Lower back pain (LBP) is one of the most common
reasons for seeking medical advice in orthopedic clinics. World
Health Organization statistics revealed that patients with LBP cost
up to 100 billion dollars each year in the United States (1). According to a report, 68–85% of
individuals worldwide experience LBP at least once in their
lifetime (2) and 5% ultimately go
on to develop chronic LBP (CLBP) (3). This is associated with severe pain and
disability, and is a heavy economic burden on society (4). Increasingly, studies have shown that
one of the main symptoms associated with intervertebral disc (IVD)
degeneration (IDD) is CLBP (5–8). A
number of researchers have reported that genetic factors accounted
for >68% of the pathogenic factors that are associated with IDD,
although a variety of factors can lead to IDD (9–11). IDD
is the pathological basis of disc herniation, and it is likely to
increase the susceptibility of disc herniation (12,13).
The spontaneous disappearance, or decrease in size, of a herniated
disc in patients displaying clinical symptoms have been reported on
numerous times in the past, but there has been an increasing number
of cases, even in patients showing large herniated discs (14–16).
One of the common features of these patients is that the
individuals are relatively young and it was observed that herniated
disc tissue retraction, or even disappearance through MRI (14–16).
Recently, Gao et al (16)
reported on a woman in her 40s whose herniated disc tissue
self-absorbed 8 months after the initial diagnosis of lumbar disc
herniation. This phenomenon cannot be explained by biomechanics.
Therefore, exploring the mechanism of IDD from a genetic
perspective is necessary in order to solve the current clinical
problems of CLBP
Anatomically, the IVD consists of the central
nucleus pulposus (NP) enriched in proteoglycans, the peripheral
annulus fibrosus (AF), and the endplates of hyaline cartilage at
the upper and lower ends (17).
From the cross-section, IVD can be observed as four concentric
areas: The outer AF; the inner AF; the transition zone; and the
central NP (18). The IVD is
essentially an avascular structure because vascular structures are
only visible in the outer AF; they are not in NP tissue and these
structures are separated by a thick layer of avascular AF (19). Blood vessels from the lumbar artery
terminate at the cartilage endplate, with only a small amount of
blood vessels infiltrating the cartilage endplate and the outer
third of the AF zone, but none of these vessels penetrate the NP
and inner AF (17,20,21).
NP cells (NPCs) are morphologically analogous to chondrocytes, and
have similar histological and physiological structures to the
articular cartilage (22).
Therefore, NPCs are largely dependent on capillary diffusion
through the cartilage endplates for oxygen exchange, nutrient
acquisition and metabolic waste transport (23,24).
In addition to the complex structure, there is an oxygen
concentration gradient around the avascular IVD where the partial
pressure of oxygen in the IVD central region is as low as 1%
(25). The oxygen partial pressure
of degenerate tissue is lower than that of normal IVD tissue
(26). Accumulating evidence has
revealed that hypoxic environments play a crucial role in
maintaining the physiological functions of IVDs, including cell
metabolism and matrix synthesis (4,21,27–29).
Therefore, NPCs adapt to survive in hypoxic microenvironments under
physiological conditions (23).
This hypoxic environment may affect the activity of a series of
genes related to cell adaptation for survival in hypoxic
microenvironments (24).
Hypoxia-inducible factor-1α (HIF-1α) is one of the most essential
transcriptional regulators of cell adaptation to hypoxic
environments (30). Feng et
al (28,29) found that the expression of HIF-1α in
human NPCs was significantly increased under hypoxic conditions
compared with normoxic conditions. Richardson et al
(31) demonstrated that HIF-1α was
markedly expressed in degenerative IVD tissue.
HIFs belong to the Per-Arnt-Sim family; they are
heterodimeric proteins composed of α and β subunits. The α subunit
predominantly determines the activity of HIF, which is regulated by
the oxygen-dependent degradation (ODD) domain in its central
region, and thus can regulate gene expression depending on the
level of oxygen tension (54). The
β subunit is expressed in the nucleus, where it has roles in
maintaining the stability of HIF structure and biofunctions, but it
is not regulated by oxygen tension (55). There are three isoforms of HIF-α,
HIF-1α, HIF-2α and HIF-3α (56–58).
HIF-1α and HIF-2α have similar mechanisms of action in different
cell types because they have extensive sequence homology and
contain two ODD domains (56).
Therefore, they are closely related to each other and can activate
gene transcription that is dependent on the hypoxia responsive
elements (HREs) (56). HIF-3α has a
unique structure, containing only one ODD domain; it is spliced
selectively and modulated via HIF-1α (57,58). A
number of studies have shown that the metabolism of HIF-α is
associated with oxygen concentration in numerous cell types
(33,34,36).
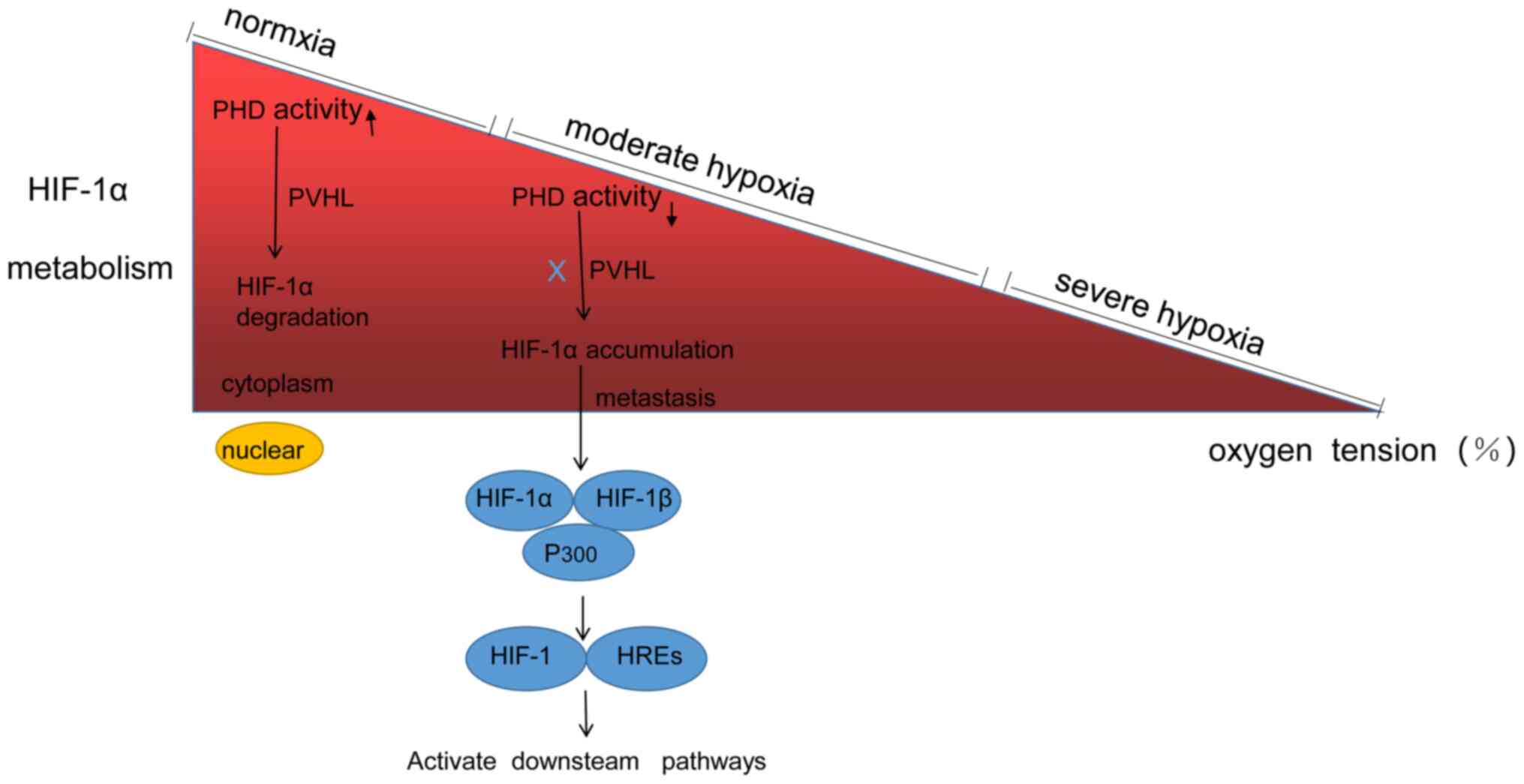
Under normoxic conditions, the α subunit is hydroxylated and
subsequently activates prolyl hydroxylase (PHD), which is then
rapidly degraded via the ubiquitin-proteasome pathway (33). However, when the cells are exposed
to hypoxic-ischaemic conditions, PHD activity is reduced and the
degradation of the α subunit is inhibited (33,59).
Thus, HIF-1α rapidly expands and gradually accumulates in the
cytoplasm (33,59). Then, it enters the nucleus and binds
to HIF-1β to form an ectopic dimer, HIF-1, which recognizes and
binds to the conserved sequence of HREs to activate the
transcription of target genes (60). The α subunits of HIF-1α and HIF-2α
have non-overlapping regions that have different biofunctions and
even completely converse effects. Firstly, HIF-1α is ubiquitously
expressed in almost all cells in the human body, while HIF-2α
appears to be expressed in specific tissues, mainly the lung,
heart, kidneys and liver in adults (61,62).
Secondly, HIF-1α, but not HIF-2α, has been shown to markedly
regulate the expression of the key enzymes involved in the
glycolytic pathway (44).
Furthermore, HIF-1α exclusively regulates adenosine triphosphate
(ATP) production and mediates macrophage migration, infiltration
and bacterial killing during acute inflammation responses, while
HIF-2α mainly mediates tumour-associated inflammation and appears
to play a greater role than HIF-1α in cytokine production (63). Thirdly, the metabolism of HIF-1α and
HIF-2α in NPCs is also different. PHD family members include PHD1,
PHD2 and PHD3 (64). The
degradation of HIF-1α and HIF-2α is mainly mediated by the 26S
proteasome, which is not associated with oxygen tension (65). In addition, HIF-1α is mainly
degraded by the oxy-PHD2 pathways, whereas the degradation of
HIF-2α is associated with PHD3 pathways (21,65).
Lastly, HIF-1α has an antitumour effect, but HIF-2α mainly promotes
tumour progression (44,66). Therefore, it is more meaningful to
study the mechanism of HIF-1α in IDD, and the metabolism of HIF-1α
is shown in Fig. 1.
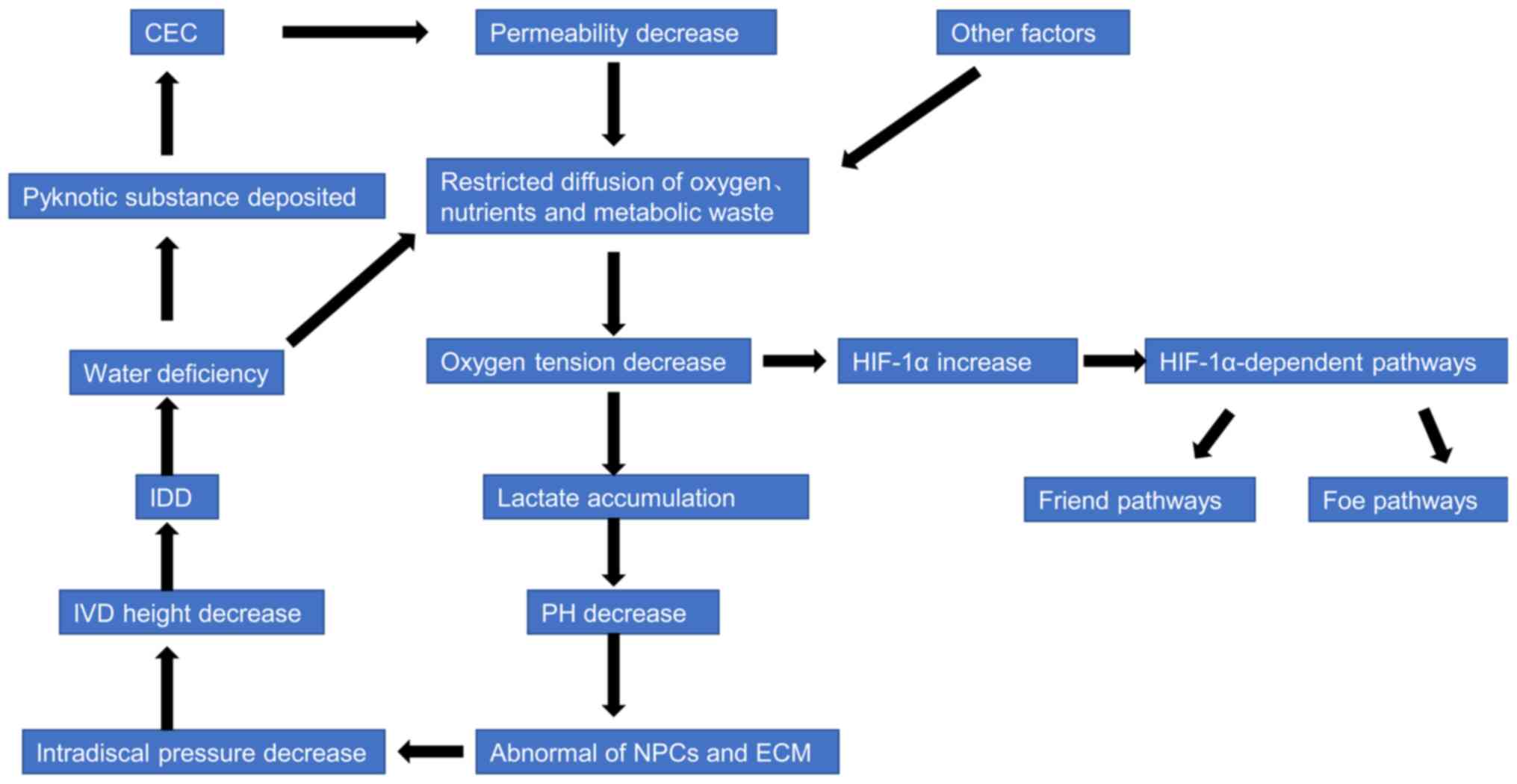
It is generally hypothesized that disrupting the
diffusion of oxygen, nutrients and metabolic waste in the IVD space
is the most critical mechanism in the occurrence and progression of
IDD (24,67). With the occurrence of IDD, the
amount of water in the NP tissue is reduced, following which the
diffusion of oxygen, nutrients and metabolic waste is restricted.
The oxygen tension is lower in the IVD microenvironment in the wake
of poor diffusion, following which the production of lactate is
increased through anaerobic metabolism (24). The excretion of lactate is blocked
so it gradually accumulates, decreasing the pH of the IVD
microenvironment, which further affects cellular metabolism and
biofunction (24). Thus, NPC
quantity and viability is decreased, and the synthesis and
decomposition of ECM are unbalanced; this imbalance decreases the
intradiscal pressure, transforms the biomechanics from hydrostatic
stress to shear stress and decreases the IVD height, which further
aggravates the progression of IDD (24,40).
Therefore, the first important vicious cycle is established. The
low oxygen tension activates the expression of HIF-1α, which
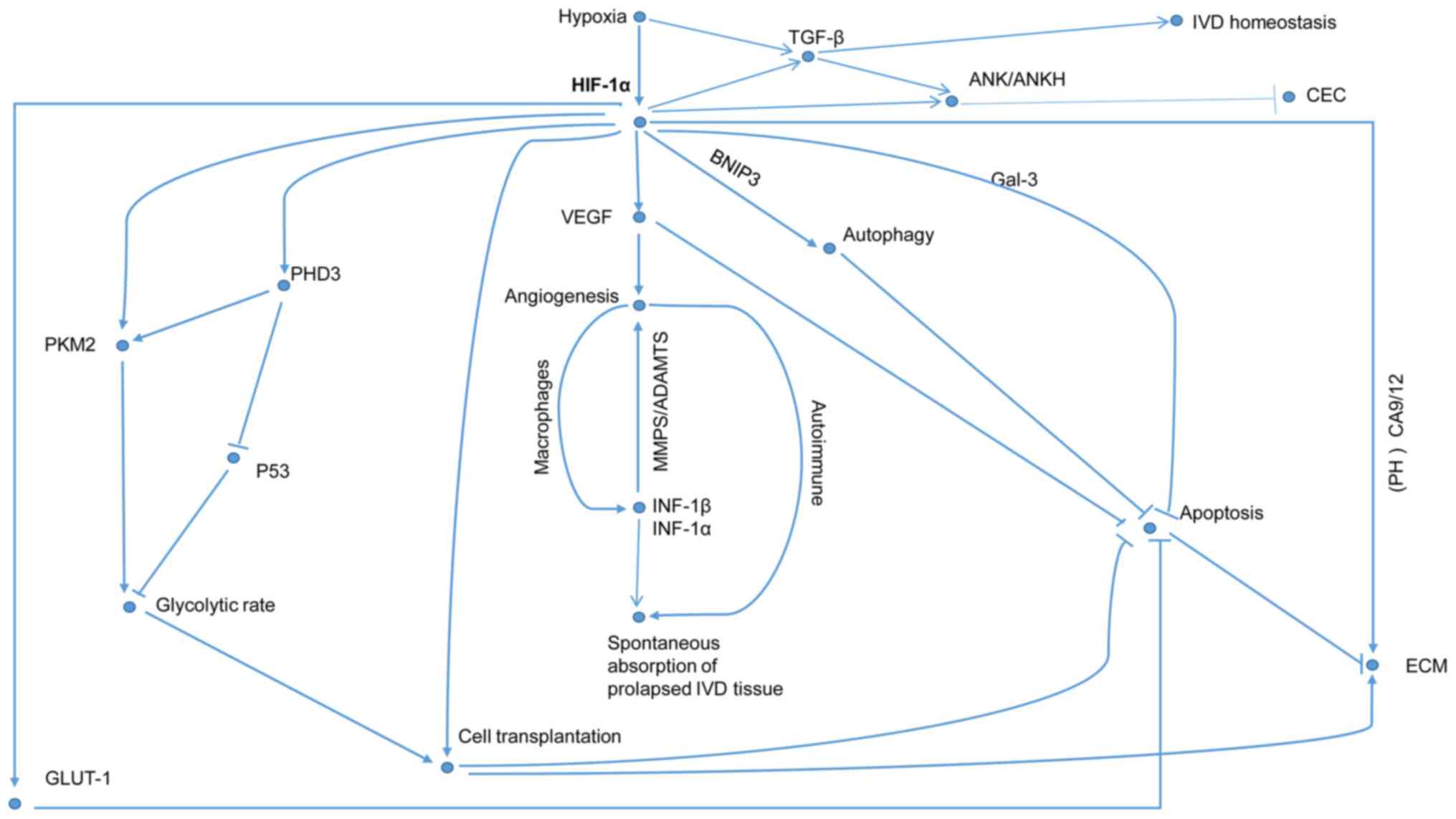
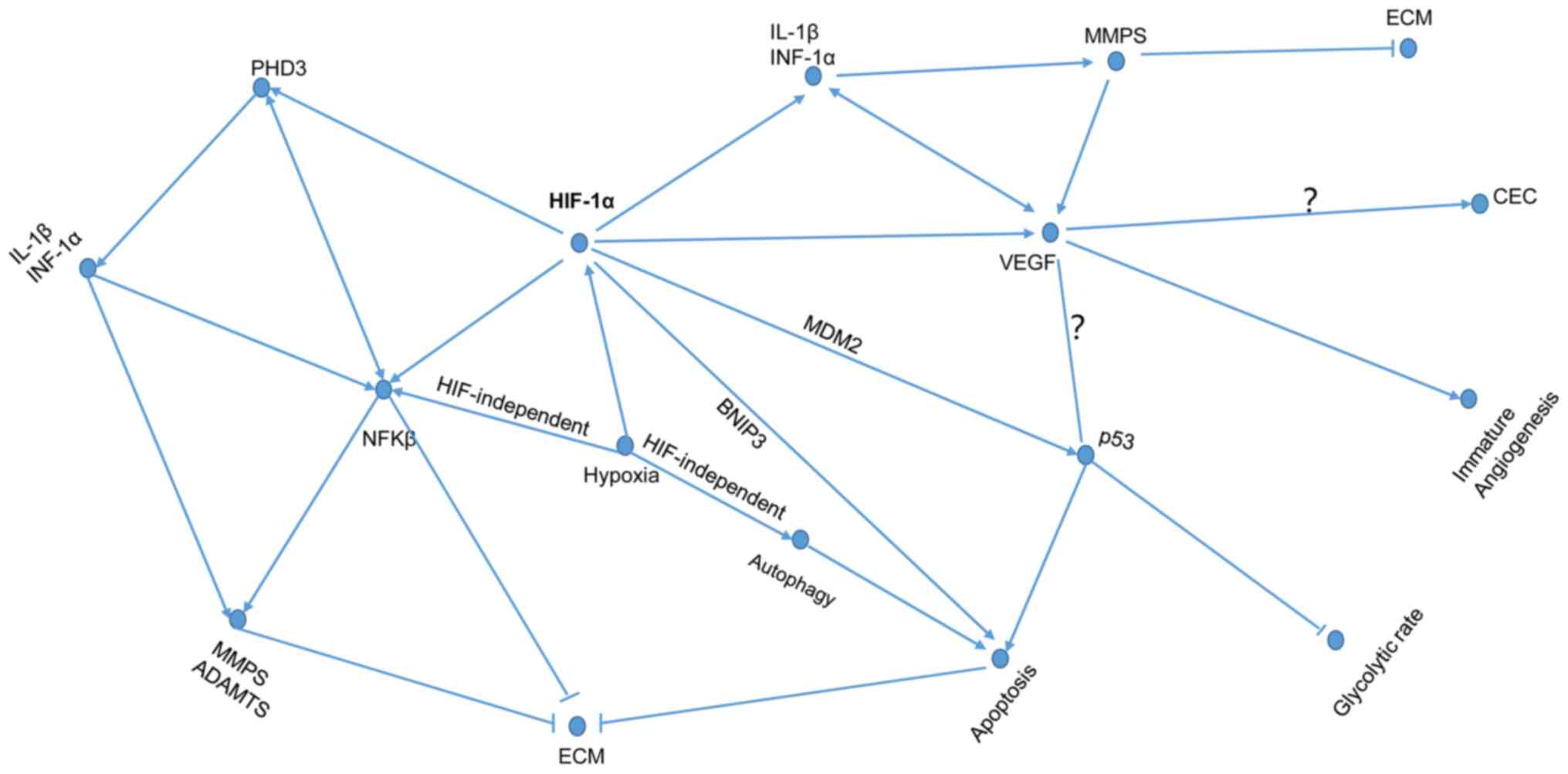
subsequently enters into the hypoxia-induced HIF pathways (Fig. 2). The HIF pathways are dichotomized
as friend and foe pathways according to the oxygen tension of the
IVD microenvironment (Figs. 3 and
4).
The second vicious cycle centers on cartilage
endplate calcification (CEC). A previous study indicated that the
degree of CEC is associated with the severity of IDD (68). When the water content in NP tissue
decreases, pyknotic substances are deposited in the cartilage
endplate, which gradually calcifies; the permeability of cartilage
endplate declines and then enters into the vicious cycle (Fig. 2) (25,69).
Notably, there is an association between angiogenesis and CEC, but
the mechanism by which angiogenesis aggravates CEC remains unclear
(70).
An IVD is a fibrous cartilage pad between the
vertebral bodies that resists pressure from the spine and allows
for slight movement of the spine (71). Peripheral AF cells and central NPCs
from IVDs exhibit different morphology and functional expression.
AF cells are similar to fibroblasts in morphology and contain
higher levels of collagen type I (COL1), which accounts for ~68% of
the dry weight of AF (67,71,72).
NP is enriched in collagen type II (COL2) and proteoglycans; COL2
accounts for ~20% of the dry weight of NP, and aggrecan (ACAN) is
the major component of proteoglycans, which constitute 50% of the
wet weight of NP (22,71,72).
ACAN contains a large quantity of chondroitin sulfate (CS), which
primarily binds to water molecules and is responsible for
maintaining the moisture and mechanical load of IVD; it is
necessary for IVD to exert its physiological functions and bear
stress (73–75). Collagen imparts tensile strength to
IVD by forming a network of collagen fibres that encloses the
proteoglycans (72). Therefore, it
is more meaningful to study NPCs than AF cells. Interestingly, the
content and distribution of various collagens changes with IDD,
while IVD ageing will not cause this change. In the early stages of
degeneration, collagen content usually increases in the region of
its distribution (76,77). COL1 begins to appear in the nucleus,
and collagen types IV and X can be detected with the aggravation of
IDD; the expression of COL2 was not detected in the endplate
(76,77). Accumulating evidence suggests that
ECM plays an important role in supporting the NP-integrated
structure and biofunctions, as well as mediating NP tissue
morphogenesis, homeostasis, repair and remodelling (25,77,78). A
number of factors cause the degradation and decreased synthesis of
the ECM, leading to a downregulation in the water content of NP
tissue; thus, the IVD height and the capability of bearing
mechanical loads decrease further, which will ultimately trigger
IDD (79).
Hypoxia significantly increased the phenotype of
NPCs while having little effect on AF, indicating that HIF-1α can
be used as marker of NPCs (28,29).
Richardson et al (31) found
that HIF-1α is expressed in NPCs rather than AF cells, further
demonstrating that the use of HIF-1α for phenotypic identification
of NPCs is scientific and justified. However, there is still
controversy surrounding the findings concerning the regulation of
ECM metabolism by HIF-1α. Over the years, an increasing number of
studies have shown that hypoxia can induce numerous cells to
upregulate HIF-1α to promote ECM synthesis, including NPCs
(4,28,29,80),
chondrocytes (81–83), fibroblasts (84) and mesenchymal stem cells (83). Animal and human studies have
suggested that the content of ECM synthesis has a negative
association with the degree of hypoxia (Figs. 3 and 4) (28,29,85–87).
The ideal level of hypoxia for reinforcing NPC survival is 1%
physiological oxygen concentration (85). Ishihara and Urban (87) reported that the synthesis of
glycosaminoglycan (GAG) in bovine NPCs significantly increased as
the oxygen tension in the medium gradually decreased. Furthermore,
Liu et al (80) found that
HIF-1α increases the expression of COL2 and ACAN via the mediation
of the NOTCH1 signalling pathway. Interestingly, however, Cigognini
et al (88) found that
although HIF-1α was activated at 2% oxygen tension, no increase in
ECM synthesis was observed, which suggested that HIF-1α is not the
only factor that promotes ECM synthesis. Another study showed that
O2 is essential for the synthesis of GAG in bovine NPCs
(89). In complete anaerobic
conditions, bovine NPCs express little GAG (89). These two different results may be
attributed to different cell-specific endogenous factors, including
age, sex, histologic origin and the severity of the damage model
(88). Exploring and analysing the
ways in which HIF-1α promotes or inhibits ECM synthesis in NPCs,
and finding the most critical mechanism for regulating HIF-1α
should be the focus of future research.
The process of forming new capillaries from existing
blood vessels is called angiogenesis (90). Vascular endothelial growth factor
(VEGF) is the most important factor in angiogenesis (91). VEGF regulates vascular maturation
(92), alters ECM expression
(93), increases vascular
permeability (94), promotes
endothelial cell proliferation and maintains vascular function
(94). The oxygen tension of the
cell survival environment is an important factor in the regulation
of VEGF expression and angiogenesis (41). Hypoxia can stabilize the
post-transcriptional levels of HIF-1α, and HIF-1α directly induces
VEGF expression under hypoxic conditions (41,95,96).
HIF1-α can also indirectly regulate VEGF expression via modulating
its own transcriptional activity through E/D-rich carboxy-terminal
domain-2 (a p300 binding protein) (97). In addition, interleukin (IL)-8
(98), basic fibroblast growth
factor (bFGF) (99,100) and transforming growth factor-β
(TGF-β) (100) also have strong
angiogenic effects, and these factors are downstream target genes
of HIF-1α. TGF-β also activates bFGF and maintains IVD homeostasis
(101,102). Interestingly, TNF-α (103,104), IL-1β (41,105)
and MMPs (106,107) also play important and complex
roles in the regulation of angiogenesis. In endothelial cells,
TNF-α promotes angiogenesis by increasing the expression of VEGF,
IL-8 and bFGF (103). MMP-2 may
promote angiogenesis, whereas MMP-9 may block angiogenesis by
converting plasminogen into an angiogenesis inhibitor (106,107). It is noteworthy that hypoxia has a
dual role in angiogenesis to avoid over-activation of the
HIF-1α/VEGFA axis during hypoxia in order to maintain the
surrounding environment and biofunction of cells (108).
Studies have indicated that HIF-1α and VEGF are
directly involved in the entire process of angiogenesis (109). The VEGF gene may increase
the susceptibility to IVD degeneration, and VEGF expression is also
positively correlated with the severity of IVD degeneration
(110). With regard to the role of
angiogenesis in IDD, different scholars hold different
perspectives. Some scholars hypothesize that angiogenesis may be
the adaptive response of the body to deal with various stresses or
injuries to repair degenerative IVD tissue (Fig. 3). Long et al (111) found that the expression of VEGF in
human IVD tissues is time-specific, and that there is differential
expression in different developmental stages of IVD. This is
clearly related to the observation that IVD undergoes partial
vascularization, devascularization and revascularization (111). Vascularized IVD tissue not only
increases blood circulation and improves the nutrition of NPCs, but
also plays an important role in the spontaneous absorption of
prolapsed IVD tissue (104,107,112).
Neovascularization facilitates the infiltration of inflammatory
cells, such as macrophages, into NP tissues (9,112,113). The prolapsed IVD tissue can be
phagocytosed by macrophages or dissolved via autoimmune reactions
(112,113). Various cytokines, such as TNF-α
and IL-1β, can also be synthesized by macrophages and other cells
to promote both MMPs and a disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS), in order to digest ECM components
of prolapsed tissues to mitigate IDD (112,113). Activated MMPs can stimulate VEGF
expression and promote the vascularization of IVD (107). Other scholars hypothesize that
angiogenesis may accelerate IDD (Fig.
4). Uncontrolled and immature angiogenesis is the cause of a
variety of diseases (89). Although
HIF-1α promotes angiogenesis, it may promote immature angiogenesis
to trigger IDD. VEGF may have a combinatory effect with the
p53 gene to promote IDD (114). p53 induces apoptosis by directly
combining with HIF-1α (115) and
is ubiquitously considered a pro-apoptotic gene (116). The association between HIF-1α and
p53 is so complex that it has not been elucidated, but it is clear
that p53 is motivated by HIF-1α to reduce glycolytic rates under
severe or chronic hypoxia (36).
Pyruvate kinase M2 (PKM2) reinforces the transactivation of HIF-1α
and glycolytic rates via interaction with PHD3 (117). It could be that HIF-1α safeguards
p53 against degradation via mouse double minute 2 homolog (MDM2)
(81). Furthermore, in mild hypoxic
conditions, HIF-1α degradation of p53 is associated with PHD3,
while MDM2 is not involved in this process (118). Interestingly, p53-enhanced HIF-1α
ubiquitination and degradation is involved in the malfunction of
cell mitochondria (119). PHD3 is
a critical regulator of HIF-1α, p53 and NF-κB pathways (9,21,118,120). HIF-1α upregulates the expression
of PHD3, while PHD3 consolidates the transcriptional activity of
HIF-1α (21). PHD3 upregulates the
TNF-α-induced NF-κB/p65 signaling activity to reinforce the
expression of ADAMTS-5 and MMP-13; additionally it can downregulate
the expression of the genes ACAN and COL2 (9,121).
Fujita et al (120) first
showed that the expression of PHD3 is mediated by NF-κB-dependent,
but not HIF-1α-dependent inflammatory cytokines in NPCs. PHD3
decreases the activity of p53, playing a crucial role in the
reciprocal negative correlation between the p53 and NF-κB pathways
(118). Another possible mechanism
is that activated MMPs and ADAMTS may digest ECM components in
non-prolapsed IVD tissues (111).
In addition, angiogenesis can also aggravate IDD by promoting CEC
(113).
During IDD, the accompanying aseptic inflammatory
response within the NP is another important pathological phenomenon
(121). The expression of a number
of pro-inflammatory cytokines secreted by IVDs and other cells,
such as TNF-α (8,74,121),
IL-1β (8,74,121–125) and IL-6 (121) are upregulated in IDD. These
cytokines promote the progression of IDD by increasing the
expression of MMPs (8,40,125),
degrading ECM (8,74,125),
promoting angiogenesis (103–105), enhancing NPC apoptosis (123–125) and deteriorating IVD tissue
(Fig. 4) (8). IL-1β is considered to be the most
important cytokine involved in multiple pathological processes of
IDD (8,122). IL-1β and TNF-α can reduce the
biosynthesis of ECM (including CS, COL2 and ACAN), and enhance the
expression of MMPs and pro-apoptotic proteins by stimulating
non-coding RNAs (74,125,126). Additionally they can also
upregulate the expression of ADAMTS-4/5 to enhance ECM degradation
through MAPK and NF-κB signaling pathways (8,125,127). Interestingly, they also activate
the Notch pathway to maintain NPC homeostasis by mediating MAPK and
NF-κB signaling pathways (128).
In addition, IL-1β induces NPC apoptosis and autophagy through the
mitochondrial pathway, as well as activating the NF-κB signaling
pathway to promote NPC apoptosis, and inhibit the expression of
ACAN and COL2 (123–125). Notably, Risbud and Shapiro
(8) elucidated that there are three
distinct but overlapping inflammation response stages in IDD, of
which the second stage emphasizes the role of angiogenesis in
transporting various inflammatory cytokines into IVD tissue to
further amplify the inflammatory responses that were established in
the first stage. Here, we propose a hypothesis that HIF-1α may be
predominantly involved in the second stage to regulate the
inflammation response of the IVD microenvironment (129).
Increasingly, research suggests that there is a
sophisticated and tight relationship between hypoxia, HIF-1α,
inflammation and angiogenesis (Fig.
4) (39,130). The NF-κB cascade is an
inflammatory signaling pathway. Hypoxia activates both the
canonical HIF-dependent pathway and the NF-κB signaling pathway by
enhancing inhibitor of NF-κB kinase subunit β (IKKβ) activity (an
NF-κB inhibitor) and decreasing PHD-dependent hydroxylation, as
IKKβ contains sequences that are similar to hydroxylation sites for
binding of PHD to HIF-1α (39,130).
Notably, IKKβ is also potentially hydroxylated by PHD on account of
its particular structure (39).
From this perspective, HIF-1α is involved in activating the NF-κB
signalling pathway (130).
Moreover, NF-κB also stabilizes HIF-1α expression under normoxic
conditions and activates HIF-related pathways (39,130).
Konisti et al (39) and
Oliver et al (130)
demonstrated the interaction between the NF-κB signalling pathway
and HIF-related pathways, which together induce and amplify the
inflammatory cascade, and also promote angiogenesis. Previously, it
has been confirmed that the expression of HIF-1α is upregulated in
inflammatory diseases, such as osteoarthritis (37,131)
and rheumatoid arthritis (37,39,131).
In 2003, Cramer et al (129) conditionally knocked out HIF-1α in
mouse bone marrow cells, and found that the production of ATP and
biofunction of macrophages decreased significantly, thus further
demonstrating that HIF-1α controls the inflammatory response via
regulating the glycolytic metabolic pathway and plays a key role in
the early stage of inflammatory cell infiltration. Subsequently, in
2010, Imtiyaz et al (63)
found that the production of ATP is exclusively regulated via
HIF-1α, but not HIF-2α. Firstly, NPCs survive in a hypoxic
microenvironment, and the expression of HIF-1α is upregulated in
IDD (23,28,29).
Secondly, NPCs are primarily powered via the anaerobic glycolysis
pathway (132). Finally, IDD is
often accompanied by aseptic inflammatory response in NPCs
(122). Therefore, in this article
it is hypothesized that the hypoxia-HIF-inflammatory pathway is
involved in the regulation of IDD. Further investigation is needed
to elucidate the above hypothesis.
MMPs are endopeptidases of the ECM that have the
ability to degrade almost all known components of the ECM in IVDs
(133,134). Structurally, MMPs can be divided
into six main sections: Gelatinase (MMP-2 and MMP-9); collagenase
(MMP-1, MMP-8, MMP-13 and MMP-18); matrix degradation element
(MMP-3, MMP-10 and MMP-11); matrilysins (MMP-7 and MMP-26);
transmembrane and GPI-linked MT-MMP (MMP-14, MMP-15, MMP-16,
MMP-17, MMP-24 and MMP-25); and glassy lectin (MMP-21), as well as
other types of MMPs (MMP-24) (133,135–137). Functionally, a variety of studies
have shown that different types of MMPs, including MMP-2 (134,138–142), MMP-9 (134,139,143), MMP-13 (144), MMP-14 (145) and MMP-16 (146) are negatively correlated with the
ECM level of degenerated NP and can promote IDD via the induction
of ECM degradation. The most studied MMP is MMP-2, and upregulation
of its expression is significantly associated with IDD (137–140). As early as 1997, Crean et
al (134) found that the
expression levels of MMP-2 and MMP-9 were related to the extent of
IVD degeneration. Kozaci et al (138) found that MMP-2 was upregulated in
the early stages of degenerative disc disease, and could promote
IDD by degrading COL2. Roberts et al (139) found that the expression of MMPs
was the most abundant in blood vessels and cell clusters of
degenerative IVDs, and was associated with the grade of IDD on the
macroscopic level. Rutges et al (140) reported that increased MMP-2
activity during disc degeneration was associated with MMP-14
expression. Micro (mi)RNAs are a class of small non-coding RNA
regulators. A number of miRNAs have distinct expression profiles in
human normal and degenerative IVDs (143,144). They promote the apoptosis or ECM
degradation of NPCs in IVD to regulate IDD progression via the
targeted regulation of MMPs (144,145). Xu et al (143) found that miRNA-133a was involved
in the regulation of IVD degeneration by negatively regulating the
expression of MMP9 indirectly, and outlined the role of the
miRNA-133a/MMP9/COL2 axis in the pathophysiology of IDD. Ji et
al (145) demonstrated that
miRNA-193a-3p may participate in disc degeneration via the targeted
regulation of MMP-14. Zhang et al (146) reported that miRNA-155 inhibits the
ECM degradation of NP and reverses IVD degeneration by targeting
negative regulation of MMP-16 expression. At the same time, MMP-16
is involved in MMP-2 activation (147). The expression of MMP-2 was
upregulated by HIF-1α in numerous cell types (49–52,148).
Wu et al (52) found that
the expression of HIF-1α and MMP-2 increased in degenerated lumbar
IVD, and that there was a significant association between them.
Therefore, in the present review, it is hypothesized that HIF-1α
may also increase MMP-2 activity in degenerated NPCs. However, the
mechanism by which HIF-1α promotes MMP-2 expression remains
unclear. Inflammatory cytokines such as IL-1β (121,149) and TNF-α (121,142,149) stimulate the expression of MMP-2 in
the degenerative tissues of IVD. In summary, it is hypothesized
that the HIF-1α/IL-1β and TNF-α/MMP-2/ECM axes may play an
important role in the development of IDD (Fig. 4).
NPCs are mainly dependent on anaerobic glycolysis
to synthesize ATP to maintain energy metabolism in the absence of
oxygen (132). HIF-1α regulates
ATP production and can be involved in the regulation of glycolytic
metabolism by stimulating the expression of glycolytic genes
(43,63,129).
Glucose transporter (GLUT), located on the cell membrane, assists
cells to take up glucose and plays an important role in glycolysis
(31,150–152). There are numerous subunits of
GLUT, of which GLUT-1 is the most widely studied in IDD. It has
been shown that the overexpression of GLUT-1 reduced
hypoxia-induced apoptosis (151).
In addition, GLUT-1 provides energy for transplanted cells under
hypoxia (Fig. 3) (150). Richardson et al (31) found that the expression of GLUT in
NPCs is positively correlated with the expression of HIF-1α and the
severity of IDD. The upregulation of HIF-1α increased the
expression of GLUT-1, GLUT-3 and GLUT-9 in NPCs; however, this
association has not been observed in AF cells (31). Furthermore, HIF-1α is involved in
regulating the expression of a series of genes in the anaerobic
glycolysis pathway, such as 6-phosphofructo-2-kinase (152), PKM2 (117), phosphoglycerate kinase-1 (119) and lactate dehydrogenase (119). HIF-1α is also involved in
regulating mitochondrial energy metabolism (153,154). Papandreou et al (154) found that HIF-1α also activates
pyruvate dehydrogenase kinase-1 in hypoxia and thereby inhibits
mitochondrial function. In addition, HIF-1α-induced mitochondrial
autophagy decreases the quantity of mitochondria to reduce oxygen
consumption (155). However,
non-mitochondrial oxygen consumption was found to be regulated via
protein-tyrosine phosphatase-1B in breast cancer under hypoxia
(156). Whether HIF-1α is involved
in the above process remains unknown.
Autophagy is a non-apoptotic, programmed cell death
that leads to the self-digestion and degradation of unwanted
proteins and organelles to prevent cellular stress and maintain
cell function (157). Cells can
resist apoptosis and senescence by activating autophagy, which
alleviates the progression of certain chronic degenerative diseases
(158–162). Autophagy plays a crucial role in
the pathogenesis of a number of degenerative diseases, including
osteoarthritis (158) and IDD
(159–162). The autophagy-related genes or
proteins beclin 1, cathepsin B, damage-regulated autophagy
modulator 1 and p65 were highly expressed in degenerated IVD
tissues compared with normal, healthy IVD tissues (163). Autophagy is a physiological
response to hypoxia in cells, and has a complicated relationship
with hypoxia (164,165). HIF-dependent autophagy enhances
cell survival during physiological hypoxia or mild hypoxia
(Fig. 3), while autophagy
reinforces cell death via a HIF-independent pathway in severe
hypoxia or even anoxia (Fig. 4)
(164). Accumulating evidence
indicates that HIF-1α induces autophagy via the activation of BCL2
interacting protein 3 (BNIP3) in different mammalian cell types
(155,164,166,167). Follicle-stimulating hormone can
induce autophagy of mouse granulosa cells through the AKT-mTOR
signalling pathway, and HIF-1α is a key factor in mediating this
process (167).
The level of cellular autophagy changes accordingly
as IVD degenerates and ages (160,164,168). There has been preliminary progress
in the study of whether HIF-1α regulates IDD through autophagy in
IVD cells under hypoxic conditions. On the one hand, Ye et
al (168) found that the level
of autophagy in rat NP tissues increased with age, and the level of
autophagy was higher in the IDD model group compared with the
control group. A number of studies have shown that NPCs exhibit
lower autophagy activity in culture under hypoxia than in normoxia,
which is contrary to studies in most other types of cells (45,164,168). Considering the special
physiological environment and energy metabolism of NPCs, there is
reason to consider that autophagy in NPCs under hypoxia should be
different from that of other types of cells. Autophagy has a dual
effect on the survival of NPCs in serum-free culture conditions;
proper autophagy activity of cells promotes NPC survival, while
excessive autophagy promotes NPC death (45). Hypoxia enhances NPC survival under
serum-free culture conditions via the downregulation of excessive
autophagy activity, including limiting the generation of reactive
oxygen species and promoting the inactivation of the AMPK/mTOR
signalling pathway; this process may be controlled by HIF-1α
(45). On the other hand, contrary
to previous investigations, Choi et al (169) confirmed that hypoxia enhances the
non-classical autophagy of NPCs, and this process is not related to
the mTOR and HIF-1α signalling pathways. This process does not
affect the glycolytic metabolism of NPCs (169), which suggests that autophagy in
NPCs may have other effects besides its classical degradation and
recapture function. Therefore, further studies are needed to
determine the physiological role of autophagy in NPCs and how it
contributes to alleviate IDD.
The biological activity of NPCs is indispensable
for maintaining the stability and function of the IVD. Accumulating
evidence suggests that excessive apoptosis and senescence of NPCs
can lead to ECM degeneration in the NP, and it has been considered
that it could be a therapeutic target in IDD (170,171). Gruber et al (171) studied postoperative specimens and
found that the apoptosis of a large number of cells was detected in
IVD, while active cells synthesize degraded ECM components,
impeding cell growth and communication by altering the environment
surrounding NP. It is becoming increasingly evident that HIF-1α is
widely involved in the regulation of NPC survival. The positive
correlation between the expression of HIF-1α and NPC apoptosis was
analyzed in patients with disc herniation (43). In this review it is hypothesized
that the survival state of NPCs is related to the severity of
hypoxia under hypoxic environments; there is an oxygen threshold
beyond which the expression of pro-apoptotic proteins and VEGF is
reinforced. Moritz et al (59) and Liu et al (35) also found that HIF-1α has a dual
function in vivo, and the alternative function of HIF-1α
depends on the oxygen tension (Figs.
3 and 4). Some pro-apoptotic
proteins, such as BNIP3, are downstream target genes of HIF-1α
(166). BNIP3 can induce the
apoptosis of NPCs via the mitochondrial pathway, and it is also
involved in inducing mitochondrial autophagy, promoting cells to
adapt to various metabolic reactions and exerting anti-apoptotic
benefits (155). Forkhead box O3
(FOXO3a), which regulates resistance to various stresses and
promotes cell survival, decreases the expression of BNIP3 by
promoting the activity of the transcription cofactor cbp/P33
interacting transactivator 2 to inhibit apoptosis (172). In addition, inflammatory
cytokines, decreased energy metabolism, ECM imbalance and
angiogenesis, all promote apoptosis in varying degrees.
Increasingly, investigations have tended to support
that HIF-1α enhances the NPCs adaptability to hypoxia and
consolidates NPC survival under hypoxic conditions. The HIF-1α/VEGF
pathway plays a vital role in maintaining the ECM balance of NPCs
and promoting NPC survival (42).
The function of VEGF is also restricted to the VEGF/membrane bound
VEGF receptor-1 axis (173). The
NPCs derived from progenitor cells were vulnerable to apoptosis in
hypoxic conditions due to their inability to induce the expression
of HIF-1α (174). After
proteoglycans were added, the activation of HIF-1α was subsequently
consolidated, and the susceptibility of progenitor cells to
apoptosis induced by hypoxia was reduced (174). HIF-1α inhibits Fas ligand-mediated
apoptosis by reinforcing the activation of the galectin-3 promoter
(175). In addition, HIF-1α can
enhance the expression of carbonic anhydrase (CA)-12 to increase
the synthesis of the ECM of NP (4).
HIF-1α is also involved in CA9/12-mediated bicarbonate cycling to
maintain the pH stability of NP (176).
Although IVD is enriched in collagen fibrin and
moisture, abnormal pyknotic substances are not deposited within NP
under physiological conditions (48). The dysregulation of inorganic
phosphate metabolism plays a key role in CEC (177), and its expression level can be
used to assess the degree of CEC (178). The progressive ankylosis protein
homolog gene (ANK) is a phosphate transporter that can prevent
mineralization by regulating the transport of inorganic phosphates
(48,179). ANK is involved in the local
control of mineralization in bone, cartilage and the calcified
tissue area of growth plates (179,180). Malnutrition mineralization caused
by abnormal expression of ANK can lead to the occurrence of chronic
diseases such as osteoarthritis (179,180) and IDD (48,181).
Sohn et al (182) found
that ANK is mainly expressed on the surface and hypertrophic areas
of joints and cartilage, which indicates that the expression of ANK
is oxygen-dependent. Zaka et al (183) demonstrated that the expression of
ANK in growth plate chondrocytes depends on the regulation
of HIF-1α. However, Skubutyte et al (48) found that ANK was highly expressed in
calcified areas of cartilage endplates enriched in blood supply and
in NP tissues lacking blood supply, suggesting that there is an
intrinsic difference in the regulation of ANK between the NP and
the growth plate. A previous study found that HIF-1α or HIF-2α
directly negatively regulate the expression of ANK, indicating that
the expression of HIF-1α or HIF-2α can maintain the physiological
level of ANK under physiological conditions, thereby preventing the
occurrence of dystrophic mineralization and promoting NPCs to adapt
to the hypoxic environment (48).
Interestingly, although the expression of HIF proteins is not
related to oxygen tension, silencing HIF further increases the
expression of ANK during hypoxia, indicating that hypoxia can also
regulate can regulate TGF-β expression to modulate ANK promoter
activity in a HIF-independent manner (Fig. 3) (48).
Cell transplantation repair or even reconstruction
of degenerate IVD could be used to treat IDD. Wang et al
(184) injected NPCs into an
established IDD mouse model and found that transplanted NPCs can
counteract apoptosis, increase the expression of ECM and promote
NPC migration to the damaged area, thereby alleviating IDD. Yang
et al (46,47) found that the isolation, expansion
and culture of human degenerate NPCs can successfully preserve the
regenerative capacity of NPCs during hypoxia. The expression of
HIF-1α is enhanced under hypoxia, which can promote cell
proliferation and maintain the functional phenotype of NPCs.
Therefore, it is necessary to isolate and amplify NPCs during
hypoxia to pave the way for post-cultivation or cell
transplantation. The energy metabolism of transplanted NPCs relies
on the HIF-1α-GLUT1 pathway (Fig.
3) (150).
An increasing number of studies have reported
herniated disc tissue retraction or even disappearance in patients
with lumbar disc herniation previously diagnosed by MRI (14–16).
Macrophages, angiogenesis, MMPs and inflammatory cytokines play an
important role in this process (104,107,112,113). HIF-1α mediates macrophage
infiltration, and their role in acute inflammation response, via
the regulation of ATP production (63). HIF-1α also enhances the phagocytic
activity of M1 macrophages and the expression of pro-inflammatory
cytokines and VEGF (8,130,185). Due to the network regulatory
relationships among HIF-1α and angiogenesis, MMPs and inflammatory
cytokines (Fig. 3), in this review
it is hypothesized that HIF-1α may be involved in regulating the
spontaneous absorption of prolapsed IVD tissue. The possible
mechanisms need further research.
Numerous studies have reported that non-coding
RNAs, including miRNAs, long non-coding (lnc)RNAs and newly
discovered circular (circ)RNAs, play an important role in the
occurrence and progression of IDD. More importantly, numerous
non-coding RNAs are associated with HIF-1α (35,53,108,186–191), as shown in Table I. Liu et al (35) confirmed that miR-335 can directly
regulate the expression of HIF-1α, and found that HIF-1α has a dual
role in the regulation of cerebral ischaemia. Wang et al
(53) found that
lncRNA-RP11-296A18.3 enhances the expression of HIF-1α by
sequestering miR-138, which in turn promotes human NPC
proliferation and ECM synthesis. Serocki et al (188) reviewed the miRNAs and lncRNAs that
can regulate the HIF-1α switch and found that they are mainly
involved in process of cell proliferation, migration, angiogenesis
and hypoxia. lncRNAs regulate HIF-1α directly (188) and can indirectly regulate HIF-1α
expression by sequestering miRNAs (53,188,189). In the present review, the focus is
mainly on the circRNAs that are associated with HIF-1α (180–191). Liang et al (186) found that the expression of
circDENND4C was enhanced in breast cancer cells under hypoxia, and
reduced after HIF-1α knockdown. Thus, indicating that HIF-1α
mediates cell metabolism via regulating the expression of circRNA.
Interestingly, circRNA mediates HIF-1α metastasis to reverse its
biofunction (188). Du et
al (187) reported that
circ-Foxo3 was enriched in the cytoplasm and absorbs HIF-1α, which
was originally highly expressed in the nucleus and translocated
into the cytoplasm to promote cell senescence. Dang et al
(190) found that circ_0010729 can
sequester miR-186 to relieve the inhibitory effect of miR-186 on
HIF-1α, thereby promoting vascular endothelial cell proliferation
and migration, and inhibiting apoptosis. Liu et al (191) first studied the expression profile
of circRNAs in osteosarcoma and found that circRNA_103801
expression was markedly upregulated. Functional enrichment analysis
showed that it could play a role in cancer signalling pathways,
such as HIF-1α, VEGF and angiogenesis (191). circRNAs have an effect on
mitigating IDD (Table II)
(125,126,192). Cheng et al (125) demonstrated that circVMA21 reduces
inflammatory cytokine-induced NPC death and the expression of MMPs
and ADAMTSs, as well as increasing the synthesis of COL2 and ACAN
in in vitro experiments. Subsequently, the results of in
vivo experiments were in accordance with the in vitro
experiments, suggesting that the circVMA21/miR-200c/XIAP axis plays
a crucial role in mitigating IDD (126). Guo et al (192) further demonstrated that the
circ-GRB10/miR-328-5p/erb-B2 receptor tyrosine kinase 2 axis can
also regulate NPC apoptosis. Wang et al (126) reported that TNF-α enhances the
expression of circ-4099, which promotes the synthesis of SOX9 via
the sequestering of miR-616-5p. However, these three experiments do
have similar limitations; the key genes researched do not
necessarily play a major role in IDD. As the purpose of studying
the genes involved in IDD is so that the results can go on to be
applied in the clinic, the key genes should be selected for study.
In this review, it is questioned whether circRNAs participate in
the regulation of IDD via modulating the HIF-1α switch. In summary,
it is further speculated that HIF-1α may be a common relay site for
non-coding RNAs regulating IDD. It is necessary to further
investigate the role of the circRNA-miRNA-HIF-1α-MMP-ECM signalling
pathway in regulating the progression of IDD.
Increasingly, patients with LBP in the clinic are
shown to have protrusive or degenerative IVD via MRI imaging
(14–16). Pfirrmann et al (193) mainly used MRI to classify the
grade of the severity of IDD, without considering the
microenvironmental factors of IVD. The IVD microenvironment has
already become dysregulated before MRI shows positive results, such
as protrusion or degeneration of the IVD (25,40,77).
Thus, even if a negative result is detected by MRI, it cannot be
ruled out that the IVD microenvironment has already deteriorated.
Therefore, compared with Pfirrmann's ‘external grading’, the
‘internal grading’ of the degree of microenvironmental disturbance
within IVD can easily be neglected before this type of patient has
a positive result. Consequently, it is crucial to determine the
‘internal grading’ of the severity of IDD as soon as possible and
to intervene early to block the vicious cycle of degeneration. This
also opens up alternative avenues for patients who have results
contradictory to MRI imaging. In view of the fact that IVD has no
vascular structures (19,22), the diagnosis of the ‘internal
grading’ of IDD relies on puncture only. Ultrasound-guided puncture
has the advantages of accurate positioning, convenient operation,
economy, and both diagnosis and treatment, as well as having
clinical applicability (194).
HIF-1α, as the most important molecule regulating the degeneration
of human IVD (30), is a common
relay station for the regulation of a number of non-coding RNA
molecules (35,53,108,186–191). The various mechanisms affecting
IDD interact with each other and ultimately regulate ECM metabolism
(25,77–79).
Therefore, the dysregulation of ECM metabolism is the most
important mechanism affecting IDD. Water loss within the NP is an
important feature of IDD (24,40,77).
Although MMPs (49–52), inflammatory factors (8,103,125) and various related proteins
(90,114,120,155) also play an important role in IDD,
they are indirectly reflected by HIF-1α and ECM and may not be
included in the evaluation index. Therefore, HIF-1α, ECM (the
content and distribution of ACAN and COL2 and other collagens) and
H2O can be used as the evaluation criteria for the
pathological diagnosis of the ‘internal grading’ of the degree of
microenvironmental disorder in IVD. However, the challenges faced
by this method should be noted. This includes how to puncture for
the biopsy and its feasibility (why patients should take an
invasive examination and where is the value). Therefore, further
research is needed to demonstrate that early precaution and
intervention can avoid advanced surgery. Additionally, another
challenge concerns the determination of reference values of various
evaluation indicators. circRNAs are tissue-specific and can serve
as markers of disease (195) so
greater challenges include further screening of circRNAs with
markers that indirectly reflect the expression of HIF-1α and ECM,
and then diagnose the degree of IDD through the drawing of
blood.
In summary, this review put forward three
invaluable and strongly indispensable research questions. Firstly,
HIF-1α is a double-edged sword in modulating the occurrence and
progression of IDD, which is related to the oxygen tension of the
IVD microenvironment. The oxygen tension is gradually decreased
with the progression of the vicious cycle. The HIF-dependent
pathways play a friendly role during moderate hypoxia, while
playing a foe role in severe hypoxia. Is it possible to alter the
oxygen tension indirectly, in order to control the expression of
HIF-1α and to treat IDD? Secondly, HIF-1α has the ability to
regulate all other mechanisms involved in IDD, and it may also be a
common relay station for a number of non-coding RNAs that regulate
the progression of IDD. Does the circRNA-miRNA-HIF-1α-MMP2-ECM
signalling pathway play a crucial role in regulating the
progression of IDD? Thirdly, puncturing the IVD to obtain NP tissue
for biopsy, guided by B-ultrasound, could be a novel and vital
method for early diagnosis and treatment in cases where the patient
is displaying symptoms but MRI is showing a negative result. So,
how feasible is this diagnosis method? Why undergo an invasive
examination, and where is the value? Can levels of HIF-1α, ACAN,
COL2, VEGF and H2O be used as evaluation criteria for
the pathological diagnosis of the ‘internal grading’ of the degree
of IVD microenvironmental dysregulation? Further research is needed
to answer these questions in order to aid the clinical diagnosis
and treatment of IDD.
The authors would like to thank Professor Shiqing
Feng, for his guidance on article design and critically review the
article.
This study was supported by the State Key Program
of the National Natural Science Foundation of China (grant no.
81330042), the Special Program for Sino-Russian Joint Research
Sponsored by the Ministry of Science and Technology, China (grant
no. 2014DFR31210), and the International Cooperation Program of the
National Natural Science Foundation of China (grant no.
81620108018).
Not applicable.
YL, SL and DP contributed to the concept and the
design of the review. YL and DP wrote the manuscript. SL, GN and XX
helped draft the manuscript and drew the figures. BX provided
significant suggestions for the study. HZ, BZ, SZ searched the
literature and collated important reference information. SF
critically reviewed the manuscript. All authors read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Deyo RA and Tsui-Wu YJ: Descriptive
epidemiology of low-back pain and its related medical care in the
United States. Spine (Phila Pa 1976). 12:264–268. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersson GB: Epidemiological features of
chronic low-back pain. Lancet. 354:581–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Fang XQ, Wang Q, Wang SW, Wang SW,
Hu ZJ, Zhou ZJ, Xu WB, Wang JY, Qin A and Fan SW: PHD/HIF-1
upregulates CA12 to protect against degenerative disc disease: A
human sample, in vitro and ex vivo study. Lab Invest. 96:561–569.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samartzis D, Karppinen J, Mok F, Fong DY,
Luk KD and Cheung KM: A population-based study of juvenile disc
degeneration and its association with overweight and obesity, low
back pain, and diminished functional status. J Bone Joint Surg Am.
93:662–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verrills P, Nowesenitz G and Barnard A:
Prevalence and characteristics of discogenic pain in tertiary
practice: 223 consecutive cases utilizing lumbar discography. Pain
Med. 16:1490–1499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bijkerk C, Houwing-Duistermaat JJ,
Valkenburg HA, Meulenbelt I, Hofman A, Breedveld FC, Pols HA, van
Duijn CM and Slagboom PE: Heritabilities of radiologic
osteoarthritis in peripheral joints and of disc degeneration of the
spine. Arthritis Rheum. 42:1729–1735. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sambrook PN, MacGregor AJ and Spector TD:
Genetic influences on cervical and lumbar disc degeneration: A
magnetic resonance imaging study in twins. Arthritis Rheum.
42:366–372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Battié MC, Videman T, Gibbons LE, Fisher
LD, Manninen H and Gill K: 1995 Volvo award in clinical sciences:
Determinants of lumbar disc degeneration. A study relating lifetime
exposures and magnetic resonance imaging findings in identical
twins. Spine (Phila Pa 1976). 20:2601–2612. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van den Eerenbeemt KD, Ostelo RW, van
Royen BJ, Peul WC and van Tulder MW: Total disc replacement surgery
for symptomatic degenerative lumbar disc disease: A systematic
review of the literature. Eur Spine J. 19:1262–1280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacobs WC, van der Gaag NA, Kruyt MC,
Tuschel A, de Kleuver M, Peul WC, Verbout AJ and Oner FC: Total
disc replacement for chronic discogenic low back pain: A Cochrane
review. Spine (Phila Pa 1976). 38:24–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nozawa S, Nozawa A, Kojima H and Shimizu
K: Spontaneous disappearance of lumbar disk herniation within 3
months. Orthopedics. 32:8522009.PubMed/NCBI
|
|
15
|
Ryu SJ and Kim IS: Spontaneous regression
of a large lumbar disc extrusion. J Korean Neurosurg Soc.
48:285–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao S, Geng X and Fang Q: Spontaneous
disappearance of large lumbar disk herniation. JAMA Neurol.
75:123–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Humzah MD and Soames RW: Human
intervertebral disc: Structure and function. Anat Rec. 220:337–356.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nerlich AG, Boos N, Wiest I and Aebi M:
Immunolocalization of major interstitial collagen types in human
lumbar intervertebral discs of various ages. Virchows Arch.
432:67–76. 1988. View Article : Google Scholar
|
|
19
|
Ogata K and Whiteside LA: 1980 Volvo award
winner in basic science. Nutritional pathways of the intervertebral
disc. An experimental study using hydrogen washout technique. Spine
(Phila Pa 1976). 6:211–216. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gruber HE, Ashraf N, Kilburn J, Williams
C, Norton HJ, Gordon BE and Hanley EN Jr: Vertebral endplate
architecture and vascularization: Application of micro-computerized
tomography, a vascular tracer, and immunocytochemistry in analyses
of disc degeneration in the aging sand rat. Spine (Phila Pa 1976).
30:2593–2600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujita N, Markova D, Anderson DG, Chiba K,
Toyama Y, Shapiro IM and Risbud MV: Expression of prolyl
hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2
proteins in nucleus pulposus cells of the intervertebral disc:
Distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α
activity in hypoxia. J Biol Chem. 287:16975–16986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K
and An HS: The origin of chondrocytes in the nucleus pulposus and
histologic findings associated with the transition of a notochordal
nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact
rabbit intervertebral discs. Spine (Phila Pa 1976). 28:982–990.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urban JP, Smith S and Fairbank JC:
Nutrition of the intervertebral disc. Spine (Phila Pa 1976).
29:2700–2709. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guiot BH and Fessler RG: Molecular biology
of degenerative disc disease. Neurosurgery. 47:1034–1040. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng G, Jin X, Hu J, Ma H, Gupte MJ, Liu H
and Ma PX: Effects of hypoxias and scaffold architecture on rabbit
mesenchymal stem cell differentiation towards a nucleus
pulposus-like phenotype. Biomaterials. 32:8182–8189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakai D and Grand S: Advancing the
cellular and molecular therapy for intervertebral disc disease. Adv
Drug Deliv Rev. 84:159–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Risbud MV, Schipani E and Shapiro IM:
Hypoxic regulation of nucleus pulposus cell survival: From niche to
notch. Am J Pathol. 176:1577–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng G, Li L, Liu H, Song Y, Huang F, Tu
C, Shen B, Gong Q, Li T, Liu L, et al: Hypoxia differentially
regulates human nucleus pulposus and annulus fibrosus cell
extracellular matrix production in 3D scaffolds. Osteoarthritis
Cartilage. 21:582–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng G, Li L, Hong Y, Liu H, Song Y, Pei
F, Ma PX, Gong Q and Gupte MJ: Hypoxia promotes nucleus pulposus
phenotype in 3D scaffolds in vitro and in vivo: Laboratory
investigation. J Neurosurg Spine. 21:303–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Semenza GL: Life with oxygen. Science.
318:62–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Richardson SM, Knowles R, Tyler J,
Mobasheri A and Hoyland JA: Expression of glucose transporters
GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate
human intervertebral disc. Histochem Cell Biol. 129:503–511. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benita Y, Kikuchi H, Smith AD, Zhang MQ,
Chung DC and Xavier RJ: An integrative genomics approach identifies
Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core
response to hypoxia. Nucleic Acids Res. 37:4587–4602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semenza GL: Hypoxia-inducible factor 1:
Control of oxygen homeostasis in health and disease. Pediatr Res.
49:614–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharp FR and Bernaudin M: HIF1 and oxygen
sensing in the brain. Nat Rev Neurosci. 5:437–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu FJ, Kaur P, Karolina DS, Sepramaniam
S, Armugam A, Wong PT and Jeyaseelan K: MiR-335 regulates hif-1α to
reduce cell death in both mouse cell line and rat ischemic models.
PLoS One. 10:e01284322015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eales KL, Hollinshead KE and Tennant DA:
Hypoxia and metabolic adaptation of cancer cells. Oncogenesis.
5:e1902016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giatromanolaki A, Sivridis E, Maltezos E,
Athanassou N, Papazoglou D, Gatter KC, Harris AL and Koukourakis
MI: Upregulated hypoxia inducible factor-1alpha and −2alpha pathway
in rheumatoid arthritis and osteoarthritis. Arthritis Res Ther.
5:R193–R201. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim
BJ, Min BH and Chun JS: Hypoxia-inducible factor-2alpha is a
catabolic regulator of osteoarthritic cartilage destruction. Nat
Med. 16:687–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Konisti S, Kiriakidis S and Paleolog EM:
Hypoxia-a key regulator of angiogenesis and inflammation in
rheumatoid arthritis. Nat Rev Rheumatol. 8:153–162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kwon WK, Moon HJ, Kwon TH, Park YK and Kim
JH: The role of hypoxia in angiogenesis and extracellular matrix
regulation of intervertebral disc cells during inflammatory
reactions. Neurosurgery. 81:867–875. 2017.PubMed/NCBI
|
|
42
|
Wu WJ, Zhang XK, Zheng XF, Yang YH, Jiang
SD and Jiang LS: SHH-dependent knockout of HIF-1 alpha accelerates
the degenerative process in mouse intervertebral disc. Int J
Immunopathol Pharmacol. 26:601–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ha KY, Koh I, Kirpalani PA, Kim YY, Cho
YK, Khang GS and Han CW: The expression of hypoxia inducible
factor-1alpha and apoptosis in herniated discs. Spine (Phila Pa
1976). 31:1309–1313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu CJ, Wang LY, Chodosh LA, Keith B and
Simon MC: Differential roles of hypoxia-inducible factor 1alpha
(HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell
Biol. 23:9361–9374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen JW, Ni BB, Zheng XF, Li B, Jiang SD
and Jiang LS: Hypoxia facilitates the survival of nucleus pulposus
cells in serum deprivation by down-regulating excessive autophagy
through restricting ROS generation. Int J Biochem Cell Biol.
59:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang SH, Hu MH, Sun YH and Lin FH:
Differential phenotypic behaviors of human degenerative nucleus
pulposus cells under normoxic and hypoxic conditions: Influence of
oxygen concentration during isolation, expansion, and cultivation.
Spine J. 13:1590–1596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang SH, Hu MH, Lo WY, Sun YH, Wu CC and
Yang KC: The influence of oxygen concentration on the extracellular
matrix production of human nucleus pulposus cells during
isolation-expansion process. J Biomed Mater Res A. 105:1575–1582.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Skubutyte R, Markova D, Freeman TA,
Anderson DG, Dion AS, Williams CJ, Shapiro IM and Risbud MV:
Hypoxia-inducible factor regulation of ANK expression in nucleus
pulposus cells: Possible implications in controlling dystrophic
mineralization in the intervertebral disc. Arthritis Rheum.
62:2707–2715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jing SW, Wang YD, Kuroda M, Su JW, Sun GG,
Liu Q, Cheng YJ and Yang CR: HIF-1α contributes to hypoxia-induced
invasion and metastasis of esophageal carcinoma via inhibiting
E-cadherin and promoting MMP-2 expression. Acta Med Okayama.
66:399–407. 2012.PubMed/NCBI
|
|
50
|
Rodrigues M, Xin X, Jee K,
Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ,
Daoud Y, Baranano D, et al: VEGF secreted by hypoxic Müller cells
induces MMP-2 expression and activity in endothelial cells to
promote retinal neovascularization in proliferative diabetic
retinopathy. Diabetes. 62:3863–3873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsai SH, Huang PH, Hsu YJ, Peng YJ, Lee
CH, Wang JC, Chen JW and Lin SJ: Inhibition of hypoxia inducible
factor-1α attenuates abdominal aortic aneurysm progression through
the down-regulation of matrix metalloproteinases. Sci Rep.
6:286122016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu WP, Jiang JM, Qu DB, Wei QZ and Jiang
H: Expression of hypoxia-inducible factor-1alpha and matrix
metalloproteinase-2 in degenerative lumbar intervertebral disc. Nan
Fang Yi Ke Da Xue Xue Bao. 30:1152–1155. 2010.(In Chinese).
PubMed/NCBI
|
|
53
|
Wang X, Lv G, Li J, Wang B, Zhang Q and Lu
C: LncRNA-RP11-296A18.3/miR-138/HIF1A pathway regulates the
proliferation ECM synthesis of human nucleus pulposus cells
(HNPCs). J Cell Biochem. 118:4862–4871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2-dependent degradation domain via the
ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 95:7987–7992.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wenger RH: Cellular adaptation to hypoxia:
O2-sensing protein hydroxylases, hypoxia-inducible transcription
factors, and O2-regulated gene expression. FASEB J. 16:1151–1162.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Makino Y, Kanopka A, Wilson WJ, Tanaka H
and Poellinger L: Inhibitory PAS domain protein (IPAS) is a
hypoxia-inducible splicing variant of the hypoxia-inducible
factor-3alpha locus. J Biol Chem. 277:32405–32408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pasanen A, Heikkilä M, Rautavuoma K,
Hirsilä M, Kivirikko KI and Myllyharju J: Hypoxia-inducible factor
(HIF)-3alpha is subject to extensive alternative splicing in human
tissues and cancer cells and is regulated by HIF-1 but not HIF-2.
Int J Biochem Cell Biol. 42:1189–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Moritz W, Meier F, Stroka DM, Giuliani M,
Kugelmeier P, Nett PC, Lehmann R, Candinas D, Gassmann M and Weber
M: Apoptosis in hypoxic human pancreatic islets correlates with
HIF-1alpha expression. FASEB J. 16:745–747. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wenger RH and Gassmann M: Oxygen(es) and
the hypoxia-inducible factor-1. Biol Chem. 378:609–616.
1997.PubMed/NCBI
|
|
61
|
Ema M, Taya S, Yokotani N, Sogawa K,
Matsuda Y and Fujii-Kuriyama Y: A novel bHLH-PAS factor with close
sequence similarity to hypoxia-inducible factor 1alpha regulates
the VEGF expression and is potentially involved in lung and
vascular development. Proc Natl Acad Sci USA. 94:4273–4278. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tian H, McKnight SL and Russell DW:
Endothelial PAS domain protein 1 (EPAS1), a transcription factor
selectively expressed in endothelial cells. Genes Dev. 11:72–82.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Imtiyaz HZ, Williams EP, Hickey MM, Patel
SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B and Simon
MC: Hypoxia-inducible factor 2alpha regulates macrophage function
in mouse models of acute and tumor inflammation. J Clin Invest.
120:2699–2714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Madsen CD, Pedersen JT, Venning FA, Singh
LB, Moeendarbary E, Charras G, Cox TR, Sahai E and Erler JT:
Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease
tumour stiffness and metastasis. EMBO Rep. 16:1394–1408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fujita N, Chiba K, Shapiro IM and Risbud
MV: HIF-1α and HIF-2α degradation is differentially regulated in
nucleus pulposus cells of the intervertebral disc. J Bone Miner
Res. 27:401–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Raval RR, Lau KW, Tran MG, Sowter HM,
Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL and Ratcliffe
PJ: Contrasting properties of hypoxia-inducible factor 1 (HIF-1)
and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol
Cell Biol. 25:5675–5686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Eyre DR, Benya P, Buckwalter JA, Caterson
B, Heinegard D, Oegema T, Pearce R, Pope M and Urban J: Basic
Sciences Perspectives: Part B-Intervertebral Discs. Park Ridge:
American Academy of Orthopaedic Surgeons; pp. 147–207. 1989
|
|
68
|
Shao J, Yu M, Jiang L, Wei F, Wu F, Liu Z
and Liu X: Differences in calcification and osteogenic potential of
herniated discs according to the severity of degeneration based on
Pfirrmann grade: A cross-sectional study. BMC Musculoskelet Disord.
17:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grant MP, Epure LM, Bokhari R, Roughley P,
Antoniou J and Mwale F: Human cartilaginous endplate degeneration
is induced by calcium and the extracellular calcium-sensing
receptor in the intervertebral disc. Eur Cell Mater. 32:137–151.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Karamouzian S, Eskandary H, Faramarzee M,
Saba M, Safizade H, Ghadipasha M, Malekpoor AR and Ohadi A:
Frequency of lumbar intervertebral disc calcification and
angiogenesis, and their correlation with clinical, surgical, and
magnetic resonance imaging findings. Spine (Phila Pa 1976).
35:881–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Eyre DR and Muir H: Quantitative analysis
of types I and II collagens in human intervertebral discs at
various ages. Biochim Biophys Acta. 492:29–42. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Roughley P, Martens D, Rantakokko J, Alini
M, Mwale F and Antoniou J: The involvement of aggrecan polymorphism
in degeneration of human intervertebral disc and articular
cartilage. Eur Cell Mater. 11:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu B, Xu C, Tian Y, Shi C, Zhang Y, Deng
L, Zhou H, Cao P, Chen H and Yuan W: Inflammatory microRNA-194 and
−515 attenuate the biosynthesis of chondroitin sulfate during human
intervertebral disc degeneration. Oncotarget. 8:49303–49317. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Poole AR: Biologic markers and disc
degeneration. J Bone Joint Surg Am. 88 (Suppl 2):S72–S75. 2006.
View Article : Google Scholar
|
|
76
|
Duance VC, Crean JK, Sims TJ, Avery N,
Smith S, Menage J, Eisenstein SM and Roberts S: Changes in collagen
cross-linking in degenerative disc disease and scoliosis. Spine
(Phila Pa 1976). 23:2545–2551. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vo NV, Hartman RA, Patil PR, Risbud MV,
Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA and Kang
JD: Molecular mechanisms of biological aging in intervertebral
discs. J Orthop Res. 34:1289–1306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Peck SH, McKee KK, Tobias JW, Malhotra NR,
Harfe BD and Smith LJ: Whole transcriptome analysis of
notochord-derived cells during embryonic formation of the nucleus
pulposus. Sci Rep. 7:105042017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu Z, Li C, Meng X, Bai Y, Qi J, Wang J,
Zhou Q, Zhang W and Zhang X: Hypoxia-inducible factor-lα mediates
aggrecan and collagen Π expression via NOTCH1 signaling in nucleus
pulposus cells during intervertebral disc degeneration. Biochem
Biophys Res Commun. 488:554–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen D, Li M, Luo J and Gu W: Direct
interactions between HIF-1 alpha and Mdm2 modulate p53 function. J
Biol Chem. 278:13595–13598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Aro E, Khatri R, Gerard-O'Riley R,
Mangiavini L, Myllyharju J and Schipani E: Hypoxia-inducible
factor-1 (HIF-1) but not HIF-2 is essential for hypoxic induction
of collagen prolyl 4-hydroxylases in primary newborn mouse
epiphyseal growth plate chondrocytes. J Biol Chem. 287:37134–37144.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Meretoja VV, Dahlin RL, Wright S, Kasper
FK and Mikos AG: The effect of hypoxia on the chondrogenic
differentiation of co-cultured articular chondrocytes and
mesenchymal stem cells in scaffolds. Biomaterials. 34:4266–4273.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D
and Semenza GL: Hypoxia-inducible factor 1 (HIF-1) promotes
extracellular matrix remodeling under hypoxic conditions by
inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol
Chem. 288:10819–10829. 2014. View Article : Google Scholar
|
|
85
|
Mwale F, Ciobanu I, Giannitsios D,
Roughley P, Steffen T and Antoniou J: Effect of oxygen levels on
proteoglycan synthesis by intervertebral disc cells. Spine (Phila
Pa 1976). 36:E131–E138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Pei M, Shoukry M, Li J, Daffner SD, France
JC and Emery SE: Modulation of in vitro microenvironment
facilitates synovium derived stem cell-based nucleus pulposus
tissue regeneration. Spine (Phila Pa 1976). 37:1538–1547. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ishihara H and Urban JP: Effects of low
oxygen concentrations and metabolic inhibitors on proteoglycan and
protein synthesis rates in the intervertebral disc. J Orthop Res.
17:829–835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cigognini D, Gaspar D, Kumar P, Satyam A,
Alagesan S, Sanz-Nogués C, Griffin M, O'Brien T, Pandit A and
Zeugolis DI: Macromolecular crowding meets oxygen tension in human
mesenchymal stem cell culture-A step closer to physiologically
relevant in vitro organogenesis. Sci Rep. 6:307462016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Obradovic B, Carrier RL, Vunjak-Novakovic
G and Freed LE: Gas exchange is essential for bioreactor
cultivation of tissue engineered cartilage. Biotechnol Bioeng.
63:197–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Senger DR, Van de Water L, Brown LF, Nagy
JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM and Dvorak HF:
Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer
Metastasis Rev. 12:303–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Marsano A, Maidhof R, Luo J, Fujikara K,
Konofagou EE, Banfi A and Vunjak-Novakovic G: The effect of
controlled expression of VEGF by transduced myoblasts in a cardiac
patch on vascularization in a mouse model of myocardial infarction.
Biomaterials. 34:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial growth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Saharinen P, Eklund L and Alitalo K:
Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug
Discov. 16:635–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: Role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 38:721–732. 2003. View Article : Google Scholar
|
|
97
|
Agrawal A, Gajghate S, Smith H, Anderson
DG, Albert TJ, Shapiro IM and Risbud MV: Cited2 modulates
hypoxia-inducible factor-dependent expression of vascular
endothelial growth factor in nucleus pulposus cells of the rat
intervertebral disc. Arthritis Rheum. 58:3798–3808. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ahn JK, Koh EM, Cha HS, Lee YS, Kim J, Bae
EK and Ahn KS: Role of hypoxia-inducible factor-1alpha in
hypoxia-induced expressions of IL-8, MMP-1 and MMP-3 in rheumatoid
fibroblast-like synoviocytes. Rheumatology (Oxford). 47:834–839.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Calvani M, Rapisarda A, Uranchimeg B,
Shoemaker RH and Melillo G: Hypoxic induction of an
HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in
human endothelial cells. Blood. 107:2705–2712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bos R, van Diest PJ, de Jong JS, van der
Groep P, van der Valk P and van der Wall E: Hypoxia-inducible
factor-1alpha is associated with angiogenesis, and expression of
bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology.
46:31–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bian Q, Ma L, Jain A, Crane JL, Kebaish K,
Wan M, Zhang Z, Edward Guo X, Sponseller PD, Séguin CA, et al:
Mechanosignaling activation of TGFβ maintains intervertebral disc
homeostasis. Bone Res. 5:170082017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
oshida S, Ono M, Shono T, Izumi H,
Ishibashi T, Suzuki H and Kuwano M: Involvement of interleukin-8,
vascular endothelial growth factor, and basic fibroblast growth
factor in tumor necrosis factor alpha-dependent angiogenesis. Mol
Cell Biol. 17:4015–4023. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Haro H, Kato T, Komori H, Osada M and
Shinomiya K: Vascular endothelial growth factor (VEGF)-induced
angiogenesis in herniated disc resorption. J Orthop Res.
20:409–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lee JM, Song JY, Baek M, Jung HY, Kang H,
Han IB, Kwon YD and Shin DE: Interleukin-1β induces angiogenesis
and innervation in human intervertebral disc degeneration. J Orthop
Res. 29:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sang Q: Complex role of matrix metal
oproteinases in angiogenesis. Cell Res. 8:171–177. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ebrahem Q and Anand A: Induction of
angiogenesis by active matrix metalloproteinases-2 and 9: Role of
VEGF. Invest Ophthalmol Vis Sci. 44:445–452. 2003.
|
|
108
|
Liang H, Xiao J, Zhou Z, Wu J, Ge F, Li Z,
Zhang H, Sun J, Li F, Liu R and Chen C: Hypoxia induces miR-153
through the IRE1α-XBP1 pathway to fine tune the HIF1α/VEGFA axis in
breast cancer angiogenesis. Oncogene. 37:1961–1975. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mazure NM, Brahimi-Horn MC and Pouysségur
J: Protein kinases and the hypoxia-inducible factor-1, two switches
in angiogenesis. Curr Pharm Des. 9:531–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Han IB, Ropper AE, Teng YD, Shin DA, Jeon
YJ, Park HM, Shin DE, Park YS, Kim KN and Kim NK: Association
between VEGF and eNOS gene polymorphisms and lumbar disc
degeneration in a young Korean population. Genet Mol Res.
12:2294–2305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Long H and Hu Y: Expression of vascular
endothelial growth factor in lumbar intervertebral disc and its
significance. Chin J Spinal Cord. 12:280–282. 2002.
|
|
112
|
Doita M, Kanatani T, Ozaki T, Matsui N,
Kurosaka M and Yoshiya S: Influence of macrophage infiltration of
herniated disc tissue on the production of matrix
metalloproteinases leading to disc resorption. Spine (Phila Pa
1976). 26:1522–1527. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kobayashi S, Meir A, Kokubo Y, Uchida K,
Takeno K, Miyazaki T, Yayama T, Kubota M, Nomura E, Mwaka E and
Baba H: Ultrastructural analysis on lumbar disc herniation using
surgical specimens: Role of neovascularization and macrophages in
hernias. Spine (Phila Pa 1976). 34:655–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lu XY, Ding XH, Zhong LJ, Xia H, Chen XD
and Huang H: Expression and significance of VEGF and p53 in
degenerate intervertebral disc tissue. Asian Pac J Trop Med.
6:79–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sánchez-Puig N, Veprintsev DB and Fersht
AR: Binding of natively unfolded HIF-1alpha ODD domain to p53. Mol
Cell. 17:11–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ullah K, Rosendahl AH, Izzi V, Bergmann U,
Pihlajaniemi T, Mäki JM and Myllyharju J: Hypoxia-inducible factor
prolyl-4-hydroxylase-1 is a convergent point in the reciprocal
negative regulation of NF-κB and p53 signaling pathways. Sci Rep.
7:172202017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chowdhury AR, Long A, Fuchs SY, Rustgi A
and Avadhani NG: Mitochondrial stress-induced p53 attenuates HIF-1α
activity by physical association and enhanced ubiquitination.
Oncogene. 36:397–409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Fujita N, Gogate SS, Chiba K, Toyama Y,
Shapiro IM and Risbud MV: Prolyl hydroxylase 3 (PHD3) modulates
catabolic effects of tumor necrosis factor-α (TNF-α) on cells of
the nucleus pulposus through co-activation of nuclear factor κB
(NF-κB)/p65 signaling. J Biol Chem. 287:39942–39953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Johnson ZI, Schoepflin ZR, Choi H, Shapiro
IM and Risbud MV: Disc in flames: Roles of TNF-α and IL-1β in
intervertebral disc degeneration. Eur Cell Mater. 30:104–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Phillips KL, Cullen K, Chiverton N,
Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK
and Le Maitre CL: Potential roles of cytokines and chemokines in
human intervertebral disc degeneration: Interleukin-1 is a master
regulator of catabolic processes. Osteoarthritis Cartilage.
23:1165–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Shen J, Xu S, Zhou H, Liu H, Jiang W, Hao
J and Hu Z: IL-1β induces apoptosis and autophagy via mitochondria
pathway in human degenerative nucleus pulposus cells. Sci Rep.
7:410672017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Shen J, Fang J, Hao J, Zhong X, Wang D,
Ren H and Hu Z: SIRT1 inhibits the catabolic effect of IL-1β
through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus
pulposus cells. Pain Physician. 19:E215–E226. 2016.PubMed/NCBI
|
|
125
|
Cheng X, Zhang L, Zhang K, Zhang G, Hu Y,
Sun X, Zhao C, Li H, Li YM and Zhao J: Circular RNA VMA21 protects
against intervertebral disc degeneration through targeting miR-200c
and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis.
77:770–779. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang H, He P, Pan H, Long J, Wang J, Li Z,
Liu H, Jiang W and Zheng Z: Circular RNA circ-4099 is induced by
TNF-α and regulates ECM synthesis by blocking miR-616-5p inhibition
of Sox9 in intervertebral disc degeneration. Exp Mol Med.
50:272018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tian Y, Yuan W, Fujita N, Wang J, Wang H,
Shapiro IM and Risbud MV: Inflammatory cytokines associated with
degenerative disc disease control aggrecanase-1 (ADAMTS-4)
expression in nucleus pulposus cells through MAPK and NF-κB. Am J
Pathol. 182:2310–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang H, Tian Y, Wang J, Phillips KL, Binch
AL, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, et al:
Inflammatory cytokines induce NOTCH signaling in nucleus pulposus
cells: Implications in intervertebral disc degeneration. J Biol
Chem. 288:16761–16774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cramer T, Yamanishi Y, Clausen BE, Förster
I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V,
et al: HIF-1alpha is essential for myeloid cell-mediated
inflammation. Cell. 112:645–657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Oliver KM, Taylor CT and Cummins EP:
Hypoxia. Regulation of NFkappaB signalling during inflammation: The
role of hydroxylases. Arthritis Res Ther. 11:2152009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Malandrino A, Noailly J and Lacroix D: The
effect of sustained compression on oxygen metabolic transport in
the intervertebral disc decreases with degenerative changes. PLoS
Comput Biol. 7:e10021122011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Mannello F and Medda V: Nuclear
localization of matrix metalloproteinases. Prog Histochem Cytochem.
47:27–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Crean JK, Roberts S, Jaffray DC,
Eisenstein SM and Duance VC: Matrix metalloproteinases in the human
intervertebral disc: Role in disc degeneration and scoliosis. Spine
(Phila Pa 1976). 22:2877–2884. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Weiler C, Nerlich AG, Zipperer J,
Bachmeier BE and Boos N: 2002 SSE award competition in basic
science: Expression of major matrix metalloproteinases is
associated with intervertebral disc degradation and resorption. Eur
Spine J. 11:308–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Xie Y, Mustafa A, Yerzhan A, Merzhakupova
D, Yerlan P, N Orakov A, Wang X, Huang Y and Miao L: Nuclear matrix
metalloproteinases: Functions resemble the evolution from the
intracellular to the extracellular compartment. Cell Death Discov.
3:170362017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kozaci LD, Guner A, Oktay G and Guner G:
Alterations in biochemical components of extracellular matrix in
intervertebral disc herniation: Role of MMP-2 and TIMP-2 in type II
collagen loss. Cell Biochem Funct. 24:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Roberts S, Caterson B, Menage J, Evans EH,
Jaffray DC and Eisenstein SM: Matrix metalloproteinases and
aggrecanase: Their role in disorders of the human intervertebral
disc. Spine (Phila Pa 1976). 25:3005–3013. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rutges JP, Kummer JA, Oner FC, Verbout AJ,
Castelein RJ, Roestenburg HJ, Dhert WJ and Creemers LB: Increased
MMP-2 activity during intervertebral disc degeneration is
correlated to MMP-14 levels. J Pathol. 214:523–530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yurube T, Takada T, Suzuki T, Kakutani K,
Maeno K, Doita M, Kurosaka M and Nishida K: Rat tail static
compression model mimics extracellular matrix metabolic imbalances
of matrix metalloproteinases, aggrecanases, and tissue inhibitors
of metalloproteinases in intervertebral disc degeneration.
Arthritis Res Ther. 14:R512012. View
Article : Google Scholar : PubMed/NCBI
|
|
142
|
Séguin CA, Pilliar RM, Madri JA and Kandel
RA: TNF-alpha induces MMP2 gelatinase activity and MT1-MMP
expression in an in vitro model of nucleus pulposus tissue
degeneration. Spine (Phila Pa 1976). 33:356–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xu YQ, Zhang ZH, Zheng YF and Feng SQ:
Dysregulated miR-133a mediates loss of type II collagen by directly
targeting matrix metalloproteinase 9 (MMP9) in human intervertebral
disc degeneration. Spine (Phila Pa 1976). 41:E717–E724. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hua WB, Wu XH, Zhang YK, Song Y, Tu J,
Kang L, Zhao KC, Li S, Wang K, Liu W, et al: Dysregulated
miR-127-5p contributes to type II collagen degradation by targeting
matrix metalloproteinase-13 in human intervertebral disc
degeneration. Biochimie. 139:74–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ji ML, Zhang XJ, Shi PL, Lu J, Wang SZ,
Chang Q, Chen H and Wang C: Downregulation of microRNA-193a-3p is
involved in invertebral disc degeneration by targeting MMP14. J Mol
Med (Berl). 94:457–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang WL, Chen YF, Meng HZ, Du JJ, Luan
GN, Wang HQ, Yang MW and Luo ZJ: Role of miR-155 in the regulation
of MMP-16 expression in intervertebral disc degeneration. J Orthop
Res. 35:1323–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jung JC, Wang PX, Zhang G, Ezura Y, Fini
ME and Birk DE: Collagen fibril growth during chicken tendon
development: Matrix metalloproteinase-2 and its activation. Cell
Tissue Res. 336:79–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang B, Ding YM, Fan P, Wang B, Xu JH and
Wang WX: Expression and significance of MMP2 and HIF-1α in
hepatocellular carcinoma. Oncol Lett. 8:539–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wu X, Liu W, Duan Z, Gao Y, Li S, Wang K,
Song Y, Shao Z, Yang S and Yang C: The involvement of protease
nexin-1 (PN1) in the pathogenesis of intervertebral disc (IVD)
degeneration. Sci Rep. 6:305632016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Huang Z, Zhang L, Feng X, Chen T and Bi S:
A new in vivo method to retard progression of intervertebral disc
degeneration through stimulation of endogenous stem cells with
simvastatin. Med Hypotheses. 101:65–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Lin Z, Weinberg JM, Malhotra R, Merritt
SE, Holzman LB and Brosius FC III: GLUT-1 reduces hypoxia-induced
apoptosis and JNK pathway activation. Am J Physiol Endocrinol
Metab. 278:E958–E966. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Agrawal A, Guttapalli A, Narayan S, Albert
TJ, Shapiro IM and Risbud MV: Normoxic stabilization of HIF-1alpha
drives glycolytic metabolism and regulates aggrecan gene expression
in nucleus pulposus cells of the rat intervertebral disk. Am J
Physiol Cell Physiol. 293:C621–C631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Maher JC, Wangpaichitr M, Savaraj N,
Kurtoglu M and Lampidis TJ: Hypoxia-inducible factor-1 confers
resistance to the glycolytic inhibitor 2-deoxy-D-glucose. Mol
Cancer Ther. 6:732–741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang H, Bosch-Marce M, Shimoda LA, Tan
YS, Baek JH, Wesley JB, Gonzalez FJ and Semenza GL: Mitochondrial
autophagy is an HIF-1-dependent adaptive metabolic response to
hypoxia. J Biol Chem. 283:10892–10903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Banh RS, Iorio C, Marcotte R, Xu Y,
Cojocari D, Rahman AA, Pawling J, Zhang W, Sinha A, Rose CM, et al:
PTP1B regulates non-mitochondrial oxygen consumption via RNF213 to
promote tumour survival during hypoxia. Nat Cell Biol. 18:803–813.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Dutta D, Xu J, Kim JS, Dunn WA Jr and
Leeuwenburgh C: Upregulated autophagy protects cardiomyocytes from
oxidative stress-induced toxicity. Autophagy. 9:328–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Caramés B, Taniguchi N, Otsuki S, Blanco
FJ and Lotz M: Autophagy is a protective mechanism in normal
cartilage, and its aging-related loss is linked with cell death and
osteoarthritis. Arthritis Rheum. 62:791–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu
Y, Tian N, Huang Y, Xue E, Wang X and Xu H: Apoptosis, senescence,
and autophagy in rat nucleus pulposus cells: Implications for
diabetic intervertebral disc degeneration. J Orthop Res.
31:692–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tu J, Li W, Li S, Liu W, Zhang Y, Wu X,
Luo R, Hua W, Wang K, Song Y, et al: Sestrin-mediated inhibition of
stress-induced intervertebral disc degradation through the
enhancement of autophagy. Cell Physiol Biochem. 45:1940–1954. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chen J, Xie JJ, Jin M, Gu YT, Wu CC, Guo
WJ, Yan YZ, Zhang ZJ, Wang JL, Zhang XL, et al: Sirt6
overexpression suppresses senescence and apoptosis of nucleus
pulposus cells by inducing autophagy in a model of intervertebral
disc degeneration. Cell Death Dis. 9:562018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y,
Cai N, Tang Q, Wang C, Yan M, et al: Metformin protects against
apoptosis and senescence in nucleus pulposus cells and ameliorates
disc degeneration in vivo. Cell Death Dis. 7:e24412016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Gruber HE, Hoelscher GL, Ingram JA, Bethea
S and Hanley EN Jr: Autophagy in the degenerating human
intervertebral disc: In vivo molecular and morphological evidence,
and induction of autophagy in cultured annulus cells exposed to
proinflammatory cytokines-implications for disc degeneration. Spine
(Phila Pa 1976). 40:773–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Mazure NM and Pouysségur J:
Hypoxia-induced autophagy: Cell death or cell survival? Curr Opin
Cell Biol. 22:177–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Scherz-Shouval R and Elazar Z: Regulation
of autophagy by ROS: Physiology and pathology. Trends Biochem Sci.
36:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia
J, Sun ZJ, Wang YN and Zhao YF: Autophagy regulates hypoxia-induced
osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J
Cell Physiol. 227:639–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhou J, Yao W, Li C, Wu W, Li Q and Liu H:
Administration of follicle-stimulating hormone induces autophagy
via upregulation of HIF-1α in mouse granulosa cells. Cell Death
Dis. 8:e30012017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ye W, Zhu W, Xu K, Liang A, Peng Y, Huang
D and Li C: Increased macroautophagy in the pathological process of
intervertebral disc degeneration in rats. Connect Tissue Res.
54:22–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Choi H, Merceron C, Mangiavini L, Seifert
EL, Schipani E, Shapiro IM and Risbud MV: Hypoxia promotes
noncanonical autophagy in nucleus pulposus cells independent of
MTOR and HIF1A signaling. Autophagy. 12:1631–1646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ding F, Shao Z-w and Xiong L-m: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Gruber HE and Hanley EN Jr: Analysis of
aging and degeneration of the human intervertebral disc. Comparison
of surgical specimens with normal controls. Spine (Phila Pa 1976).
23:751–757. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Bakker WJ, Harris IS and Mak TW: FOXO3a is
activated in response to hypoxic stress and inhibits HIF1-induced
apoptosis via regulation of CITED2. Mol Cell. 28:941–953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Fujita N, Imai J, Suzuki T, Yamada M,
Ninomiya K, Miyamoto K, Iwasaki R, Morioka H, Matsumoto M, Chiba K,
et al: Vascular endothelial growth factor-A is a survival factor
for nucleus pulposus cells in the intervertebral disc. Biochem
Biophys Res Commun. 372:367–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Huang S, Leung VY, Long D, Chan D, Lu WW,
Cheung KM and Zhou G: Coupling of small leucine-rich proteoglycans
to hypoxic survival of a progenitor cell-like subpopulation in
rhesus macaque intervertebral disc. Biomaterials. 34:6548–6558.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zeng Y, Danielson KG, Albert TJ, Shapiro
IM and Risbud MV: HIF-1 alpha is a regulator of galectin-3
expression in the intervertebral disc. J Bone Miner Res.
22:1851–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Silagi ES, Schoepflin ZR, Seifert EL,
Merceron C, Schipani E, Shapiro IM and Risbud MV: Bicarbonate
recycling by HIF-1-dependent carbonic anhydrase isoforms 9 and 12
is critical in maintaining intracellular pH and viability of
nucleus pulposus cells. J Bone Miner Res. 33:338–355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Guicheux J, Palmer G, Shukunami C, Hiraki
Y, Bonjour JP and Caverzasio J: A novel in vitro culture system for
analysis of functional role of phosphate transport in endochondral
ossification. Bone. 27:69–74. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hristova GI, Jarzem P, Ouellet JA,
Roughley PJ, Epure LM, Antoniou J and Mwale F: Calcification in
human intervertebral disc degeneration and scoliosis. J Orthop Res.
29:1888–1895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zaka R and Williams CJ: Role of the
progressive ankylosis gene in cartilage mineralization. Curr Opin
Rheumatol. 18:181–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Gurley KA, Reimer RJ and Kingsley DM:
Biochemical and genetic analysis of ANK in arthritis and bone
disease. Am J Hum Genet. 79:1017–1029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Liu MH, Sun C, Yao Y, Fan X, Liu H, Cui
YH, Bian XW, Huang B and Zhou Y: Matrix stiffness promotes
cartilage endplate chondrocyte calcification in disc degeneration
via miR-20a targeting ANKH expression. Sci Rep. 6:254012016.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sohn P, Crowley M, Slattery E and Serra R:
Developmental and TGF-beta-mediated regulation of Ank mRNA
expression in cartilage and bone. Osteoarthritis Cartilage.
10:482–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zaka R, Dion AS, Kusnierz A, Bohensky J,
Srinivas V, Freeman T and Williams CJ: Oxygen tension regulates the
expression of ANK (progressive ankylosis) in an HIF-1-dependent
manner in growth plate chondrocytes. J Bone Miner Res.
24:1869–1878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Wang W, Deng G, Qiu Y, Huang X, Xi Y, Yu
J, Yang X and Ye X: Transplantation of allogenic nucleus pulposus
cells attenuates intervertebral disc degeneration by inhibiting
apoptosis and increasing migration. Int J Mol Med. 41:2553–2564.
2018.PubMed/NCBI
|
|
185
|
Sica A, Schioppa T, Mantovani A and
Allavena P: Tumour-associated macrophages are a distinct M2
polarised population promoting tumour progression: Potential
targets of anti-cancer therapy. Eur J Cancer. 42:717–727. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Liang G, Liu Z, Tan L, Su AN, Jiang WG and
Gong C: HIF1α-associated circDENND4C promotes proliferation of
breast cancer cells in hypoxic environment. Anticancer Res.
37:4337–4343. 2017.PubMed/NCBI
|
|
187
|
Du WW, Yang W, Chen Y, Wu ZK, Foster FS,
Yang Z, Li X and Yang BB: Foxo3 circular RNA promotes cardiac
senescence by modulating multiple factors associated with stress
and senescence responses. Eur Heart J. 38:1402–1412.
2017.PubMed/NCBI
|
|
188
|
Serocki M, Bartoszewska S,
Janaszak-Jasiecka A, Ochocka RJ, Collawn JF and Bartoszewski R:
miRNAs regulate the HIF switch during hypoxia: A novel therapeutic
target. Angiogenesis. 21:183–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Bao MH, Li GY, Huang XS, Tang L, Dong LP
and Li JM: Long noncoding RNA LINC00657 acting as a miR-590-3p
sponge to facilitate low concentration oxidized low-density
lipoprotein-induced angiogenesis. Mol Pharmacol. 93:368–375. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Dang RY, Liu FL and Li Y: Circular RNA
hsa_circ_0010729 regulates vascular endothelial cell proliferation
and apoptosis by targeting the miR-186/HIF-1α axis. Biochem Biophys
Res Commun. 490:104–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Liu W, Zhang J, Zou C, Xie X, Wang Y, Wang
B, Zhao Z, Tu J, Wang X, Li H, et al: Microarray expression profile
and functional analysis of circular RNAs in osteosarcoma. Cell
Physiol Biochem. 43:969–985. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Guo W, Zhang B, Mu K, Feng SQ, Dong ZY,
Ning GZ, Li HR, Liu S, Zhao L, Li Y, et al: Circular RNA GRB10 as a
competitive endogenous RNA regulating nucleus pulposus cells death
in degenerative intervertebral disk. Cell Death Dis. 9:3192018.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
2:1873–1878. 2001. View Article : Google Scholar
|
|
194
|
Martin JT, Gorth DJ, Beattie EE, Harfe BD,
Smith LJ and Elliott DM: Needle puncture injury causes acute and
long-term mechanical deficiency in a mouse model of intervertebral
disc degeneration. J Orthop Res. 31:1276–1282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016. View Article : Google Scholar : PubMed/NCBI
|