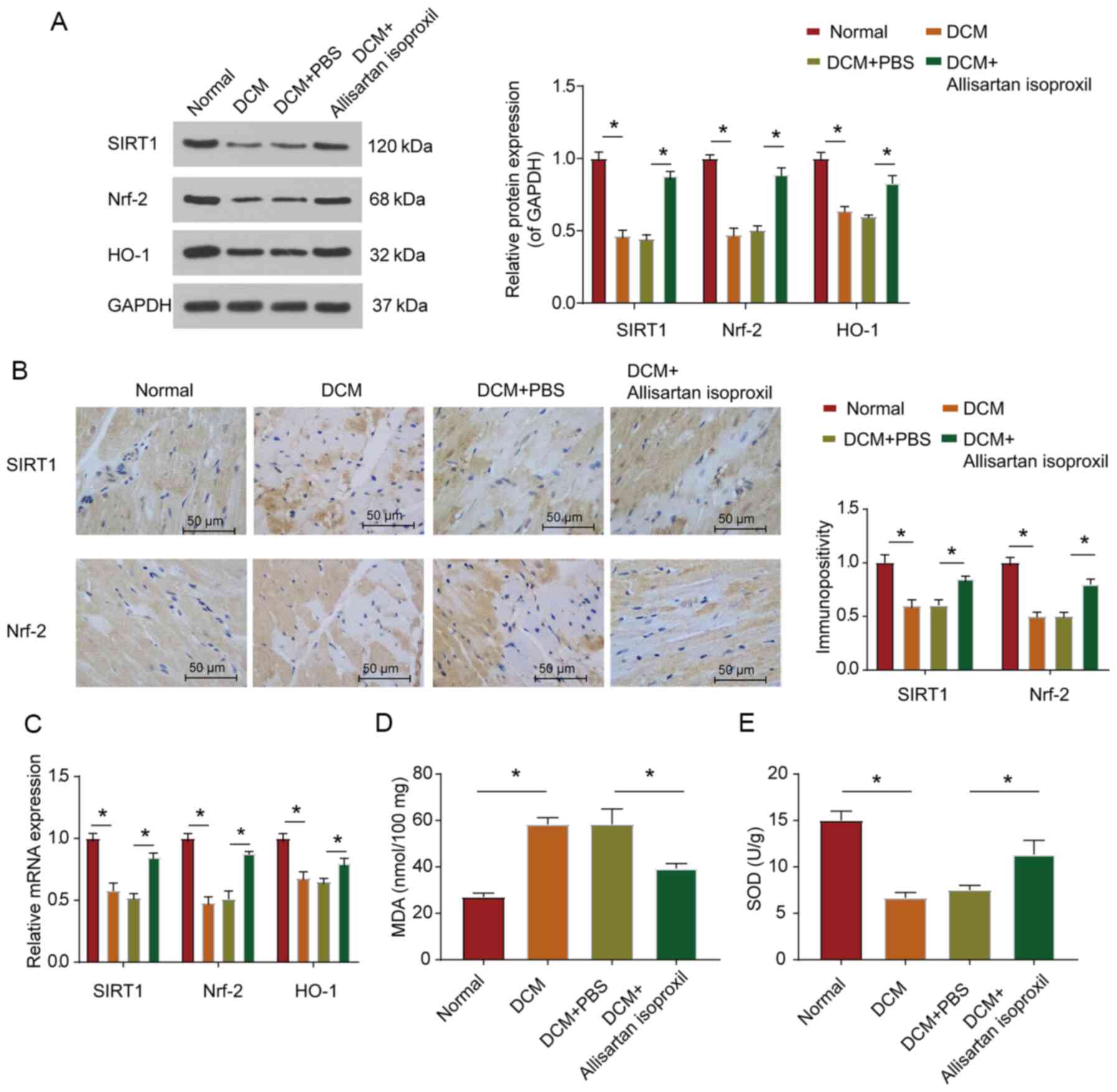 | Figure 3.Allisartan isoproxil can inhibit
diabetes-induced myocardial oxidative stress in rats. (A) Western
blot analysis of SIRT1, Nrf2 and HO-1 expression in lysates
prepared from heart tissues. The myocardial tissues were subjected
to immunohistochemical analysis as described in the Materials and
methods. (B) Representative images were acquired and quantified for
SIRT1 and Nrf2. (C) The expression levels of SIRT1, Nrf2 and HO-1
were determined by reverse transcription-quantitative PCR, and the
grouping was the same as in B. (D and E) Oxidative stress in
myocardial tissues was determined by measuring the levels of (D)
MDA and (E) SOD in lysates prepared from heart tissues, and the
grouping was the same as in B. The values are the mean ± SD of n=6
in each group. *P<0.05. SIRT1, silent information regulator 2
homologue 1; Nrf2, nuclear factor erythroid 2-related factor 2;
HO-1, heme oxygenase-1; MDA, malondialdehyde; SOD, superoxide
dismutase; DCM, diabetic cardiomyopathy. |
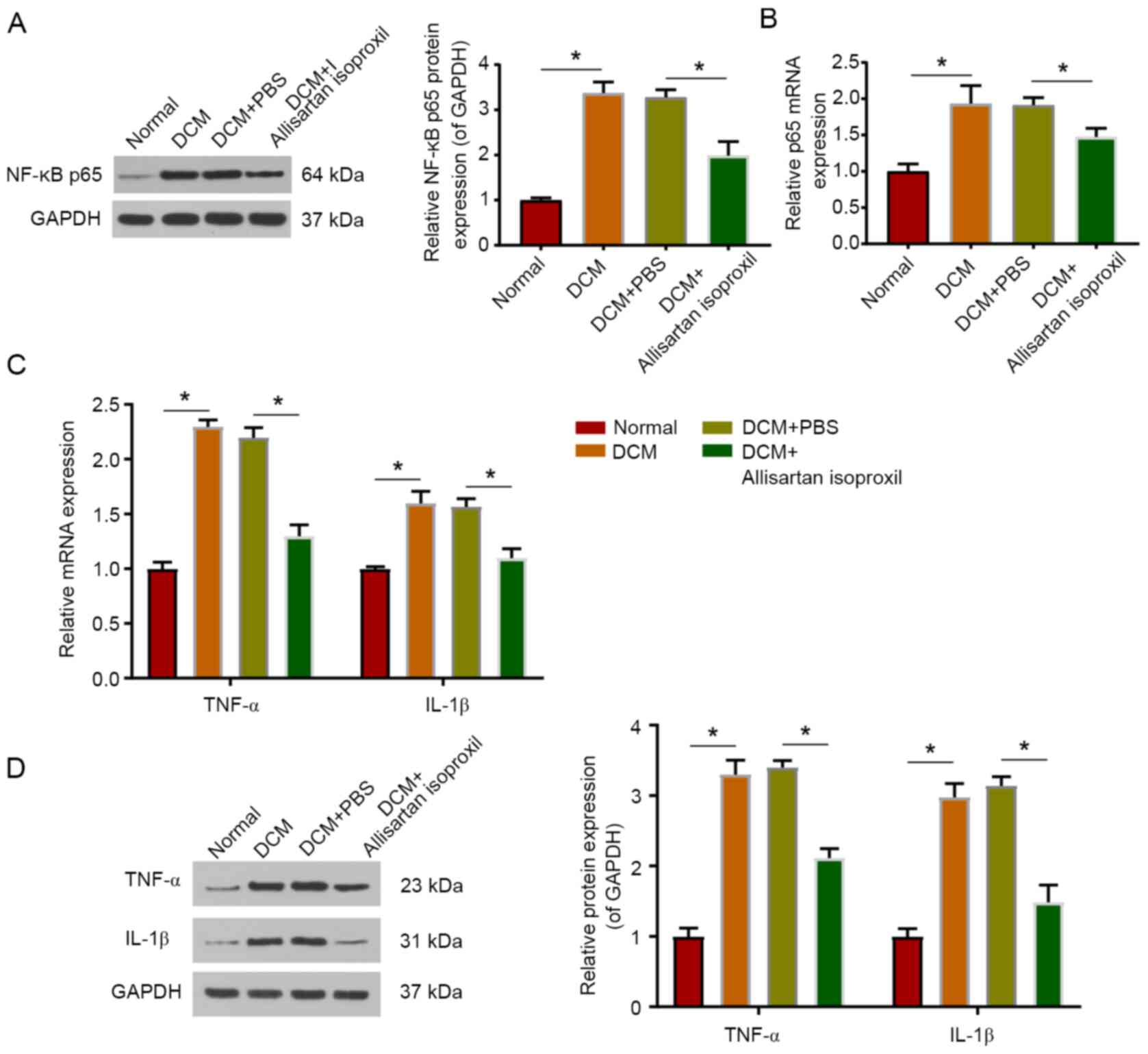
Allisartan isoproxil can attenuate
inflammation in DCM rats
As a transcription factor, NF-κB is activated by
phosphorylation and transfers into the nucleus to regulate the
transcription of inflammatory cytokine genes, including TNF-α and
IL-1β (40,41). The protein level of phosphorylated
NF-κB p65 was examined by western blotting, and the mRNA level was
measured by RT-qPCR. The present data indicated that the protein
level of NF-κB p65 was significantly upregulated in DCM rats, while
allisartan isoproxil treatment partially restored the protein and
mRNA levels of NF-κB p65 (Fig. 4A and
B). In addition, allisartan isoproxil significantly reduced the
upregulation of TNF-α and IL-1β protein expression in DCM rats
(Fig. 4D). Consistent results were
also obtained by RT-qPCR (Fig.
4C).
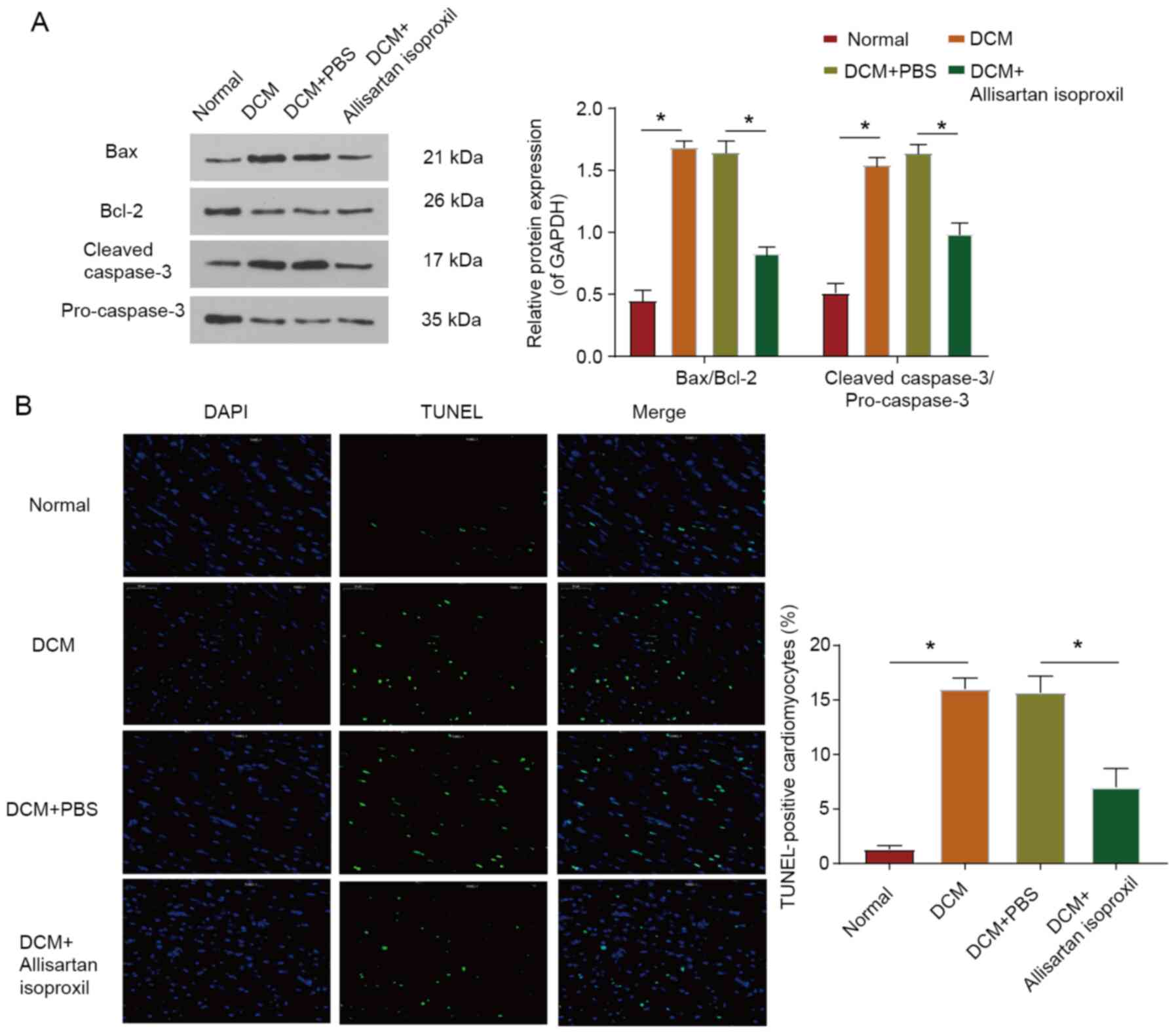
Cardiomyocyte apoptosis in DCM rats is
reduced by allisartan isoproxil
Myocardial cell injury leads to cell apoptosis
(6). To investigate the protective
effect of allisartan isoproxil on myocardial apoptosis in DCM rats,
the expression levels of Bax, Bcl-2, pro-caspase-3 and cleaved
caspase-3 were assessed by western blotting (Fig. 5A). The Bax/Bcl-2 and cleaved
caspase-3/pro-caspase-3 ratios were significantly decreased in DCM
rats treated with allisartan isoproxil (Fig. 5A). Moreover, cardiomyocytes
underwent apoptotic death in diabetic cardiomyopathy, as evidenced
by increased positive TUNEL staining, but this effect was reversed
by allisartan isoproxil (Fig. 5B).
In summary, the decrease in proapoptotic proteins, the increase in
antiapoptotic proteins and the decrease in apoptotic cardiomyocytes
all indicated that allisartan isoproxil protected cardiomyocytes
from apoptosis in DCM.
Discussion
Diabetes is known to cause numerous complications,
including cardiomyopathy. Persistent high blood glucose levels
cause oxidative stress, inflammation, and apoptosis, and these
pathological reactions are related to the pathogenesis of DCM
(42,43). Long-term diabetes is associated with
persistently high blood glucose levels and may be associated with
myocardial tissue damage. The exacerbation of oxidative stress
injury and increased inflammatory response under sustained
hyperglycaemia are considered to be the key mechanisms of DCM
induction (6,8). These two harmful mechanisms promote
myocardial tissue fibrosis, activate the apoptosis pathway, and
ultimately damage the structure and function of the heart (44,45).
Allisartan isoproxil is a new nonpeptide angiotensin II receptor
blocker precursor drug. Compared with losartan, allisartan
isoproxil has higher absorption efficiency, better blood pressure
lowering effect and lower adverse reactions (25,26).
In the present study, it was revealed that treatment of DCM with
allisartan isoproxil in diabetic rats alleviated inflammation and
oxidative stress injury. The protective effect of allisartan ester
was achieved by inhibiting the aberrant activation of NF-κB and
activating the antioxidant Nrf2 system. However, it should be noted
that when designing the experiment, we neglected to set a positive
reference group. Nonetheless, our results provide insights into the
future exploration of allisartan isoproxil in the treatment of
DCM.
A rat model of diabetes induced by STZ and an HFD
was first established. The effect of allisartan isoproxil on
cardiac function in diabetic rats was investigated by
echocardiography, and the results revealed that diabetic rats
exhibited cardiac dysfunction. However, allisartan isoproxil
protected the heart functions of the rats by increasing their heart
EF%, E™/A™ ratio and LVPWs. Notably, LVPWs in our diabetic rat
model was reduced, which is consistent with the results of Zhao
et al (8). Histological
examination of the diabetic heart revealed increased myocardial
damage and collagen deposition (33), consistent with the present results.
Furthermore, aberrant expression of apoptosis-related proteins was
also revealed in the heart tissues of DCM rats.
Hyperglycaemia-induced oxidative stress, inflammation and cell
damage are the main causes of myocardial fibrosis (46,47).
NF-κB, a key transcription factor associated with
inflammatory responses, activates NF-κB translocation into the
nucleus and regulates the expression of related genes, including
TNF-α, IL-1β and IL-6 (48,49). Moreover, NF-κB has been revealed to
play a pivotal role in the development of DCM (50). A recent study reported that
activation of NF-κB induced increased oxidative stress, which could
lead to heart dysfunction in DCM (51). Notably, inflammatory pathways in DCM
are closely related to oxidative stress signalling and ultimately
promote the development of myocardial injury (52,53).
In the present study, DCM was associated with increased cardiac
NF-κB p65 and TNF-α expression, while allisartan isoproxil
treatment partially restored the protein and mRNA levels of NF-κB
p65 and IL-1β. Allisartan isoproxil treatment prevented cardiac
injury in diabetic rats, and this effect may be attributed to
attenuated inflammation.
A growing body of evidence has revealed that
oxidative stress caused by excessive ROS in hyperglycaemia is
associated with the progression of myocardial injury (54,55).
Cardiac oxidative stress is closely related to myocardial fibrosis
and cardiomyocyte apoptosis, and excessive oxidative stress can
lead to severe cardiac dysfunction and heart failure (8,11).
Nrf2, a member of the nuclear factor erythroid 2 family of nuclear
basic leucine-zipper transcription factors, activates the
transcription and translation of several antioxidant genes and
proteins by binding to AREs (19).
As one of the key regulators of the antioxidant defence system,
Nrf2 can mediate the expression of HO-1 and NQO1, thus playing an
antioxidative stress role (14,15).
Civantos et al (56)
revealed that sitagliptin treatment could downregulate the
expression of miR-200a, thereby abrogating its inhibitory effect on
the Keap-1/Nrf2 pathway and ultimately alleviating diabetic
nephropathy in rats. In STZ-induced diabetic mouse models,
activated Nrf2 helped prevent the occurrence and progression of
diabetic retinopathy (57).
Moreover, existing research has reported that SIRT1 could activate
the Nrf2/ARE pathway in glomerular mesangial cells (19). The present results revealed that
allisartan isoproxil treatment could partially reverse the
downregulation of both SIRT1 and Nrf2 protein expression in the
heart tissue of diabetic rats. Moreover, the trend in the
expression of HO-1 was consistent with that of Nrf2. The expression
level of MDA and the activity of SOD were also measured and the
results demonstrated that allisartan isoproxil could reduce
oxidative stress in DCM rats, at least in part, through the
restoration of Nrf2.
The present results indicated that allisartan
isoproxil alleviated DCM by attenuating diabetes-induced oxidative
stress and inflammation through the SIRT1/Nrf2/NF-κB signalling
pathway. In conclusion, allisartan isoproxil had a protective
effect on DCM. Therefore, allisartan isoproxil may also be useful
in the treatment of DCM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Provincial
Basic Public Welfare Research Projects (grant no. LGC20H020001),
the Zhejiang Medical and Health Science and Technology Project
(grant no. 2017KY200) and the Chinese Medicine Research Programme
of Zhejiang Province (grant no. 2016ZB016).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author upon reasonable
request.
Authors contributions
XL, QJ and QZ contributed to the conception and
design of the study. KW and MC performed the data analysis and
interpretation of the data. QJ and QZ drafted the manuscript. XL
and QJ performed critical revisions for important intellectual
content of the manuscript. All authors approved the final version
to be published.
Ethics approval and consent to
participate
All animal study protocols were approved by the
Institutional Ethics Committee of The First Affiliated Hospital
with Nanjing Medical University (approval no. IACUC-1803019) and
were performed according to the guidelines of the US Department of
Health (NIH Publication no. 85-23, revised 1996) for the use and
care of laboratory animals. This article does not contain any
studies with human participants performed by any of the
authors.
Patient consent for publication
Not applicable.
Competing of interests
All authors declare that they have no competing
interests.
References
|
1
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Xiao J, Zhu H, Wei X, Platt C,
Damilano F, Xiao C, Bezzerides V, Boström P, Che L, et al: miR-222
is necessary for exercise-induced cardiac growth and protects
against pathological cardiac remodeling. Cell Metab. 21:584–595.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tribouilloy C, Rusinaru D, Mahjoub H,
Tartière JM, Kesri-Tartière L, Godard S and Peltier M: Prognostic
impact of diabetes mellitus in patients with heart failure and
preserved ejection fraction: A prospective five-year study. Heart.
94:1450–1455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devereux RB, Roman MJ, Paranicas M,
O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER and
Howard BV: Impact of diabetes on cardiac structure and function:
The strong heart study. Circulation. 101:2271–2276. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howard BV, Cowan LD, Go O, Welty TK,
Robbins DC and Lee ET: Adverse effects of diabetes on multiple
cardiovascular disease risk factors in women. The Strong Heart
Study. Diabetes Care. 21:1258–1265. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma ZG, Yuan YP, Xu SC, Wei WY, Xu CR,
Zhang X, Wu QQ, Liao HH, Ni J and Tang QZ: CTRP3 attenuates cardiac
dysfunction, inflammation, oxidative stress and cell death in
diabetic cardiomyopathy in rats. Diabetologia. 60:1126–1137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou X, An G and Lu X: Hydrogen sulfide
attenuates the development of diabetic cardiomyopathy. Clin Sci
(Lond). 128:325–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao MX, Zhou B, Ling L, Xiong XQ, Zhang
F, Chen Q, Li YH, Kang YM and Zhu GQ: Salusin-β contributes to
oxidative stress and inflammation in diabetic cardiomyopathy. Cell
Death Dis. 8:e26902017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajesh M, Bátkai S, Kechrid M,
Mukhopadhyay P, Lee WS, Horváth B, Holovac E, Cinar R, Liaudet L,
Mackie K, et al: Cannabinoid 1 receptor promotes cardiac
dysfunction, oxidative stress, inflammation, and fibrosis in
diabetic cardiomyopathy. Diabetes. 61:716–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajesh M, Mukhopadhyay P, Bátkai S, Patel
V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B,
Becker L, et al: Cannabidiol attenuates cardiac dysfunction,
oxidative stress, fibrosis, and inflammatory and cell death
signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol.
56:2115–2125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duecker R, Baer P, Eickmeier O, Strecker
M, Kurz J, Schaible A, Henrich D, Zielen S and Schubert R:
Oxidative stress-driven pulmonary inflammation and fibrosis in a
mouse model of human ataxia-telangiectasia. Redox Biol. 14:645–655.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rajasekaran NS, Varadharaj S, Khanderao
GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL and Benjamin IJ:
Sustained activation of nuclear erythroid 2-related factor
2/antioxidant response element signaling promotes reductive stress
in the human mutant protein aggregation cardiomyopathy in mice.
Antioxid Redox Signal. 14:957–971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Cheng Y, Wu H, Kong L, Wang S, Xu Z,
Zhang Z, Tan Y, Keller BB, Zhou H, et al: Metallothionein is
downstream of Nrf2 and partially mediates sulforaphane prevention
of diabetic cardiomyopathy. Diabetes. 66:529–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu MC, Ji JA, Jiang ZY and You QD: The
Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic
target: An update. Med Res Rev. 36:924–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Magesh S, Chen Y and Hu L: Small molecule
modulators of Keap1-Nrf2-ARE pathway as potential preventive and
therapeutic agents. Med Res Rev. 32:687–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L
and Peng J: MicroRNA-140-5p aggravates doxorubicin-induced
cardiotoxicity by promoting myocardial oxidative stress via
targeting Nrf2 and Sirt2. Redox Biol. 15:284–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karbasforooshan H and Karimi G: The role
of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother.
90:386–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang K, Huang J, Xie X, Wang S, Chen C,
Shen X, Liu P and Huang H: Sirt1 resists advanced glycation end
products-induced expressions of fibronectin and TGF-β1 by
activating the Nrf2/ARE pathway in glomerular mesangial cells. Free
Radic Biol Med. 65:528–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Mingo Á, de Gregorio E, Moles A,
Tarrats N, Tutusaus A, Colell A, Fernandez-Checa JC, Morales A and
Marí M: Cysteine cathepsins control hepatic NF-κB-dependent
inflammation via sirtuin-1 regulation. Cell Death Dis. 7:e24642016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong B, Yu QT, Dai HY, Gao YY, Zhou ZL,
Zhang L, Jiang H, Gao F, Li SY, Zhang YH, et al:
Angiotensin-converting enzyme-2 overexpression improves left
ventricular remodeling and function in a rat model of diabetic
cardiomyopathy. J Am Coll Cardiol. 59:739–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mori J, Basu R, McLean BA, Das SK, Zhang
L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD and Oudit GY:
Agonist-induced hypertrophy and diastolic dysfunction are
associated with selective reduction in glucose oxidation: A
metabolic contribution to heart failure with normal ejection
fraction. Circ Heart Fail. 5:493–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu MY, Ma XJ, Yang C, Tao X, Liu AJ, Su DF
and Liu JG: Effects of allisartan, a new AT(1) receptor blocker, on
blood pressure and end-organ damage in hypertensive animals. Acta
Pharmacol Sin. 30:307–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JQ, Yang GH, Zhou X, Liu JX, Shi R,
Dong Y, Chen SB and Li YM: Effects of allisartan isoproxil on blood
pressure and target organ injury in patients with mild to moderate
essential hypertension. Medicine (Baltimore). 98:e149072019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Li XH, Huang ZJ, Yang GP, Zhang GG,
Zhao SP, Guo Y, Lu SJ, Ma JL, Meng FB, et al: A randomized, double
blind, placebo-controlled, multicenter phase II trial of Allisartan
Isoproxil in essential hypertensive population at low-medium risk.
PLoS One. 10:e01175602015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Os I, Franco V, Kjeldsen SE, Manhem K,
Devereux RB, Gerdts E, Hille DA, Lyle PA, Okin PM, Dahlöf B and
Oparil S: Effects of losartan in women with hypertension and left
ventricular hypertrophy: Results from the Losartan Intervention for
Endpoint Reduction in Hypertension Study. Hypertension.
51:1103–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bokma JP, Winter MM, van Dijk AP, Vliegen
HW, van Melle JP, Meijboom FJ, Post MC, Berbee JK, Boekholdt SM,
Groenink M, et al: Effect of losartan on right ventricular
dysfunction: Results from the double-blind, randomized REDEFINE
Trial (Right ventricular dysfunction in tetralogy of fallot:
Inhibition of the renin-angiotensin-aldosterone system) in adults
with repaired tetralogy of fallot. Circulation. 137:1463–1471.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Li J and Li D: Losartan reduces
myocardial interstitial fibrosis in DCMrats by inhibiting JAK/STAT
signaling pathway. Int J Clin Exp Pathol. 8:466–473.
2015.PubMed/NCBI
|
|
30
|
Axelsson A, Iversen K, Vejlstrup N, Ho CY,
Havndrup O, Kofoed KF, Norsk J, Jensen M and Bundgaard H:
Functional effects of losartan in hypertrophic cardiomyopathy-a
randomised clinical trial. Heart. 102:285–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimada YJ, Passeri JJ, Baggish AL,
O'Callaghan C, Lowry PA, Yannekis G, Abbara S, Ghoshhajra BB,
Rothman RD, Ho CY, et al: Effects of losartan on left ventricular
hypertrophy and fibrosis in patients with nonobstructive
hypertrophic cardiomyopathy. JACC Heart Fail. 1:480–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carswell CI and Goa KL: Losartan in
diabetic nephropathy. Drugs. 63:407–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ti Y, Xie GL, Wang ZH, Bi XL, Ding WY,
Wang J, Jiang GH, Bu PL, Zhang Y, Zhong M and Zhang W: TRB3 gene
silencing alleviates DCMin a type 2 diabetic rat model. Diabetes.
60:2963–2974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bati K, Kwape TE and Chaturvedi P:
Anti-Diabetic effects of an ethanol extract of cassia abbreviata
stem bark on diabetic rats and possible mechanism of its action:
-Anti-diabetic properties of cassia abbreviata. J Pharmacopuncture.
20:45–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng D, Zhang Y, Hu Y, Guan J, Xu L, Xiao
W, Zhong Q, Ren C, Lu J, Liang J and Hou J: Long noncoding RNA
Crnde attenuates cardiac fibrosis via Smad3-Crnde negative feedback
in diabetic cardiomyopathy. FEBS J. 286:1645–1655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghorbanzadeh V, Mohammadi M, Dariushnejad
H, Chodari L and Mohaddes G: Effects of crocin and voluntary
exercise, alone or combined, on heart VEGF-A and HOMA-IR of HFD/STZ
induced type 2 diabetic rats. J Endocrinol Invest. 39:1179–1186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou Y, Shao J, Fu Q, Li J, Sun J and He Z:
Spray-dried nanocrystals for a highly hydrophobic drug: Increased
drug loading, enhanced redispersity, and improved oral
bioavailability. Int J Pharm. 516:372–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai Y, Cui W, Xin Y, Miao X, Barati MT,
Zhang C, Chen Q, Tan Y, Cui T, Zheng Y and Cai L: Prevention by
sulforaphane of DCMis associated with up-regulation of Nrf2
expression and transcription activation. J Mol Cell Cardiol.
57:82–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koller WC and Vetere-Overfield B:
Usefulness of a writing aid in writer's cramp. Neurology.
39:149–150. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Czarnecka AK, Milewski K and Zielińska M:
Asymmetric dimethylarginine and hepatic encephalopathy: Cause,
effect or association? Neurochem Res. 42:750–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roul D and Recchia FA: Metabolic
alterations induce oxidative stress in diabetic and failing hearts:
Different pathways, same outcome. Antioxid Redox Signal.
22:1502–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Palomer X, Pizarro-Delgado J and
Vázquez-Carrera M: Emerging actors in diabetic cardiomyopathy:
Heartbreaker biomarkers or therapeutic targets? Trends Pharmacol
Sci. 39:452–467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu Y, Qian X, Li J, Lin X, Luo J, Huang J
and Jin Z: Astragaloside-IV protects H9C2(2-1) cardiomyocytes from
high glucose-induced injury via miR-34a-mediated autophagy pathway.
Artif Cells Nanomed Biotechnol. 47:4172–4181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsai TH, Lin CJ, Chua S, Chung SY, Chen
SM, Lee CH and Hang CL: Deletion of RasGRF1 attenuated interstitial
fibrosis in streptozotocin-induced DCMin mice through affecting
inflammation and oxidative stress. Int J Mol Sci. 19:30942018.
View Article : Google Scholar
|
|
46
|
Li L, Luo W, Qian Y, Zhu W, Qian J, Li J,
Jin Y, Xu X and Liang G: Luteolin protects against DCMby inhibiting
NF-κB-mediated inflammation and activating the Nrf2-mediated
antioxidant responses. Phytomedicine. 59:1527742019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Biernacka A, Cavalera M, Wang J, Russo I,
Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW,
et al: Smad3 signaling promotes fibrosis while preserving cardiac
and aortic geometry in obese diabetic mice. Circ Heart Fail.
8:788–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rojas A, Delgado-López F, González I,
Pérez-Castro R, Romero J and Rojas I: The receptor for advanced
glycation end-products: A complex signaling scenario for a
promiscuous receptor. Cell Signal. 25:609–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lorenzo O, Picatoste B, Ares-Carrasco S,
Ramírez E, Egido J and Tuñón J: Potential role of nuclear factor κB
in diabetic cardiomyopathy. Mediators Inflamm. 2011:6520972011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li H, Shi Y, Wang X, Li P, Zhang S, Wu T,
Yan Y, Zhan Y, Ren Y, Rong X, et al: Piceatannol alleviates
inflammation and oxidative stress via modulation of the Nrf2/HO-1
and NF-κB pathways in diabetic cardiomyopathy. Chem Biol Interact.
310:1087542019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Westermann D, Rutschow S, Van Linthout S,
Linderer A, Bücker-Gärtner C, Sobirey M, Riad A, Pauschinger M,
Schultheiss HP and Tschöpe C: Inhibition of p38 mitogen-activated
protein kinase attenuates left ventricular dysfunction by mediating
pro-inflammatory cardiac cytokine levels in a mouse model of
diabetes mellitus. Diabetologia. 49:2507–2513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Westermann D, Rutschow S, Jäger S,
Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M
and Tschöpe C: Contributions of inflammation and cardiac matrix
metalloproteinase activity to cardiac failure in diabetic
cardiomyopathy: The role of angiotensin type 1 receptor antagonism.
Diabetes. 56:641–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu T, Sheu SS, Robotham JL and Yoon Y:
Mitochondrial fission mediates high glucose-induced cell death
through elevated production of reactive oxygen species. Cardiovasc
Res. 79:341–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guo Y, Zhuang X, Huang Z, Zou J, Yang D,
Hu X, Du Z, Wang L and Liao X: Klotho protects the heart from
hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated
inflammation both in vitro and in vivo. Biochim Biophys Acta Mol
Basis Dis. 1864:238–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Civantos E, Bosch E, Ramirez E, Zhenyukh
O, Egido J, Lorenzo O and Mas S: Sitagliptin ameliorates oxidative
stress in experimental diabetic nephropathy by diminishing the
miR-200a/Keap-1/Nrf2 antioxidant pathway. Diabetes Metab Syndr
Obes. 10:207–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung
ER, Huang H, Wu L, Eberhart C, Handa JT, et al: NRF2 plays a
protective role in diabetic retinopathy in mice. Diabetologia.
57:204–213. 2014. View Article : Google Scholar : PubMed/NCBI
|