Introduction
Bone is in a dynamic balance between bone formation
and bone resorption (1). With aging
and after the menopause, an imbalance in bone resorption relative
to formation results in osteoporosis (2). Osteoporosis is a common skeletal
disease characterized by decreased bone mass, deterioration of bone
microarchitecture and increased susceptibility to fractures. With
the gradual aging of the population in many countries, osteoporosis
has become a major global health concern (3). In the United States, osteoporosis is
projected to affect nearly 14 million adults over the age of 50 by
the year 2020 (4). Worldwide, ~200
million women have osteoporosis (5).
Skeletal development is regulated by numerous
homeodomain proteins, including the distal-less (DLX) family, which
play an important role in the development of bone tissue (6). DLX family has six transcription factor
members, known as DLX1-6 (7). DLX3
is mapped to 17q21.33 and has a notable impact on the development
of organs, including glands, teeth and hair follicles (8,9). In
humans, mutation of DLX3 can cause tricho-dento-osseous syndrome,
which is characterized by hypoplasia of the hair, enamel and
dentin, as well as a high bone density (8).
Bone marrow mesenchymal stem cells (BMSCs) are a
type of post-natal stem cell with the potential to differentiate
into different cells, such as osteoblasts, chondrocytes and
adipocytes (10). Bone formation
depends on the osteogenic potential and proliferative capability of
BMSCs, but the proliferative ability of BMSCs is limited, which has
restricted their application in clinical settings (10,11).
Therefore, there is a need for an improved cell source to overcome
the limitations of BMSCs (11).
Induced pluripotent stem cell-derived mesenchymal stem cells
(iPSC-MSCs), which are an unlimited source of MSCs, exhibit
favorable proliferation, cell viability, and osteo- and
chondrogenic differentiation potential, and thus could be used to
meet the requirement of bone regeneration (11–15).
Previously, several cell culture supplements have been utilized to
derive MSCs from iPSC (11). The
most commonly used supplements are synthetic coatings, such as
PMEDSAH (15), fibrillar collagen
(16), extracellular matrix Geltrex
(17) and fibronectin (17). Moreover, a few small molecules, such
as SB431542 (TGF-β pathway inhibitor) (18) and CHIR99021 (GSK inhibitor)
(19) have been applied to obtain
iPSC-MSCs. In the current study, iPSC-MSCs were derived using
Geltrex, which retains the complete osteogenesis function of the
cells (12–14,17).
A previous study revealed that overexpression of
DLX3 enhances the osteogenic differentiation of human BMSCs
(20). Similar results have also
been reported in human dental pulp cells (DPCs), in which
overexpressed DLX3 decreases cell proliferation and increases mRNA
expression levels of osteo- and chondrogenic markers, including
alkaline phosphatase (ALP), dentin sialophosphoprotein (DSPP) and
dentin matrix acidic phosphoprotein 1 (DMP1) (21). Thus, the DLX3 gene is crucial for
osteogenic differentiation. However, the role of DLX3 gene in
regulating iPSC-MSCs is yet to be fully elucidated.
In the present study, DLX3-overexpression iPSC-MSCs
(iPSC-MSC-DLX3) were constructed, the expression of DLX3 in
iPSC-MSCs and iPSC-MSC-DLX3 was examined by reverse
transcription-quantitative PCR (RT-qPCR) and western blotting, and
then the effects of the DLX3 gene on osteogenic differentiation of
these cells was evaluated.
Materials and methods
Derivation of iPSC-MSCs
The experiment protocol of the present study was
approved by the Ethical Review Committee of Jinan University
(approval no. 2015-045). Human iPSC line was obtained from the
South China Institute for Stem Cell Biology and Regenerative
Medicine Group of the Chinese Academy of Sciences. iPSCs were
cultured in mTeSR1 medium (Stemcell Technologies, Inc.) on Geltrex™
LDEV-Free Reduced Growth Factor Basement Membrane Matrix (Gibco;
Thermo Fisher Scientific, Inc.) coated dishes for 5 days. Then, the
medium was replaced with DMEM (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 2
mM L-glutamine, 1% penicillin/streptomycin and 0.1 mM non-essential
amino acids (Gibco; Thermo Fisher Scientific, Inc.). When these
cells reached 80% confluence, they were passaged. When most of the
cells presented spindle-like morphology, they were collected and
applied for subsequent experiments.
Flow cytometry analysis
Flow cytometry was used to evaluate surface markers
of iPSC-MSCs. Briefly, the cells were harvested, and then 3% BSA
(Gibco; Thermo Fisher Scientific, Inc.) was used to block
non-specific antigens on the cell surface at 37°C for 30 min.
Subsequently, the cells were incubated with monoclonal antibodies
(all purchased from BioLegend, Inc.) against CD73 (cat. no.
344005), CD90 (cat. no. 328113), CD105 (cat. no. 323207), CD34
(cat. no. 343607) and CD45 (cat. no. 368511). Then, the cells were
washed three times with BSA to remove non-specific antibodies. A
Guava® easyCyte™ flow cytometer and Guava®
Suite Software 3.4 (both EMD Millipore) were used to analyze
surface antigens.
Differentiation capability
analysis
The osteogenic, adipogenic and chondrogenic
differentiation capabilities of iPSC-MSCs were analyzed. To study
osteogenic differentiation, iPSC-MSCs at 1×104/well
density were seeded on 6-well plates (Corning, Inc.) and incubated
with 2 ml StemPro™ Osteogenesis Differentiation medium (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C for 3 weeks. The
Osteogenesis Differentiation medium was replaced every 3 days. The
cells were fixed with 4% paraformaldehyde for 30 min at room
temperature. The ALP activity of iPSC-MSCs was assayed using an ALP
staining kit (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's instructions, and the calcified matrix deposition
was detected using a Von Kossa staining kit (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions and observed under a light microscope (Zeiss Axio
Observer.Z1; Carl Zeiss AG).
In order to study adipogenic differentiation,
iPSC-MSCs at 1×104/well density were seeded on 6-well
plates and incubated with 2 ml StemPro™ Adipogenesis
Differentiation medium (Gibco; Thermo Fisher Scientific, Inc.) at
37°C for 3 weeks. Adipogenesis Differentiation medium was replaced
every 3 days, and subsequently the cells were assayed with an Oil
Red O kit (Nanjing Jiancheng Bioengineering Institute) for 15 min
at room temperature.
To study chondrogenic differentiation, iPSC-MSCs at
1×104/well density were seeded on 6-well plates and
incubated with 2 ml StemPro™ Chondrogenesis Differentiation medium
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C for 3 weeks. The
Chondrogenesis Differentiation medium was replaced every 3 days,
and subsequently the cells were assayed with an Alcian blue kit
(Nanjing Jiancheng Bioengineering Institute) for 30 min at room
temperature.
Lentiviral plasmid transfection
Human DLX3 gene primers (PrimerBank ID:38327640c1,
Table I) were designed according to
PrimerBank online (The Massachusetts General Hospital) (22). Subsequently, PCR amplification was
performed according to our previous study (23). The amplified DLX3 primers were
treated with EcoRI and BamHI, and then combined into the lentivirus
vector pCDH-CMV-MCS-EF1-copGFP (pCDH; System Biosciences, LLC) to
construct the recombinant plasmid pCDH-DLX3. The target gene was
transduced into 4×105 293FT cells per well in 6 well
plate using a combination of enveloping plasmid, packaging plasmid
and recombinant lentiviral plasmid (3rd generation system; Cyagen
Biosciences, Inc.) at 37°C. Then, 48 h later, 293FT cells were
centrifuged at 10,000 × g at 4°C for 4 h and the supernatant was
collected to infect the 3rd passage of iPSC-MSCs (iPSC-MSC-DLX3) at
37°C. The MOI was 50, and concentration of Polybrene was 5 µg/ml.
Cells were cultured at 37°C in DMEM containing 10% FBS with 1 µg/ml
puromycin for 3 days in order to select puromycin-resistant cells
and 0.25 µg/ml puromycin was used for maintenance. Similarly,
iPSC-MSCs transfected with blank lentivirus vector (iPSC-MSC-GFP)
were generated and used as the control. After 2 days, reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis
were performed to evaluate the expression of DLX3 in the two
groups.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequences
(5→3) | Size (bp) |
|---|
| DLX3 | F:
TACCCTGCCCGAGTCTTCTG | 111 |
|
| R:
TGGTGGTAGGTGTAGGGGTTC |
|
| ALP | F:
ACCACCACGAGAGTGAACCA | 79 |
|
| R:
CGTTGTCTGAGTACCAGTCCC |
|
| OPN | F:
CTCCATTGACTCGAACGACTC | 230 |
|
| R:
CAGGTCTGCGAAACTTCTTAGAT |
|
| OCN | F:
CACTCCTCGCCCTATTGGC | 112 |
|
| R:
CCCTCCTGCTTGGACACAAAG |
|
| COL-1 | F:
GAGGGCCAAGACGAAGACATC | 140 |
|
| R:
CAGATCACGTCATCGCACAAC |
|
| GAPDH | F:
GGAGCGAGATCCCTCCAAAAT | 197 |
|
| R:
GGCTGTTGTCATACTTCTCATGG |
|
Cell proliferation assay
Proliferation of iPSC-MSC-GFP and iPSC-MSC-DLX3 was
assessed using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.). On the first day, cells were seeded on 96-well
plates at 2×103 cells/well, and then cultured with DMEM
plus 10% FBS. Cell viability was evaluated on day 1, 3, 5 and 7
post-transfection. Optical density (OD) at 450 nm was recorded
using an enzyme immunoassay reader (Bio-Rad Laboratories,
Inc.).
RT-qPCR analysis
Total RNA was isolated from iPSC-MSC-GFP and
iPSC-MSC-DLX3 using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) on day 7. NanoDrop™ 2000 system (Thermo
Fisher Scientific, Inc.) was used to test RNA concentrations. The
extracted RNA was reverse-transcribed into cDNA using iScript™ gDNA
Clear cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. qPCR analysis was
operated using PowerUp SYBR-Green Master Mix (Invitrogen; Thermo
Fisher Scientific, Inc.) and measured by spectrofluorimetric iQ5
Thermal iCycler (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. The primer sequences of DLX3, ALP,
osteopontin (OPN), osteocalcin (OCN), Collagen Type I (COL-1) and
GAPDH are presented in Table I. The
relative expression of target genes was determined using the
2−ΔΔCq method (24) and
normalized to GAPDH. Each sample was tested three times.
Western blot analysis
A total of 7 days after transfection, iPSC-MSC-GFP
and iPSC-MSC-DLX3 were rinsed with PBS and lysed in 0.1 ml RIPA
buffer containing 10 mg/ml proteinase inhibitor PMSF (Invitrogen;
Thermo Fisher Scientific, Inc.) on ice for 30 min. The lysed cells
were centrifuged at 10,000 × g for 10 min at 4°C, and the
supernatant was collected. During electrophoresis, 20 µg target
total protein/lane was separated via SDS-PAGE on a 10% gel
(Beyotime Institute of Biotechnology), which were subsequently
transferred to PVDF membranes (Thermo Fisher Scientific, Inc.) at
200 mA for 2 h. PVDF membranes were blocked using 5% non-fat milk
with TBS with 0.1% Tween-20 at room temperature for 2 h, and then
incubated with the primary antibodies at 4°C overnight. The primary
antibodies used were as follows: DLX3 (1:500; cat. no. ab64953;
Abcam), ALP (1:500; cat. no. ab83259; Abcam), OPN (1:500; cat. no.
ab8448; Abcam), OCN (1:500; cat. no. ab93876; Abcam), COL-1 (1:500;
cat. no. ab34710; Abcam) and GAPDH (1:2,500; cat. no. ab9485;
Abcam). Then, PVDF membranes were incubated with a secondary
antibody (1:2,500; cat. no. ab97051; Abcam) at 37°C for 2 h. GAPDH
was used as the control. The blotting results were visualized with
chemiluminescent western blotting detection reagents (Pierce;
Thermo Fisher Scientific, Inc.) and measured by Image-Pro Plus
version 6.0 (Media Cybernetics, Inc.).
ALP staining and mineralized nodule
counting
The transfected cells were seeded on 6-well plates
at an initial density of 3×104 cells/well and cultured
for 14 days to 80% confluence in DMEM containing 10% FBS. The cells
were fixed with 4% paraformaldehyde for 30 min at room temperature.
The ALP Staining kit (Nanjing Jiancheng Bioengineering Institute)
was used to stain cells for 30 min at room temperature on day 14
after transfection. In total, three randomized observation views
were selected, and the number of mineralized nodules was counted
under a microscope (Zeiss Axio Observer.Z1; Carl Zeiss AG).
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used to analyze
the data, which are presented as the mean ± SD. Statistical
significance was calculated using an unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
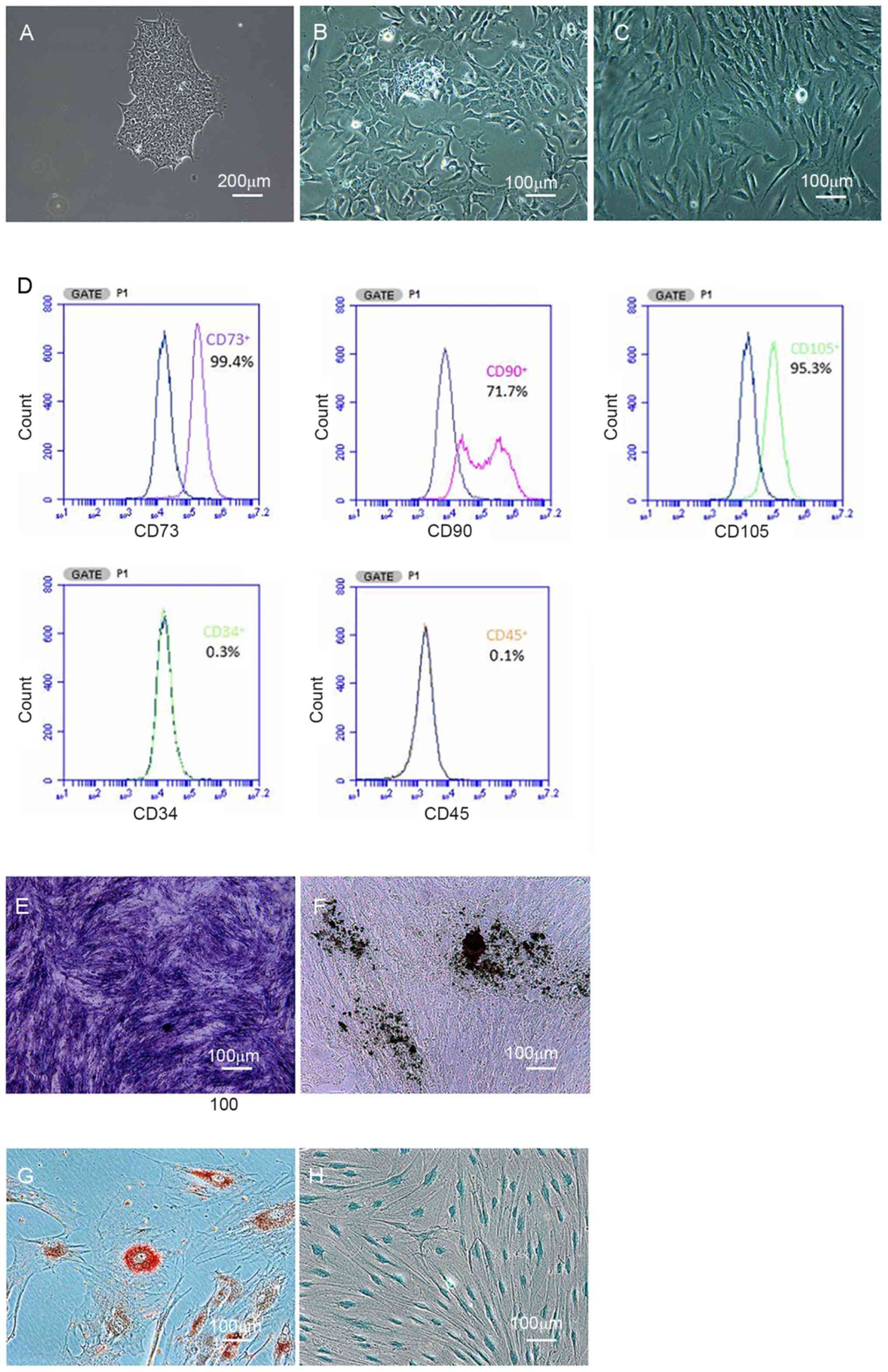
Characterization of iPSC-MSCs
As illustrated in Fig.
1A, human iPSCs presented as packed clones with clear margins.
After culturing in MSC medium for 14 days, iPSCs were gradually
induced into MSCs. Under the microscope, borders of the colonies
were eliminated (Fig. 1B), and
cells exhibited homogeneous spindle-like morphology (Fig. 1C). The iPSC-derived cells were
positive for CD73 (99%), CD90 (71%) and CD105 (95%), and negative
for CD34 and CD45, as determined by flow cytometric analysis
(Fig. 1D).
After osteogenic induction, iPSC-derived cells
displayed positive ALP staining (Fig.
1E) and Von Kossa staining (Fig.
1F). Additionally, iPSC-derived cells had positive Oil Red O
and Alcian blue staining after adipogenic (Fig. 1G) and chondrogenic induction
(Fig. 1H). Thus, iPSCs were
successfully induced into iPSC-MSCs.
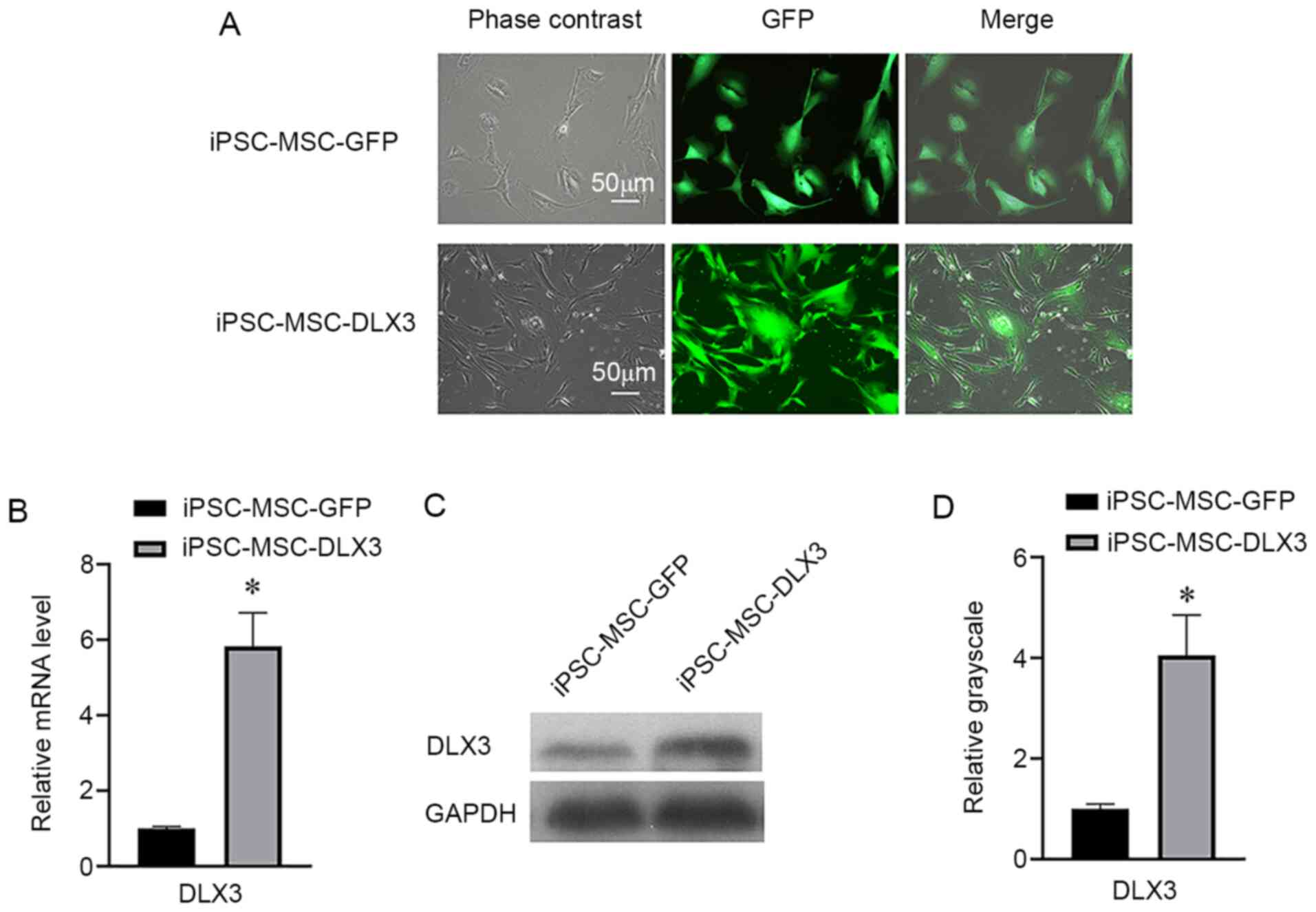
Expression of DLX3 in iPSC-MSCs
Both iPSC-MSC-GFP and iPSC-MSC-DLX3 groups had a GFP
expression percentage of ~100% (Fig.
2A). RT-qPCR results indicated that the relative mRNA
expression of DLX3 in iPSC-MSC-GFP and iPSC-MSC-DLX3 was 1.00±0.05
and 5.84±0.89, respectively (Fig.
2B). Moreover, the western blotting results demonstrated that
the relative DLX3 expression in iPSC-MSC-GFP and iPSC-MSC-DLX3 was
1.00±0.10 and 4.05±0.81, respectively (Fig. 2C and D). Based on these results, it
was suggested that the DLX3 gene was successfully transfected into
iPSC-MSCs.
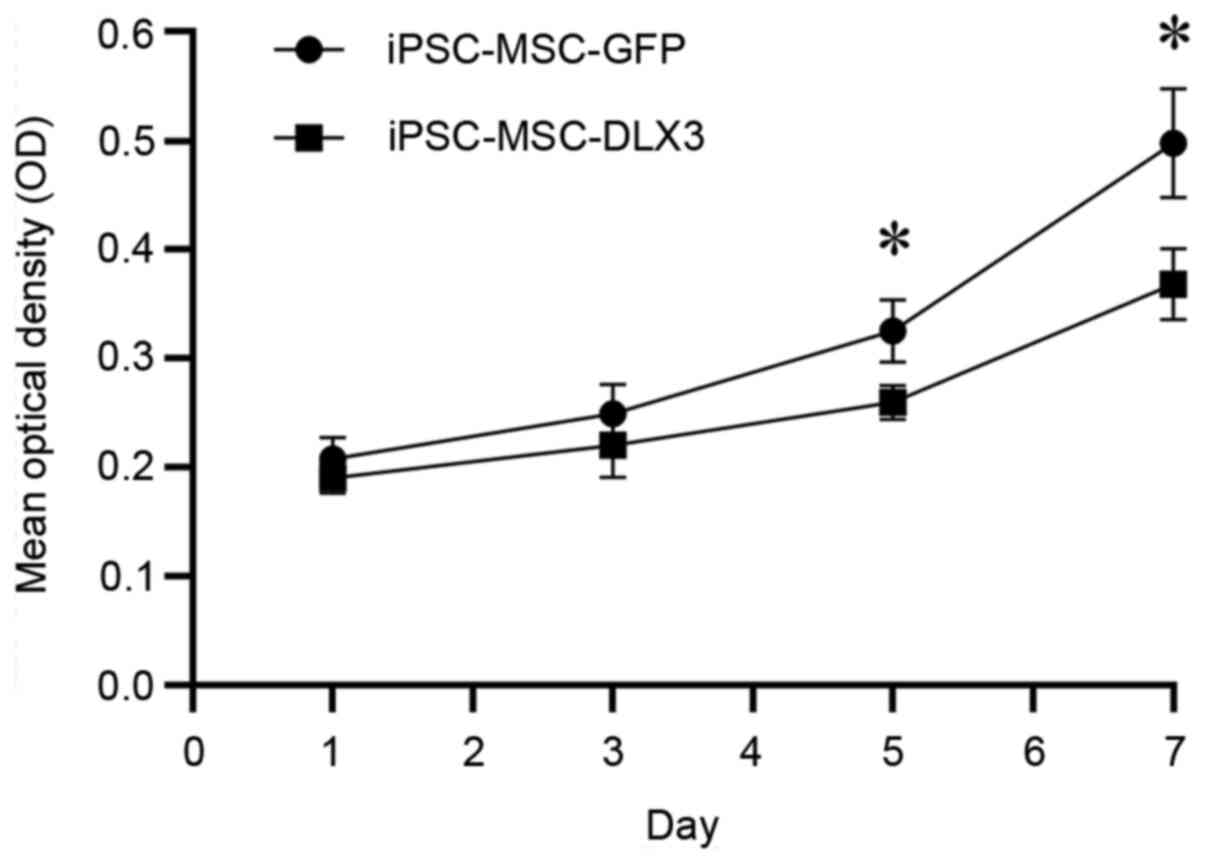
DLX3 regulates proliferation of
iPSC-MSCs
The OD values of iPSC-MSC-GFP on day 1, 3, 5 and 7
were 0.21±0.02, 0.25±0.03, 0.33±0.03 and 0.50±0.05, while the OD
values of iPSC-MSC-DLX3 on day 1, 3, 5 and 7 were 0.19±0.01,
0.22±0.03, 0.26±0.02 and 0.37±0.03, respectively (Fig. 3). There was no significant
difference in cell numbers between the two groups on days 1 and 3
(P>0.05). However, iPSC-MSC-DLX3 demonstrated significantly
lower proliferative activity compared with the iPSC-MSC-GFP group
on days 5 and 7 (P<0.05).
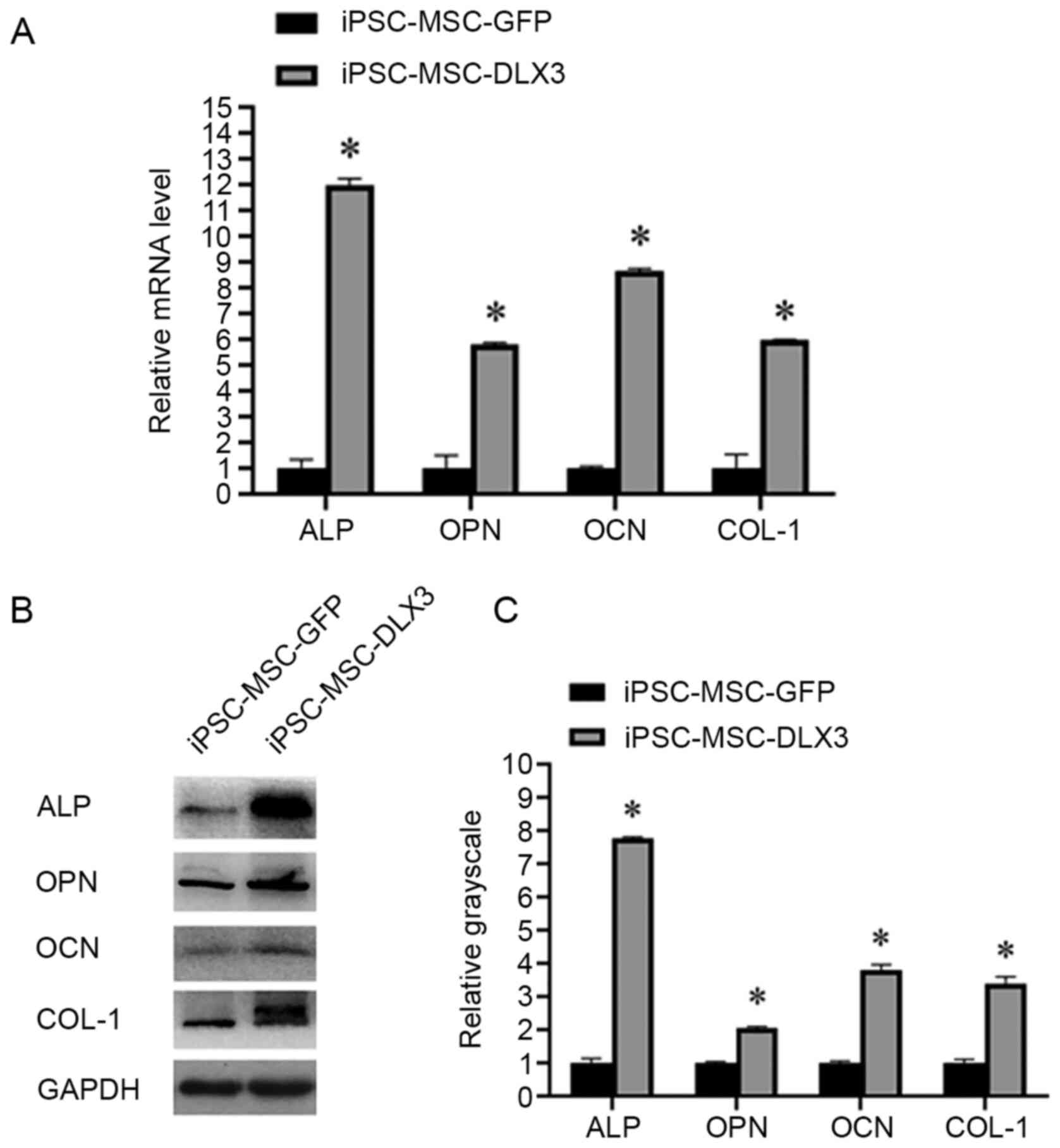
Expression of osteogenesis related
genes and proteins
After 7 days of transfection of iPSC-MSC-GFP and
iPSC-MSC-DLX3, the relative expression levels of ALP were 1.00±0.33
and 11.99±0.24, those of OPN were 1.00±0.49 and 5.80±0.07, those of
OCN were 1.00±0.06 and 8.64±0.11, those of COL-1 were 1.00±0.53 and
5.98±0.02. The results indicated that the mRNA expression levels of
osteogenic markers in iPSC-MSC-DLX3 were significantly higher
compared with those in iPSC-MSC-GFP (P<0.05; Fig. 4A).
Similarly, the relative expression levels of ALP
were 1.00±0.13 and 7.77±0.05, those of OPN were 1.00±0.04 and
2.05±0.04, those of OCN were 1.00±0.05 and 3.79±0.17, and those of
COL-1 were 1.00±0.11 and 3.38±0.22. The expression levels of the
osteogenic proteins in iPSC-MSC-DLX3 were significantly increased
compared with those in iPSC-MSC-GFP (P<0.05; Fig. 4B and C).
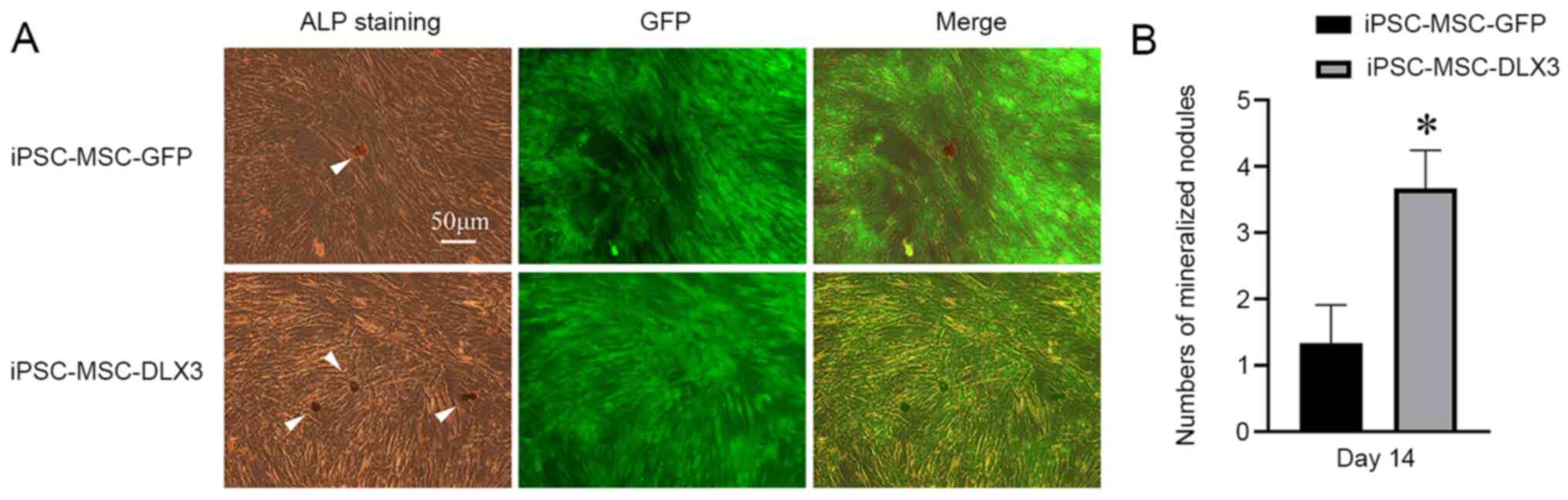
ALP activity and mineralization
As presented in Fig.
5A, ALP was stained as a golden color. ALP staining in the
iPSC-MSC-DLX3 group was increased and brighter compared with that
in the iPSC-MSC-GFP group. Mineralized nodules were stained as
black/brown in color. The number of mineralized nodules in
iPSC-MSC-DLX3 was significantly higher compared with that in
iPSC-MSC-GFP (P<0.05; Fig.
5B).
Discussion
iPSC-MSCs have been considered as a novel cell
resource for bone tissue engineering (11–14,17).
The present study used Geltrex as a cell culture coating to
generate MSCs from iPSCs, as Geltrex can enhance cell attachment
and improve the efficacy to generate iPSC-MSCs (17). The current results suggested that
iPSC-MSCs presented a spindle-like morphology, which was consistent
with a previous study on BMSCs (17). Moreover, the current results
demonstrated that iPSC-MSC attained 99% positive for CD73, 71%
positive for CD90 and 95% positive for CD105, which were typical
cell markers comparable to BMSCs (25).
While iPSC-MSCs have similar surface markers as
BMSCs, their gene expression profile differs (26), and iPSC-MSCs are genetically related
to vascular progenitor cells. Additionally, iPSC-MSCs have similar
osteogenic and chondrogenic differentiation properties as BMSCs,
but their adipogenic differentiation potential is significantly
lower compared with that of BMSCs (12,26).
Therefore, iPSC-MSCs are not entirely equivalent to BMSCs.
Consistent with the aforementioned findings, the present results
indicated that iPSC-MSCs had a satisfactory osteogenic and
chondrogenic differentiation potential, which suggested that
iPSC-MSCs are appropriate for bone tissue engineering.
At present, the role of DLX3 on cell proliferation
has not been sufficiently investigated. A previous research group
pointed out that stable overexpression of DLX3 gene inhibited the
proliferation of human DPCs by inactivating the canonical Wnt
pathway (21,27). In the current study, a CCK-8 assay
was used to assess the proliferation of iPSC-MSCs after
transfection of DLX3 gene on days 1, 3, 5 and 7. Compared with the
control group, the iPSC-MSC-DLX3 group demonstrated a significant
decreased proliferative rate on days 5 and 7. These results were
consistent with the reported studies regarding human DPCs.
DLX3, expressed in osteo-/odontogenic lineages, is a
crucial transcription factor for osteo-/odontogenic
differentiation, mineralization and skeletal development (21,28–30).
In BMSCs, transfection of DLX3 gene promotes the expression of ALP,
Runt-related transcription factor 2 (RUNX2), OSX and OCN, as well
as the formation of calcified matrix (20). Overexpression of DLX3 stimulates
osteoprogenitor cells to express bone matrix proteins, such as
COL-1, bone sialoprotein, OCN and ALP (9). Moreover, DLX3 is strongly expressed in
differentiating and differentiated osteoblasts, and particularly
upregulates OCN and COL-1 (31). An
in vitro study also revealed that DLX3 could promote
odontoblastic differentiation of human DPCs, and could increase the
expression levels of ALP, DSPP, DMP1 and Nestin (21). However, one study provided the
opposite evidence in vivo, and reported that neural crest
deletion of DLX3 increased bone formation and mineralization in
craniofacial bones, which suggested an inhibitory role for DLX3 in
osteoblastic differentiation (32).
RNA sequencing and chromatin immunoprecipitation-Seq analyses have
further demonstrated that DLX3 regulates transcription factors
crucial for bone formation, such as DLX5, DLX6, RUNX2 and Sp7, as
well as genes important for mineral deposition (bone sialoprotein
2, ectonucleotide pyrophosphatase/phosphodiesterase family member 1
and matrix extracellular phosphoglycoprotein) and bone turnover
(tumor necrosis factor receptor superfamily member 11B) (33). Furthermore, with the knockdown of
DLX3, researchers have observed increased occupancy of DLX5, as
well as enhanced and earlier occupancy of RUNX2 on the
bone-specific osteocalcin promoter (33). Taken together, these findings
provide evidence that DLX3 attenuates bone mass accrual to support
bone homeostasis via osteogenic gene pathway regulation. In the
present study, it was identified that DLX3 overexpression
significantly upregulated osteogenic differentiation of iPSC-MSC by
activating ALP, OCN, OPN and COL-1 in vitro. In addition,
iPSC-MSC-DLX3 formed significantly more mineralized nodules
compared with the control group. These results were consistent with
a number of previous studies (9,20,21,31).
Osteoporosis (OP) is a bone metabolic disease that
is characterized by the degeneration of bone structure and
decreased bone mass (34). OP
occurs in >1/3 of women and 1/5 of men >50 years old, and
affects the health and lives of these individuals (35). The primary mechanism of OP is the
dysregulation of the dynamic balance between bone formation and
resorption, resulting in higher bone resorption than bone
formation, which may lead to bone metabolism disorder (36). The present results suggested that
DLX3 was a positive transcription factor in regulating osteogenic
differentiation. Previous studies have reported that some
osteogenic stimulators and transcription factors can induce the
protein expression of DLX3. It has been revealed that DLX3 is a
novel target of Estrogen receptor α (ER-α), and ER-α regulates
osteoblast differentiation via the modulation of DLX3 expression
and/or interaction with DLX3 (30).
DLX3 is also a novel target of protein kinase A (PKA), and PKA
mediates bone morphogenetic protein signaling during osteoblast
differentiation, at least in part, by phosphorylating DLX3 and
modulating the protein stability and function of DLX3 (37). The present results may facilitate
the development of novel strategies for the targeted therapy of
OP.
However, the present study has some limitations. It
was assumed that multilineage induction experiments were enough to
demonstrate the successful induction of human iPSCs into iPSC-MSCs,
but the relative marker genes were not analyzed pre/post-osteogenic
and chondrogenic induction. Therefore, it is hoped that these
limitations can be improved in future studies.
In conclusion, the present study demonstrated that
DLX3 exerted a positive role in regulating the osteogenesis of
iPSC-MSCs. However, the specific underlying mechanism of DLX3
affecting osteogenic differentiation in iPSC-MSCs is yet to be
fully elucidated. Further investigations, both in vitro and
in vivo, are required to confirm its effect on osteogenic
differentiation. Therefore, future studies will focus on the
specific mechanism of DLX3 affecting osteogenic differentiation of
iPSC-MSCs. Moreover, in vivo assays of transgenic DLX3
should be performed to further confirm its effect on
osteogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangdong
Basic and Applied Basic Research Foundation (grant no.
2020A1515010239), National Natural Science Foundation of China
(grant no. 81500825), Medical Scientific Research Foundation of
Guangdong Province (grant no. A2015423), Special Fund for Public
Welfare Research and Capacity Building of Guangdong Province (grant
no. 2014A020212211), and Science and Technology Program of
Guangzhou (grant no. 201607010205).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and RL designed the study, prepared the figures
and drafted the manuscript. JL and QL performed the experiments. YL
conceptualized the study and drafted and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Committee of Jinan University (approval no. 2015-045).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cummings SR and Melton LJ: Epidemiology
and outcomes of osteoporotic fractures. Lancet. 359:1761–1767.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Genant HK, Cooper C, Poor G, Reid I,
Ehrlich G, Kanis J, Nordin BE, Barrett-Connor E, Black D, Bonjour
JP, et al: Interim report and recommendations of the World Health
Organization Task-Force for Osteoporosis. Osteoporos Int.
10:259–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aspray TJ and Hill TR: Osteoporosis and
the Ageing Skeleton. Subcell Biochem. 91:453–476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassan MQ, Javed A, Morasso MI, Karlin J,
Montecino M, van Wijnen AJ, Stein GS, Stein JL and Lian JB: Dlx3
transcriptional regulation of osteoblast differentiation: Temporal
recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to
chromatin of the osteocalcin gene. Mol Cell Biol. 24:9248–9261.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao N, Zeng L, Liu Y, Han D, Liu H, Xu J,
Jiang Y, Li C, Cai T, Feng H, et al: DLX3 promotes bone marrow
mesenchymal stem cell proliferation through H19/miR-675 axis. Clin
Sci (Lond). 131:2721–2735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Han D, Zhang H, Liu H, Wong S, Zhao
N, Qiu L and Feng H: Morphological analyses and a novel de novo
DLX3 mutation associated with tricho-dento-osseous syndrome in a
Chinese family. Eur J Oral Sci. 123:228–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi SJ, Song IS, Ryu OH, Choi SW, Hart
PS, Wu WW, Shen RF and Hart TC: A 4 bp deletion mutation in DLX3
enhances osteoblastic differentiation and bone formation in vitro.
Bone. 42:162–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin H, Sohn J, Shen H, Langhans MT and
Tuan RS: Bone marrow mesenchymal stem cells: Aging and tissue
engineering applications to enhance bone healing. Biomaterials.
203:96–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabapathy V and Kumar S: hiPSC-derived
iMSCs: NextGen MSCs as an advanced therapeutically active cell
resource for regenerative medicine. J Cell Mol Med. 20:1571–1588.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang R, Zhou Y, Tan S, Zhou G, Aagaard L,
Xie L, Bünger C, Bolund L and Luo Y: Mesenchymal stem cells derived
from human induced pluripotent stem cells retain adequate
osteogenicity and chondrogenicity but less adipogenicity. Stem Cell
Res Ther. 6:1442015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie J, Peng C, Zhao Q, Wang X, Yuan H,
Yang L, Li K, Lou X and Zhang Y: Osteogenic differentiation and
bone regeneration of iPSC-MSCs supported by a biomimetic
nanofibrous scaffold. Acta Biomater. 29:365–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jungbluth P, Spitzhorn LS, Grassmann J,
Tanner S, Latz D, Rahman MS, Bohndorf M, Wruck W, Sager M, Grotheer
V, et al: Human iPSC-derived iMSCs improve bone regeneration in
mini-pigs. Bone Res. 7:322019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villa-Diaz LG, Brown SE, Liu Y, Ross AM,
Lahann J, Parent JM and Krebsbach PH: Derivation of mesenchymal
stem cells from human induced pluripotent stem cells cultured on
synthetic substrates. Stem Cells. 30:1174–1181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Goldberg AJ, Dennis JE, Gronowicz
GA and Kuhn LT: One-step derivation of mesenchymal stem cell
(MSC)-like cells from human pluripotent stem cells on a fibrillar
collagen coating. PLoS One. 7:e332252012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
TheinHan WW, Liu J, Tang M, Chen W, Cheng
L and Xu HH: Induced pluripotent stem cell-derived mesenchymal stem
cell seeding on biofunctionalized calcium phosphate cements. Bone
Res. 1:371–384. 2013. View Article : Google Scholar
|
|
18
|
Chen YS, Pelekanos RA, Ellis RL, Horne R,
Wolvetang EJ and Fisk NM: Small molecule mesengenic induction of
human induced pluripotent stem cells to generate mesenchymal
stem/stromal cells. Stem Cells Transl Med. 1:83–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng X, Su RJ, Baylink DJ, Neises A,
Kiroyan JB, Lee WY, Payne KJ, Gridley DS, Wang J, Lau KH, et al:
Rapid and efficient reprogramming of human fetal and adult blood
CD34+ cells into mesenchymal stem cells with a single
factor. Cell Res. 23:658–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun S, Yu M, Fan Z, Yeh IT, Feng H, Liu H
and Han D: DLX3 regulates osteogenic differentiation of bone marrow
mesenchymal stem cells via Wnt/β-catenin pathway mediated histone
methylation of DKK4. Biochem Biophys Res Commun. 516:171–176. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Yang G and Fan M: Effects of
homeobox gene distal-less 3 on proliferation and odontoblastic
differentiation of human dental pulp cells. J Endod. 38:1504–1510.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spandidos A, Wang X, Wang H and Seed B:
PrimerBank: A resource of human and mouse PCR primer pairs for gene
expression detection and quantification. Nucleic Acids Res. 38
(Suppl 1):D792–D799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Zhang X, Li J, Zheng J, Hu X, Xu
M, Mao X and Ling J: Foxc2 and BMP2 induce osteogenic/odontogenic
differentiation and mineralization of human stem cells from Apical
Papilla. Stem Cells Int. 2018:23639172018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou L, Luo Y, Chen M, Wang G, Ding M,
Petersen CC, Kang R, Dagnaes-Hansen F, Zeng Y, Lv N, et al: A
simple method for deriving functional MSCs and applied for
osteogenesis in 3D scaffolds. Sci Rep. 3:22432013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu M, Shaw G, Murphy M and Barry F:
Induced pluripotent stem cell-derived mesenchymal stromal cells are
functionally and genetically different from bone marrow-derived
mesenchymal stromal cells. Stem Cells. 37:754–765. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhan Y, Li X, Gou X, Yuan G, Fan M and
Yang G: Dlx3 inhibits the proliferation of human dental pulp cells
through inactivation of canonical wnt/beta-catenin signaling
pathway. Front Physiol. 9:16372018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beanan MJ and Sargent TD: Regulation and
function of Dlx3 in vertebrate development. Dev Dyn. 218:545–553.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghoul-Mazgar S, Hotton D, Lézot F,
Blin-Wakkach C, Asselin A, Sautier JM and Berdal A: Expression
pattern of Dlx3 during cell differentiation in mineralized tissues.
Bone. 37:799–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SH, Oh KN, Han Y, Choi YH and Lee KY:
Estrogen Receptor α Regulates Dlx3-Mediated Osteoblast
Differentiation. Mol Cells. 39:156–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Marijanovic I, Kronenberg MS, Erceg
I, Stover ML, Velonis D, Mina M, Heinrich JG, Harris SE, Upholt WB,
et al: Expression and function of Dlx genes in the osteoblast
lineage. Dev Biol. 316:458–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duverger O, Isaac J, Zah A, Hwang J,
Berdal A, Lian JB and Morasso MI: In vivo impact of Dlx3
conditional inactivation in neural crest-derived craniofacial
bones. J Cell Physiol. 228:654–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Isaac J, Erthal J, Gordon J, Duverger O,
Sun HW, Lichtler AC, Stein GS, Lian JB and Morasso MI: DLX3
regulates bone mass by targeting genes supporting osteoblast
differentiation and mineral homeostasis in vivo. Cell Death Differ.
21:1365–1376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sözen T, Özışık L and Başaran NC: An
overview and management of osteoporosis. Eur J Rheumatol. 4:46–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou Z, Liu W, Cao L, Liu Y, He T, Peng S
and Shuai C: Advances in the occurrence and biotherapy of
osteoporosis. Biochem Soc Trans. 48:1623–1636. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin X, Zhou C, Li J, Liu R, Shi B, Yuan Q
and Zou S: Autophagy in bone homeostasis and the onset of
osteoporosis. Bone Res. 7:282019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Jeong HM, Choi YH, Kim JH, Choi JK,
Yeo CY, Jeong HG, Jeong TC, Chun C and Lee KY: Protein kinase a
phosphorylates Dlx3 and regulates the function of Dlx3 during
osteoblast differentiation. J Cell Biochem. 115:2004–2011.
2014.PubMed/NCBI
|