Introduction
Proximal tubular epithelial cells (PTECs) are the
most abundant cell type in the kidney, and have an important role
in renal repair and/or the progression of chronic kidney diseases.
PTECs exert immunological functions by expressing multiple
Toll-like receptors (TLRs), such as TLR 1, 2, 3, 4 and 9 (1,2), and
molecules associated with antigen-presenting cell function,
including MHCII, CD74, CD80 and CD86 (3). These innate immune characteristics of
PTECs enable them to act as immune responders to a wide range of
stimuli, with the consequent production and release of bioactive
mediators, including proinflammatory cytokines, chemokines and
complement components, which drive interstitial inflammation and
fibrosis (4). PTECs also express
neonatal Fc receptor, and preserve the capacity of specific
pH-dependent binding and transcytosis of immunoglobulin (Ig)G
(5). However, to the best of our
knowledge, it remains unknown as to whether PTECs express Igs.
It was previously hypothesized that Igs are produced
solely by mature B cells and plasma cells, and that Igs act as
antibodies to recognize and neutralize various pathogens. However,
this theory has been challenged in recent decades, as increasing
evidence has reported that Igs, including IgA, IgG and IgM, can be
produced and secreted by non-B cells, such as human epithelial
cancer cells (6,7) and normal non-B cells (8,9), as
well as in immune-privileged sites, such as the eyes (10), central neurons (11,12),
placenta (13), and the testis and
epididymis (14).
Similar to B cell-derived Igs (B-Igs), non-B-Igs are
also the products of Ig gene transcription and rearrangement, and
display classic V(D)J recombination patterns with nucleotide
additions at the junctions and somatic hypermutations (7,11,14).
In contrast to B-Igs, non-B-Igs displays limited V(D)J
recombination patterns and less diversity (7). Functionally, the non-B-Igs not only
exert natural antibody activity in the skin and mucosa (8) but can also act as growth factors to
promote cell proliferation and adhesion, and may enhance the
initiation and metastasis of cancer by binding to integrins
(15–17). For example, RP215 recognized cancer
IgG executes its oncogenic function by interacting with the
integrin α6β4 complex and activating the FAK and Src pathways
(15).
Our previous study demonstrated that mesangial cells
(18) and podocytes (19) can synthesize and secrete IgA and
IgG, and participate in cell growth and cell adhesion in
vitro. The present study aimed to evaluate the expression
levels of Igs in PTECs and investigate its potential role in
epithelial-mesenchymal transition (EMT).
Materials and methods
Cell culture and treatment
An immortalized PTEC line HK-2 was purchased from
American Type Culture Collection. HK-2 cells were cultured in
DMEM/F12 supplemented with 100 U/ml penicillin, 0.1 mg/ml
streptomycin (all Gibco; Thermo Fisher Scientific, Inc.) and 10%
fetal bovine serum (FBS; Australian origin; Biological Industries
USA, Inc.) at 37°C in an atmosphere containing 95% air and 5%
CO2. In order to avoid the interference of Ig in FBS,
the medium was replaced with serum-free medium 24–48 h prior to
cell harvest. HK-2 cells were treated with different concentrations
(2, 5 and 10 ng/ml) of TGF-β1 (Sigma-Aldrich; Merck KGaA).
Single PTEC isolation and cDNA
synthesis
A human kidney sample of macroscopically normal
cortical tissue was obtained from a patient (male, 31 years old)
undergoing nephrectomy as a result of renal carcinoma without
obvious renal dysfunction. A single-cell suspension was prepared by
digesting renal cortex with 1 mg/ml collagenase I (Sigma-Aldrich;
Merck KGaA) at 37°C for 20 min. PTECs were sorted using
phycoerythrin (PE)-conjugated anti-CD10 (cat. no. 312203) and
allophycocyanin (APC)-conjugated anti-CD13 (cat. no. 301705; both
BioLegend, Inc.) by fluorescence-activated cell sorting (BD
FACSAria II Special Order System) as previously described (20). The corresponding isotype control
antibodies (cat. nos. 400111 and 400119; both BioLegend, Inc.) were
used to exclude non-specific staining. Double positively labeled
living cells were isolated as PTECs. A single PTEC was manually
selected under an inverted light microscope using a capillary
pipette and was then transferred to a 0.2-ml thin-wall PCR tube
containing lysis buffer (21).
Single PTEC RNA extraction and cDNA synthesis were carried out
according to previously described methods (21). A total of five single PTECs were
used to detect Ig gene transcription and rearrangement.
PCR amplification
Total RNA was extracted from HK-2 cells, peripheral
blood mononuclear cells [PBMCs, isolated from a 31-year-old female
healthy donor using Ficoll (cat. no. 7111011; Dakewe Biotech.,
Ltd.)] and kidney cortex (from the same patient used in single PTEC
isolation) using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and the RNA concentration was assessed
using a NanoDrop spectrophotometer (NanoDrop; Thermo Fisher
Scientific, Inc.). Subsequently, 2 µg total RNA was
reverse-transcribed to cDNA using the RevertAid First Strand cDNA
Synthesis kit (cat. no. K1622; Thermo Fisher Scientific, Inc.). PCR
was performed using primers targeting the constant regions of Igγ,
Igκ, Igλ and activation-induced cytidine deaminase (AID). Nested
PCR was performed to amplify the variable region of Igγ,
low-density lipoprotein receptor-related protein 2 (LRP2) and
recombination activating gene (RAG)1 and RAG2. The PCR products
were separated by electrophoresis on a 1.0% agarose gel and was
visualized using GelRed (cat. no. 41003; Biotium, Inc.). The
primers of AID, RAG1/2 and the constant regions of Igγ, Igκ, Igλ
used in this study refer to primers used by Jing et al
(19). The primers of Igγ variable
region refer to primers used by van Dongen et al (22). The other primers used for PCR are
listed in Table SI. The
thermocycling conditions are listed in Table SII.
Sanger sequencing and analyses of
sequencing data
PCR products of the Igγ variable region obtained
from single PTECs, HK-2 cells and PBMCs were respectively cloned
into a pGEM-T Easy Vector system I (cat. no. A1360; Promega
Corporation), which was transformed into TOP10 Competent cells
(CB104; Tiangen Biotech Co., Ltd.). Briefly, 5 µl ligation products
were added to 30 µl TOP10 competent cells, incubated on ice for 30
min, heat shocked at 42°C for 90 sec and incubated on ice for 5
min. Then, 500 µl LB was added and left to stand at 37°C for 40 min
before inoculating part of the bacterial liquid on Petri dishes
coated with 0.1 mmol/l IPTG and 20 µg/ml X-Gal. Dishes were
inverted at 37°C overnight. In all, 5–16 white colonies per sample
were chosen randomly, and sequenced using an ABI 3730XL Genetic
Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
rearranged V(D)J sequences were compared with those in the basic
local alignment search tool (https://www.ncbi.nlm.nih.gov/igblast/) to identify the
best matching germline gene segments and junctions following primer
trimming.
Western blot analysis
HK-2 cells were lysed in TSD lysis buffer [1% SDS,
50 mM Tris-HCl (pH 7.5), 50 mM DTT] containing a protease inhibitor
cocktail (Applygen Technologies Inc.), sonicated at ice-water for 1
min (working 5 sec and resting 15 sec; 3 times) and lysed for 30
min at room temperature. Following centrifugation at 12,000 × g for
10 min at 4°C, the protein concentration of the cell lysate was
determined using a BCA kit (Applygen Technologies Inc.).
Subsequently, 5X reducing loading buffer was added to the lysate,
boiled at 100°C for 10 min, and the samples were immediately used
for western blot analysis. Serum, used as a positive control for
Ig, was isolated from a healthy donor (the same donor as used in
PBMCs) by centrifugation at 2,103 × g for 10 min at room
temperature.
Western blotting was carried out according to
standard procedures. Briefly, 30 µg proteins were separated by
SDS-PAGE on 10% gels and were transferred onto a nitrocellulose
membrane. Subsequently, the membrane was blocked in 5% skimmed milk
at room temperature for 1 h and was incubated with primary
antibodies at 4°C overnight, including rabbit anti-human Igγ (cat.
no. ab109489; 1:1,000), rabbit anti-human Igγ4 (cat. no. ab109493;
1:1,000), anti-Igκ (cat. no. ab124727; 1:10,000), anti-Igλ (cat.
no. ab124719; 1:20,000), rabbit anti-human β-actin (cat. no.
ab8227; 1:2,000) (all from Abcam), and RP215 monoclonal antibody
(mAb) (donated by Professor Xiaoyan Qiu, Peking University,
Beijing, China; 1:1,000), which specifically identified a
carbohydrate-associated epitope on non-B-Igγ. The membrane was then
incubated with goat anti-rabbit (cat. no. 926-32211) or anti-mouse
(cat. no. 926-32210) IgG-IRDyeTM680CW secondary antibodies (both
1:10,000; both LI-COR Biosciences) at room temperature for 1 h. The
signal was detected using the Odyssey Imaging system and Odyssey
V3.0 software (both LI-COR Biosciences). ImageJ software (version
1.8.0; National Institutes of Health) was used for
semi-quantification.
IgG purification and mass
spectrometry
After HK-2 cells had been cultured in DMEM/F12
without FBS for 48 h, the culture supernatant was collected after
centrifugation at 2,103 × g for 10 min at 4°C. The cell supernatant
was purified by affinity chromatography using protein G Sepharose,
according to the manufacturer's instructions (cat. no. 17-0618-02;
Thermo Fisher Scientific, Inc.). The eluent was ultra-filtered to
replace the elution buffer (0.1 M Glycine; pH 2.4) with PBS. The
purified proteins were separated by SDS-PAGE on 10% gels, detected
by western blotting, and further analyzed by mass spectrometry,
performed by Beijing Protein Innovation Co., Ltd.
Immunofluorescence
HK-2 cells were cultured on cover-slips, which were
fixed in cold undiluted acetone for 5 min at room temperature.
Subsequently, the slides were washed twice in PBS and blocked with
5% FBS/PBS at room temperature for 20 min, after which they were
incubated with primary antibodies at 4°C overnight. The antibodies
were the same as those used in western blotting: Rabbit anti-human
Igγ (1:150), anti-human Igκ (1:250), anti-human Igλ (1:250) and
RP215 mAb (1:200); PBS was used as a negative control. After
washing in PBS, the slides were incubated with fluorescein
isothiocyanate-labeled goat anti-rabbit (cat. no. A11008) or goat
anti-mouse (cat. no. A11001) IgG antibodies (1:1,000; Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h. Nuclei
were stained with DAPI. Images were captured under a Leica DFC300
FX fluorescence microscope (Leica Microsystems GmbH).
Immunohistochemical staining
Paracancerous renal cortices were collected from
four male patients (age, 38–49 years) with renal carcinoma. Normal
paracancerous renal cortices following nephrectomy were fixed with
10% formalin for 48 h at room temperature, and then embedded in
paraffin. Paraffin-embedded human kidney samples were cut into 3-µm
sections, and deparaffinized and rehydrated through a series of
graded ethanol concentrations. Antigen retrieval was performed by
boiling in 0.05 M Tris-EDTA (pH 9.0) in a pressure cooker for 3
min. The sections were then incubated with 3%
H2O2 solution for 10 min at room temperature
to eliminate endogenous peroxidase and incubated with normal goat
serum (cat. no. ZLI-9022, ZSGB-BIO, China) for 30 min at room
temperature to block nonspecific antibody binding sites at room
temperature. Subsequently, indirect immunohistochemical staining
was performed with primary antibodies at 4°C overnight, including
RP215 mAb (1:200; 5 µg/ml), rabbit anti-human Igγ (1:2,000),
anti-human Igκ (1:1,000), anti-human Igλ (1:1,000) (the same
antibodies as used in western blotting). Sections without primary
antibodies were used as negative controls. The slides were then
incubated with undiluted horseradish peroxidase-labelled secondary
antibodies (cat. nos. PV-6001 and PV-6002; both OriGene
Technologies, Inc.) at room temperature for 30 min. Bound
antibodies were detected using diaminobenzidine. Finally, the
slides were counterstained with hematoxylin. Images were captured
using a light microscope (×200 magnification).
Statistical analysis
Data are presented as the means ± standard deviation
and were analyzed using SPSS 20.0 for Windows (IBM Corp.). All
experiments were repeated 3 times. The differences among multiple
groups were analyzed using one-way ANOVA and Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
IgG expression in renal tubular
epithelial cells of the kidney cortex
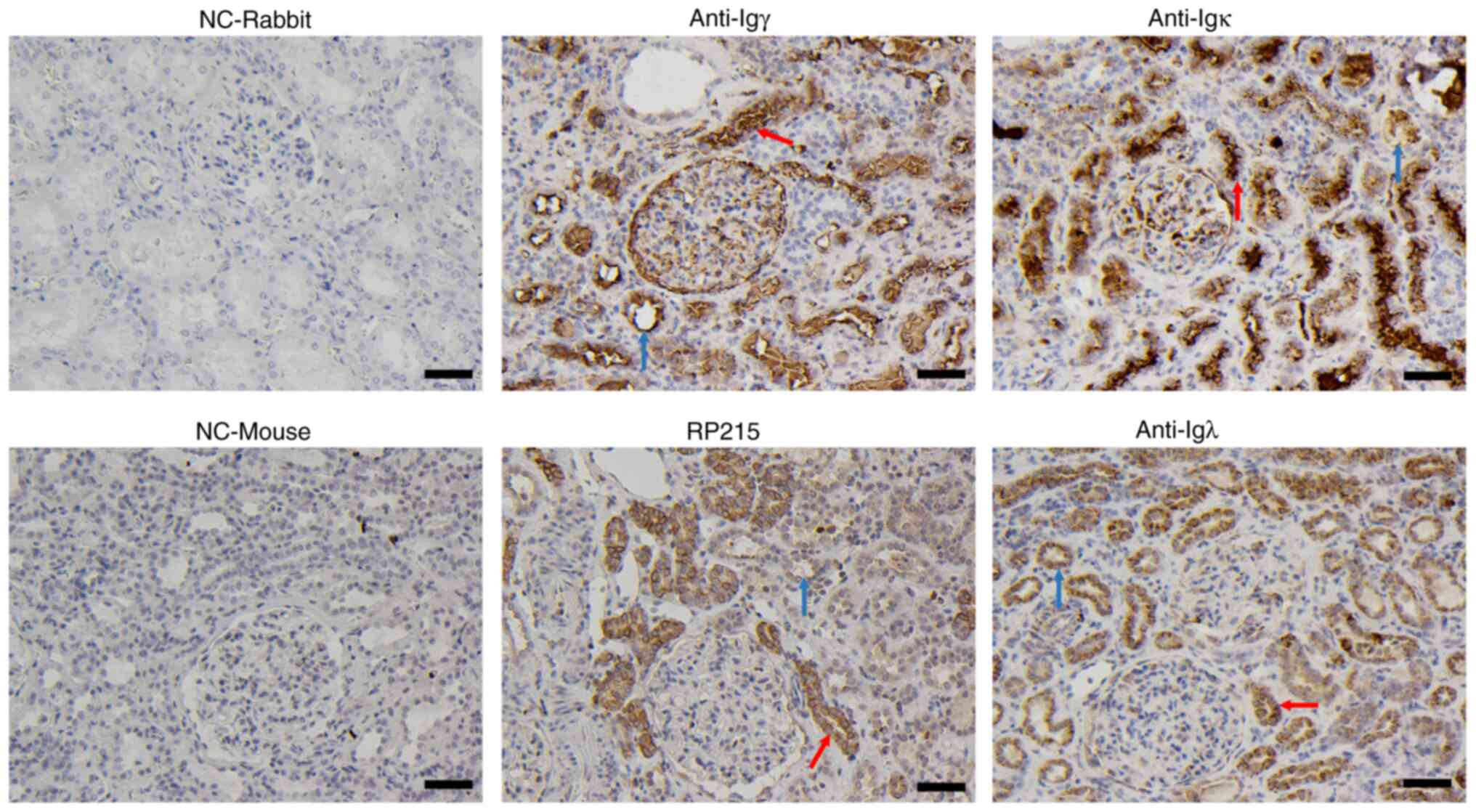
Normal renal cortexes, which were collected from
donors undergoing nephrectomy as a result of renal cell carcinoma,
were used to determine the IgG expression in renal tubular
epithelial cells by immunohistochemistry using antibodies against
human Igγ, Igκ, Igλ and RP215 (an antibody that predominantly binds
to non-B-IgG). Positive staining of IgG heavy and light chains was
not only detected in the PTECs but also in distal convoluted tubule
epithelial cells, either in the cytoplasm, cell membrane or tubular
lumen (Fig. 1).
Transcription and V(D)J recombination
of IgG in single PTECs
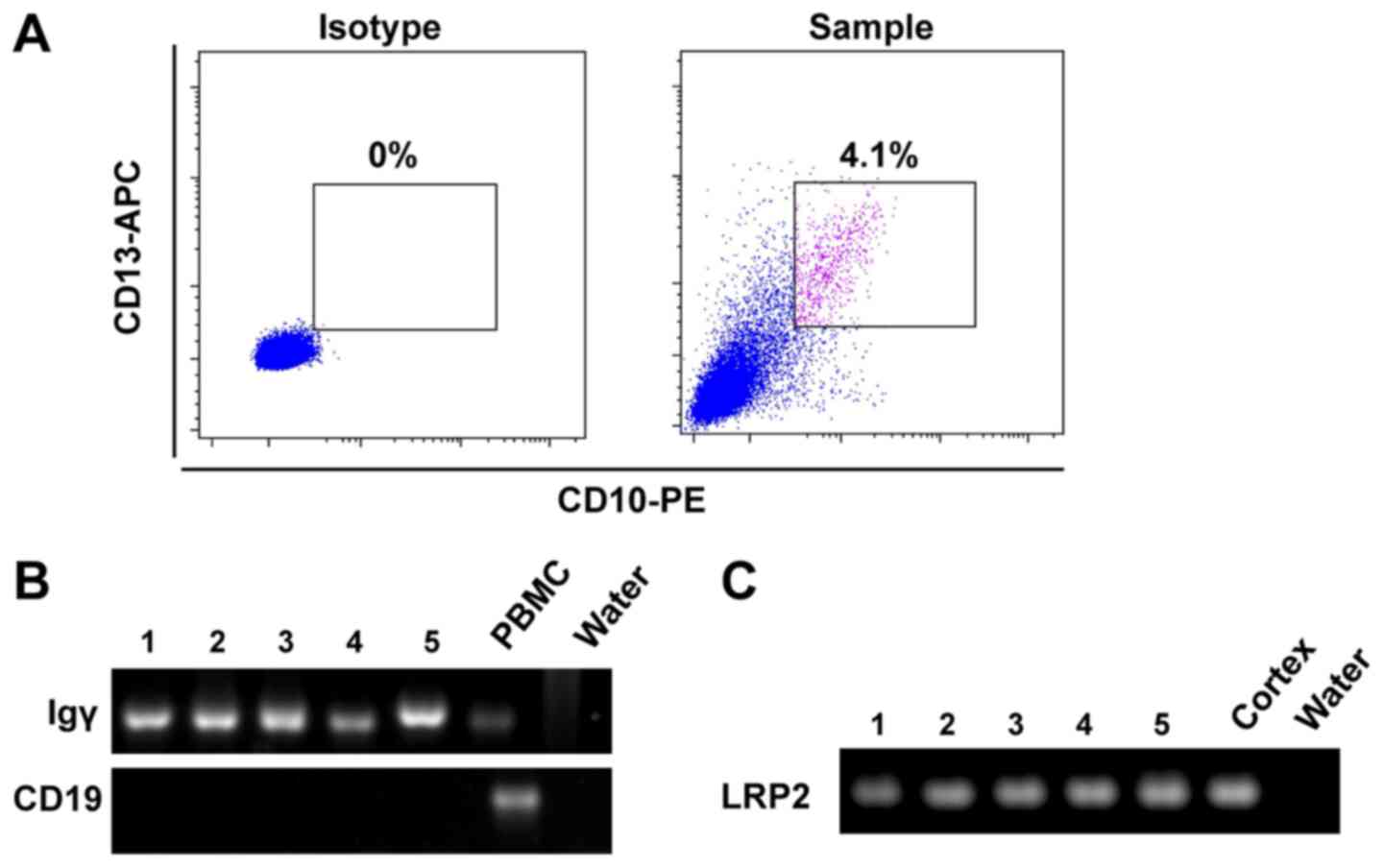
To avoid the interference of residual blood, binding
and transcytosis of IgG by PTECs in the kidney cortex, and to
obtain direct evidence of IgG expression in PTECs, single PTECs
were sorted from human kidney cortex using CD10 and CD13
co-labeling by flow cytometry (20). As shown in Fig. 2A, CD10/CD13 double-positive PTECs
accounted for 4.1% of viable cells in the sample. The isolated
PTECs were further confirmed using the specific marker gene LRP2,
and B-cell contamination was eliminated by CD19. Igγ variable
region transcripts were amplified in five single PTECs by nested
PCR (Fig. 2B and C). Sanger
sequencing results revealed that PTECs exhibited functional and
conservative VDJ recombination of IgG heavy chain (Table I), indicating that IgG expression
may occur in PTECs.
 | Table I.VHDJH
recombination patterns of IgG in single PTECs. |
Table I.
VHDJH
recombination patterns of IgG in single PTECs.
| Cell ID | Clones |
VHDJH usage | Productive | Identity with
germlines (%) |
|---|
| 1 | 6/6 |
IGHV1-46/IGHD5-12/IGHJ4 | Yes | 86.5–87.1 |
| 2 | 6/6 |
IGHV1-18/IGHD6-19/IGHJ4 | Yes | 91.5–92.7 |
| 3 | 6/6 |
IGHV1-2/IGHD1-1/IGHJ5 | Yes | 89.5–90.6 |
| 4 | 6/6 |
IGHV4-59/IGHD3-22/IGHJ3 | Yes | 91.1–91.7 |
| 5 | 6/6 |
IGHV1-46/IGHD5-12/IGHJ4 | Yes | 88.3–88.9 |
IgG heavy and light chain expression
in HK-2 cells
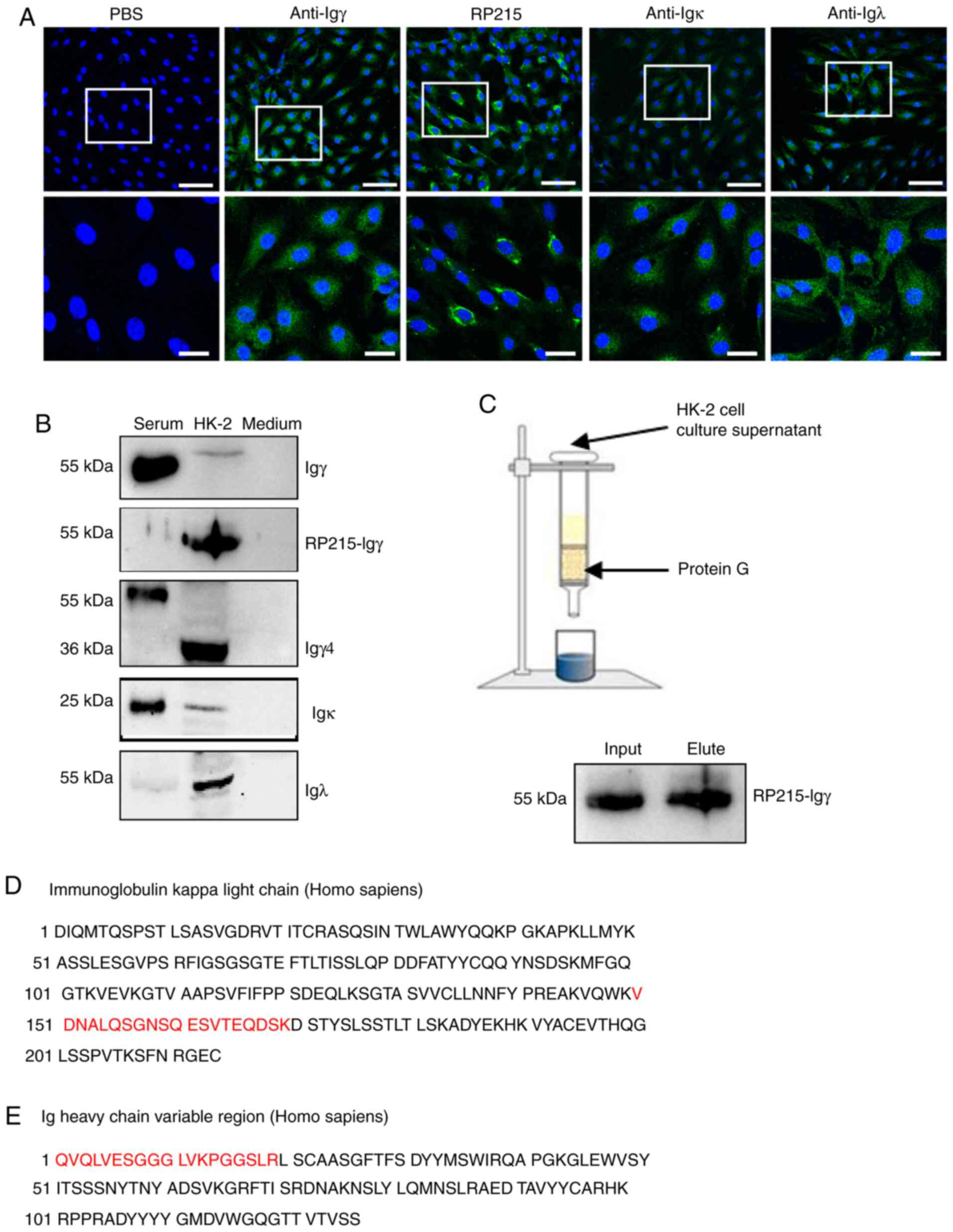
Since it was difficult to detect IgG protein
expression in a single PTEC and to obtain enough PTECs for western
blotting, the HK-2 cell line, which is comprised of immortalized
PTECs, was selected to further confirm IgG protein expression.
Immunofluorescence analysis demonstrated positive staining of Igγ,
Igκ and Igλ in the cytoplasm, and stronger positive staining of
RP215 predominantly in the cytoplasm and cell membrane (Fig. 3A).
Subsequently, the expression of IgG heavy and light
chains in HK-2 cells were detected by western blotting under
reducing conditions. To eliminate FBS interference in the culture
medium, medium containing FBS was blotted with corresponding
antibodies and stained negative. A commercial IgG antibody was able
to detect serum-derived IgG but not HK-2-derived IgG. By contrast,
RP215 could detect HK-2-derived IgG, but not serum IgG. Both
commercial IgG antibody and RP215 were able to detect Igγ at 55
kDa. An Igγ4 (36 kDa) band was detected in HK-2 cells, consistent
with the predicted molecular weight. Furthermore, Igκ (25 kDa) and
Igλ (50 kDa, dimer) expression was observed in the cell lysates
(Fig. 3B). Subsequently, IgG in the
cell supernatant was purified by protein G, confirmed by western
blotting and sequenced by mass spectrometry, which demonstrated
that the 55-kDa band contained fragments of the Igκ chain and Ig
heavy chain variable region, according to the National Center for
Biotechnology Information (NCBI) database (Fig. 3C-E). These data suggested that HK-2
cells produced and secreted IgG protein.
Transcription and V(D)J recombination
of IgG heavy and light chains in HK-2 cells
Ig gene transcription and functional V(D)J
recombination is a prerequisite for Ig expression. To confirm the
expression of IgG in HK-2 cells, Igγ, Igκ and Igλ transcripts were
assessed by amplifying the constant and variable regions in HK-2
cells (Fig. 4A and B). Sequencing
of the constant PCR products exhibited high homology with the
published sequence in the NCBI database. T-A cloning and Sanger
sequencing demonstrated that the Igγ in HK-2 cells displayed
conservative V(D)J recombination with VH4-4/D2-8/JH5. By contrast,
the diversity of V(D)J recombination was observed in PBMCs, which
eliminated primer bias (Table II).
Similar to B cells, HK-2-derived IgG displayed typical productive
V(D)J recombination with the V-D and D-J junctions (Fig. 4C). Moreover, the somatic
hypermutations were detected in HK-2-derived Igγ (range, 6.8–8.4%)
with 7 out of 15 hotspot motifs (RGYW/WRCY, W=A/T, R=A/G, Y=C/T)
presenting with mutations.
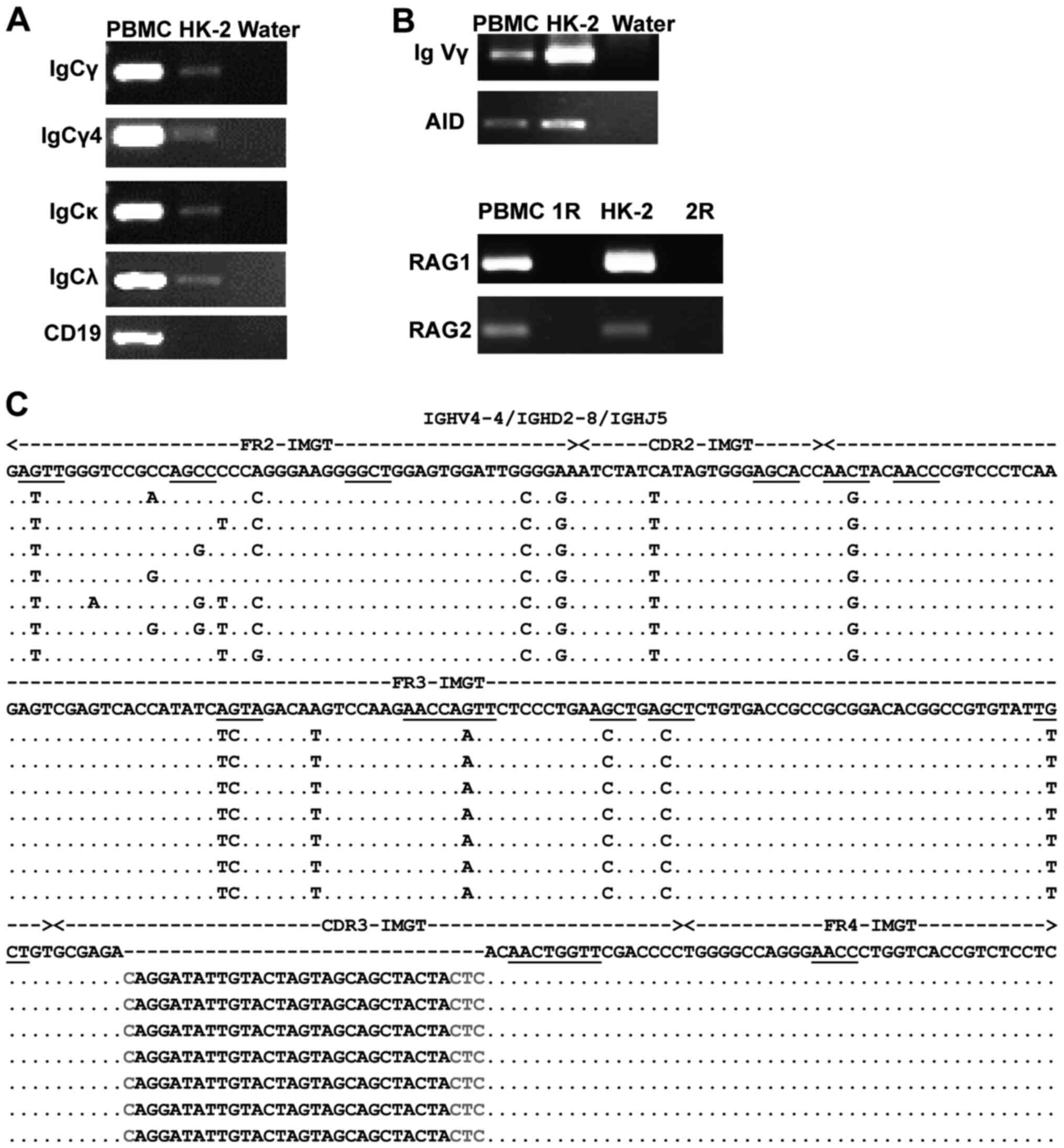 | Figure 4.Transcription and V(D)J recombination
of IgG heavy and light chains in HK-2 cells. (A) Transcription of
IgCγ, IgCγ4, IgCκ and IgCλ were amplified by RT-PCR. CD19 was used
to eliminate B-cell interference. (B) IgVγ and RAG1/RAG2 mRNA
expression was amplified by nested RT-PCR, whereas the AID mRNA was
amplified by RT-PCR. PBMCs acted as a positive control. Water acted
as a negative control. R acted as a negative control. (C) Variable
region sequences and mutations of IGHV4-4/IGHD2-8/IGHJ5. Identity
with the homologous germline sequence is indicated by dots. Each
nucleotide mutation is indicated. The mutation hotspots in germline
genes are underlined. The red letters refer to the junctions. CDR,
complementarity determining region; FR, framework region; R, cDNA
template replaced by DNase-treated RNA; RT-PCR, reverse
transcription-PCR; AID, activation-induced cytidine deaminase; RAG,
recombination activating genes; IgC, immunoglobulin constant
region; IgV, immunoglobulin variable region. |
 | Table II.VHDJH
recombination patterns of IgG in HK-2 cells and PBMCs. |
Table II.
VHDJH
recombination patterns of IgG in HK-2 cells and PBMCs.
| Name of cells | Clones |
VHDJH usage | Productive | Identity with
Germlines (%) |
|---|
| HK-2 | 16/16 |
IGHV4-4/IGHD2-8/IGHJ5 | Yes | 91.6–93.2 |
| PBMCs | 2/10 |
IGHV3-7/IGHD4-17/IGHJ4 | Yes | 92.6 |
|
| 1/10 |
IGHV3-7/IGHD3-10/IGHJ4 | Yes | 93.7 |
|
| 1/10 |
IGHV3-23/IGHD3-10/IGHJ3 | Yes | 95.2 |
|
| 2/10 |
IGHV3-30/IGHD3-10/IGHJ4 | Yes | 89.5–91.1 |
|
| 1/10 |
IGHV3-33/IGHD3-22/IGHJ1 | Yes | 85.9 |
|
| 1/10 |
IGHV4-4/IGHD6-19/IGHJ4 | Yes | 93.7 |
|
| 1/10 |
IGHV4-59/IGHD6-19/IGHJ5 | Yes | 98.9 |
|
| 1/10 |
IGHV5-51/IGHD3-22/IGHJ3 | Yes | 96.9 |
In addition, AID transcripts (essential element for
somatic hypermutation and class switch recombination in B cells),
and RAG1 and RAG2 [necessary for V(D)J recombination] were detected
in HK-2 cells (Fig. 4B), indicating
that the mechanism underlying Ig synthesis in HK-2 cells may be
similar to that in B cells.
TGF-β1 upregulates IgG expression in
HK-2 cells
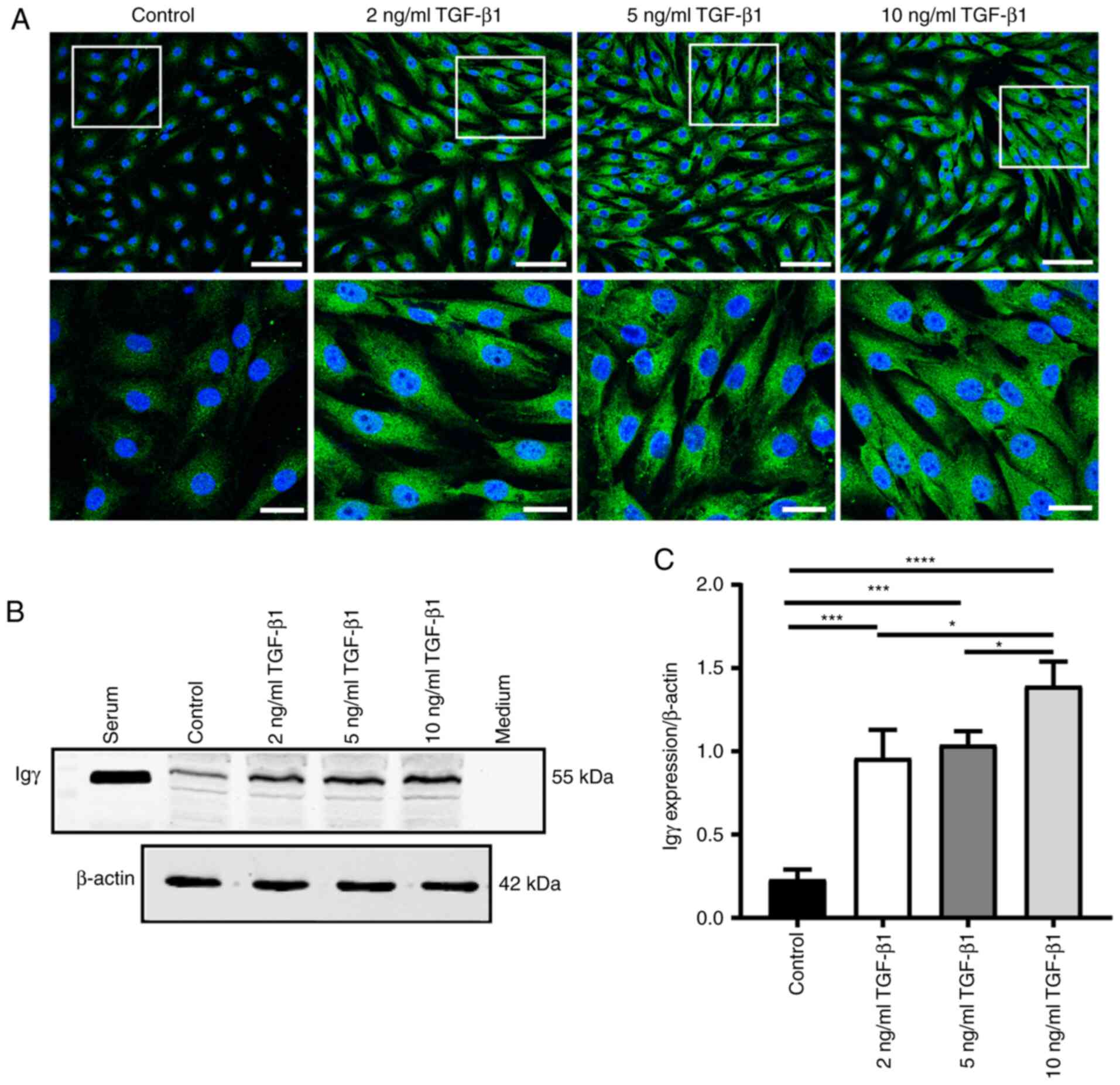
HK-2 cells were stimulated with various
concentrations of TGF-β1 for 48 h. Immunofluorescence staining
revealed that cytoplasmic IgG exhibited enhanced positive staining
compared with the control group (Fig.
5A). Western blotting confirmed that IgG was significantly
upregulated by TGF-β1 (P<0.05; Fig.
5B and C).
Discussion
The present study demonstrated that PTECs expressed
IgG with gene transcription and functional conservative V(D)J
recombination, which was similar to non-B-IgG. TGF-β1 upregulated
IgG expression in HK2 cells.
To investigate whether PTECs expressed IgG, IgG was
first detected in the cytoplasm, cell membrane and lumen of PTECs
in the normal human renal cortex by immunohistochemistry; the
results indicated that PTECs produced and secreted IgG. This is in
contrast to our routine pathological examination, where no apparent
IgG was detected in PTECs. This could be due to very weak PTEC
staining, which was too low to be detected, particularly in
immune-related glomerular diseases in which Igs are strongly
positive in the glomeruli and PTEC staining may be unintentionally
but artificially lost. IgG transcytosis from the circulation by
PTECs via the Fc receptor was partially ruled out, as clear
staining of IgG by RP215 was observed in the cell membrane and
cytoplasm. These findings suggested that PTEC-derived IgG was
similar to other non-B-IgG and may have unique glycosylated
epitopes, which can be specifically recognized by RP215 instead of
a commercial anti-IgG antibody (15). The finding that only part of tubular
epithelial cells express IgG may be explained by dynamic expression
at different cell cycles, which was similar to the expression
pattern of mesangial cell-derived IgA (18). Co-staining of IgG with tubular
markers (such as aquaporin 1 and aquaporin 3) would be useful for
further studies to enhance the findings of the present study.
Single cell RNA sequencing can clearly display the
transcription of a specific gene in a given cell. Transcripts of
the Igγ chain and V(D)J recombination were detected in single
PTECs. Although only five single PTECs were used, the process was
rigorous as the kidney cortex was collected far from the tumor, and
each single cell was sorted by flow cytometry with two
PTEC-specific marker genes, reconfirmed using a third specific
marker gene (LRP2) and B-cell contamination was eliminated. The
results revealed that PTECs do not only present with IgG gene
transcripts but also the classical V(D)J recombination in the
variable region as B cells, such as the productive V(D)J
recombination with the V-D and D-J junctions (Fig. 4), indicating that PTECs have the
potential to produce IgG. In addition, the more conservative V(D)J
recombination illustrated that PTECs present with IgG gene
transcripts and V(D)J recombination that are similar to other non-B
cells.
HK-2, an immortalized PTEC line, is easy to culture
in large quantities. The present study confirmed IgG protein
expression in cultured HK-2 cells; IgG was detected in HK-2 cells
by both immunofluorescence and western blotting. Igγ4, a subclass
of Igγ, was also detected at a band size consistent with the
predicted molecular weight. Mass spectrometry revealed that the
protein purified from cell supernatant using protein G contained
fragments of the Ig heavy chain variable region and Igκ chain,
providing evidence for IgG secretion by PTECs. IgG transcription in
HK-2 cells further supported IgG expression in PTECs, and the
conservative V(D)J recombination with IGHV4-4/IGHD2-8/IGHJ5 in HK-2
cells further supported the presence of IgG in HK-2 cells similar
to other non-B cells.
The present study also investigated the underlying
mechanism of IgG production in PTECs by examining the transcription
of RAG1, RAG2 and AID in HK-2 cells. It was revealed that RAG1,
RAG2 and AID were transcribed in HK-2 cells. Additionally, RAG1,
RAG2 or AID transcripts have previously been detected in numerous
other non-B cells, such as podocytes (19) and several cancer cell lines
(23). These results suggested that
non-B cells, including PTECs, may have similar mechanisms of Ig
synthesis to B cells. However, whether AID and/or RAG1/2 are
necessary genes for PTEC-derived IgG requires further
investigation. In addition, follicular helper CD4 T cells are
important to regulate germinal center B-cell differentiation into
plasma cells and support the production of Igs (24). Our unpublished data revealed that
non-B-Igs were still detected in T and B cell-deficient NOD-SCID
mice, indicating that non-B-Igs were not entirely dependent on T
helper cells. Whether T helper cells are required for PTECs to
express IgG requires further research.
TGF-β1 has a key immunomodulatory role in Ig
production. For example, TGF-β1 induced IgA class switching and
secretion in stimulated B cells in mouse spleen (25) and human tonsil B cells (26). McIntyre et al (27) demonstrated that TGF-β1 selectively
stimulated IgG2b secretion by lipopolysaccharide-activated B cells
most likely by inducing an IgM to IgG2b class switch. Duan et
al (28) demonstrated that
TGF-β1 increased IgA expression by upregulating the transcription
factor Ets-1 in epithelial cancer cells. The present study
demonstrated that TGF-β1 upregulated IgG expression in HK-2 cells.
Given that only IgG was detected in HK-2 cells, it was hypothesized
that TGF-β1 induced IgG expression independent of Ig class
switching. The regulatory mechanism of IgG production by TGF-β1
requires further investigation.
PTECs serve an important role in tubular
interstitial fibrosis through EMT and TGF-β1 acts as a master
profibrotic mediator. In the present study, the addition of TGF-β1
to cultured HK-2 cells increased IgG expression, indicating that
HK-2-derived IgG may be positively associated with EMT. Previous
studies have reported that cancer-IgG was associated with
metastasis and promoted EMT by decreasing E-cadherin in salivary
adenoid cystic carcinoma (29) and
lung cancer (30). It is worth
investigating whether PTEC-derived IgG may serve a role in renal
tubular EMT and interstitial fibrosis under disease conditions,
such as ischemia/reperfusion injury or chronic kidney disease.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that PTECs can express
and secrete IgG, and TGF-β1 can upregulate IgG expression in HK-2
cells. However, the potential role of PTEC-derived IgG in
tubulointerstitial fibrosis requires further investigation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 82070736, 91642109
and 81870488), the Hainan Natural Science Foundation (grant no.
819QN355), the Hainan Medical University Scientific Research
Cultivation Foundation (grant no. HYPY201926) and the Key Support
Projects of the National Natural Science Foundation's Major
Research Program (grant no. 91642206).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
As the corresponding authors, YW and XQ conceived
and designed the study. ZD participated in the research design,
performed histological and single-cell experiments, prepared
samples for mass spectrometry, analyzed data and wrote the
manuscript. ZJ participated in the research design, most
experiments regarding IgG expression in HK-2 cells and relevant
data analysis. YG, JM, HD and YL performed partial cell line
experiments. ZC and YP selected appropriate cases for single-cell
experiments according to clinical characteristics. HY and ZS
participated in single-cell experiments. SW participated in
immunohistochemical staining, analyzed tissue staining data and
drafted and revised the manuscript. YW, ZD and ZJ confirmed the
authenticity of all the raw data. All authors reviewed the
manuscript and revised data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study conformed to the principles of the
Declaration of Helsinki, and was approved by the Medical Ethics
Committee of Peking University Third Hospital (approval no.
S2020121) and conducted in accordance with the protocol. All donors
voluntarily donated kidney cortexes and provided written informed
consent prior to donating the kidney cortex to the study. All
methods were carried out in accordance with relevant guidelines and
regulations. These samples were strictly anonymized. Human serum
and PBMCs, used as positive controls in western blotting or reverse
transcription-PCR in the present study, were obtained from the
blood of a healthy volunteer, who provided written informed consent
for sampling.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schlondorff DO: Overview of factors
contributing to the pathophysiology of progressive renal disease.
Kidney Int. 74:860–866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donadio ME, Loiacono E, Peruzzi L, Amore
A, Camilla R, Chiale F, Vergano L, Boido A, Conrieri M, Bianciotto
M, et al: Toll-like receptors, immunoproteasome and regulatory T
cells in children with Henoch-Schonlein purpura and primary IgA
nephropathy. Pediatr Nephrol. 29:1545–1551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Breda PC, Wiech T, Meyer-Schwesinger C,
Grahammer F, Huber T, Panzer U, Tiegs G and Neumann K: Renal
proximal tubular epithelial cells exert immunomodulatory function
by driving inflammatory CD4+ T cell responses. Am J
Physiol Renal Physiol. 317:F77–F89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu BC, Tang TT, Lv LL and Lan HY: Renal
tubule injury: A driving force toward chronic kidney disease.
Kidney Int. 93:568–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi N, Suzuki Y, Tsuge T, Okumura K,
Ra C and Tomino Y: FcRn-mediated transcytosis of immunoglobulin G
in human renal proximal tubular epithelial cells. Am J Physiol
Renal Physiol. 282:F358–F365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao
P, Li G, Lv P, Li Z, Sun X, et al: Human epithelial cancers secrete
immunoglobulin g with unidentified specificity to promote growth
and survival of tumor cells. Cancer Res. 63:6488–6495.
2003.PubMed/NCBI
|
|
7
|
Zheng J, Huang J, Mao Y, Liu S, Sun X, Zhu
X, Ma T, Zhang L, Ji J, Zhang Y, et al: Immunoglobulin gene
transcripts have distinct VHDJH recombination characteristics in
human epithelial cancer cells. J Biol Chem. 284:13610–13619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang D, Ge J, Liao Q, Ma J, Liu Y, Huang
J, Wang C, Xu W, Zheng J, Shao W, et al: IgG and IgA with potential
microbial-binding activity are expressed by normal human skin
epidermal cells. Int J Mol Sci. 16:2574–2590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Z, Zhang M, Shao W, Wang P, Gong X, Ma
J, Qiu X and Wang B: Immunoglobulin M, a novel molecule of
myocardial cells of mice. Int J Biochem Cell Biol. 88:172–180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu N, Zhang J, Sun Y, Wang S, Sun Y,
Korteweg C, Gao W and Gu J: Expression and distribution of
immunoglobulin G and its receptors in an immune privileged site:
The eye. Cell Mol Life Sci. 68:2481–2492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J, Sun X, Mao Y, Zhu X, Zhang P,
Zhang L, Du J and Qiu XY: Expression of immunoglobulin gene with
classical V-(D)-J rearrangement in mouse brain neurons. Int J
Biochem Cell Biol. 40:1604–1615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu N, Zhang J, Guo Y, Zhao Y, Korteweg C
and Gu J: Expression and distribution of immunoglobulin G and its
receptors in the human nervous system. Int J Biochem Cell Biol.
43:556–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Korteweg C, Qiu Y, Luo J, Chen Z,
Huang G, Li W and Gu J: Two ultrastructural distribution patterns
of immunoglobulin G in human placenta and functional implications.
Biol Reprod. 91:1282014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Zhang L, Ma T, Zhang P and Qiu X:
Expression of immunoglobulin gene with classical V-(D)-J
rearrangement in mouse testis and epididymis. J Histochem Cytochem.
57:339–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang J, Zhang J, Liu Y, Liao Q, Huang J,
Geng Z, Xu W, Sheng Z, Lee G, Zhang Y, et al: Lung squamous cell
carcinoma cells express non-canonically glycosylated IgG that
activates integrin-FAK signaling. Cancer Lett. 430:148–159. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui M, You L, Zheng B, Huang X, Liu QF,
Huang J, Pan B, Qiu X, Liao Q and Zhao Y: High expression of
cancer-derived glycosylated immunoglobulin G predicts poor
prognosis in pancreatic ductal adenocarcinoma. J Cancer.
11:2213–2221. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui M, Hu Y, Zheng B, Zhang S, Zhang X,
Wang M, Qiu XY, Liao Q and Zhao YP: Cancer-derived immunoglobulin
G: A novel marker for differential diagnosis and relapse prediction
in parathyroid carcinoma. Clin Endocrinol (Oxf). 92:461–467. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng H, Ma J, Jing Z, Deng Z, Liang Y, A
L, Liu Y, Qiu X and Wang Y: Expression of immunoglobulin A in human
mesangial cells and its effects on cell apoptosis and adhesion. Mol
Med Rep. 17:5272–5282. 2018.PubMed/NCBI
|
|
19
|
Jing Z, Deng H, Ma J, Guo Y, Liang Y, Wu
R, A L, Geng Z, Qiu X and Wang Y: Expression of immunoglobulin G in
human podocytes, and its role in cell viability and adhesion. Int J
Mol Med. 41:3296–3306. 2018.PubMed/NCBI
|
|
20
|
Van der Hauwaert C, Savary G, Gnemmi V,
Glowacki F, Pottier N, Bouillez A, Maboudou P, Zini L, Leroy X,
Cauffiez C, et al: Isolation and characterization of a primary
proximal tubular epithelial cell model from human kidney by
CD10/CD13 double labeling. PLoS One. 8:e667502013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang F, Barbacioru C, Nordman E, Li B, Xu
N, Bashkirov VI, Lao K and Surani MA: RNA-Seq analysis to capture
the transcriptome landscape of a single cell. Nat Protoc.
5:516–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Dongen JJ, Langerak AW, Bruggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z and Gu J: Immunoglobulin G
expression in carcinomas and cancer cell lines. FASEB J.
21:2931–2938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coffman RL, Lebman DA and Shrader B:
Transforming growth factor beta specifically enhances IgA
production by lipopolysaccharide-stimulated murine B lymphocytes. J
Exp Med 170: 1039, 1989. J Immunol. 182:8–13. 2009.PubMed/NCBI
|
|
26
|
van Vlasselaer P, Punnonen J and de Vries
JE: Transforming growth factor-beta directs IgA switching in human
B cells. J Immunol. 148:2062–2067. 1992.PubMed/NCBI
|
|
27
|
McIntyre TM, Klinman DR, Rothman P, Lugo
M, Dasch JR, Mond JJ and Snapper CM: Transforming growth factor
beta 1 selectively stimulates immunoglobulin G2b secretion by
lipopolysaccharide-activated murine B cells. J Exp Med.
177:1031–1037. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duan Z, Deng H, Xu S, Jiang Y, Liu H, Li
M, Hu D, Li W, Bode AM, Dong Z and Cao Y: Activation of the Ig Iα1
promoter by the transcription factor Ets-1 triggers Ig Iα1-Cα1
germline transcription in epithelial cancer cells. Cell Mol
Immunol. 11:197–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng J, Wang HC, Liu Y, Jiang JH, Lv WQ,
Yang Y, Li CY and Qiu XY: Involvement of non-B cell-derived
immunoglobulin G in the metastasis and prognosis of salivary
adenoid cystic carcinoma. Oncol Lett. 14:4491–4498. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang C, Huang T, Wang Y, Huang G, Wan X
and Gu J: Immunoglobulin G expression in lung cancer and its
effects on metastasis. PLoS One. 9:e973592014. View Article : Google Scholar : PubMed/NCBI
|