Introduction
Leukemias, presenting with increased numbers of
leucocytes, are a group of malignant disorders. Globally, the
number of newly diagnosed leukemia patients increased from
3,545,000 in 1990 to 5,185,000 in 2017 (1). It is estimated there were a total of
4,370,000 new cases of and 3,090,000 deaths from leukemia worldwide
in 2018 (2). Myeloid leukemia,
including acute myelogenous leukemia (AML) and chronic myelogenous
leukemia (CML), is the most common hematological malignancy. CML,
which is characterized by the Philadelphia chromosome (a fusion of
chromosomes 9 and 22), gives rise to the Bcr/Abl oncogene (3,4).
Although Bcr/Abl kinase inhibitors have achieved a promising
clinical response in CML, certain patients progressing to the blast
phase may develop resistance to Bcr/Abl kinase inhibitors (5). Furthermore, AML is frequently
accompanied by a poor prognosis. The DA regimen, mainly comprising
cytarabine and doxorubicin (Doxo), has been the standard treatment
for AML (6). However, the majority
of the patients with AML will experience recurrence due to the
development of multiple drug resistance, with decreasing cancer
cell capability for apoptosis (7).
Therefore, novel therapeutic targets must be investigated to
enhance the therapeutic efficacy against leukemia.
It was previously demonstrated that the mTOR pathway
is upregulated in CML, and rapamycin can arrest cells at the
G0/G1 phase and promote apoptosis of K562
cells (8). In addition, rapamycin
enhances the antitumor effects of Doxo by downregulating the
mTOR/ribosomal protein S6 kinase (p70S6K) pathway (9). Therefore, targeting the mTOR pathway
may increase cell sensitivity to Doxo and serve as an effective
therapeutic approach to leukemia. NVP-BEZ235 (BEZ235), a dual
inhibitor of the PI3K/mTOR pathway, was reported to induce cancer
cell apoptosis in multiple studies (10,11).
It has been established that the PI3K/AKT/mTOR signaling pathway
regulates cell survival, proliferation and metabolism under
physiological and pathological conditions (12,13).
At present, previous studies have revealed that BEZ235 exerts an
inhibitory effect on cancer cell proliferation by downregulating
the PI3K/AKT/mTOR pathway (14–19).
Moreover, BEZ235 was observed to reverse chemoresistance in several
human cancer types, such as ovarian, pancreatic, prostate and
gastric cancer (14–17). BEZ235 can also induce apoptosis and
enhance autophagic activity, as well as inhibit the proliferation
of leukemia cells (18,19). Furthermore, BEZ235 was identified to
enhance the accumulation of Doxo in drug-resistant ovarian and
pancreatic cancer cells (9).
However, the effect of BEZ235 on Doxo-resistant K562 cells (K562/A)
has not been extensively reported.
In the present study, the proliferation and
apoptosis of K562/A cells were investigated following BEZ235
treatment. In addition, the effects of BEZ235 on the PI3K/AKT/mTOR
signaling pathway in K562/A cells were examined, with the aim of
determining the underlying mechanism and identifying a novel
therapeutic target for clinical application in hematological
malignancies.
Materials and methods
Chemicals and reagents
The K562 human erythromyeloblastoid leukemia cell
line was purchased from the Beijing Institute for Cancer Research,
and K562/A cells were obtained from the Institute of Hematology and
Blood Diseases Hospital, Chinese Academy of Medical Sciences.
The primary antibodies used in the present study
included rabbit anti-human AKT (1:1,000; cat. no. 4685),
phosphorylated (p)-AKT (1:2,000; cat. no. 4060), mTOR (1:1,000;
cat. no. 2983), p-mTOR (1:1,000; cat. no. 5536), eukaryotic
translation initiation factor 4E binding protein 1 (4E-BP1)
(1:1,000; cat. no. 9644), p-4E-BP1 (1:1,000; cat. no. 2855), p70S6K
(1:800, cat. no. 2708) and p-p70S6K (1:500; cat. no. 9234), which
were purchased from Cell Signaling Technology, Inc. Rabbit
anti-human β-actin (1:10,000; cat. no. AC026) monoclonal antibody
was purchased from ABclonal Biotech Co., Ltd., while rabbit
anti-human Bax (1:500; cat. no. sc-23959) and mouse anti-human
Bcl-2 (1:500; cat. no. sc-509) antibodies were obtained from Santa
Cruz Biotechnology, Inc. Rabbit anti-human CDK4 (1:5,000; cat. no.
ab108357), CDK6 (1:20,000; cat. no. ab124821), cyclin B1 (1:10,000;
cat. no. ab32053) and cyclin D1 (1:200; cat. no. ab16663) were
purchased from Abcam. Goat anti-mouse IgG (H&L), horseradish
peroxidase (HRP)-conjugated secondary antibody (1:5,000; cat. no.
S0002) and goat anti-rabbit IgG (H&L), HRP-conjugated secondary
antibody (1:5,000; cat. no. S0001) were obtained from Affinity
Biosciences.
Cell culture and treatment
K562 and K562/A cells were cultured in RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with
penicillin (100 U/ml), streptomycin (100 U/ml) and 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2. Doxo (cat. no. 22386; Cayman Chemical
Company) and BEZ235 (cat. no. 21185; Cayman Chemical Company) were
diluted in DMSO (Merck KGaA) at a concentration of 1 mM as the
primary stock solution and stored at 4°C, respectively.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay (Dojindo Molecular Technologies, Inc.)
was used to examine the viability of K562 and K562/A cells,
according to the manufacturer's instructions. BEZ235 was dissolved
in DMSO at a concentration of 1 mM as the primary stock solution
and stored at 4°C. K562 and K562/A cells were cultured at 37°C and
treated with various concentrations of BEZ235 (25, 50, 100, 200,
400, 800 and 1,600 nM) for 24 h or with 200 nM BEZ235 for 0.5, 1, 2
and 3 days. Meanwile, the cells were treated with various
concentrations of Doxo (31.25, 62.5, 125, 250, 500, 1,000 and 2,000
nM) according to our previously published paper (9). After the cells were incubated with 10
µl CCK-8 reagent at 37°C for 4 h, the cytotoxic effect of BEZ235 on
K562/A cells was measured on a spectrophotometer microplate reader
(BioTek Instruments, Inc.) at a wavelength of 450 nm. Cell
viability was assayed and compared with the control group. Based on
the results of CCK-8 and our previous study, 200 nM BEZ235 and 250
nM Doxo were chosen for the following experiments.
Flow cytometry
K562/A cells were cultured as described above and
treated with BEZ235 (200 nM) for 24 h, then were harvested in the
buffer (PBS-0.05% trypsin). K562/A cells were incubated with 5 µl
Annexin V and 10 µl PI in the dark for 5 min at room temperature,
and then resuspended in 1X binding buffer. The fluorescence of the
cells was analyzed on a FACSCalibur (BD Biosciences). Cell
apoptosis was analyzed using an Annexin V/PI apoptosis detection
kit [Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.], according
to the manufacturer's protocol. The frequency of intact cells
(annexin V−/PI−), early apoptotic cells
(annexin V+/PI−) and late apoptotic/necrotic
cells (annexin V+/PI+) were analyzed using
FlowJo 10 software (FlowJo LLC).
For cell cycle analysis, flow cytometry analysis
with propidium iodide staining was performed on a FACSCalibur (BD
Biosciences). After 24-h treatment with 200 nM BEZ235, cells were
fixed with 70% ethanol and incubated with PI/RNase Staining Buffer
(BD Biosciences; cat. no. 550825) for 15 min at room temperature,
according to the manufacturer's instructions. Cells in the
G2/M phases of the cell cycle have twice the DNA content
of those in G0/G1 phases. Cells in the S
phase have a DNA content lying between these extremes. The data
were analyzed using FlowJo 10 software (FlowJo LLC).
Western blot analysis
K562/A were cultured as aforementioned and treated
with 200 nM BEZ235 for 24 h. Total protein was extracted from
K562/A cells using lysis buffer (1% Triton X-100; 150 mM NaCl; 2 mM
EDTA; 50 mM Tris-HCl) supplemented with phosphatase inhibitor
cocktail. Total protein concentration was measured using Gen5 1.0
software (BioTek Instruments, Inc.) on a spectrophotometer
microplate reader (BioTek Instruments, Inc.). A total of 20–50 µg
protein was separated by SDS-PAGE using 10–15% gels and transferred
onto a PVDF membrane. Following blocking non-specific binding sites
with 5% non-fat dry milk in Tris-buffered saline with 0.05%
Tween-20 at room temperature for 60 min, the membranes were
incubated with primary antibodies at 4°C overnight. Next, the
membranes were probed with HRP-conjugated secondary antibody
(1:5,000) for 60 min at 37°C. Subsequently, the immunoreactive
membranes were developed using Pierce™ Electrochemiluminescent
Western Bloting Substrate (cat. no. UC280185, Thermo Fisher
Scientific, Inc.) on ImageQuant™ LAS 4000 (GE Healthcare Life
Sciences) and the density was quantified and normalized to
β-actin.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. After cDNA was obtained by GoScript™
Reverse Transcription System (Promega Corporation), PCR
amplification was performed with forward and reverse primers using
the SYBR PrimeScript RT-PCR kit (Takara Bio, Inc.) in an Mx3005p
instrument (Agilent Technologies GmbH). RT was performed under the
following conditions: 25°C for 5 min, 42°C for 60 min and 70°C for
15 min. The thermocycling conditions of PCR amplification consisted
of an initial denaturation at 95°C for 10 min, followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30
sec and elongation at 60°C for 30 sec. The Cq values of the test
genes were normalized to the mean Cq values of β-actin. Fold change
was calculated using the 2−ΔΔCq method (20). The primers used in the present study
are listed in Table I.
 | Table I.Primers for reverse
transcription-quantitative PCR amplification. |
Table I.
Primers for reverse
transcription-quantitative PCR amplification.
| Target | Sequence | Product length,
bp |
|---|
| CDK4 | F:
5′-AGTTCGTGAGGTGGCTTTAC-3′ | 162 |
|
| R:
5′-GCCTTGTCCAGATATGTCCTTAG-3′ |
|
| CDK6 | F:
5′-CTGCCTTGTTGGCAAAGTATC-3′ | 229 |
|
| R:
5′-CCAGGTAGAAGGACTGCATTAG-3′ |
|
| cyclin D1 | F:
5′-CCACTCCTACGATACGCTACTA-3′ | 219 |
|
| R:
5′-GGACTGAAAGTGCTTGGAAATG-3′ |
|
| cyclin B1 | F:
5′-GGTGTCACTGCCATGTTTATTG-3′ | 179 |
|
| R:
5′-CGAAGGAAGTGCAAAGGTAGA-3′ |
|
| Bcl-2 | F:
5′-GCCAGGGTCAGAGTTAAATAGAG-3′ | 143 |
|
| R: 5′-
GCCTCTCTTGCGGAGTATTT-3′ |
|
| Bax | F:
5′-GTCACTGAAGCGACTGATGT-3′ | 128 |
|
| R:
5′-CTTCTTCCAGATGGTGAGTGAG-3′ |
|
| β-actin | F:
5′-AGCGAGCATCCCCCAAAGTT-3′ | 285 |
|
| R:
5′-GGGCACGAAGGCTCATCATT-3′ |
|
Statistical analysis
All experiments were performed ≥3 times. Data are
presented as the mean ± standard deviation. Statistically
significant differences were evaluated using an unpaired Student's
t-test or the comparative data among groups was analyzed using
one-way ANOVA followed by Tukey's test, using SPSS 21.0 for Windows
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
BEZ235 inhibits the viability of K562
and K562/A cells
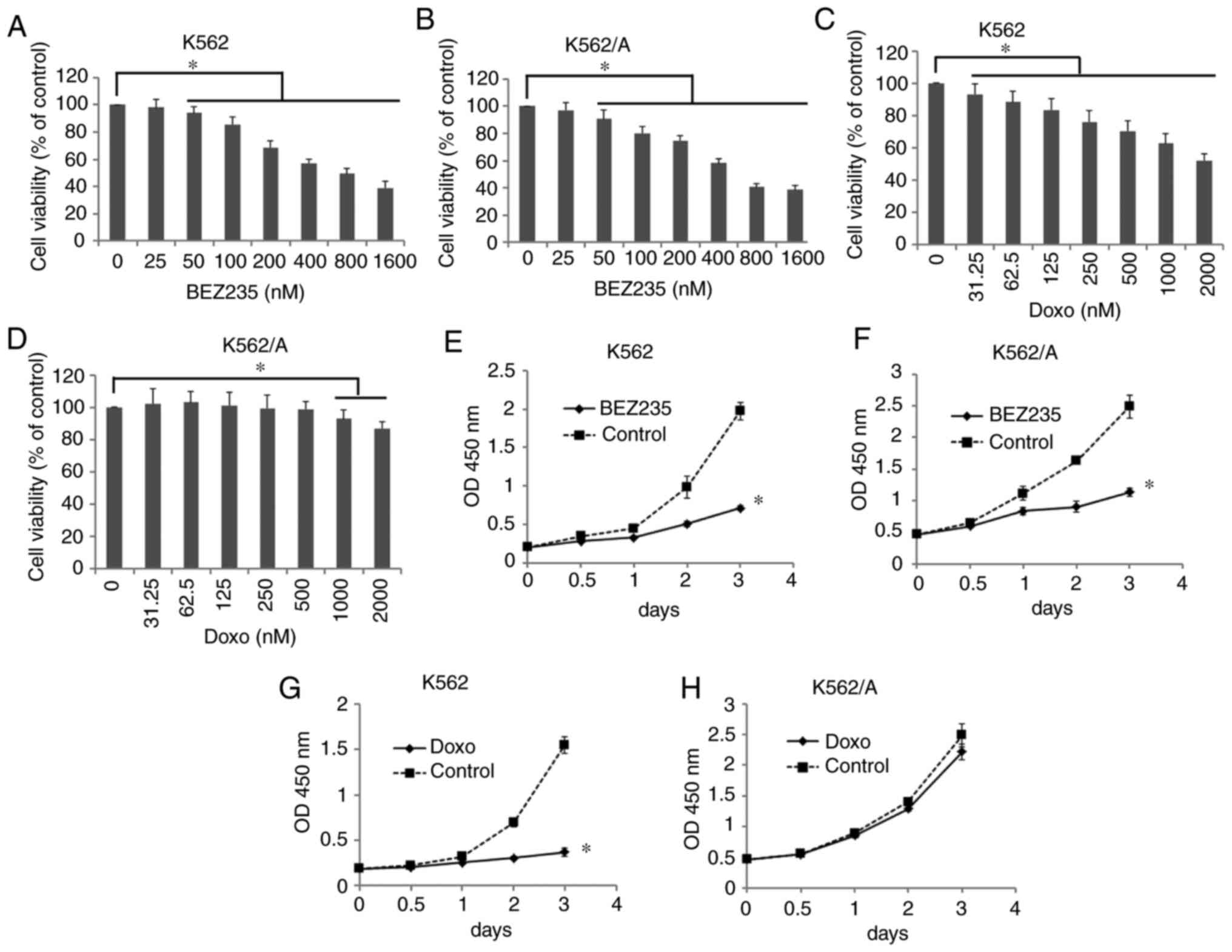
The cytotoxic effect of BEZ235 and Doxo on K562/A
cells was measured using a CCK-8 assay after treating cells with
increasing concentrations of each reagent for 24 h. The viability
of K562 cells was significantly inhibited in the Doxo-treated group
from 31.25–2,000 nM (P<0.05); however, that of K562/A cells was
decreased in only the 1,000 and 2,000 nM treatment groups
(P<0.05; Fig. 1C and D). In
addition, cell viability was significantly decreased in a
dose-dependent manner in K562 and K562/A cells treated with BEZ235
from 25–1,600 nM (P<0.05; Fig. 1A
and B).
Based on the aforementioned results and our previous
study (9), 200 nM BEZ235 and 250 nM
Doxo were used to treat K562 and K562/A cells for 0.5, 1, 2 or 3
days. At these concentrations, the effect of BEZ235 was observed to
be time-dependent (P<0.05; Fig.
1E-G). However, Doxo exerted little effect on the viability of
K562/A cells over time (P>0.05, Fig.
1H). These findings suggested that K562/A cells were resistant
to Doxo, while BEZ235 significantly inhibited the viability of
K562/A cells.
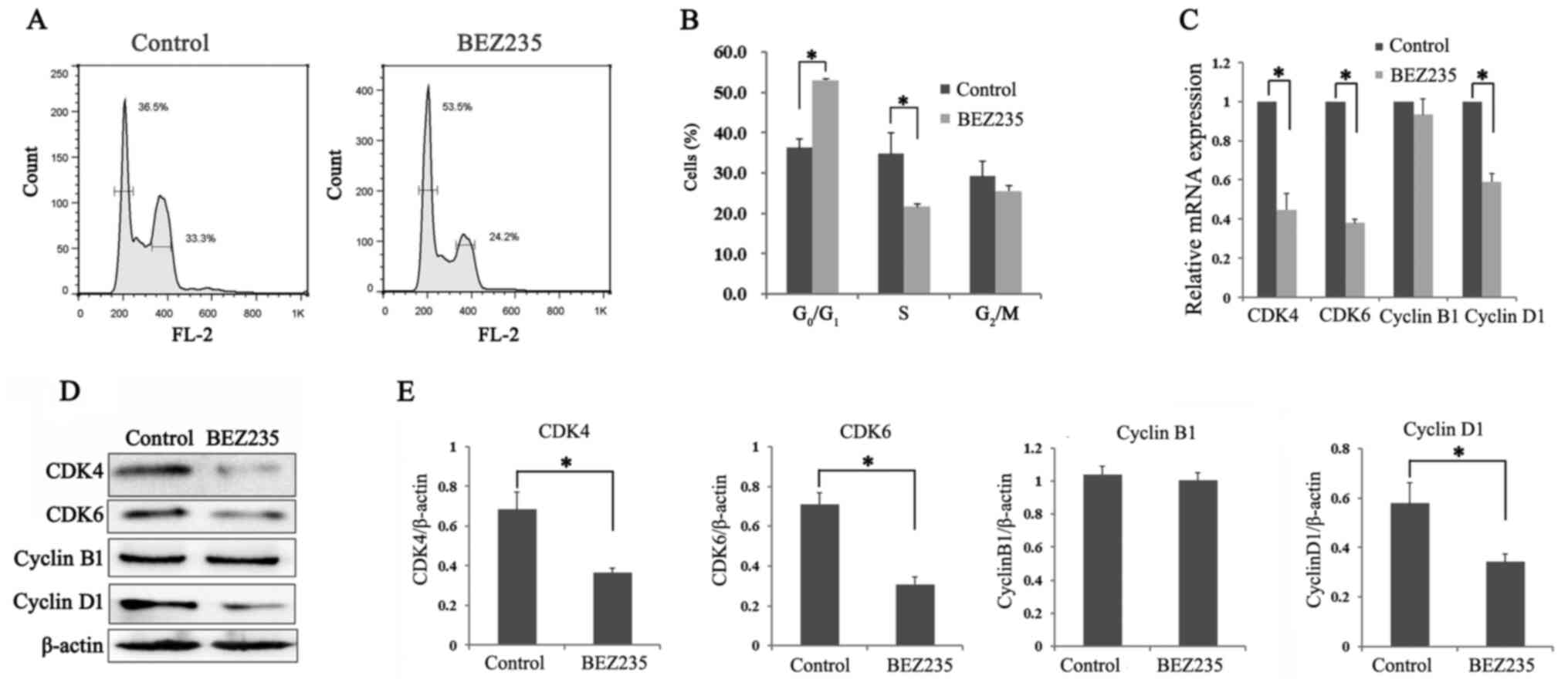
BEZ235 arrests K562/A cells at the
G0/G1 phase
To investigate the inhibitory effects of BEZ235 on
the proliferation of Doxo-resistant cells, cell cycle progression
was evaluated following BEZ235 treatment. The proportion of K562/A
cells at the G0/G1 phase was 52.97±0.47% in
the BEZ235 treatment group, which was significantly higher compared
with that in the control group (36.17±2.31%; P<0.05; Fig. 2A and B). Consistent with the
increasing number of G0/G1 phase cells, the
proportion of S phase cells was significantly decreased in the
BEZ235 treatment group (P<0.05; Fig.
2A and B).
The protein and mRNA expression levels of several
cell cycle-related molecules were further assessed following BEZ235
treatment (Fig. 2C-E). The protein
expression levels of CDK4, CDK6 and cyclin D1 were significantly
decreased in the BEZ235 group compared with the control group, as
indicated via western blotting (P<0.05; Fig. 2D and E). However, cyclin B1
expression displayed no change in K562/A cells after incubation
with BEZ235 (P>0.05). The RT-qPCR results demonstrated the same
trend (Fig. 2C). Thus, these
observations suggested that BEZ235 induced
G0/G1 arrest in K562 cells.
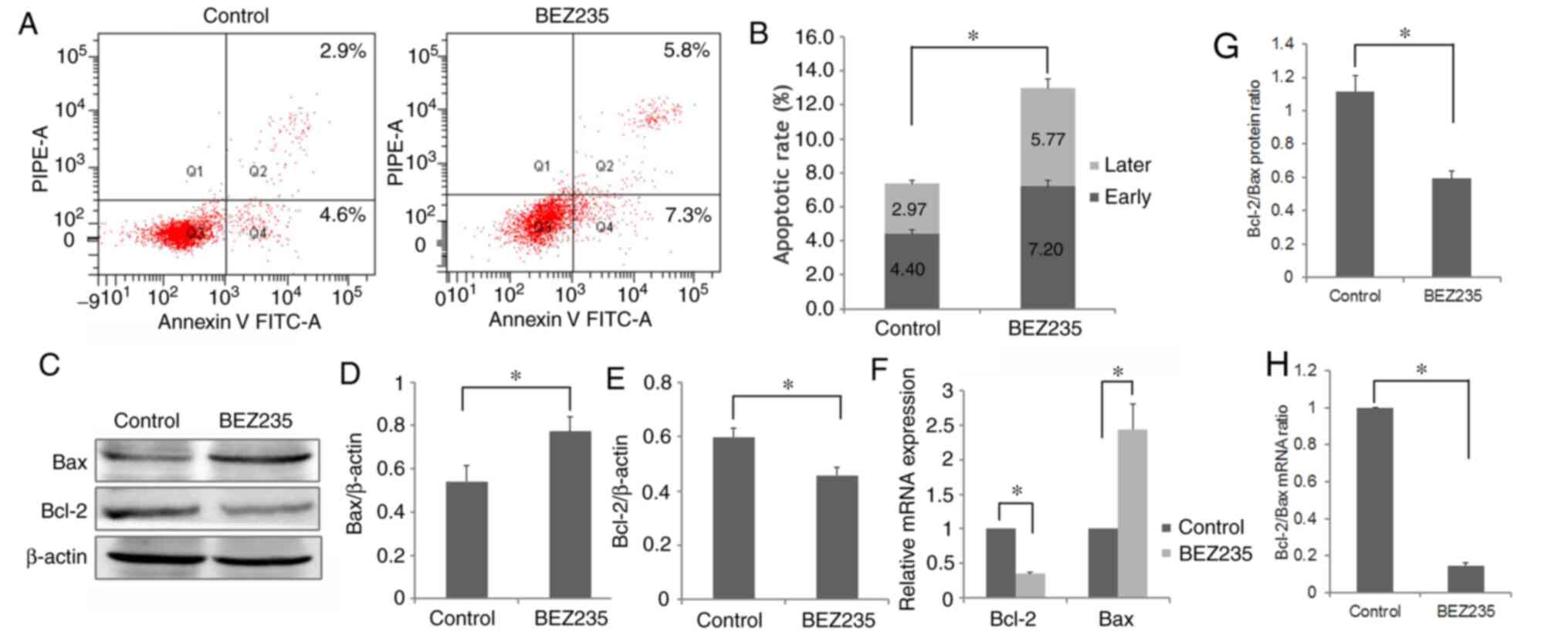
BEZ235 induces apoptosis of K562/A
cells
To assess whether apoptosis contributed to cell
proliferation inhibition in the BEZ235 treatment group, the effects
of BEZ235 on K562/A cell apoptosis were examined. The total
apoptotic rate following treatment with BEZ235 was 12.97±0.91%,
which was significantly higher compared with that in the control
group (7.37±0.42%; P<0.05; Fig. 3A
and B). In addition, the both rates of early and late apoptosis
were significantly increased in BEZ235-treated cells (P<0.05;
Fig. 3B).
To investigate the apoptotic mechanism induced by
BEZ235, the expression levels of Bcl-2 and Bax, two pivotal
apoptosis-related proteins, were detected using western blotting
and RT-qPCR analysis. Downregulation of Bcl-2 and upregulation of
Bax were observed in the BEZ235 treatment group (P<0.05;
Fig. 3C-F). Thus, compared with the
control group, a significantly lower Bcl-2/Bax ratio was observed
in the BEZ235-treated cells (P<0.05, Fig. 3G and H), indicating that BEZ235 may
induce cell apoptosis by downregulating the Bcl-2/Bax ratio in
K562/A cells. Therefore, these findings demonstrated that BEZ235
could inhibit K562/A cell proliferation via inducing apoptosis.
BEZ235 downregulates the PI3K/AKT/mTOR
pathway and its downstream proteins in K562/A cells
The effects of BEZ235 on the PI3K/AKT/mTOR pathway
in K562/A cells were also investigated using western blot analysis.
The phosphorylation levels of AKT (Ser473) and mTOR (Ser-2448) were
significantly lower in the BEZ235 group compared with those in the
control group (P<0.05; Fig. 4A and
B). The two major downstream targets of mTOR, p70S6K and
4E-BP1, were examined, and the expression levels of p-p70S6K
(Thr-389) and p-4E-BP1 (Thr37/46) were significantly decreased in
K562/A cells treated with BEZ235 (P<0.05; Fig. 4A and B). Furthermore, the ratio of
p-/total protein for these factors were significantly downregulated
in BEZ235-treated cells, compared with the control group
(P<0.05; Fig. 4C). Collectively,
the results demonstrated that BEZ235 inhibits cell proliferation
via downregulating the PI3K/AKT/mTOR pathway in K562/A cells.
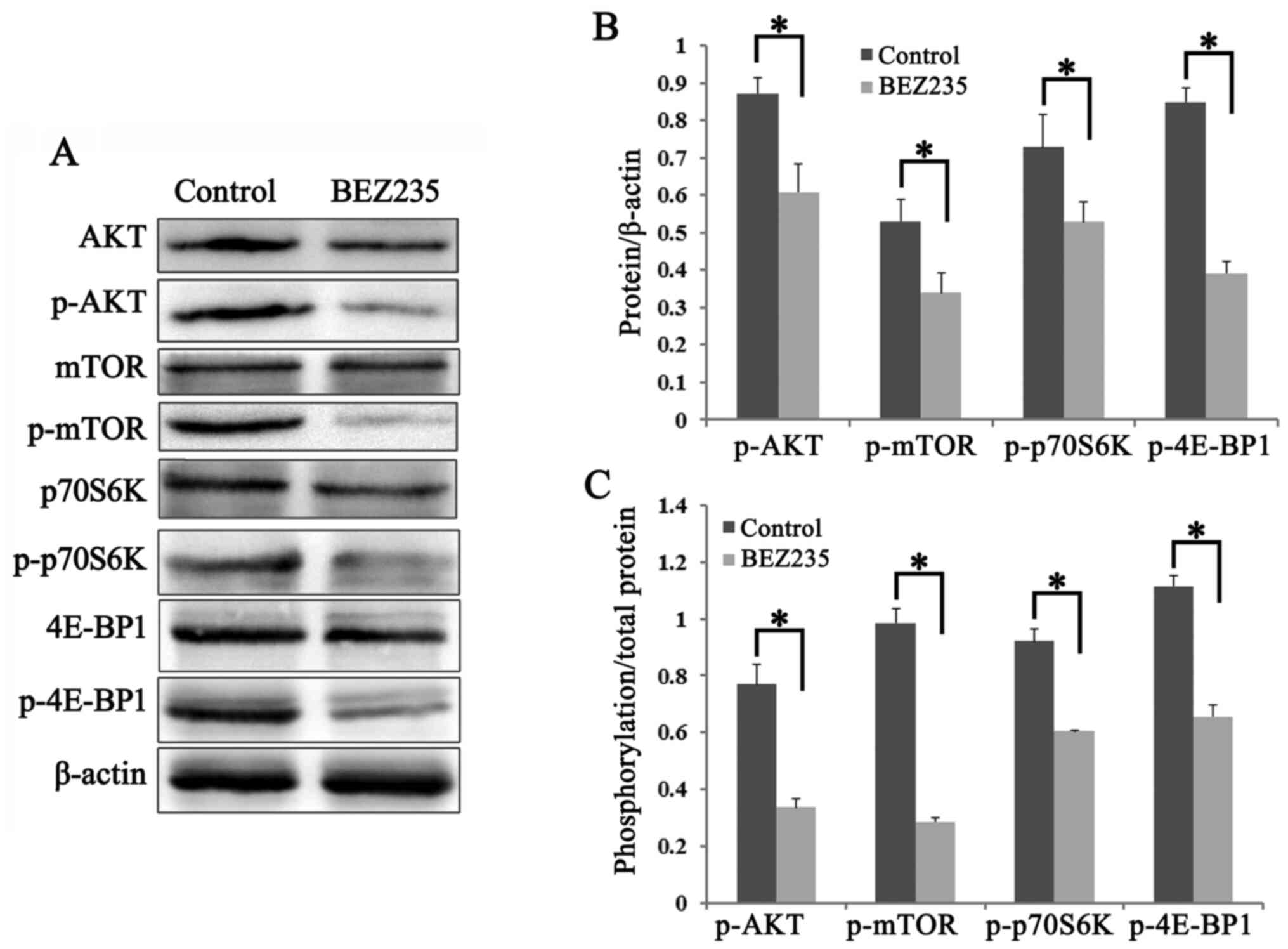 | Figure 4.BEZ235 downregulates the
PI3K/AKT/mTOR signaling pathway in K562/A cells. Cells were
cultured with BEZ235 (200 nM) for 24 h. Total protein was
extracted, followed by immunoblotting with the antibodies against
the PI3K/AKT/mTOR pathway. Experiments were repeated three times
and data are presented as mean ± standard deviation. (A) Protein
expression levels of AKT, mTOR, 4E-BP1 and p70S6K, and their
phosphorylation levels were detected in K562/A cells using western
blot analysis. (B) Semi-quantification of the results demonstrated
lower expression levels of p-AKT (Ser-473), p-mTOR (Ser-2448),
p-p70S6K (Thr-389) and p-4E-BP1 (Thr37/46) in K562/A cells with
BEZ235 treatment. (C) Ratio of p-/total protein were significantly
downregulated in BEZ235 treated cells, as compared with the control
group. *P<0.05. BEZ235, NVP-BEZ235; 4E-BP1, eukaryotic
translation initiation factor 4E binding protein 1; p-,
phosphorylated; p70S6K, ribosomal protein S6 kinase. |
Discussion
Despite Doxo-induced rapid and durable responses in
patients with myelogenous leukemia, a proportion of patients will
develop drug resistance and therefore treatment failure (21). Thus, it is important to identify an
alternative novel target that can achieve a satisfactory clinical
response in patients who experience relapse and have a poor
prognosis. In the present study, a Doxo-resistant cell line
(K562/A) was selected and the viability of K562/A cells after Doxo
treatment was detected. The results demonstrated that K562/A cells
exhibited lower sensitivity to Doxo, which provided an opportunity
to search for new drugs that are potentially effective against
Doxo-resistant leukemia cells.
PI3K/AKT/mTOR is a central signaling pathway
involved in the regulation of cell proliferation, differentiation
and survival (12,13). Moreover, the BCR/ABL protein encoded
by the Bcr/Abl oncogene can activate PI3K/AKT/mTOR via its
regulatory subunit (22). mTOR
activation further phosphorylates its two downstream targets,
p70S6K and 4E-BP1. Phosphorylated p70S6K affects cell proliferation
by inducing protein synthesis and cell survival, while 4E-BP1
phosphorylation results in the release of the eukaryotic initiation
factor 4E (23,24). Our previous study reported that the
mTOR pathway was upregulated in CML, and rapamycin arrested cells
at the G0/G1 phase and increased apoptosis in
K562 cells (8). In addition, a mTOR
inhibitor, everolimus is currently being investigated as an
anticancer agent due to its potential action by inhibiting tumor
cell proliferation, and has been used in clinical trials (25–27).
However, it has been reported that everolimus plus only or Imatinib
demonstrate limited activity in progressing advanced cancer types
(28,29), and the clinical application of
everolimus does not exert the same effect as that in in
vitro experiments. Thus, this may explain why inhibition of
mTOR complex 1 (mTORC1), which is sensitive to rapamycin, may
paradoxically enhance the PI3K/AKT axis (30,31).
Therefore, a dual PI3K/mTOR inhibitor may have improved anticancer
activity compared with a mTORC1 inhibitor alone (32,33).
It was reported that BEZ235 inhibits the activity of PI3K, mTOR and
Rad3-related protein, as well as possesses an anticancer potential
(34). Therefore, BEZ235 has been
used to reverse temozolomide resistance in glioma and to suppress
paclitaxel-resistant gastric cancer (35,36).
In the present study, BEZ235 was found to significantly inhibit the
viability of K562/A cells, which suggested that BEZ235 may reverse
Doxo resistance of leukemia cells.
To identify the mechanism via which BEZ235 regulates
cell proliferation, the present study further detected cell cycle
progression following BEZ235 treatment of K562/A cells. The present
results demonstrated that BEZ235 arrested K562/A cells at the
G0/G1 phase. In addition, the expression
levels of several cell cycle-related proteins, including CDK4, CDK6
and cyclin D1, were significantly decreased in BEZ235-treated
cells, indicating that BEZ235, as an inhibitor of PI3K/mTOR,
blocked cell cycle progression from the G1 to the S
phase, ultimately arresting cell proliferation. To assess whether
apoptosis contributes to cell proliferation inhibition following
BEZ235 treatment, cell apoptosis was further investigated. The
present study identified an increase in apoptotic K562/A cells
following treatment with BEZ235. Moreover, significantly lower
Bcl-2 and higher Bax expression levels were observed in
BEZ235-treated cells. Bcl-2 family members are key regulators of
cell apoptosis, and an increasing Bax expression and/or decreasing
Bcl-2 expression have been reported to induce apoptosis (37). Therefore, in the current study, it
was suggested that BEZ235 may induce cell apoptosis by
downregulating the Bcl-2/Bax ratio in K562/A cells. These findings
indicated that BEZ235 may reverse Doxo resistance via inducing cell
apoptosis and G0/G1 arrest.
The present study demonstrated that p-AKT and p-mTOR
expression levels were significantly decreased in BEZ235-treated
K562/A cells, as well as those of p-4E-BP1 and p-p70S6K compared
with the control group. These findings suggested that BEZ235
inhibited the PI3K/mTOR pathway and decreased the phosphorylation
levels of its downstream proteins. Previous studies have revealed
that suppression of AKT/mTOR pathway activation, acting additively
or synergistically with trastuzumab, carboplatin and Doxo, may
suppress cancer cell proliferation in vitro (38–40).
In addition, rapamycin may enhance the antitumor effects of Doxo by
inhibiting mTOR/p70S6K (9).
Collectively, the present results suggested that BEZ235 can inhibit
the proliferation of Doxo-resistant leukemia cells by
downregulating the PI3K/mTOR pathway, and thus targeting PI3K/mTOR
may be a novel approach to leukemia treatment.
In conclusion, the present findings indicated that
BEZ235 inhibited cell proliferation, induced apoptosis and
G0/G1 arrest and decreased PI3K/mTOR
signaling in K562/A cells, suggesting that BEZ235 may reverse
resistance of K562/A cells to Doxo by inhibiting the PI3K/mTOR
pathway. One limitation of the study is that the results were
derived from in vitro studies rather than an appropriate
animal model of leukemia. Moreover, the mechanism related to
PI3K/mTOR in leukemia remains unclear. Nevertheless, the present
findings may provide insight into the underlying mechanism and
identification of a novel therapeutic target for clinical
application in myelogenous leukemia.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Natural
Science Foundation of Hebei Province (grant no. H2017307024) and
the Project of Health Department of Hebei Province (grant no.
20170030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and LX designed the study, analyzed the data and
wrote the manuscript. XW, CM, SX and MX performed the experiments
and prepared the figures. RW and JY prepared the figures and
analyzed the data. JL and LX performed critical revision of the
manuscript and supervised the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hebei General Hospital (Shijiazhuang, China; approval
no. 202041).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dong Y, Shi O, Zeng Q, Lu X, Wang W, Li Y
and Wang Q: Leukemia incidence trends at the global, regional, and
national level between 1990 and 2017. Exp Hematol Oncol. 9:142020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rowley JD: Ph1-positive leukaemia,
including chronic myelogenous leukaemia. Clin Haematol. 9:55–86.
1980.PubMed/NCBI
|
|
4
|
Groffen J, Stephenson JR, Heisterkamp N,
de Klein A, Bartram CR and Grosveld G: Philadelphia chromosomal
breakpoints are clustered within a limited region, bcr, on
chromosome 22. Cell. 36:93–98. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorre ME, Mohammed M, Ellwood K, Hsu N,
Paquette R, Rao PN and Sawyers CL: Clinical resistance to STI-571
cancer therapy caused by BCR-ABL gene mutation or amplification.
Science. 293:876–880. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amico D, Barbui AM, Erba E, Rambaldi A,
Introna M and Golay J: Differential response of human acute myeloid
leukemia cells to gemtuzumab ozogamicin in vitro: Role of Chk1 and
Chk2 phosphorylation and caspase 3. Blood. 101:4589–4597. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Xue L, Hao H, Han Y, Yang J and Luo
J: Rapamycin provides a therapeutic option through inhibition of
mTOR signaling in chronic myelogenous leukemia. Oncol Rep.
27:461–466. 2012.PubMed/NCBI
|
|
9
|
Li J, Liu W, Hao H, Wang Q and Xue L:
Rapamycin enhanced the antitumor effects of doxorubicin in
myelogenous leukemia K562 cells by downregulating the mTOR/p70S6K
pathway. Oncol Lett. 18:2694–2270. 2019.PubMed/NCBI
|
|
10
|
Seo BR, Min KJ, Cho IJ, Kim SC and Kwon
TK: Curcumin significantly enhances dual PI3K/Akt and mTOR
inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma
Caki cells through down-regulation of p53-dependent Bcl-2
expression and inhibition of Mcl-1 protein stability. PLoS One.
9:e955882014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie G, Wang Z, Chen Y, Zhang S, Feng L,
Meng F and Yu Z: Dual blocking of PI3K and mTOR signaling by
NVP-BEZ235 inhibits proliferation in cervical carcinoma cells and
enhances therapeutic response. Cancer Lett. 388:12–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi X, Wang J, Lei Y, Cong C, Tan D and
Zhou X: Research progress on the PI3K/AKT signaling pathway in
gynecological cancer (Review). Mol Med Rep. 19:4529–4535.
2019.PubMed/NCBI
|
|
13
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Durrant DE, Das A, Dyer S and Kukreja RC:
A dual PI3 kinase/mTOR inhibitor BEZ235 reverses doxorubicin
resistance in ABCB1 overexpressing ovarian and pancreatic cancer
cell lines. Biochim Biophys Acta Gen Subj. 1864:1295562020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park H, Kim Y, Sul JW, Jeong IG, Yi HJ,
Ahn JB, Kang JS, Yun J, Hwang JJ and Kim CS: Synergistic anticancer
efficacy of MEK inhibition and dual PI3K/mTOR inhibition in
castration-resistant prostate cancer. Prostate. 75:1747–1759. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Zhang S, Xie D, Chen H, Zheng X and
Pan D: Dual inhibitor of PI3K and mTOR (NVP-BEZ235) augments the
efficacy of fluorouracil on gastric cancer chemotherapy. Onco
Targets Ther. 11:6111–6118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia A, Li H, Li R, Lu L and Wu X:
Co-treatment with BEZ235 enhances chemosensitivity of A549/DDP
cells to cisplatin via inhibition of PI3K/Akt/mTOR signaling and
downregulation of ERCC1 expression. Oncol Rep. 40:2353–2362.
2018.PubMed/NCBI
|
|
18
|
Deng L, Jiang L, Lin XH, Tseng KF, Liu Y,
Zhang X, Dong RH, Lu ZG and Wang XJ: The PI3K/mTOR dual inhibitor
BEZ235 suppresses proliferation and migration and reverses
multidrug resistance in acute myeloid leukemia. Acta Pharmacol Sin.
38:382–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xin P, Xu W, Zhu X, Li C, Zheng Y, Zheng
T, Cheng W and Peng Q: Protective autophagy or autophagic death:
Effects of BEZ235 on chronic myelogenous leukemia. Cancer Manag
Res. 11:7933–7951. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Estey EH: Acute myeloid leukemia: 2019
update on risk-stratification and management. Am J Hematol.
93:1267–1291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ly C, Arechiga AF, Melo JV, Walsh CM and
Ong ST: Bcr-Abl kinase modulates the translation regulators
ribosomal protein S6 and 4E-BP1 in chronic myelogenous leukemia
cells via the mammalian target of rapamycin. Cancer Res.
63:5716–5722. 2003.PubMed/NCBI
|
|
23
|
Harada H, Andersen JS, Mann M, Terada N
and Korsmeyer SJ: p70S6 kinase signals cell survival as well as
growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad
Sci USA. 98:9666–9670. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miklja Z, Yadav VN, Cartaxo RT, Siada R,
Thomas CC, Cummings JR, Mullan B, Stallard S, Paul A, Bruzek AK, et
al: Everolimus improves the efficacy of dasatinib in PDGFRα-driven
glioma. J Clin Invest. 130:5313–5325. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JS, Yost SE, Blanchard S, Schmolze D,
Yin HH, Pillai R, Robinson K, Tang A, Martinez N, Portnow J, et al:
Phase I clinical trial of the combination of eribulin and
everolimus in patients with metastatic triple-negative breast
cancer. Breast Cancer Res. 21:1192019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Sorafenib and everolimus for
patients with unresectable high-grade osteosarcoma progressing
after standard treatment: A non-randomised phase 2 clinical trial.
Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stacchiotti S, Morosi C, Lo Vullo S,
Casale A, Palassini E, Frezza AM, Dinoi G, Messina A, Gronchi A,
Cavalleri A, et al: Imatinib and everolimus in patients with
progressing advanced chordoma: A phase 2 clinical study. Cancer.
124:4056–4063. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim ST, Lee J, Park SH, Park JO, Park YS,
Kang WK and Lim HY: Prospective phase II trial of everolimus in
PIK3CA amplification/mutation and/or PTEN loss patients with
advanced solid tumors refractory to standard therapy. BMC Cancer.
17:2112017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fruman DA and Rommel C: PI3K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aylett CH, Sauer E, Imseng S, Boehringer
D, Hall MN, Ban N and Maier T: Architecture of human mTOR complex
1. Science. 351:48–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hall CP, Reynolds CP and Kang MH:
Modulation of glucocorticoid resistance in pediatric T-cell acute
lymphoblastic leukemia by increasing BIM expression with the
PI3K/mTOR inhibitor BEZ235. Clin Cancer Res. 22:621–632. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gazi M, Moharram SA, Marhäll A and Kazi
JU: The dual specificity PI3K/mTOR inhibitor PKI-587 displays
efficacy against T-cell acute lymphoblastic leukemia (T-ALL).
Cancer Lett. 392:9–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiarini F, Evangelisti C, McCubrey JA and
Martelli AM: Current treatment strategies for inhibiting mTOR in
cancer. Trends Pharmacol Sci. 36:124–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu Z, Xie G, Zhou G, Cheng Y, Zhang G, Yao
G, Chen Y, Li Y and Zhao G: NVP-BEZ235, a novel dual PI3K-mTOR
inhibitor displays anti-glioma activity and reduces chemoresistance
to temozolomide in human glioma cells. Cancer Lett. 367:58–68.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li
Z, Wang J, Li B, Hu Y, Dong B, et al: Dual PI3K/mTOR inhibitor
BEZ235 as a promising therapeutic strategy against
paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR
pathway. Cell Death Dis. 9:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng JH, Viacava Follis A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mondesire WH, Jian W, Zhang H, Ensor J,
Hung MC, Mills GB and Meric-Bernstam F: Targeting mammalian target
of rapamycin synergistically enhances chemotherapy-induced
cytotoxicity in breast cancer cells. Clin Cancer Res. 10:7031–7042.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen YQ, Guerra-Librero A, Fernandez-Gil
BI, Florido J, García-López S, Martinez-Ruiz L, Mendivil-Perez M,
Soto-Mercado V, Acuña-Castroviejo D, Ortega-Arellano H, et al:
Combination of melatonin and rapamycin for head and neck cancer
therapy: Suppression of AKT/mTOR pathway activation, and activation
of mitophagy and apoptosis via mitochondrial function regulation. J
Pineal Res. 64:Jun 9–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu PY, Wu VS, Kanaya N, Petrossian K, Hsu
HK, Nguyen D, Schmolze D, Kai M, Liu CY, Lu H, et al: Dual mTOR
Kinases MLN0128 inhibitor sensitizes
HR+/HER2+ breast cancer patient-derived
xenografts to trastuzumab or fulvestrant. Clin Cancer Res.
24:395–406. 2018. View Article : Google Scholar : PubMed/NCBI
|